Introduction
Glioblastoma (GBM), a common primary malignant brain
tumor, accounts for 70% of total brain tumors and demonstrates
aggressive proliferation, metastasis and recurrence (1–3).
These biological behaviors contribute to its poor prognosis and
high mortality with 15–20% patients surviving >3 months
post-diagnosis (4,5). Due to simultaneous occurrence of
multiple lesions, it is challenging to treat patients with GBM and
obtain a desirable clinical outcome (6). At present, a clinical therapeutic
regimen for patients with GBM is to coordinate several methods
including neurosurgery, radiotherapy and chemotherapy (7). Despite the progress made in these
treatment methods, the outcome for patients with GBM remains poor
due to disease recurrence, with 12–15 months median survival time
(8). Therefore, there is a need to
investigate more effective therapies targeting aggressive
biological processes associated with GMB to improve prognosis.
Vitexin, an apigenin-8-C-D-glucopyranoside, is a
flavonoid compound derived from natural products, serving the role
of active ingredient in a number of traditional Chinese medicines
(9,10). Numerous studies have demonstrated
that vitexin has anti-oxidative, anti-inflammatory,
anti-hyperalgesic and neuroprotective effects (11–14).
It has also been reported that vitexin may suppress cell growth and
induce cell apoptosis in a number of cancer cell lines including
hepatocellular carcinoma, oral and esophageal cancer (15–17).
However, to the best of the authors knowledge, whether vitexin
exhibits an effect on GBM remains to be elucidated.
Cell proliferation requires completion of a cell
cycle, and cell apoptosis results from cell cycle arrest (18). A complete cell cycle is composed of
G1, S, G2 and M phases, in which a successful G2/M transition is
important. It has been previously demonstrated that uncontrolled
cell proliferation is a consequence of imbalanced cell cycle
regulation, which is a characteristic of cancer cells (19,20).
Numerous anticancer drugs have been reported to induce G2/M cell
cycle arrest which may effectively repress proliferation of cancer
cells (21–23).
Apoptosis, also known as type I programmed cell
death, is a regulated process triggered in response to cell damage
(24). It is primarily triggered
by caspase-dependent intrinsic or extrinsic pathways and may be
predictive factor for the cytotoxicity of anti-cancer drugs
(25). During apoptosis, a
characteristic morphological alteration in cells is observed,
including cell shrinkage, chromatin condensation and nuclear
fragmentation (26). Additionally,
poly(ADP-ribose) polymerase (PARP), a component of the DNA damage
response mechanism elicited by cytotoxic agents, is cleaved by
functional caspases activated in the apoptotic process (27). Phosphatidylinositol
4,5-bisphosphate 3-kinase (PI3K)/RAC-α serine/threonine-protein
kinase (Akt)/mechanistic target of rapamycin kinase (mTOR)
signaling pathway is a common cancer-associated pathway and
previous studies have reported that it is negatively associated
with cell apoptosis in various cancers (28–30).
To the best of the authors knowledge, the present
study was the first to investigate the anti-cancer effect of
vitexin on human GBM cells and to attempt to elucidate the
underlying mechanisms. The present study demonstrated that vitexin
induced G2/M cell cycle arrest and cell apoptosis by inhibiting
Akt/mTOR signaling in human GBM cells.
Materials and methods
Cell line and culture
The human GBM cell line LN-18 was purchased from
American Type Culture Collection (ATCC; Manassas, VA, USA). These
cells were cultured in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% fetal bovine serum (FBS), 1% penicillin (100
U/ml) and 1% streptomycin (100 µg/ml). The culture was maintained
at 37°C, in a humid atmosphere containing 5% CO2.
Reagents
DMEM and FBS were acquired from Gibco (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Vitexin was purchased from
Selleck Chemicals (Houston, TX, USA) and stored at −20°C following
preparation of the stock solution at 300 mM vitexin dissolved in
dimethyl sulfoxide (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany).
Antibodies against cleaved-PARP (cat. no. 5625), Akt (cat. no.
4685), mTOR (cat. no. 2983), phosphor (p)-Akt (cat. no. 4060),
p-mTOR (cat. no. 5536), GAPDH (cat. no. 5174) and anti-rabbit
immunoglobulin G, horseradish peroxidase-linked antibody (cat. no.
14708) were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA).
Cell viability assay
Viability of GBM cells following treatment with
vitexin was assessed using Cell Counting Kit-8 (CCK8; Dojindo
Molecular Technologies, Inc., Kumamoto, Japan). Cells were plated
in 96-well plates at a density of 5×105 cells/ml for 24
h and subsequently treated with different concentrations of vitexin
(0, 10, 20, 40, 80 and 160 µM). Following incubation for another 24
or 48 h at 37°C, cells were washed with PBS twice and cultured with
CCK8 solution for 2 h according to the manufacturer's instructions.
