Introduction
Denervated-dependent skeletal muscle atrophy (DSMA)
is a disorder caused by the peripheral neuro-disconnection of
skeletal muscle (1,2). The skeletal muscle atrophy (SMA) is
triggered by pharmacological drug treatment, certain diseases
(including cancer, end stage pulmonary disorders and heart failure)
(3) and traumatic peripheral nerve
injury, all of which can lead to the immediate loss or damage of
the voluntary contractile function of the skeletal muscle (4). The muscle denervation mainly occurs
following several clinical syndromes, including degenerative disc
disorder, diabetic neuropathy, alcoholic neuropathy, trauma, spinal
muscular atrophy, viral infection and amyotrophic lateral sclerosis
(2,5). Thus, when the above pathogenic
factors that cause SMA and the muscle denervation are combined, the
DSMA occurs, and ultimately results in irreversible muscle
dysfunction (6,7). Although DSMA has become prevalent in
recent years, the molecular mechanism is elusive and there is no
complete therapeutic strategy, which prevents the development of
the pharmacological therapies for used in the clinic.
Recently, a study reported that angiopoietin-like
protein 4 (ANGPTL4) is secreted by the human forearm muscle after
eating a meal high in saturated fatty acids (8). ANGPTL4 is also thought to be
important mediators of muscle atrophy, and to have an important
role in muscle and wound repair (9). However, whether ANGPTL4 has a key
role in DSMA and the mechanism by which denervation causes ANGPTL4
changes have not been fully investigated.
Buyang Huanwu Tang (BYHWT) has been used for
treating hemiplegia, and is composed of Radix astragali,
Semen Persicae, Carthami flos, Radix Paeoniae Rubra, Rhizoma
Chuanxiong, Radix Angelicae Sinensis and Pheretima
(10). The BYHWT has been
demonstrated to improve the blood circulation, inhibit fibroblast
proliferation and decrease liquefaction and wallerian degeneration,
as well as enhance nerve cell growth (11). Therefore, it is speculated that the
BYHWT may participate in the pathogenesis of DSMA.
The current study aimed to investigate the role of
BYHWT in the development of DSMA and the mechanism that underlines
the ANGPTL4 changes in a DSMA model. Thus, an ANGPTL-4-targeting
siRNA was generated and used in an established DSMA rat model to
determine the role and mechanism ANGPTL4 in the pathogenesis of
DSMA. The findings demonstrated that ANGPTL4 has an important role
in DSMA via triggering of nuclear factor-κB (NF-κB) and muscle
RING-finger protein-1 (MURF1) pathways.
Materials and methods
Reagents and materials
Male Sprague-Dawley (SD) rats (body weight, 230–250
g; 8–10 weeks old) were purchased from Beijing HFK Bioscience Co.,
Ltd., (Beijing, China). The rats were housed in the specific cages
received free access to the water and food, and at a constant
temperature. All of the experiments were performed according to the
Guidelines of the Institutional Animal Care and approved by the
Ethics Committee of the Nanjing University of Traditional Chinese
Medicine (Nanjing, China).
The components of BYHWT, including Astragalus
(480 g), Tangkuei tail (24 g), Paeoniae (20 g),
Pheretima (12 g), Rhizoma Chuanxiong (12 g), Flos Carthami
Tinctorii (12 g) and peach seeds (12 g), were purchased from
Beijing Tong Ren Tang Co., Ltd. (Beijing China). The above Chinese
medicine components were mixed together and obtained by boiling to
a final volume of 1,500 ml (supplementing with the distilled
water), which was assigned as the BYHWT stock solution for the
subsequent experiments. The chemical reagents, including
K2HPO4, K3PO4 and
MgCl2, were purchased from Kelong Co., Ltd. (Chengdu,
China).
Establishment of DSMA rat model
SD rats (n=8) were used to establish the DSMA model.
Meanwhile, the other eight rats were selected as the sham operation
group (Sham group). The rats were fixed in the prone position
during the surgical process and then the rats were anesthetized by
injecting intraperitoneally with 7% chloral hydrate at the
concentration of 0.5 ml/100 g. The surgery was conducted only on
the left side of lower limb by cutting at the dorsolateral skin
incision. Then, between the gluteus muscle and the biceps femoris,
the common peroneal nerve was exposed, which was also separated and
extracted from the connective tissues in the surrounding area, and
exposing ~1.5 cm of common peroneal nerve. The two ends of the
common peroneal nerve were turned by ~180°. In order to prevent the
reconnection between the nerves, the nerves were sewn on the muscle
membranes by using a 9-0 non-destructive wound suture (nylon). The
DSMA model has been successfully established according to
previously published studies (3,4,6). For
the sham group, the left sciatic nerves were only mildly exposed
and separated from the surrounding tissues, and were not sewn by
the 9-0 non-destructive wound suture.
Lentiviral vectors expressing
ANGPTL4-siRNA
The high titer lentiviral vectors, which deliver the
ANGPTL4-specific small interfering RNA (siRNA) and control siRNA
were generated according to the previous published reports
(12,13). The following siRNA sequences
targeting the ANGPTL-4 was designed and assigned as ANGPTL4-siRNA:
Sense, 5′-AAAGCTGCAAGATGACCTCAGATGGAGGCTG-3′; and anti-sense,
5′-AAAAGGCTTAAGAAGGGAATCTTCTGGAAGAC-3′. The following control siRNA
sequences was also designed and assigned as CON-siRNA: Sense,
5′-AAAGCTGTCTTCAAGATTGATATCGAAGACTA-5′; and anti-sense,
5′-AAAATAGTCTTCGATATCAAGCTTGAAGACA-3′. Then, the ANGPTL4-siRNA and
CON-siRNA were cloned into the lentiviral vectors to synthesize the
ANGPTL-4 siRNA lentiviral vector, which was used to infect the DSMA
rat models.
