Introduction
Atherosclerosis is a disease in which the inside of
an artery narrows due to the build-up of plaque. Atherosclerosis
may result in the development of cardiovascular disease and is a
leading cause of morbidity and mortality in developed countries
(1,2). Atherosclerotic lesions are
characterized by a thin fibrous cap, large lipid core and the
accumulation of macrophages. Lesions with abundant macrophage
accumulation are referred to as ‘vulnerable plaques’ (3), as these lesions are susceptible to
rupture, which results in thrombosis and eventually leads to
myocardial and cerebral infarction (4). Endothelial function impairment is a
primary pathological factor in the development of atherosclerotic
lesions (5). Oxidative stress,
endoplasmic reticulum (ER) stress and inflammation have emerged as
primary contributors to endothelial cell dysfunction (6–8).
Oxidized low-density lipoprotein (oxLDL) results
from one of the biologically relevant modifications in LDL in the
form of oxidation, since LDL particles are markedly sensitive to
oxidative damage. oxLDL accumulation is one of the numerous causes
of atherosclerosis initiation and progression (9). Initially, oxLDL binds to scavenger
receptors on the vascular endothelial cell surface, inducing
adhesion molecule expression that facilitates monocyte adhesion and
migration into the sub-endothelial layer (10). OxLDL transverses the endothelial
barrier and enters the sub-endothelium, where it induces the
differentiation of monocytes into macrophages, which attempt to
phagocytose the oxLDL and become lipid-laden foam cells (11). The latter contributes to the
expansion of the atherosclerotic plaque lipid core and exacerbates
oxidative stress (12). Macrophage
accumulation is regarded as a crucial step in atherosclerotic
plaque formation and development. However, the interactions that
occur between macrophages and endothelial cells in atherosclerosis
are still unclear.
Exosomes are nano-sized membrane vesicles with a
diameter of 30–100 nm (13–15).
Macrophage-derived exosomes may regulate the cellular functions of
fibroblasts, vascular smooth muscle and endothelial cells through
the delivery of microRNAs (miRNAs), mRNAs and proteins (16–19).
However, the function of exosomes secreted by oxLDL-stimulated
macrophages in atherosclerosis is yet to be elucidated.
The present study demonstrated that oxLDL-stimulated
macrophages attenuated the growth and tube formation of endothelial
cells, in part through exosomal transfer. Exosomes from
oxLDL-treated macrophages and comparable controls were collected
and co-cultured with endothelial cells and the exosomes were
subsequently endocytosed. Cell Counting Kit-8 (CCK8) and tube
formation assays revealed that exosomes derived from
oxLDL-stimulated macrophages reduced the growth and tube formation
abilities of endothelial cells, compared with control macrophages.
Suppression of exosome secretion by oxLDL-stimulated macrophages
rescued the growth and tube formation abilities of endothelial
cells. The results indicated that oxLDL-stimulated macrophages
attenuated the growth and tube formation of endothelial cells,
partially through exosomal transfer. This may provide novel targets
for atherosclerosis therapy.
Materials and methods
Cell culture
THP-1 human monocytic cells were purchased from
American Type Culture Collection (ATCC; Manassas, VA, USA) and
maintained in RPMI-1640 medium (Thermo Fisher Scientific Inc.,
Waltham, MA, USA) and human umbilical vein endothelial cells
(HUVECs) were purchased from ATCC and maintained in Dulbecco's
modified Eagle's medium(DMEM)/F12 (Thermo Fisher Scientific Inc.).
The two media were supplemented with 10% heat-inactivated fetal
bovine serum (Invitrogen; Thermo Fisher Scientific Inc.), 100
units/ml penicillin and 100 µg/ml streptomycin in a humidified
atmosphere of 5% CO2 at 37°C. All cells were confirmed
to be free of mycoplasma contamination.
Electron microscopic observation of
exosomes
The exosome suspension was added to an equal volume
of 4% paraformaldehyde (Nacalai Tesque, Inc., Kyoto, Japan), and
the mixture was applied to a Formvar/Carbon film-coated
transmission electron microscope (TEM) grid (Alliance Biosystems,
Osaka, Japan). Then, the sample was fixed by incubation with 1%
glutaraldehyde for 5 min, washed with PBS, and incubated with 1%
uranyl acetate for 5 min at 4°C. The sample was observed under a
TEM (Hitachi H7650; Hitachi, Ltd., Tokyo, Japan).
