Introduction
Despite the decline in the incidence of gastric
cancer, it remains one of the most common malignant tumors
worldwide and is one of the leading causes of cancer-associated
mortality worldwide (1,2). This is a result of malignant
behaviors, including rapid progression, and ease of metastasis and
recurrence, and the poor prognosis of patients with gastric cancer
(3). In previous years, due to
improvements in early diagnosis and the development of combined
therapy, the mortality rates of patients with gastric cancer have
declined to certain degree. However, for a substantial proportion
of patients with progressive gastric cancer, the prognosis remains
poor (4). Therefore, there is an
urgent requirement to identify more effective targets to improve
therapy outcomes.
Progranulin (PGRN) is a secretory protein. Previous
studies have suggested that it is mainly expressed in specific
neuron cells (5), immune cells
(6), chondrocytes (7) and epithelial cells (8), mediating the prevention of
neurodegeneration, wound healing and cartilage development
(5–9). High expression levels of PGRN, and
its correlation with tumor progression and poor prognosis, have
been reported in different types of tumor, including breast cancer
(10,11), ovarian cancer (12,13)
and liver cancer (14). However,
the role of PGRN in gastric cancer remains to be elucidated.
In the present study, the expression levels of PGRN
in gastric cancer tissues were detected using immunohistochemistry
(IHC), and PGRN was confirmed to correlate with lymph node
metastasis, lymphatic invasion, advanced clinical stage and poor
prognosis for the first time, to the best of our knowledge. In
addition, investigation of the molecular mechanism showed that the
level of PGRN in gastric cancer cells was regulated by
extracellular PGRN via the AKT and extracellular signal-regulated
kinase (ERK) signaling pathways in a positive feedback loop.
Materials and methods
Cell lines and cell culture
The MKN-45 and MGC-803 human gastric cancer cell
lines were purchased from American Type Culture Collection
(Manassas, VA, USA). These cell lines were cultured in RMPI-1640
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.). All cells were cultured in 5% CO2 at
37°C.
To determine the underlying molecular mechanism of
PGRN regulation, an AKT inhibitor (LY294002) and ERK inhibitor
(U0126) were used. After resuspending in dimethyl sulfoxide,
LY294002 or U0126 was added to medium at a final concentration of
50 µmol/l or 10 µmol/l, respectively. Then, recombinant PGRN
(rPGRN) was added and cells were cultured for 2 days.
IHC
Following approval from the review board and ethics
committee, 120 retrospective primary gastric cancer tissue samples
were collected from patients who accepted surgical treatment
between 2007 and 2010 in Jinan Central Hospital Affiliated to
Shandong University (Jinan, China). None of the patients received
chemotherapy or radiotherapy prior to surgery. Follow-up was
continued until 31st December 2015.
The tissue samples were cut into sections (3-µm
thick) and incubated with primary antibodies against PGRN (1:400;
cat no. ALX-804-737-C100, Enzo life science, Farmingdale, NY, USA)
at 4°C overnight. Normal rabbit IgG, in place of primary
antibodies, was used as a negative control. The sections were then
incubated with HRP-conjugated goat anti-rabbit IgG polymer (cat no.
9902, Fuzhou Maixin Biotech Co., Ltd., Fuzhou, China) at room
temperature for 30 min and stained with 3,3′-diaminobenzidin. The
cell nuclei were stained using hematoxylin. The expression scores
were evaluated by two independent pathologists simultaneously under
a microscope (magnification, ×400). The fraction of
positively-stained tumor cells was evaluated using proportion
scores (0, none; 1, <25%; 2, 26–75%; 3, >75%). The intensity
score represented the average staining intensity (0, none; 1, weak;
2, intermediate; 3, strong). The expression of PGRN was evaluated
by combining the proportion score and intensity score. Scores ≥4
were considered as high expression and those <4 was considered
as low expression.
Reverse transcription polymerase chain
reaction (RT-PCR) analysis
Total RNA from the gastric cancer cells was
extracted using TRIzol (Invitrogen; Thermo Fisher Scientific,
Inc.). The PrimeScript RT-PCR kit (Takara Biotechnology Co., Ltd.,
Dalian, China) was used to synthesize cDNAs. The primers were as
follows: PGRN, forward 5′-ATCTTTACCGTCTCAGGGACTT-3′ and reverse
5′-CCATCGACCATAACACAGCAC-3′; GAP DH, forward
5′-AGAAGGCTGGGGCTCATTTG-3′ and reverse 5′-AGGGGCCATCCACAGTCTTC-3′.
The amplification mixture was as follows: 1.4 µl cDNA, 1.6 µl
forward primer, 1.6 µl reverse primer, 5.4 µl double distilled
dH2O and 10 µl buffer was incubated at 94°C for 5 min,
followed by 30 cycles at 94°C for 30 sec, 53°C for 30 sec, and 72°C
for 30 sec, at last in 72°C for 5 min. PCR products were
electrophoretically separated on 1.0% agarose gels. The results
were analyzed by Labwork software version 4.0 (UVP, Inc., Upland,
CA, USA) (15).
