Introduction
Stroke has previously been demonstrated to be one of
the most important underlying etiologies of the development of
seizures (1). The brain comprises
only 2% of human body mass, however consumes 25% of the body's
total glucose and 20% of oxygen (2). Stroke results in glucose and oxygen
deficiency for brain cells, leading to brain dysfunction and injury
(1,2). The specific mechanisms by which
stroke results in the occurrence and development of seizures remain
to be fully elucidated, however abnormal neuronal hyperexcitability
is commonly observed following stroke (3). A total of 50–70% of seizures are
observed in children <1 year of age and adults >60 years
(4). Clinical data suggest that
seizures are a commonly observed complication following perinatal
and childhood stroke (5,6). In addition, stroke, whether ischemic
or hemorrhagic, is an independent risk factor for seizures in the
elderly population (7). Seizures
occurring in this age group are associated with an increased risk
of mortality during the first year following the onset of the
seizure (4), therefore a better
understanding regarding the pathological mechanisms and further
improvements in treatment modalities are of primary concern.
Mild hypothermia of the brain or the whole body is a
therapeutic strategy to alleviate stroke burden by enhancing
tolerance of neurons to glucose and oxygen deficiency. Mild
hypothermia of the whole body (rectal or esophageal temperature
33–34°C), which starts within 6 h following birth and protracts for
48–72 h, significantly reduces the incidences of death or
disability at 18 months, with improved neurological outcome in
survivors (8,9). Ongoing studies reveal that
hypothermia is able to inhibit the occurrence and frequency of
seizures induced by stroke. Seizures following human-perinatal
stroke are effectively avoided when neonates are born in a cooling
environment (10). Evidence from
animal data indicates that hypothermia is significantly associated
with a lower frequency of seizures in acute stroke (11). The mechanism by which hypothermia
conveys anti-seizure effects remains to be fully elucidated,
although it is known that hypothermia attenuates excitotoxicity,
specifically the glutamate- and dopamine-associated cerebral
cytotoxicity of global ischemia (12).
The mammalian target of rapamycin (mTOR) is a
serine/threonine kinase involved in the highly conserved
phosphatidylinositol-3-kinase (PI3K)-AKT serine/threonine kinase
(AKT) signaling pathway (13).
mTOR has been reported to regulate multiple physiological processes
of neurons and glia, including their growth and survival,
metabolism and autophagy, in addition to structure and cell-cell
interactions. However, hyperactivation of mTOR signaling has been
associated with certain types of seizures resulting from genetic
mutation and brain injury (13).
Excessive activation of mTOR is usually the consequence of
mutations in negative regulators of mTOR, including phosphatase and
tensin homolog and tuberous sclerosis complexes 1 and 2 (TSC1 and
TSC2), in a class of human neurological diseases collectively
referred to as ‘TORopathies’ (13). mTOR overactivation results in
cortical malformations and epileptic phenotypes. Inhibition of mTOR
by rapamycin, everolimus, tamoxifen or SB-399885 consistently
attenuates seizures in various genetic and acquired seizure animal
models, which suggests a close association between mTOR and these
types of seizures (1,14,15).
However, it is currently unclear whether mTOR is implicated in the
pathogenesis of stroke-induced seizures.
Glucose is the primary energy source for the brain,
however, due to its hydrophilic property, it needs to be
transported across the cell membrane via glucose transporters
(GLUTs), prior to being utilized by neurons and glial cells
(16). Solute carrier family 2,
facilitated glucose transporter member (GLUT)-1 is the predominant
transporter responsible for glucose transport in the brain
(16). GLUT-1 deficiency (G1D)
syndrome, which is induced by GLUT-1 mutation, is associated with
neurological dysfunction and paradoxically excessive neuronal
activation (presented as spike-wave seizures) (17). Ullner et al (18) constructed a haploinsufficient
GLUT-1 mouse model (Glut-1+/−), in which epileptiform
discharges were observed on the electroencephalogram (EEG). These
data suggest that GLUT-1 deficiency is an important contributor to
seizures. Expression and intracellular translocation of GLUT-1 are
reported to be regulated by the mTOR signaling pathway (19). Various types of cancers result in
overactivation of PI3K/AKT/mTOR cascades that are associated with
upregulated GLUT-1 in cells and the increased glucose uptake
(20). mTOR activation induces
upregulation of hypoxia inducible factor and vascular endothelial
growth factor and acceleration of protein synthesis, which may
increase GLUT-1 abundance in cells (21). However, it has additionally been
demonstrated that mTOR activation resulting from loss of TSC2
function (Tsc2−/−) diminishes glucose uptake by the
embryonic fibroblasts via inhibition of GLUT translocation from the
cytoplasm to plasma membrane (22). Therefore, mTOR promotes or inhibits
glucose uptake by cells through different mechanisms regulating
GLUTs. mTOR regulation of GLUTs and the glucose uptake is dependent
on the cell type and surrounding environments.
The present study aimed to investigate whether
stroke-induced seizures are associated with hyperactivation of mTOR
in neurons. The study also aimed to determine whether the
protective effect of hypothermia against seizures is associated
with mTOR inhibition. Finally, the mechanism underlying how mTOR
regulates GLUT-1 in stroke-induced seizures and the hypothermic
condition was investigated. Overall, the study aimed to facilitate
further understanding of the pathogenesis of stroke-induced
seizures and the improvement of hypothermic therapy for the
future.
Materials and methods
Animals
A total of 105 Sprague-Dawley male rats (8–10 weeks,
~280 g; Central South University, Changsha, China) were separately
housed in four vivariums that were maintained at a fixed
temperature (22–23°C) and moisture (70%), with a 12-h light on/off
cycle. Food and water were provided ad libitum. All
procedures were approved by the Animal Care and Use Committee of
the Central South University and adhered to National Institutes of
Health Guidelines for the Care and Use of Animals (23). In line with the guidelines, animals
with severe seizures were treated with clinically appropriate
anticonvulsants, however these animals were excluded from the
experiments.
Global cerebral ischemia (GCI)
GCI was performed using an ‘L’ shape stick, the
method of which was described in a previous study (24). Briefly, animals were anesthetized
with 2% isoflurane and subsequently placed in a supine position
with the four extremities fastened to the table. The ‘L’ shape
stick was inserted into the mediastinum at the level of the second
intercostal segment and the distal end of the stick was twisted 45°
for positioning under the bundle of primary cardiac blood vessels.
To interrupt blood flow to the brain, the stick was lifted up with
finger pressure from outside of the chest. The compression for 2–3
min resulted in cardiac arrest. Rats were immediately treated with
a rodent respirator to help recover heart beat and blood supply to
the brain. Rats (n=9) in the sham group (Nor) were subjected to the
same procedure without compression of the carotid arteries and
served as the control.
Experimental design
Following establishment of GCI, rats with convulsive
seizures were randomly selected and humanely euthanized at
indicated time-points (24, 48 h, and 7 days, n=3 each time).
Protein levels of mTOR and GLUT-1 in specific regions of the brain
were tested using a western blot analysis. In addition, following
GCI, a group of rats were randomly divided into 4 groups: i) GCI
group (no further treatment, n=18); ii) mild hypothermia group
(n=24); iii) rapamycin group (n=24); and iv) mild
hypothermia+rapamycin group (n=30). Rats in the last three groups
were subjected to treatments with mild hypothermia and rapamycin
injection, alone or in combination.
