Introduction
Lung cancer is a commonly diagnosed cancer in
developed countries and has poor rate of prognosis (1). The treatments currently available for
lung cancer include surgical extraction at an early stage and the
use of the chemotherapeutic agents (2–4).
However, despite these strategies, tumor recurrence is reported in
the majority of patients (2–4).
Therefore, the development of novel efficient treatment strategies
for lung cancer is warranted. A tumor mass consists of a
combination of carcinoma cells, fibroblasts and inflammatory cells
(5). Stromal fibroblasts possess
markedly higher levels of a protein known as α-smooth muscle actin
(α-SMA) (6,7). These stromal fibroblasts secrete
various factors, which enhance the rate of carcinoma cell
proliferation, and increase the invasive and migration potential of
cancer cells (8–11). Studies have revealed that stromal
formation in various types of malignant carcinoma is facilitated by
platelet-derived growth factor (PDGF) (12–14),
and there are interactions between receptors for tyrosine kinase
and PDGF (15).
Natural products offer promise in the treatment of
various types of diseases without producing harmful side effects
due to their modification in the living systems over millions of
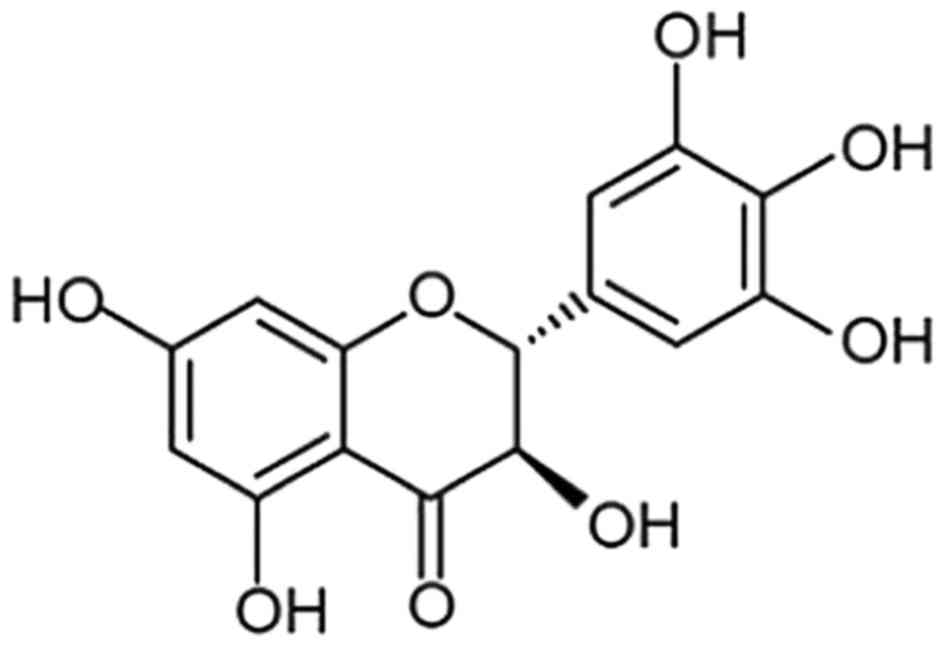
years. Dihydromyricetin (Fig. 1),
a member of the flavonoid family, was obtained following the
phytochemical investigation of Ampelopsis grossedentata
(16). The biological evaluation
of dihydromyricetin has shown its promising potential as an
anti-oxidant, antithrombolytic and anti-inflammatory agent
(16–18). Dihydromyricetin has been shown to
inhibit the peroxidation of membrane lipids in various types of
cell and also act as hepatoprotective agent (19,20).
The present study was performed to investigate the effect of
dihydromyricetin on the growth of fibroblasts in lung carcinoma.
The results demonstrated that dihydromyricetin inhibited the
proliferation of fibroblasts through the inhibition of
extracellular signal-regulated kinase (Erk)1/2 and Akt.
Materials and methods
Reagents and drugs
Dihydromyricetin and dimethyl sulfoxide (DMSO) were
purchased from Sigma-Aldrich; Merck Millipore (Darmstadt, Germany).
PDGF-BB was obtained from Gibco; Thermo Fisher Scientific, Inc.
(Waltham, MA USA).
