Introduction
Liver cancer is one of the leading causes of
cancer-associated mortality worldwide, and hepatocellular carcinoma
(HCC) is the primary type of liver cancer, accounting for ~90% of
liver cancer cases (1). Previous
studies have indicated that the incidence rate of HCC is increasing
with a poor 5-year survival rate of ~7% (2,3). In
the early stages of HCC, the tumor can be removed effectively with
a relatively long survival time, while advanced HCC is associated
with poor survival, due to distant organ metastasis (4,5).
Therefore, further investigation of HCC diagnostic biomarkers is
required. However, an early diagnosis of HCC is not always
effaceable. In addition, to the best of our knowledge, an effective
HCC treatment has not yet been established.
Long non-coding RNAs (lncRNAs) are a group of
non-coding RNAs >200 nucleotides and have emerged as important
regulators in the majority of biological processes (6). Recent advances have suggested that
lncRNA dysregulation is linked to the development of
pathophysiological processes, including development, invasion and
metastasis of cancer (7,8).
Increasing evidence suggests that the expression of
dysregulated lncRNA has been implicated in the development and
progression of tumors (9–12). The emerging roles of lncRNAs in HCC
have also been investigated in previous studies. For example, the
downregulated expression of the lncRNA, growth arrest specific 5
(GAS5), promotes cell invasion in HCC, by regulating vimentin, and
is associated with a relatively poor prognosis (9). Colon cancer associated transcript 1
(CARLo-5) has been considered as an independent risk factor for
disease-free survival in HCC as it may accelerate the metastasis of
HCC, making it a potential novel therapeutic biomarker (10). Prostate cancer associated
transcript 1 (PCAT-1) has been reported to be significantly
upregulated in HCC tissues, and significantly associated with the
overall survival time of patients with HCC (13). ZNFX1 antisense RNA 1 (ZFAS1) may
function as a cancer gene in HCC progression, as it has been proved
to bind microRNA-150 and abolish its tumor suppressive function,
promoting the expression of matrix metalloproteinase (MMP14), MMP16
and zinc finger E-box-binding homeobox 1 (14). The upregulated expression of the
lncRNAs metastasis associated lung adenocarcinoma transcript 1 and
hepatocellular carcinoma upregulated long non-coding RNA (HULC) in
HCC tissue may represent a good prognostic biomarker for curative
resected HCC (15).
In the present study, lncRNA and mRNA expression
data of a large number of patients with HCC was investigated using
data from The Cancer Genome Atlas (TCGA). In addition, the study
attempted to identify the optimal diagnostic lncRNA biomarkers by
using feature selection and classification models. The functions of
the potential lncRNA diagnostic biomarkers in HCC were further
analyzed by the functional annotation of their co-expressed
mRNAs.
Materials and methods
Eligible lncRNA and mRNA gene
expression profiles of HCC in TCGA
From the database set-up until April 28th 2018, TCGA
was searched for the genomic data of HCC. From TCGA data portal
(tcga-data.nci.nih.gov/), lncRNA and mRNA
gene expression profiles and clinical data of HCC and normal
samples were downloaded. The present study included only patients
who were histologically diagnosed as HCC. Finally, 361 HCC tissues
and 50 normal adjacent samples from patients with HCC were included
in this study.
Identification of differentially
expressed mRNAs and lncRNAs between HCC and normal tissues
Undetectable lncRNAs and mRNAs, with a read count
quantification of 0 in >20% HCC case or in >20% normal
tissues were filtered and deleted. The differentially expressed
lncRNAs (DElncRNAs) and mRNAs (DEmRNAs) in HCC compared to normal
tissues were calculated using the R (version 3.3.3) package DESeq2
1.28.0 (16). The Benjamini and
Hochberg multiple testing method (17) was applied to acquire the false
discovery rate (FDR). FDR thresholds <0.05 and a log2-fold
change >1 were used to define DElncRNAs and DEmRNAs. Using the R
package pheatmap 0.7.4 (https://cran.r-project.org/web/packages/pheatmap/index.html),
the hierarchical clustering analysis of DElncRNAs and DEmRNAs was
performed.
Identification of the optimal
diagnostic lncRNA biomarkers for HCC
To identify optimal diagnostic lncRNA biomarkers for
HCC, feature selection procedures were performed as follows: The
LASSO algorithm analysis was conducted using the ‘glmnet’ package
(https://cran.r-project.org/web/packages/glmnet/) to
decrease data dimensions. Single 10-fold cross-validation cycles
were carried out using the coordinate descent algorithm for each
fold and regularization parameters were indicated, resulting in the
smallest average mean squared error across all folds. The optimal
DElncRNAs were selected in HCC and normal tissue.
To further identify the optimal diagnostic lncRNA
biomarkers for HCC, feature selection procedures were performed as
follows. The importance value of each lncRNA was ranked from large
to small, according to the decrease of mean accuracy using the
random forest algorithm (randomForest; http://cran.r-project.org/web/packages/randomForest/);
the optimum number of features was indicated by adding one DElncRNA
at a time in the top down forward-wrapper packaging method; by
using a support vector machine (SVM) at each increment.
The randomForest package was used to establish the
random forest model. The ‘rpart’ package (https://cran.r-project.org/web/packages/rpart/)
was used to build the decision tree mode. The e1071 package
(https://cran.r-project.org/web/packages/e1071/index.html)
in R was used to establish the SVM model. The diagnostic ability of
these three models and each lncRNA biomarker was evaluated by the
receiver operating characteristic (ROC) area under curve (AUC),
sensitivity and specificity.
Correlation between the four optimal
diagnostic lncRNAs and clinical features
Analysis of the correlation between the four
optimal lncRNAs and the clinical features, age and gender, was
performed using Pearson's correlation coefficient. Analysis of the
correlation between the four optimal lncRNAs and the clinical
features, stage, grade and race, was performed using Spearman's
correlation. r represents the correlation coefficient. The
threshold for correlation was r>0.5 and P<0.05.
Survival analysis of optimal
diagnostic lncRNA biomarkers for HCC
To determine potential associations between
identified DElncRNAs and the survival of patients with HCC,
survival analysis was performed using the R survival package
(version 2.44; http://cran.r-project.org/web/packages/survival/index.html).
Univariate Cox regression analysis was performed for each
DElncRNAs. P<0.05 was considered statistically significant.
DEmRNAs co-expressed with the
identified optimal diagnostic lncRNAs
The correlation between the optimal diagnostic
lncRNAs and DEmRNAs were analyzed by the pairwise Pearson
correlation coefficient. The threshold for DElncRNA-DEmRNA
co-expression pairs was P<0.05 and r>0.6. Cytoscape software
3.5.0 (cytoscape.org/) was used to construct
the DElncRNA-DEmRNA co-expression network.