Mild hypothermia
Active whole-body cooling was performed via a
blanket cooling device (Cincinnati Subzero Blanketrol III,
Cincinnati, OH, USA) immediately following the GCI. Core
temperatures of rats, as measured by a rectal probe, were
maintained at 33.5°C for 30 min. At the end of the cooling period,
the animal was transferred to a heating pad and allowed to warm up
to normal body temperature. Following this, the animal was released
into the normal housing cage.
Rapamycin injection
Rapamycin injections were administered following the
method described by Butler et al (15). Rapamycin (LC Laboratories, Woburn,
MA, USA) was initially dissolved in 100% ethanol (20 mg/ml) and
then diluted in a vehicle solution containing 5% Tween-80, 5%
PEG400, and 4% ethanol (Thermo Fisher Scientific, Inc., Waltham,
MA, USA) dissolved in distilled, deionized water. Rapamycin (3
mg/kg) or vehicle was injected intraperitoneally when the mice
regained consciousness following the GCI injury (20–30 min) and the
treatment was continued once daily until rats were sacrificed.
Seizure severity scores and EEG
recordings
To detect seizures following GCI, animals were
placed under continuous visual surveillance with concurrent EEG
recordings. The appearance of seizures was exemplified by rapid
running, jumping, barrel rolling (≥3 turns), falling (loss of
righting reflex) with tonic limb flexion, and repetitive tail
erection. The seizure severity was evaluated with a scoring method
(25): 0 = normal behavior; 1 =
immobility; 2 = spasm, tremble, or twitch; 3 = tail extension; 4 =
forelimb clonus; 5 = generalized clonic activity; 6 = jumping or
running seizures; 7 = full tonic extension and 8 = death. The
seizure severity scores were given by a neurologist blinded to
treatments and to time post-injury.
EEG recordings were performed in free-moving animals
using an amplitude-integrated EEG monitor as previously described
(26). A rat restrainer was used
when rats exhibited vigorous convulsive behavior, including jumping
and rapid running. EEG recordings were performed using a
dual-channel AC microelectrode amplifier connected to a
custom-built digital video-EEG monitoring system (model 1,800; AM
Systems, Carlsborg, WA, USA). Electrodes were implanted bilaterally
into the hippocampal CA1 (bregma-2.3 mm, lateral 2.0 mm and depth
2.0 mm) and parietal cortex (bregma-0.6 mm, lateral 1.5 mm and
depth 1 mm). Signals were collected in a frequency bandwidth of
0.1–1,000 Hz, amplified 1,000 times and then digitized at ≥5 KHz
(Digidata 1,300; Molecular Devices, LLC, Sunnyvale, CA, USA). Data
were analyzed using pClamp software, version 10 (Molecular Devices,
LLC).
Immunohistochemistry (IHC)
Following sacrifice of rats, the cerebral cortex and
hippocampus were separated from the brain, fixed in 10% formalin
with 20% sucrose at room temperature for 12 h and finally embedded
in paraffin. The sections (5 µm) were blocked with PBS containing
0.3% Triton X-100/5% bovine serum albumin (w/v, Beijing Solarbio
Science and Technology, Co., Ltd., Beijing, China) for 1 h at room
temperature, prior to incubation with primary antibodies specific
for phospho(p)-mTOR (Ser2448; 1:1,000 dilution; cat. no. 2971; Cell
Signaling Technology, Inc., Danvers, MA, USA) and GLUT-1 (1:1,000
dilution, ab32551; Abcam Cambridge, UK) for 2 h at room
temperature. The sections were then incubated with horseradish
peroxidase (HRP)-labeled anti-IgG secondary antibody (1:2,000
dilution; PA128664, Invitrogen; Thermo Fisher Scientific, Inc.) for
30 min at room temperature, and treated with Thermo
Scientific™; Pierce™ ECL solution (Pierce;
Thermo Fisher Scientific, Inc.). All the sections were
counterstained with haematoxylin for 30 min at room temperature and
analyzed using a microscope (Axio Imager 2; Carl Zeiss AG,
Oberkochen, German).
Western blot analysis
Cell membrane proteins of the cerebral cortex and
hippocampus tissues were extracted using a membrane protein
extraction kit (Biovision, Inc., Milpitas, CA, USA). The membrane
proteins were subsequently used for western blot analysis of GLUT-1
and Na+/K+-ATPase. Detection of proteins in
the cells of the cerebral cortex and hippocampus tissues occurred
via homogenizing the tissues directly using a lysis buffer (10 mM
HEPES pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 12% glycerol, 0.1
mM EGTA, 0.5 mM DTT, and 0.5 mM spermidine) with the addition of a
protease inhibitor cocktail (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany). Proteins were quantified via a Bicinchoninic Acid assay
(Pierce; Thermo Fisher Scientific, Inc.). Proteins (20 µg) were
separated by 12% SDS-PAGE in Tris buffer, prior to being
transferred to nitrocellulose membranes. Following blocking with 5%
non-fat milk overnight at 4°C, membranes were incubated with an
anti-GLUT-1 antibody (cat. no. ab32551, 1:1,000 dilution; Abcam),
anti-p-AMP-activated protein kinase (AMPK) antibody (Thr172, cat.
no. ab133448, 1:1,000 dilution; Abcam), anti-mTOR antibody (cat.
no. 2972; 1:1,000 dilution), and anti-p-mTOR antibody (Ser2448,
cat. no. 2971; 1:1,000 dilution) (both from Cell Signaling
Technology, Inc.), anti-p-p70S6 kinase (S6K; Thr229) antibody (cat.
no. GTX25231, 1:1,000 dilution; GeneTex, Inc., Irvine, CA, USA),
anti-p-AKT (T308) antibody (cat. no. ab38449, 1:1,000 dilution;
Abcam), anti-p-p70S6 kinase antibody (cat. no. ab65753, 1:500
dilution; Abcam), or anti-GAPDH antibody (cat. no. SC-365062, 1:800
dilution; Santa Cruz Biotechnology, Inc., Dallas, TX USA) overnight
at 4°C. Then, membranes were incubated with a HRP-conjugated
secondary antibody that targeted mouse IgG (cat. no. ab97040;
Abcam), rabbit IgG (cat. no. A0545), or goat IgG (cat. no. A5420)
(both from Sigma-Aldrich; Merck KGaA) at a 1:2,000 dilution for 2 h
at room temperature. Reactive proteins were detected using Enhanced
Chemiluminescent and SuperSignal™ Chemiluminescent
substrates (Pierce; Thermo Fisher Scientific, Inc.), and quantified
using Optiquant 3.0 software (PerkinElmer, Inc., Waltham, MA,
USA).
Statistical analysis
Statistical tests were conducted using SPSS
software, version 12.0 (SPSS, Inc., Chicago, IL, USA). Data are
presented as the mean ± standard deviation. A Student's t-test was
used for two group comparisons. For multiple group comparisons, a
one-way analysis of variance followed by Dunn's test was used.
P<0.05 was considered to indicate a statistically significant
difference.
