Introduction
The nucleotide-binding oligomerization domain-like
receptor family pyrin domain-containing 3 (NLRP3) inflammasome can
stimulate the innate or adaptive immune system in response to
certain signals, including endogenous metabolites and
microorganisms. It is understood that the underlying mechanism
employed by the NLRP3 inflammasome involves the recruitment of
apoptosis-associated speck-like protein containing a CARD (ASC) and
caspase-1. As a result of inflammasome activation, the
proinflammatory cytokines interleukin (IL)-1β and IL-18 are
released via pyroptosis and proteolytic cleavage (1). Activators of the NLRP3 inflammasome
are heterogeneous, ranging from self-originating crystals, such as
monosodium urate monohydrate, uric acid, glucose and adenosine
5′triphosphate (ATP) to environment-derived aluminum hydroxide,
silica and asbestos, as well as pathogenic molecules (2,3). How
such structurally diverse molecules activate the NLRP3 inflammasome
is yet to be determined; however, it appears that K+
efflux and reactive oxygen species (ROS) may serve pivotal roles in
activation of the NLRP3 inflammasome (3,4).
NLRP3 is usually expressed in myeloid cells, including monocytes,
macrophages and dendritic cells; recently it has been revealed that
NLRP3 is also expressed in T helper (Th) 1 (5), Th2 (6) and Th17 (7) cells. Providing the association
between NLRP3 activation and numerous pathological conditions,
including Muckle-Wells syndrome (8), Alzheimer's disease (9), type 2 diabetes (10), as well as autoimmune disorders,
such as experimental autoimmune encephalitis (7) and systemic lupus erythematosus
(11), increasing efforts to
clarify the underlying molecular mechanism are being made.
Secreted by adipocytes, leptin is a cytokine-like
hormone that has been reported to control energy expenditure and
metabolism, and modulate the innate and adaptive immune responses.
The activation of natural killer cells, chemotaxis of neutrophils,
and secretion of tumor necrosis factor (TNF)-α, IL-6 and IL-12 from
macrophages (12) also involves
leptin. Additionally, leptin also promotes Th17 cell responses
(13) and downregulates the number
of T regulatory cells (14).
Previously, it was demonstrated that the mRNA and protein
expression levels of caspase-1 and ASC were significantly reduced,
in addition to reduced expression levels of IL-18, IL-1β, leptin
and macrophage infiltration markers, within white adipose tissue of
nonsteroidal anti-inflammatory drug-activated gene-1 transgenic
mice (15). These findings have
indicated an association between leptin and the NLRP3 inflammasome
within macrophage cells. The present study demonstrated that
activation of the NLRP3 inflammasome was promoted by an increase in
ROS synthesis and K+ efflux in response to leptin, which
resulted in an increase in IL-18 secretion within RAW 264.7 cells.
Leptin may therefore be considered a novel activator and a
potential modulator of the NLRP3 inflammasome.
Materials and methods
Cell culture
RAW 264.7 murine macrophage cells (The Cell Bank of
Type Culture Collection of the Chinese Academy of Sciences,
Shanghai, China) were cultured in Dulbecco's modified Eagle's
medium (DMEM, low glucose) supplemented with 10% fetal bovine
serum, 100 ng/ml streptomycin and 100 U/ml penicillin (Gibco;
Thermo Scientific, Inc., Waltham, MA, USA) at 37°C, 5%
CO2 and humidity. Various doses of leptin (10, 100 and
500 ng/ml; PeproTech China, Suzhou, China) were added and the cells
were cocultured for 24 h in the presence or absence of Ac-YVAD-cmk
(18.4 µM; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), KCl (100
µM) or diphenyleneiodonium chloride (DPI; 50 µM, Sigma-Aldrich;
Merck KGaA). Lipopolysaccharide (LPS, 100 ng/ml, Sigma-Aldrich;
Merck KGaA) and/or adenosine 5′triphosphate (ATP, 5 mM,
Sigma-Aldrich; Merck KGaA) were cultured for 3 h respectively and
used as positive controls. Cells were centrifuged at 400 × g, for 5
min, 4°C, and supernatants and sediments were collected and
analyzed by ELISA or reverse transcription-quantitative polymerase
chain reaction (RT-qPCR).
Flow cytometric analysis
Caspase-1 activity was detected using a
Fluorochrome-Labeled Inhibitor of caspase-1 kit:
FAM-FLICA®Caspase assay kit (cat. no. 655)
(ImmunoChemistry Technologies, LLC, Bloomington, MN, USA). ROS
synthesis was investigated using a ROS detection assay kit, CFDA
Cellular ROS Detection assay kit (cat. no. ab113851, Abcam,
Shanghai, China). THBP was used as a positive control. All kits
were performed according to the manufacturer's protocol. Results
were analyzed using a FACSCanto FlowJo 7.6 (BD Biosciences,
Franklin Lakes, CA, USA).
Cytokine measurement
IL-1β and IL-18 expression levels in the supernatant
of RAW 264.7 cells were measured using ELISA kits (Mouse IL-18 cat.
no. BMS618, Mouse IL-1β cat. no. BMS6002) and were purchased from
eBioscience; Thermo Fisher Scientific, Inc., according to the
manufacturer's protocols.
