Introduction
Acute liver injury induced by drugs, poisons or
infections is a common health problem worldwide, which remains one
of the leading causes of death (1). To alleviate the liver damage and
improve the outcomes, extensive studies have been conducted to
investigate the mechanisms underlying the development of liver
injury (2–5). Importantly, the liver has strong
regenerative activity after injury to compensate liver cell loss
(6–8). The regeneration process is crucial
for the recovery of hepatic structure and function (9–12).
AMP-activated protein kinase (AMPK) is a
serine/threonine kinase. It is usually regarded as a sensor of
cellular energy status used to keep up a delicate balance by
monitoring both the short- and long-term total body energy
requirements (13). Interestingly,
recent studies have found that AMPK was extensively involved in
various energy-intensive physiological and pathophysiological
processes, such as inflammation (14), autophagy (15–17)
and proliferation (18–21). Therefore, AMPK is rapidly emerging
as a novel target for pathophysiological and pharmacological
research (22).
There is increasing evidence that AMPK is involved
in the development of hepatic disorders. Several studies have found
that AMPK plays crucial roles in the development of cholestatic
liver diseases, nonalcoholic fatty liver disease and liver fibrosis
(23–25). In addition, AMPK also have
potential value for the pharmacological intervention of liver
injury induced by carbon tetrachloride (CCl4), endotoxin
or ischemia (26–28). Although the crucial roles of AMPK
in liver injury have been reported, the pathological significance
of AMPK in the regeneration stage post acute liver injury was
unclear.
Because liver regeneration includes a serial of
highly active molecular responses, which requires intensive energy
supply (9), we then suspected that
AMPK might regulate the procedure of liver regeneration. In the
present study, the potential role of AMPK in liver regeneration was
investigated in mice with CCl4-induced toxic liver
injury (13). In this widely used
animal model, the phosphorylation status of AMPK post
CCl4 exposure was detected. Then, the activity of AMPK
was inhibited by a selective AMPK inhibitor, compound C (29,30),
and the degree of liver regeneration was determined.
Materials and methods
Experimental animals
The male KM mice (Mus musculus Km) weighing
18–22 g were purchased from the Experimental Animal Center of
Chongqing Medical University (Chongqing, China). Mice were kept in
a 12-h light/dark cycle with ad libitum water and food. All
experimental protocols involving the animals were approved by the
Institutional Animal Care and Use Committee of Chongqing Medical
University.
Reagents
CCl4 was obtained from Chengdu Kelong
Chemical Reagent Factory (Chengdu, China). The AMPK inhibitor
F6-[4-[2-(1-piperidinyl)ethoxy]phenyl]-3-
(4-pyridinyl)-pyrazolo[1,5-a]pyrimidine (compound C) was purchased
from Cayman Chemical (Ann Arbor, Michigan, MI, USA). The alanine
aminotransferase (ALT) detection kit was purchased from Nanjing
Jiancheng Bioengineering Institute (Nanjing, China). Rabbit
anti-mouse antibodies against AMPK, phosphorylated AMPK (p-AMPK,
Thr172) and β-actin were purchased from Cell signaling
Technology (Danvers, MA, USA). Rabbit anti-mouse antibody against
proliferating cell nuclear antigen (PCNA) antibody was purchased
from Abcam (Cambridge, UK). The BCA protein assay kit, the
horseradish peroxidase-conjugated goat anti-rabbit antibody and the
enhanced chemiluminescence (ECL) reagents were obtained from Pierce
Biotechnology (Rockford, IL, USA).
Experimental design
To induce acute liver injury, the mice received
intraperitoneal injection of CCl4 (1%, dissolved in
olive oil, 0.8 ml/kg). To determine the phosphorylation status of
hepatic AMPK, the mice were anesthetized and sacrificed at various
timepoints post CCl4 exposure (0, 24 and 48 h; n=8 for
each group), the liver and plasma samples were harvested for
further experiments. To investigate the potential roles of AMPK in
acute liver injury, the mice were randomly divided into 4 groups
(n=8 for each group): i) the control (CON) group, mice received
vehicle only; ii) the compound C group, mice received the AMPK
inhibitor compound C (15 mg/kg, i.p.) only; iii) the
CCl4 group, mice exposed to CCl4; iv) the
CCl4 + compound C group, mice received compound C 0.5 h
prior to CCl4 challenge. The animals were sacrificed 24
h post CCl4 exposure, the liver and plasma samples were
harvested. To investigate the potential roles of AMPK in liver
injury, the mice were randomly divided into 4 groups (n=8 for each
group): i) the CON group, mice received vehicle only; ii) the
compound C group, mice received the AMPK inhibitor compound C (15
mg/kg, i.p.) only; iii) the CCl4 group, mice exposed to
CCl4; iv) the CCl4 + compound C group, mice
received compound C 24 h post CCl4 challenge. The
animals were sacrificed 48 h post CCl4 exposure, the
liver and plasma samples were harvested. The plasma samples were
collected in the heparin tubes and were then centrifuged at 5,000
RPM for 15 min at 4°C. The supernatants were collected for further
examinations.
Determination of liver enzymes
The plasma samples were collected with the method
described above. The levels of ALT in plasma samples were
determined following the manufacturer's instructions. The enzymatic
activities were calculated according to the standard curve.
Histochemistry
The liver samples were fixed in formalin, embedded
in paraffin, and the liver sections were evaluated with hematoxylin
and eosin staining under light microscope (Olympus Corp., Tokyo,
Japan).
Immunoblot analysis
The proteins were extracted from the liver samples
and the concentration of the protein samples was determined by BCA
protein assay kit (Pierce Biotechnology). Protein extracts were
separated on 10 or 8% SDS-PAGE gels, then transferred to
nitrocellulose membrane (Millipore, Billerica, MA, USA). After
incubation with the 5% (w/v) skimmed milk in Tris-buffered saline
(TBST) containing 0.1% Tween-20 for 2 h at room temperature, the
membranes were incubated with primary antibodies overnight at 4°C.
And then, the membranes were washed by TBST (containing 0.05%
Tween-20), then incubated with secondary antibody for 1.5 h at 37°C
and then washed by TBST. Antibody binding was visualized with an
ECL chemiluminescence system (Pierce Biotechnology).