Cell lines and culture
The A549 lung carcinoma cell line was obtained from
the China Center for Type Culture Collection (Wuhan, China). The
A549 cells were cultured in Dulbecco's modified Eagle's medium
(Gibco; Thermo Fisher Scientific, Inc.) containing 10% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc.). The medium was
supplemented with antibiotics streptomycin (50 mg/ml) and
penicillin (50 IU/ml), and glutamine (2 mM). The cells were
cultured in a humidified atmosphere of 5% CO2 and 95%
air at 37°C.
Fibroblast culture
The lung carcinoma tissue samples were obtained
immediately following collection and were stored under a liquid
nitrogen atmosphere. Written consent was obtained from the patients
(4 patients, 3 males and 1 female in the age group of 45–60 years
with lung cancer admitted in the PLA General Hospital, Beijing
China on 23-04-2016) confirming that they were aware of the type of
investigation being performed. The study was approved by the local
ethics committee of PLA General Hospital (Beijing, China). The thin
lung tissue samples (30 µM3 thickness) were subjected to
incubation for 1 h in DMEM/F-12 supplemented with dispase (2,500
U/ml) and collagenase Type IV (450 U/ml). Following incubation, the
primary fibroblasts were then cultured in DMEM/F-12 supplemented
with penicillin (50,000 U/ml) and streptomycin (50,000 µg/ml). The
fibroblasts were cultured overnight in a humidified atmosphere of
5% CO2 and 95% air at 37°C. The fibroblasts obtained
from the normal and carcinoma tissues were designated as FKs and
CKs, respectively.
Immunohistochemistry
The lung cancer tissue samples were washed with PBS
and then embedded in paraffin. The tissues were sectioned into
2-µm-thin sections, boiled in xylene and rehydrated in gradient
ethanol. The activity of endogenous peroxidase in the sections was
quenched by treatment with 0.3% H2O2. The
sections were then incubated with primary antibodies against α-SMA
(cat. no. A03744; dilution 1:200; Boster Biological Technology,
Wuhan, China) for 2 h at room temperature. Visualization of the
samples was performed using a Histofine Simple Stain MAX-PO (Multi)
and DAB substrate kit (Nichirei Biosciences, Inc., Tokyo, Japan).
The counterstaining was performed using hematoxylin at 37°C for 45
min and images were captured with a light microscope (Nikon Eclipse
E800; Berlin, Germany) equipped with a Spot RT digital camera
(Diagnostic Instruments Inc, Sterling Heights, CA, USA).
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
From the fibroblasts and the A549 cells treated with
dihydromyricetin for 48 h, total RNA was isolated using TRIzol
reagent (Beyotime Institute of Biotechnology, Shanghai, China). The
first-strand cDNA synthesis was performed using reverse
transcriptase (Beyotime Institute of Biotechnology) with 2 µg
samples of RNA. The Premix Ex Taq kit (Takara Biotechnology Co.,
Ltd., Dalian, China) was used for performing the RT-PCR analysis.
Transcript amplification was carried out by PCR as follows: 2 µl
template complimentary DNA (cDNA), 0.2 mM dNTPs, 1.25 U/µl Go Taq
polymerase (Promega, Corporation, Madison, WI, USA) and various
primer sets (0.5 µM each) in 50 µl water. The denaturation was
performed for 5 min prior to 40 thermal cycles of 95°C for 1 min,
52°C for 25 sec and 75°C for 1 min, with a final extension step at
75°C for 10 min. Following amplification, the PCR products were
subjected to electrophoresis on 1% agarose gels at 80 V for 45 min
and were subsequently visualized using ethidium bromide staining.
The primer sequences are as follows: α-SMA forward,
5′-CCAACCGGGAGAAAATGACTCAAATT-3′ and reverse,
5′-AGGCAACTCGTAACTCTTCTCAAGG-3′; FAP forward,
5′-CCAGCAATGATAGCCTCAA-3′ and reverse, 5′-TAACACACTTCTTGCTTGGA-3′;
PDGFRα forward, 5′-GAAGCTGTCAACCTGCATGA-3′ and reverse,
5′-CTTCCTTAGCACGGATCAGC-3′; PDGFRβ forward,
5′-GTGAACGCAGTGCAGACTGT-3′ and reverse, 5′-AGGTGTAGGTCCCCGAGTCT-3′;
GAPDH Forward, 5′-TGAAGGTCGGAGTCAACGGATTTGGT-3′ and reverse,
5′-CATGTGGGCCATGAGGTCCACCAC−3′.
