Introduction
Prostate cancer has been the most prevalent disease
among men for decades and estimated 180,890 new prostate cancer
cases have been diagnosed in 2016 which accounts for 21% of all
cancer diagnoses that year (1).
Androgen expression or androgen receptor (AR) activation serve a
role in the prostate cancer (PCa) and androgen deprivation therapy
(ADT) has been extensively applied as a treatment. The progress of
battling PCa was made substantially based on ADT, such as combining
radical radiotherapy with ADT may improve overall survival outcome
in localized PCa patients (2).
Following a median treatment of 24 months, almost all prostate
cancer cases invariably progress to castration resistant prostate
cancer (CRPC), maintaining AR activity and continuation of ADT is
recommended for treatment (3,4).
In the process of ADT, adverse effects on bone,
metabolic, cardiovascular, sexual and cognitive health as well as
body composition are known by clinicians (5). To alleviate pain and improve the
quality of life, glucocorticoids (GCs) combined with antitumor or
antiandrogen agents (including Docetaxel and Abiraterone acetate)
are a common class of adjuvant drugs for treatment of CRPC
(6,7). Apart from ameliorating side effects
caused by antitumor or antiandrogen agents, a direct effect has
been identified on prostate cancer cell proliferation (8,9). GC
inhibits prostate cancer cell proliferation in vivo and
vitro (8,9). By contrast, an increasing amount of
evidence demonstrates that glucocorticoid receptor confers
resistance to antiandrogens (10,11).
Therefore, GC serves a complex role in treatment, management and
progression of CRPC and understanding the biological role of
glucocorticoids in patients with prostate cancer is of major
importance (12).
The effects of GC on prostate cancer cells,
especially the castration resistance prostate cancer cells, remain
to be elucidated. An association between AR and glucocorticoid
receptor (GR) has been previously demonstrated and GR was
negatively regulated by active androgen receptor signaling
(13). Dexamethasone is a common
GC agent used in the clinic (14,15).
A recent study demonstrated that dexamethasone may be more
effective compared with prednisolone for prostate cancer treatment
(14). Therefore, the present
study aimed to determine the effects of dexamethasone on prostate
cancer cells. Recently, it has been demonstrated that
dihydrotestosterone (DHT) can promote prostate cancer cell
proliferation via glucocorticoid receptor (GR) (16). In the present study, prostate
cancer cells were cultured in RPMI-1640 with 10% charcoal-stripped
serum to investigate the effects of dexamethasone on prostate
cancer cell proliferation and migration ability, and the role of AR
in the effects.
Materials and methods
Cell culture
Human PCa cell lines, PC3-AR9 was provided as a gift
from professor Niu (Tianjin Institute of Urology, the Second
Hospital of Tianjin Medical University, Tianjin, China) (17), LNCaP, CWR22Rv1, C4-2, Du145 and PC3
(American Type Culture Collection; Manassas, VA, USA) were
routinely maintained in RPMI-1640 medium (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) with 10% FBS (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), 100 U/ml of penicillin
and 100 mg/ml of streptomycin in a 5% CO2 atmosphere at
37°C. For glucocorticoid-induced experiments, the cells were
transferred and cultured in RPMI-1640 with 10% charcoal-stripped
serum (HyClone; GE Healthcare Life Sciences, Logan, UT, USA) for 24
h in a 5% CO2 atmosphere at 37°C. The charcoal-stripped
serum contains reduced hormone levels and is suitable for AR or GR
signaling studies. DHT (MedChemExpress Co., Ltd., Princeton, New
Jersey, USA) was dissolved in ethanol in 10−5 M
concentration, DHT was diluted 1,000-fold in RPMI-1640 (Invitrogen;
Thermo Fisher Scientific, Inc.) with 10% charcoal-stripped serum
(HyClone; GE Healthcare Life Sciences) to reach a final
concentration of 10 nM. Dexamethasone was dissolved in DMSO at
2×10−2 M final concentration, which was further diluted
100,000-fold in the RPMI-1640 medium with 10% charcoal-stripped
serum to reach a final concentration of 100 nM.
Measurement of cell viability
Cell viability was assessed by MTT assay. MTT assay
was modified (150 µl DMSO were used and a microplate reader at a
wavelength of 490 nm instead of 50 ul DMSO at 570 nm) and performed
to quantify cell proliferation (18). Briefly, LNCaP (3×104),
C4-2 (104), 22Rv1 (104), PC-3
(103), Du145 (103) and PC3-AR9
(3×104). Cells were maintained in RPMI-1640 medium
(Invitrogen; Thermo Fisher Scientific, Inc.) with 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.) and were incubated in 96-well
microplates with RPMI-1640 medium supplemented with 10%
charcoal-stripped serum. Following 24 h, the medium was removed and
replaced by either a medium containing different concentration of
drug (RPMI-1640 medium supplemented with 10% charcoal-stripped
serum with 10-8 M DHT, 10 nM dexamethasone, or 10 uM MDV3100;
Selleck Chemicals, Shanghai, China) or a drug-free medium (control
condition: DMSO or ethanol diluted in RPMI-1640 medium supplemented
with 10% charcoal-stripped serum). Following 24, 48, 72 and 96 h or
on day 2, 4 and 6 the media were removed and repla ced with 100 µl
of 1 mg/ml MTT (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) in
RPMI-1640. Following a 4-h incubation in a 5% CO2
atmosphere at 37°C, the MTT solution was removed and replaced with
150 µl DMSO, and the plates were shaken for 3 min on an oscillator.
