Introduction
Cardiac arrest (CA) is a major cause of mortality
worldwide. There are approximately 544,000 cases of CA each year in
China (1). Although the
preliminary rate of return of spontaneous circulation (ROSC) is
40–50%, the majority of CA survivors succumb to this event before
discharge, resulting in less than 10% in-hospital survival rate
(2). High in-hospital mortality in
the early stages after ROSC is most often associated with
multisystem organ failure due to myocardial dysfunction (3,4).
Although a great amount of effort has been made to improve PRMD, no
treatment, including drugs that have demonstrated translational
efficacy in animal models, have exhibited clinical efficacy in
humans to date.
The pathogenesis of PRMD is multifactorial and
complex. To date, the mechanism underlying its pathogenesis remains
to be deciphered. In rodent CA models, after ROSC, there were
significant reductions in the activity of mitochondrial complex I,
a marked production of ROS, mitochondrial protein oxidation, and
tyrosine nitrosylation (5).
Several studies have suggested that nitric oxide (NO)-derived
reactive nitrogen species (RNS) contributes to pathologic tissue
injury by nitrative protein modification (nitrative stress). This
may serve as a possible target for therapeutic intervention
(6,7). NO is produced from the conversion of
L-arginine by nitric oxide synthase (NOS). Previous studies have
demonstrated that a high pathological concentration of NO, produced
by inducible NOS (iNOS), results in nitrative stress and tissue
injury (8). NO is not toxic and
does not cause significant tissue injury even at high
concentrations; however, NO and superoxide can combine in a
diffusion-limited reaction to generate peroxynitrite
(ONOO−), resulting in nitrative molecules that are toxic
(9). Studies have demonstrated
that ONOO− plays a critical role in myocardial
ischemia/reperfusion injury (10).
Hence, drugs that suppress ONOO− formation or antagonize
its activity may protect the heart from reperfusion injury
(11). However, it is not clear
whether nitrative stress is involved in PRMD or whether inhibitors
of nitrative stress could alleviate PRMD.
Resveratrol is a natural polyphenol phytoalexin that
is present in numerous plant species and is thought to be
responsible for the ‘French paradox’. Several studies have
demonstrated that resveratrol has beneficial effects in several
cardiovascular diseases. These benefits may be due to its versatile
biological effects such as attenuation of oxidative and nitrative
stress, which are involved in the pathophysiologic mechanisms of
PRMD (12,13). In a rat model of local myocardial
ischaemia-reperfusion, resveratrol preconditioning inhibited
excessive NO expression that resulted after reperfusion (14). However, to date, there are limited
studies regarding the use of resveratrol in post-resuscitation
models.
Preconditioning and postconditioning with
resveratrol have revealed beneficial effects in some local ischemia
studies (15,16). Resveratrol had a dose-dependent
effect in the previous studies, where low doses (5–20 µM) protected
cells while higher doses (10–40 mM) induced apoptosis (17,18).
However, these results have not been ascertained in CA models,
where the optimal dose is unknown.
A rat CA model was used to determine whether
resveratrol could attenuate PRMD via its inhibitory effect on
myocardial nitrative stress.
Materials and methods
Ethical statement
The present study was performed in strict accordance
to the recommendations by the Guide for the Care and Use of
Laboratory Animals, National Institutes of Health. The
Institutional Animal Care and Use Committee of West China Hospital
(Sichuan University; approval no 2016045A) approved the study
protocol. All surgeries were performed under sodium pentobarbital
anaesthesia and every effort was made to minimize suffering.
Animals and preparatory
procedures
Specific pathogen-free Sprague-Dawley (SD) male rats
12 weeks of age and weighing 350–400 g were used for the study. The
animals were fasted on the night before the procedure but were
allowed free access to water. The animals were prepared as
previously described (19,20). Briefly, rats were anaesthetized
with intra-peritoneal injections of 45 mg/kg 1.5% pentobarbital,
placed in a supine position and immobilized. They were then
intubated with a standard endotracheal tube and anaesthesia was
maintained with 1.5% pentobarbital at 15 mg/kg/h intraperitoneally.