Western blot analysis
The expression levels of factors, including PDGFRβ,
p-PDGFRβ, Akt, p-Akt Erk1/2 and p-Erk1/2, were analyzed in the
fibroblasts following treatment with dihydromyricetin. The FKs and
CKs were treated with lysis buffer under ice-cold conditions for 45
min. The lysates were then centrifuged at 12,000 × g for 20 min at
4°C and the concentration of proteins in the supernatants was
determined using a bicinchoninic assay. Equal quantities of the
cell lysates (40 µg protein) were subjected to separation via
SDS-PAGE on 10% a gel and subsequently transferred onto
polyvinylidene difluoride membranes. Blocking of the membranes was
performed by incubation with 5% non-fat milk at 37°C for 1 h. The
membranes were then incubated with PDGF receptor β (PDGFRβ; cat.
no. P3082; dilution 1:1,000), Erk1/2 (cat. no. 62206; dilution
1:1,000), phosphorylated (p)-Akt (cat. no. KHO0201; dilution
1:1,000; all the three from Thermo Fisher Scientific Inc.; MK3),
Akt (cat. no. 200-401-N98; dilution 1:1,000; Rockland,
Gilbertsville, PA, USA), p-PDGFRβ (ab16868; dilution 1:1,000;
Abcam) and p-Erk1/2 (FCMAB100P; dilution 1:1,000; EMD Millipore
(Darmstadt, Germany) primary antibodies (at 1:1000 dilution)
overnight at 4°C and washed with TBST for 5 min. β-actin (1:500,
sc-47778; Santa Cruz Biotechnology Inc., Dallas, TX, USA) was used
as an internal loading control. The membranes were then incubated
with horseradish peroxidase-conjugated secondary antibodies (cat.
no. MPX-2402; 1:5,000 dilution in TBST containing 1% bovine serum
albumin; Santa Cruz Biotechnology, Inc.) at room temperature for 1
h. Analysis of the bands was performed using an enhanced
chemiluminescence blotting detection system (FluorChem E;
Proteinsimple, Santa Clara, CA, USA).
Cell proliferation assay
The effects of dihydromyricetin treatment on the
rates of lung cancer cell and fibroblast proliferation were
analyzed using an MTT assay. The cells were distributed at a
density of 2×106 cells per well in 96-well culture
plates and incubated for 24 h at 37°C. Following incubation, the
medium was replaced with medium containing various concentrations
(1, 5, 10 µM) of dihydromyricetin. The cells in the control wells
were treated with DMSO alone. After incubation for 48 h at 37°C,
MTT solution (5 mg/ml) was added into each well of the plate and
the plates were incubated at a temperature of 37°C for 4 h. The
culture medium was removed carefully and DMSO was added to
solubilize the formazan crystals. The absorbance measurement for
each well was performed at 570 nm using a microplate reader
(PerkinElmer, Inc., Waltham, MA, USA). All experiments were
performed in triplicate.
Statistical analysis
All data are presented as the mean ± standard
deviation of experiments performed in triplicate. Comparisons among
multiple groups were performed using a one-way analysis of
variance. Statistical analysis was performed using SPSS 180
statistical software (SPSS, Inc., Chicago, IL, USA). In all cases,
comparisons were made relative to untreated controls. P<0.05 was
considered to represent a statistically significant difference.
Results
Levels of PDGFR and PDGF in lung
carcinoma cells and fibroblasts
The results from the RT-PCR analysis showed markedly
higher expression of α-SMA and fibroblast activation protein in the
fibroblasts (Fig. 2). The
expression of PDGF-A and PDGF-B were markedly lower in the
fibroblasts compared to lung carcinoma cells. In the lung cancer
cells, the expression levels of PDGFRα and PDGFRβ were markedly
lower in comparison to the levels in the fibroblasts (Fig. 2).