Results
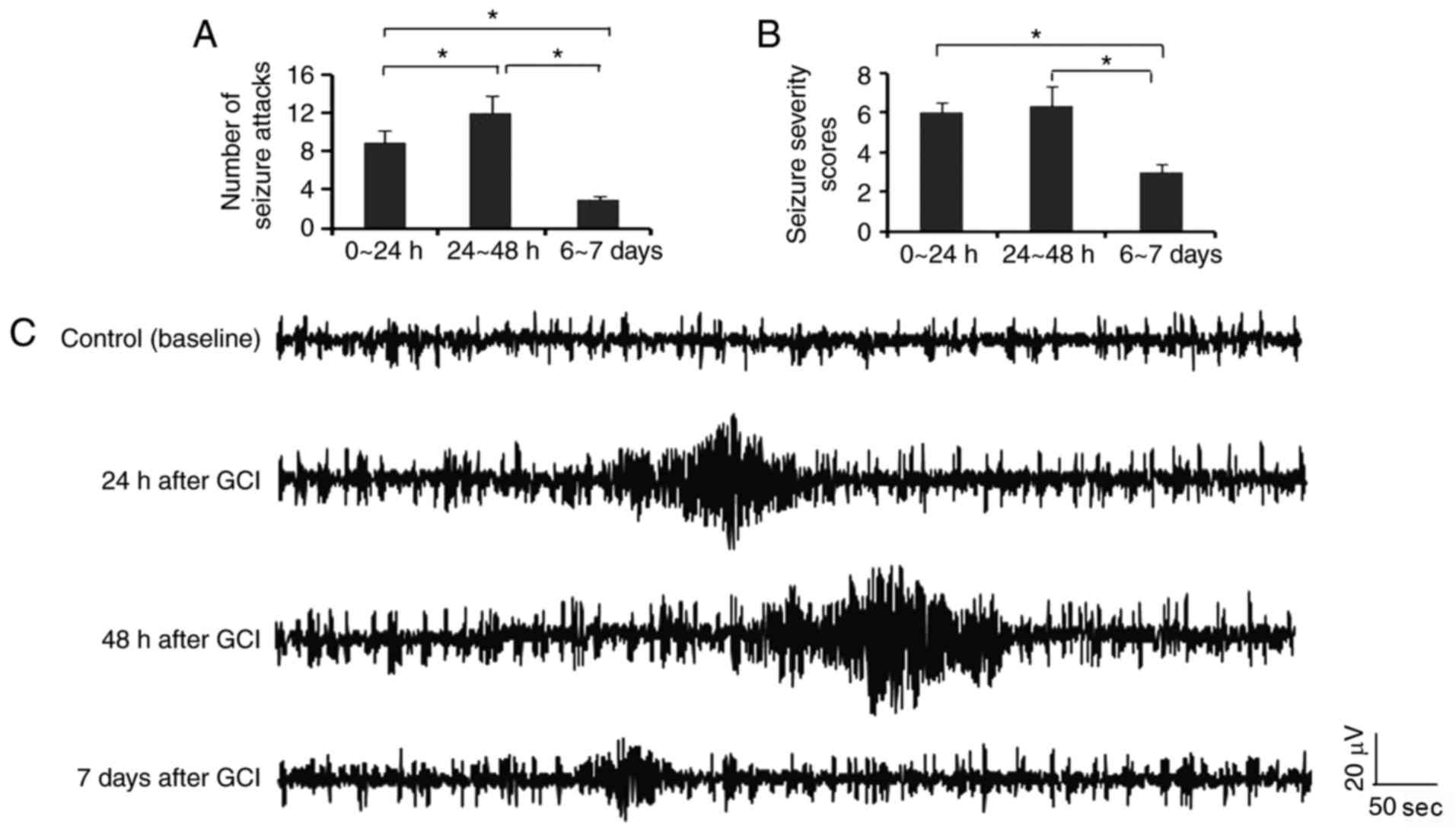
GCI provokes seizures in rats
In the present study, seizures were observed during
the first 24 h following GCI, however not in the sham surgery
group. The number of seizure attacks was increased during the
second 24 h following GCI (P<0.05; Fig. 1A), compared with the first 24 h
following GCI. The number of seizure attacks from day 6 to 7
following GCI was reduced compared with the first and the second 24
h following GCI. Rats gained relative high seizure severity scores
during the first and the second 24 h following GCI, however the
scores were decreased from day 6 to 7 following GCI (P<0.05;
Fig. 1B). EEG discharges of
seizures were defined as repetitive single-spike or poly-spike
waveforms lasting ≥25 sec in duration with amplitudes ≥x2 that of
the background signals. As presented in Fig. 1C, typical EEG discharges of
seizures were observed following GCI, with the longest duration and
highest amplitudes presented during 24–48 h following GCI. The
duration and amplitudes of EEG discharges of seizures were
decreased 7 days following GCI, compared with those during 24–48 h
following GCI.
Upregulation of mTOR and GLUT-1 in
neurons following stroke
Immunohistochemistry was conducted to detect
expression of p-mTOR and GLUT-1 in neurons from the cerebral cortex
and hippocampus of rats at three time-points, 24, 48 h and 7 day
post-surgery. Samples from rats that were not subjected to GCI were
treated as control (Nor) group. Compared with Nor group, p-mTOR
staining was notably increased in the cytoplasm and nucleus of
neurons in the cerebral cortex and hippocampus at 24 h and 48 h
following GCI (Fig. 2). A total of
7 day following GCI, a small fraction of neurons in the cerebral
cortex and hippocampus still revealed moderate p-mTOR staining.
GLUT-1 staining presented outside of neuron nucleus. GLUT-1
staining in neurons in the cerebral cortex was increased following