The rats were mechanically ventilated using a volume-controlled
ventilator (HX-100E; Chengdu TME Technology & Market Co., Ltd.)
with a tidal volume of 6 ml/kg and a fraction of inspired oxygen
(FiO2) of 1.0 (21).
Electrocardiograms (ECGs) were monitored with the aid of
subcutaneous needles throughout the procedure. A 24G catheter was
introduced into the right femoral artery to measure arterial blood
pressure, while drug infusion was performed through a 20G catheter
inserted into the right femoral vein. A saline-filled PE-50
catheter (BD Biosciences) was advanced from the right carotid
artery into the left ventricle cavity for measurement of left
intraventricular pressure. The ECG, arterial blood pressure and
left intraventricular pressure were monitored continuously via a
4-channel physiological recorder (BL-420F Data Acquisition &
Analysis System; Chengdu TME Technology Co., Ltd.).
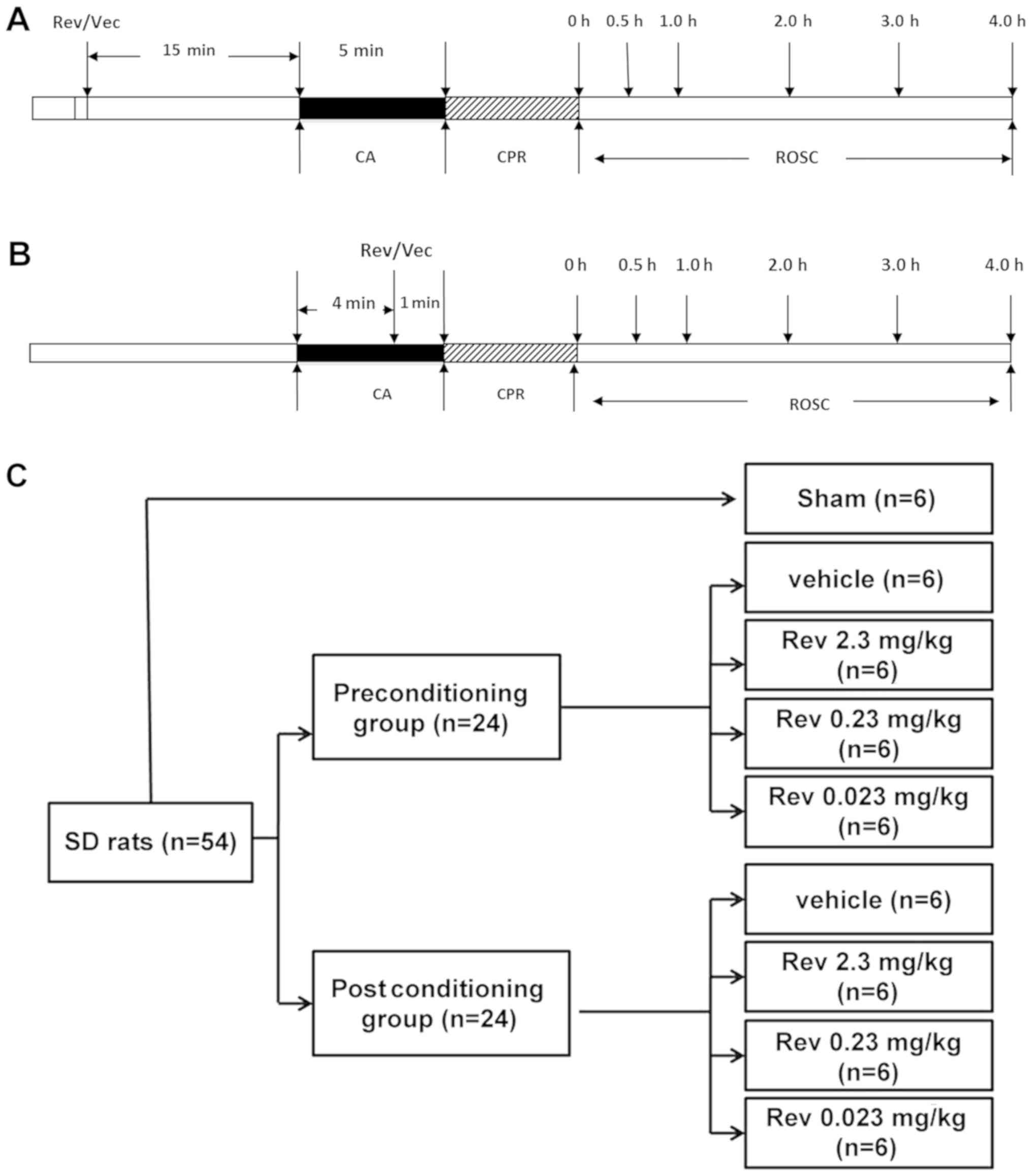
Experimental protocol and
grouping
A pacing electrode (model 3830; Medtronic, Inc.)