Trial grouping and treatment
In this study, the DSMA rat models were divided into
three groups, including DSMA + saline, assigned as model group,
DSMA + CON-siRNA lentiviral vector [tail vein injection with 100 µl
(100 multiplicity of infection) at 14th day] + BYHWT (twice diluted
BYHWT stock solution, 2 ml every day per os), assigned as Con-siRNA
group, and DSMA + ANGPTL4-siRNA lentiviral vector (tail vein
injection with 100 µl at 14th day) + BYHWT (twice diluted BYHWT
stock solution, 2 ml every day), assigned as ANGPTL4-siRNA
group.
The BYHWT was also divided into three groups,
including high-dose BYHWT group (BYHWT-high, BYHWT stock solution
diluted 2X), moderate-dose BYHWT group (BYHWT-moderate, BYHWT stock
solution diluted 4X) and low-dose BYHWT group (BYHWT-low, BYHWT
stock solution diluted 8X).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) gene expression assay
Total RNA was extracted from the anterior cervical
muscle samples using TRIzol reagent (Thermo Fisher Scientific,
Inc., Waltham, MA, USA). Briefly, the sciatic nerves were lysed by
using 1 ml TRIzol. Subsequently, the RNAs were extracted by using
200 µl chloroform (Western Biotech. Co., Ltd., Chongqing, China),
and the supernatant was stored for the further experiments. Then,
the RNAs were precipitated by using isopropanol (1 ml), followed
with washing in 70% ethanol (1 ml, twice). Finally, the RNA
precipitations were diluted in the diethylpyrocarbonate-treated
water. The cDNA was synthesized by using the RT method in a 10 µl
system including oligo dT, total RNA, RT buffer (5X), random primer
mix, RNase inhibitor, RT enzyme mix (Beyotime Institute of
Biotechnology, Shanghai, China) and ddH2O. The RT-qPCR
process was performed at 37°C for 10 min, followed by 95°C for 5
min. Amplification of ANGPTL4 was performed by using the
synthesized cDNA as the template. The primers for ANGPTL4 (129 bp)
were as follows: Forward, 5′-AGAAGTTGGAGATGCAGAGGGAC-3′ and
reverse, 5′-CCACAAGAGCACCATTGAGTGTAT-3′. The primers for the
internal control β-actin (150 bp) were as follows: Forward,
5′-CCCATCTATGAGGGTTACGC-3′ and reverse,
5′-TTTAATGTCACGCACGATTTC-3′. The total volume of the PCR system was
a 10 µl reaction, which included forward primer (1 µl), reverse
primer (1 µl), 2X SYBR-Green Mixture (4.5 µl; Invitrogen; Thermo
Fisher Scientific, Inc.), cDNA (1 µl), and ddH2O (2.5
µl). The PCR conditions were as follows: 95°C for 10 sec, 55°C for
40 sec and 75°C for 30 sec. The RT-qPCR was performed on the
FTC2000 fluorescent qPCR cycler for 40 cycles. Finally, the
amplified products were loaded onto 1.5% agarose gels, and the
images were analyzed by using the Quantity One image analysis
software (version 4.6.5; Bio-Rad Laboratories, Inc., Hercules, CA,
USA). The relative mRNA expression of targeting genes was
normalized to the β-actin gene by using the comparative threshold
cycle (2−ΔΔCq) method (6).
Western blotting
The anterior cervical muscle samples were lysed by
using the radioimmunoprecipitation assay lysis buffer
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), and centrifuged at
12,000 × g for 30 min. Then, the supernatant (50 µg), the protein
concentration was quantified by using the bicinchoninic assay
method according to the manufacturer's protocol (Beyotime Institute
of Biotechnology) were separated by using 10% SDS-PAGE, and then
were transferred on to a polyvinylidene fluoride membrane (Dupont,
Wilmington, DE, USA) by using the Trans-Blot SD cell instrument
(Bio-Rad Laboratories, Inc.). The membrane was blocked by using 5%
non-fat milk at room temperature for 2 h, followed by incubation
with the rabbit anti-rat ANGPTL4 polyclonal antibody (1:2,000; cat.
no. AF3485; R&D Systems, Inc., Minneapolis, MN, USA), rabbit
anti-rat NF-κB p65 polyclonal antibody (1:1,000; cat. no. MAB50781;
R&D Systems, Inc.), mouse anti-rat MURF1 monoclonal antibody
(1:3,000; cat. no. AF5366; R&D Systems, Inc.) and the rabbit
anti-rat actin (internal control, cat. no. MAB1420; R&D
Systems, Inc.) polyclonal antibody at 4°C overnight. The membranes
were washed by using the PBS Tween-20 (PBST) buffer three times for
5 min. The membranes were incubated with the horseradish peroxidase
(HRP)-labeled goat anti-rabbit IgG (1:1,000; cat. no. AQ132P;
Sigma-Aldrich; Merck KGaA) and HRP-labeled goat anti-mouse IgG
(1:1,000; cat. no. AP308P; Sigma-Aldrich; Merck KGaA) for 60 min at
37°C, and washed with PBST buffer three times for 5 min. Finally,
the enhanced chemiluminescence reagent (Pierce; Thermo Fisher
Scientific, Inc.) was used to treat the membrane for 2 min in the
dark. The relative grey density of the bands were analyzed by using
the Labworks Analysis Software (version 4.5; UVP, Inc., Upland, CA,
USA; www.uvp.com).