Western blot analysis
Cells were washed twice in PBS and lysed in
radioimmunoprecipitation assay buffer (cat no. P0013B, Beyotime
Institute of Biotechnology, Haimen, China) containing 1% protease
inhibitor. Protein concentration was measured by spectrophotometry
(ND-1000; NanoDrop Technologies; Thermo Fisher Scientific, Inc.). A
total of 200 µg protein was loaded per well in 5% acrylamide and
separated by 10% separating gel and transferred onto a PVDF
membrane (EMD Millipore, Billerica, MA, USA). The membrane was then
incubated with the following primary antibodies at 4°C overnight:
PGRN (cat no. ALX-804-737-C100, Enzo life science); phosphorylated
(p-)ERK (cat no. 2219-1, Epitomics, Burlingame, CA, USA); ERK (cat
no. 12950, Cell Signaling Technology, Inc., Danvers, MA, USA);
p-AKT (cat no. 2938, Cell Signaling Technology, Inc.); AKT (cat no.
4685, Cell Signaling Technology, Inc.); GAPDH (cat no. 10494-1-AP,
Proteintech Group, Inc., Wuhan, China). This was followed by
incubation with HRP-conjugated goat anti-rabbit-IgG (cat no.
SA00001-2, 1:4,000, Proteintech Group, Inc.) at room temperature
for 1 h. Signals on the membrane were visualized using
chemiluminescence reagents (EMD Millipore).
Statistical analysis
All data are expressed as the mean ± standard
deviation. SPSS 11.0 software, (SPSS, Inc., Chicago, IL, USA) was
used for analysis. The correlation of PGRN with clinical parameters
was analyzed using the χ2 test. Survival curves were
drawn using the Kaplan-Meier method, and means were compared using
the log-rank test. Cox regression analysis was performed to confirm
potential prognostic factors of gastric cancer. The differences
between two groups were analyzed using Student's two-tailed t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
High expression of PGRN is positively
correlated with lymph node metastasis, lymphatic invasion and
advanced clinical stage
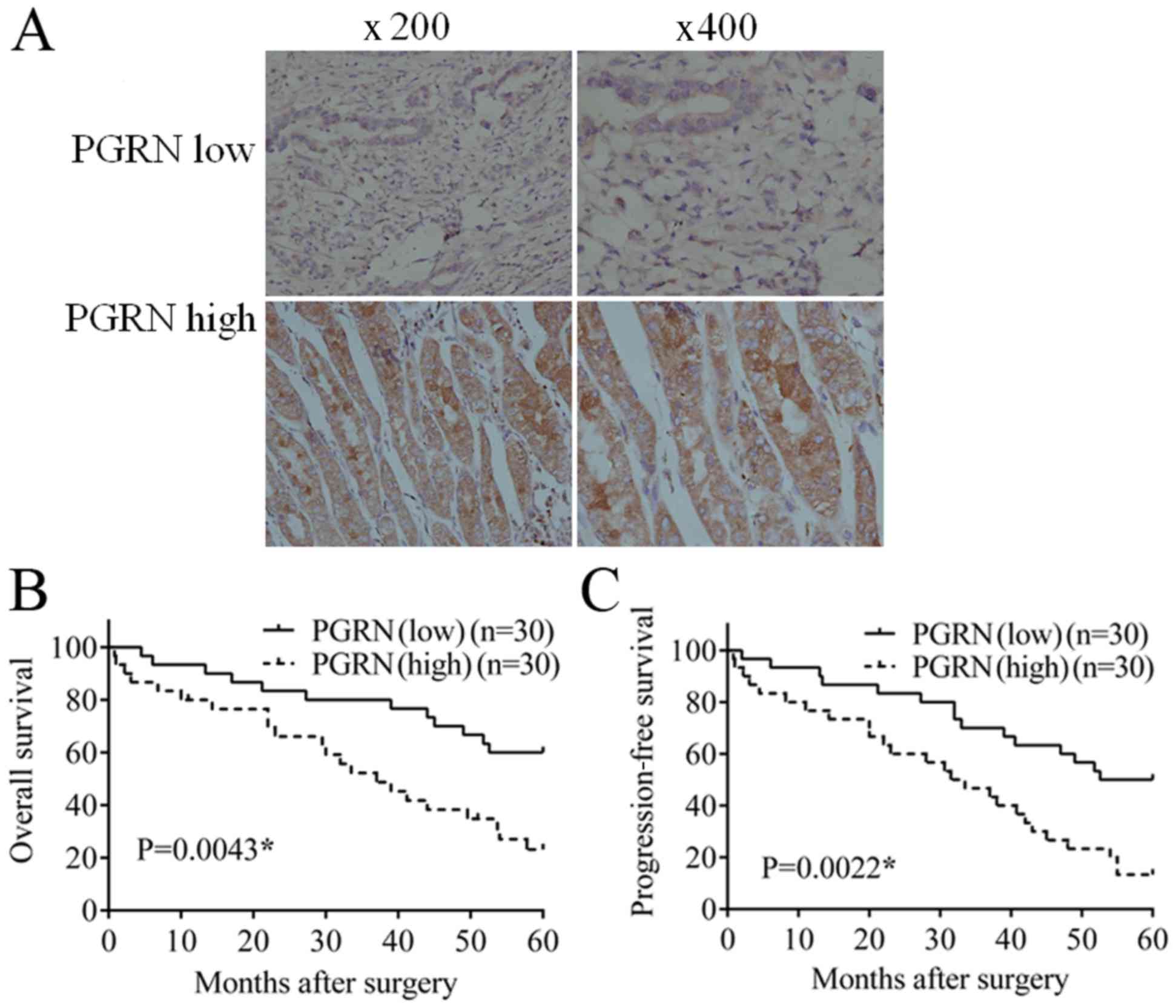
As shown in Fig.
