Introduction
Cerebral ischemia injury is a common and serious
neurological disease, leading to causes of death long-term
disability worldwide (1). Despite
hundreds of preclinical trials demonstrating efficacy of
neuron-targeted therapies in vivo and vitro models of
stroke, the only clinical treatment remains early restoration of
blood flow with thrombolysis (2).
The failure to translate neuron-targeted approaches to beneficent
clinical therapy indicates that alternative cellular targets in
brain may more effectively coordinate the complex intracellular and
extracellular signaling cascades which contribute to neuronal
injury. Emerging evidences reveal that one of the most widely
accepted pathophysiological mechanisms of cerebral ischemia involve
oxidative DNA damage (3,4).
Oxidative DNA damage is an early event following
cerebral ischemia-reperfusion injury, resulting from direct or
indirect attacks by reactive oxygen species (ROS) during
reperfusion (4–6). Oxidative DNA damage consists of
DNA-protein crosslinks, 8-hydroxy-2¢-deoxyguanosine (8-OHdG)
formation and apurinic/apyrimidinic (AP) sites (4,5).
Apurinic/apyrimidinic endonuclease 1 (APE1) is a multifunctional
enzyme that participates in base-excision repair of oxidative DNA
damage and in the redox activation of transcription factors
(7,8). Neurons with decreased APE1 expression
and endonuclease activity were found to be extremely vulnerable to
cell death induced by in vitro ischemia, indicating that
oxidative base lesions and AP sites can trigger ischemic cell death
(9). Furthermore, a strong
correlation exists between loss of APE1 expression in ischemic
neurons and neuronal cell death after ischemia (10,11).
Energy failure after ischemia has been speculated to deplete APE1
expression, thereby triggering neuronal death (12). In another study, APE1 is required
for pituitary adenylate cyclase-activating polypeptide
(PACAP)-induced neuroprotection against global cerebral ischemia
(13). However, the role of
endogenous APE1 in cellular protection from ischemic injury has not
been unequivocally established.
Resveratrol (3,5,4′-trihydroxy-trans-stilbene) is a
naturally occurring phytoalexin that is found abundantly in the
skin of red grapes and is a component of red wine (14). Several studies have demonstrated
that resveratrol elicits a variety of biological and
pharmacological functions, including cardioprotective,
anti-oxidant, anti-apoptotic, and anti-inflammatory activities
(15–18). In addition, resveratrol is also
regarded as a natural antioxidant, has been suggested to reduce DNA
damage and oxidative organ injury (19). Importantly, increasing studies have
identified a neuroprotective role of resveratrol in animal models
of cerebral ischemia/reperfusion injury (20–22).
However, the potential neuroprotective effects of resveratrol
against hypoxic-ischemic brain injury and the underlying mechanisms
remain clear unknown.
Therefore, the present study attempts to investigate
the role of APE1 in the protective effect of resveratrol against
oxygen-glucose deprivation and re-oxygenation (OGD/R)-induced HT22
cells injury which is an effective in vitro model of
cerebral ischemia. We found that resveratrol prevents OGD/R-induced
cytotoxicity and oxidative stress accompanied by increasing APE1
activity and protein and mRNA levels, resulting in neuroprotective
effect under cerebral ischemic environment, while these effects are
blocked by APE1 shRNA. The present study reveals a central role of
DNA repair induced by APE1 in resveratrol-induced neuroprotection
after cerebral ischemia.
Materials and methods
Reagents
Resveratrol (RSV) was purchased from Sigma-Aldrich
(St. Louis, MO, USA). Dulbecco's Modified Eagle's Medium (DMEM) and
fetal bovine serum (FBS) were obtained from GIBCO Life Technologies
(Paisley, Scotland).
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay kit (C0009) and lactate dehydrogenase (LDH) cytotoxicity
assay kit (C0016) were from Beyotime Institute of Biotechnology
(Jiangsu, China). The primary antibodies against APE1, tubulin and
HRP conjugated goat anti-rabbit IgG were obtained from Cell
Signaling Technology (Beverly, MA, USA). ROS detection reagent,
5-(and-6)-carboxy-2′, 7′-dichlorodihydrofluorescein diacetate
(carboxy-H2DCFDA, C400), was purchased from Invitrogen
(Carlsbad, CA, USA).
Cell culture
HT22 cells were obtained from the National Cell Bank
of Iran (Pasteur Institute of Iran, Tehran, Iran). The cells were
cultured in DMEM containing 10% FBS, 100 U/ml of penicillin and 100
µg/ml of streptomycin under normal culture condition (5%
CO2 and 95% humidified air at 37°C).
Oxygen-glucose Deprivation and
Re-oxygenation (OGD/R) model
After grown to 60–70% confluency in a standard cell
culture incubator (humidified 5% CO2 athmosphere and
37°C), the HT22 cells were exposed to OGD/R process. Briefly,
cultured medium was instead of glucose-free Neurobasal A medium
(Life Tech, USA) and the cells were put in a hypoxic chamber at
37°C with a mix gas containing 1% O2, 5% CO2
and 94% N2, which was monitored with O2analyzer (GODEE,
China). After incubation for 2 h, cells were returned to normal
cultured conditions for re-oxygenation (48 h) according to the
instructions of the manufacturers.
Constructs and transfection
The lentiviral vectors expressing APE1-targeting
sequence (APE1t) or the scrambled sequence (APE1s) were constructed
using pDC315-eGFP adenovirus vector. shRNA sequence for huamn APE1
were inserted into pDC315-eGFP adenovirus vector. The recombinant
adenoviral vector (pDC315-eGFP-APE1 shRNA and pDC315-eGFP-APE1
scramble) was packaged and amplified in HEK 293A cells. The
following oligonucleotides were used for the APE1 shRNA: sense,
5′-GATCCCCCCTGCCACACTCAAGATCTGCTTCAAGAGAGCAGATCTTGAGTGTGGCAGGTTTTTGGAAA-3′;
and
antisense,5′-AGCTTTTCCAAAAACCTGCCACACTCAAGATCTGCTCTCTTGAAGCAGATCTTGAGTGTGGCAGGGGG-3′;
and scrambled oligonucleotide sequences: Sense,
5′-GATCCCCAGTCTAACTCGCCACCCCGTATTCAAGAGATACGGGGTGGCGAGTTAGACTTTTTTGGAAA-3′;
antisense,
5′-AGCTTTTCCAAAAAAGTCTAACTCGCCACCCCGTATCTCTTGAATACGGGGTGGCGAGTTAGACTGGG-3′.
The transfections were performed using Lipofectamine™ 3000
Transfection Reagent (L3000001, Invitrogen) following the
manufacturer's protocol for transient transfection of HT22 cells.
The transfection efficiency was detected using Real-time
quantitative PCR (RT-PCR) and western blot analysis.