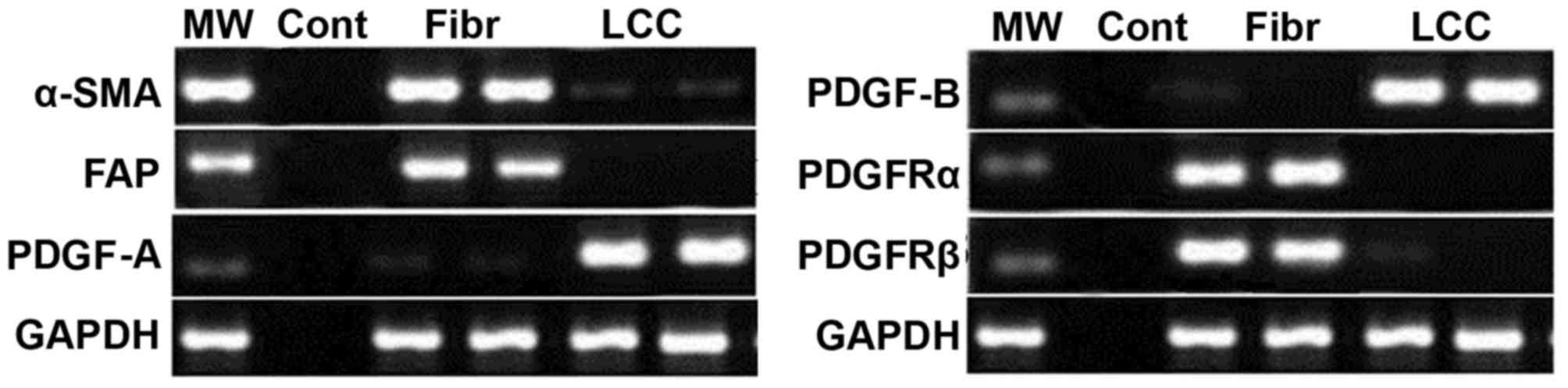 | Figure 2.Expression levels of α-SMA, PDGF-A,
PDGF-B and its PDGFR in fibroblasts and lung cancer cells. The
expression of the negative control, fibroblasts and lung cancer
cells were analyzed using reverse transcription-polymerase chain
reaction analysis to determine the expression levels of these
factors. α-SMA, α-smooth muscle actin; FAP, fibroblast activation
protein; PDGF, platelet-derived growth factor; PDGFR, PDGF
receptor; MW, marker; Con, negative control; Fibr, fibroblasts;
LCC, lung cancer cells. |
Effect of dihydromyricetin on the
PDGFR activity of tyrosine kinase in fibroblasts
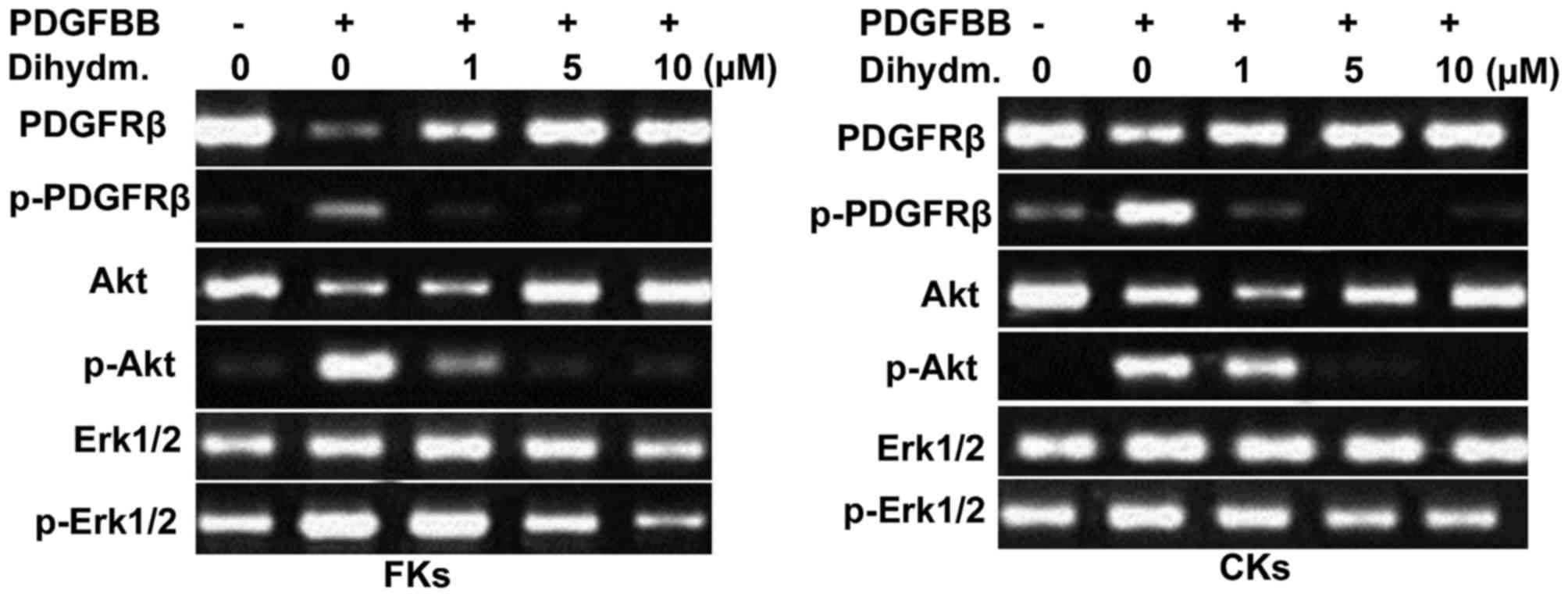
The results from western blot analysis of the
lysates from the FK and CK fibroblasts showed markedly higher
activity of PDGFRβ (Fig. 3).