The absorbance was measured at a wavelength of 450 nm using a
microplate reader iMark (Molecular Devices, LLC, Sunnyvale, CA,
USA) and IC50 values were calculated using SPSS software
19.0 statistical software package (IBM Corp., Armonk, NY, USA).
Cell cycle analysis by flow
cytometry
The influence of vitexin on the proportion of cells
at differing stages of the cell cycle was examined using flow
cytometry. Cells were plated in a 6-well plate at a density of
5×106 cells/ml and treated with different concentrations
of vitexin (0, 20, 40 and 80 µM) for 24 h at 37°C. Cells were
digested using trypsin at 37°C for 1 min and centrifuged at 600 × g
for 5 min at room temperature following washing with PBS, fixed in
75% ethanol at 4°C overnight and subsequently stained with
propidium iodide assay kit (BD Biosciences, Franklin Lakes, NJ,
USA) for 15 min in the dark. The cell cycle distribution was
analyzed using an Accuri C6 flow cytometer (BD Biosciences) and the
resulting data were presented following analysis with ModFit LT
software v3.1 (FACSCalibur; BD Biosciences).
Morphological apoptosis
Morphological characteristics of apoptotic cells
were observed using Hoechst 33342 staining. Cells were incubated in
6-well plates with a coverslip at a density of 5×106
cells/ml and treated with 40 µM vitexin for 24 h at 37°C. Following
incubation, cells on the coverslip were washed with PBS three times
and fixed in 4% paraformaldehyde for 20 min at room temperature and
washed again with PBS. Subsequently, 0.1% Triton X-100 was used to
permeabilize the cells and Hoechst 33342 solution (5 µg/ml) was
added to stain the nucleus of apoptotic cells, for 10 min at room
temperature without light. A fluorescence microscope (Olympus
Corporation, Tokyo, Japan) was used to observe morphological
alterations in the stained cells.
Flow cytometric analysis of
apoptosis
Apoptosis detection was identified using a Annexin
V-fluorescein isothiocyanate (FITC)/PI assay kit (Beyotime
Institute of Biotechnology, Beijing, China) according to the
manufacturer's instructions. In brief, the GBM cells were incubated
in 6-well plates at a density of 5×106 cells/ml and
treated with 0, 20, 40 and 80 µM vitexin. Following incubation for
24 h at 37°C and two washes in ice-cold PBS, cells were collected
and re-suspended in annexin-binding buffer. Subsequently, cells
were stained by adding Annexin V-FITC and PI at room temperature
for 15 min without light. Apoptosis analysis of each sample was
performed using an flow cytometer with Accuri C6 software (BD
Biosciences).
Western blotting
Protein samples were extracted by adding
radioimmunoprecipitation assay buffer (Sigma-Aldrich; Merck KGaA)
to lyse GBM cells. Protein concentrations were quantified using a
Bicinchoninic Acid Protein Assay kit (Pierce; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
Proteins, 30 µg/lane, were added and separated on 8–12% SDS-PAGE
prior to being transferred onto polyvinylidene fluoride membranes
(EMD Millipore, Billerica, MA, USA). Subsequently, membranes were
blocked using 5% skimmed milk at room temperature for 1 h. Primary
antibodies (1:1,000; cleaved-PARP, Akt, phospho-Akt, mTOR,
phospho-mTOR and GAPDH) were added to the membranes and incubated
at 4°C overnight. Subsequently, horseradish peroxidase-conjugated
secondary antibody (1:5,000) was added for 1 h at room temperature.
Detection of proteins was performed using an Enhanced
Chemiluminescence kit (EMD Millipore).
Statistical analysis
Data are presented as the mean ± standard deviation
of three independent experiments and analyzed by one-way analysis
of variance and Dunnett's post hoc test were used in order to
compare differences among groups. P<0.05 was considered to
indicate a statistically significant difference. All statistical
analyses were performed using SPSS software (version 18.0; SPSS,
Inc., Chicago, IL, USA).