1A, PGRN was mainly expressed in the cytoplasm of tumor cells,
with sporadic weak staining also present in the tumor mesenchyme.
The cases were divided into two groups according to different
expression levels of parenchymal PGRN; there were 49 cases with low
expression of PGRN, which was markedly lower than the 71 cases with
high expression of PGRN.
As shown in Table
I, in the PGRN low expression group, only 20 of the 49 cases
exhibited lymph node metastasis, which was significantly lower than
the high expression group, in which 42 of the 71 cases exhibited
lymph node metastasis. In addition, 28 of the 49 cases exhibited
lymphatic invasion, which was significantly lower than the high
expression group, in which 55 of the 71 cases exhibited lymphatic
invasion. A total of 24 of the 49 cases were at clinical stage III,
which was significantly lower than the 49 of 71 cases in the group
expressing a high level of PGRN. The results of the χ2
test showed that PGRN was positively correlated with lymph node
metastasis (P=0.048), lymphatic invasion (P=0.018) and clinical
stage (P=0.027).
 | Table I.Correlation between PGRN and clinical
parameters of patients with gastric cancer. |
Table I.
Correlation between PGRN and clinical
parameters of patients with gastric cancer.
|
|
| PGRN |
|
|
|---|
|
|
|
|
|
|
|---|
| Parameter | Cases (n) | Low (n) | High (n) |
χ2-value | P-value |
|---|
| Age (years) |
|
|
|
|
|
| ≥60 | 58 | 28 | 30 | 2.574 | 0.109 |
|
<60 | 62 | 21 | 41 |
|
|
| Sex |
|
|
|
|
|
| Male | 86 | 36 | 50 | 0.133 | 0.716 |
|
Female | 34 | 13 | 21 |
|
|
| Differentiation |
|
|
|
|
|
|
High/moderate | 73 | 29 | 44 | 0.095 | 0.758 |
| Poor | 47 | 20 | 27 |
|
|
| Invasion depth |
|
|
|
|
|
|
T1/T2 | 34 | 18 | 16 | 2.879 | 0.090 |
|
T3/T4 | 86 | 31 | 55 |
|
|
| Lymph node
metastasis |
|
|
|
|
|
| + | 62 | 20 | 42 | 3.904 | 0.048a |
| − | 58 | 29 | 29 |
|
|
| Lymphatic
invasion |
|
|
|
|
|
| + | 83 | 28 | 55 | 5.614 | 0.018a |
| − | 37 | 21 | 16 |
|
|
| Vascular
invasion |
|
|
|
|
|
| + | 56 | 28 | 28 | 3.652 | 0.056 |
| − | 64 | 21 | 43 |
|
|
| Clinical stage |
|
|
|
|
|
|
I–II | 47 | 25 | 22 | 4.884 | 0.027a |
|
III | 73 | 24 | 49 |
|
|
PGRN is positively correlated with
poor prognosis
In 30 follow-up cases with low expression of PGRN,
12 cases succumbed to mortality and the overall survival (OS) rate
was 60%; 15 cases showed disease progression, and the
progression-free survival (PFS) rate was 50%. In 30 follow-up cases
with high expression of PGRN, 22 cases succumbed to mortality and
the OS rate was 26.7%, which was substantially lower, compared with
that in the low expression group. There were 26 cases of disease
progression and the PFS rate was 13.3%, which was substantially
lower, compared with that in the low expression group. Statistical
analysis confirmed that the OS (Fig.
1B; P=0.0043) and PFS (Fig.