Cell viability assay
HT22 cell survival was measured using a MTT assay.
At the end of the incubation period, the culture medium was removed
and HT22 cells were incubated with MTT solution (0.5 mg/ml) in a
dark place for 4 h at 37°C. Then, cells were treated with dimethyl
sulfoxide (DMSO) in order to dissolve the formazan crystals.
Absorbance at 595 nm was assessed using a microplate reader
(PowerWave XS; BioTek Instruments, Wincoski, VT, USA). Viability
was assessed by comparison of the absorbance of different treatment
of the samples with control group according to the following
formula and each experiment was carried out for triplicate: Cell
viability (%) = (ODexperimental
group-ODblack/ODcontrol
group-ODblack) × 100%.
LDH assay
The cytotoxicity of HT22 cells following the
exposures was assessed by measuring the amount of released lactate
dehydrogenase (LDH) enzyme from cells using lactate dehydrogenase
(LDH) cytotoxicity assay kit according to the manufacturer's
instructions. In brief, HT22 cells were seeded into 6-well plate at
a density of 1×106 cells/well. At the end of the
treatment, LDH from the culture medium was measured at 340 nm. The
cells were lysed in 0.5 ml of lysis buffer provided within the
assay. The amount of intracellular LDH released into the
extracellular medium is expressed as a fold of total LDH activity
(LDH in the medium + LDH in the cell) according to the following
equation: LDH released = LDH activity in the medium/total LDH
activity.
Measurement of APE1 levels using ELISA
kit
The culture supernatant of HT22 cells treated with
indicated regents were collected. Prepare and mix all reagents
thoroughly before use. Sample or standard (50 µl) were added to the
wells of the conjugate coated plate, respectively. After incubated
for 10 min at room temperature, the diluted anti-APE1 antibody (50
µl) was added to each well and co-incubated at room temperature for
1 h. After washed three times with 1× Wash Buffer (250 µl) per well
with thorough aspiration between each wash, the diluted secondary
antibody-enzyme conjugate (100 µl) was added to all wells and
incubated at room temperature for 1 h. Following washed three
times, warm substrate solution (100 µl) was added to each well and
incubated at room temperature for 30 min. Finally, stop solution
(100 µl) was added into each well plate to stop the enzyme
reaction. Results should be read immediately at 450 nm wave length
by Mithras LB 940 Multimode Microplate Reader (Berthold
Technologies GmbH & Co., Bad Wildbad, Germany).
8-hydroxydeoxyguanosine (8-OHdG) level
measurement
8-OHdG, a marker of oxidative stress to DNA, was
also measured by an enzyme linked immunosorbent assay (ELISA;
YLA0016HU; Hangzhou Eastbiopharm Co., Ltd., Hangzhou, China),
according to the manufacturer's instructions. The absorbance was
measured at 450 nm. The results were represented as the number of
8-OHdG per 106 nucleotides.
AP Sites of DNA measurement
Nuclear DNA of HT22 cells was freshly isolated after
treatment for indicated time. AP sites were tested by the
colorimetric assay kit (CAS:154-21-2; Integrated Device Technology,
San Jose, CA, USA). Biotin labeled aldehyde reactive probe (ARP) in
the ring-opened AP site was detected for AP sites. The purified DNA
of HT22 cells was dissolved in TE at 100 µg/ml, and then DNA
solution (10 µl) was incubated with ARP solution (5 mM, 10 µl) at
37°C for 1 h. The ARP-labeled DNA was then precipitated with
ethanol, and the DNA pellet was resuspended in TE. ARP in the
labeled DNA was measured using an ELISA-like assay in a microtiter
plate according to the manufacturer's instructions. OD values of
each well were measured by Mithras LB 940 Multimode Microplate
Reader (Berthold Technologies GmbH & Co.) at 650 nm. All ARP
assays were performed in triplicate. The results were represented
as the number of AP sites per 106 nucleotides, were
normalized with calibration curve based on ARP-DNA standard
solutions.
Intracellular reactive oxygen species
(ROS) measurement
ROS generation was determined using the oxidative
conversion of cell-permeable DCFH-DA to fluorescent DCF. HT22 cells
were cultured in 6-well plates at a density of 1×106
cells/well. After indicated treatments, cells were harvested,
resuspended in 1 ml PBS with 20 µM of carboxy-H2DCFDA, and then
incubated for 1 h at 37°C. After washing, DCF fluorescence was
measured using a Coulter CyFlow Cytometer (Partec). The results
were expressed as the mean DCFH-DA fluorescence intensity over that
of the control.
Cellular SOD and GSH activity
analysis
Oxidative stress damage is prevented by the rapid
scavenging of O2− by the mitochondrial enzyme manganese
superoxide dismutase (SOD). Glutathione (GSH) is an intracellular
antioxidant and plays an important role in the detoxification of
various electrophilic compounds. The activity of SOD and the level
of GSH were detected using SOD activity kit (KT-219) and GSH
measurement kit (K251-100) supplied by Assay Designs (Ann Arbor,
MI, USA), respectively. Briefly, HT22 Cells were seeded in 6-well
plates (1×106 cells/well) and subjected to the
treatment. Then, the cells were harvested and protein was
extracted. SOD activity was measured by adding the master mix and
xanthine supplied in the kit, followed by incubation and
measurement by ELISA reader at 450 nm for 10 min at 1-min
intervals. GSH was determined by adding the reaction mix and GSH
reductase supplied in the kit, followed by incubation and
measurement by ELISA reader at 405 nm for 20 min at 1-min
intervals. Protein concentration was quantified by using a Bio-Rad
protein assay kit. Then, SOD activity in the cell extracts was
calculated vs. a SOD standard curve and normalized to the protein
concentration. The total amount of GSH was determined by means of a
calibration curve and normalized to the protein concentration.
Real Time RT-PCR
Total RNA was extracted and purified from treated
HT22 cells using the RNA isolator Total RNA Extraction Reagent
(TaKaRa, Kusatsu, Japan) in accordance with manufacturer's
instructions. Total RNA was subjected to reverse transcription
using iScript™ cDNA Synthesis kit and Real-time quantitative PCR
(RT-PCR) was performed according to the AceQ® qPCR
SYBR® Green Master Mix kit (TaKaRa) on ABI 7500 system
(ABI, New York, NY, USA). Glyceraldehyde-3-phosphate dehydrogenase
(GAPDH) was used to normalize levels of specific mRNA between
samples. Primer pairs were designed as follows: APE1:
5′-CTGCCTGGACTCTCTCATCAATAC-3′ and 5′-GAATGCCGTATCCGCTACTCC-3′;
GAPDH: 5′-GCACCGTCAAGGCTGAGAAC-3′, GAPDH R:
5′-TGGTGAAGACGCCAGTGGA-3′. Relative quantification of the indicated
mRNA normalized against GAPDH mRNA was calculated by using the
2−ΔΔCT methods.