Transmission electron microscopy
(TEM)
Anterior cervical muscles tissues were fixed in 10%
neutral buffered formalin for 15 min at 37°C and minced into the
pieces of 1 mm3. Then, the pieces were immersed in 2.5%
glutaraldehyde for 2 h at 37°C (dissolved into the 0.1 M PBS, and
adjusted to pH 7.4). The formalin-fixed tissues were embedded in
paraffin and mounted on glass slides. Then, the tissues were
stained with 0.5% periodic acid-Schiff reagent for 15 min at 37°C,
following the diastase treatment. The status of the coverslips was
examined using a light microscope. The muscle tissues incubated in
glutaraldehyde were rinsed thoroughly with PBS. The muscle tissues
were continuously post-fixed in 2% OsO4 at room
temperature for 2 h, and dehydrated and embedded Embed 812. The
muscle tissues were embedded and polymerized overnight at 60°C in
the flat embedding molds. The areas of interest were selected using
0.5% toluidine blue-stained sections (0.5 µm) at room temperature
for 15 min. Then, ultrathin sections (0.1-µm) were cut and mounted
on the grids, and then stained using 2% uranyl acetate for 10 min
at 37°C. Finally, the sections were examined by using a Tecnal-10
TEM (Philips Medical Systems B.V., Eindhoven, The Netherlands).
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) assay
In order to observe the possible DNA fragmentation
in the anterior cervical muscle tissues, TUNEL was performed by
using a TUNEL kit (Roche Diagnostics, Indianapolis, IN, USA) in the
present study. In brief, the isolated anterior cervical muscle
tissues were fixed with 4% paraformaldehyde for 10 min at room
temperature in NaH2PO4 (0.1 M, adjusted to pH
7.4). Endogenous peroxidase activity in the anterior cervical
muscle tissues were inactivated using 3% H2O2
for 10 min at room temperature. The tissues were incubated with
biotin-dUTP solution and incubated with the terminal
deoxynucleotidyl transferase (20 U/µl) at 37°C for 1 h. After the
tissues were treated using the end-horseradish peroxidase, the
anterior cervical muscle tissues were stained with the
diaminobenzidine (final concentration of 0.05%, at room temperature
for 10 min) and counterstained with the ethyl green (room
temperature for 10 min) to detect the stained and biotin-labeled
cell nuclei. The apoptotic bodies stained brown and the cell nuclei
were observed, and counted under the light microscope. For the
quantitative analysis, the apoptosis index was evaluated as the
percentage of apoptotic cells. The positively-stained nuclei were