RNA isolation, cDNA synthesis and
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR)
Total cellular RNA was isolated using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. cDNA was synthesized from 500 ng total RNA
in 10 ul volume using a Superscript kit (Invitrogen; Thermo Fisher
Scientific, Inc.). The conditions used were 37°C for 15 min and
85°C for 5 sec. RT-qPCR reactions were performed using 1ul cDNA, 10
ul SYBR Green mater mix (Bio-Rad Laboratories, Inc., Hercules, CA,
USA), 2 ul of primer mix in a total volume of 20 ul. Thermocycling
conditions were set up as follows: 5 min at 95°C, 40 cycles of
denaturation (5 sec at 95°C), and combined annealing/extension (34
sec at 64°C). The housekeeping gene GAPDH was used as the internal
standard. Analysis of relative gene expression data using the
2−ΔΔCq method (16).
RT-qPCR was performed on an ABI Prism 7500 Real-Time PCR system
(Thermo Fisher Scientific, Inc.). Primer sequences were as follows:
NLRP3-forward, 5′-ATTACCCGCCCGAGAAAGG-3′, and reverse,
5′-CATGAGTGTGGCTAGATCCAAG-3′; IL-1β forward,
5′-GTACAAGGAGAACCAAGCAA-3′ and reverse, 5′-CCGTCTTTCATTACACAGGA-3′;
IL-18 forward, 5′-AGGACACTTTCTTGCTTGCC-3′, and reverse,
5′-CACAAACCCTCCCCACCTAA-3′; GAPDH forward
5′-TTCACCACCATGGAGAAGGC-3′ and reverse
5′-GGCATGGACTGTGGTCATGA-3′.
NLRP3 gene knockdown
RAW 264.7 cells were nucleofected with 20 µM NLRP3
specific small interfering (si)RNA (Shanghai Biotend, Shanghai,
China) or negative control (NC) siRNA (Shanghai Biotend) using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. Nucleofected cells were
incubated for 24 h at 37°C, 5% CO2 and humidity in the
presence or absence of leptin, 6 h post-transfection. Knockdown of
the NLRP3 gene was determined via RT-qPCR with the following
primers: NLRP3 siRNA forward, 5′-GCAGGUUCUACUCUAUCAAdTdT-3′ and
reverse, 5′-UUGAUAGAGUAGAACCUGCdTdT-3′. The NC siRNA sequences were