GCI, with a peak level at 48 h. On day 7, ~70% neurons demonstrated
relatively deep GLUT-1 staining. GLUT-1 staining in neurons in the
hippocampus was increased at 24 and 48 h, however not on day 7,
following GCI.
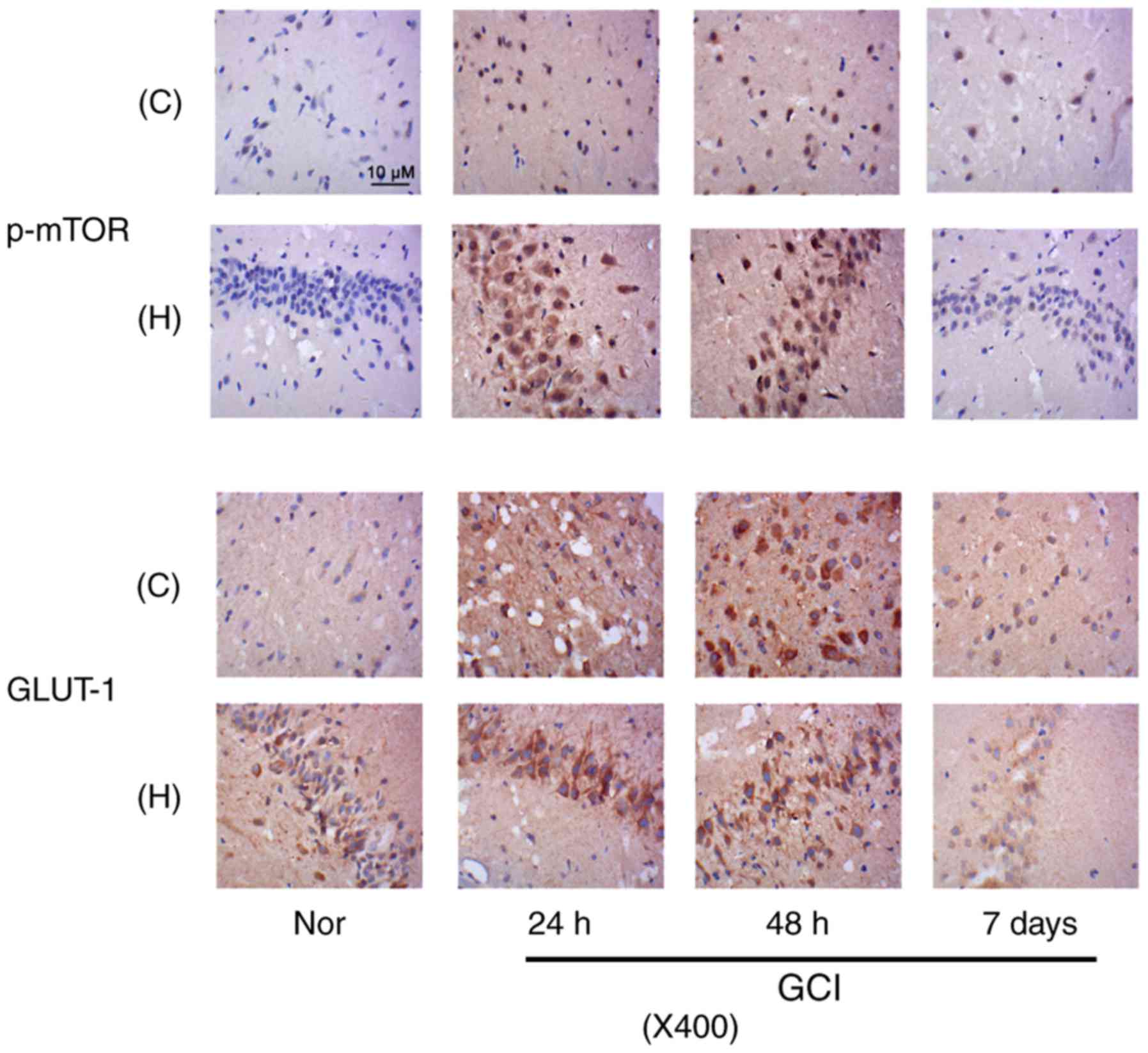 | Figure 2.p-mTOR and GLUT-1 expression in
neurons following stroke. Immunohistochemistry was conducted to
detect expression of p-mTOR and GLUT-1 in neurons in cerebral
cortex and hippocampus of rats at three time-points, 24, 48 h and 7
day post-surgery. Magnification, ×400. C, cerebral cortex tissues;
H, hippocampus; Nor, normal group; GCI, global cerebral ischemia;
p, phosphorylated; mTOR, mammalian target of rapamycin; GLUT-1,
solute carrier family 2, facilitated glucose transporter member
1. |
Furthermore, mTOR, p-mTOR and GLUT-1 protein levels
in the cerebral cortex and hippocampus detected via western
blotting were quantified. As presented in Fig. 3, mTOR and p-mTOR protein levels
were increased at 24 and 48 h post-surgery, compared with Nor group
(P<0.05). mTOR protein level was still increased on day 7
following GCI compared with Nor group. GLUT-1 functions as a
glucose transporter following its transfer to the cell membrane
from the cytoplasm. Therefore, the present study examined the
abundance of GLUT-1 only in neuronal membranes, to evaluate the
ability to transport glucose. The GLUT-1 protein level in the cell
membrane was increased at 24 and 48 h following GCI (P<0.05),
compared with Nor group. There was no significant difference
between control value and the GLUT-1 protein level 7 day following
GCI.
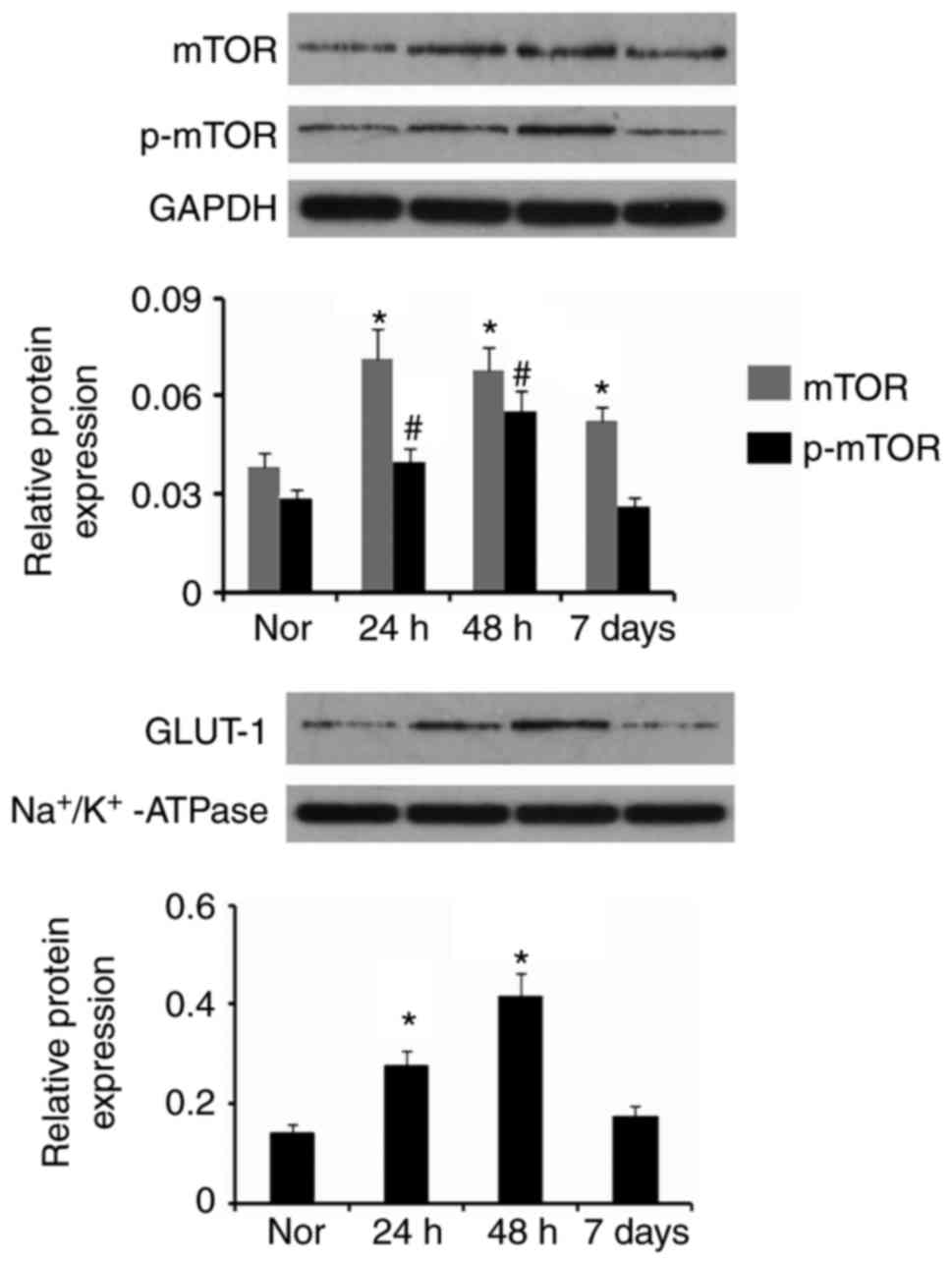 | Figure 3.mTOR, p-mTOR and GLUT-1 expression in
neurons following stroke. Western blotting was conducted to detect
the expression levels of mTOR, p-mTOR and GLUT-1 in neurons and the
cell membrane, respectively, in the cerebral cortex and hippocampus
of rats at three time-points, 24, 48 h and 7 day post-surgery.