1C; P=0.0022) in the low PGRN group were significantly higher
than the rates in the high PGRN group.
Regression analysis for potential
prognostic factors
To confirm the potential prognostic factors for
gastric cancer in the present study, Cox regression analysis was
performed. The univariate Cox regression analysis showed that the
expression of PGRN (P=0.002) and clinical stage (P<0.001) had a
significant effect on the OS rate of the patients with gastric
cancer (Table II). The PGRN
(P=0.003) and clinical stage (P=0.004) also had a significant
effect on the PFS rate of the patients with gastric cancer
(Table III).
 | Table II.Univariate and multivariate analyses
of factors affecting OS rates in patients with gastric cancer. |
Table II.
Univariate and multivariate analyses
of factors affecting OS rates in patients with gastric cancer.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | RR | 95% CI | P-value | RR | 95% CI | P-value |
|---|
| PGRN | 2.952 | 1.468–5.937 | 0.002a | 2.258 | 1.008–5.056 | 0.048a |
| Lymph node
involvement | 1.158 | 0.602–2.228 | 0.660 | 1.061 | 0.533–2.109 | 0.867 |
| Lymphatic
invasion | 1.842 | 0.887–3.826 | 0.101 | 1.247 | 0.532–2.922 | 0.611 |
| Vascular
invasion | 1.141 | 0.591–2.203 | 0.695 | 0.923 | 0.463–1.839 | 0.820 |
|
Differentiation | 1.547 | 0.803–2.98 | 0.192 | 1.549 | 0.753–3.185 | 0.234 |
| Clinical stage | 3.783 | 1.847–7.75 |
<0.001a | 3.493 | 1.691–7.218 | 0.001a |
 | Table III.Univariate analysis and multivariate
analysis of factors affecting PFS rates in patients with gastric
cancer. |
Table III.
Univariate analysis and multivariate
analysis of factors affecting PFS rates in patients with gastric
cancer.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | RR | 95% CI | P-value | RR | 95% CI | P-value |
|---|
| PGRN | 2.626 | 1.382–4.988 | 0.003a | 2.215 | 1.075–4.562 | 0.031a |
| Lymph node
involvement | 1.220 | 0.660–2.256 | 0.526 | 1.043 | 0.552–1.973 | 0.896 |
| Lymphatic
invasion | 1.909 | 0.971–3.755 | 0.061 | 1.287 | 0.592–2.797 | 0.524 |
| Vascular
invasion | 1.022 | 0.553–1.888 | 0.946 | 0.886 | 0.466–1.684 | 0.711 |
|