Functional annotation
To disclose the biological functions and the
potential pathways of the genes co-expressed with optimal DElncRNAs
with diagnostic value for HCC, Gene Ontology (GO) classification
(18,19) and Kyoto Encyclopedia of Genes and
Genomes (KEGG) (20–22) (https://www.genome.jp/kegg/docs/relnote.html) pathway
enrichment analysis were performed using GeneCodis3
(genecodis.cnb.csic.es/analysis) online software. FDR<0.05 was
considered to indicate a statistically significant difference.
Results
Characteristics of patients with
HCC
The clinical data of 361 patients with HCC were
downloaded from TCGA. In the present study, 21.3 and 3.3% of
patients had hepatitis B infection and hepatitis C infection,
respectively. The incidence rate of HCC was indicated in 67.6 and
32.4%, in male and female patients, respectively. The incidence
rate of HCC in Caucasians, Asians, and Black or African American
was 48.7, 43.2 and 4.7%, respectively. The tumor stages were as
follows: Stage I, 46.2%; stage II, 22.7%; stage III, 23.2%; and
stage IV, 1.1% (Table I).
 | Table I.HCC patient characteristics
(n=361). |
Table I.
HCC patient characteristics
(n=361).
| Parameter | Patients
(n=361) |
|---|
| Gender |
|
|
Male | 244 |
|
Female | 117 |
| Age |
|
|
<60 | 165 |
|
≥60 | 195 |
|
Unknown | 1 |
| Race |
|
|
Asian | 156 |
| Black
or African American | 17 |
|
White | 176 |
|
Unknown | 12 |
| Tumor histologic
grade |
|
| G1 | 53 |
| G2 | 171 |
| G3 | 121 |
| G4 | 11 |
|
Unknown | 5 |
| Tumor histologic
stage |
|
| Stage
I | 167 |
| Stage
II | 82 |
| Stage
III | 84 |
| Stage
IV | 4 |
DEmRNAs and DElncRNAs between HCC and
adjacent normal liver tissues
A total of 3,177 lncRNAs and 15,183 mRNAs were
available for analysis following the exclusion of the minimally
detected lncRNAs and mRNAs. The criteria of FDR<0.05 and
log2-fold change >1 were used to identify DElncRNAs and DEmRNAs.