Treatment of the fibroblasts with different concentrations of
dihydromyricetin caused suppression in the activity of PDGFRß in a
dose-dependent manner. The inhibited activation of PDGFRβ was
maximal following treatment at a dihydromyricetin concentration of
10 µM for 48 h. However, dihydromyricetin caused no alteration in
the expression of the protein, PDGFRβ in the fibroblasts (Fig. 3).
Effect of dihydromyricetin on the
activation of phosphoinositide 3-kinase-Akt and
Ras-mitogen-activated protein kinase (MAPK) Erk1/2
Incubation of the FK and CK fibroblasts with PDGF-BB
caused a significant increase in the activation of Erk1/2 and Akt.
Dihydromyricetin treatment for 48 h led concentration-dependent
inhibition of the activation of Erk1/2 and Akt in the fibroblasts
(Fig. 3). The activation of Erk1/2
and Akt in the fibroblasts was completely inhibited following 48 h
of treatment with a 10 µM concentration of dihydromyricetin.
Effect of dihydromyricetin on the
proliferation of fibroblasts induced by PDGF
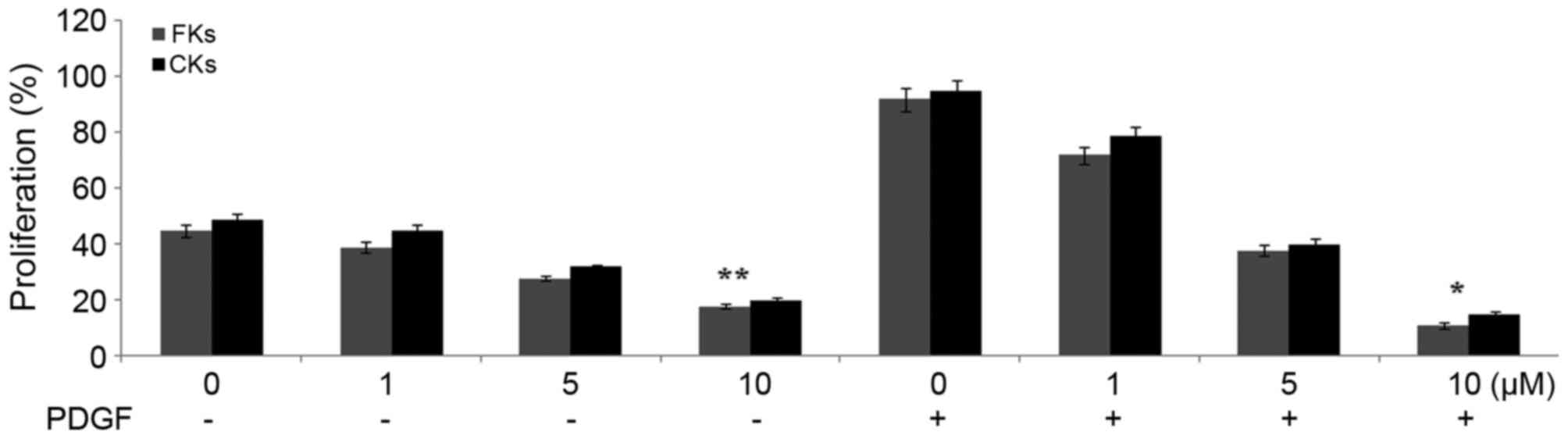
The results of the MTT assay showed a significant
increase in the proliferation rates of the FK and CK fibroblasts
following incubation with PDGF. Treatment of the fibroblasts with
dihydromyricetin reduced the PDGF-mediated increase in the rates of
proliferation (Fig. 4). The
fibroblasts were treated with various concentrations of
dihydromyricetin and the maximum inhibition of proliferation was
observed with a concentration of 10 µM.