*P<0.05 and #P<0.05 vs. control (Nor). Nor, normal
group; p, phosphorylated; mTOR, mammalian target of rapamycin;
GLUT-1, solute carrier family 2, facilitated glucose transporter
member 1. |
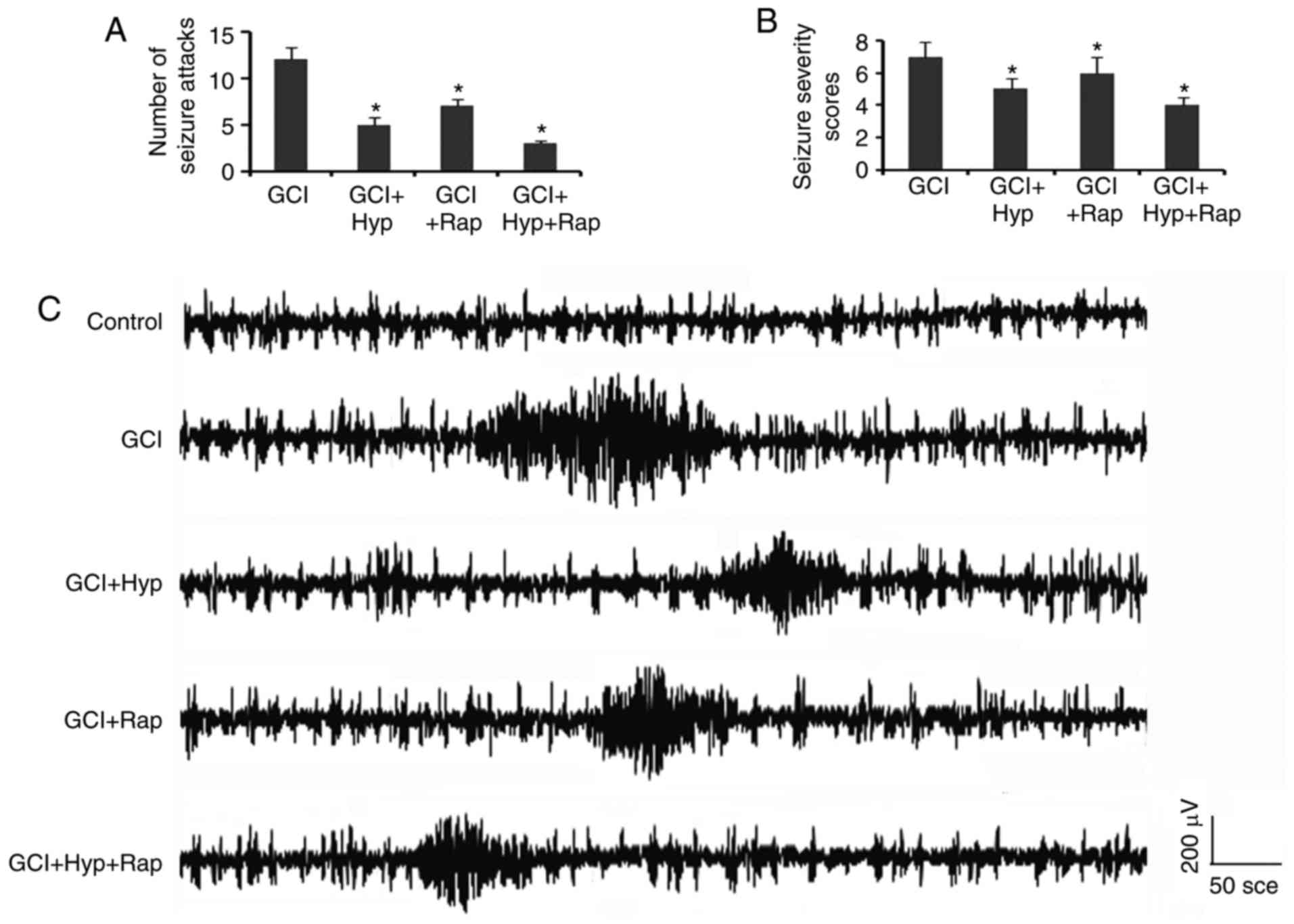
Mild hypothermia and rapamycin
treatments alleviate seizures induced by GCI
The number of epileptic attacks and seizure severity
scores during the second 24 h following GCI were decreased by mild
hypothermia and rapamycin treatments, alone or in combination
(P<0.05; Fig. 4A and B). In
addition, EEG recordings revealed that mild hypothermia and
rapamycin treatments suppressed the burst of the epileptic
discharge, and resulted in a reduction in the spike frequency and
amplitude (Fig. 4C).
Hypothermia and rapamycin treatments
inhibit upregulation of p-mTOR and GLUT-1 following stroke
Immunohistochemistry demonstrated that immediate
hypothermia following GCI prevented the increase in p-mTOR staining
in neurons from the cerebral cortex and hippocampus, at 48 h
following GCI (Fig. 5). The
injection of rapamycin more effectively inhibited the increase in
the staining of p-mTOR than hypothermia; the effects of hypothermia
against seizures may be associated with other mechanisms in
addition to mTOR inhibition. Combination of the hypothermia and
rapamycin injection demonstrated a synergistic effect on the
inhibition. The hypothermic strategy also hindered the increase in
GLUT-1 staining in neurons in the cerebral cortex and hippocampus.
Furthermore, the mTOR inhibitor rapamycin exerted a strong
suppressive effect on the GLUT-1 staining, particularly in neurons
from the hippocampus. The combination of hypothermia and rapamycin
injection demonstrated a synergistic effect on the inhibition,
particularly in neurons from the cerebral cortex.
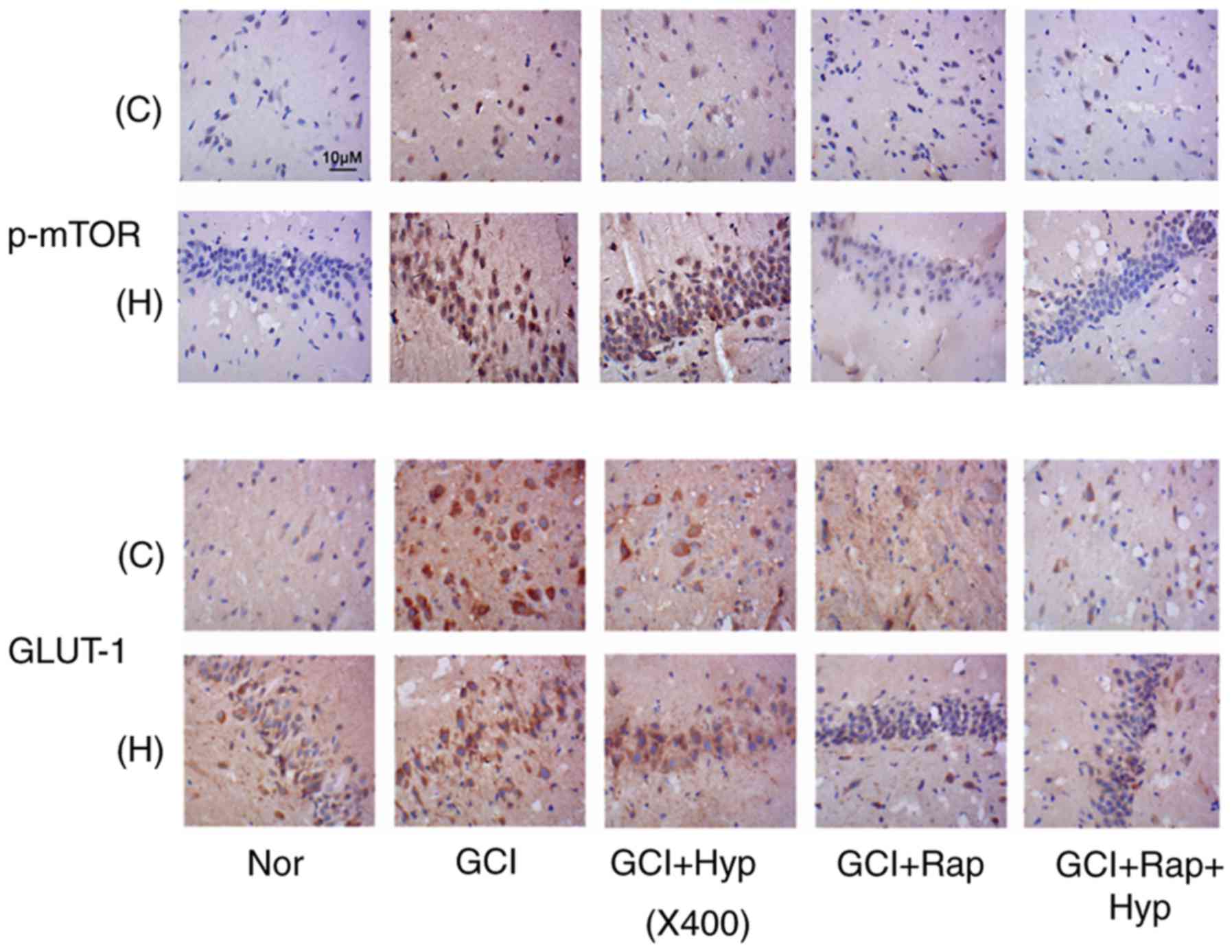 | Figure 5.p-mTOR and GLUT-1 expression following
hypothermia and rapamycin treatments. Following GCI, rats were
subjected to active whole-body cooling and/or rapamycin injection
protocols. Immunohistochemistry was conducted to detect expression
of p-mTOR and GLUT-1 in neurons in the cerebral cortex and
hippocampus of rats at 48 h following GCI. C, cerebral cortex
tissues; H, hippocampus; Nor, normal group; GCI, global cerebral
ischemia; Hyp, mild hypothermia treatment; Rap, rapamycin
injection; p, phosphorylated; mTOR, mammalian target of rapamycin;
GLUT-1, solute carrier family 2, facilitated glucose transporter
member 1. |