counted by at least three investigators and in at least three
different fields.
Hematoxylin and eosin (H&E)
staining assay
Anterior cervical muscles tissues were fixed with
the 4% paraformaldehyde at room temperature for 24 h. Then, the
tissues were embedded in paraffin, and sectioned into 4-µm thick
sections. The paraffin was removed using the regular method
(6), and the sections were use for
the H&E staining as previously described (6). The images were captured by the
inverted fluorescence microscope (Olympus Corporation, Tokyo,
Japan), and were analyzed by employing the Medical Image Analysis
system (cat. no. HMIAS22000; Qianping Image Engineering Company,
Tongji Medical University, Wuhan, China). The percentage of
inflammatory cells was calculated as follows: (The number of
inflammatory cells/the total number of cells) ×100 (%). The
inflammatory cells were stained with the blue and the normal cells
were without staining.
Statistical analysis
All data were analyzed by with the SPSS software
20.0 (IBM Corp., Armonk, NY, USA). The data are presented as the
mean ± standard deviation obtained from at least three independent
experiments. The Student's t-test was performed to evaluate the
statistical significance between the two groups, and the ANOVA test
was performed to assess statistical significance among the multiple
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
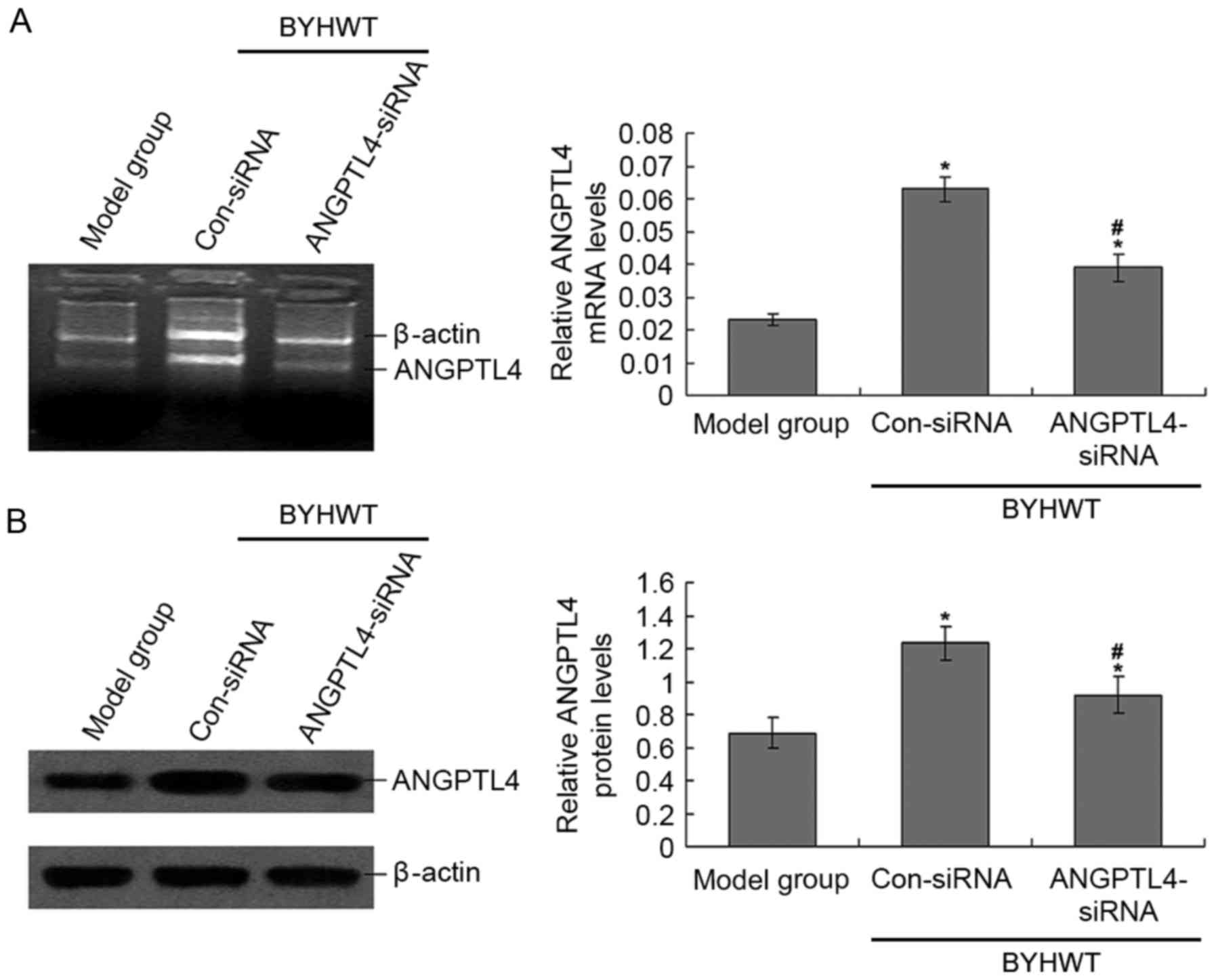
BYHWT treatment increases ANGPTL4 mRNA
levels
In order to confirm the successful transfection of
the ANGPTL-4 siRNA lentiviral vector to the rats, the ANGPTL4 mRNA
and protein levels were observed by using RT-PCR and western
blotting (Fig. 1). ANGPTL4 mRNA
and protein levels in the ANGPTL4-siRNA group were significantly
lower than the Con-siRNA group (P<0.05; Fig. 1), which suggests that the ANGPTL4
siRNA lentiviral vector was efficiently transfected and expressed.
Additionally, the ANGPTL4 levels in the Con-siRNA group were also
significantly higher compared with the model group (P<0.05;
Fig. 1), which suggests that BYHWT
significantly increased the levels of ANGPTL4. In the
pre-experiment, the data of Sham rats compared with the normal rats
were examined. The results demonstrated that there were no
differences between the Sham rats and the normal rats (data not
shown).
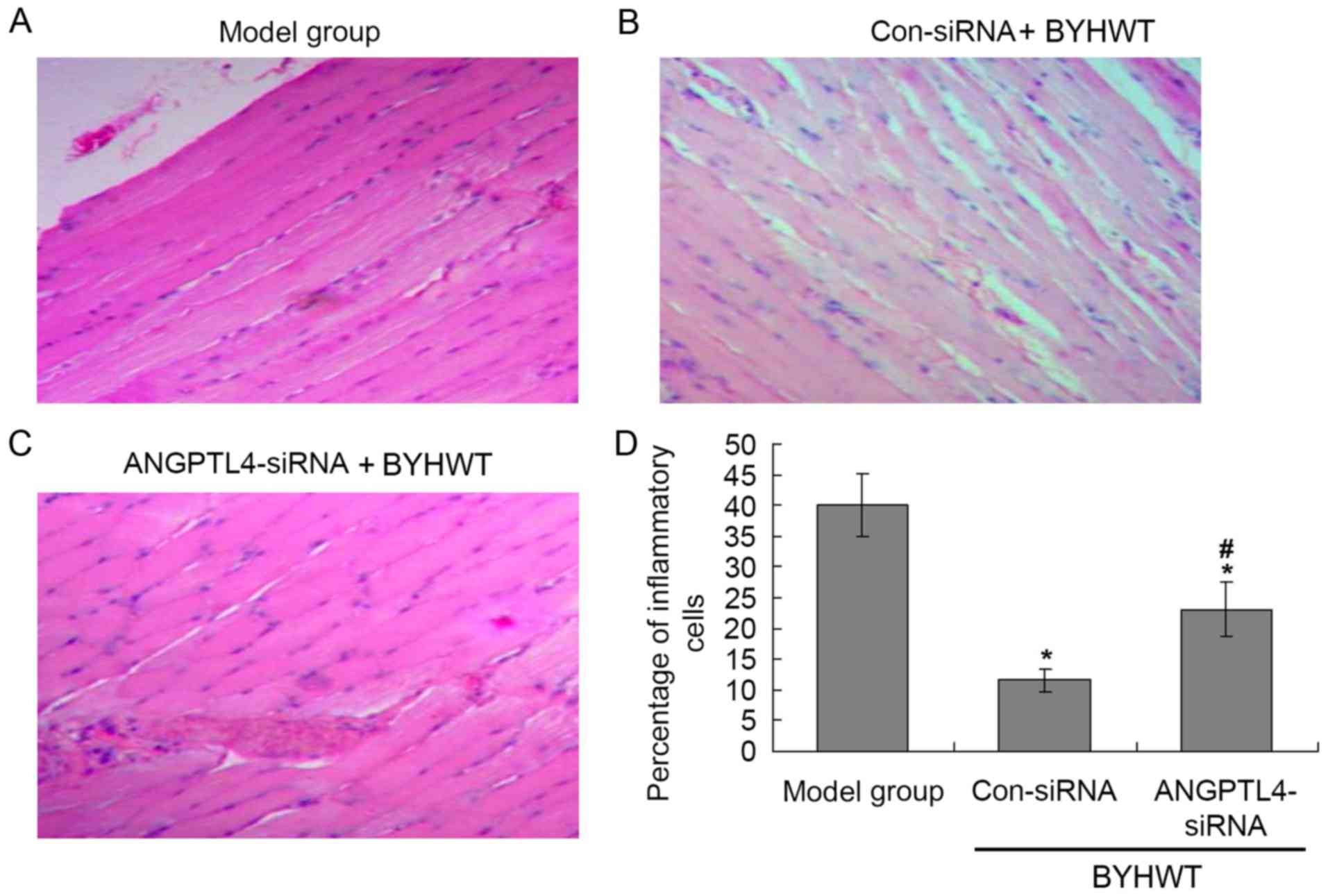
H&E staining
According to the H&E staining results (Fig. 2), there were many inflammatory
cells in the model group (Fig.