The optical density of each sample was determined using a
microplate reader at a wavelength of 490 nm. Each experiment was
performed in triplicate.
Western blot analysis
Harvested cells following the aforementioned
treatment, were washed with PBS and lysed in RIPA buffer (50 mM
Tris-HCl, pH 7.4; 1% NP-40; 150 mM NaCl; 1 mM EDTA; 1 mM proteinase
inhibitor; 1 mM Na3VO4; 1 mM NaF; 1 mM
okadaic acid; and 1 mg/ml aprotinin, leupeptin and pepstatin).
Cytoplasmic and nuclear extracts were prepared as previously
described (19). Prior to the
procedure, the following compounds were prepared: Buffer A: 10 mM
HEPES (Sigma-Aldrich; Merck KGaA), pH 7.9, 1.5 mM MgCl2
(Sigma-Aldrich; Merck KGaA), 10 mM KCl (Sigma-Aldrich; Merck KGaA),
300 mM sucrose (Sigma-Aldrich; Merck KGaA), 0.5% NP-40
(Sigma-Aldrich; Merck KGaA), stored at 4°C; Buffer B: 20 mM HEPES,
pH 7.9, 1.5 mM MgCl2, 420 mM NaCl (Sigma-Aldrich; Merck
KGaA), 0.2 mM EDTA (Invitrogen; Thermo Fisher Scientific, Inc.),
2.5% glycerol (Sigma-Aldrich; Merck KGaA), stored at 4°C; and
Buffer D: 20 mM HEPES, pH 7.9, 100 mM KCl, 0.2 mM EDTA, 8%
glycerol, stored at 4°C. Medium was removed from the cultures and
cells were washed in cold PBS, and harvested with a rubber scraper.
Subsequently, cells were centrifuged at 550 × g for 5 min at 4°C
and supernatant was discarded. The following inhibitors were added
to buffers A, B and D: 0.5 mM PMSF (Sigma-Aldrich; Merck KGaA), 1
mM Na3VO4 (Sigma-Aldrich; Merck KGaA), 0.5 mM
DTT (Invitrogen; Thermo Fisher Scientific, Inc.), 1 µg/ml leupeptin
(Sigma-Aldrich; Merck KGaA), 25 mM β-glycerophosphate
(Sigma-Aldrich; Merck KGaA), 10 mM NaF (Sigma-Aldrich; Merck KGaA).
The pellet was resuspended in 2X cell volume of buffer A with
inhibitors and the solution was kept on ice for 10 min. Samples
were vortexed briefly and centrifuged at 2,600 × g for 30 sec at
4°C. The supernatant was collected which corresponds to cytoplasm
proteins. The pellet was resuspended in buffer B with inhibitors.
The mixture was sonicated for 5 sec at 4°C and centrifuge at 10,400
× g for 5 min at 4°C. The supernatant was diluted with equal volume
of buffer D with inhibitors, and nuclear protein was extracted.
Protein concertation was measured by coomassie brilliant blue.
Samples (30 µg protein/lane) were separated on 8% SDS-PAGE gel and
transferred to polyvinylidene fluoride membranes at 4°C (250 mA, 2
h). Membranes were blocked in 5% fat-free milk in TBS with 1%
Tween-20 for 1 h at room temperature and incubated with primary
antibodies: Anti-GAPDH (Sanjian, Tianjin, China; cat. no. KM9002;
1:5,000; www.sungenebiotech.com), anti-GR (BIOSS, Beijing,
China; cat. no. bs-0252R; 1:500), anti-Histone3 (used as nuclear
control; Abcam, Cambridge, UK; cat. no. ab8580; 1:1,000), AR
(Abcam; cat. no. ab9474; 1:1,000), Akt (Abcam; cat. no. ab8805;
1:1,000), p-Akt (Abcam; cat. no. ab81283; 1:1,000), vimentin
(Abcam; cat. no. ab92547; 1:1,000) overnight at 4°C. Subsequently,
the membranes were washed in TBS with 1% Tween (TBST) for 10
min/wash 3 times and incubated with horseradish peroxidase
conjugated anti-rabbit or anti-mouse antibodies (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA; cat. no.) for 1 h at
room temperature and washed for 10 min/wash 3 times. The blots were
developed in Enhanced Chemiluminescence mixture (Amersham
Biosciences; GE Healthcare, Chicago, IL, USA) and visualized by
Imager (Image J, National Institutes of Health, version 1.48).
Migration assay
Cells (105 of PC3-AR9 and
5×104 of PC3) following different treatments (DMSO or
dexamethasone) were re-suspended with RPMI-1640 with 10%
charcoal-stripped serum (HyClone; GE Healthcare Life Sciences,
Logan, UT, USA) and seeded in the upper chambers of the transwells
(Corning Inc., Corning, NY, USA). A 10% solution of FBS (Gibco;
Thermo Fisher Scientific, Inc.) with or without 100 nM
dexamethasone was applied in the lower chamber. As described
previously (17), following a 24-
or 48-h incubation (with PC3 or PC3-AR9 cells, respectively), the
cells that invaded to the lower part of the membrane were
harvested, fixed with 75% ethanol and stained with 0.1% crystal
violet at room temperature for 25 min in PBS. Invaded cells were
counted under a light microscope (magnification, ×100). The
standard deviation was calculated from three independent wells.