Results
Vitexin inhibits cell viability in
human GBM cells
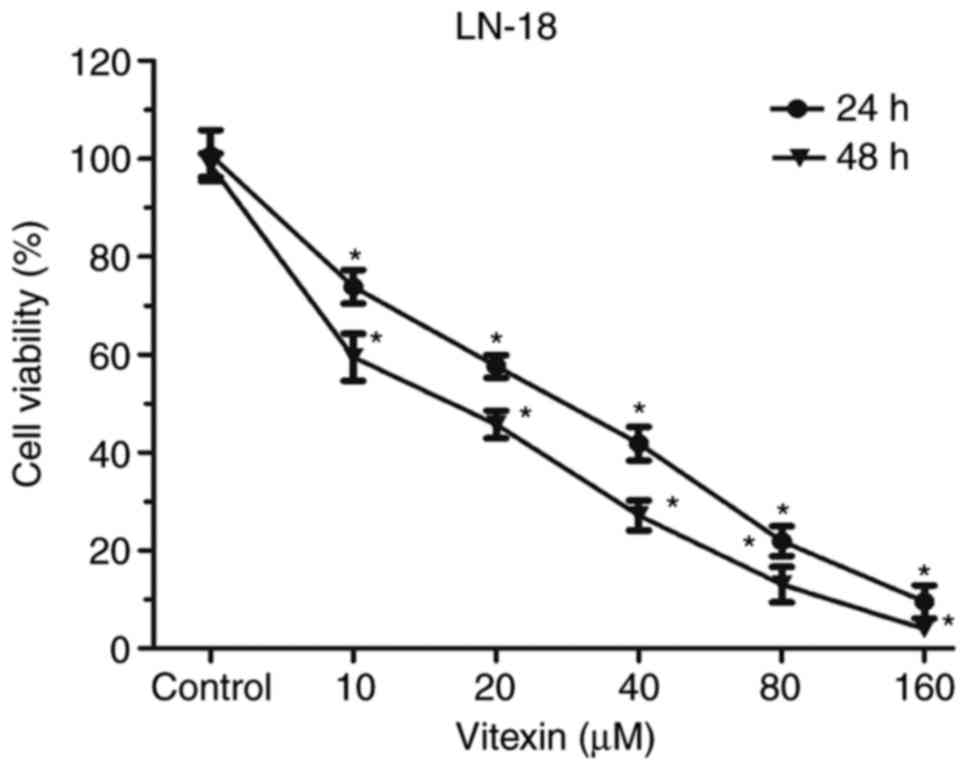
The influence of vitexin on proliferation of human
GBM cells was assessed by CCK-8 assay (Fig. 1). Cells were cultured with
different concentrations of vitexin (0, 10, 20, 40, 80 and 160 µM)
for 24 or 48 h. Following incubation, cell viability significantly
decreased in a dose and time dependent manner. IC50
values for GBM cells were 32.32 and 25.32 µM following 24 and 48 h
of incubation, respectively. The aforementioned results indicated
that vitexin suppressed cell proliferation in human GBM cells in a
dose- and time-dependent manner.
Vitexin induces G2/M cell cycle arrest
in human GBM cells
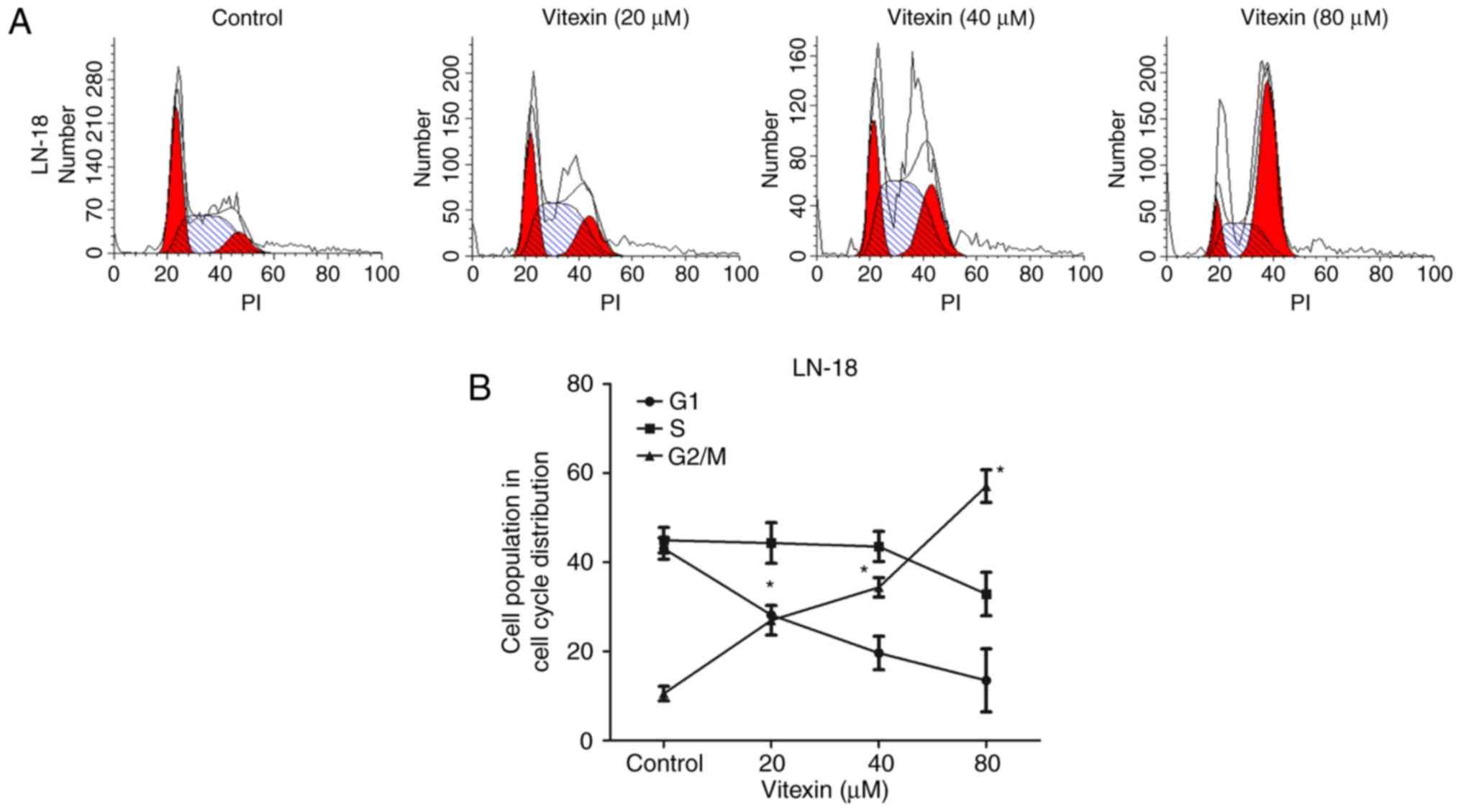
To investigate whether vitexin inhibited GBM cell
proliferation by inducing cell cycle arrest, cell cycle progression
was assessed by flow cytometry. Following treatment with vitexin at
20–80 µM for 24 h, cells were collected to perform cell cycle
analysis (Fig. 2A and B). Compared
with the control group, a significant increase in the percentage of
the cell population in the G2/M phase, and a decrease in the number
of cells in G1 phase, were observed in cells treated with vitexin.