Western blot analysis
The cells were homogenized with protein extraction
solution (lysis in RIPA). The homogenate was centrifuged at 12,000
rpm at 4°C for 30 min and total protein were quantified by using a
Bio-Rad protein assay kit. Equal amounts of proteins were separated
by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) and then transferred to polyvinylidene fluoride
membranes (PVDF, Millipore, Billerica, MA, USA). After blocked with
5% skim milk in PBST for 2 h at room temperature, followed by
overnight exposure to primary antibody against APE1 (cat. no. 4128,
1:2,000; Cell Signaling Technology) or -tubulin (cat. no. 5335,
1:2,000; Cell Signaling Technology). Membranes were then incubated
with appropriate horseradish peroxidase (HRP)-conjugated antibody
(cat. no. 7074, 1:5,000; Cell Signaling Technology) for 2 h at room
temperature. Tubulin was performed as an internal loading control.
Bands were visualized using an enhanced chemiluminescence system
(Pierce Biotech, Rockford, IL, USA) and imaged using ImageJ
software (NIH). Results are representative of at least three
experiments and defined as the percentage of the control group
after being normalized against tubulin.
Statistical analysis
Statistical analyses were conducted with GraphPad
Prism 5 (Graphpad Software, San Diego, CA, USA) and expressed as
the mean ± standard deviation (SD). Differences between the groups
were analyzed with the one-way ANOVA test followed by Dunnett's
post hoc tests. P<0.05 was considered significant.
Results
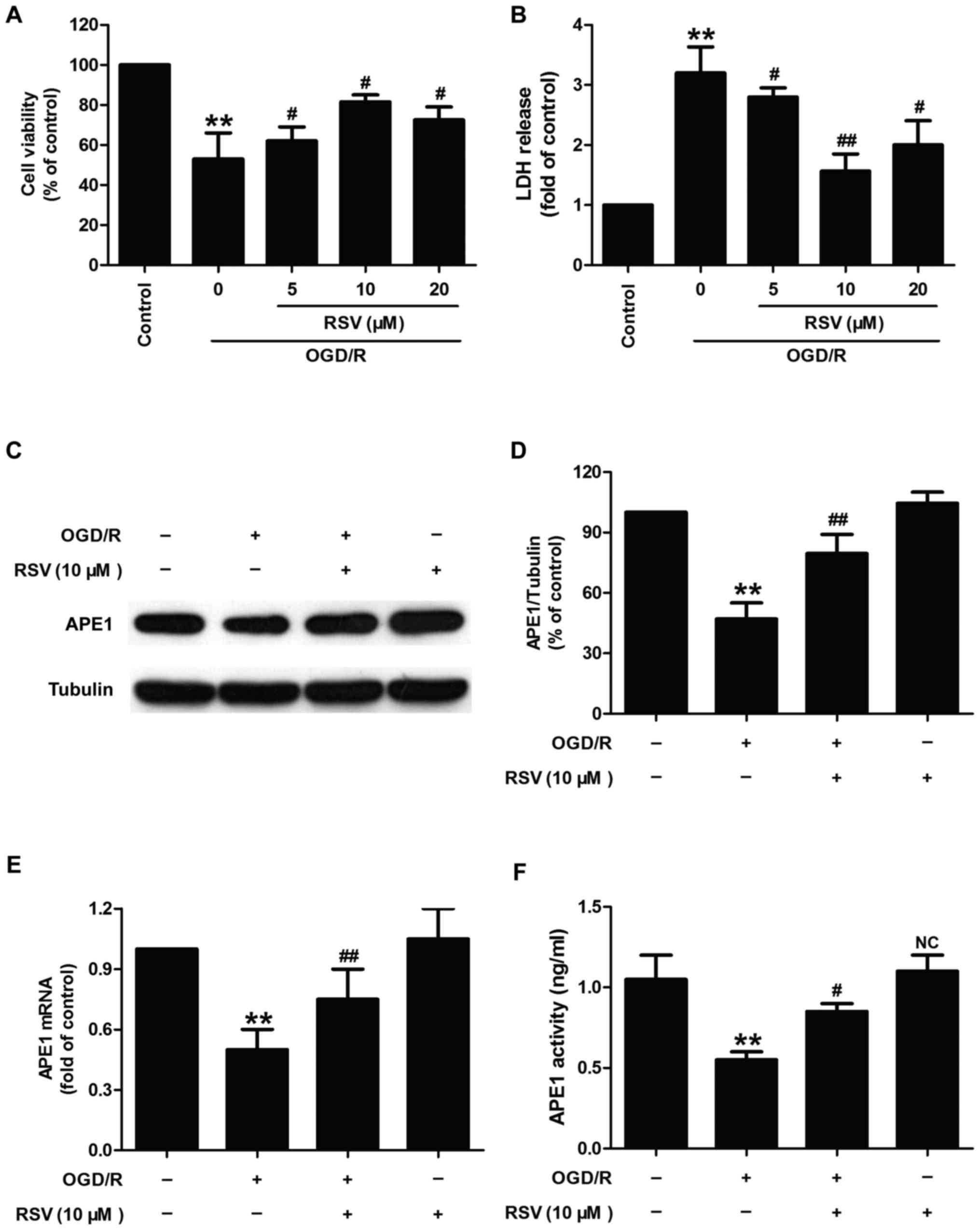
Resveratrol decreases neurotoxicity
and increases APE1 activity and APE1 levels in oxygen-glucose
deprivation and re-oxygenation (OGD/R)-treated HT22 cells
Firstly, we investigated whether resveratrol (RSV)
had protection and influence on APE1 in OGD/R-treated HT22 cells.
As shown in Fig. 1, OGD/R
condition induced the decrease in cell viability (Fig. 1A) and the increase in LDH release
(Fig. 1B) in HT22 cells. However,
the effects were reversed by different doses of RSV (5, 10, and 20
µM) pretreatment. The cell viability significantly increased
in response to the concentration of RSV (10 µM) which may
reach saturation values compared to RSV (20 µM), so 10
µM of RSV was selected as the optimal concentration for
subsequent experiments to demonstrate the effect of RVS on APE1
level and activity in HT22. Next, western blot analysis result
(Fig. 1C) reveled that RVS (10
µM) pretreatment mitigated OGD/R-induced the down-regulation
of APE1 protein level in HT22 cells (Fig. 1D). In addition, RVS pretreatment
also abolished the decreases in the level of APE1 mRNA (Fig. 1E) and the activity of APE1
(Fig. 1F) induced by OGD/R
treatment in HT22 cells. RVS treatment alone had no effect on the
activity and level of APE1. Take together, these results suggested
that APE1 may contribute to the protective effects of RVS against
OGD/R-induced nerve damage.