Statistical analysis
All values are presented as the mean ± standard
error of the mean. Statistical evaluation of the results was
performed by one-way analysis of variance followed by the
Newman-Keuls method (SPSS software; version 18.0; SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Dexamethasone affects prostate cancer
cell proliferation
Glucocorticoids exert effects through non-genomic
action or genomic action mediated by GR (15,20).
To investigate the effects of glucocorticoids on prostate cancer
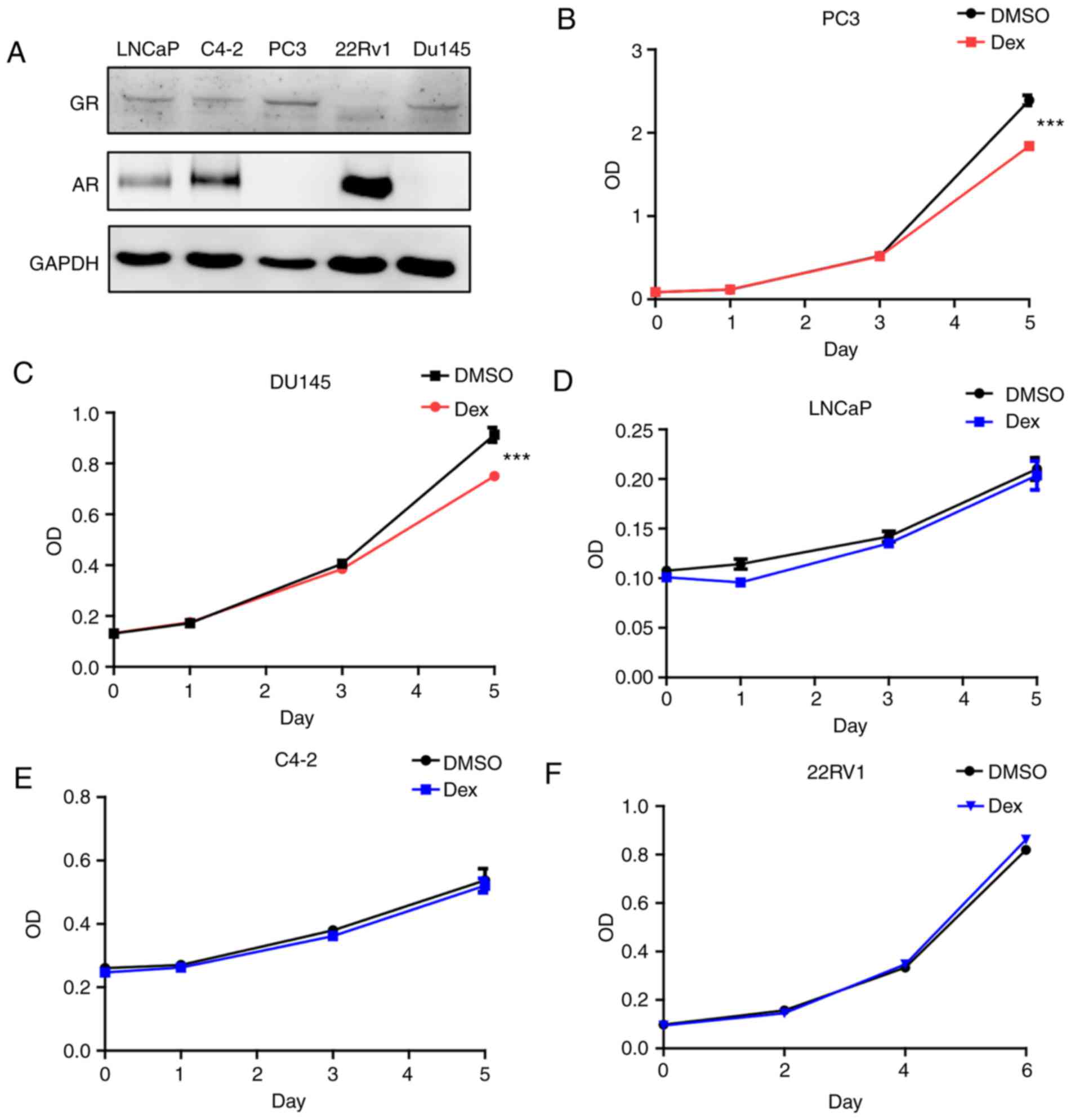
cells, GR expression was initially assessed in various cell lines.
PC3 and Du145 demonstrated elevated GR expression compared with
other cells, whereas no AR expression was detected in these cells
(Fig. 1A). AR was expressed in
LNCaP, C4-2 and 22Rv1 cells, whereas GR expression was low or
hardly detectable in these cells. Dexamethasone was used to treat
the cell lines in the present study. Dexamethasone treatment
inhibited the PC3 and Du145 cell proliferation (Fig. 1B and C), but exerted no effect on
LNCaP, C4-2 and 22Rv1 cells (Fig.
1D-F). Therefore, dexamethasone exhibits distinct effects on
different prostate cancer cells. Based on the above results, it can
be hypothesized that the presence of AR reduced the effect of
dexamethasone on cell proliferation.