The aforementioned data indicated that vitexin exhibited an effect
on cell cycle progression by inducing cell cycle arrest at G2/M
phase.
Vitexin induces apoptosis in human GBM
cells
Cell apoptosis is a consequence of cell cycle
arrest. Therefore, the present study further investigated the
influence of vitexin on cell apoptosis of human GBM cells. Hoechst
33258 staining was performed to observe nuclear morphological
alterations characteristic of apoptotic cells, following treatment
of GBM cells with 40 µM vitexin for 24 h. GBM cells treated with
vitexin exhibited features including cell shrinkage, chromatin
condensation and nuclear fragmentation, compared with the control
group (Fig. 3A). Subsequently,
Annexin V/PI double staining was used to assess the proportion of
apoptotic cells. The results indicated a dose-dependent increase in
the number of early and late apoptotic cells (Fig. 3B and C). Western blotting was
performed to determine the expression level of the intracellular
apoptosis-associated protein, cleaved-PARP. PARP recruits DNA
repair proteins by binding to DNA breaks. Levels of cleaved-PARP
markedly increased following vitexin treatment. The aforementioned
results demonstrated that vitexin induced cell apoptosis to inhibit
cell proliferation.
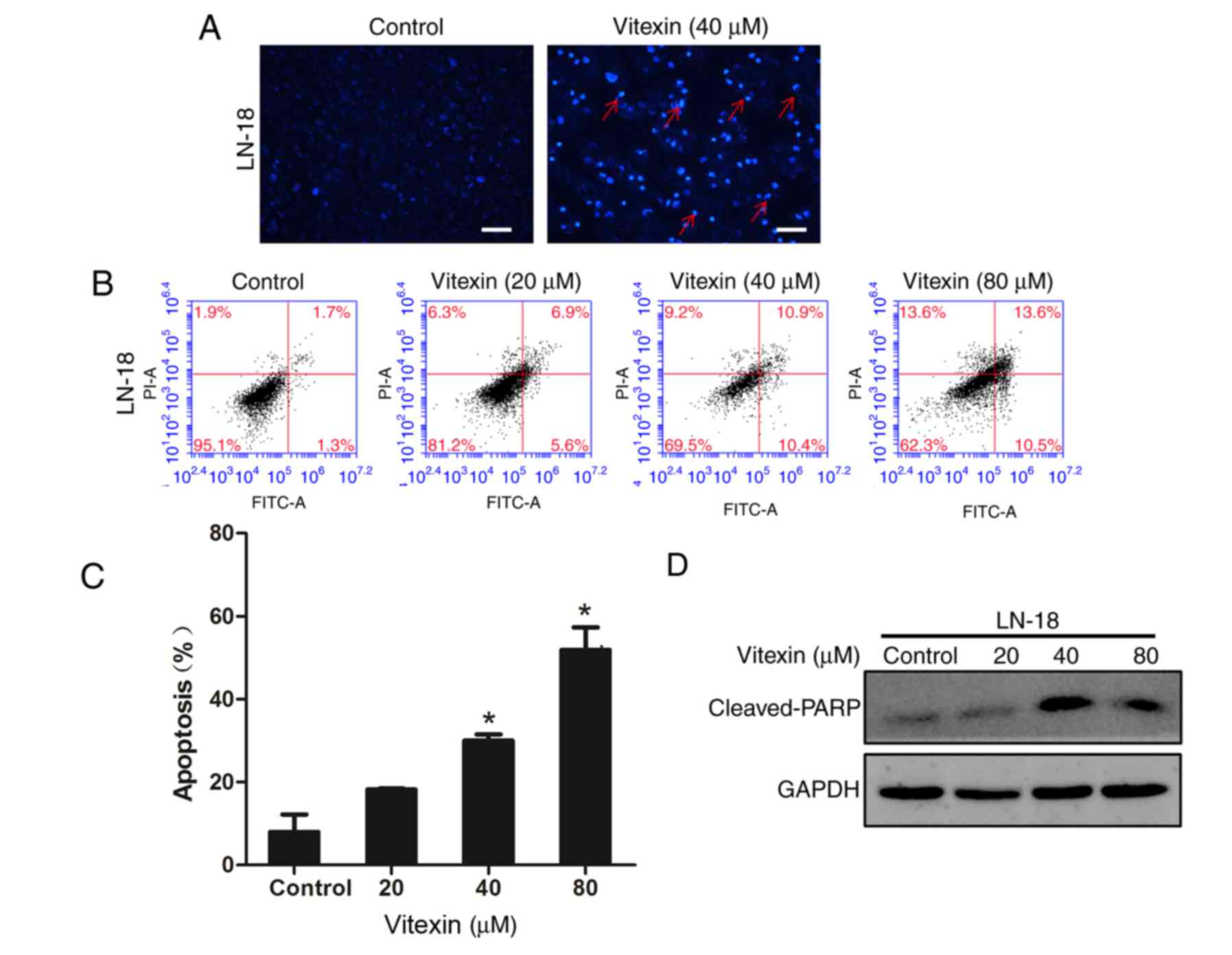 | Figure 3.Vitexin induces apoptosis of human GBM
cells. GBM cells were treated with vitexin for 24 h. (A) Nuclear
morphological alterations characteristic of apoptotic cells