Statistical analysis
Data were presented as mean ± SD and analyzed by
one-way ANOVA with Turkey's post hoc test in SPSS13.0. P<0.05
was considered to indicate a statistically significant
difference.
Results
AMPK was activated post
CCl4 challenge
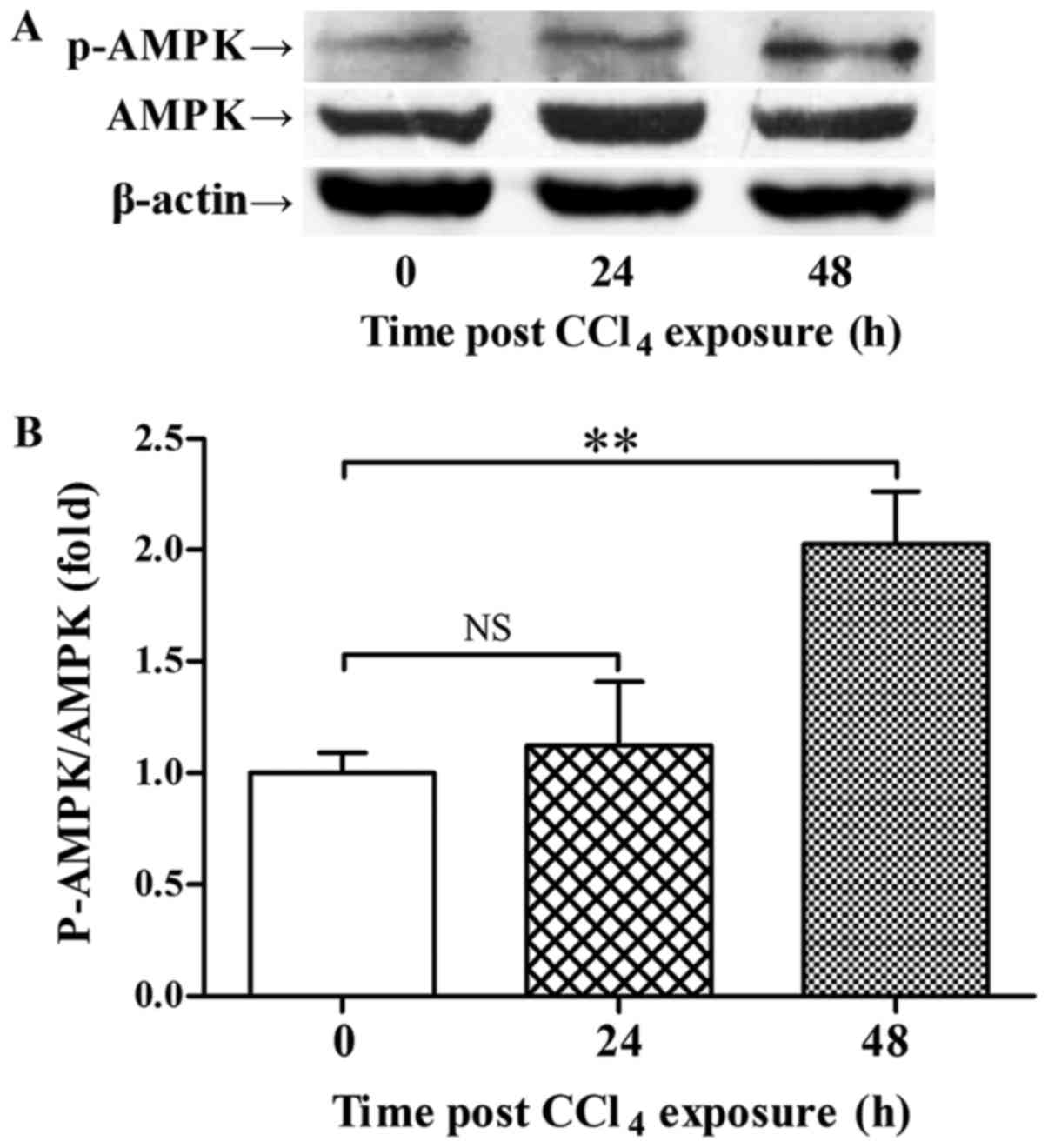
The immunoblot analysis showed that CCl4
exposure did not stimulate the phosphorylation of AMPK 24 h