AR reverts the inhibition of
dexamethasone on prostate cancer cell proliferation
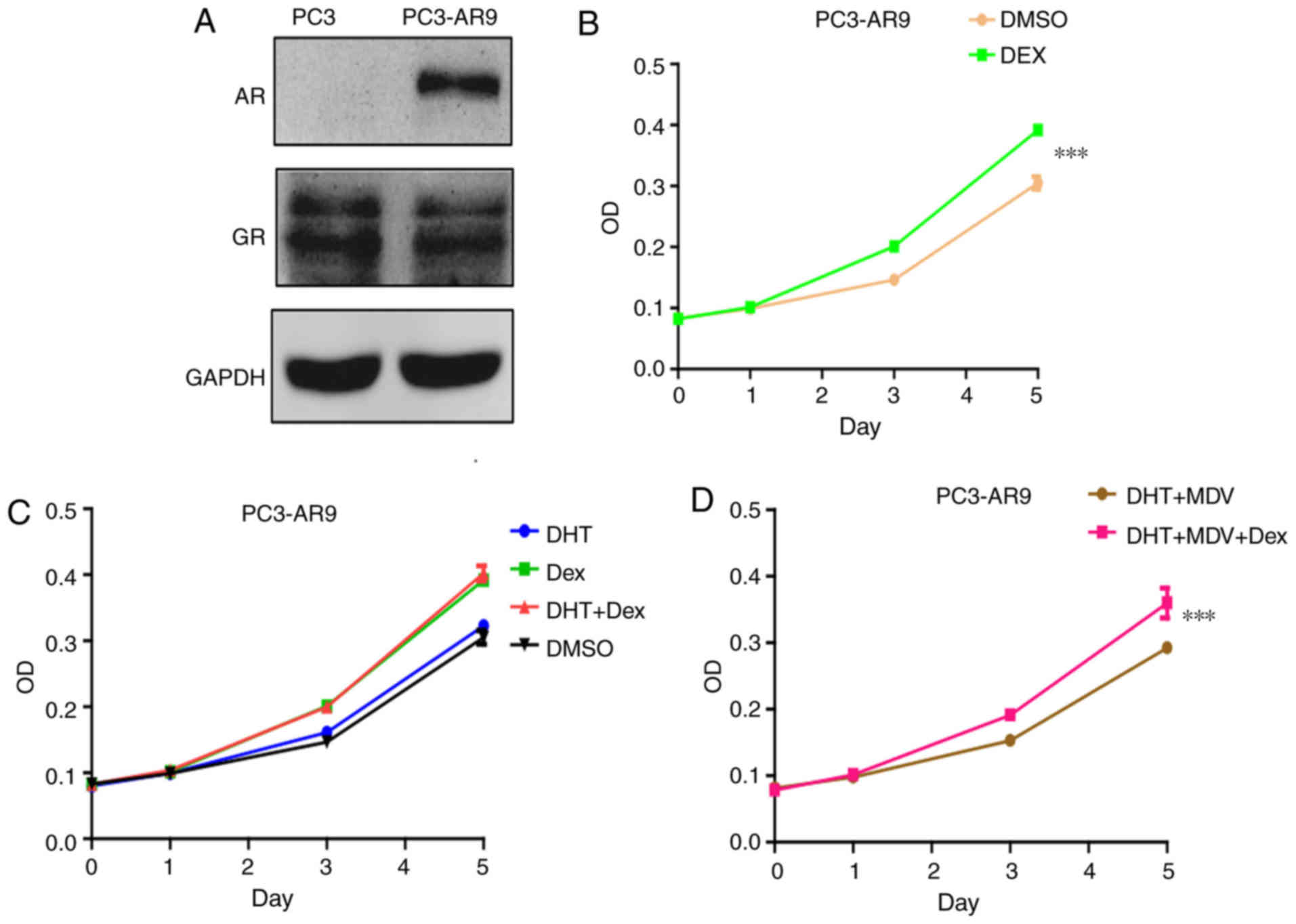
To determine the role of AR in the effect of
dexamethasone on prostate cancer proliferation, PC3 cells were
transfected with AR in to establish a PC3-AR9 cell line, as
previously described (21). The
transfected cell line expressed both AR and GR (Fig. 2A). By contrast to the PC3 cells,
dexamethasone exerted positive effect on PC3-AR9 cell proliferation
(Fig. 2B). This effect was not
affected by AR agonist DHT (Fig.
2C) or antagonist MDV3100 (Fig.
2D). Therefore, AR reverted the inhibition of dexamethasone on
prostate cancer cell proliferation. The treatment with an agonist
and antagonist indicated that this alteration was depended on AR
protein but not AR signal.