(indicated by red arrows), including chromatin condensation and
nuclear fragmentation, were observed under the fluorescence
microscope through Hoechst 33258 staining. Scale bar, 50 µm. (B)
Cells were harvested, stained by Annexin V and PI, and subsequently
analyzed by flow cytometry. (C) Apoptotic cell proportion was
presented in the histogram. Data are presented as the mean ±
standard deviation. *P<0.05 vs. the untreated control group. (D)
Following incubation, expression level of cleaved-PARP, an
apoptosis-associated protein, was assessed by western blotting.
PARP, poly(ADP-ribose) polymerase; GBM, glioblastoma; PI, propidium
iodide. |
Vitexin inhibits Akt/mTOR signaling
pathway in human GBM cells
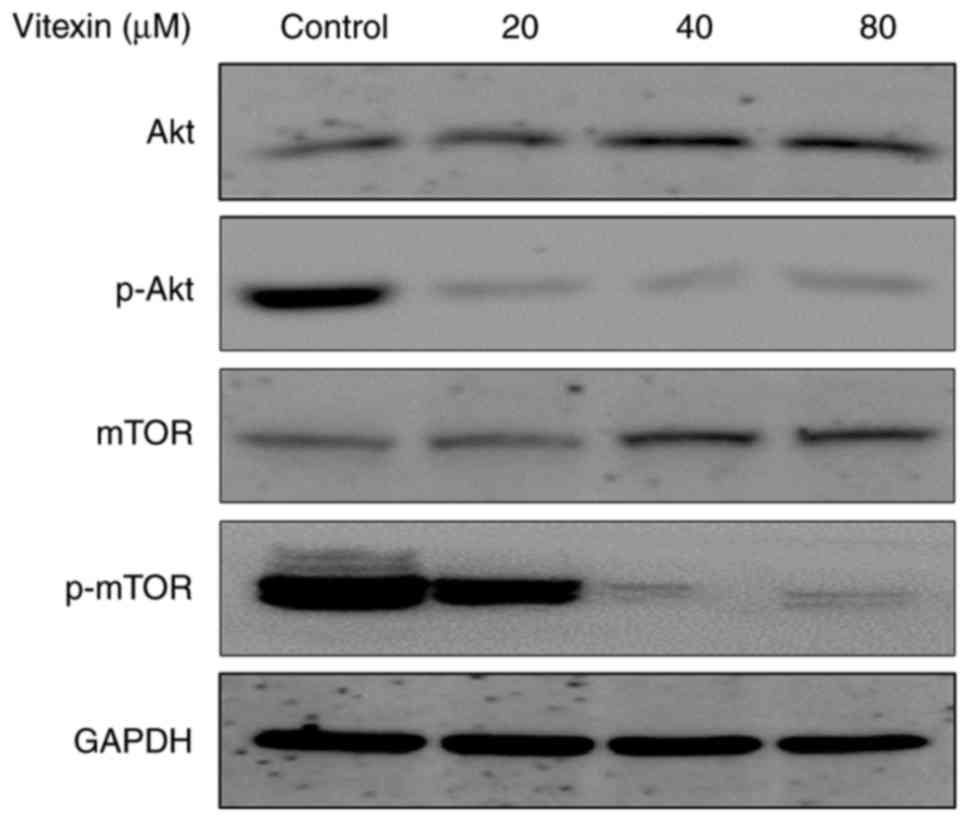
The aforementioned in vitro results indicated
that vitexin treatment resulted in cell cycle arrest and cell
apoptosis to inhibit cell viability in human GBM cells. To further
investigate the underlying molecular mechanism of the anti-tumor
effect of vitexin, the present study investigated the alterations
in the Akt/mTOR signaling pathway. Western blotting was performed
to measure the expression levels of Akt, mTOR, p-Akt, and p-mTOR
proteins. The results demonstrated a dose-dependent decrease in
phosphorylation of Akt and mTOR following treatment with vitexin
(Fig. 4). These results indicated
that vitexin inhibited activation of the Akt/mTOR signaling
pathway, which may contribute to vitexin-induced cell cycle arrest
and apoptosis.