Exosome isolation and co-culture with
HUVECs
To isolate the exosomes, THP-1 exosomes were first
treated with 50 µg/ml oxLDL (Shanghai Leuven Biotechnology Co.,
Ltd., Shanghai, China) for 48 h. The supernatant was subsequently
collected and centrifuged at 1,000 × g for 10 min, then 3,000 × g
for 30 min at 4°C to remove cell components and fragments. A total
exosome isolation kit (Thermo Fisher Scientific Inc.) was then
added to the sample overnight at 4°C, prior to centrifugation at
10,000 × g for 1 h at 4°C. Exosomes were re-suspended in PBS and
stored at −80°C. The exosome concentration was detected using a
bicinchoninic acid protein assay kit (Beyotime Institute of
Biotechnology, Hangzhou, China). Exosomes were then co-cultured
with 105 HUVECs in 50 ng/ml DMEM for 24 h at 37°C.
Western blot analysis
Western blot analysis was performed to analyse the
expression of the exosomal marker cluster of differentiation
(CD)63. Cells were lysed with radioimmunoprecipitation assay buffer
(50 mM Tris-HCl, pH 7.5; 150 mM NaCl; 1% Triton X-100 and 0.5%
sodium deoxycholate) and blocked with the complete mini protease
inhibitor cocktail 30 min at 4°C (Roche Diagnostics GmbH, Mannheim,
Germany). The protein concentration was detected using a
bicinchoninic acid protein kit (Beyotime Institute of
Biotechnology). A total of 20–30 µg lysate samples were
subsequently separated on 8–12% SDS-PAGE gel and transferred onto
polyvinylidene membranes. The membranes were incubated with the
following primary antibodies overnight at 4°C: rabbit anti-human
CD63 (cat. no. ab59479, 1:1,000; Abcam, Cambridge, UK), rabbit
anti-human CD9 (cat. no. ab92726, 1:1,000; Abcam), mouse anti-human
CD81 (cat. no. H00000975-B01P, 1:500; Novus Biologicals, LLC,
Littleton, CO, USA) and mouse anti-Actin (cat. no. CP01-1EA,
1:10,000; Merck KGaA, Darmstadt, Germany). Membranes were then
incubated with the following horse radish peroxidase
(HRP)-conjugated anti-rabbit secondary antibody (cat. no. 7074;
1:10,000; CST Biological Reagents Co., Ltd., Shanghai, China) and
HRP-anti-mouse antibody (cat. no. 7076; 1:10,000; CST Biological
Reagents Co., Ltd.) at room temperature for 1 h. The antibodies
were detected using an enhanced chemiluminescence kit (cat. no.
PI32209; Pierce; Thermo Fisher Scientific Inc.).
PHK67 stained exosomes co-culture with
HUVECs
Purified exosomes stained with PHK67 (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) according to the manufacture's
protocols. Briefly, exosomes resuspended in a buffer provided in
the kits were mixed with the PKH dyes and were incubated for 5 min
at room temperature. Next, the samples were added to PBS
supplemented with 5% bovine serum albumin and were ultracentrifuged
at 100,000 g for 1 h at 4°C to remove free dyes. Then, the stained
exosomes were co-cultured with HUVECs which were then fixed with 4%
paraformaldehyde at 25°C for 20 min.
GW4869 treatment of macrophages
Macrophages were treated with or without 5 µg/ml
GW4869 (dissolved in DMSO; MedChem Express, USA) for 24 h at 37°C
to inhibit exosome secretion.
CCK8 assay
The CCK8 assay was conducted according to the
manufacturer's protocol (CK04; Dojindo Molecular Technologies,
Inc., Kumamoto, Japan). Exosome-treated cells were plated at equal
cell density (2,000 cells/100 µl well) in 96-well plates with 300
µg/ml cetuximab (Merck KGaA) for continuous detection over a 5-day
period. At the beginning of the second day, the culture was
terminated with the addition of 10 µl CCK8 (5 mg/ml) to the
original culture medium. Following 2 h, plates were measured using
a microplate reader (Biotek Elx800, Biotek Instruments, Inc.,
Winooski, VT, USA). Cell proliferation was measured using optical
density at 450 nm.
Tube formation assay
The exosome-treated HUVECs (5×104/96 well
plate) were re-suspended in the conditioned medium and seeded into
growth factor-reduced Matrigel (356234; BD Biosciences, Franklin
Lakes, NJ, USA). Following incubation for 6 h images of the tubes
formed were captured under an inverted microscope at ×100
magnification. The length of tubes was measured using ImageJ
software version 1.47 (National Institutes of Health, Bethesda, MD,
USA).