as follows: NC siRNA forward, 5′-UUCUCCGAACGUGUCACGUdTdT-3′ and
reverse, 5′-ACGUGACACGUUCGGAGAAdTdT-3′.
Statistical analysis
A paired t-test was employed for two group analyses
and Kruskal-Wallis one-way analysis of variance was used for
analyses of >3 groups using GraphPad Prism 5 software (GraphPad
Software, Inc., La Jolla, CA, USA). Results are expressed as the
mean ± standard deviation. P<0.05 was considered to indicate a
statistically significant difference and the experiments were
repeated three times.
Results
Leptin promotes IL-18 secretion in RAW
264.7 cells
To study the effects of leptin on macrophages,
leptin was applied at increasing doses to RAW 264.7 cells for 24 h,
using LPS and/or ATP as positive controls. Supernatants or cell
sediments were collected and analyzed by ELISA or RT-qPCR. The
present study reported a leptin-induced increase in IL-1β mRNA
expression levels only, whereas a dose-dependent increase was
observed in IL-18 mRNA and protein expression levels (Fig. 1)
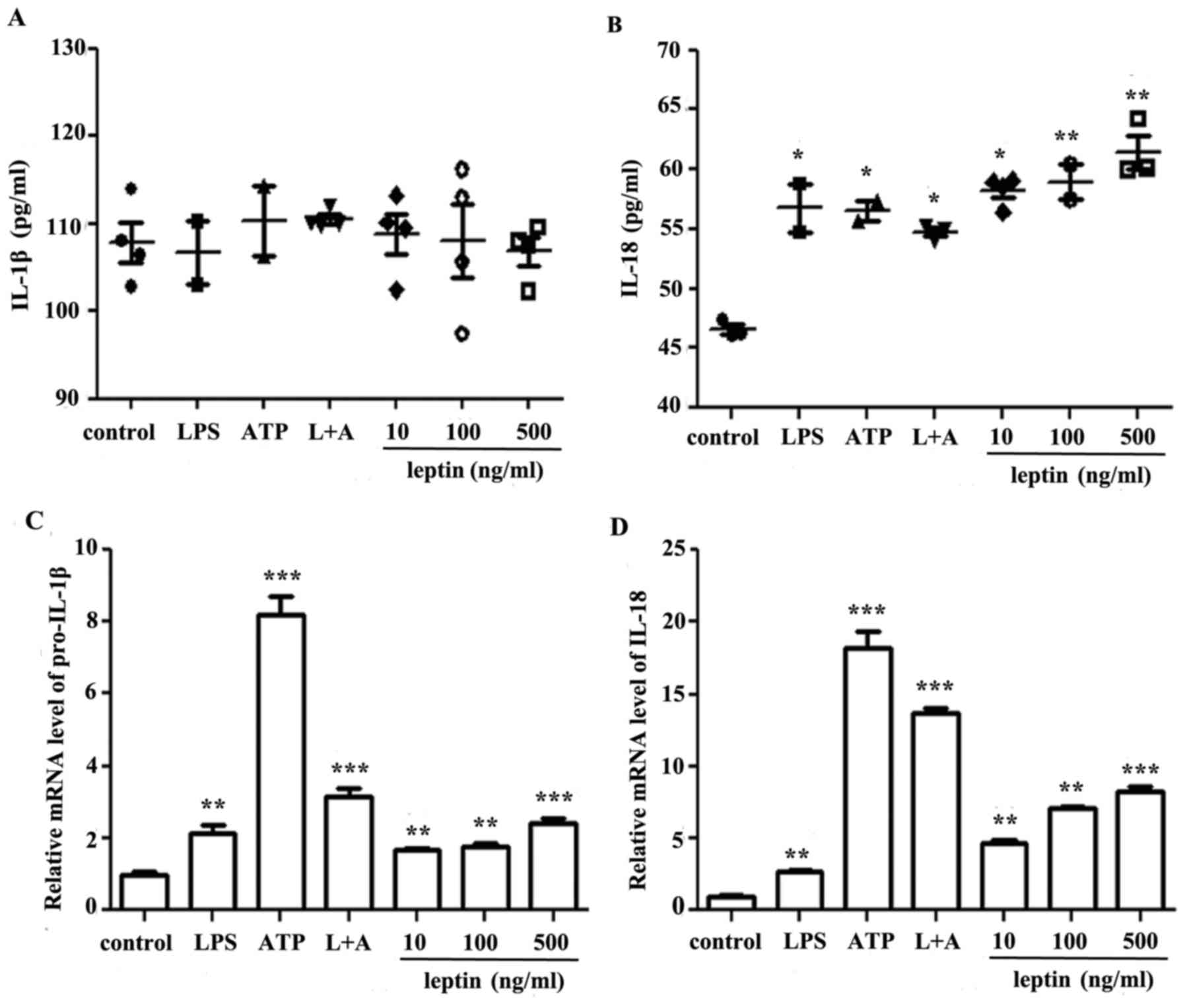 | Figure 1.Leptin promotes IL-18, but not IL-1β
secretion, in RAW 264.7 cells. (A) IL-1β and (B) IL-18 ELISA of
culture supernatants from RAW 264.7 cells incubated with LPS, ATP,
L+A or leptin at increasing doses (10, 100 and 500 ng/ml) for 24 h.
Results are presented as the mean ± standard error of the mean from
four independent experiments. Relative mRNA expression levels of
(C) IL-1β and (D) IL-18 in RAW 264.7 cells treated as
aforementioned. Results are from three independent experiments.
*P<0.05, **P<0.01, ***P<0.001 vs. the control. ATP,
adenosine 5′triphosphate; IL, interleukin; LPS, lipopolysaccharide;
L+A, LPS and ATP. |
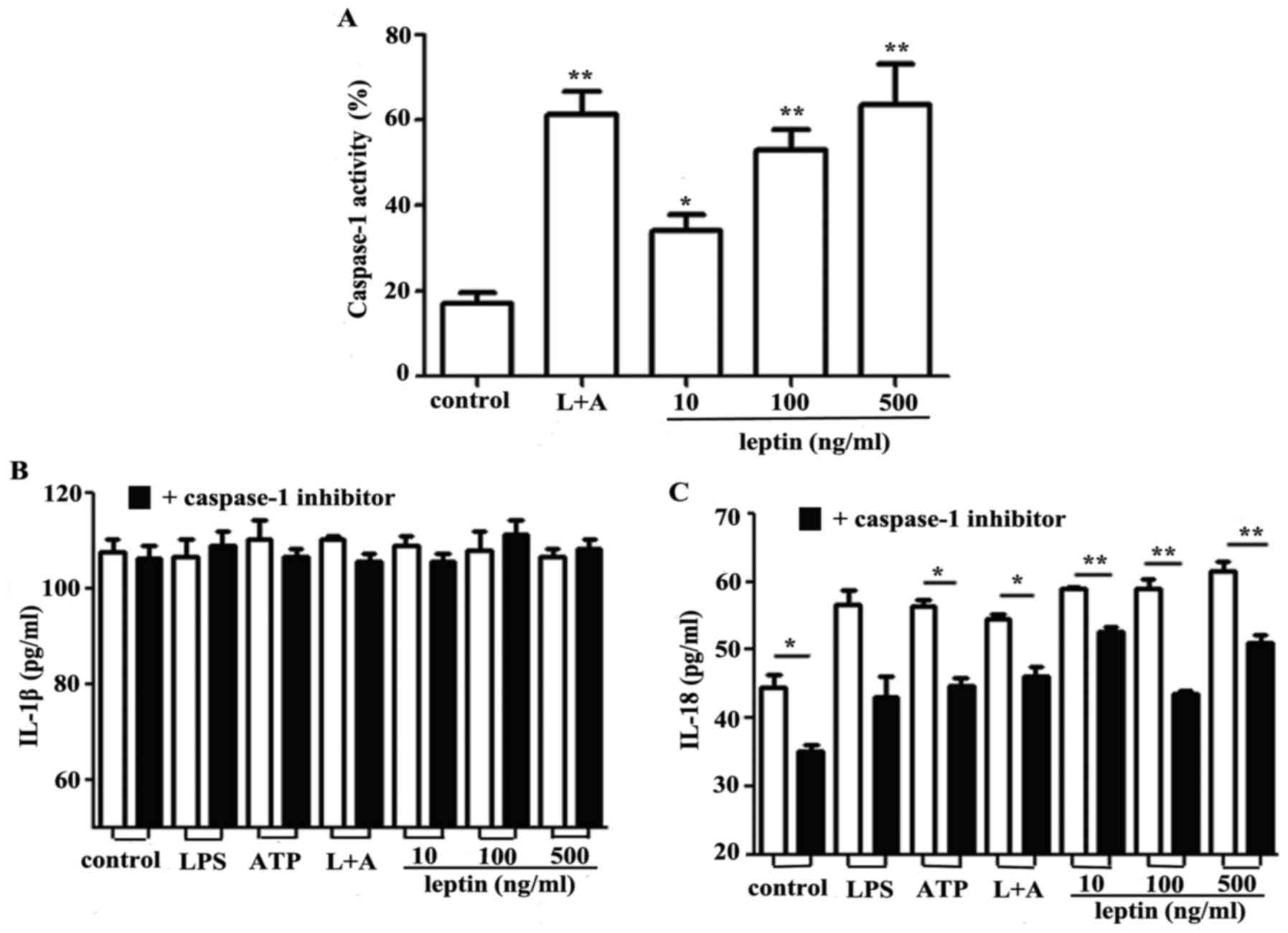
Leptin activates caspase-1 and NLRP3
to promote IL-18 secretion
Caspase-1 contributes to the NLRP3 inflammasome
complex and regulates the synthesis and secretion of IL-18 by
proteolytically digesting pro-IL-18 (17,18).