with two 1-mm ring electrodes and an inter-electrode distance of 5
mm was inserted orally into the oesophagus to a depth of 4 cm.
After a 30-min stabilization period, CA was induced via an
oesophageal electrode connected to a cardiac electrophysiological
stimulation apparatus (BL-420F Data Acquisition & Analysis
System). The current had an amplitude of 6 mA, with a frequency of
50 Hz, and a wave width of 10 msec (19).
CA was defined as an ECG having waveforms of
ventricular fibrillation, pulseless electricity activity, or
asystole with a rapid decline in systolic pressure to below 25
mmHg. Artificial ventilation was paused while inducing CA.
Cardiopulmonary resuscitation was initiated after 5 min of CA. A
mechanical chest compressor (College of Electrical Engineering,
Sichuan University) with artificial ventilation of 80 breaths/min,
tidal volume of 6 ml/kg, and FiO2 of 1.00 was used to
deliver 200 chest compressions/min. Epinephrine was administered
(20 µg/kg) immediately and one dose every 5 min after the
compressions. After the first 5 min, a 2J electrical shock was
delivered if ventricular fibrillation was present. Subsequently, an
electrical shock was delivered every 1 min if necessary, however,
the number of shocks were no more than 3 times in the whole
experiment. ROSC was characterized by spontaneous cardiac rhythms
with a mean arterial pressure (MAP) ≥60 mmHg and persisting for
more than 10 min. Rats were included for further study if ROSC
persisted for at least 30 min. Left ventricular function
(+dP/dtmax and -dP/dtmin) was recorded at
0.5, 1, 2, 3 and 4 h after ROSC, after which the animals were
euthanized and their hearts were rapidly removed for analyses.
Post-resuscitation care was similar for all rats in the different
groups. Sham control animals were similarly treated except they
received ventilation without CA induction (Fig. 1A and B). In the experiment, all
rats were sacrificed by cervical dislocation under anesthetic
conditions maintained by 1.5% pentobarbital at 15 mg/kg/h
intraperitoneally. All experiments were performed at room
temperature.
Animals which met the inclusion criteria as stated
before were randomly assigned to one of two groups: A
preconditioning or postconditioning group, based on different
intervention times. Prior to administration, resveratrol (Sigma
Aldrich; Merck KGaA) was dissolved in dimethyl sulfoxide (DMSO) and
then in sterile water containing 0.01% DMSO. Resveratrol or vehicle
was administered by bolus injection 15 min before CA to the
preconditioning group and 1 min before CPR to the postconditioning
group. Each group was then subdivided into four groups (6
rats/group) and administered different doses of resveratrol: i)
Vehicle (DMSO); ii) 2.3 mg/kg resveratrol; iii) 0.23 mg/kg
resveratrol, and iv) 0.023 mg/kg resveratrol (Fig. 1C). Resveratrol doses that were
administered were based on data from several studies on the aqueous
solubility of resveratrol (12,22).
The 6 rats in the sham group underwent surgical procedures without
induced cardiac arrest and showed no differences between
thepreconditioning and postconditioning groups.
Based on the preliminary results, the resveratrol
group that exhibited the greatest significant myocardial protective
effect was selected and it was investigated whether wortmannin
(Sigma Aldrich; Merck KGaA) inhibited its protective effect. This
part of the study comprised of four groups (6 rats/group): i) Sham;
ii) vehicle; iii) resveratrol; iv) resveratrol + 15 µg/kg
wortmannin (Rev+Wom). Resveratrol and wortmannin were administered
simultaneously to the 4-h group.