post-CCl4 administration, but the phosphorylation level
of AMPK was upregulated 48 h post-CCl4 exposure
(Fig. 1). The upregulation of
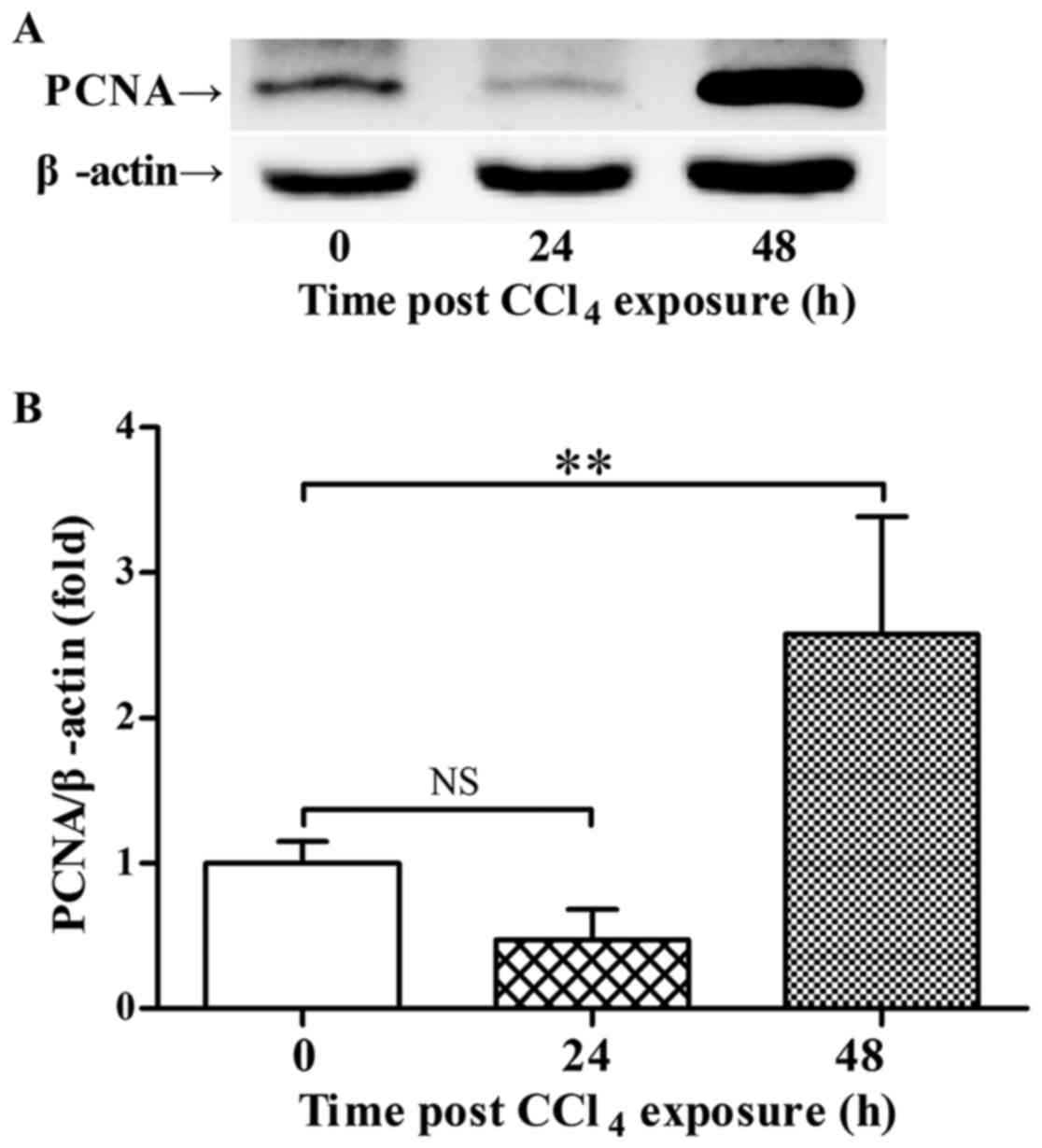
p-AMPK 48 h post-CCl4 exposure was accompanied with
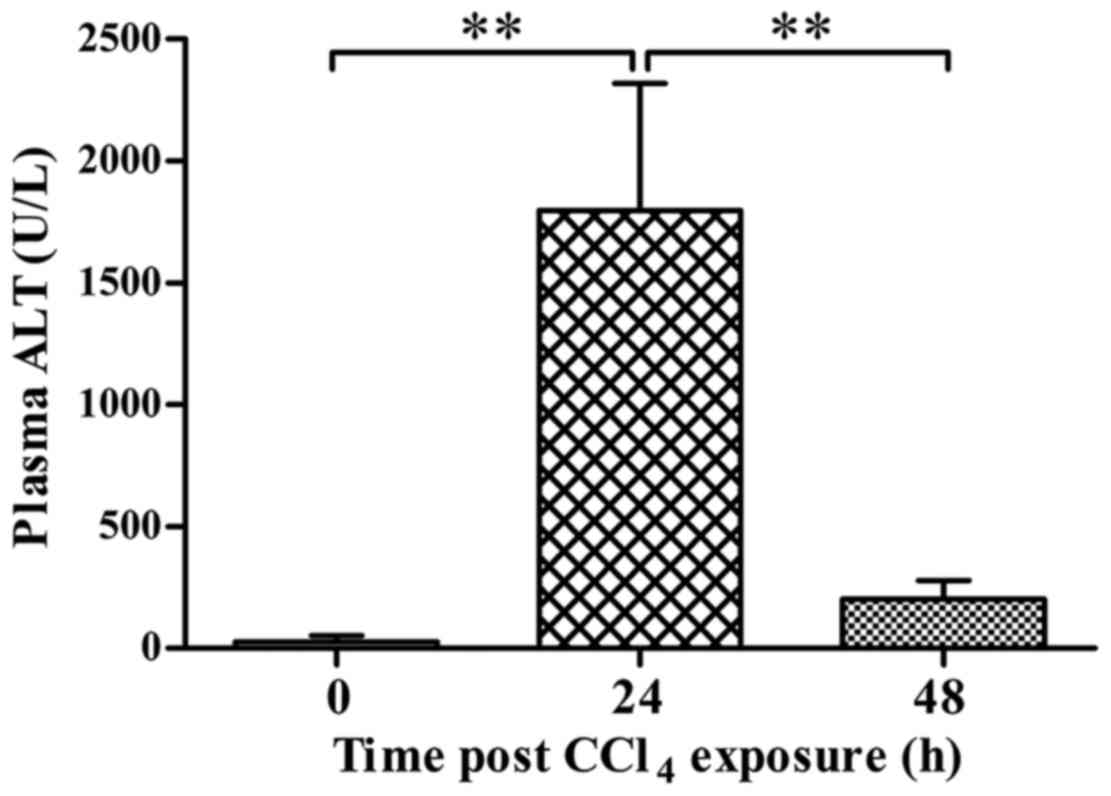
increased expression of PCNA and recovery of ALT level (Figs. 2 and 3).