Effect of dihydromyricetin on the
proliferation rate of lung cancer cells cultured with
fibroblasts
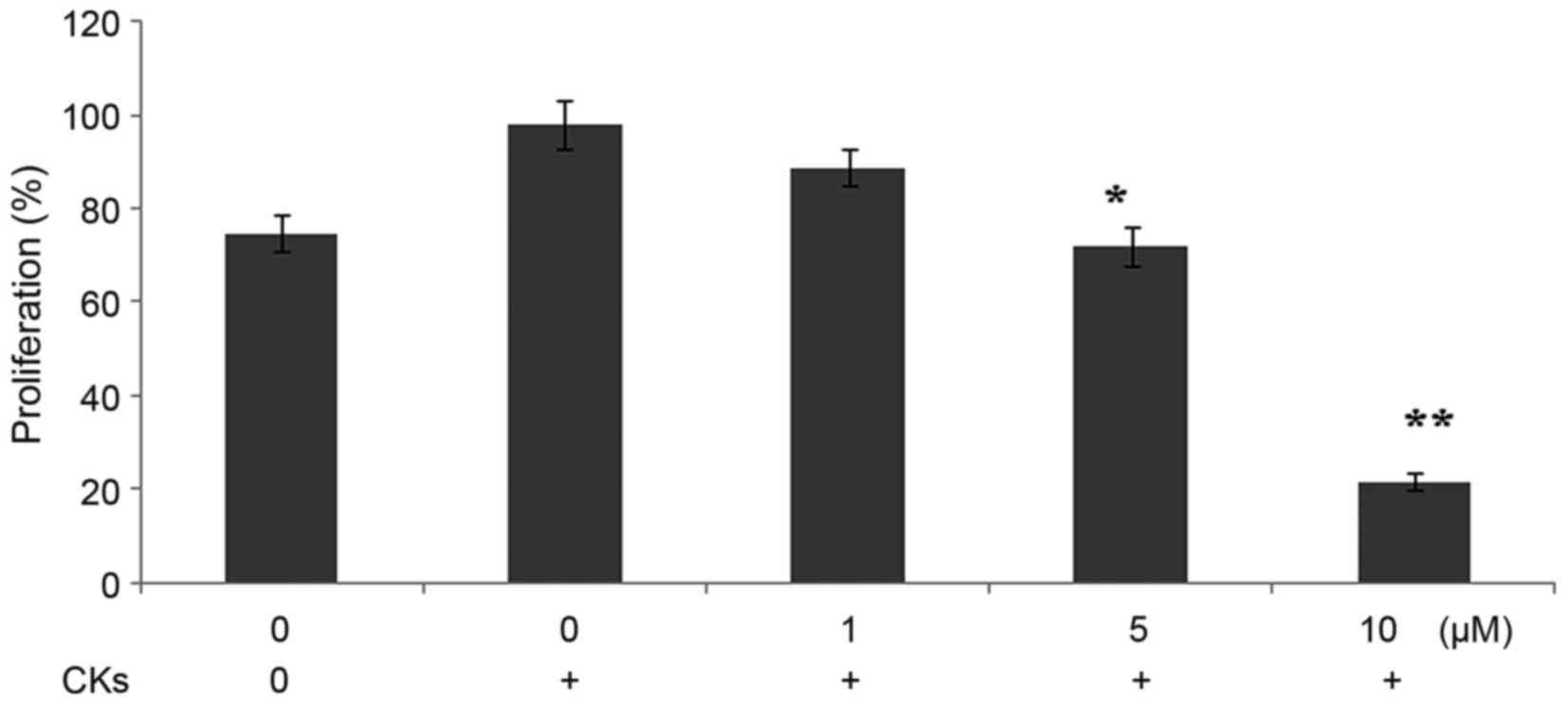
The rate of proliferation of the A549 lung cancer
cells cultured with CK fibroblasts was higher, compared with that
in A549 cells cultured alone. However, dihydromyricetin
significantly (P<0.05) inhibited the rate of proliferation of
A549 cells cultured with CKs, compared with the untreated cultures
(Fig. 5). The proliferation rates
of the A549 cancer cells, A549 cells cultured with CK fibroblasts
and A549 cells cultured with CK fibroblasts and treated with
dihydromyricetin were 78.45, 98.45 and 21.37%, respectively.