Phosphatidylinositol 4,5-bisphosphate
3-kinase (PI3K)-RAC-alpha serine/threonine-protein kinase (Akt)
pathway is involved in the distinct effect of dexamethasone on
various prostate cancer cells
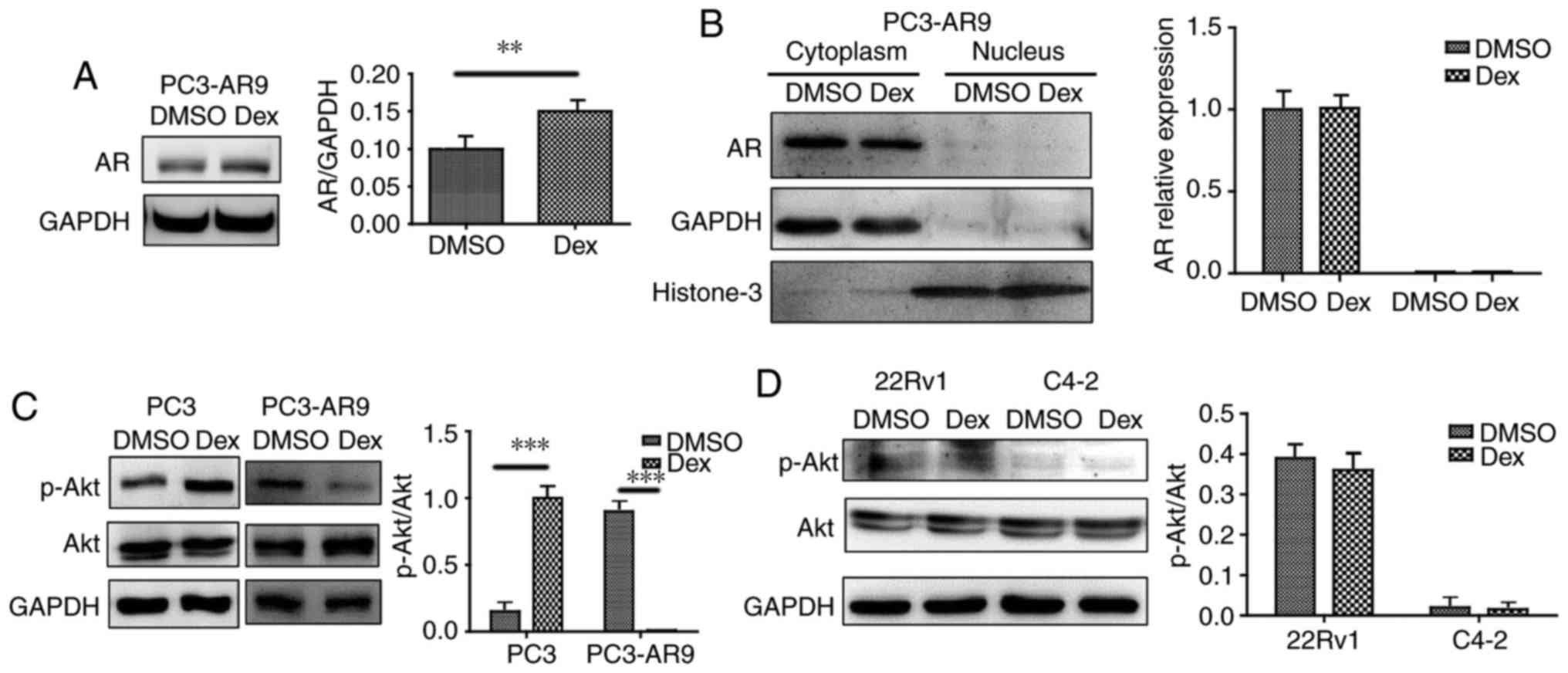
It has been previously demonstrated that AR
expression and translocation influence prostate cancer cell
proliferation (22). AR expression
and distribution were investigated upon dexamethasone treatment;
however, AR expression was not significantly affected by
dexamethasone treatment (Fig. 3A)
and no AR protein was detected in the nuclei (Fig. 3B). A direct interaction between Akt
and AR was previously demonstrated by co-immunoprecipitation, and
increased phosphorylation of Akt (Ser-473 and Thr-308) was
associated with phosphorylation at Ser213 and Ser791, and AR
degradation (22). The present
study demonstrated that p-Akt473 increased following
dexamethasone treatment in PC3 cells, but decreased in PC3-AR9
cells (Fig. 3C).
p-Akt473 level was unaltered despite dexamethasone
treatment in 22Rv1 and C4-2 cells (Fig. 3D). The present data are consistent
with a previous study where Akt-AR interaction in androgen
dependent prostate cancer (ADPC) and androgen independent prostate
cancer (AIPC) was different (23).
AR rescues the inhibition of
dexamethasone on prostate cancer migration
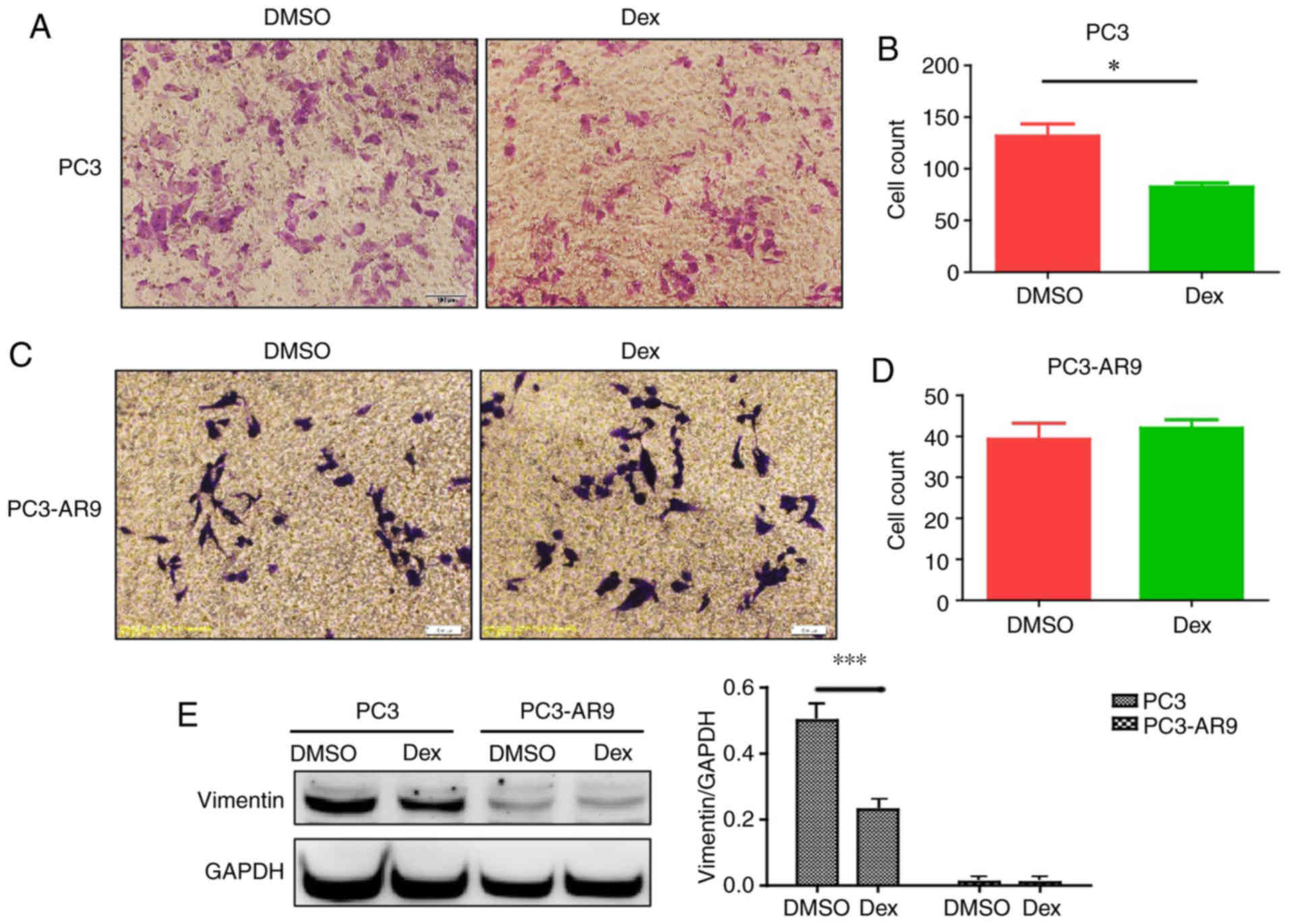
To assess the effect of dexamethasone on prostate
cancer migration, a migration assay was performed using PC3 cells.
Dexamethasone inhibited cell migration in PC3 cells (Fig. 4A and B). However, dexamethasone
exhibited no influence on PC3-AR9 cell migration ability (Fig. 4C and D). Therefore, AR rescued the
inhibition of dexamethasone on prostate cancer migration. The
expression of mesenchymal marker vimentin was decreased following
dexamethasone treatment in PC3 cells and unaltered in PC3-AR9 cells
(Fig. 4E), which may indicate that
dexamethasone may be linked with epithelial mesenchymal transition
(EMT) and AR may alter dexamethasone's effect.
Discussion
It has been previously hypothesized that
dexamethasone may inhibit prostate cancer proliferation and that
the underlining mechanisms may involve interleukin (IL)-6, nuclear
factor-κB inhibition (8,24). A previous study suggested that
glucocorticoids exhibit a distinct effect on prostate cancer cells,
suppress PC3 and Du145 cell proliferation and induce no effect on