Differentiation | 1.262 | 0.682–2.334 | 0.458 | 1.398 | 0.721–2.708 | 0.321 |
| Clinical stage | 2.540 | 1.348–4.786 | 0.004a | 2.474 | 1.300–4.707 | 0.006a |
To further ascertain the potential prognostic
factors, multivariate Cox regression analysis was also performed
and showed that the expression of PGRN (P=0.048) and clinical stage
(P=0.001) had a significant effect on the OS rates of patients with
gastric cancer (Table II); PGRN
(P=0.031) and clinical stage (P=0.006) also had significant effects
on the PFS rates of patients with gastric cancer (Table III). Lymph node involvement,
lymphatic invasion, vascular invasion and differentiation had no
prognostic significance, when evaluated using univariate Cox
regression analysis and multivariate Cox regression analysis.
Extracellular PGRN promotes the
intracellular expression of PGRN via the AKT and ERK signaling
pathways
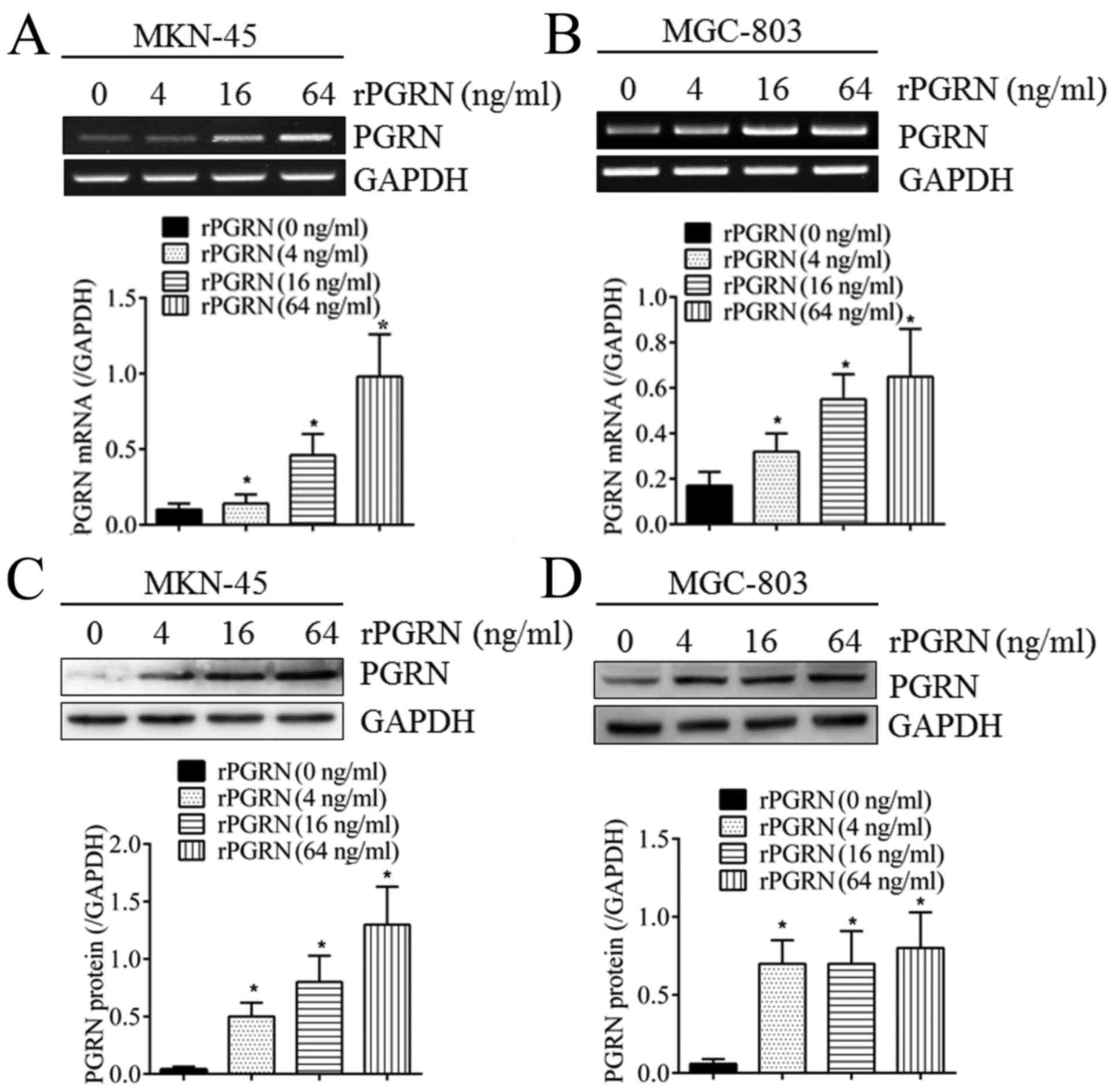
As shown in Fig. 2,
when stimulated with different concentrations of recombinant PGRN
(rPGRN) for 2 days, the expression of intracellular PGRN was
significantly upregulated at the mRNA level in the MKN-45 and
MGC-803 cells (Fig. 2A and B) in a
concentration-dependent manner. The same was true of the protein
levels (Fig. 2C and D). This
confirmed the positive feedback regulatory mechanism of PGRN in
gastric cancer cells.
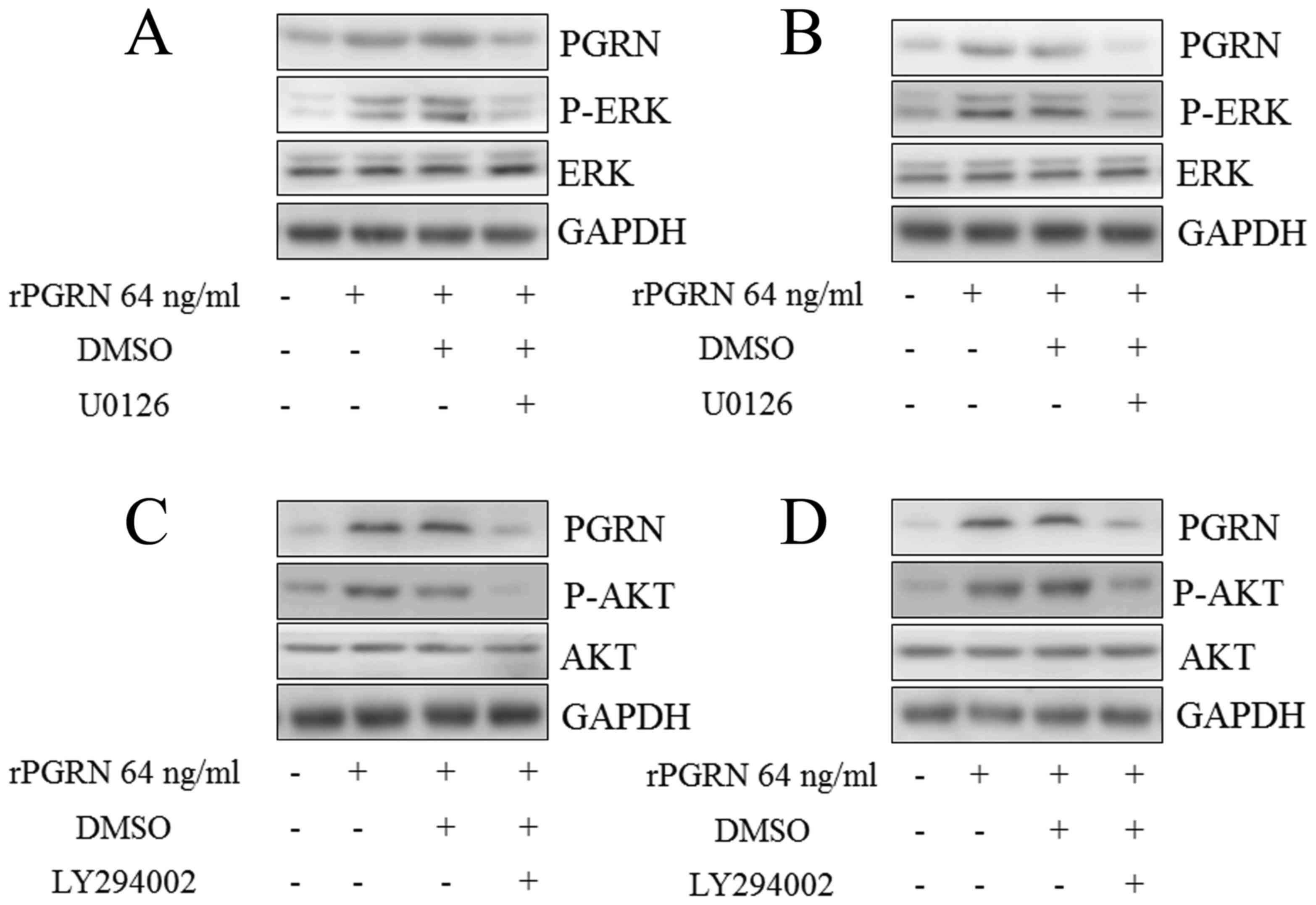
Subsequent investigation into the molecular
mechanism underlying the above process showed that the
phosphorylation of EKT was activated by rPGRN in the MKN-45
(Fig. 3A) and MGC-803 (Fig. 3B) cells, and the same was observed
for the phosphorylation of AKT (Fig.
3C and D). The ERK inhibitor (U0126) and AKT inhibitor
(LY294002) abrogated the upregulation of intracellular PGRN induced
by rPGRN in the MKN-45 (Fig. 3A and
C) and MGC-803 (Fig. 3B and D)
cells. This confirmed the roles of AKT and ERK in the positive
feedback regulation of the expression of PGRN.
Discussion
The PGRN gene is localized on human chromosome 17
and the encoding region is composed of 12 exons. PGRN protein
weighs 68.5 kDa and is composed of 7.5 tandem repeats, which are
rich in serine (16). In previous