Similar to the immunohistochemistry outcome, western
blotting demonstrated that hypothermia prevented the increase in
mTOR protein levels in the cerebral cortex and hippocampus from
GCI, and even reversed the p-mTOR protein level (P<0.05 vs. Nor
group; Fig. 6A). Rapamycin
abolished the increase in p-mTOR protein level by GCI, however only
moderately inhibited the increase in mTOR. The combination of
hypothermia and rapamycin injection decreased protein levels of
mTOR and p-mTOR, compared with Nor group (P<0.05). Protein
levels of p-p70S6 and p-AKT, which are the downstream effecter and
upstream regulator, respectively, of mTOR were additionally
detected. p-p70S6 protein level in cerebral cortex and hippocampus
was increased by GCI. Hypothermia following GCI decreased the
p-p70S6 protein level compared with Nor group (P<0.05).
Treatment with rapamycin abolished increase in p-p70S6 protein
level by GCI. Co-treatment of hypothermia and rapamycin following
GCI significantly decreased the p-p70S6 protein level. GCI
upregulated p-AKT protein level in cerebral cortex and hippocampus.
Although hypothermia to a certain extent decreased the p-AKT
protein level, it was still significantly increased compared with
Nor group (P<0.05). Treatment with rapamycin further increased
the p-AKT protein level (P<0.05). Combination of hypothermia and
rapamycin treatments exerted a modest effect on the increase in
p-AKT protein level. p-AMPK protein level demonstrated no
significant alterations between groups.
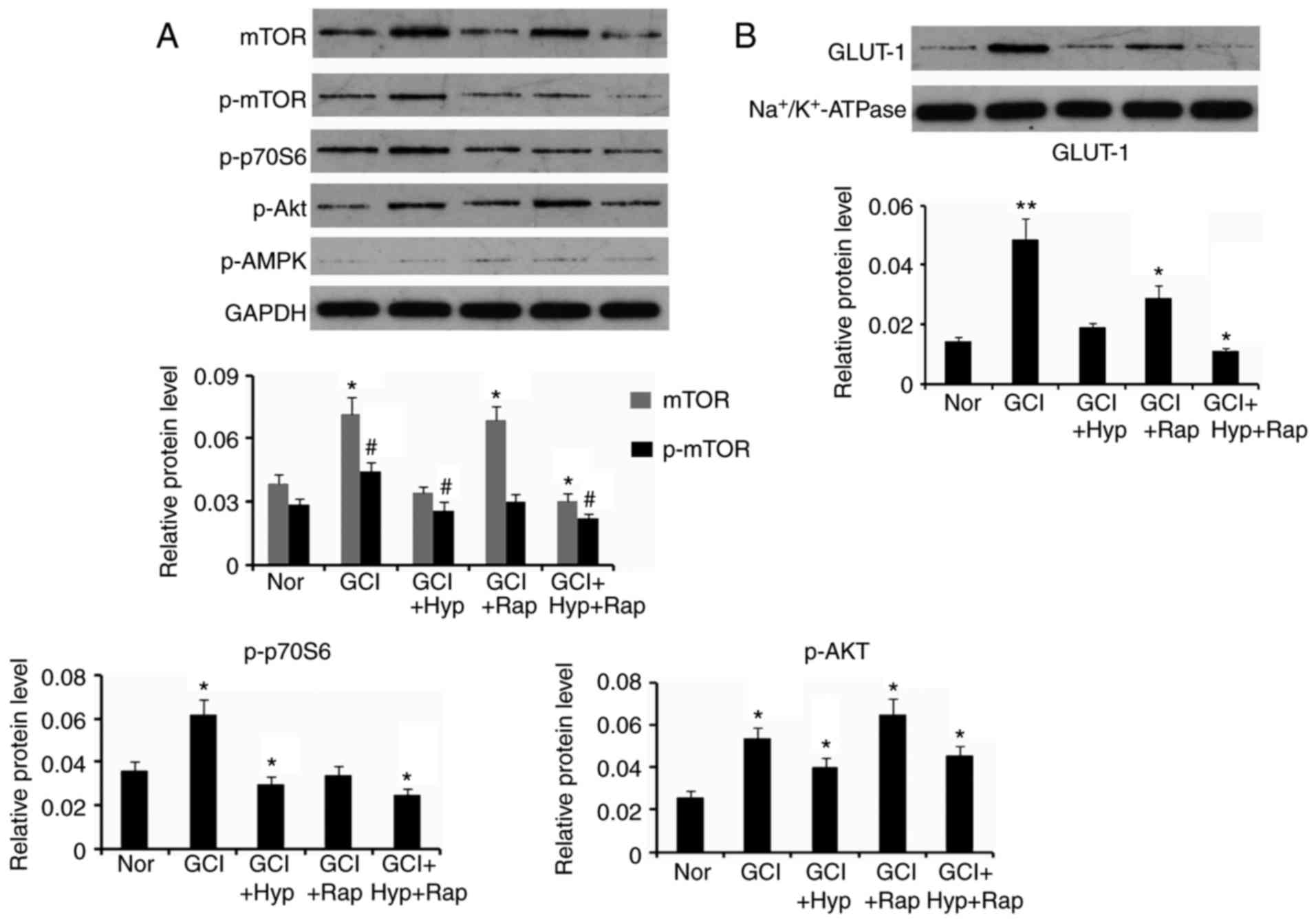 | Figure 6.Protein expression levels following
hypothermia and rapamycin treatments. Following GCI, rats were
subjected to active whole-body cooling and/or rapamycin injection
protocols. (A) Expression levels of proteins were detected in
neurons in cerebral cortex and hippocampus of rats, (B) except for
GLUT-1 protein levels that were detected in the cell membrane of
neurons, at 48 h following GCI by western blotting. Nor, normal
group; GCI, global cerebral ischemia; Hyp, mild hypothermia
treatment; Rap, rapamycin injection; p, phosphorylated; mTOR,
mammalian target of rapamycin; GLUT1, solute carrier family 2,
facilitated glucose transporter member 1; Akt, AKT serine/threonine
kinase; AMPK, AMP-activated protein kinase. *P<0.05, **P<0.01
and #P<0.05 vs. control (Nor). |
GLUT-1 protein level was significantly increased in
cell membrane of neurons in cerebral cortex and hippocampus
following GCI (P<0.01; Fig.