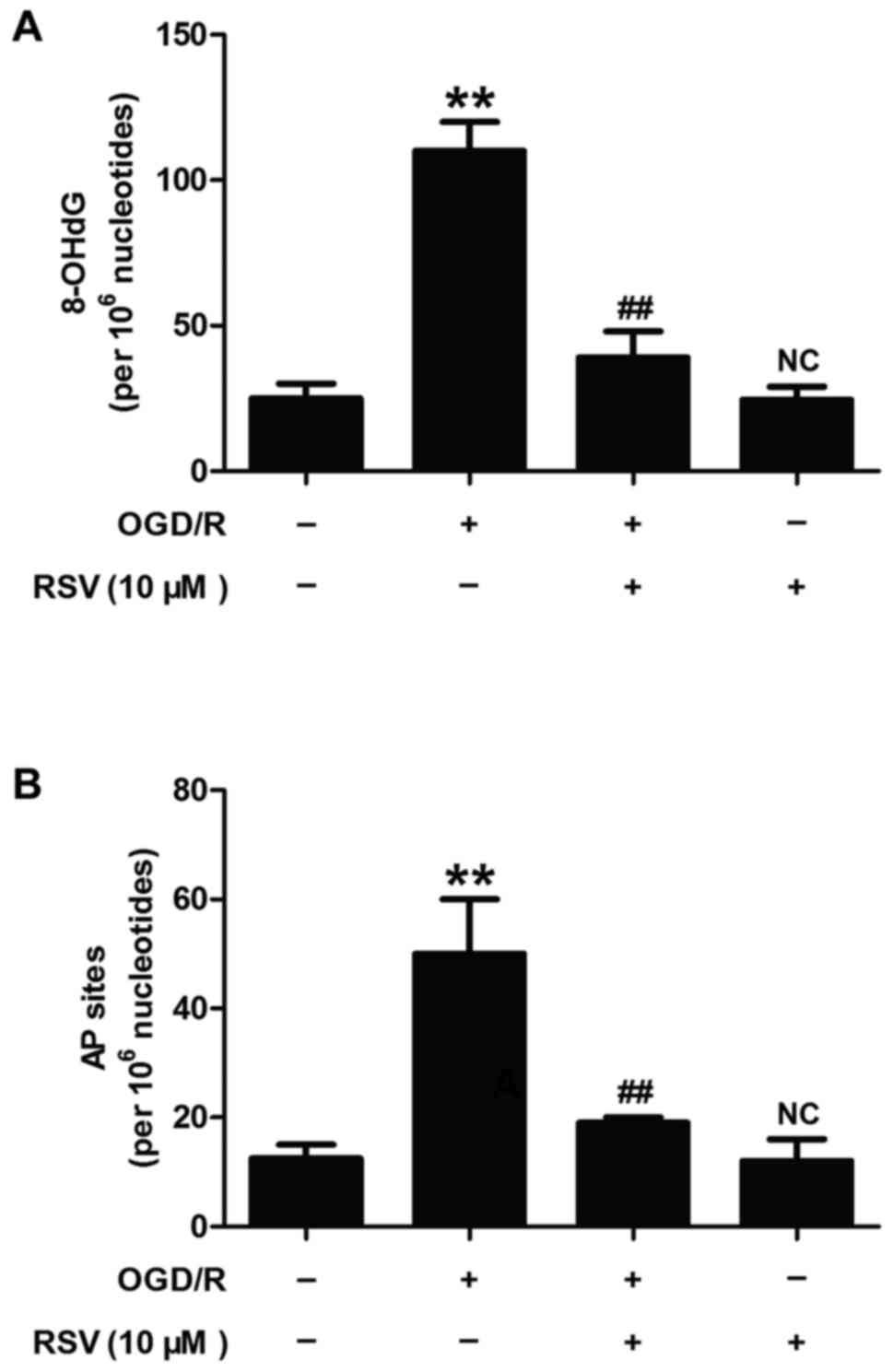
Resveratrol alleviates OGD/R-induced
oxidative DNA damage and repair activity in HT22 cells
APE1 is a multifunctional enzyme that plays a part
in base-excision repair of oxidative DNA injury and in the redox
activation of transcription factors (7). To further determine the role of APE1
in RVS-elicited beneficent effect on cerebral ischemia, we detected
the levels of 8-hydroxy-2¢-deoxyguanosine (8-OHdG) and
apurinic/apyrimidinic (AP) sites which were considered as oxidative
DNA damage markers. As illustrated in Fig. 2, OGD/R treatment significantly
increased the levels of 8-OHdG (Fig.
2A) and AP sites (Fig. 2B) in
HT22 cells, while these effects were obviously blocked by RVS (10
µM) pretreatment. RVS itself did not induce the changes of
8-OHdG and AP sites levels. These results indicated that RSV
alleviates OGD/R-induced oxidative DNA damage and repair activity,
which may mean up-regulation of APE1 activity and level.