Since leptin was observed to promote IL-18 secretion in RAW 264.7
cells, the effects of leptin on caspase-1 were investigated. The
findings of the present study indicated that activation of
caspase-1 was induced by leptin in a dose-dependent manner
(Fig. 2A). Inhibition of caspase-1
via Ac-YVAD-cmk had no effect on IL-1β secretion (Fig. 2B); however, IL-18 secretion was
markedly decreased in response to the caspase-1 inhibitor (Fig. 2C).
The NLRP3 inflammasome comprises NLRP3, and the
adapter and effector proteins, ASC and caspase-1, respectively. The
effects of leptin on capsase-1 activation prompted an investigation
into the association between leptin and the NLRP3 inflammasome. The
results demonstrated that leptin upregulated the mRNA expression
levels of NLRP3 (Fig. 3A).
Conversely, NLRP3, IL-1β and IL-18 expression levels were reduced
following nucleofection with NLRP3-specific siRNA (Fig. 3B, C and D respectively).
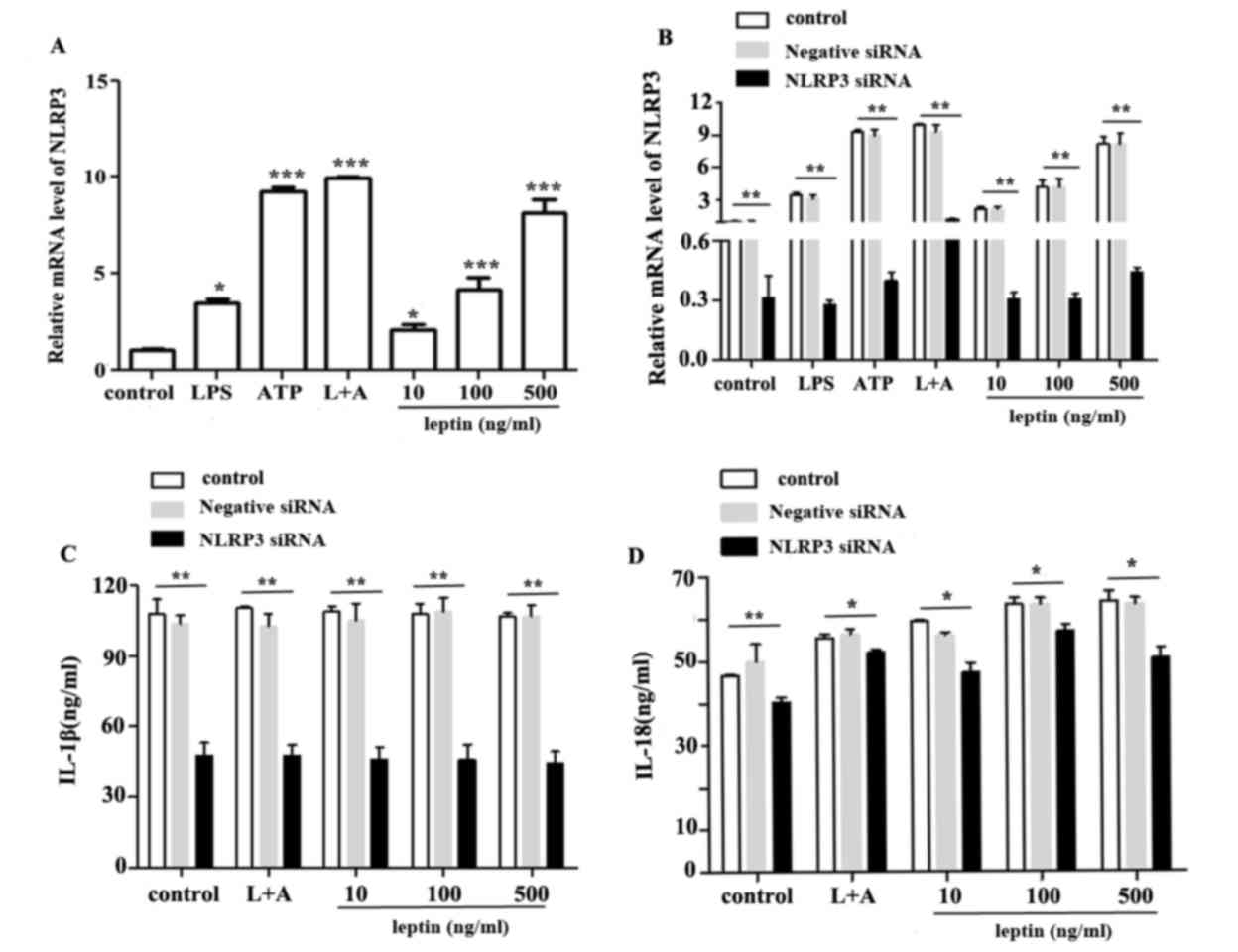 | Figure 3.NLRP3 upregulation promotes IL-18
expression in response to leptin. (A) Relative mRNA expression
levels of NLRP3 in RAW 264.7 cells incubated with LPS, ATP, L+A or
leptin at increasing doses (10,100 and 500 ng/ml) for 24 h. (B)
Relative mRNA expression levels of NLRP3 in RAW 264.7 cells
following nucleofection with NLRP3 specific siRNA or negative
control siRNA. (C) IL-1β or (D) IL-18 expression in the culture
supernatants from RAW 264.7 cells treated as aforementioned, as
determined by ELISA. *P<0.05, **P<0.01, ***P<0. 001 vs.