6B), however this increase was abolished by post-treatment with
hypothermia. Post-treatment with rapamycin additionally inhibited
the increase in GLUT-1 protein level, however it was still
increased compared with Nor group (P<0.05). The combination of
hypothermia and rapamycin treatments decreased the GLUT-1 protein
level (P<0.05).
Discussion
Stroke has been identified as an important causative
factor for seizures, however the underlying mechanisms have not
been fully elucidated. It is known that brain cells are very
vulnerable to energy deficiency in stroke, therefore it is
reasonable to hypothesize that signaling molecules that participate
in regulation of energy metabolism may be involved in the
pathological mechanism of post-stroke seizures. mTOR is an
intriguing candidate due to its critical role in energy and protein
metabolism. In addition to this, loss of function of inhibitors
upstream of the mTOR pathway have previously been demonstrated to
be associated with genetically acquired development of seizures
(13–15). The present study aimed to
investigate the involvement of the mTOR pathway in
stroke-associated seizures. A rat stroke model was first
established via the arrest of primary vessels that supply blood to
the brain. Convulsive seizure behaviors frequently occurred during
the first and the second 24 h following GCI, which were accompanied
with seizure discharge reflected in the EEG monitor. This evidence
indicated that stroke resulted in seizure occurrences. mTOR was
upregulated in neurons in the cerebral cortex and hippocampus in
response to GCI. Parenteral administration of the mTOR inhibitor
rapamycin repressed phosphorylation levels of mTOR and its
downstream target S6 protein, and inhibited post-ischemic
seizure-associated characteristics, which suggested that mTOR was
involved in stroke-associated seizures.
The outcome of the increased mTOR activity following
stroke did not conform to anticipated results, as stroke has
previously been demonstrated to activate AMPK through decreasing
ATP/AMP ratio in neurons (27).
Activated AMPK is associated with diminished mTOR activity due to
the stimulatory effect of AMPK on TSC1/2. However, the results of
the western blotting assay demonstrated that the phosphorylation
level of AMPK at Thr172 in the cerebral cortex and hippocampus
tissues did not increase at 48 h following stroke. Fu et al
(28) using western blotting and
immunohistochemistry suggest that phosphorylated AMPK significantly
increases at 3 and 6 h following focal ischemia in the cerebral
cortex of mice, however decreases at 12 and 24 h. This indicates
that increased AMPK activity is observed a short time period
following stroke. PI3K-AKT represents a key signaling pathway
positively regulating mTOR activity. It was observed that AKT
phosphorylation was significantly elevated at 48 h following
stroke, which indicated that the increased mTOR activity was
associated with the stimulation of AKT.
Mild hypothermia of the brain following stroke has
been verified to effectively attenuate the severity and frequency
of seizures (29). It is generally
believed that the inhibitory effect of hypothermia on seizures is
associated with the reduction of energy metabolism which enhances
the tolerance of neurons under the status of glucose and oxygen
deficiency and prevents disorders of transmission of neural signals
in stroke conditions (29–31). According to previous studies,
cerebral metabolic rate is reduced by 6–7% per degree Celsius
reduction. Dropping temperatures to 20°C leads to cerebral
metabolic rate of one fifth of that at normothermic values
(29–31). As a consequence, the release of
excitatory neurotransmitters is slowed and the function of ion
pumps in the neuron membrane is altered, which are proposed to
contribute to seizure suppression (30–32).
However, the molecular basis underlying how hypothermia alleviates
seizures has rarely been investigated. In the present study,
hypothermia prevented mTOR activation with functional consequence
equivalent to treatment with rapamycin. Furthermore, hypothermia in
combination with rapamycin treatment exhibited a synergistic effect
on mTOR suppression, which resulted in a more efficient therapeutic
effect on seizures compared with hypothermia and rapamycin
treatments alone. Decreased mTOR activity from hypothermia may be
involved in its protective effect against seizures post-stroke.
The critical role of GLUT-1 in delivering glucose to
neurons is well established. GLUT-1 deficiency in brain cells is
associated with a series of neurological diseases including
seizures (19). The present study
detected the abundance of GLUT-1 in neurons via
immunohistochemistry and in the cell membrane via western blotting.
Notably, GLUT-1 expression was upregulated in neurons at 24 and 48
h following stroke. This alteration may have been an adaptive
response for neurons that underwent GCI. Inhibition of metabolism
in the brain by hypothermia treatment did not result in a notable
increase in GLUT1 expression following stroke. GLUT1 expression and
translocation from cytoplasm to cell membrane are regulated by mTOR
via its complicated biological functions (19). Treatment of the rats with mTOR
inhibitor following stroke resulted in decreased GLUT1 abundance in
the neurons and the cell membrane, suggesting that mTOR promoted
GLUT1 amplification following stroke and facilitated glucose uptake
by neurons. It has been hypothesized that blood supply to the brain
was recovered 3 min following vascular compression, thus the
situation of glucose and oxygen deficiencies in cerebral neurons at
24 and 48 h following stroke did not occur. However, damages
induced by preceding stroke were not eliminated at these
time-points, and GLUT1 was unable to repair the detriments just
through replenishing energy for neurons. Therefore, GLUT1
upregulation triggered by mTOR would result in a weak effect on
seizure suppression. Conversely, various biological functions of
mTOR, may contribute to seizures (14,15),
thus inhibition of mTOR activity either by drug-like molecules or a
physical approach (hypothermia) is desirable to attenuate seizures
following stroke.
In conclusion, the present study revealed that the
seizures induced by stroke were associated with mTOR activation,
and hypothermia protected against the seizures via repressing mTOR.
Although mTOR increased GLUT-1 abundance in cell membrane of
neurons following stroke, GLUT-1 had a limited effect on seizures
following stroke. Results obtained in the present study contribute
to further understanding of pathogenesis of stroke-induced seizures
and improvement of hypothermic therapy in the seizures.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Key Program
of Science and Technology Planning of Haikou (grant no. 2014-73)
and Hainan Provincial Natural Science Foundation (grant no.
20158272).
Availability of data and methods
All datasets used in the current study are available
upon request from the corresponding author
Authors' contributions
GY and XZ took the responsibility of the
experimental design. GY, XA, XL, YZ and DY performed the study and
wrote the manuscript. All authors have read and approved of the
final manuscript.
Ethics approval and consent to
participate
All procedures were approved by the Animal Care and
Use Committee of the Central South University and adhered to
National Institutes of Health Guidelines for the care and use of
animals (23).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Stefanidou M, Das RR, Beiser AS, Sundar B,
Kelly-Hayes M, Kase CS, Devinsky O, Seshadri S and Friedman D:
Incidence of seizures following initial ischemic stroke in a
community-based cohort: The Framingham Heart Study. Seizure.