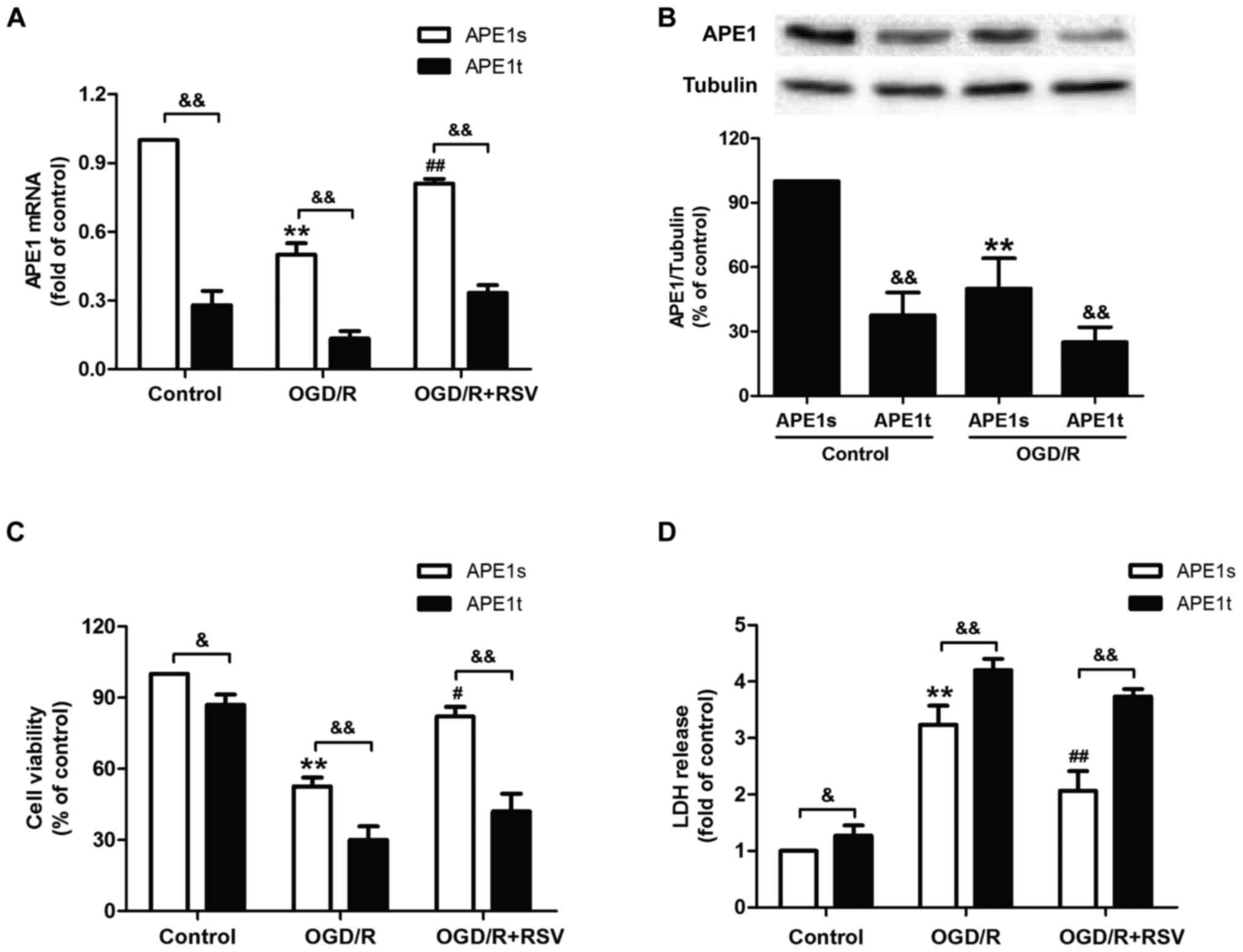
APE1 knockdown abrogates
resveratrol-induced neuroprotection against cytotoxicity in
OGD/R-treated HT22 cells
Given the obvious increase in DNA repair activity
induced by APE1 under OGD/R treatment, we hypothesized that
enhancement of DNA repair significantly contributes to RVS-mediated
neuroprotection. To confirm this hypothesis directly, we transduced
lentiviral vectors containing either shRNA targeted to APE1 (APE1t)
or a scrambled control shRNA (APE1s) into HT22 cells. Results from
RT-PCR and western blot assasy reveled that the level of APE1 mRNA
(Fig. 3A) and protein were
(Fig. 3B) decreased in APE1t
transfected cells compared to APE1s transfected cells with or
without OGD/R treatment, indicating the successful knockdown of
APE1 gene in HT22 cells. Next, we found that APE1t transfection
remarkably reduced the viability of HT22 cells (Fig. 3C) and increased the LDH leakage
(Fig. 3D) compared to RVS
pretreatment group, indicating that the down-regulation of APE1
abolished RVS-mediated neuroprotection against OGD/R-induced
injuryies in HT22 cells. These dada indicated that APE1 is required
for RVS-induced neuroprotection against cerebral ischemia
injury.
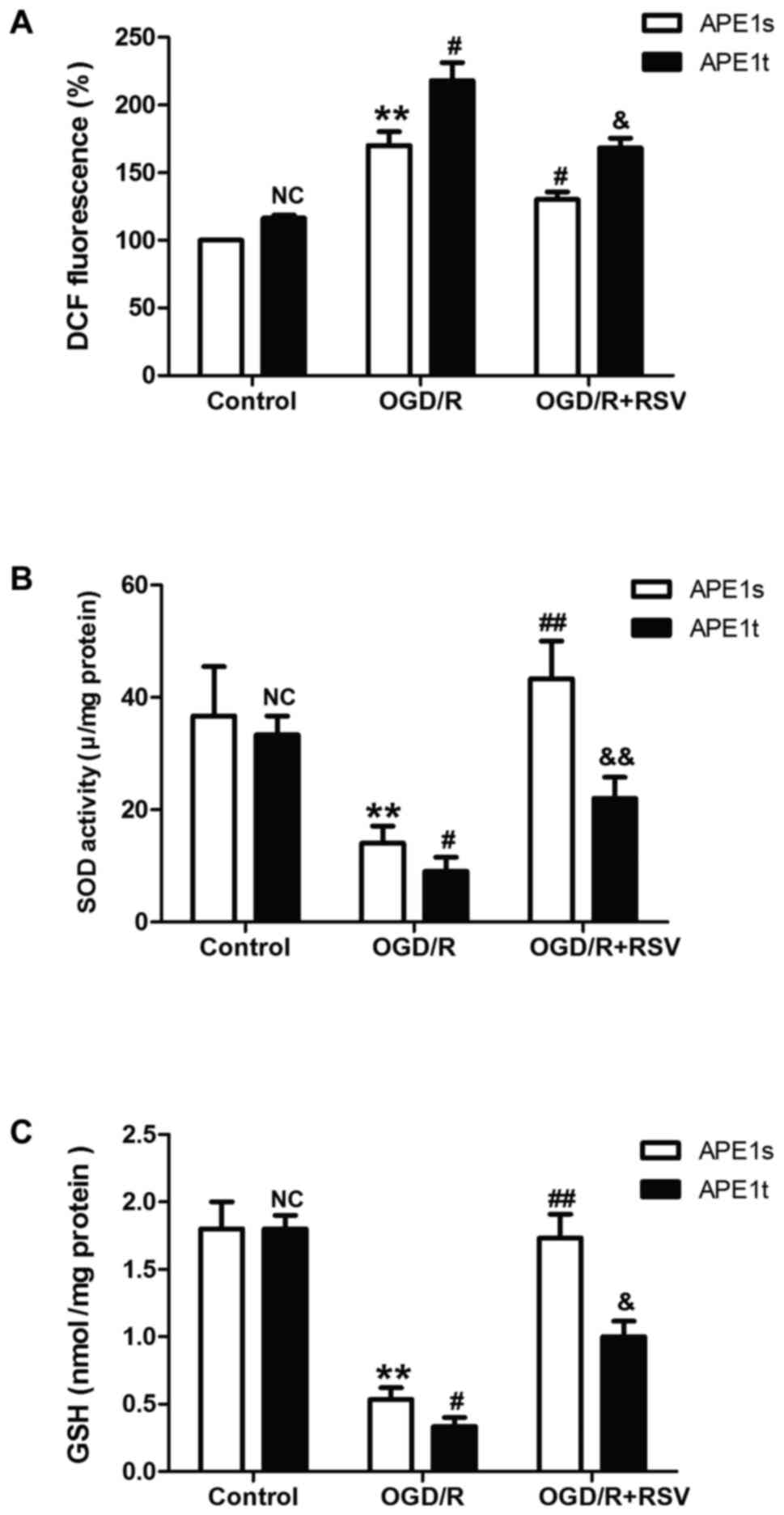
APE1 knockdown alleviates
resveratrol-elicited beneficent effects on oxidative stress under
OGD/R condition in HT22 cells
Emerging evidence reveals that APE1 functions in the
DNA base excision repair pathway, the redox regulation of several
transcription factors, and the control of intracellular redox
status through the inhibition of reactive oxygen species (ROS)
production. Therefore, we further demonstrated the effect of
resveratrol on OGD/R-induced oxidative stress and the role of APE1
in this process. As shown in Fig.