the control. ATP, adenosine 5′triphosphate; IL, interleukin; LPS,
lipopolysaccharide; L+A, LPS and ATP; NLRP3, nucleotide-binding
oligomerization domain-like receptor family pyrin domain-containing
3; siRNA, small interfering RNA. |
ROS synthesis and K+ efflux
is involved in leptin-induced IL-18
ROS have been reported to be key mediators in the
activation of the NLRP3 inflammasome (19–21).
Intracellular ROS generation was measured in response to leptin
within RAW 264.7 cells. Leptin-induced ROS generation was observed
compared with in the control group (Fig. 4A and B). In addition, a significant
decrease in IL-18 production was observed in response to the NADPH
oxidase inhibitor DPI (Fig.
4C).
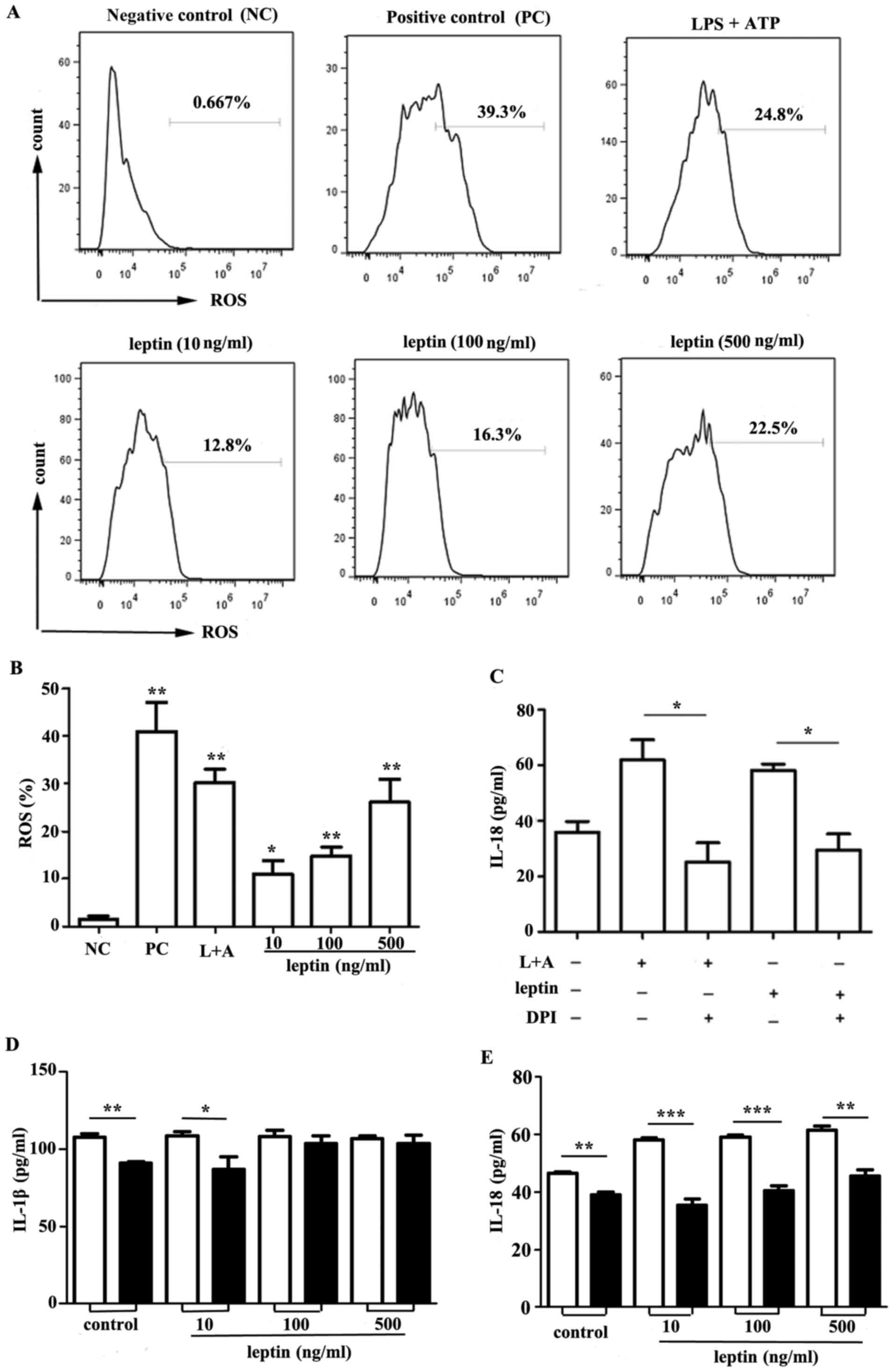 | Figure 4.ROS synthesis and K+ efflux
is involved in producing IL-18 in response to leptin. (A) Flow
cytometric analysis of ROS in RAW 264.7 cells incubated with leptin
at increasing doses (10, 100 and 500 ng/ml) for 24 h. THBP was used
as a positive control. Representative data from three independent
experiments are presented. (B) Cumulative data of ROS in RAW 264.7
cells from three experiments. (C) IL-18 ELISA of culture
supernatants from RAW 264.7 cells incubated with leptin (500 ng/ml)
for 24 h in the presence or absence of the ROS inhibitor DPI (50
µM). (D) IL-1β or (E) IL-18 expression in the culture supernatants
from RAW 264.7 cells incubated with leptin at increasing doses (10,
100 and 500 ng/ml) for 24 h in the presence or absence of KCl (100
mM), as determined by ELISA. *P<0.05, **P<0.01, ***P<0.001
vs. the control. ATP, adenosine 5′triphosphate; DPI,
diphenyleneiodonium chloride; IL, interleukin; LPS,
lipopolysaccharide; L+A, LPS and ATP; ROS, reactive oxygen
species. |
Previous studies have suggested that K+
efflux appears to be an important mediator in the activation of the
NLRP3 inflammasome (22–24). RAW 264.7 cells were incubated with
leptin at increasing doses for 24 h in the presence or absence of
100 mM KCl. The results of the present study indicated that
although IL-1β secretion was unaffected (Fig. 4D), an impairment in IL-18 secretion
following elimination of K+ efflux was observed
(Fig. 4E).
Discussion
A previous study revealed that leptin induced the
upregulation of phagocytic function-associated markers, and
stimulated the secretion of proinflammatory cytokines, including
TNF-α, IL-6 and IL-12 (12). The
results of the present study suggested an association between
leptin-induced IL-18 secretion and activation of the NLRP3
inflammasome, via increases in ROS generation and K+
efflux within RAW 264.7 cells. As a member of the IL-1 cytokine
family, IL-18 possesses similarities to IL-1 with regards to
structure and receptor utilization. IL-18 serves a key