Discussion
Natural products may serve as a source of flavonoids
used for cancer prevention and treatment (31,32).
The flavonoid vitexin has previously gained attention due to its
multiple pharmacological effects, including neuroprotective and
anti-cancer properties. Vitexin was reported to markedly inhibit
cell growth and induce cell apoptosis in a number of cancer cell
lines, including hepatocellular carcinoma, oral and esophageal
cancer (33,34). However, whether it exhibits an
effect on malignant GBM remains to be elucidated. The present study
demonstrated that vitexin induced G2/M cell cycle arrest and cell
apoptosis by inhibiting Akt/mTOR signaling in human GBM cells.
The cell cycle is a precisely regulated process
composed of G1, S, G2 and M phases. A successful transition between
two phases serves a role in cell proliferation, particularly the
G2/M transition, which promotes the symmetric division of a cell
(35). Disruption of the cell
cycle is characteristic of cancer cells and results in uncontrolled
cell proliferation. Therefore, the therapeutic aim of anti-cancer
agents is to suppress cell proliferation, via an induction of G2/M
cell cycle arrest. The present study demonstrated that vitexin
exhibited an effect on cell cycle progression by inducing cell
cycle arrest at G2/M transition phase. Compared with the control
group, the percentage of cells at G2/M phase increased markedly,
whereas the percentage of cells at G1 phase decreased.
Apoptosis is a result of cell cycle arrest and its
rate may be used to predict the cytotoxicity of anti-cancer agents
(36,37). In the presence of pro-death
stimuli, including cytotoxic agents, cells respond by initiating
programmed death, characterized by certain detectable morphological
alterations, including nuclear fragmentation, chromatin
condensation, cell shrinkage, membrane blebbing and formation of
apoptotic bodies (26,38). The aforementioned morphological
alterations serve as indicators of cell apoptosis. In the present
study, morphological alterations indicated cell apoptosis following
treatment with vitexin. A number of studies have demonstrated that
cell apoptosis is triggered by caspase-dependent or
caspase-independent pathways and the former is more common
(39–42). In the caspase-dependent apoptotic
process, caspases-3 and −8, and other downstream caspases are
activated and subsequently cleave their common substrate, PARP,
inducing apoptosis. Therefore, an increased expression level of
cleaved-PARP is an indicator of cell apoptosis. Western blotting
results demonstrated an increase in the expression level of
cleaved-PARP in human GBM cells.
It has previously been demonstrated that certain
signaling pathways are involved in the cell apoptosis process,
including the PI3K/Akt/mTOR and mitogen-activated protein kinase
pathways (29,43,44).
The present study investigated Akt/mTOR signaling due to its
association with cell apoptosis in various cancer diseases
(45). In the present study,
phosphorylation of Akt and mTOR molecules was suppressed compared
with the control group, which indicated negative regulation of
Akt/mTOR signaling during vitexin-induced cell apoptosis.
In conclusion, the present study demonstrated that
vitexin induced G2/M cell cycle arrest and cell apoptosis by
inhibiting the Akt/mTOR signaling pathway in human GBM cells. To
the best of the authors knowledge, the present study is the first
to investigate anticancer effects of vitexin on human GBM cells and
the underlying molecular mechanisms. Since flavonoids have been
extensively used for cancer prevention and treatment, vitexin may
in the future be used for GBM chemotherapeutic treatment, which
requires further investigation.
Acknowledgements
The present study was supported by the Natural
Science Foundation of Shandong Province (grant no. S20163721).
References
|
1
|
Kubelt C, Hattermann K, Sebens S, Mehdorn
HM and Held-Feindt J: Epithelial-to-mesenchymal transition in
paired human primary and recurrent glioblastomas. Int J Oncol.
46:2515–2525. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Omuro A and DeAngelis LM: Glioblastoma and
other malignant gliomas: A clinical review. JAMA. 310:1842–1850.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Woehrer A, Bauchet L and Barnholtz-Sloan
JS: Glioblastoma survival: Has it improved? Evidence from
population-based studies. Curr Opin Neurol. 27:666–674.