A total of 601 DElncRNAs, including 407 upregulated and 194
downregulated lncRNAs, and 2,920 DEmRNAs, including 1,930
upregulated and 990 downregulated mRNAs, were identified comparing
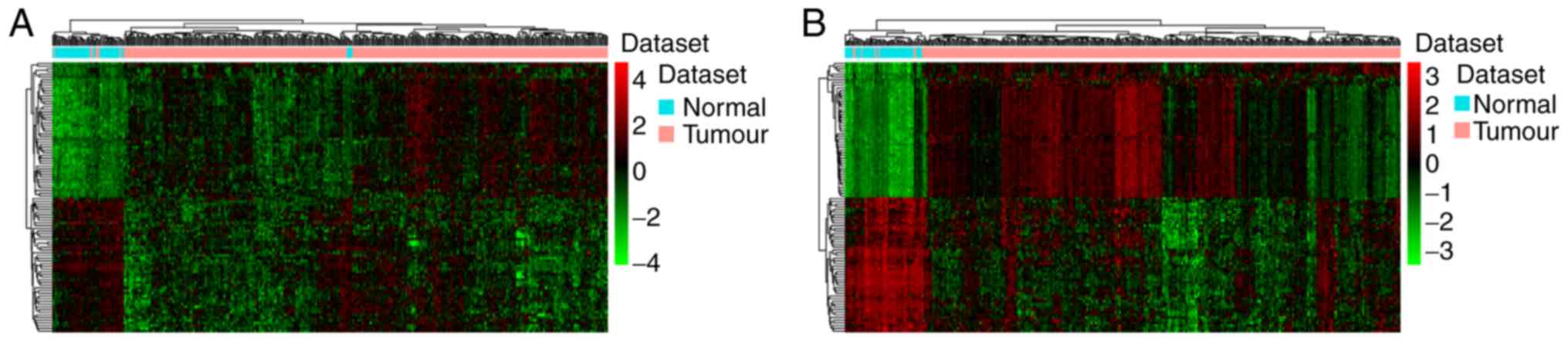
HCC and adjacent normal tissues. Hierarchical clustering analysis
of the top 100 DElncRNAs and DEmRNAs is presented in Fig. 1A and B, respectively.
Identification of the optimal
diagnostic lncRNA biomarkers for HCC
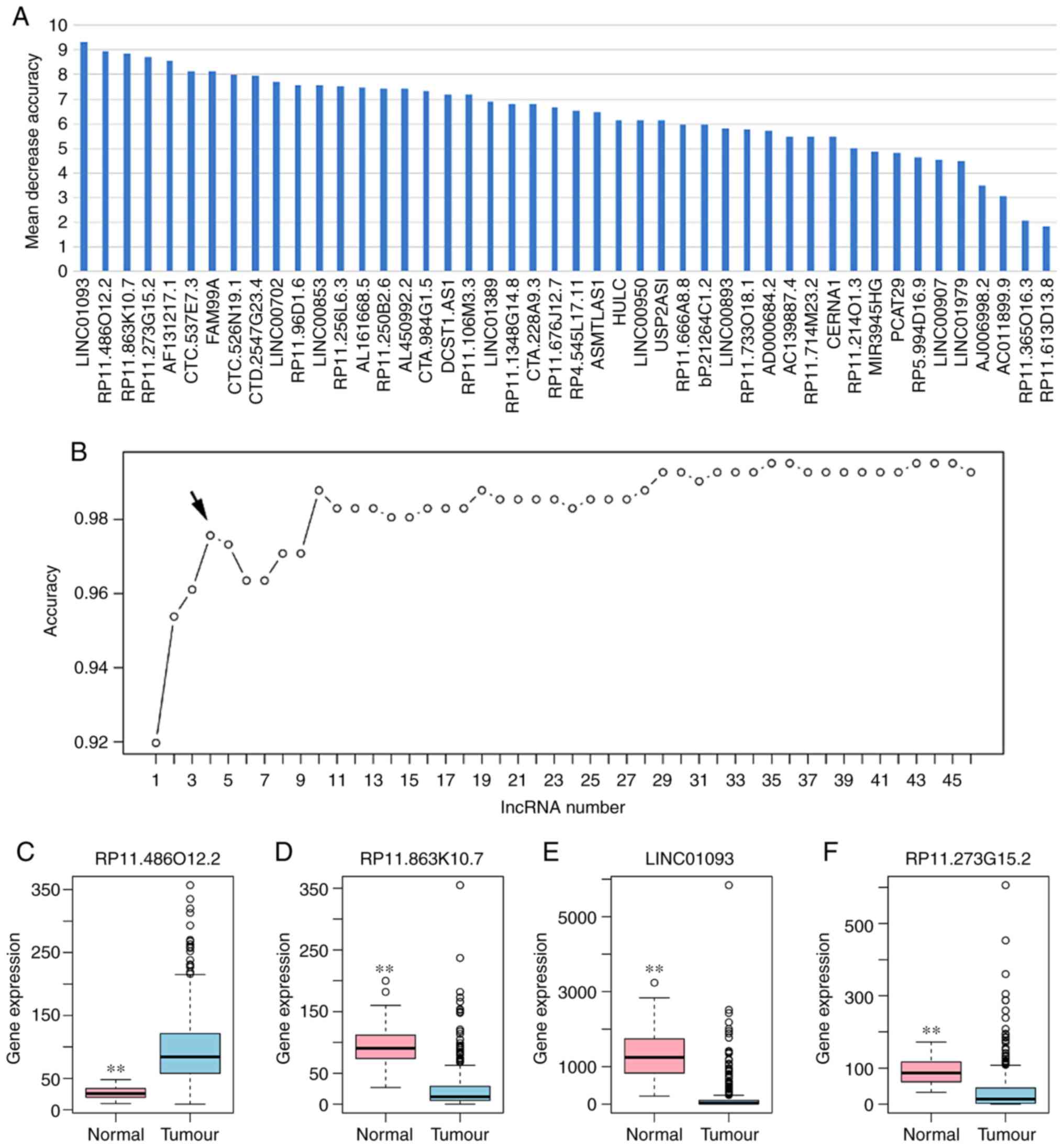
Based on the reduced dimension of the data,
comparing HCC and adjacent normal tissues identified 45 DElncRNAs
using LASSO algorithm analysis (Table
II). The random forest analysis was used to rank the 45
DElncRNAs, according to the decrease in mean accuracy (Fig. 2A). A 10-fold cross-validation
result demonstrated that the average accuracy rate of four
DElncRNAs, including RP11-486O12.2, RP11-863K10.7, LINC01093 and
RP11-273G15.2, exhibited the highest score (Fig. 2B). Therefore, these four DElncRNAs
were selected as the potential optimal diagnostic lncRNA biomarkers
for HCC and were used to establish the random forests, decision
tree and SVM models. Box-plot displayed the expression levels of
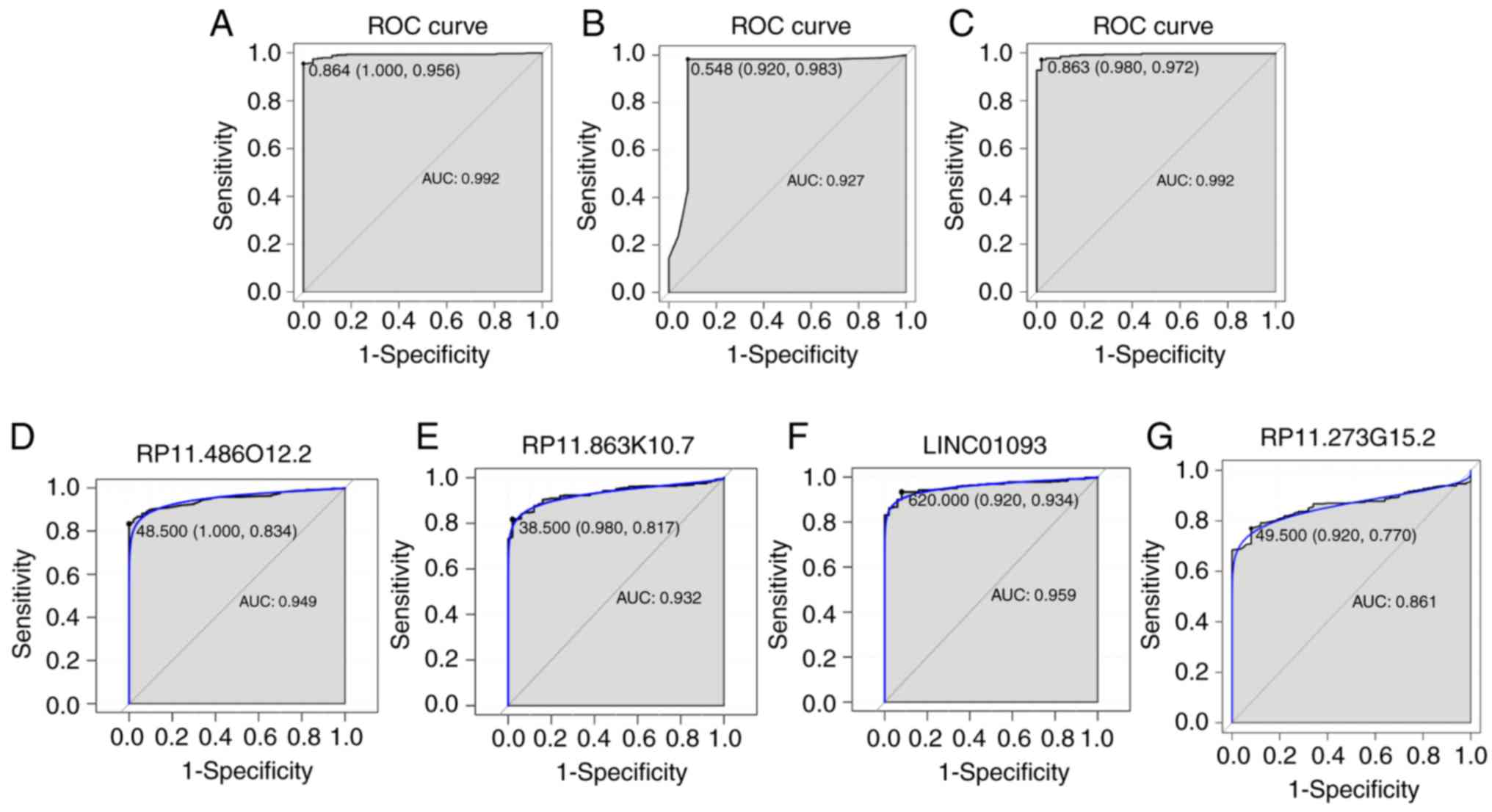
four DElncRNAs between HCC and normal tissues (Fig. 2C-F). The AUC of the random forests
model was 0.992 and the specificity and sensitivity of this model
were 100.0 and 95.6%, respectively (Fig. 3A). The AUC of the decision tree
model was 0.927 and the specificity and sensitivity of this model
were 92.0% and 98.3, respectively (Fig. 3B). The AUC of the SVM model was
0.992, and the specificity and sensitivity of this model were 98.0
and 97.2% (Fig. 3C). The AUC of
the combination of these four lncRNAs was >0.85, which indicated
that the combination of these four lncRNAs was associated with HCC
and serves a potential role in predicting the occurrence of HCC.