Pre-insult treatment with an AMPK
inhibitor had no obvious effects on liver injury
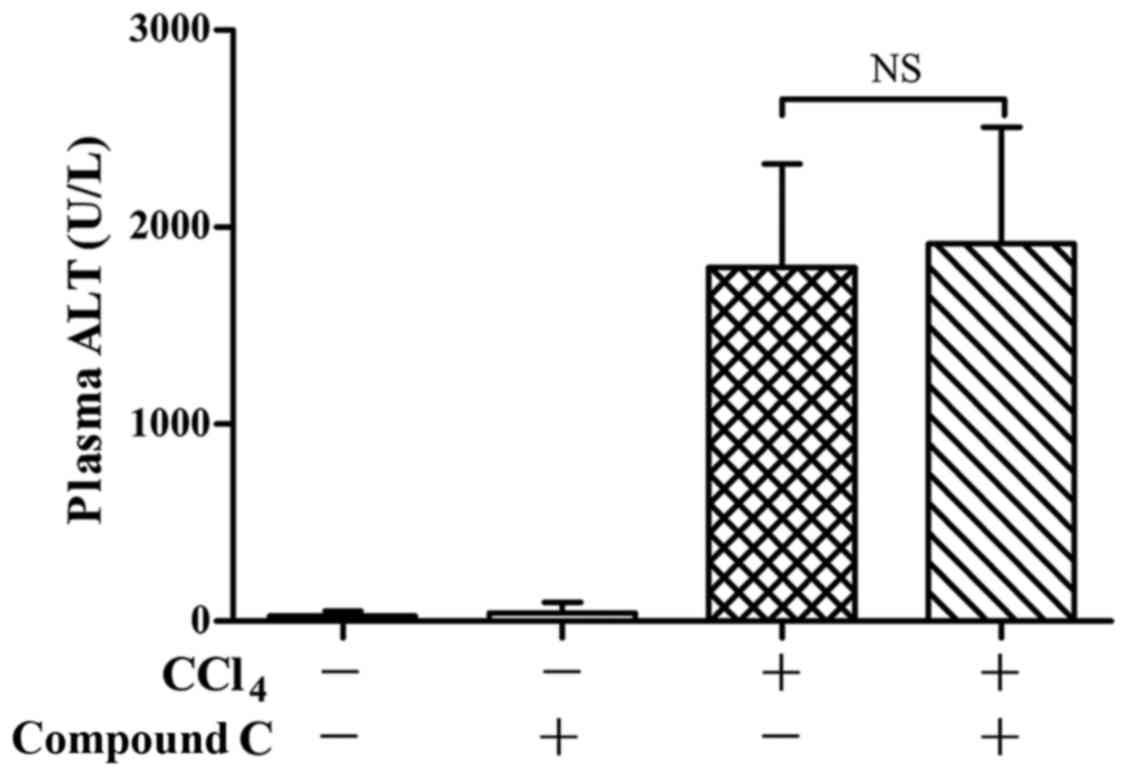
To determine the potential roles of AMPK on
CCl4-induced liver injury, the AMPK inhibitor compound C
was administered before CCl4 exposure. The experimental
data shown that pretreatment with compound C had no significant
effects on CCl4-induced elevation of ALT in plasma
(Fig. 4). Consistently, the
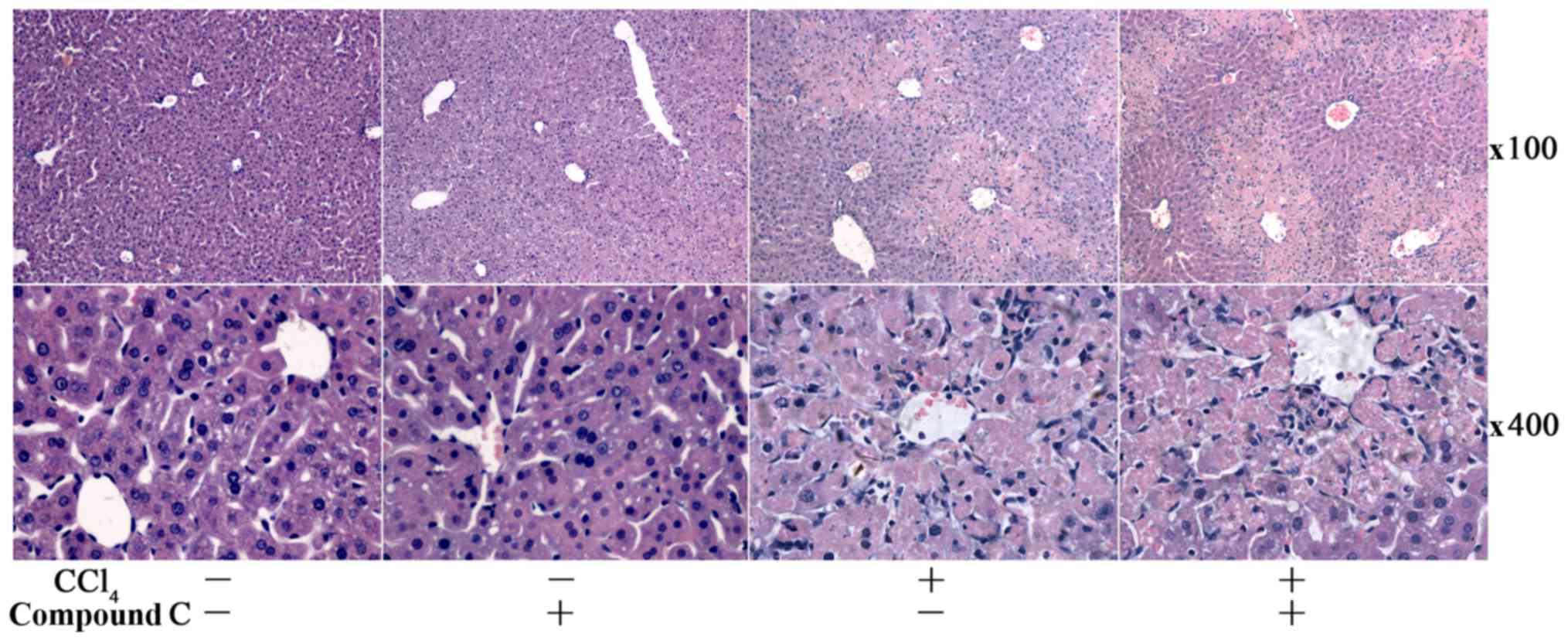
histopathological examination found no obvious difference in
CCl4-challenged mice with or without compound C
administration (Fig. 5).