Statistical analysis
Statistical analysis was performed using the
Student's t-test to compare between groups. Data were presented as
mean ± standard error of the mean. A Tukey test was conducted for
multiple comparisons in conjunction with one way analysis of
variance. P<0.05 was considered to indicate a statistically
significant difference. SPSS version 13.0 was used for analysis
(SPSS, Inc., Chicago, IL, USA).
Results
Exosomes derived from oxLDL-stimulated
macrophages
To investigate the function of oxLDL-stimulated
macrophage-derived exosomes in atherosclerosis, exosomes were
isolated from oxLDL-treated or untreated THP-1 cells. The
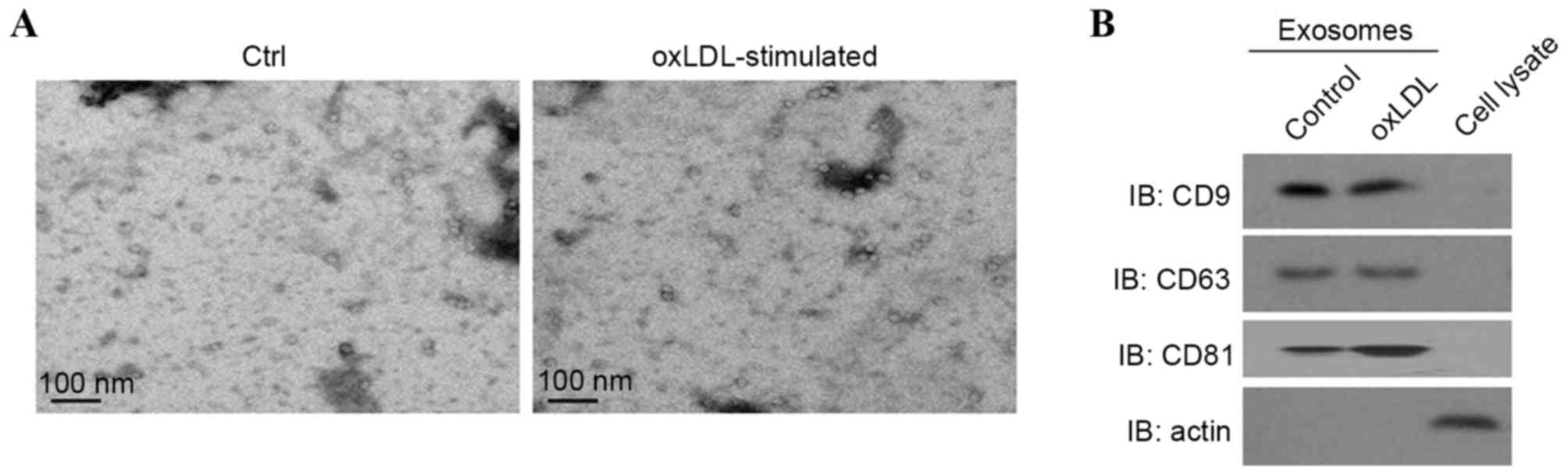
morphology of exosomes collected was observed with scanning
electron microscopy. The diameters of the exosomes ranged from
30–120 nm (Fig. 1A). Western blot
analysis revealed that exosomes were enriched with the exosomal
markers CD9, CD63 and CD81 whereas actin was enriched in the
cellular lysate (Fig. 1B),
indicating an effective isolation of exosomes.