years, the importance of PGRN has been widely reported in different
types of tumor. In 2014, Edelman et al reported that PGRN
was expressed at high levels in non-small cell lung carcinoma
(NSCLC) tissues and was correlated with poor OS and PFS rates in
patients with NSCLC (17). In our
previous study, PGRN not only promoted the proliferation and
angiogenesis of colorectal cancer, but also was positively
correlated with lymph node metastasis, advanced clinical stage and
poor PFS rates (18).
Cancer progression is a complex process and its
prognosis can be affected by different factors. Patients at an
advanced clinical stage usually have a poorer prognosis, compared
with patients at an early stage. In the present study, it was
confirmed that PGRN and clinical stage had potential effects on the
prognosis of patients with gastric cancer through Cox regression
analysis. This evidence supporting the effects of PGRN and clinical
stage on the prognosis of patients with gastric cancer emphasizes
the importance of early screening and treatment. Lymph node
involvement, lymphatic invasion, vascular invasion and
differentiation had no significant effects in the present study,
however, it is not possible to dismiss the importance of these
factors in cancer progression and prognosis. The present study
included a limited number of cases and a study comprising an
increased number of cases may provide different results.
Until now, studies investigating the roles of PGRN
in gastric cancer have been limited. In 2011, Wang et al
reported that PGRN was expressed at a high level in gastric cancer
tissue, however, no further retrospective analysis or cell
experiments were performed (19).
In the present study, the expression of PGRN in gastric cancer was
examined, and it was confirmed that PGRN was positively correlated
with lymph node metastasis, lymphatic invasion, advanced clinical
stage, and poor OS and PFS rats in patients with gastric cancer.
This confirmed the importance of PGRN in the progression and
prognosis of gastric cancer. This was consistent with the
tumor-promoting roles of PGRN reported in previous studies.