Delayed inhibition of AMPK suppressed
liver regeneration
To investigate whether AMPK is involved in liver
regeneration post CCl4 challenge, compound C was
administered 24 h post CCl4 exposure. The experimental
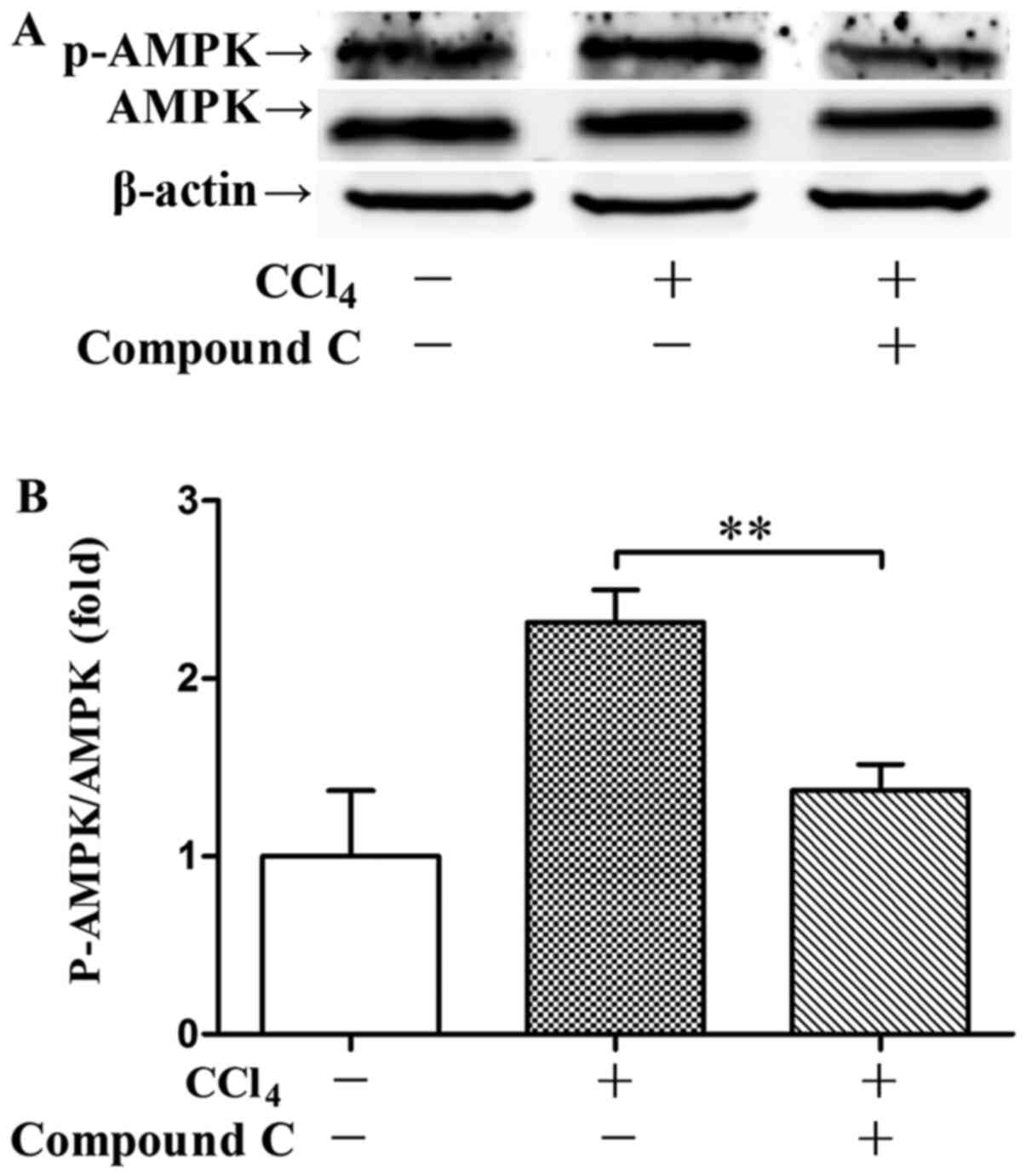
data shown that post-treatment with compound C significantly
suppressed AMPK phosphorylation 48 h post CCl4 exposure
(Fig. 6). Post-treatment with
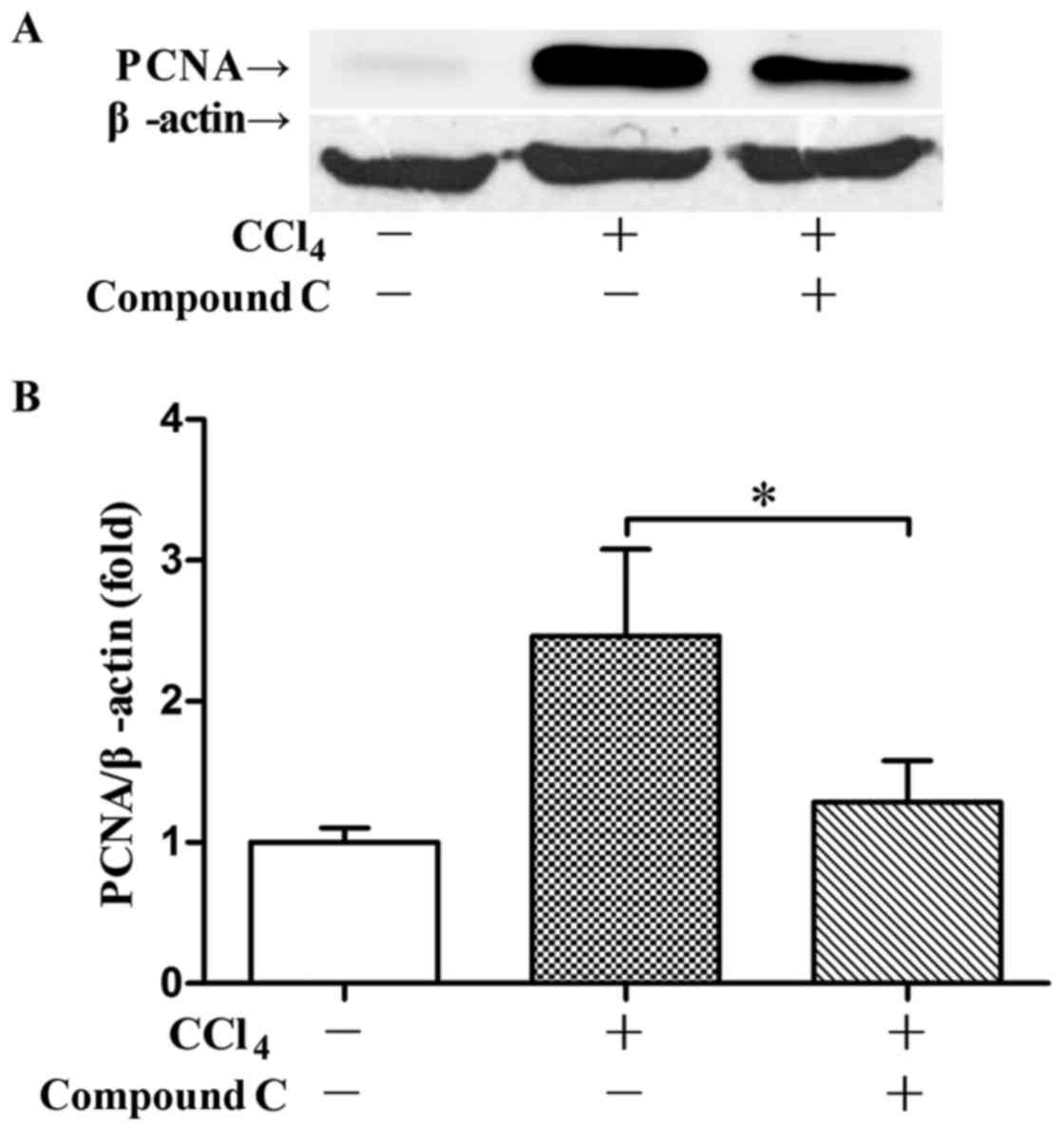
compound C also suppressed CCl4-induced expression of
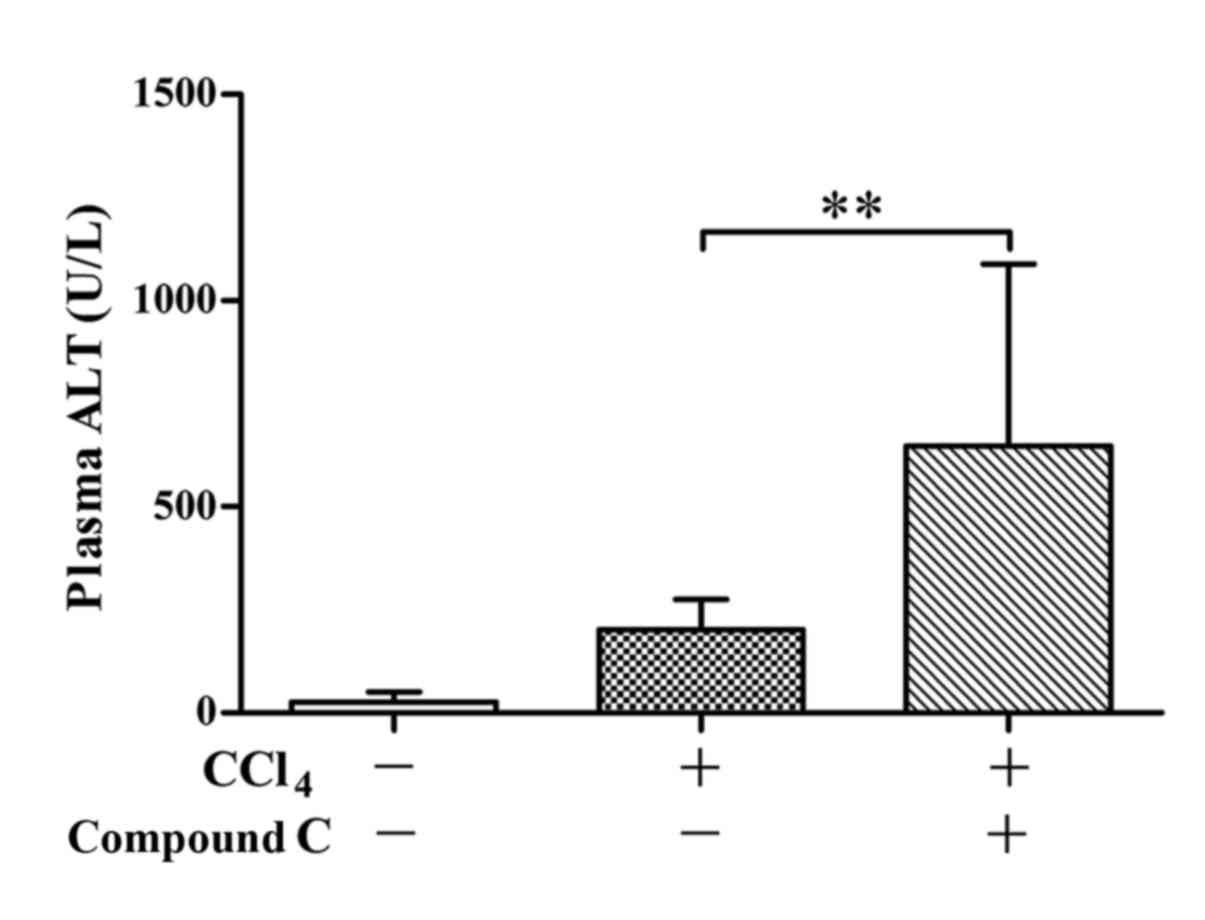
PCNA 48 h post CCl4 exposure (Fig. 7). Meanwhile, the recovery of ALT
level 48 h post CCl4 exposure was impaired by compound C
administered post CCl4 exposure (Fig. 8).
Discussion
AMPK has been regarded as a crucial metabolic
regulator which plays central roles in the maintenance of energy
homeostasis (22). Our previous
studies have found that AMPK provided anti-inflammatory benefits in
mice with acute hepatitis induced by carbon tetrachloride or
endotoxin (26,31). Several studies have found that AMPK
was involved in the regulation of cellular proliferation (32–34).
In the present study, we found that pharmacological inhibition of
AMPK suppressed liver regeneration post CCl4-induced
acute liver injury. Consistently, similar results have been
obtained in a regenerative model with partial hepatectomy (11,18,35).
These data suggests that AMPK might have positive roles in liver
regeneration.
CCl4 is a representative hepatotoxin
which induces severe liver injury quickly, the degree of liver
damage usually peaks at 24 h after CCl4 exposure,
followed by liver regeneration and recovery (13). In the present study, the immunoblot
analysis indicated that the phosphorylation level of AMPK
significantly increased 48 h post CCl4 challenge. In
another model with endotoxin-induced acute liver injury, we also
found that endogenous AMPK was mainly phosphorylated at the late
stage (36). In the present study,
pretreatment with the AMPK inhibitor compound C had little effects
on the elevation of ALT in plasma and the degree of histological
abnormalities in liver. Therefore, endogenous AMPK mainly functions
at the regeneration stage in CCl4-exposed mice.