The AUCs of these four lncRNAs, RP11-486O12.2, RP11-863K10.7,
LINC01093 and RP11-273G15.2, were also >0.85 (Fig. 3D-G). Correlation between these four
lncRNAs and clinical features was also performed using Pearson's
correlation coefficient or Spearman's correlation, and results
showed that the four DElncRNAs were not associated with age,
gender, stage, grade or race (Table
III).
 | Table II.Differentially expressed lncRNAs
between HCC and normal tissues following reduced dimensions of the
data. |
Table II.
Differentially expressed lncRNAs
between HCC and normal tissues following reduced dimensions of the
data.
| lncRNA | log2 fold
change | P-value | Regulation |
|---|
| CTD-2547G23.4 | 1.661885351 |
3.18×10−38 | Up |
| CTC-526N19.1 | −2.226501065 |
8.58×10−38 | Down |
| RP11-250B2.6 | −1.735228252 |
4.38×10−34 | Down |
|
RP11-486O12.2 | 1.085632129 |
1.24×10−29 | Up |
|
RP11-863K10.7 | −2.44537751 |
5.53×10−28 | Down |
| AF131217.1 | −2.550790337 |
7.72×10−28 | Down |
|
LINC01093 | −3.43026059 |
8.33×10−26 | Down |
| LINC00853 | 2.046868315 |
1.74×10−25 | Up |
| RP5-994D16.9 | −1.016054876 |
7.17×10−24 | Down |
| LINC00907 | −2.889956524 |
4.49×10−22 | Down |
| MIR3945HG | −2.002666478 |
1.58×10−19 | Down |
| CTC-537E7.3 | −2.630756292 |
2.58×10−19 | Down |
| RP11-733O18.1 | −1.727966554 |
1.26×10−18 | Down |
| ASMTL-AS1 | 1.304698293 |
1.80×10−18 | Up |
| AL161668.5 | −2.049712877 |
2.28×10−18 | Down |
| RP11-106M3.3 | 1.079926794 |
3.88×10−18 | Up |
| CTA-228A9.3 | 1.604725390 |
4.56×10−18 | Up |
| RP11-96D1.6 | −1.922229332 |
3.17×10−16 | Down |
| DCST1-AS1 | 1.241184606 |
1.48×10−15 | Up |
| LINC00893 | 1.211220275 |
5.90×10−15 | Up |
| RP11-365O16.3 | −1.828187085 |
4.13×10−14 | Down |
| CTA-984G1.5 | 1.347562311 |
4.19×10−14 | Up |
| LINC00950 | 1.227184171 |
5.67×10−14 | Up |
| LINC01389 | 1.558778315 |
2.04×10−13 | Up |
| RP11-676J12.7 | −2.447116555 |
8.57×10−13 | Down |
| RP11-1348G14.8 | 1.017320557 |
2.62×10−12 | Up |
| AD000684.2 | 1.15389113 |
1.22×10−11 | Up |
|
RP11-273G15.2 | −1.877345455 |
5.25×10−11 | Down |
| RP4-545L17.11 | 1.052121463 |
1.04×10−10 | Up |
| RP11-666A8.8 | 1.026612682 |
1.81×10−10 | Up |
| RP11-613D13.8 | −1.567841389 |
3.61×10−10 | Down |
| RP11-214O1.3 | −1.537151619 |
1.54×10−9 | Down |
| RP11-256L6.3 | −1.692081916 |
1.58×10−9 | Down |
| LINC01979 | −1.709561819 |
3.31×10−9 | Down |
| bP-21264C1.2 | 1.063155352 |
7.78×10−9 | Up |
| FAM99A | −1.893766593 |
2.04×10−8 | Down |
| LINC00702 | 1.138170496 |
2.97×10−8 | Up |
| AC139887.4 | 1.064667365 |
6.72×10−8 | Up |
| CERNA1 | 1.430153282 |
8.35×10−8 | Up |
| AC011899.9 | −1.006302718 |
5.80×10−7 | Down |
| HULC | 1.117705678 |
1.11×10−6 | Up |
| USP2-AS1 | 1.009672100 |
6.87×10−6 | Up |
| PCAT29 | 1.048101691 |
2.05×10−4 | Up |
| AJ006998.2 | 1.090865825 |
7.06×10−4 | Up |
| RP11-714M23.2 | −1.009911294 |
2.28×10−8 | Down |
 | Table III.Correlation between four lncRNA and
clinical features. |
Table III.
Correlation between four lncRNA and
clinical features.