LNCaP cells (8). Glucocorticoids
can also promote prostate cancer cell proliferation and previous
data demonstrated that dexamethasone promotes proliferation of
22Rv1 cells (11,25). Recently published data indicated
that DHT can affect AR− prostate cancer cell
proliferation via GR (16).
Therefore, the authors of the present study evaluated dexamethasone
action in the absence of androgen.
In the present study, it was demonstrated that
dexamethasone exhibits different action on LNCaP, PC3, 22Rv1 and
C4-2 cells. Dexamethasone inhibited PC3 proliferation but did not
affect the proliferation of LNCaP, 22Rv1, C4-2 cells. For the
purpose of glucocorticoid-induced experiments, the cells were
transferred and cultured in RPMI-1640 with 10% charcoal-stripped
serum for at least 24 h to abolish the action exerted by androgen.
Previous data suggested that glucocorticoids exert a negative
effect on proliferation of androgen-independent prostate cancer and
LNCaP-GR (LNCaP transfected with GR) cells (8,9).
Increasing amount of evidence suggests that glucocorticoid receptor
confers resistance to antiandrogens (10,11).
The authors of the present study hypothesized that the action of
dexamethasone on prostate cancer cells may depends on the presence
of AR. Dexamethasone promoted cancer cell proliferation in PC3
cells transfected with AR (Fig.
2B).
The present study investigated the effect of
dexamethasone on various prostate cancer cells. The association
between AR and GR is complex and their interaction is influenced by
disease progression (12). The
expression of GR is negatively regulated by active androgen
receptor signaling (13). By
contrast, androgen receptor activity was inhibited by
glucocorticoid action in human adipocytes (26). Whether androgen receptor activity
was inhibited by glucocorticoid action has not been fully
demonstrated in prostate cancer cells (12). Nevertheless, the immunophilin
FKBP51 which is the downstream of AR regulates the function of GR
(26,27). Increased expression of FKBP51 by AR
inhibits GR nuclear translocation and therefore suppresses the
function of GR (27,28). Therefore, prostate cancer cells
should be divided into four types according to AR and GR expression
(double AR/GR+ or – and AR/GR single
positive).
Clinically, multiple studies investigated the
utility of dexamethasone in CRPC (14,29–34).