2A), and BYHWT significantly decreased the levels of
inflammatory cells compared with the model group (P<0.05;
Fig. 2B). Additionally, the
suppression ANGPTL4 significantly increased the number of
inflammatory cells compared with the Con-siRNA group (P<0.05;
Fig. 2C and D).
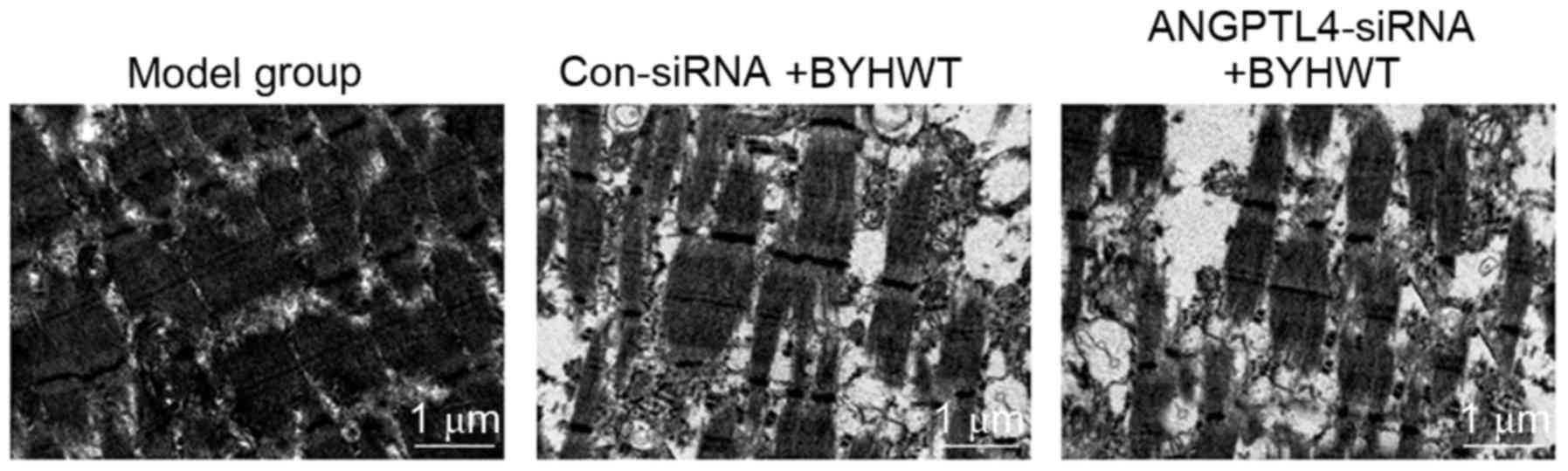
BYHWT protects the ultrastructure of
muscle tissues
In the current study, TEM was performed to analyze
the ultrastructure of the muscle tissues. The TEM findings
demonstrated that the ultrastructure of muscle was seriously
damaged in the model group (Fig.
3A). However, the BYHWT treatment (Con-siRNA group) observably
improved the ultrastructure of muscle, including the muscle fibers
being more ordered with fewer spaces between the fibers (Fig. 3B). Furthermore, the inhibition of
ANGPTL4 (ANGPTL4-siRNA group) suppressed the effects of BYHWT on
the ultrastructure of muscle tissues (Fig. 3C), which suggests that the ANGPTL-4
may participate in the repair of the muscle tissues.
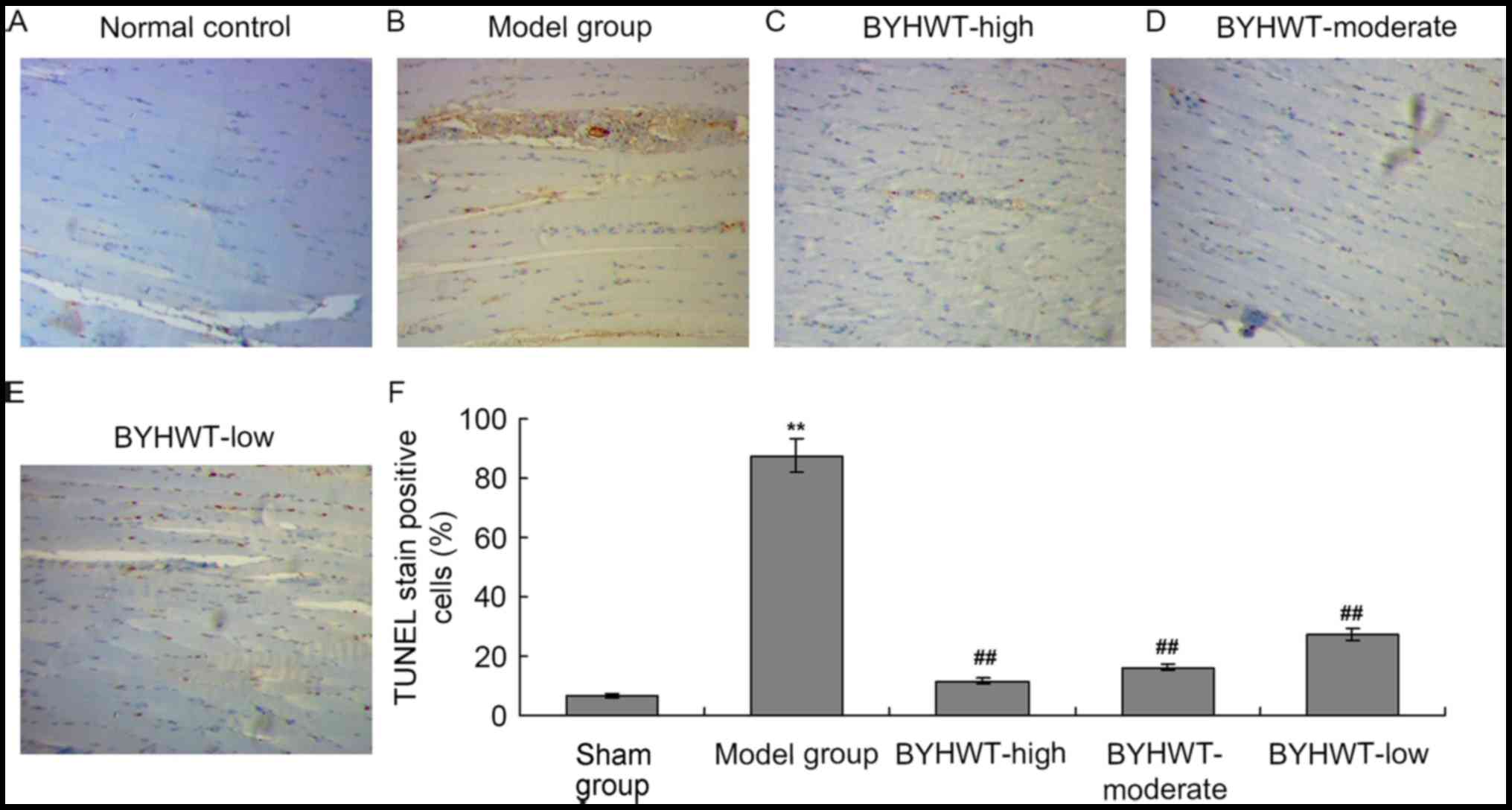
BYHWT inhibits cell apoptosis in DSMA
model
According to the TEM findings, the ultrastructure of
muscle tissues was damaged in the DSMA model, therefore, the muscle