47:105–110. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang H, Wang B, Normoyle KP, Jackson K,
Spitler K, Sharrock MF, Miller CM, Best C, Llano D and Du R: Brain
temperature and its fundamental properties: A review for clinical
neuroscientists. Front Neurosci. 8:3072014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lei Z, Zhang H, Liang Y and Xu ZC: Reduced
expression of IA channels is associated with post-ischemic
seizures. Epilepsy Res. 124:40–48. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Assis TM, Bacellar A, Costa G and
Nascimento OJ: Mortality predictors of epilepsy and epileptic
seizures among hospitalized elderly. Arq Neuropsiquiatr.
73:510–515. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Robertson CM and Perlman M: Follow-up of
the term infant after hypoxic-ischemic encephalopathy. Paediatr
Child Health. 11:278–282. 2006.PubMed/NCBI
|
|
6
|
Glass HC, Glidden D, Jeremy RJ, Barkovich
AJ, Ferriero DM and Miller SP: Clinical neonatal seizures are
independently associated with outcome in infants at risk for
hypoxic-ischemic brain injury. J Pediatr. 155:318–323. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Procaccianti G, Zaniboni A, Rondelli F,
Crisci M and Sacquegna T: Seizures in acute stroke: Incidence, risk
factors and prognosis. Neuroepidemiology. 39:45–50. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jacobs SE, Berg M, Hunt R, Tarnow-Mordi
WO, Inder TE and Davis PG: Cooling for newborns with hypoxic
ischaemic encephalopathy. Cochrane Database Syst Rev: CD003311.
2013. View Article : Google Scholar
|
|
9
|
Azzopardi DV, Strohm B, Edwards AD, Dyet
L, Halliday HL, Juszczak E, Kapellou O, Levene M, Marlow N, Porter
E, et al: Moderate hypothermia to treat perinatal asphyxial
encephalopathy. N Engl J Med. 361:1349–1358. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Harbert MJ, Tam EW, Glass HC, Bonifacio
SL, Haeusslein LA, Barkovich AJ, Jeremy RJ, Rogers EE, Glidden DV
and Ferriero DM: Hypothermia is correlated with seizure absence in
perinatal stroke. J Child Neurol. 26:1126–1130. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
D'Ambrosio R, Eastman CL, Darvas F, Fender
JS, Verley DR, Farin FM, Wilkerson HW, Temkin NR, Miller JW,
Ojemann J, et al: Mild passive focal cooling prevents epileptic
seizures after head injury in rats. Ann Neurol. 73:199–209. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dietrich WD and Bramlett HM: The evidence
for hypothermia as a neuroprotectant in traumatic brain injury.
Neurotherapeutics. 7:43–50. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lasarge CL and Danzer SC: Mechanisms
regulating neuronal excitability and seizure development following
mTOR pathway hyperactivation. Front Mol Neurosci. 7:182014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wong M: mTOR as a potential treatment
target for epilepsy. Future Neurol. 7:537–545. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Butler CR, Boychuk JA and Smith BN:
Effects of rapamycin treatment on neurogenesis and synaptic
reorganization in the dentate gyrus after controlled cortical
impact injury in mice. Front Syst Neurosci. 9:1632015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Marin-Valencia I, Good LB, Ma Q, Duarte J,
Bottiglieri T, Sinton CM, Heilig CW and Pascual JM: Glut1
deficiency (G1D): Epilepsy and metabolic dysfunction in a mouse
model of the most common human phenotype. Neurobiol Dis. 48:92–101.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pascual JM, Campistol J and Gil-Nagel A:
Epilepsy in inherited metabolic disorders. Neurologist. 14 6 Suppl
1:S2–S14. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ullner PM, Di Nardo A, Goldman JE, Schobel
S, Yang H, Engelstad K, Wang D, Sahin M and De Vivo DC: Murine
Glut-1 transporter haploinsufficiency: Postnatal deceleration of
brain weight and reactive astrocytosis. Neurobiol Dis. 36:60–69.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liemburg-Apers DC, Wagenaars JA, Smeitink
JA, Willems PH and Koopman WJ: Acute stimulation of glucose influx
upon mitoenergetic dysfunction requires LKB1, AMPK, Sirt2 and
mTOR-RAPTOR. J Cell Sci. 129:4411–4423. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Demel HR, Feuerecker B, Piontek G, Seidl
C, Blechert B, Pickhard A and Essler M: Effects of topoisomerase
inhibitors that induce DNA damage response on glucose metabolism
and PI3K/Akt/mTOR signaling in multiple myeloma cells. Am J Cancer
Res. 5:1649–1664. 2015.PubMed/NCBI
|
|
21
|
Inglis DJ, Lavranos TC, Beaumont DM, Leske
AF, Brown CK, Hall AJ and Kremmidiotis G: The vascular disrupting
agent BNC105 potentiates the efficacy of VEGF and mTOR inhibitors
in renal and breast cancer. Cancer Biol Ther. 15:1552–1560. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jiang X, Kenerson H, Aicher L, Miyaoka R,
Eary J, Bissler J and Yeung RS: The tuberous sclerosis complex
regulates trafficking of glucose transporters and glucose uptake.
Am J Pathol. 172:1748–1756. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Committee for the Update of the Guide for
the Care and Use of Laboratory Animals, Institute for Laboratory
Animal Research, National Research Council, . Guide for the Care
and Use of Laboratory Animals. 8th. The National Academies Press;
Washington, DC: 2011, PubMed/NCBI
|
|
24
|
Kawai K, Nitecka L, Ruetzler CA, Nagashima
G, Joó F, Mies G, Nowak TS Jr, Saito N, Lohr JM and Klatzo I:
Global cerebral ischemia associated with cardiac arrest in the rat:
I. Dynamics of early neuronal changes. J Cereb Blood Flow Metab.
12:238–249. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cao L, Tian Y, Jiang Y, Zhang GJ, Lei H
and Di ZL: Down-regulation of Homer1b/c protects against chemically
induced seizures through inhibition of mTOR signaling. Cell Physiol
Biochem. 35:1633–1642. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu C, Wang J, Peng J, Patel N, Huang Y,
Gao X, Aljarallah S, Eubanks JH, McDonald R and Zhang L: Modeling
early-onset post-ischemic seizures in aging mice. Exp Neurol.
271:1–12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu F, Benashski SE, Persky R, Xu Y, Li J
and McCullough LD: Age-related changes in AMP-activated protein
kinase after stroke. Age (Dordr). 34:157–168. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fu L, Huang L, Cao C, Yin Q and Liu J:
Inhibition of AMP-activated protein kinase alleviates focal
cerebral ischemia injury in mice: Interference with mTOR and
autophagy. Brain Res. 1650:103–111. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Andresen M, Gazmuri JT, Marín A, Regueira
T and Rovegno M: Therapeutic hypothermia for acute brain injuries.
Scand J Trauma Resusc Emerg Med. 23:422015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Luo M, Li Q, Dong W, Zhai X and Kang L:
Evaluation of mild hypothermia therapy for neonatal
hypoxic-ischaemic encephalopathy on brain energy metabolism using
18F-fluorodeoxyglucose positron emission computed tomography. Exp
Ther Med. 8:1219–1224. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bakhsheshi MF, Diop M, Morrison LB, St
Lawrence K and Lee TY: Coupling of cerebral blood flow and oxygen
consumption during hypothermia in newborn piglets as measured by
time-resolved near-infrared spectroscopy: A pilot study.
Neurophotonics. 2:0350062015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Feng JZ, Wang WY, Zeng J, Zhou ZY, Peng J,
Yang H, Deng PC, Li SJ, Lu CD and Jiang H: Optimization of brain
metabolism using metabolic-targeted therapeutic hypothermia can
reduce mortality from traumatic brain injury. J Trauma Acute Care
Surg. 83:296–304. 2017. View Article : Google Scholar : PubMed/NCBI
|