4, resveratrol pretreatment obviously abolished OGD/R-induced
the increase in the level of ROS (Fig.
4A). However, this effect was abolished by knockdown of APE1
with APE1t. The antioxidant system has been shown to be crucial for
detoxification in many cellular organ systems, so the status of the
enzymatic antioxidant superoxide dismutase (SOD) and nonenzymatic
antioxidant glutathione (GSH) were evaluated. We found that APE1t
transfection remarkably reversed resveratrol-prevented the
up-regulation of SOD (Fig. 4B) and
GSH activities (Fig. 4C) induced
by OGD/R treatment. These results suggested that APE1 contributes
to the protective effects of resveratrol against OGD/R-induced
oxidative stress, which may be related to attenuation of oxidative
stress and enhancement of antioxidant defense.
Discussion
Hypoxic-ischemic brain injury is an important
neurological disorder associated with neonatal death and long-term
disability, leading to approximately 6 million deaths every year
(23). As the standard of clinical
treatment, however, hypothermia has limited utility (24,25).
Recent research reveals that APE1-modulated oxidative DNA damage
plays an important role in cerebral ischemia injury (13,26,27).
Hence, the present study characterizes the beneficent impact of
APE1 on resveratrol (a neuroprotective agent)-mediated
neuroprotective effect in a cell model of cerebral ischemia injury.
Three major findings from this study contribute to our
understanding of the role of APE1 in the protective effects of
resveratrol against oxygen-glucose deprivation and re-oxygenation
(OGD/R)-induced HT22 cells injury. First, resveratrol reversed
OGD/R-induced cytotoxicity accompanied by increasing APE1 activity
and levels. Second, resveratrol alleviated OGD/R-induced oxidative
DNA damage as evidenced by the decreases in the levels of
8-hydroxy-2¢-deoxyguanosine (8-OHdG) and apurinic/apyrimidinic (AP)
sites. Third, APE1 knockdown blocked resveratrol-induced protective
effects on cytotoxicity and oxidative stress under OGD/R condition.
Our data suggests a promising therapeutic strategy to the cerebral
ischemia and reperfusion injury.
Resveratrol, a stilbene formed in many plants in
response to various stressors, elicits multiple beneficial effects
including anti-oxidative, anti-apoptotic and anti-inflammatory
properties in dozens of diseases (28). Particularly, resveratrol was shown
to have therapeutic properties in neuronal following ischemia
reperfusion injury (29,30). Resveratrol post-treatment protects
against neonatal brain injury after hypoxia-ischemia (20). Resveratrol can ameliorate oxidative
stress following rat cerebral ischemia-reperfusion injury (31). In present study, we also found that
resveratrol also reversed OGD/R-induced neurotoxicity in HT22
cells. Notably, the present show that the concentration of
resveratrol (10 µM) significantly increased the cell
viability compared to resveratrol (20 µM), which may be due
to that 10 µM reaches saturation value of resveratrol and
resveratrol may have inhibition on cell viability with the increase
of concentration.
Zaky et al confirmed that a reactive oxygen
species (ROS)-scavenger, resveratrol, attenuates central
inflammation and modulate APE1 expression in aluminum chloride
(AlCl3)-induced neurotoxicity (32). APE1, a multifunctional protein,
functions in DNA repair and plays a vital role in cell survival vs.
death upon stimulation with cytotoxic agents, making it an
attractive emerging therapeutic target (32). Emerging evidence confers the
beneficent effects of APE1 on cerebral ischemia. Stetler et
al prove that endogenous APE1 protects against ischemic
infarction in gray and white matter and facilitates the functional
recovery of central nervous system (CNS) after mild stroke injury
(26). Leak et al also
prove that APE1 up-regulation, either endogenously or through
transgene overexpression, reduce oxidative DNA damage and protect
hippocampal neurons from ischemic injury (27). In addition, some other studies
reveal that APE1 is involved in neuroprotective agents such as
17β-estradiol (E2) and pituitary adenylate cyclase-activating
polypeptide (PACAP) against ischemia-induced damage (13,33).
Therefore, we put forward the hypothesis that APE1 may contribute
to the neuroprotective effect of resveratrol against cerebral
ischemic injury.
Consistent with these studies, in current study, we
found that resveratrol pretreatment significantly increased the
activity and the level of APE1. A major hallmark of oxidative DNA
damage after stroke is the induction of apurinic/apyrimidinic (AP)
sites and strand breaks (4,6).
APE1 can repair AP sites during base-excision repair (BER)
(3). Research has shown that
adenovirus-mediated APE1 upregulation reduces 8-OHdG formation, AP
sites, DNA fragmentation, and infarct volume after
ischemia-reperfusion injury (34).
In agreement with these studies, the present study found that
resveratrol treatment decreased the level of 8-OHdG and AP sites
under ODG/R condition in HT22 cells, further indicating the
inconvenient role of APE1 in resveratrol-offered protective effect
against oxidative DNA damage in cerebral ischemia reperfusion
injury.
Furthermore, we found that APE1 knockdown induced by
APE1 shRNA abolished resveratrol-induced the increases in the cell
viability and decreases in the LDH release, indicating the
contribution of APE1 to the protective effect of resveratrol
against OGD/R injury. Increasing evidence confirms that inhibition
of oxidative stress of and promotion of antioxidant signaling
contributes to neuroprotective agent against ischemia-reperfusion
damage to rat brains (35,36). APE1 is a multifunctional protein
that plays a vital role in the cellular response to DNA injury and
redox regulation against oxidative stress through the inhibition of
reactive oxygen species (ROS) production (37). The present study also further
showed that APE1 knockdown increased the level of ROS and increased
the activity of SOD and GSH compared to OGD/R treatment in HT22
cells. Combined with the previous research related to the
relationship between APE1 and oxidative stress, these results
suggested that APE1 contributes to the protective effects of
resveratrol against OGD/R-induced oxidative stress, which may be
related to attenuation of oxidative stress and enhancement of
antioxidant defense. However, the protective effect of resveratrol
was not completely inhibited by APE1 shRNA. These might be because
APE1 shRNA did not completely reduced the APE1 shRNA. In addition,
many other studies reveal that resveratrol-activated pathways have
been shown to protect against ischemia through modulating SIRT1
activity (38), autophagy
(39) and NO signaling (40), further implying that there are
further mechanisms involved the protective effects of resveratrol
against cerebral ischemia reperfusion injury in independent of APE1
pathway.