Endothelial cells endocytose exosomes
derived from oxLDL-stimulated macrophages
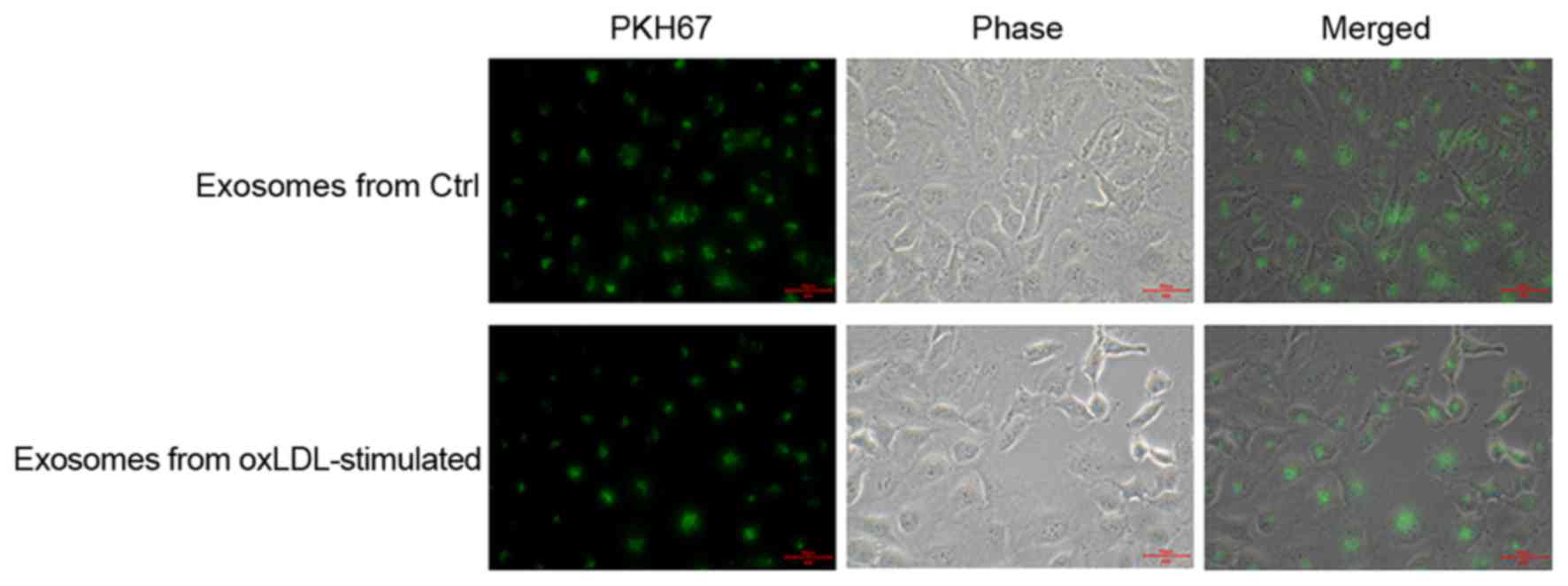
PHK67 labeled exosomes were co-cultured with HUVECs.
Following 24 h, co-cultured HUVECs were fixed and observed by
fluorescent microscopy. Exosomes were observed inside the
co-cultured HUVECs (Fig. 2),
verifying that endothelial cells endocytosed the exosomes derived
from oxLDL-stimulated macrophages.
Exosomes derived from oxLDL-stimulated
macrophages reduce the growth and tube formation abilities of
endothelial cells
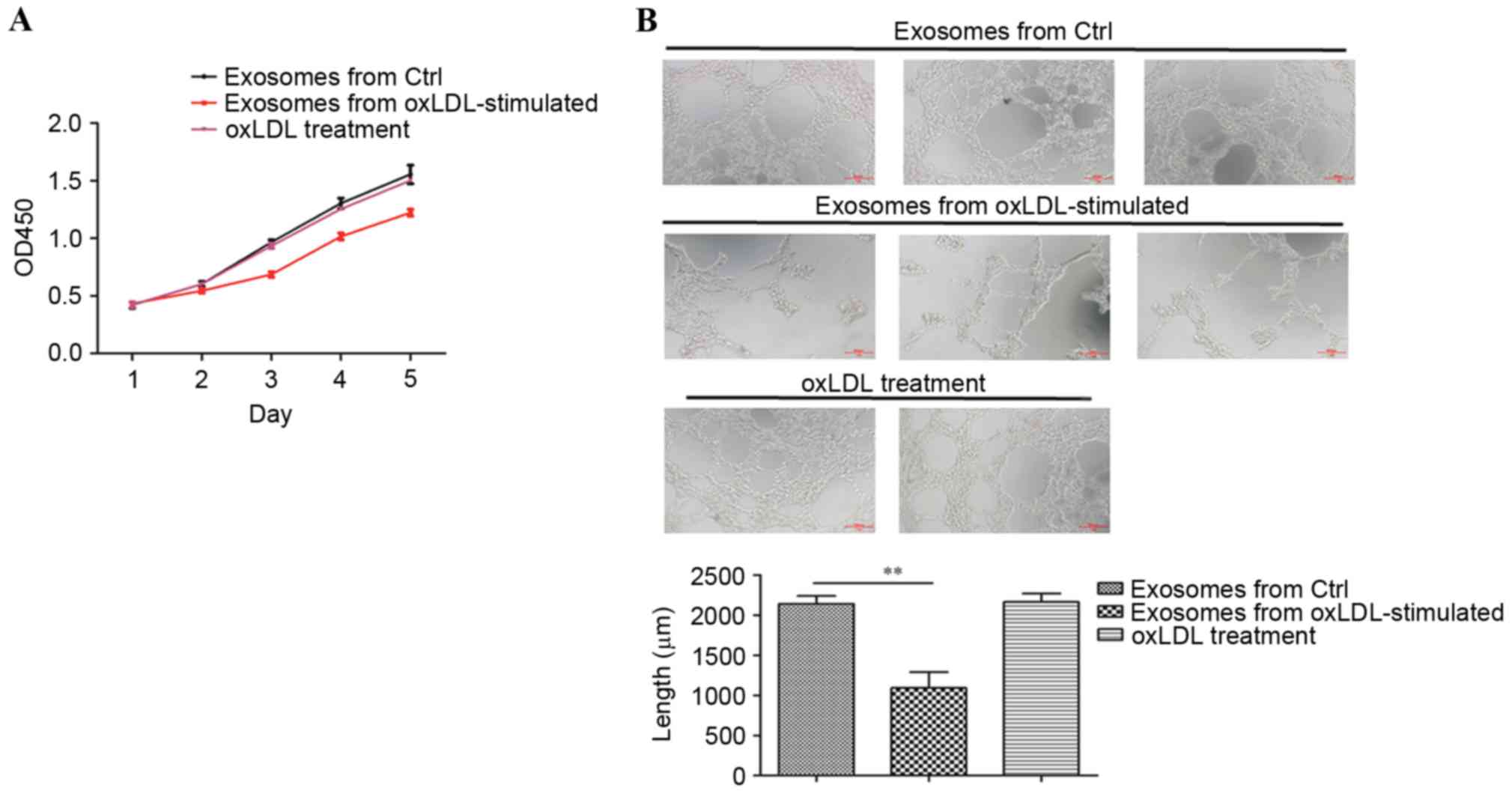
The growth and tube formation abilities of HUVECs
co-cultured with exosomes isolated from oxLDL-treated or untreated
macrophages were analysed to explore the effects of
oxLDL-stimulated macrophage-derived exosomes on endothelial cells.
CCK8 assay results revealed that oxLDL-stimulated
macrophage-derived exosomes decreased the cell growth ability of
HUVECs significantly, compared with exosomes isolated from
untreated macrophages (Fig. 3A).
Furthermore, tube formation assay results demonstrated that
incubation with oxLDL-stimulated macrophage-derived exosomes
reduced the tube formation ability of HUVECs compared with to the
untreated control (Fig. 3B).
HUVECs were also treated with 50 ng/ml oxLDL instead of exosomes.
The CCK8 assay and tube formation assay results revealed that 50
ng/ml oxLDL treatment did not suppress the growth and tube
formation (Fig. 3), indicating
that specifically the exosomes derived from oxLDL-stimulated
macrophages reduced the growth and tube formation abilities of
endothelial cells.