proinflammatory role by inducing interferon-γ expression (25). The expression, synthesis and
processing of IL-18 however, is distinct compared with other
members of the IL-1 cytokine family (26–28)
and is regulated by caspase-1. Following processing, IL-18 is
released into the extracellular milieu and can also be expressed as
a membrane-bound form. Active IL-18 attaches to the IL-18 receptor,
which is expressed on various cells, including macrophages. Myeloid
differentiation primary response gene 88-IRAF6-nuclear factor-κB
and signal transduction and activator of transcription 3-mitogen
activated protein kinase signaling pathways are two major pathways
for IL-18 (25). Recently, it has
been reported that breast cancer cell metastasis may be associated
with leptin-induced secretion of IL-18 (29), which requires future
investigation.
In the present study, leptin was reported to
increase IL-1β gene expression; however, protein secretion remained
unaffected within RAW 264.7 cells. Additionally, IL-1β was
unaffected by caspase-1 inhibition. Martin et al (7) detected IL-1β secretion within Th17
cells in a NLRP3-ASC-caspase-8-dependent manner in the absence of
caspase-1. These findings suggested an association between
caspase-8 and the leptin-induced effects observed in macrophage
cells. In addition, the secreted protein p60 from Listeria
monocytogenes serves as a ‘non-canonical’ stimulus, which
activates the NLRP3 inflammasome. In the present study, leptin
mainly promoted IL-18 secretion and caspase-1 inhibitor mainly
inhibited IL-18. It could be hypothesized that other caspases, such
as caspase-8 may influence IL-1β response to leptin. The production
of IL-1β or IL-18 is independently regulated following activation
of the inflammasome. Inhibitors of ROS production inhibited
secretion of IL-1β, but did not impair IL-18 secretion.
Furthermore, DCs from caspase-11 (casp11)-deficient mice failed to
secrete IL-1β in response to p63 but were fully responsive for
IL-18 secretion. Therefore, other disparate licensing factors may
control IL-18 vs. IL-1β within dendritic cells (30). Consistent with previous studies of
human monocytes, the present study demonstrated that leptin
augmented the secretion of IL-18, but not IL-1β (31). Conversely, leptin has been reported
to induce IL-1β secretion within human peripheral blood mononuclear
cells (PBMCs) (32,33); elevated IL-1β mRNA and IL-18
protein expression levels, and increased cytokine responses to LPS
have also been observed in monocyte-derived dendritic cells
(34). In addition, it has been
reported that leptin may expedite IL-1β secretion within bovine
PBMCs; however, it appears that the mRNA expression levels of IL-1β
and IL-18 were unaffected (35).