Of course, there are many deficiencies in preset
article. We discussed the role of APE1 in resveratrol-elicited
protective effect in cerebral ischemia injury only in terms of its
antioxidant stress activity. Many researches revel that both APE1
and resveratrol have anti-apoptotic and anti-inflammatory
activities (41–43), implying that apoptosis may also
contribute to the role of APE1 in these. In addition, we only use
HT22 cells to test the hypothesis. In the next experiment, we can
further investigate the underlying mechanism in in vivo or
primary cell models.
In conclusion, in the current study, it was observed
that resveratrol significantly decreases OGD/R-induced
neurotoxicity through increasing APE1 level and activity. The
results further indicate the neuroprotective effects of resveratrol
against cerebral ischemia injury are associated with APE1-elicited
reduction of oxidative DNA damage involved in attenuation of
oxidative stress and enhancement of antioxidant defense. Some other
antioxidants such as Picroside II (44), nanomelatonin (45), and A water-soluble polysaccharide
(LJPB2) (46) have been proved to
protect against cerebral ischemia-reperfusion injury dependent on
strong antioxidant capacity. Our study further provide new
perspectives that APE1 may also be involved in the neuroprotective
effect of these antioxidants.
References
|
1
|
Roger VL, Go AS, Lloyd-Jones DM, Adams RJ,
Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, et
al: Heart disease and stroke statistics-2011 update: A report from
the American Heart Association. Circulation. 123:e18–e209. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Blakeley JO and Llinas RH: Thrombolytic
therapy for acute ischemic stroke. J Neurol Sci. 261:55–62. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li P, Hu X, Gan Y, Gao Y, Liang W and Chen
J: Mechanistic insight into DNA damage and repair in ischemic
stroke: Exploiting the base excision repair pathway as a model of
neuroprotection. Antioxid Redox Signal. 14:1905–1918. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen J, Jin K, Chen M, Pei W, Kawaguchi K,
Greenberg DA and Simon RP: Early detection of DNA strand breaks in
the brain after transient focal ischemia: Implications for the role
of DNA damage in apoptosis and neuronal cell death. J Neurochem.
69:232–245. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen H, Yoshioka H, Kim GS, Jung JE, Okami
N, Sakata H, Maier CM, Narasimhan P, Goeders CE and Chan PH:
Oxidative stress in ischemic brain damage: Mechanisms of cell death
and potential molecular targets for neuroprotection. Antioxid Redox
Signal. 14:1505–1517. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lan J, Li W, Zhang F, Sun FY, Nagayama T,
O'Horo C and Chen J: Inducible repair of oxidative DNA lesions in
the rat brain after transient focal ischemia and reperfusion. J
Cereb Blood Flow Metab. 23:1324–1339. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dyrkheeva NS, Lebedeva NA and Lavrik OI:
AP Endonuclease 1 as a key enzyme in repair of
apurinic/apyrimidinic sites. Biochemistry (Mosc). 81:951–967. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Laev SS, Salakhutdinov NF and Lavrik OI:
Inhibitors of nuclease and redox activity of apurinic/apyrimidinic
endonuclease 1/redox effector factor 1 (APE1/Ref-1). Bioorg Med
Chem. 25:2531–2544. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu PK: DNA damage and repair in the brain
after cerebral ischemia. Curr Top Med Chem. 1:483–495. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vasko MR, Guo C and Kelley MR: The
multifunctional DNA repair/redox enzyme Ape1/Ref-1 promotes
survival of neurons after oxidative stress. DNA Repair (Amst).
4:367–379. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ludwig DL, MacInnes MA, Takiguchi Y,
Purtymun PE, Henrie M, Flannery M, Meneses J, Pedersen RA and Chen
DJ: A murine AP-endonuclease gene-targeted deficiency with
post-implantation embryonic progression and ionizing radiation
sensitivity. Mutat Res. 409:17–29. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Singh S and Englander EW: Nuclear
depletion of apurinic/apyrimidinic endonuclease 1 (Ape1/Ref-1) is
an indicator of energy disruption in neurons. Free Radic Biol Med.
53:1782–1790. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stetler RA, Gao Y, Zukin RS, Vosler PS,
Zhang L, Zhang F, Cao G, Bennett MV and Chen J:
Apurinic/apyrimidinic endonuclease APE1 is required for
PACAP-induced neuroprotection against global cerebral ischemia.
Proc Natl Acad Sci USA. 107:pp. 3204–3209. 2010; View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakata R, Takahashi S and Inoue H: Recent
advances in the study on resveratrol. Biol Pharm Bull. 35:273–279.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yin K, Zhao L, Feng D, Ma W, Liu Y, Wang
Y, Liang J, Yang F, Bi C, Chen H, et al: Resveratrol attenuated low
ambient temperature-induced myocardial hypertrophy via inhibiting
cardiomyocyte apoptosis. Cell Physiol Biochem. 35:2451–2462. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nie P, Hu W, Zhang T, Yang Y, Hou B and
Zou Z: Synergistic induction of erlotinib-mediated apoptosis by
resveratrol in human non-small-cell lung cancer cells by
down-regulating survivin and up-regulating PUMA. Cell Physiol
Biochem. 35:2255–2271. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mokni M, Elkahoui S, Limam F, Amri M and
Aouani E: Effect of resveratrol on antioxidant enzyme activities in
the brain of healthy rat. Neurochem Res. 32:981–987. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hung LM, Su MJ and Chen JK: Resveratrol
protects myocardial ischemia-reperfusion injury through both
No-dependent and NO-independent mechanisms. Free Radic Biol Med.
36:774–781. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Eybl V, Kotyzova D, Cerná P and Koutensky
J: Effect of melatonin, curcumin, quercetin, and resveratrol on
acute ferric nitrilotriacetate (Fe-NTA)-induced renal oxidative
damage in rats. Hum Exp Toxicol. 27:347–353. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pan S, Li S, Hu Y, Zhang H, Liu Y, Jiang
H, Fang M, Li Z, Xu K, Zhang H, et al: Resveratrol post-treatment
protects against neonatal brain injury after hypoxia-ischemia.