Suppression of exosomal secretion in
oxLDL-stimulated macrophages rescues the growth and tube formation
abilities of endothelial cells
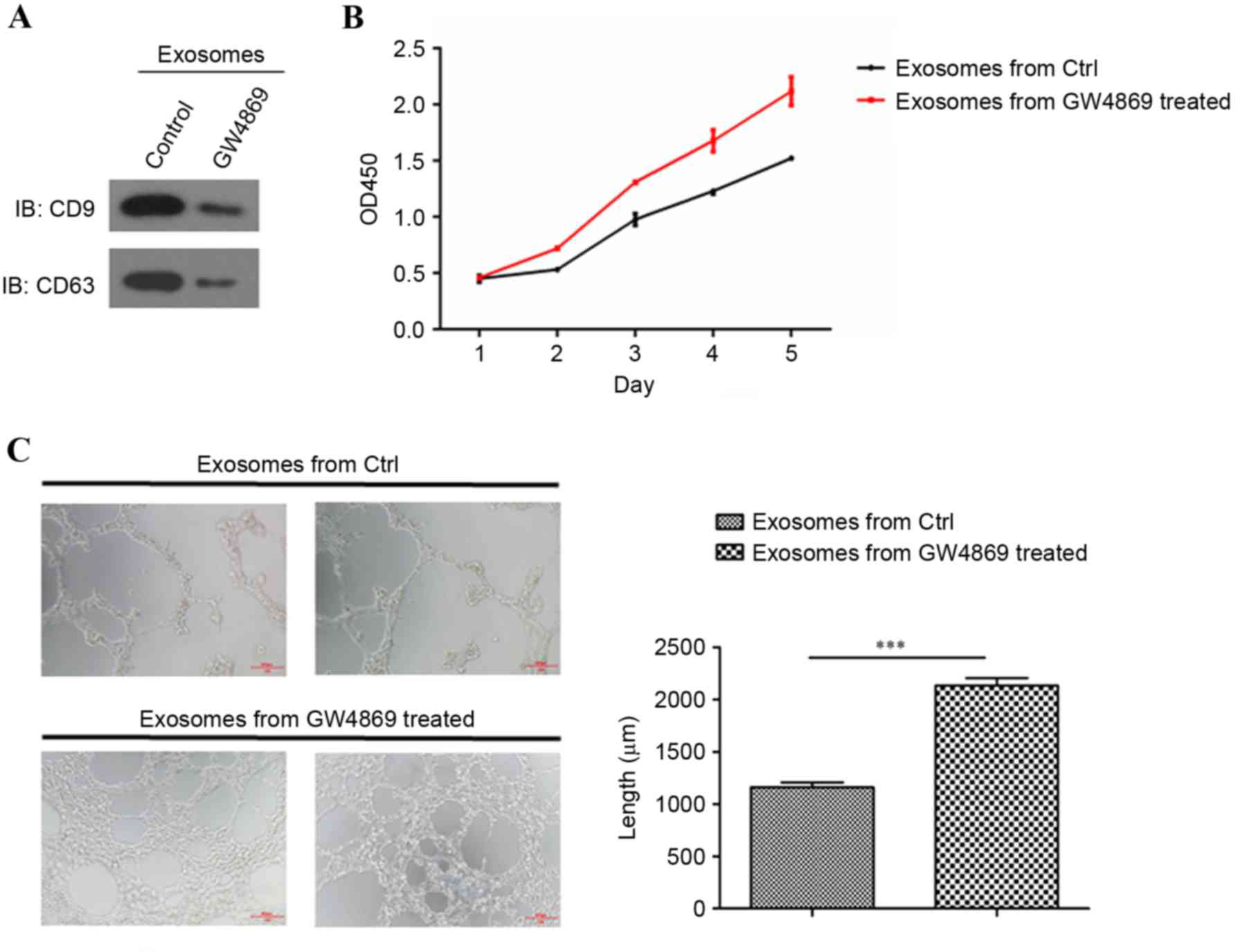
To further verify the effects of oxLDL-stimulated
macrophage-derived exosomes on endothelial cells, exosome
generation was blocked with exosomal release inhibitors GW4869
(20,21) (Fig.
4A). CCK8 assay results revealed that exosomes collected from
oxLDL-stimulated macrophages treated with the GW4869 inhibitor
recovered the cell growth ability of HUVECs compared with the
dimethyl sulfoxide (DMSO) control (Fig. 4B). The tube formation assay results
also indicated that the exosomes collected from the
oxLDL-stimulated macrophages treated with the GW4869 inhibitor
regained tube formation ability compared with the DMSO control
(Fig. 4C). These results indicated
that the blockade of exosomal secretion in oxLDL-stimulated
macrophages rescued normal endothelial function.
Discussion
It has previously been demonstrated that exosomes
mediate cell-cell communication through the transfer of RNAs and
proteins, which contributes to a variety of physiological and
pathological processes, including cardiovascular disease (22–24).
The function of exosomes secreted by oxLDL-stimulated macrophages
in atherosclerosis was investigated in the present study. Exosomes
derived from oxLDL-stimulated macrophages were demonstrated to
reduce the growth and tube formation abilities of endothelial
cells. To the best of the author's knowledge, this is the first
study to demonstrate that oxLDL-stimulated macrophage-derived
exosomes mediate endothelial cell function.
OxLDL contributes to atherosclerotic plaque
formation and progression through several mechanisms, including the
formation of macrophage foam cells and the induction of endothelial
cell activation and dysfunction (25,26).
OxLDL stimulates the differential regulation of acid
sphingomyelinase in macrophages. Pro-inflammatory responses to
oxLDL-immune complexes are mediated by the prolonged activation of
acid sphingomyelinase (26).