2014.PubMed/NCBI
|
|
5
|
Cuddapah VA, Robel S, Watkins S and
Sontheimer H: A neurocentric perspective on glioma invasion. Nat
Rev Neurosci. 15:455–465. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wirsching HG, Galanis E and Weller M:
Glioblastoma. Handb Clin Neurol. 134:381–397. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Arévalo ÁST, Erices JI, Uribe DA, Howden
J, Niechi I, Muñoz S, Martín RS and Monrás CAQ: Current therapeutic
alternatives and new perspectives in glioblastoma multiforme. Curr
Med Chem. 24:2781–2795. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Desjardins A, Rich JN, Quinn JA,
Vredenburgh J, Gururangan S, Sathornsumetee S, Reardon DA, Friedman
AH, Bigner DD and Friedman HS: Chemotherapy and novel therapeutic
approaches in malignant glioma. Front Biosci. 10:2645–2668. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gaitan E, Cooksey RC, Legan J and Lindsay
RH: Antithyroid effects in vivo and in vitro of vitexin: A
C-glucosylflavone in millet. J Clin Endocrinol Metab. 80:1144–1147.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
He M, Min JW, Kong WL, He XH, Li JX and
Peng BW: A review on the pharmacological effects of vitexin and
isovitexin. Fitoterapia. 115:74–85. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Y, Zhen Y, Wu X, Jiang Q, Li X, Chen
Z, Zhang G and Dong L: Vitexin protects brain against
ischemia/reperfusion injury via modulating mitogen-activated
protein kinase and apoptosis signaling in mice. Phytomedicine.
22:379–384. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Che X, Wang X, Zhang J, Peng C, Zhen Y,
Shao X, Zhang G and Dong L: Vitexin exerts cardioprotective effect
on chronic myocardial ischemia/reperfusion injury in rats via
inhibiting myocardial apoptosis and lipid peroxidation. Am J Transl
Res. 8:3319–3328. 2016.PubMed/NCBI
|
|
13
|
An F, Yang G, Tian J and Wang S:
Antioxidant effects of the orientin and vitexin in Trollius
chinensis Bunge in D-galactose-aged mice. Neural Regen Res.
7:2565–2575. 2012.PubMed/NCBI
|
|
14
|
Yang L, Yang ZM, Zhang N, Tian Z, Liu SB
and Zhao MG: Neuroprotective effects of vitexin by inhibition of
NMDA receptors in primary cultures of mouse cerebral cortical
neurons. Mol Cell Biochem. 386:251–258. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
He JD, Wang Z, Li SP, Xu YJ, Yu Y, Ding
YJ, Yu WL, Zhang RX, Zhang HM and Du HY: Vitexin suppresses
autophagy to induce apoptosis in hepatocellular carcinoma via
activation of the JNK signaling pathway. Oncotarget. 7:84520–84532.
2016.PubMed/NCBI
|
|
16
|
Yang SH, Liao PH, Pan YF, Chen SL, Chou SS
and Chou MY: The novel p53-dependent metastatic and apoptotic
pathway induced by vitexin in human oral cancer OC2 cells.
Phytother Res. 27:1154–1161. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
An F, Wang S, Tian Q and Zhu D: Effects of
orientin and vitexin from Trollius chinensis on the growth and
apoptosis of esophageal cancer EC-109 cells. Oncol Lett.
10:2627–2633. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schwartz GK and Shah MA: Targeting the
cell cycle: A new approach to cancer therapy. J Clin Oncol.
23:9408–9421. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stewart ZA, Westfall MD and Pietenpol JA:
Cell-cycle dysregulation and anticancer therapy. Trends Pharmacol
Sci. 24:139–145. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim G, Kim TH, Hwang EH, Chang KT, Hong JJ
and Park JH: Withaferin A inhibits the proliferation of gastric
cancer cells by inducing G2/M cell cycle arrest and apoptosis.
Oncol Lett. 14:416–422. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liberal J, Carmo A, Gomes C, Cruz MT and
Batista MT: Urolithins impair cell proliferation, arrest the cell
cycle and induce apoptosis in UMUC3 bladder cancer cells. Invest
New Drugs. Jun 20–2017.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Silva IT, Geller FC, Persich L, Dudek SE,
Lang KL, Caro MS, Durán FJ, Schenkel EP, Ludwig S and Simões CM:
Cytotoxic effects of natural and semisynthetic cucurbitacins on
lung cancer cell line A549. Invest New Drugs. 34:139–148. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
King KL and Cidlowski JA: Cell cycle and
apoptosis: Common pathways to life and death. J Cell Biochem.
58:175–180. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kiraz Y, Adan A, Kartal Yandim M and Baran
Y: Major apoptotic mechanisms and genes involved in apoptosis.
Tumour Biol. 37:8471–8486. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Burgess DJ: Apoptosis: Refined and lethal.