Prostate-specific antigen (PSA) response rate of dexamethasone is
~41–62%, median time to PSA progression of dexamethasone is
~5.4–9.7 months. Dexamethasone induces distinct effect in various
clinical studies (reviewed in 15). In order to confirm the present
findings, future in vivo experiments and experiments using
more cell lines are needed.
When PCa is a localized disease, five year survival
rates can be 100% but once it has spread, the survival rates
decrease to 28% (35). Cancer
metastases markedly decrease patients' survival time. The present
study performed a migration assay to determine the effects of
dexamethasone on prostate cancer migration. Dexamethasone inhibited
PC3 cell migration but did not affect PC3-AR9 cell migration. In
bladder cancer, dexamethasone increased glucocorticoid
receptor-mediated reporter activity and cell proliferation;
however, dexamethasone induced mesenchymal-to-epithelial transition
by suppressing the expression of MMP-2/MMP-9, IL-6, VEGF, and the
activity of MMP-2/MMP-9, thus inhibited bladder cell invasion
(36). Although the mechanism of
dexamethasone activity on PC3 migration remains to be elucidated,
it appears that dexamethasone inhibited cell migration of
AR− but not AR+ cancer cells. As demonstrated
in previous studies, dexamethasone was sufficient to confer
enzalutamide resistance, and substituted for AR to activate genes
involved in proliferation and metastasis and were necessary for
maintenance of the resistant phenotype (10).
There are certain limitations of the present study.
A serial concentration of dex may be included in the future studies
to support the initial results. Additionally, the potential
mechanism was proposed based on experimental results from one pair
of prostate cell lines and other cell lines should be added in the
future. The experiments preformed in the present study also need to
be repeated to confirm the results. There is also no loss of
function studies to support the hypothesis that the AR protein
itself is critical. The aforementioned issues should be addressed
in future studies.
In conclusion, the present study suggested that
dexamethasone positively or negatively regulated proliferation of
various prostate cancer cells according to AR and GR
expression.
Acknowledgements
The present study was supported by the Project of
the Education Department of Jiangxi Province (grant no.
GJJ160036).
Competing interests
The authors declare thay they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Attard G, Parker C, Eeles RA, Schröder F,
Tomlins SA, Tannock I, Drake CG and de Bono JS: Prostate cancer.
Lancet. 387:70–82. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Perner S, Cronauer MV, Schrader AJ,
Klocker H, Culig Z and Baniahmad A: Adaptive responses of androgen
receptor signaling in castration-resistant prostate cancer.
Oncotarget. 6:35542–35555. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saad F and Fizazi K: Androgen deprivation
therapy and secondary hormone therapy in the management of
hormone-sensitive and castration-resistant prostate cancer.
Urology. 86:852–861. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nguyen PL, Alibhai SM, Basaria S, D'Amico
AV, Kantoff PW, Keating NL, Penson DF, Rosario DJ, Tombal B and
Smith MR: Adverse effects of androgen deprivation therapy and
strategies to mitigate them. Eur Urol. 67:825–836. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tagawa ST, Posadas EM, Bruce J, Lim EA,
Petrylak DP, Peng W, Kheoh T, Maul S, Smit JW, Gonzalez MD, et al:
Phase 1b study of abiraterone acetate plus prednisone and docetaxel
in patients with metastatic castration-resistant prostate cancer.
Eur Urol. 70:718–721. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Teply BA, Luber B, Denmeade SR and
Antonarakis ES: The influence of prednisone on the efficacy of
docetaxel in men with metastatic castration-resistant prostate
cancer. Prostate Cancer Prostatic Dis. 19:72–78. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nishimura K, Nonomura N, Satoh E, Harada
Y, Nakayama M, Tokizane T, Fukui T, Ono Y, Inoue H, Shin M, et al:
Potential mechanism for the effects of dexamethasone on growth of
androgen-independent prostate cancer. J Natl Cancer Inst.
93:1739–1746. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yemelyanov A, Czwornog J, Chebotaev D,
Karseladze A, Kulevitch E, Yang X and Budunova I: Tumor suppressor
activity of glucocorticoid receptor in the prostate. Oncogene.
26:1885–1896. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Arora VK, Schenkein E, Murali R, Subudhi
SK, Wongvipat J, Balbas MD, Shah N, Cai L, Efstathiou E, Logothetis
C, et al: Glucocorticoid receptor confers resistance to
antiandrogens by bypassing androgen receptor blockade. Cell.
155:1309–1322. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Isikbay M, Otto K, Kregel S, Kach J, Cai
Y, Vander Griend DJ, Conzen SD and Szmulewitz RZ: Glucocorticoid
receptor activity contributes to resistance to androgen-targeted
therapy in prostate cancer. Horm Cancer. 5:72–89. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Narayanan S, Srinivas S and Feldman D:
Androgen-glucocorticoid interactions in the era of novel prostate
cancer therapy. Nat Rev Urol. 13:47–60. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xie N, Cheng H, Lin D, Liu L, Yang O, Jia
L, Fazli L, Gleave ME, Wang Y, Rennie P and Dong X: The expression
of glucocorticoid receptor is negatively regulated by active
androgen receptor signaling in prostate tumors. Int J Cancer.
136:E27–E38. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Venkitaraman R, Lorente D, Murthy V,
Thomas K, Parker L, Ahiabor R, Dearnaley D, Huddart R, De Bono J
and Parker C: A randomised phase 2 trial of dexamethasone versus
prednisolone in castration-resistant prostate cancer. Eur Urol.