Oncotarget. 7:79247–79261. 2016.PubMed/NCBI
|
|
21
|
Abdel-Aleem GA, Khaleel EF, Mostafa DG and
Elberier LK: Neuroprotective effect of resveratrol against brain
ischemia reperfusion injury in rats entails reduction of DJ-1
protein expression and activation of PI3K/Akt/GSK3b survival
pathway. Arch Physiol Biochem. 122:200–213. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Feng Y, Cui Y, Gao JL, Li MH, Li R, Jiang
XH, Tian YX, Wang KJ, Cui CM and Cui JZ: Resveratrol attenuates
neuronal autophagy and inflammatory injury by inhibiting the
TLR4/NF-κB signaling pathway in experimental traumatic brain
injury. Int J Mol Med. 37:921–930. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hua C, Ju WN, Jin H, Sun X and Zhao G:
Molecular chaperones and hypoxic-ischemic encephalopathy. Neural
Regen Res. 12:153–160. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu Q, Chen W, Sinha B, Tu Y, Manning S,
Thomas N, Zhou S, Jiang H, Ma H, Kroessler DA, et al:
Neuroprotective agents for neonatal hypoxic-ischemic brain injury.
Drug Discov Today. 20:1372–1381. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Silveira RC and Procianoy RS: Hypothermia
therapy for newborns with hypoxic ischemic encephalopathy. J
Pediatr (Rio J). 91(6 Suppl 1): S78–S83. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Stetler RA, Gao Y, Leak RK, Weng Z, Shi Y,
Zhang L, Pu H, Zhang F, Hu X, Hassan S, et al: APE1/Ref-1
facilitates recovery of gray and white matter and neurological
function after mild stroke injury. Proc Natl Acad Sci USA. 113:pp.
E3558–E3567. 2016; View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Leak RK, Li P, Zhang F, Sulaiman HH, Weng
Z, Wang G, Stetler RA, Shi Y, Cao G, Gao Y and Chen J:
Apurinic/apyrimidinic endonuclease 1 upregulation reduces oxidative
DNA damage and protects hippocampal neurons from ischemic injury.
Antioxid Redox Signal. 22:135–148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lopez MS, Dempsey RJ and Vemuganti R:
Resveratrol neuroprotection in stroke and traumatic CNS injury.
Neurochem Int. 89:75–82. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kizmazoglu C, Aydin HE, Sevin IE, Kalemci
O, Yüceer N and Atasoy MA: Neuroprotective Effect of Resveratrol on
Acute Brain Ischemia Reperfusion Injury by Measuring Annexin V,
p53, Bcl-2 Levels in Rats. J Korean Neurosurg Soc. 58:508–512.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang R, Liu YY, Liu XY, Jia SW, Zhao J,
Cui D and Wang L: Resveratrol protects neurons and the myocardium
by reducing oxidative stress and ameliorating mitochondria damage
in a cerebral ischemia rat model. Cell Physiol Biochem. 34:854–864.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li W, Tan C, Liu Y, Liu X, Wang X, Gui Y,
Qin L, Deng F, Yu Z, Hu C and Chen L: Resveratrol ameliorates
oxidative stress and inhibits aquaporin 4 expression following rat
cerebral ischemia-reperfusion injury. Mol Med Rep. 12:7756–7762.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zaky A, Mohammad B, Moftah M, Kandeel KM
and Bassiouny AR: Apurinic/apyrimidinic endonuclease 1 is a key
modulator of aluminum-induced neuroinflammation. BMC Neurosci.
14:262013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dietrich AK, Humphreys GI and Nardulli AM:
17β-estradiol increases expression of the oxidative stress response
and DNA repair protein apurinic endonuclease (Ape1) in the cerebral
cortex of female mice following hypoxia. J Steroid Biochem Mol
Biol. 138:410–420. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim HW, Cho KJ, Park SC, Kim HJ and Kim
GW: The adenoviral vector-mediated increase in
apurinic/apyrimidinic endonuclease inhibits the induction of
neuronal cell death after transient ischemic stroke in mice. Brain
Res. 1274:1–10. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li Y and Liu S: The effect of
dexmedetomidine on oxidative stress response following cerebral
ischemia-reperfusion in rats and the expression of intracellular
adhesion molecule-1 (ICAM-1) and S100B. Med Sci Monit. 23:867–873.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xiao H, Deng M, Yang B, Hu Z and Tang J:
Pre-treatment of 17β-estradiol attenuates cerebral-ischemia-induced
blood-brain barrier disruption in aged rats: Involvement of
antioxidant signaling. Neuroendocrinology. Feb 15–2017.(Epub ahead
of print). View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Choi S, Joo HK and Jeon BH: Dynamic
regulation of APE1/Ref-1 as a therapeutic target protein. Chonnam
Med J. 52:75–80. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Howitz KT, Bitterman KJ, Cohen HY, Lamming
DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL,
et al: Small molecule activators of sirtuins extend Saccharomyces
cerevisiae lifespan. Nature. 425:191–196. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gurusamy N, Lekli I, Mukherjee S, Ray D,
Ahsan MK, Gherghiceanu M, Popescu LM and Das DK: Cardioprotection
by resveratrol: A novel mechanism via autophagy involving the
mTORC2 pathway. Cardiovasc Res. 86:103–112. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Malhotra A, Bath S and Elbarbry F: An
organ system approach to explore the antioxidative,
anti-inflammatory and cytoprotective actions of resveratrol. Oxid
Med Cell Longev. 2015:8039712015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Thakur S, Sarkar B, Cholia RP, Gautam N,
Dhiman M and Mantha AK: APE1/Ref-1 as an emerging therapeutic
target for various human diseases: Phytochemical modulation of its
functions. Exp Mol Med. 46:e1062014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xu Y, Zhang B, Xie D, Hu Y, Li HL, Zhong
LL, Wang HW, Jiang W, Ke ZP and Zheng DH: Nanoparticle-mediated
dual delivery of resveratrol and DAP5 ameliorates kidney
ischemia/reperfusion injury by inhibiting cell apoptosis and
inflammation. Oncotarget. 8:39547–39558. 2017.PubMed/NCBI
|
|
43
|
Kolahdouz Mohammadi R and Arablou T:
Resveratrol and endometriosis: In vitro and animal studies and
underlying mechanisms (Review). Biomed Pharmacother. 91:220–228.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhai L, Liu M, Wang T, Zhang H, Li S and
Guo Y: Picroside II protects the blood-brain barrier by inhibiting
the oxidative signaling pathway in cerebral ischemia-reperfusion
injury. PLoS One. 12:e01744142017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sarkar S, Mukherjee A, Das N and Swarnakar
S: Protective roles of nanomelatonin in cerebral
ischemia-reperfusion of aged brain: Matrixmetalloproteinases as
regulators. Exp Gerontol. 92:13–22. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Su D, Li S, Zhang W, Wang J, Wang J and Lv
M: Structural elucidation of a polysaccharide from Lonicera
japonica flowers and its neuroprotective effect on cerebral
ischemia-reperfusion injury in rat. Int J Biol Macromol.
99:350–357. 2017. View Article : Google Scholar : PubMed/NCBI
|