Therefore, these findings indicated that leptin-mediated responses
within myeloid immune cells may differ between species, lineage and
differentiation state.
Reactive oxygen species and K+ efflux
appear to be important mediators for the activation of the NLRP3
inflammasome. The present study revealed that leptin promotes IL-18
secretion by increasing ROS synthesis and K+ efflux,
which may activate the NLRP3 inflammasome in RAW 264.7 cells.
Numerous studies have indicated that ROS and K+ efflux
may serve as strong activators for the NLRP3 inflammasome (36,37).
In conclusion, the findings of the present study
revealed that leptin promotes IL-18 secretion by enhancing ROS
synthesis and K+ efflux, which activates the NLRP3
inflammasome in RAW 264.7 cells. Leptin may therefore serve as a
novel activator of the NLRP3 inflammasome through blocking leptin
may be considered as a target for future antimicrobial and
anti-inflammatory treatment.
Acknowledgements
The present study was supported by grants from the
National Science Foundation for Young Scholars of China (grant no.
81401345) and the Young Physician Training Plan of North Huashan
Hospital, Fudan University (grant no. 0000077).
References
|
1
|
Próchnicki T, Mangan MS and Latz E: Recent
insights into the molecular mechanisms of the NLRP3 inflammasome
activation. F1000Res 5: pii: F1000 Faculty Rev. 14692016.
|
|
2
|
Kim Y, Wang W, Okla M, Kang I, Moreau R
and Chung SJ: Suppression of NLRP3 inflammasome by γ-tocotrienol
ameliorates type 2 diabetes. Lipid Res. 57:66–76. 2016. View Article : Google Scholar
|
|
3
|
Shin MS, Kang Y, Lee N, Wahl ER, Kim SH,
Kang KS, Lazova R and Kang I: Self double-stranded (ds)DNA induces
IL-1β production from human monocytes by activating NLRP3
inflammasome in the presence of anti-dsDNA antibodies. J Immuno.
190:1407–1415. 2013. View Article : Google Scholar
|
|
4
|
Tschopp J and Schroder K: NLRP3
inflammasome activation: The convergence of multiple signalling
pathways on ROS production? Nat Rev Immunol. 10:210–215. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Arbore G, West EE, Spolski R, Robertson
AA, Klos A, Rheinheimer C, Dutow P, Woodruff TM, Yu ZX, O'Neill LA,
et al: T helper 1 immunity requires complement-drivenNLRP3
inflammasome activity in CD4+ T cells. Science.
352:aad12102016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bruchard M, Rebé C, Derangère V, Togbé D,
Ryffel B, Boidot R, Humblin E, Hamman A, Chalmin F, Berger H, et
al: The receptor NLRP3 is a transcriptional regulator of TH2
differentiation. Nat Immunol. 16:859–870. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Martin BN, Wang C, Zhang CJ, Kang Z, Gulen
MF, Zepp JA, Zhao J, Bian G, Do JS, Min B, et al: T cell-intrinsic
ASC critically promotes T(H)17-mediated experimental autoimmune
encephalomyelitis. Nat Immunol. 17:583–592. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mortimer L, Moreau F, MacDonald JA and
Chadee K: NLRP3 inflammasome inhibition is disrupted in a group of
auto-inflammatory disease CAPS mutations. Nat Immunol.
17:1176–1186. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
White CS, Lawrence CB, Brough D and
Rivers-Auty J: Inflammasomes as therapeutic targets for Alzheimer's
disease. Brain Pathol. 27:223–234. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim Y, Wang W, Okla M, Kang I, Moreau R
and Chung S: Suppression of NLRP3 inflammasome by γ-tocotrienol
ameliorates type 2 diabetes. J Lipid Res. 57:66–76. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lu A, Li H, Niu J, Wu S, Xue G, Yao X, Guo
Q, Wan N, Abliz P, Yang G, et al: Hyperactivation of the NLRP3
inflammasome in myeloid cells leads to severe organ damage in
experimental lupus. J Immunol. 198:1119–1129. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fernández-Riejos P, Najib S,
Santos-Alvarez J, Martín-Romero C, Pérez-Pérez A, González-Yanes C
and Sánchez-Margalet V: Role of leptin in the activation of immune
cells. Mediators Inflamm. 2010:5683432010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu Y, Liu Y, Shi FD, Zou H, Matarese G and
La Cava A: Cutting edge: Leptin-induced RORγt Expression in
CD4+ T cells promotes Th17 responses in systemic lupus
erythematosus. Immunol. 190:3054–3058. 2013. View Article : Google Scholar
|
|
14
|
Liu Y, Yu Y, Matarese G and La Cava A:
Cutting edge: Fasting-induced hypoleptinemia expands functional
regulatory T cells in systemic lupuserythematosus. J Immunol.