|
| r (P-value) |
|---|
|
|
|
|---|
| lncRNA | Age | Gender | Stage | Grade | Race |
|---|
| LINC0109 | 0.2181856 | 0.01921931 | −0.135419 | −0.3284388 | −0.124623 |
|
|
(5.793×10−5)a | (0.7264) |
(0.01325)a |
(7.68×10−10)a |
(0.02273)a |
| RP11-486O12.2 | 0.0327529 | −0.1374603 | 0.1646208 | 0.1652273 | −0.08335334 |
|
| (0.5508) |
(0.01191)a |
(0.002545)a |
(0.002452)a | (0.1284) |
| RP11-863K10.7 | 0.02148461 | 0.1633948 | −0.07297289 | −0.244541 | −0.1271082 |
|
| (0.6956) |
(0.002743)a | (0.1834) |
(6.148×10−6)a |
(0.02014)a |
| RP11-273G15.2 | −0.07088287 | 0.02640504 | 0.06047835 | 0.07930304 | 0.06394619 |
|
| (0.1963) | (0.6306) | (0.2704) | (0.1481) | (0.2438) |
Co-expression of DEmRNAs and the
identified optimal diagnostic lncRNA
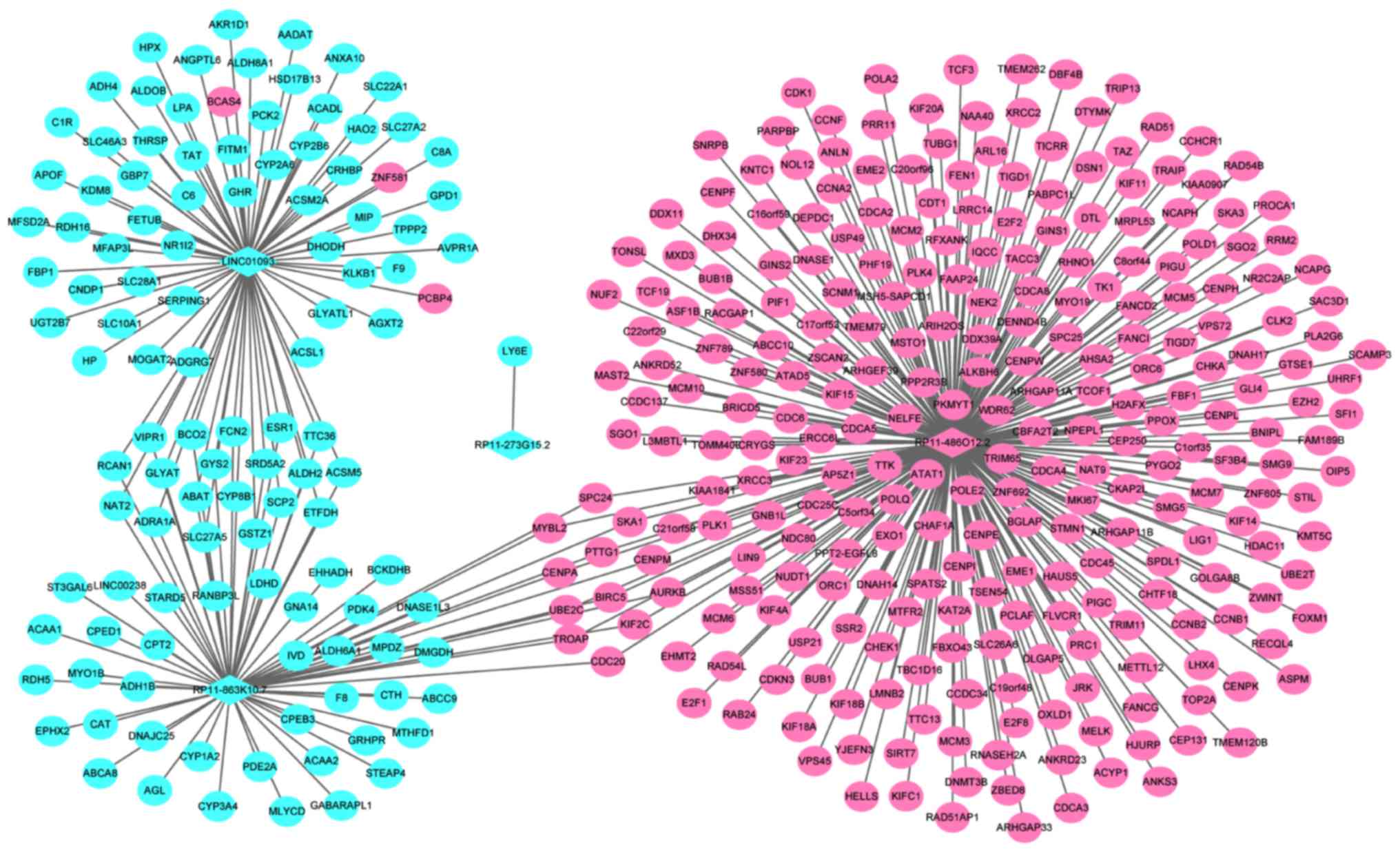
A total of four optimal DElncRNA biomarkers for HCC
were co-expressed with 388 DEmRNAs, accounting for 419
DElncRNA-DEmRNA co-expression pairs. RP11-486O12.2, RP11-863K10.7,
LINC01093 and RP11-273G15.2 were co-expressed with 273, 69, 76 and
1 DEmRNAs, respectively (Fig.
4).
Functional annotation
The aforementioned co-expression of 388 DEmRNAs with
four optimal DElncRNA biomarkers was used to perform the GO and
KEGG enrichment analysis. According to GO enrichment analysis,
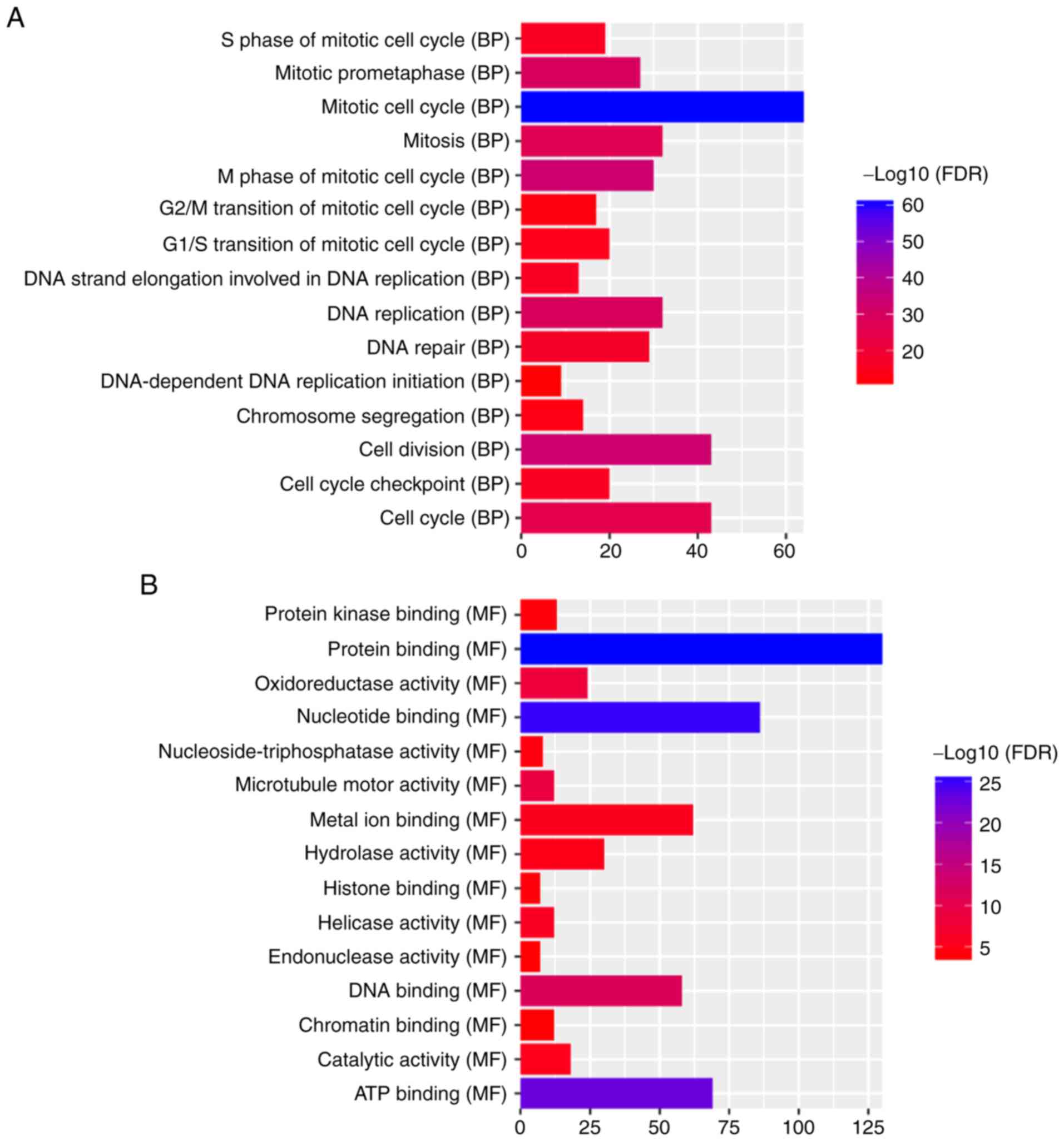
‘mitotic cell cycle’ (FDR=3.59×10−61), ‘nucleus’
(FDR=2.63×10−38) and ‘protein binding’
(FDR=1.62×10−26) were significantly enriched GO terms
associated with the 388 DEmRNAs (Fig.