tissues may be experiencing apoptosis. In the present study, the
TUNEL assay was performed to investigate the apoptosis of the
muscle tissue cells. The results indicated that there was obvious
TUNEL staining in the model group compared with the normal control
group (P<0.01; Fig. 4A and B).
Additionally, the number of TUNEL-positive cells were also
significantly decreased in the BYHWT-treated groups in a
dose-dependent manner (from high to low; P<0.05; Fig. 4C-F).
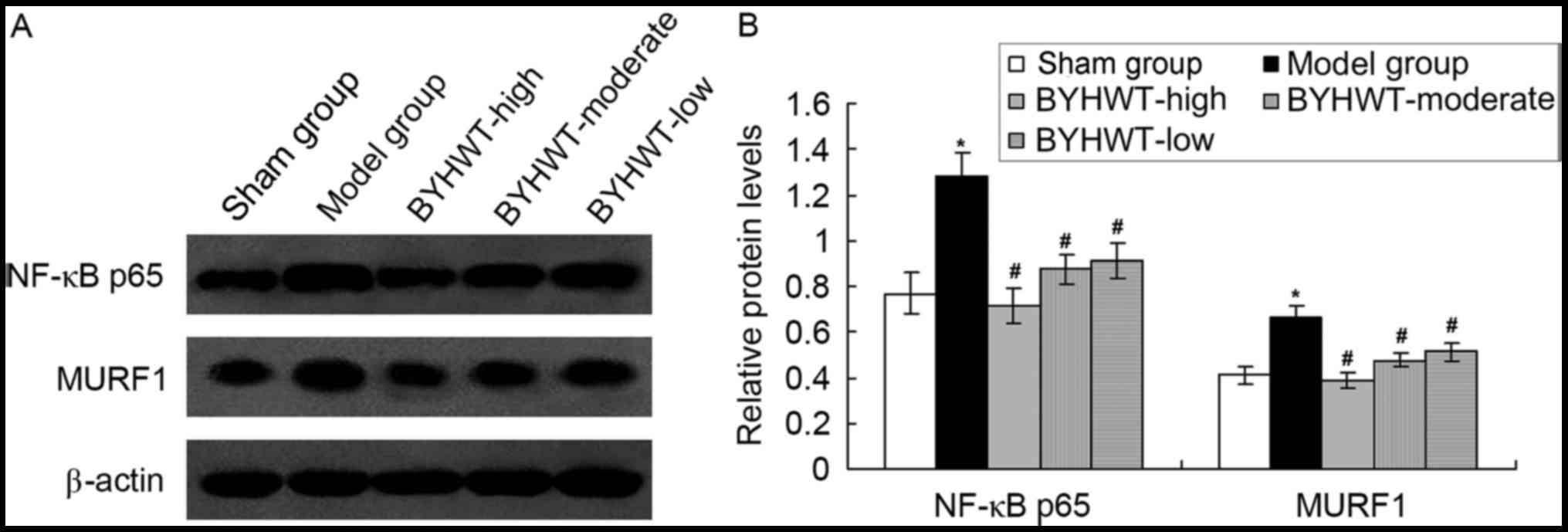
BYHWT reduces NF-κB p65 and MURF1
expression in a DSMA model
In order to investigate the mechanism of the
BYHWT-activated protective effects, the inflammatory signaling
pathway factor, NF-κB p65, and the SMA-associated factor, MURF1,
were detected by using the western blot assay. The results
indicated that the establishment of the DSMA model increased the
expression of NF-κB p65 and MURF1 compared with the normal rats
(P<0.05; Fig. 5). Furthermore,
BYHWT treatment significantly decreased the levels of NF-κB p65 and
MURF1 compared to the model group (P<0.05; Fig. 5). Furthermore, the change in of
NF-κB p65 and MURF1 induced by BYHWT was dose-dependent.