67:673–679. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu J and Chen Q: The role of
glucocorticoid receptor in prostate cancer progression: From bench
to bedside. Int Urol Nephrol. 49:369–380. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Song C, Kim Y, Min GE and Ahn H:
Dihydrotestosterone enhances castration-resistant prostate cancer
cell proliferation through STAT5 activation via glucocorticoid
receptor pathway. Prostate. 74:1240–1248. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wen S, Shang Z, Zhu S, Chang C and Niu Y:
Androgen receptor enhances entosis, a non-apoptotic cell death,
through modulation of Rho/ROCK pathway in prostate cancer cells.
Prostate. 73:1306–1315. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kan SF, Huang WJ, Lin LC and Wang PS:
Inhibitory effects of evodiamine on the growth of human prostate
cancer cell line LNCaP. Int J Cancer. 110:641–651. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu KK: Analysis of protein-DNA binding by
streptavidin-agarose pulldown. Methods Mol Biol. 338:281–290.
2006.PubMed/NCBI
|
|
20
|
Ramamoorthy S and Cidlowski JA: Exploring
the molecular mechanisms of glucocorticoid receptor action from
sensitivity to resistance. Endocr Dev. 24:41–56. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yuan S, Trachtenberg J, Mills GB, Brown
TJ, Xu F and Keating A: Androgen-induced inhibition of cell
proliferation in an androgen-insensitive prostate cancer cell line
(PC-3) transfected with a human androgen receptor complementary
DNA. Cancer Res. 53:1304–1311. 1993.PubMed/NCBI
|
|
22
|
Hu J, Wang G and Sun T: Dissecting the
roles of the androgen receptor in prostate cancer from molecular
perspectives. Tumour Biol. 39:10104283176922592017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Paliouras M and Diamandis EP: An AKT
activity threshold regulates androgen-dependent and
androgen-independent PSA expression in prostate cancer cell lines.
Biol Chem. 389:773–780. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yano A, Fujii Y, Iwai A, Kageyama Y and
Kihara K: Glucocorticoids suppress tumor angiogenesis and in vivo
growth of prostate cancer cells. Clin Cancer Res. 12:3003–3009.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Attardi BJ, Burgenson J, Hild SA and Reel
JR: Steroid hormonal regulation of growth, prostate specific
antigen secretion, and transcription mediated by the mutated
androgen receptor in CWR22Rv1 human prostate carcinoma cells. Mol
Cell Endocrinol. 222:121–132. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hartig SM, He B, Newberg JY, Ochsner SA,
Loose DS, Lanz RB, McKenna NJ, Buehrer BM, McGuire SE, Marcelli M
and Mancini MA: Feed-forward inhibition of androgen receptor
activity by glucocorticoid action in human adipocytes. Chem Biol.
19:1126–1141. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stechschulte LA and Sanchez ER: FKBP51-a
selective modulator of glucocorticoid and androgen sensitivity.
Curr Opin Pharmacol. 11:332–337. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jääskeläinen T, Makkonen H and Palvimo JJ:
Steroid up-regulation of FKBP51 and its role in hormone signaling.
Curr Opin Pharmacol. 11:326–331. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Storlie JA, Buckner JC, Wiseman GA, Burch
PA, Hartmann LC and Richardson RL: Prostate specific antigen levels
and clinical response to low dose dexamethasone for
hormone-refractory metastatic prostate carcinoma. Cancer.
76:96–100. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nishimura K, Nonomura N, Yasunaga Y,
Takaha N, Inoue H, Sugao H, Yamaguchi S, Ukimura O, Miki T and
Okuyama A: Low doses of oral dexamethasone for hormone-refractory
prostate carcinoma. Cancer. 89:2570–2576. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Morioka M, Kobayashi T, Furukawa Y, Jo Y,
Shinkai M, Matsuki T, Yamamoto T and Tanaka H: Prostate-specific
antigen levels and prognosis in patients with hormone-refractory
prostate cancer treated with low-dose dexamethasone. Urol Int.
68:10–15. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Venkitaraman R, Thomas K, Huddart RA,
Horwich A, Dearnaley DP and Parker CC: Efficacy of low-dose
dexamethasone in castration-refractory prostate cancer. BJU Int.
101:440–443. 2008.PubMed/NCBI
|
|
33
|
Kume H, Suzuki M, Fujimura T, Fukuhara H,
Enomoto Y, Nishimatsu H, Ishikawa A and Homma Y: Docetaxel as a
vital option for corticosteroid-refractory prostate cancer. Int
Urol Nephrol. 43:1081–1087. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shamash J, Powles T, Sarker SJ, Protheroe
A, Mithal N, Mills R, Beard R, Wilson P, Tranter N, O'Brien N, et
al: A multi-centre randomised phase III trial of dexamethasone vs
dexamethasone and diethylstilbestrol in castration-resistant
prostate cancer: Immediate vs deferred diethylstilbestrol. Br J
Cancer. 104:620–628. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hodson R: Small organ, big reach. Nature.
528:S118–S119. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zheng Y, Izumi K, Li Y, Ishiguro H and
Miyamoto H: Contrary regulation of bladder cancer cell
proliferation and invasion by dexamethasone-mediated glucocorticoid
receptor signals. Mol Cancer Ther. 11:2621–2632. 2012. View Article : Google Scholar : PubMed/NCBI
|