5A-C). KEGG pathway enrichment analysis revealed that ‘retinol
metabolism’ (FDR=2.97E-07) and ‘PPAR signaling pathway’
(FDR=9.66E-08) were two significantly enriched pathways. A total of
10 DEmRNAs, including SLC27A5, ACSL1, SCP2, SLC27A2, ACADL, EHHADH,
ACAA1, CPT2, CYP8B1 and PCK2, were enriched in the ‘PPAR signaling
pathway’. A total of nine DEmRNAs, including RDH5, CYP2A6, CYP3A4,
RDH16, CYP1A2, CYP2B6, ADH4, UGT2B7 and ADH1B were enriched in the
‘retinol metabolism pathway’.
Discussion
HCC is the most common subtype of liver cancer, with
a high worldwide incidence and mortality rate (1). Taking into consideration the
importance of early diagnosis on survival, further examination for
the identification of accurate and specific biomarkers of HCC are
required. Increasing evidence suggest that lncRNAs have a vital
role in the progress of HCC (7–9).
However, studies on lncRNAs as predictive biomarkers and
therapeutic targets remain limited.
In the present study, 601 DElncRNAs and 2,920
DEmRNAs were identified between HCC and adjacent tissues, based on
the data downloaded from TCGA from 361 patients with HCC. A total
of four optimal diagnostic lncRNA biomarkers, including
RP11-486O12.2, RP11-863K10.7, LINC01093 and RP11-273G15.2, for HCC
were identified by feature selection and classification models.
The ROC results displayed the important diagnostic
value of these four lncRNA biomarkers, and their combination, for
HCC. The integrated analysis of the four diagnostic lncRNA
biomarkers from the GEO database indicated that the lncRNA
LINC01093 is downregulated in HCC (23). It has been reported that the
downregulation of lncRNA LINC01093 is associated with poor
prognosis of patients with HCC (24). Therefore, LINC01093 may be used as
a prognostic indicator for HCC. In the present study, not only
LINC01093 was indicated to be decreased in HCC tissues compared
with adjacent tissues, the diagnostic value of HCC was also
revealed with an AUC of 0.959, and a specificity and a sensitivity
of 92.0 and 93.4%, respectively.
To the best of our knowledge, with the exception of
LINC01093, the present study was the first to identify
RP11-486O12.2 as an upregulated DElncRNA, and RP11-863K10.7 and
RP11-273G15.2 as downregulated DElncRNAs in HCC. However, their
underlying biological function remains unclear. The relationship
between these four lncRNAs (LINC01093, RP11-486O12.2, RP11-863K10.7
and RP11-273G15.2) and tumor grade is unclear. The four lncRNAs
previously reported to be associated with HCC (GAS5, CARLo-5,
PCAT-1 and ZFAS1) are not included in Table II, with the following potentially
explanation: i) The data sets used may be different; ii) the
criteria for defining differential expression may be different;
iii) the algorithm used may be different.
Co-expression analysis of lncRNA-mRNA is the most
common approach for identifying potential target genes of lncRNAs
and to further investigate the biological function of lncRNAs in a
number of diseases. A co-expression network of DElncRNAs-DEmRNAs
was constructed using these four specific lncRNA biomarkers of HCC,
and the functional annotation of DEmRNAs co-expressed with four
lncRNAs was carried out. A total of six DEmRNAs, including SLC27A5,
ACSL1, SCP2, SLC27A2, ACADL and PCK2, co-expressed with LINC01093,
and four DEmRNAs, including EHHADH, ACAA1, CPT2 and CYP8B1
co-expressed with RP11-863K10.7, were significantly enriched in the
PPAR signaling pathway. In addition, three DEmRNAs, including
SLC27A5, SCP2 and CYP8B1, enriched in the PPAR pathway were
co-expressed with LINC01093 and RP11-863K10.7. PPARs is a
transcription factor activated by ligands, and is a member of the
nuclear hormone receptor superfamily (25). PPAR signaling pathways are involved
in the development and progression of multiple cancers (26–28).
Previous studies have confirmed that PPAR signaling pathway serves
a crucial role in regulating HCC tumorigenesis, and has the ability
to mediate HCC apoptosis depending on the regulation of the PI3K
pathway (29,30). ACSL1 has been reported to serve as
a classic target gene of PPARα (31,32).
lncRNAs HULC activates ACSL1 by upregulating the transcription
factor PPARα in HCC (33).
Therefore, the present study suggests that LINC01093 and
RP11-863K10.7 may be involved in the occurrence of HCC by
regulating the PPAR signaling pathway.
A total of five DEmRNAs, including CYP2A6, RDH16,
CYP2B6, ADH4 and UGT2B7, co-expressed with LINC01093 and four
DEmRNAs, including RDH5, CYP3A4, CYP1A2 and ADH1B co-expressed with
RP11-863K10.7 were significantly enriched in the ‘retinol
metabolism’ signaling pathway. Cytochrome P450 proteins (CYPs) are
a family enzymes localized in the endoplasmic reticulum and the
mitochondrial membrane with important roles in metabolism (34). The most significant drug
metabolizing CYPs in humans, include CYP2A6, CYP3A4 and CYP1A2,
which are responsible for the metabolism of >90% of medicines
and for the metabolic activation of procarcinogens (35,36).