Nat Rev Cancer. 13:792013. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Germain M, Affar EB, D'Amours D, Dixit VM,
Salvesen GS and Poirier GG: Cleavage of automodified
poly(ADP-ribose) polymerase during apoptosis. Evidence for
involvement of caspase-7. J Biol Chem. 274:28379–28384. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Granato M, Rizzello C, Gilardini Montani
MS, Cuomo L, Vitillo M, Santarelli R, Gonnella R, D'Orazi G,
Faggioni A and Cirone M: Quercetin induces apoptosis and autophagy
in primary effusion lymphoma cells by inhibiting PI3K/AKT/mTOR and
STAT3 signaling pathways. J Nutr Biochem. 41:124–136. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ding L, Ding L, Wang S, Wang S, Wang W,
Wang W, Lv P, Lv P, Zhao D, Zhao D, et al: Tanshinone IIA affects
autophagy and apoptosis of glioma cells by inhibiting
phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin
signaling pathway. Pharmacology. 99:188–195. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang Y, Sun Y, Wu Y and Zhang J:
Cucurbitacin E inhibits osteosarcoma cells proliferation and
invasion through attenuation of PI3K/AKT/mTOR signaling. Biosci
Rep. BSR201601652016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li Y, Li S, Meng X, Gan RY, Zhang JJ and
Li HB: Dietary natural products for prevention and treatment of
breast cancer. Nutrients. 9:E7282017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kou X, Fan J and Chen N: Potential
molecular targets of ampelopsin in prevention and treatment of
cancers. Anticancer Agents Med Chem. Apr 12–2017.(Epub ahead of
print). PubMed/NCBI
|
|
33
|
Zhou J, Hu H, Long J, Wan F, Li L, Zhang
S, Shi YE and Chen Y: Vitexin 6, a novel lignan, induces autophagy
and apoptosis by activating the Jun N-terminal kinase pathway.
Anticancer Drugs. 24:928–936. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tan Z and Zhang Y, Deng J, Zeng G and
Zhang Y: Purified vitexin compound 1 suppresses tumor growth and
induces cell apoptosis in a mouse model of human choriocarcinoma.
Int J Gynecol Cancer. 22:360–366. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xia Y, Lei Q, Zhu Y, Ye T, Wang N, Li G,
Shi X, Liu Y, Shao B, Yin T, et al: SKLB316, a novel small-molecule
inhibitor of cell-cycle progression, induces G2/M phase arrest and
apoptosis in vitro and inhibits tumor growth in vivo. Cancer Lett.
355:297–309. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bendale Y, Bendale V and Paul S:
Evaluation of cytotoxic activity of platinum nanoparticles against
normal and cancer cells and its anticancer potential through
induction of apoptosis. Integr Med Res. 6:141–148. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cao FJ, Zhu LF, Kuang Q, Li XQ, Zhou BH,
Yang XJ and Zhou L: Cytotoxic activity, apoptosis induction and
structure-activity relationship of
8-OR-2-aryl-3,4-dihydroisoquinolin-2-ium salts as promising
anticancer agents. Bioorg Med Chem Lett. 27:55–60. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ivanovska I, Muhoro CN and Irusta PM:
Anti-tumor therapeutic molecules that target the programmed cell
death machinery. Mini Rev Med Chem. 6:1033–1042. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kolenko VM, Uzzo RG, Bukowski R and Finke
JH: Caspase-dependent and -independent death pathways in cancer
therapy. Apoptosis. 5:17–20. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang H, Zhang T, Sun W, Wang Z, Zuo D,
Zhou Z, Li S, Xu J, Yin F, Hua Y and Cai Z: Erianin induces
G2/M-phase arrest, apoptosis, and autophagy via the ROS/JNK
signaling pathway in human osteosarcoma cells in vitro and in vivo.
Cell Death Dis. 7:e22472016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Croce CM and Reed JC: Finally, an
apoptosis-targeting therapeutic for cancer. Cancer Res.
76:5914–5920. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Su Z, Yang Z, Xu Y, Chen Y and Yu Q:
Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol
Cancer. 14:482015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Huang G, Zou B, Lv J, Li T, Huai G, Xiang
S, Lu S, Luo H, Zhang Y, Jin Y and Wang Y: Notoginsenoside R1
attenuates glucose-induced podocyte injury via the inhibition of
apoptosis and the activation of autophagy through the PI3K/Akt/mTOR
signaling pathway. Int J Mol Med. 39:559–568. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ko JH, Lee JH, Jung SH, Lee SG,
Chinnathambi A, Alharbi SA, Yang WM, Um JY, Sethi G and Ahn KS:
2,5-Dihydroxyacetophenone induces apoptosis of multiple myeloma
cells by regulating the MAPK activation pathway. Molecules.
22:E11572017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fulda S: Synthetic lethality by
co-targeting mitochondrial apoptosis and PI3K/Akt/mTOR signaling.
Mitochondrion. 19:85–87. 2014. View Article : Google Scholar : PubMed/NCBI
|