Discussion
In the present study, it was demonstrated that
ANGPTL4 expression is involved in the therapeutic effect of BYHWT
in a DSMA model. BYHWT could improve the blood circulation, inhibit
fibroblast proliferation and decrease liquefaction and Wallerian
degeneration, as well as enhance the nerve cells growth according
to the previously published studies (11,14,15).
ANGPTL4 is best known for its postprandial function in skeletal
muscle lipid metabolism following a meal high in saturated fatty
acids (8). Staiger et al
(16) reported that the
muscle-derived ANGPTL4 was induced by fatty acids viaperoxisome
proliferator activated receptor-γ in humans. Stapleton et al
(17) also reported that ANGPTL4
is expressed in human airway smooth muscle cells.
The current study investigated the expression of
ANGPTL4 in muscle tissues, and evaluated the hypothesis that DSMA
may mediate the depletion of ANGPTL4, and ANGPTL4 may have a
protective role against DSMA. Indeed, the findings suggest that
ANGPTL4 mRNA and protein were significantly increased in
BYHWT-treated DSMA model rats, which suggests that the BYHWT
significantly increased the levels of ANGPTL4 levels.
In order to confirm the role of NF-κB in the
therapeutic effects of BYHWT, H&E staining and TEM analysis
were performed on samples of the anterior cervical muscle. Previous
studies (18–20) demonstrated that there was obvious
inflammation and serious ultrastructure changes in the muscle of
the DSMA model. The present results indicated that the model group
muscle exhibited serious inflammatory cell infiltration; however,
the BYHWT treatment (Con-siRNA group) significantly decreased the
inflammatory cell infiltration. Furthermore, when ANGPTL4
expression was suppressed using ANGPTL4-siRNA viral vector, the
inflammatory cell infiltration was aggravated, and ultrastructural
damaged was observed. These results also suggest that the BYHWT
increases the expression of ANGPTL4, which may be critical for the
improvement of the inflammation and ultrastructure, and ultimately
improve the denervation-induced muscle atrophy.
In addition to the inflammation and ultrastructure
changes, cell apoptosis may also participate in the processes of
DSMA. Therefore, cell apoptosis of muscle cells was determined
using a TUNEL assay. The result indicated that there was an
increase in TUNEL-stained cells in the model group compared with
the, normal control group, and the number of TUNEL-positive cells
as significantly decreased in BYHWT treated groups, which suggest
that BYHWT inhibits the apoptosis of muscle cells. These results
are consistent with the previously published studies (21,22).
In order to clarify the mechanism of the
DSMA-associated cell apoptosis and the therapeutic effects of
BYHWT, the inflammation-associated factor, NF-κB p65, and the
atrophying skeletal muscle-associated factor, MURF1, were detected
by western blot assay. Bodine et al (23) reported that MURF1 was induced in
atrophying skeletal muscle, and exhibits reduced DSMA.
Additionally, a previous study (20) demonstrated that the NF-κB signaling
pathway regulates MURF1 expression when undergoing apoptotic
assaults. The results of the current study demonstrated that the
NF-κB p65 and MURF1 expression were increased in the DSMA model,
and were inhibited by treatment with BYHWT. Consistent with the
previous studies (24–27), these results suggest that BYHWT may
mediate its effects on the denervation-induced muscle atrophy by
regulating MURF1 protein expression, induced via changes in the
NF-κB signaling pathway.
Although the results of the current study are of
interest, there are also certain limitations in this study. The
ANGPTL4-siRNA did not produce an efficient to knockdown of ANPGTL4
expression. In future studies, a successful ANGPTL4 gene knockdown
or gene knockout animal model should be established to investigate
further.
In conclusion, BYHWT improves DSMA, potentially by
increasing ANGPTL4 expression, involving NF-κB signaling and
MURF1.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China For Youth (grant no. 81302890), Natural
Science Foundation of Colleges and Universities in Jiangsu Province
(grant no. 13KJB360003), Natural Science Foundation of Jiangsu
Province (grant no. BK2011816) and Natural Science Foundation of
Doctoral point of Colleges and Universities in China (New Teachers;
grant no. 20123237120004).
References
|
1
|
Tajrishi MM, Shin J, Hetman M and Kumar A:
DNA methyltransferase 3a and mitogen-activated protein kinase
signaling regulate the expression of fibroblast growth
factor-inducible 14 (Fn14) during denervation-induced skeletal
muscle atrophy. J Biol Chem. 289:19985–19999. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bongers KS, Fox DK, Ebert SM, Kunkel SD,
Dyle MC, Bullard SA, Dierdorff JM and Adams CM: Skeletal muscle
denervation causes skeletal muscle atrophy through a pathway that
involves both Gadd45a and HDAC4. Am J Physiol Endocrinol Metab.