Discussion
The aim of the present study was to investigate the
effect of dihydromyricetin on the proliferation of fibroblasts in
lung carcinoma. The study demonstrated the inhibition of fibroblast
proliferation by dihydromyricetin via targeting the activation of
Erk1/2 and Akt.
The tumor stroma exhibits a promoting effect on the
development of carcinoma, in which fibroblasts are the major
contributing factor (8–11). It has been suggested that
fibroblasts constitute a vital target for the treatment of tumors
(21–24). The present study revealed that
incubation of lung cancer cells with fibroblasts markedly increased
the rate of proliferation, compared with cancer cells cultured
alone. However, the fibroblast-induced proliferation of lung cancer
cells was significantly inhibited following treatment with
dihydromyricetin, in a concentration-dependent manner. There are
reports that carcinoma cells produce PDGFs, which enhance the
proliferation rate of fibroblasts in various tumor xenograft
carcinoma models, including breast cancer, lung cancer, melanoma
and colorectal cancer (12,13,25,26).
The results of the present study demonstrated markedly higher
expression levels of α-SMA and fibroblast activation protein in the
fibroblasts. The expression of PDGF-A was also markedly higher in
the fibroblasts. The activities of PDGFRβ in the lysates from FK
and CK fibroblasts were also markedly higher, as revealed using
western blot analysis. Treatment of the fibroblasts with different
concentrations of dihydromyricetin caused suppression of PDGFRβ in
a dose-dependent manner. However, dihydromyricetin did not alter
the protein expression of PDGFRβ in fibroblasts.
The incubation of FK and CK fibroblasts with PDGF-BB
caused a significant increase in the activation of Erk1/2 and Akt.
Dihydromyricetin treatment for 48 h led to a
concentration-dependent inhibition of the activation of Erk1/2 and
Akt in the fibroblasts. The MTT assay showed a significant increase
in the proliferation rates of the FK and CK fibroblasts following
incubation with PDGF. Treatment of the fibroblasts with
dihydromyricetin reduced the PDGF-mediated increase in the rate of
proliferation. The fibroblasts were treated with various
concentrations of dihydromyricetin and the maximum inhibition of
proliferation was observed at a concentration of 10 µM.
In conclusion, dihydromyricetin inhibited the
proliferative potential of fibroblasts on lung cancer cells through
targeting the activation of Erk1/2 and Akt. Therefore,
dihydromyricetin offers potential for further evaluation for the
treatment of lung cancer.
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Asamura H, Goya T, Koshiishi Y, Sohara Y,
Eguchi K, Mori M, Nakanishi Y, Tsuchiya R, Shimokata K, Inoue H, et
al: A Japanese lung cancer registry study: Prognosis of 13,010
resected lung cancers. J Thorac Oncol. 3:46–52. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mountain CF: Revisions in the
international system for staging lung cancer. Chest. 111:1710–1717.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsuboi M, Ohira T, Saji H, Miyajima K,
Kajiwara N, Uchida O, Usuda J and Kato H: The present status of
postoperative adjuvant chemotherapy for completely resected
non-small cell lung cancer. Ann Thorac Cardiovasc Surg. 13:73–77.
2007.PubMed/NCBI
|
|
5
|
Tremblay G: Stromal aspects of breast
carcinoma. Exp Mol Pathol. 31:248–260. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gabbiani G, Ryan GB and Majne G: Presence
of modified fibroblasts in granulation tissue and their possible
role in wound contraction. Experientia. 27:549–550. 1971.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rønnov-Jessen L, Petersen OW, Koteliansky
VE and Bissell MJ: The origin of the myofibroblasts in breast
cancer. Recapitulation of tumor environment in culture unravels
diversity and implicates converted fibroblasts and recruited smooth
muscle cells. J Clin Invest. 95:859–873. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Olumi AF, Grossfeld GD, Hayward SW,
Carroll PR, Tlsty TD and Cunha GR: Carcinoma-associated fibroblasts
direct tumor progression of initiated human prostatic epithelium.