Because the phosphorylation of AMPK was associated
with the expression of PCNA, a well-documented molecular marker of
cellular proliferation (37), we
also determined the roles of AMPK at the regeneration stage by
treatment with the AMPK inhibitor 24 h post CCl4
exposure. The results indicated that post-insult inhibition of AMPK
significantly suppressed the expression PCNA, which was accompanied
with delayed decline of ALT. These data suggests that
CCl4-induced activation of AMPK might act as a positive
regulator in liver regeneration.
There is a considerable amount of researches have
demonstrated a crucial link between AMPK and cellular proliferation
(18–20). A study in cardiac fibroblasts found
that AMPK suppressed cell cycle progression via modulating the
expression of p21 and p27 (38).
In addition, the suppressive effects of AMPK on tumor cell
proliferation have been observed in neuroblastoma cells, breast
cancer cells, prostate cancer cells and cervical cancer cells
(39). These data suggests that
activation of AMPK might prevent proliferation under some
circumstance.
On the contrary, it was reported that treatment with
the AMPK inhibitor compound C induced cell cycle arrest and
suppressed the growth of colorectal cancer cells (40,41).
The stimulatory actions of AMPK on proliferation were also
confirmed by molecular approaches in prostate cancer cells and
glioma cells (20,42). In addition, the in vivo
promotive activities of AMPK on regeneration were observed in mice
with mitochondrial myopathy or partial hepatectomy (19,21).
Therefore, AMPK might have positive or negative effects on cellular
proliferation in different pathological conditions.
Taken together, the present study found that
endogenous AMPK was mainly activated at the regeneration stage in
mice with CCl4-induced acute liver injury and it might
function as a positive regulator in liver regeneration. Although
the molecular mechanisms underlying the stimulatory activities of
AMPK on liver regeneration remain to be further investigated, the
present study suggests that AMPK might play crucial roles in the
recovery of liver structure and function after severe liver
damage.
Acknowledgements
The present study was supported by the grants from
the Shandong Provincial Natural Science Foundation (ZR2015HL126)
and the Training Program of Chongqing Medical University (no.
201419).
References
|
1
|
Hotchkiss RS and Nicholson DW: Apoptosis
and caspases regulate death and inflammation in sepsis. Nat Rev
Immunol. 6:813–822. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ikeda T: Idiosyncratic drug
hepatotoxicity: Strategy for prevention and proposed mechanism.
Curr Med Chem. 22:528–537. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yan J and Li S and Li S: The role of the
liver in sepsis. Int Rev Immunol. 33:498–510. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gómez-Lechón MJ, Lahoz A, Gombau L,
Castell JV and Donato MT: In vitro evaluation of potential
hepatotoxicity induced by drugs. Curr Pharm Des. 16:1963–1977.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li X, Akhtar S, Kovacs EJ, Gamelli RL and
Choudhry MA: Inflammatory response in multiple organs in a mouse
model of acute alcohol intoxication and burn injury. J Burn Care
Res. 32:489–497. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fausto N, Campbell JS and Riehle KJ: Liver
regeneration. Hepatology. 43 2 Suppl 1:S45–S53. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fausto N and Campbell JS: The role of
hepatocytes and oval cells in liver regeneration and repopulation.
Mech Dev. 120:117–130. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Michalopoulos GK and DeFrances MC: Liver
regeneration. Science. 276:60–66. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hardie DG, Ross FA and Hawley SA: AMPK: A
nutrient and energy sensor that maintains energy homeostasis. Nat
Rev Mol Cell Biol. 13:251–262. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yamamoto T, Kojima T, Murata M, Takano K,
Go M, Hatakeyama N, Chiba H and Sawada N: p38 MAP-kinase regulates
function of gap and tight junctions during regeneration of rat
hepatocytes. J Hepatol. 42:707–718. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yan XP, Wang S, Yang Y and Qiu YD: Effects
of n-3 polyunsaturated fatty acids on rat livers after partial
hepatectomy via LKB1-AMPK signaling pathway. Transplant Proc.
43:pp. 3604–3612. 2011; View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li G and G LG: Farnesoid X receptor, the
bile acid sensing nuclear receptor, in liver regeneration. Acta
Pharm Sin B. 5:93–98. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li SQ, Zhu S, Wan XD, Xu ZS and Ma Z:
Neutralization of ADAM8 ameliorates liver injury and accelerates
liver repair in carbon tetrachloride-induced acute liver injury. J
Toxicol Sci. 39:339–351. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun H, Zhu X, Lin W, Zhou Y, Cai W and Qiu
L: Interactions of TLR4 and PPARγ, dependent on AMPK signalling
pathway contribute to anti-inflammatory effects of vaccariae
hypaphorine in endothelial cells. Cell Physiol Biochem.
42:1227–1239. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang L, Fang Y, Cheng X, Lian Y, Xu H,
Zeng Z and Zhu H: TRPML1 participates in the progression of
alzheimer's disease by regulating the PPARγ/AMPK/Mtor signalling
pathway. Cell Physiol Biochem. 43:2446–2456. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang F, Zhang L, Gao Z, Sun X, Yu M, Dong
S, Wu J, Zhao Y, Xu C, Zhang W and Lu F: Exogenous H2S protects
against diabetic cardiomyopathy by activating autophagy via the
AMPK/mTOR pathway. Cell Physiol Biochem. 43:1168–1187. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kemp BE, Mitchelhill KI, Stapleton D,
Michell BJ, Chen ZP and Witters LA: Dealing with energy demand: The
AMP-activated protein kinase. Trends Biochem Sci. 24:22–25. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Varela-Rey M, Beraza N, Lu SC, Mato JM and
Martinez-Chantar ML: Role of AMP-activated protein kinase in the
control of hepatocyte priming and proliferation during liver
regeneration. Exp Biol Med (Maywood). 236:402–408. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Merlen G, Gentric G, Celton-Morizur S,
Foretz M, Guidotti JE, Fauveau V, Leclerc J, Viollet B and
Desdouets C: AMPKα1 controls hepatocyte proliferation independently
of energy balance by regulating Cyclin A2 expression. J Hepatol.