305:E907–E915. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nagpal P, Plant PJ, Correa J, Bain A,
Takeda M, Kawabe H, Rotin D, Bain JR and Batt JA: The ubiquitin
ligase Nedd4-1 participates in denervation-induced skeletal muscle
atrophy in mice. PLoS One. 7:e464272012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Batt JA and Bain JR: Tibial nerve
transection-a standardized model for denervation-induced skeletal
muscle atrophy in mice. J Vis Exp. e506572013.PubMed/NCBI
|
|
5
|
Hsieh CH, Jeng SF, Wu CJ, Lu TH, Yang JC,
Chen YC, Lin CJ and Rau CS: Altered expression of the microRNAS and
their potential target genes in the soleus muscle after peripheral
denervation and reinnervation in rats. J Trauma. 70:472–480. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li G, Li QS, Li WB, Wei J, Chang WK, Chen
Z, Qiao HY, Jia YW, Tian JH and Liang BS: miRNA targeted signaling
pathway in the early stage of denervated fast and slow muscle
atrophy. Neural Regen Res. 11:1293–1303. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
van der Kolk BW, Goossens GH, Jocken JW,
Kersten S and Blaak EE: Angiopoietin-like protein 4 and
postprandial skeletal muscle lipid metabolism in overweight and
obese prediabetics. J Clin Endocrinol Metab. 101:2332–2339. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Feingold KR, Shigenaga JK, Cross AS, Moser
A and Grunfeld C: Angiopoietin like protein 4 expression is
decreased in activated macrophages. Biochem Biophys Res Commun.
421:612–615. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Du D: Treatment of prolapse of lumbar
intervertebral disc by tuina massotherapy combined with oral
administration of buyang huanwu tang-a reprot of 75 cases. J Tradit
Chin Med. 27:43–45. 2007.PubMed/NCBI
|
|
11
|
Guo ZP, Huang MN, Liu AQ, Yuan YJ, Zhao JB
and Mei XF: Buyang Huanwu decoction up-regulates Notch 1 gene
expression in injured spinal cord. Neural Regen Res. 10:1321–1323.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tiscornia G, Singer O, Ikawa M and Verma
IM: A general method for gene knockdown in mice by using lentiviral
vectors expressing small interfering RNA. Proc Natl Acad Sci USA.
100:pp. 1844–1848. 2003; View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tiscornia G, Singer O and Verma IM: Design
and cloning of lentiviral vectors expressing small interfering
RNAs. Nat Protoc. 1:234–240. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu X, Min Y, Gu W, Wang Y and Tian Y:
Buyanghuanwu Tang therapy for neonatal rats with hypoxic ischemic
encephalopathy. Int J Clin Exp Med. 8:18448–18454. 2015.PubMed/NCBI
|
|
15
|
Jinglong T, Weijuan G, Jun L, Tao Q,
Hongbo Z and Shasha L: The molecular and electrophysiological
mechanism of buyanghuanwu decoction in learning and memory ability
of vascular dementia rats. Brain Res Bull. 99:13–18. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Staiger H, Haas C, Machann J, Werner R,
Weisser M, Schick F, Machicao F, Stefan N, Fritsche A and Häring
HU: Muscle-derived angiopoietin-like protein 4 is induced by fatty
acids via peroxisome proliferator-activated receptor (PPAR)-delta
and is of metabolic relevance in humans. Diabetes. 58:579–589.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stapleton CM, Joo JH, Kim YS, Liao G,
Panettieri RA Jr and Jetten AM: Induction of ANGPTL4 expression in
human airway smooth muscle cells by PMA through activation of PKC
and MAPK pathways. Exp Cell Res. 316:507–516. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen HX, Tang SP, Gao FT, Xu JL, Jiang XP,
Cao J, Fu GB, Sun K, Liu SZ and Shi W: Fibrosis, adipogenesis, and
muscle atrophy in congenital muscular torticollis. Medicine
(Baltimore). 93:e1382014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ohnishi Y, Iwatsuki K, Shinzawa K, Nakai
Y, Ishihara M and Yoshimine T: Disuse muscle atrophy exacerbates
motor neuronal degeneration caudal to the site of spinal cord
injury. Neuroreport. 23:157–161. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ohyama K, Koike H, Katsuno M, Takahashi M,
Hashimoto R, Kawagashira Y, Iijima M, Adachi H, Watanabe H and
Sobue G: Muscle atrophy in chronic inflammatory demyelinating
polyneuropathy: A computed tomography assessment. Eur J Neurol.
21:1002–1010. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Meneses C, Morales MG, Abrigo J, Simon F,
Brandan E and Cabello-Verrugio C: The angiotensin-(1–7)/Mas axis
reduces myonuclear apoptosis during recovery from angiotensin
II-induced skeletal muscle atrophy in mice. Pflugers Arch.
467:1975–1984. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bargiela A, Cerro-Herreros E,
Fernandez-Costa JM, Vilchez JJ, Llamusi B and Artero R: Increased
autophagy and apoptosis contribute to muscle atrophy in a myotonic
dystrophy type 1 Drosophila model. Dis Model Mech. 8:679–690. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bodine SC, Latres E, Baumhueter S, Lai VK,
Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K,
et al: Identification of ubiquitin ligases required for skeletal
muscle atrophy. Science. 294:1704–1708. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mittal A, Bhatnagar S and Kumar A,
Lach-Trifilieff E, Wauters S, Li H, Makonchuk DY, Glass DJ and
Kumar A: The TWEAK-Fn14 system is a critical regulator of
denervation-induced skeletal muscle atrophy in mice. J Cell Biol.
188:833–849. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cai D, Frantz JD, Tawa NE Jr, Melendez PA,
Oh BC, Lidov HG, Hasselgren PO, Frontera WR, Lee J, Glass DJ and
Shoelson SE: IKKbeta/NF-kappaB activation causes severe muscle
wasting in mice. Cell. 119:285–298. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mourkioti F, Kratsios P, Luedde T, Song
YH, Delafontaine P, Adami R, Parente V, Bottinelli R, Pasparakis M
and Rosenthal N: Targeted ablation of IKK2 improves skeletal muscle
strength, maintains mass, and promotes regeneration. J Clin Invest.
116:2945–2954. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Macpherson PC, Wang X and Goldman D:
Myogenin regulates denervation-dependent muscle atrophy in mouse
soleus muscle. J Cell Biochem. 112:2149–2159. 2011. View Article : Google Scholar : PubMed/NCBI
|