Furthermore, oxLDL-induced injury in retinal pigment epithelium
enhances the exosomal and apoptotic bleb release of the membrane
complement regulatory factors CD46 and CD59, indicating that oxLDL
stimulation may alter the components of exosomal release (27). Macrophage-derived exosomes have a
variety of functions in cardiovascular disease, including the
suppression of fibroblast proliferation, the induction of
inflammation and the activation of nuclear factor-κB in endothelial
cells (16). OxLDL-stimulated
macrophage-derived exosomes were demonstrated to mediate the growth
and tube formation abilities of endothelial cells in the present
study. Blockade of exosomal secretion in oxLDL-stimulated
macrophages rescued the growth and tube formation abilities of
endothelial cells. However, the observed effects may be partially
attributed to oxLDL precipitation. The inhibitor GW4869 also has
additional effects to the inhibition of vesicle release. Therefore,
further research is required to verify the proposed function of
exosomes.
Reciprocal interactions between endothelial cells
and macrophages are thought to occur through secreted microvesicles
including exosomes in angiogenic vascular niches (28). Exosomes may contain mRNAs,
non-coding RNAs and proteins. miRNA-containing microvesicles may
regulate inflammation in atherosclerotic disease (29). The horizontal transfer of
macrophage-derived exosomal miRNA-155 has been demonstrated during
cardiac injury (16). Therefore,
the content of exosomes derived from oxLDL-stimulated macrophages
requires further investigation.
In conclusion, the results of the present study
indicated that oxLDL-stimulated macrophages attenuated the growth
and tube formation of endothelial cells, in part through exosomal
transfer. This may aid in the discovery of novel targets for
atherosclerosis therapy.
Acknowledgements
The present study was supported by Zhejiang
Provincial Science Foundation of China (grant no. LY17H020003), and
Zhejiang Medical Science & Technology Project (grant no.
2015KYA070).
References
|
1
|
Wong ND: Epidemiological studies of CHD
and the evolution of preventive cardiology. Nat Rev Cardiol.
11:276–289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thomas MR and Lip GY: Novel risk markers
and risk assessments for cardiovascular disease. Circ Res.
120:133–149. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rohwedder I, Montanez E, Beckmann K,
Bengtsson E, Dunér P, Nilsson J, Soehnlein O and Fässler R: Plasma
fibronectin deficiency impedes atherosclerosis progression and
fibrous cap formation. EMBO Mol Med. 4:564–576. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Badimon L and Vilahur G: Thrombosis
formation on atherosclerotic lesions and plaque rupture. J Intern
Med. 276:618–632. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eren E, Yilmaz N and Aydin O: Functionally
defective high-density lipoprotein and paraoxonase: A couple for
endothelial dysfunction in atherosclerosis. Cholesterol.
2013:7920902013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Husain K, Hernandez W, Ansari RA and
Ferder L: Inflammation, oxidative stress and renin angiotensin
system in atherosclerosis. World J Biol Chem. 6:209–217. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu Y, Zhu J, Hu X, Wang C, Lu D, Gong C,
Yang J and Zong L: CLIC1 inhibition attenuates vascular
inflammation, oxidative stress, and endothelial injury. PLoS One.
11:e01667902016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhu L, Jia F, Wei J, Yu Y, Yu T, Wang Y,
Sun J and Luo G: Salidroside protects against homocysteine-induced
injury in human umbilical vein endothelial cells via the regulation
of endoplasmic reticulum stress. Cardiovasc Ther. 35:33–39. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pirillo A, Norata GD and Catapano AL:
LOX-1, OxLDL, and atherosclerosis. Mediators Inflamm 2013.
1527862013.
|
|
10
|
Li Z, Cheng J and Wang L: Edaravone
attenuates monocyte adhesion to endothelial cells induced by
oxidized low-density lipoprotein. Biochem Biophys Res Commun.
466:723–727. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu XH, Fu YC, Zhang DW, Yin K and Tang CK:
Foam cells in atherosclerosis. Clin Chim Acta. 424:245–252. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wallner S, Grandl M, Liebisch G, Peer M,
Orsó E, Sigrüner A, Sobota A and Schmitz G: oxLDL and eLDL induced
membrane microdomains in human macrophages. PLoS One.