188:2070–2073. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang X, Chrysovergis K, Kosak J and Eling
TE: Lower NLRP3 inflammasome activity in NAG-1 transgenic mice is
linked to a resistance to obesity and increased insulin
sensitivity. Obesity (Silver Spring). 22:1256–1263. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Scmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gross O, Thomas CJ, Guarda G and Tschopp
J: The inflammasome: An integrated view. Immunol Rev. 243:136–151.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fu S, Xu L, Li S, Qiu Y, Liu Y, Wu Z, Ye
C, Hou Y and Hu CA: Baicalin suppresses NLRP3 inflammasome and
nuclear factor-kappa B (NF-κB) signaling during Haemophilus
parasuis infection. Vet Res. 47:802016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Braga TT, Forni MF, Correa-Costa M, Ramos
RN, Barbuto JA, Branco P, Castoldi A, Hiyane MI, Davanso MR, Latz
E, et al: Soluble uric acid activates the NLRP3 inflammasome. Sci
Rep. 7:398842017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yin Y, Zhou Z, Liu W, Chang Q, Sun G and
Dai Y: Vascular endothelial cells senescence is associated with
NOD-like receptor family pyrin domain-containing 3 (NL RP3)
inflammasome activation via reactive oxygen species
(ROS)/thioredoxin-interacting protein (TXNIP) pathway. Int J
Biochem Cell Biol. 84:22–34. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Toksoy A, Sennefelder H, Adam C, Hofmann
S, Trautmann A, Goebeler M and Schmidt M: Potent NLRP3 inflammasome
activation by the HIV reverse-transcriptase inhibitor abacavir. J
Biol Chem. 292:2805–2814. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kang MJ, Jo SG, Kim DJ and Park JH: NLRP3
inflammasome mediates interleukin-1β production in immune cells in
response to Acinetobacter baumannii and contributes to pulmonary
inflammation in mice. Immunology. 150:495–505. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gong Z, Zhou J, Zhao S, Tian C, Wang P, Xu
C, Chen Y, Cai W and Wu J: Chenodeoxycholic acid activates NLRP3
inflammasome and contributes to cholestatic liver fibrosis.
Oncotarget. 7:83951–83963. 2016.PubMed/NCBI
|
|
24
|
Groß CJ, Mishra R, Schneider KS, Médard G,
Wettmarshausen J, Dittlein DC, Shi H, Gorka O, Koenig PA, Fromm S,
et al: K+ Efflux-Independent NLRP3 inflammasome
activation by small molecules targeting mitochondria. Immunity.
45:761–773. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee JH, Cho DH and Park HJ: IL-18 and
Cutaneous Inflammatory Diseases. Int J Mol Sci. 16:29357–29369.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sims JE and Smith DE: The IL-1 family:
Regulators of immunity. Nat Rev Immunol. 10:89–102. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Puren AJ, Fantuzzi G and Dinarello CA:
Gene expression, synthesis, and secretion of interleukin 18 and
interleukin 1beta are differentially regulated in human blood
mononuclear cells and mouse spleen cells. Proc Nat Acad Sci USA.
96:pp. 2256–2561. 1999; View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Arend WP, Palmer G and Gabay C: IL-1,
IL-18, and IL-33 families of cytokines. Immunol Rev. 223:20–38.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li K, Wei L, Huang Y, Wu Y, Su M, Pang X,
Wang N, Ji F, Zhong C, Chen T, et al: Leptin promotes breast cancer
cell migration and invasion via IL-18 expression and secretion. Int
J Oncol. 48:2479–2487. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schmidt RL and Lenz LL: Distinct licensing
of IL-18 and IL-1β secretion in response to NLRP3 inflammasome
activation. PLoS One. 7:e451862012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jitprasertwong P, Jaedicke KM, Nile CJ,
Preshaw PM and Taylor JJ: Leptin enhances the secretion of
interleukin (IL)-18, but not IL-1β, from human monocytes via
activation of caspase-1. Cytokine. 65:222–230. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gabay C, Dreyer M, Pellegrinelli N,
Chicheportiche R and Meier CA: Leptin directly induces the
secretion of interleukin 1 receptor antagonist in human monocytes.
J Clin Endocrinol Metab. 86:783–791. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dixit VD, Schaffer EM, Pyle RS, Collins
GD, Sakthivel SK, Palaniappan R, Lillard JW Jr and Taub DD: Ghrelin
inhibits leptin-and activation-induced proinflammatory cytokine
expression by human monocytes and T cells. J Clin Invest.
114:57–66. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mattioli B, Straface E, Quaranta MG,
Giordani L and Viora M: Leptin promotes differentiation and
survival of human dendritic cells and licenses them for Th1
priming. J Immunol. 174:6820–6828. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ahmed M, Shaban Z, Yamaji D,
Okamatsu-Ogura Y, Soliman M, Abd Eldaim M, Ishioka K, Makondo K,
Saito M and Kimura K: Induction of proinflammatory cytokines and
caspase-1 by leptin in monocyte/macrophages from holstein cows. J
Vet Med Sci. 69:509–514. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen CY, Yang CH, Tsai YF, Liaw CC, Chang
WY and Hwang TL: Ugonin U stimulates NLRP3 inflammasome activation
and enhances inflammasome-mediated pathogen clearance. Redox Biol.
11:263–274. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lordén G, Sanjuán-García I, de Pablo N,
Meana C, Alvarez-Miguel I, Pérez-García MT, Pelegrín P, Balsinde J
and Balboa MA: Lipin-2 regulates NLRP3 inflammasome by affecting
P2X7 receptor activation. J Exp Med. 214:511–528. 2016. View Article : Google Scholar : PubMed/NCBI
|