CYP2A6 is an enzyme that has a crucial role in the metabolic
activation of a number of procarcinogens, including
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, 1,3-butadiene,
aflatoxin B1 and N-nitrosodiethylamine (37,38).
The upregulated expression of CYP2A6 is associated with an
increased risk of liver cancer in individuals with frequent
aflatoxin exposure (39). CYP2A6
is also a specific biomarker for colorectal cancer (40). CYP2A6 gene deletion has been
reported to be significantly lower in patients with lung cancer
compared with healthy individuals (41). It was therefore hypothesized in the
present study that LINC01093 and RP11-863K10.7 may serve an
important role in HCC by regulating retinol metabolism. Cui et
al (42) is a more
comprehensive study, and their study predicted lncRNAs associated
with the prognosis and metastasis of patients with HCC, and the
lncRNAs were validated using cell lines in vitro. HCC
samples are currently being collected to validate the expression of
the identified key lncRNAs in subsequent research with larger
sample size. Then, the biological significances of key lncRNAs will
be investigated in model systems or cell lines.
In conclusion, the present study has indicated that
the lncRNA expression profiles are altered in HCC compared with
normal samples. A total of four DElncRNAs were identified as
potential biomarkers of HCC. Functional annotation of DEmRNAs
co-expression with the four lncRNA biomarkers of HCC provided novel
insight for examining the pathogenesis of HCC. There are
limitations of the current study. These four lncRNAs (LINC01093,
RP11-486O12.2, RP11-863K10.7 and RP11-273G15.2) were not associated
with survival (data not shown). Moreover, the expression of
specific lncRNAs in HCC induced by different factors, including
HBV, was not investigated. Finally, identifying diagnostic
biomarkers for HCC is currently in a pilot study, and further
experiments are required to determine the biological significance
of important lncRNAs in HCC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HSu and GL designed the project. HSh, XW, BW, QQ and
HG analyzed and interpreted the data. HSu and GL were major
contributors in drafting the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
lncRNAs
|
long noncoding RNAs
|
|
DEGs
|
differentially expressed genes
|
|
GO
|
Gene Ontology
|
|
KEGG
|
Kyoto Encyclopedia of Genes and
Genomes
|
|
HCC
|
hepatocellular carcinoma
|
References
|
1
|
Ma H, Yuan L, Li W, Xu K and Yang L: The
lncRNA H19/miR-193a-3p axis modifies the radio-resistance and
chemotherapeutic tolerance of hepatocellular carcinoma cells by
targeting PSEN1. J Cell Biochem. 119:8325–8335. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Merion RM: Current status and future of
liver transplantation. Semin Liver Dis. 30:411–421. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen G, Li X, He G, Yu Z, Luo J, He J and
Huang Z: Low expression of GNAI3 predicts poor prognosis in
patients with HCC. Int J Clin Exp Med. 8:21482–21486.
2015.PubMed/NCBI
|
|
4
|
Pelus LM and Fukuda S: Peripheral blood
stem cell mobilization: The CXCR2 ligand GRObeta rapidly mobilizes
hematopoietic stem cells with enhanced engraftment properties. Exp
Hematol. 34:1010–1020. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Song X, Wang Z, Jin Y, Wang Y and Duan W:
Loss of miR-532-5p in vitro promotes cell proliferation and
metastasis by influencing CXCL2 expression in HCC. Am J Transl Res.
7:2254–2261. 2015.PubMed/NCBI
|
|
6
|
Yang X, Xie X, Xiao YF, Xie R, Hu CJ, Tang
B, Li BS and Yang SM: The emergence of long non-coding RNAs in the
tumorigenesis of hepatocellular carcinoma. Cancer Lett.
360:119–124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qu Z, Yuan CH, Yin CQ, Guan Q, Chen H and
Wang FB: Meta-analysis of the prognostic value of abnormally
expressed lncRNAs in hepatocellular carcinoma. Onco Targets Ther.
9:5143–5152. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun J, Wei X and Xu L: Upregulation of
lncRNA Sox2ot indicates a poor prognosis for patients with
hepatocellular carcinoma and promotes cell invasion. Oncol Lett.
16:1189–1195. 2018.PubMed/NCBI
|
|
9
|
Chang L, Li C, Lan T, Wu L, Yuan Y, Liu Q
and Liu Z: Decreased expression of long non-coding RNA GAS5
indicates a poor prognosis and promotes cell proliferation and
invasion in hepatocellular carcinoma by regulating vimentin. Mol
Med Rep. 13:1541–1550. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang F, Xie C, Zhao W, Deng Z, Yang H and
Fang Q: Long non-coding RNA CARLo-5 expression is associated with
disease progression and predicts outcome in hepatocellular
carcinoma patients. Clin Exp Med. 17:33–43. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao J, Greene CM, Gray SG and Lawless MW:
Long noncoding RNAs in liver cancer: What we know in, 2014. Expert
Opin Ther Targets. 18:1207–1218. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu W, Qiao Y, Tang X, Ma L, Wang Y, Zhang
X, Weng W, Pan Q, Yu Y, Sun F and Wang J: Tumor suppressor long
non-coding RNA, MT1DP is negatively regulated by YAP and Runx2 to
inhibit FoxA1 in liver cancer cells. Cell Signal. 26:2961–2968.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yan TH, Yang H, Jiang JH, Lu SW, Peng CX,
Que HX, Lu WL and Mao JF: Prognostic significance of long
non-coding RNA PCAT-1 expression in human hepatocellular carcinoma.
Int J Clin Exp Pathol. 8:4126–4131. 2015.PubMed/NCBI
|
|
14
|
Li T, Xie J, Shen C, Cheng D, Shi Y, Wu Z,
Deng X, Chen H, Shen B, Peng C, et al: Amplification of long
noncoding RNA ZFAS1 promotes metastasis in hepatocellular
carcinoma. Cancer Res. 75:3181–3191. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sonohara F, Inokawa Y, Hayashi M, Yamada
S, Sugimoto H, Fujii T, Kodera Y and Nomoto S: Prognostic value of
long non-coding RNA HULC and MALAT1 following the curative
resection of hepatocellular carcinoma. Sci Rep. 7:161422017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Love MI, Huber W and Anders S: Moderated
estimation of fold change and dispersion for RNA-seq data with
DESeq2. Genome Biol. 15:5502014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Benjamini Y and Hochberg Y: Controlling
the false discovery rate: A practical and poweful approach to
multiple testing. J R Stat Soc: Ser B (Methodol). 57:289–300.