However, there were limitations in the present study. Only 120
cases were enrolled in limited quantities, and the majority of
cases were from local residents with regional patient information.
This limited number of cases and regional data restrict the
universality of the results. Multi-center and larger sample
clinical trials are likely to improve the validity of the
results.
As a secretory protein, under the guidance of
signaling peptides, PGRN is secreted to the outside of cells and
can regulate intracellular gene expression through binding to
corresponding receptors on cell membranes in an autocrine or
paracrine manner. In 2016, Liu et al reported that rPGRN
promoted the expression of Cyclin-B1 and Cyclin-D1 in HepG2
hepatocellular carcinoma cells, resulting in cell growth (20). In our previous study, PGRN was
shown to promote the expression of Ki67 and vascular endothelial
growth factor A in colorectal cancer cells (21). However, whether PGRN regulated its
expression in a positive feedback loop remained unclear. In the
present study, it was confirmed that extracellular rPGRN
efficiently promoted the intracellular expression of PGRN in MKN-45
and MGC-803 cells. This suggested that PGRN in patient blood may
affect the expression of PGRN in tumor cells and, leading to tumor
cells secreting increased PGRN into the blood, eventually resulting
in high expression levels of PGRN in the tumor and blood, offering
potential as a marker of tumor progression. Although the clinical
implication of PGRN in the blood has been reported in several types
of cancer, including breast cancer (22) and NSCLC (17), the results of the present study
indicate the importance of PGRN in the circulation from another
perspective.
A more detailed understanding of the molecular
mechanism underlying the positive feedback regulation of PGRN is
likely to improve knowledge of the role of PGRN in tumors. The AKT
and ERK signaling pathways have been commonly investigated
downstream targets of PGRN in several types of tumor (23–25).
ERK is also a crucial signal target, which is responsible for the
expression of PGRN induced by interleukin-6 in hepatocellular
carcinoma cells (20). In the
present study, it was found that extracellular PGRN efficiently
promoted the intracellular expression of PGRN via the AKT and ERK
signaling pathways. These results, together with those of the
previous reports, provide sufficient evidence supporting the close
correlation of ERK and AKT signaling pathways with the expression
and functions of PGRN in cancer.
In conclusion, the present study elucidated the
clinical implication of PGRN in the progression and prognosis of
gastric cancer using IHC analysis. In addition, it was confirmed
that PGRN regulated its own expression in a positive feedback loop
via the AKT and ERK signaling pathways. These results improve
current understanding of the role of PGRN in cancer and indicate a
novel effective target for gastric cancer therapy.
References
|
1
|
Vohlonen I, Pukkala E, Malila N, Härkönen
M, Hakama M, Koistinen V and Sipponen P: Risk of gastric cancer in
Helicobacter pylori infection in a 15-year follow-up. Scand J
Gastroenterol. 51:1159–1164. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hafez NH and Tahoun NS: Expression of
cyclooxygenase 2 and vascular endothelial growth factor in gastric
carcinoma: Relationship with clinicopathological parameters. J
Egypt Natl Canc Inst. 28:149–156. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li P, Sun D, Li X, He Y, Li W, Zhao J,
Wang Y, Wang H and Xin Y: Elevated expression of Nodal and YAP1 is
associated with poor prognosis of gastric adenocarcinoma. J Cancer
Res Clin Oncol. 142:1765–1773. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yoshino S, Nishikawa K, Morita S,
Takahashi T, Sakata K, Nagao J, Nemoto H, Murakami N, Matsuda T,
Hasegawa H, et al: Randomised phase III study of S-1 alone versus
S-1 plus lentinan for unresectable or recurrent gastric cancer
(JFMC36-0701). Eur J Cancer. 65:164–171. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Suzuki M and Nishiahara M: Granulin
precursor gene: A sex steroid-inducible gene involved in sexual
differentiation of the rat brain. Mol Genet Metab. 75:31–37. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu J, Nathan C, Jin W, Sim D, Ashcroft
GS, Wahl SM, Lacomis L, Erdjument-Bromage H, Tempst P, Wright CD
and Ding A: Conversion of proepithelin to epithelins: Roles of SLPI
and elastase in host defense and wound repair. Cell. 111:867–878.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tang W, Lu Y, Tian QY, Zhang Y, Guo FJ,
Liu GY, Syed NM, Lai Y, Lin EA, Kong L, et al: The growth factor
progranulin binds to TNF receptors and is therapeutic against
inflammatory arthritis in mice. Science. 332:478–484. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
De Muynck L and Van Damme P: Cellular
effects of progranulin in health and disease. J Mol Neurosci.
45:549–560. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu CJ and Bosch X: Progranulin: A growth
factor, a novel TNFR ligand and a drug target. Pharmacol Ther.