Cancer Res. 59:5002–5011. 1999.PubMed/NCBI
|
|
9
|
Okusa Y, Ichikura T and Mochizuki H:
Prognostic impact of stromal cell-derived urokinase-type
plasminogen activator in gastric carcinoma. Cancer. 85:1033–1038.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Orimo A, Gupta PB, Sgroi DC,
Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL
and Weinberg RA: Stromal fibroblasts present in invasive human
breast carcinomas promote tumor growth and angiogenesis through
elevated SDF-1/CXCL12 secretion. Cell. 121:335–348. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hasebe T, Sasaki S, Imoto S and Ochiai A:
Highly proliferative fibroblasts forming fibrotic focus govern
metastasis of invasive ductal carcinoma of the breast. Mod Pathol.
14:325–337. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Forsberg K, Valyi-Nagy I, Heldin CH,
Herlyn M and Westermark B: Platelet-derived growth factor (PDGF) in
oncogenesis: Development of a vascular connective tissue stroma in
xenotransplanted human melanoma producing PDGF-BB. Proc Natl Acad
Sci USA. 90:pp. 393–397. 1993; View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lindmark G, Sundberg C, Glimelius B,
Påhlman L, Rubin K and Gerdin B: Stromal expression of
platelet-derived growth factor beta-receptor and platelet-derived
growth factor B-chain in colorectal cancer. Lab Invest. 69:682–689.
1993.PubMed/NCBI
|
|
14
|
Shao ZM, Nguyen M and Barsky SH: Human
breast carcinoma desmoplasia is PDGF initiated. Oncogene.
19:4337–4345. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Betsholtz C, Karlsson L and Lindahl P:
Developmental roles of platelet-derived growth factors. Bioessays.
23:494–507. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen YQ, Ni DJ, Cheng Q, Hung HB, Meng Y
and Wu MC: Study on the hypolipidemic effect of flavones and
dihydromyricetin From Tengcha. J Tea Sci. 3:221–225, 242. 2007.
|
|
17
|
Zhong ZX, Zhou GF, Chen XF and Qin JP:
Experimental study on the protective effect of dihydromyricetin
from Guangxi Ampelopsis grossepentata on liver. Chin J Tradit Med
Sci Technol. 9:155–156. 2002.
|
|
18
|
Xu JJ, Yao MJ and Wu MC: Study on
biological efficacy of dihydromyricetin. Food Sci. 29:622–625.
2008.
|
|
19
|
Shen Y, Lindemeyer AK, Gonzalez C, Shao
XM, Spigelman I, Olsen RW and Liang J: Dihydromyricetin as a novel
anti-alcohol intoxication medication. J Neurosci. 32:390–401. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
He GX, Yang WL, Pei G, Zhu YH and Du FL:
Studies on the effect of dihydromyricetin on
antilipid-peroxidation. Zhongguo Zhong Yao Za Zhi. 28:1188–1190.
2003.PubMed/NCBI
|
|
21
|
Mueller L, Goumas FA, Himpel S, Brilloff
S, Rogiers X and Broering DC: Imatinib mesylate inhibits
proliferation and modulates cytokine expression of human
cancer-associated stromal fibroblasts from colorectal metastases.
Cancer Lett. 250:329–338. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gioni V, Karampinas T, Voutsinas G,
Roussidis AE, Papadopoulos S, Karamanos NK and Kletsas D: Imatinib
mesylate inhibits proliferation and exerts an antifibrotic effect
in human breast stroma fibroblasts. Mol Cancer Res. 6:706–714.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pietras K, Pahler J, Bergers G and Hanahan
D: Functions of paracrine PDGF signaling in the proangiogenic tumor
stroma revealed by pharmacological targeting. PLoS Med. 5:e192008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Micke P and Ostman A: Tumour-stroma
interaction: Cancerassociated fibroblasts as novel targets in
anti-cancer therapy? Lung Cancer. 45(Suppl 2): S163–S175. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kitadai Y, Sasaki T, Kuwai T, Nakamura T,
Bucana CD, Hamilton SR and Fidler IJ: Expression of activated
platelet-derived growth factor receptor in stromal cells of human
colon carcinomas is associated with metastatic potential. Int J
Cancer. 119:2567–2574. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tejada MLI, Yu L, Dong J, Jung K, Meng G,
Peale FV, Frantz GD, Hall L, Liang X, Gerber HP and Ferrara N:
Tumor-driven paracrine platelet-derived growth factor receptor
alpha signaling is a key determinant of stromal cell recruitment in
a model of human lung carcinoma. Clin Cancer Res. 12:2676–2688.
2006. View Article : Google Scholar : PubMed/NCBI
|