1995.
|
|
18
|
Ashburner M, Ball CA, Blake JA, Botstein
D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT,
et al: Gene ontology: Tool for the unification of biology. The gene
ontology consortium. Nat Genet. 25:25–29. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
The Gene Ontology Consortium: The gene
ontology resource: 20 years and still GOing strong. Nucleic Acids
Res 47D. D330–D338. 2019.
|
|
20
|
Kanehisa M, Sato Y, Furumichi M, Morishima
K and Tanabe M: New approach for understanding genome variations in
KEGG. Nucleic Acids Res 47D. D590–D595. 2019. View Article : Google Scholar
|
|
21
|
Kanehisa M, Furumichi M, Tanabe M, Sato Y
and Morishima K: KEGG: New perspectives on genomes, pathways,
diseases and drugs. Nucleic Acids Res 45D. D353–D361. 2017.
View Article : Google Scholar
|
|
22
|
Kanehisa M and Goto S: KEGG: Kyoto
encyclopedia of genes and genomes. Nucleic Acids Res. 28:27–30.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jin B, Wang W, Du G, Huang GZ, Han LT,
Tang ZY, Fan DG, Li J and Zhang SZ: Identifying hub genes and
dysregulated pathways in hepatocellular carcinoma. Eur Rev Med
Pharmacol Sci. 19:592–601. 2015.PubMed/NCBI
|
|
24
|
Dai M, Chen S, Wei X, Zhu X, Lan F, Dai S
and Qin X: Diagnosis, prognosis and bioinformatics analysis of
lncRNAs in hepatocellular carcinoma. Oncotarget. 8:95799–95809.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Antonosante A, d'Angelo M, Castelli V,
Catanesi M, Iannotta D, Giordano A, Ippoliti R, Benedetti E and
Cimini A: The involvement of PPARs in the peculiar energetic
metabolism of tumor cells. Int J Mol Sci. 19(pii): E19072018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ricci M, Miola M, Multari C, Borroni E,
Canuto RA, Congiusta N, Vernè E, Follenzi A and Muzio G: PPARs are
mediators of anti-cancer properties of superparamagnetic iron oxide
nanoparticles (SPIONs) functionalized with conjugated linoleic
acid. Chem Biol Interact. 292:9–14. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sanchez DJ, Steger DJ, Skuli N, Bansal A
and Simon MC: PPARγ is dispensable for clear cell renal cell
carcinoma progression. Mol Metab. 14:139–149. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li Q, Peng YS, Chen PJ, Wang ML, Cao C,
Xiong H, Zhang J, Chen MH, Peng XB and Zeng K: Peroxisome
proliferator-activated receptor-γ agonist-mediated inhibition of
cell growth is independent of apoptosis in human epidermoid
carcinoma A431 cells. Oncol Lett. 15:6578–6584. 2018.PubMed/NCBI
|
|
29
|
Xiao YB, Cai SH, Liu LL, Yang X and Yun
JP: Decreased expression of peroxisome proliferator-activated
receptor alpha indicates unfavorable outcomes in hepatocellular
carcinoma. Cancer Manag Res. 10:1781–1789. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu Z, Wang Y, Dou C, Sun L, Li Q, Wang L,
Xu Q, Yang W, Liu Q and Tu K: MicroRNA-1468 promotes tumor
progression by activating PPAR-γ-mediated AKT signaling in human
hepatocellular carcinoma. J Exp Clin Cancer Res. 37:492018.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Phillips CM, Goumidi L, Bertrais S, Field
MR, Cupples LA, Ordovas JM, Defoort C, Lovegrove JA, Drevon CA,
Gibney MJ, et al: Gene-nutrient interactions with dietary fat
modulate the association between genetic variation of the ACSL1
gene and metabolic syndrome. J Lipid Res. 51:1793–1800. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ong KT, Mashek MT, Bu SY, Greenberg AS and
Mashek DG: Adipose triglyceride lipase is a major hepatic lipase
that regulates triacylglycerol turnover and fatty acid signaling
and partitioning. Hepatology. 53:116–126. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cui M, Xiao Z, Wang Y, Zheng M, Song T,
Cai X, Sun B, Ye L and Zhang X: Long noncoding RNA HULC modulates
abnormal lipid metabolism in hepatoma cells through an
miR-9-mediated RXRA signaling pathway. Cancer Res. 75:846–857.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Korobkova EA: Effect of natural
polyphenols on CYP metabolism: Implications for diseases. Chem Res
Toxicol. 28:1359–1390. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zanger UM and Schwab M: Cytochrome P450
enzymes in drug metabolism: Regulation of gene expression, enzyme
activities, and impact of genetic variation. Pharmacol Ther.
138:103–141. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhou J, Wen Q, Li SF, Zhang YF, Gao N,
Tian X, Fang Y, Gao J, Cui MZ, He XP, et al: Significant change of
cytochrome P450s activities in patients with hepatocellular
carcinoma. Oncotarget. 7:50612–50623. 2016.PubMed/NCBI
|
|
37
|
Gonzalez FJ and Gelboin HV: Role of human
cytochrome P-450s in risk assessment and susceptibility to
environmentally based disease. J Toxicol Environ Health.
40:289–308. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Messina ES, Tyndale RF and Sellers EM: A
major role for CYP2A6 in nicotine C-oxidation by human liver
microsomes. J Pharmacol Exp Ther. 282:1608–1614. 1997.PubMed/NCBI
|
|
39
|
Gullstén H, Agúndez JA, Benítez J, Läärä
E, Ladero JM, Díaz-Rubio M, Fernandez-Salguero P, Gonzalez F,
Rautio A, Pelkonen O and Raunio H: CYP2A6 gene polymorphism and
risk of liver cancer and cirrhosis. Pharmacogenetics. 7:247–250.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nowell S, Sweeney C, Hammons G, Kadlubar
FF and Lang NP: CYP2A6 activity determined by caffeine phenotyping:
Association with colorectal cancer risk. Cancer Epidemiol
Biomarkers Prev. 11:377–383. 2002.PubMed/NCBI
|
|
41
|
Kamataki T, Nunoya K, Sakai Y, Kushida H
and Fujita K: Genetic polymorphism of CYP2A6 in relation to cancer.
Mutat Res. 428:125–130. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cui H, Zhang Y, Zhang Q, Chen W, Zhao H
and Liang J: A comprehensive genome-wide analysis of long noncoding
RNA expression profile in hepatocellular carcinoma. Cancer Med.
6:2932–2941. 2017. View Article : Google Scholar : PubMed/NCBI
|