11:e01667982016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lötvall J, Hill AF, Hochberg F, Buzás EI,
Di Vizio D, Gardiner C, Gho YS, Kurochkin IV, Mathivanan S,
Quesenberry P, et al: Minimal experimental requirements for
definition of extracellular vesicles and their functions: A
position statement from the international society for extracellular
vesicles. J Extracell Vesicles. 3:269132014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huber HJ and Holvoet P: Exosomes: Emerging
roles in communication between blood cells and vascular tissues
during atherosclerosis. Curr Opin Lipidol. 26:412–419. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
EV-TRACK Consortium, Van Deun J, Mestdagh
P, Agostinis P, Akay Ö, Anand S, Anckaert J, Martinez ZA, Baetens
T, Beghein E, et al: EV-TRACK: Transparent reporting and
centralizing knowledge in extracellular vesicle research. Nat
Methods. 14:228–232. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang C, Zhang C, Liu L.A.X..Chen B, Li Y
and Du J: Macrophage-derived mir-155-containing exosomes suppress
fibroblast proliferation and promote fibroblast inflammation during
cardiac injury. Mol Ther. 25:192–204. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Osada-Oka M, Shiota M, Izumi Y, Nishiyama
M, Tanaka M, Yamaguchi T, Sakurai E, Miura K and Iwao H:
Macrophage-derived exosomes induce inflammatory factors in
endothelial cells under hypertensive conditions. Hypertens Res.
40:353–360. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Niu C, Wang X, Zhao M, Cai T, Liu P, Li J,
Willard B, Zu L, Zhou E, Li Y, et al: Macrophage foam cell-derived
extracellular vesicles promote vascular smooth muscle cell
migration and adhesion. J Am Heart Assoc. 5:e0040992016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tang N, Sun B, Gupta A, Rempel H and
Pulliam L: Monocyte exosomes induce adhesion molecules and
cytokines via activation of NF-κB in endothelial cells. FASEB J.
30:3097–3106. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao L, Luo H, Li X, Li T, He J, Qi Q, Liu
Y and Yu Z: Exosomes derived from human pulmonary artery
endothelial cells shift the balance between proliferation and
apoptosis of smooth muscle cells. Cardiology. 137:43–53. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gao W, Liu H, Yuan J, Wu C, Huang D, Ma Y,
Zhu J, Ma L, Guo J, Shi H, et al: Exosomes derived from mature
dendritic cells increase endothelial inflammation and
atherosclerosis via membrane TNF-α mediated NF-κB pathway. J Cell
Mol Med. 20:2318–2327. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shen J, Huang CK, Yu H, Shen B, Zhang Y,
Liang Y, Li Z, Feng X, Zhao J, Duan L and Cai X: The role of
exosomes in hepatitis, liver cirrhosis and hepatocellular
carcinoma. J Cell Mol Med. 21:986–992. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Y, Hu YW, Zheng L and Wang Q:
Characteristics and roles of exosomes in cardiovascular disease.
DNA Cell Biol. 36:202–211. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang XC and Gao JQ: Exosomes as novel
bio-carriers for gene and drug delivery. Int J Pharm. 521:167–175.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang J, Wang D and He S: Roles of
antibody against oxygenized low density lipoprotein in
atherosclerosis: Recent advances. Int J Clin Exp Med.
8:11922–11929. 2015.PubMed/NCBI
|
|
26
|
Truman JP, Al Gadban MM, Smith KJ, Jenkins
RW, Mayroo N, Virella G, Lopes-Virella MF, Bielawska A, Hannun YA
and Hammad SM: Differential regulation of acid sphingomyelinase in
macrophages stimulated with oxidized low-density lipoprotein (LDL)
and oxidized LDL immune complexes: Role in phagocytosis and
cytokine release. Immunology. 136:30–45. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ebrahimi KB, Fijalkowski N, Cano M and
Handa JT: Oxidized low-density-lipoprotein-induced injury in
retinal pigment epithelium alters expression of the membrane
complement regulatory factors CD46 and CD59 through exosomal and
apoptotic bleb release. Adv Exp Med Biol. 801:259–265. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Baer C, Squadrito ML, Iruela-Arispe ML and
De Palma M: Reciprocal interactions between endothelial cells and
macrophages in angiogenic vascular niches. Exp Cell Res.
319:1626–1634. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hulsmans M and Holvoet P:
MicroRNA-containing microvesicles regulating inflammation in
association with atherosclerotic disease. Cardiovasc Res. 100:7–18.
2013. View Article : Google Scholar : PubMed/NCBI
|