60:152–159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park HU, Suy S, Danner M, Dailey V, Zhang
Y, Li H, Hyduke DR, Collins BT, Gagnon G, Kallakury B, et al:
AMP-activated protein kinase promotes human prostate cancer cell
growth and survival. Mol Cancer Ther. 8:733–741. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Peralta S, Garcia S, Yin HY, Arguello T,
Diaz F and Moraes CT: Sustained AMPK activation improves muscle
function in a mitochondrial myopathy mouse model by promoting
muscle fiber regeneration. Hum Mol Genet. 25:3178–3191. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cotan D, Paz MV, Alcocer-Gomez E,
Garrido-Maraver J, Oropesa-Ávila M, de la Mata M, Pavón AD, de
Lavera I, Galán F, Ybot-González P and Sánchez-Alcázar JA: AMPK as
a target in rare diseases. Curr Drug Targets. 17:921–931. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li X, Liu R, Zhang L and Jiang Z: The
emerging role of AMP-activated protein kinase in cholestatic liver
diseases. Pharmacol Res. 125:105–113. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Woods A, Williams JR, Muckett PJ, Mayer
FV, Liljevald M, Bohlooly-Y M and Carling D: Liver-specific
activation of AMPK prevents steatosis on a high-fructose diet. Cell
Rep. 18:3043–3051. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liang Z, Li T, Jiang S, Xu J, Di W, Yang
Z, Hu W and Yang Y: AMPK: A novel target for treating hepatic
fibrosis. Oncotarget. 8:62780–62792. 2017.PubMed/NCBI
|
|
26
|
Yang C, Gong X, Ai Q, Ge P, Lin L and
Zhang L: 5-Aminoimidazole-4-carboxamide-1-β-d-ribofuranoside
alleviated carbon tetrachloride-induced acute hepatitis in mice.
Int Immunopharmacol. 25:393–399. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou D, Ai Q, Lin L, Gong X, Ge P, Che Q,
Wan J, Wen A and Zhang L:
5-Aminoimidazole-4-carboxamide-1-β-D-ribofuranoside-attenuates
LPS/D-Gal-induced acute hepatitis in mice. Innate Immun.
21:698–705. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang M, Yang D, Gong X, Ge P, Dai J, Lin
L and Zhang L: Protective benefits of AMP-activated protein kinase
in hepatic ischemia-reperfusion injury. Am J Transl Res. 9:823–829.
2017.PubMed/NCBI
|
|
29
|
Jin J, Mullen TD, Hou Q, Bielawski J,
Bielawska A, Zhang X, Obeid LM, Hannun YA and Hsu YT: AMPK
inhibitor Compound C stimulates ceramide production and promotes
Bax redistribution and apoptosis in MCF7 breast carcinoma cells. J
Lipid Res. 50:2389–2397. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou G, Myers R, Li Y, Chen Y, Shen X,
Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, et al: Role of
AMP-activated protein kinase in mechanism of metformin action. J
Clin Invest. 108:1167–1174. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yuan H, Li L, Zheng W, Wan J, Ge P, Li H
and Zhang L: Antidiabetic drug metformin alleviates
endotoxin-induced fulminant liver injury in mice. Int
Immunopharmacol. 12:682–688. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shackelford DB and Shaw RJ: The LKB1-AMPK
pathway: Metabolism and growth control in tumour suppression. Nat
Rev Cancer. 9:563–575. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vazquez-Martin A, López-Bonet E,
Oliveras-Ferraros C, Pérez-Martínez MC, Bernadó L and Menendez JA:
Mitotic kinase dynamics of the active form of AMPK
(phospho-AMPKalphaThr172) in human cancer cells. Cell Cycle.
8:788–791. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vazquez-Martin A, Oliveras-Ferraros C,
Cufi S and Menendez JA: Polo-like kinase 1 regulates activation of
AMP-activated protein kinase (AMPK) at the mitotic apparatus. Cell
Cycle. 10:1295–1302. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vazquez-Chantada M, Ariz U, Varela-Rey M,
Embade N, Martínez-Lopez N, Fernández-Ramos D, Gómez-Santos L,
Lamas S, Lu SC, Martínez-Chantar ML and Mato JM: Evidence for
LKB1/AMP-activated protein kinase/endothelial nitric oxide synthase
cascade regulated by hepatocyte growth factor, S-adenosylmethionine
and nitric oxide in hepatocyte proliferation. Hepatology.
49:608–617. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cai L, Hu K, Lin L, Ai Q, Ge P, Liu Y, Dai
J, Ye B and Zhang L: AMPK dependent protective effects of metformin
on tumor necrosis factor-induced apoptotic liver injury. Biochem
Biophys Res Commun. 465:381–386. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang T, Guo P, Zhang Y, Xiong H, Yu X, Xu
S, Wang X, He D and Jin X: The antidiabetic drug metformin inhibits
the proliferation of bladder cancer cells in vitro and in vivo. Int
J Mol Sci. 14:24603–24618. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Qi H, Liu Y, Li S, Chen Y, Li L, Cao YE M,
Shi P, Song C, Li B and Sun H: Activation of AMPK attenuated
cardiac fibrosis by inhibiting CDK2 via p21/p27 and miR-29 family
pathways in rats. Mol Ther Nucleic Acids. 8:277–290. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu PF, Hsu CJ, Tsai WL, Cheng JS, Chen
JJ, Huang IF, Tseng HH, Chang HW and Shu CW: Ablation of ATG4B
suppressed autophagy and activated AMPK for cell cycle arrest in
cancer cells. Cell Physiol Biochem. 44:728–740. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yang WL, Perillo W, Liou D, Marambaud P
and Wang P: AMPK inhibitor compound C suppresses cell proliferation
by induction of apoptosis and autophagy in human colorectal cancer
cells. J Surg Oncol. 106:680–688. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Viswakarma N, Jia Y, Bai L, Gao Q, Lin B,
Zhang X, Misra P, Rana A, Jain S, Gonzalez FJ, et al: The Med1
subunit of the mediator complex induces liver cell proliferation
and is phosphorylated by AMP kinase. J Biol Chem. 288:27898–27911.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Vucicevic L, Misirkic M, Janjetovic K,
Harhaji-Trajkovic L, Prica M, Stevanovic D, Isenovic E, Sudar E,
Sumarac-Dumanovic M, Micic D and Trajkovic V: AMP-activated protein
kinase-dependent and -independent mechanisms underlying in vitro
antiglioma action of compound C. Biochem Pharmacol. 77:1684–1693.
2009. View Article : Google Scholar : PubMed/NCBI
|