133:124–132. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Swamydas M, Nguyen D, Allen LD, Eddy J and
Dréau D: Progranulin stimulated by LPA promotes the migration of
aggressive breast cancer cells. Cell Commun Adhes. 18:119–130.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Abrhale T, Brodie A, Sabnis G, Macedo L,
Tian C, Yue B and Serrero G: GP88 (PC-Cell derived growth factor,
progranulin) stimulates proliferation and confers letrozole
resistance to aromatase overexpressing breast cancer cells. BMC
Cancer. 11:2312011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Diaz-Cueto L, Arechavaleta-Velasco F,
Diaz-Arizaga A, Dominguez-Lopez P and Robles-Flores M: PKC
signaling is involved in the regulation of progranulin
(acrogranin/PC-cell-derived growth factor/granulin-epithelin
precursor) protein expression in human ovarian cancer cell lines.
Int J Gynecol Cancer. 22:945–950. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carlson AM, Maurer MJ, Goergen KM, Kalli
KR, Erskine CL, Behrens MD, Knutson KL and Block MS: Utility of
progranulin and serum leukocyte protease inhibitor as diagnostic
and prognostic biomarkers in ovarian cancer. Cancer Epidemiol
Biomarkers Prev. 22:1730–1735. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cheung PF, Cheng CK, Wong NC, Ho JC, Yip
CW, Lui VC, Cheung AN, Fan ST and Cheung ST: Granulin-epithelin
precursor is an oncofetal protein defining hepatic cancer stem
cells. PLoS One. 6:e282462011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gao J, Liu D, Li J, Song Q and Wang Q:
Effect of STK17A on the sensitivity of ovarian cancer cells to
paclitaxel and carboplatin. Oncol Lett. 12:1107–1112.
2016.PubMed/NCBI
|
|
16
|
Hu Y, Xiao H, Shi T, Oppenheim JJ and Chen
X: Progranulin promotes tumour necrosis factor-induced
proliferation of suppressive mouse CD4+
Foxp3+ regulatory T cells. Immunology. 142:193–201.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Edelman MJ, Feliciano J, Yue B, Bejarano
P, Ioffe O, Reisman D, Hawkins D, Gai Q, Hicks D and Serrero G:
GP88 (progranulin): A novel tissue and circulating biomarker for
non-small cell lung carcinoma. Hum Pathol. 45:1893–1899. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dong T, Yang D, Li R, Zhang L, Zhao H,
Shen Y, Zhang X, Kong B and Wang L: PGRN promotes migration and
invasion of epithelial ovarian cancer cells through an epithelial
mesenchymal transition program and the activation of cancer
associated fibroblasts. Exp Mol Pathol. 100:17–25. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang H, Sun Y, Liu S, Yu H, Li W, Zeng J,
Chen C and Jia J: Upregulation of progranulin by helicobacter
pylori in human gastric epithelial cells via p38MAPK and MEK1/2
signaling pathway: Role in epithelial cell proliferation and
migration. FEMS Immunol Med Microbiol. 63:82–92. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu F, Zhang W, Yang F, Feng T, Zhou M, Yu
Y, Yu X, Zhao W, Yi F, Tang W and Lu Y: Interleukin-6-stimulated
progranulin expression contributes to the malignancy of
hepatocellular carcinoma cells by activating mTOR signaling. Sci
Rep. 6:212602016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang D, Wang LL, Dong TT, Shen YH, Guo XS,
Liu CY, Liu J, Zhang P, Li J and Sun YP: Progranulin promotes
colorectal cancer proliferation and angiogenesis through TNFR2/Akt
and ERK signaling pathways. Am J Cancer Res. 5:3085–3097.
2015.PubMed/NCBI
|
|
22
|
Tkaczuk KR, Yue B, Zhan M, Tait N,
Yarlagadda L, Dai H and Serrero G: Increased circulating level of
the survival factor GP88 (progranulin) in the serum of breast
cancer patients when compared to healthy subjects. Breast Cancer
(Auckl). 5:155–162. 2011.PubMed/NCBI
|
|
23
|
He Z, Ismail A, Kriazhev L, Sadvakassova G
and Bateman A: Progranulin (PC-cell-derived growth
factor/acrogranin) regulates invasion and cell survival. Cancer
Res. 62:5590–5596. 2002.PubMed/NCBI
|
|
24
|
Kamrava M, Simpkins F, Alejandro E,
Michener C, Meltzer E and Kohn EC: Lysophosphatidic acid and
endothelin-induced proliferation of ovarian cancer cell lines is
mitigated by neutralization of granulin-epithelin precursor (GEP),
a prosurvival factor for ovarian cancer. Oncogene. 24:7084–7093.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ong CH and Bateman A: Progranulin
(granulin-epithelin precursor, PC-cell derived growth factor,
acrogranin) in proliferation and tumorigenesis. Histol Histopathol.
18:1275–1288. 2003.PubMed/NCBI
|