Histological examination of heart
sections
Left ventricular tissues were fixed in 4% buffered
formalin for 20 min at room temperature. After embedding in
paraffin, the tissues were sliced into 4-µm sections and stained
for nitrotyrosine by the following protocol. The sections were
deparaffinized and hydrated using decreasing gradients of ethanol
followed by antigen retrieval. The sections were then incubated in
0.3% H2O2 in phosphate-buffered saline (PBS)
to block endogenous peroxidase activity. The sections were then
incubated with anti-nitrotyrosine (1:1,000; 189542-50; Cayman
Chemical) antibody at 4°C overnight in a moist chamber. Goat
anti-mouse Biotinylated secondary antibodies (1:1,000; 10006617;
Cayman Chemical) were then added to the tissues according to the
manufacturer's instructions and then stained with DAPI (Sigma
Aldrich; Merck KGaA) at room temperature for 3–5 min. Subsequently,
the sections were dehydrated in ethanol, cleared in xylene and
mounted for viewing. Stained sections were visualized and images
were acquired using an IX-71 fluorescent microscope (Olympus
Corporation). Histological evaluation was performed in a blinded
manner.
Expression levels of iNOS, Akt, and
phosphorylated-Akt (p-Akt)
Left ventricle tissue samples were lysed using
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology) containing a Protease Inhibitor Cocktail (Promega
Corporation) for 30 min on ice, and after sonication were
centrifuged for 10 min at 4°C 12,000 × g. Equal amounts of protein
(30 µg protein/lane) were separated by electrophoresis on a 10%
sodium dodecyl sulphate polyacrylamide gel (SDS-PAGE) and
transferred onto polyvinylidene difluoride membranes (EMD
Millipore). After blocking with 5% skim milk in Tris-buffered
saline at room temperature (18–28°C) for 1 h, the membranes were
incubated with antibodies (1:1,000) against iNOS (ab49999; Abcam),
Akt (9272), or p-Akt (4051; both Cell Signaling Technology, Inc.)
at 4°C overnight. The membranes were then washed with
phosphate-buffered saline with Tween-20 and incubated with
horseradish peroxidase-conjugated IgG antibody (1:1,000; 7076; Cell
Signaling Technology, Inc.) at 37°C for 1 h. Blots were developed
using an enhanced chemiluminescence detection kit (Pierce
Biotechnology; Thermo Fisher Scientific, Inc.). The proteins were
visualized using ChemiDoc XRS and band densities were analysed
using Quantity One software 4.6 (both from Bio-Rad Laboratories,
Inc.).
Quantitation of myocardial
nitrotyrosine levels by enzyme-linked immunosorbent assay
(ELISA)
Cardiac nitrotyrosine, a determinant for in
vivo RNS formation, was measured using ELISA as previously
reported (18). In brief,
nitrotyrosine tissue levels were measured using a Nitrotyrosine
ELISA kit (Chemiluminescence Detection kit; EMD Millipore) in
accordance with the manufacturer's instructions and expressed as
nmol/g protein.
Immunofluorescence measurements
Paraformaldehyde-fixed myocardial tissues were cut
into semi-thin sections, of 4–5 µm thickness, and stained with a
primary antibody (1:1,000) against nitrotyrosine (189542; Cayman
Chemical) at room temperature for 1 h in a wet box and then at 4°C
overnight, followed by incubation with the goat anti-mouse
secondary antibody (1:1,000; ZK-9600; OriGene Technologies, Inc.)
at 37°C for 30 min and then counterstained with DAPI for 10 min at
37°C (Sigma Aldrich; Merck KGaA).
Statistical analysis
All data were expressed as the mean ± standard
deviation. Statistical significance was determined using one-way
analysis of variance (ANOVA) followed by the Student-Newman-Keuls'
method using SPSS 19.0 (IBM Corp.) and GraphPad Prism 5.0 software
(GraphPad Software, Inc.). P<0.05 was considered statistically
significant (two-tailed).
Results
Animals
A total of 54 rats were used in this study. All rats
attained ROSC and were sustained for over 30 min.
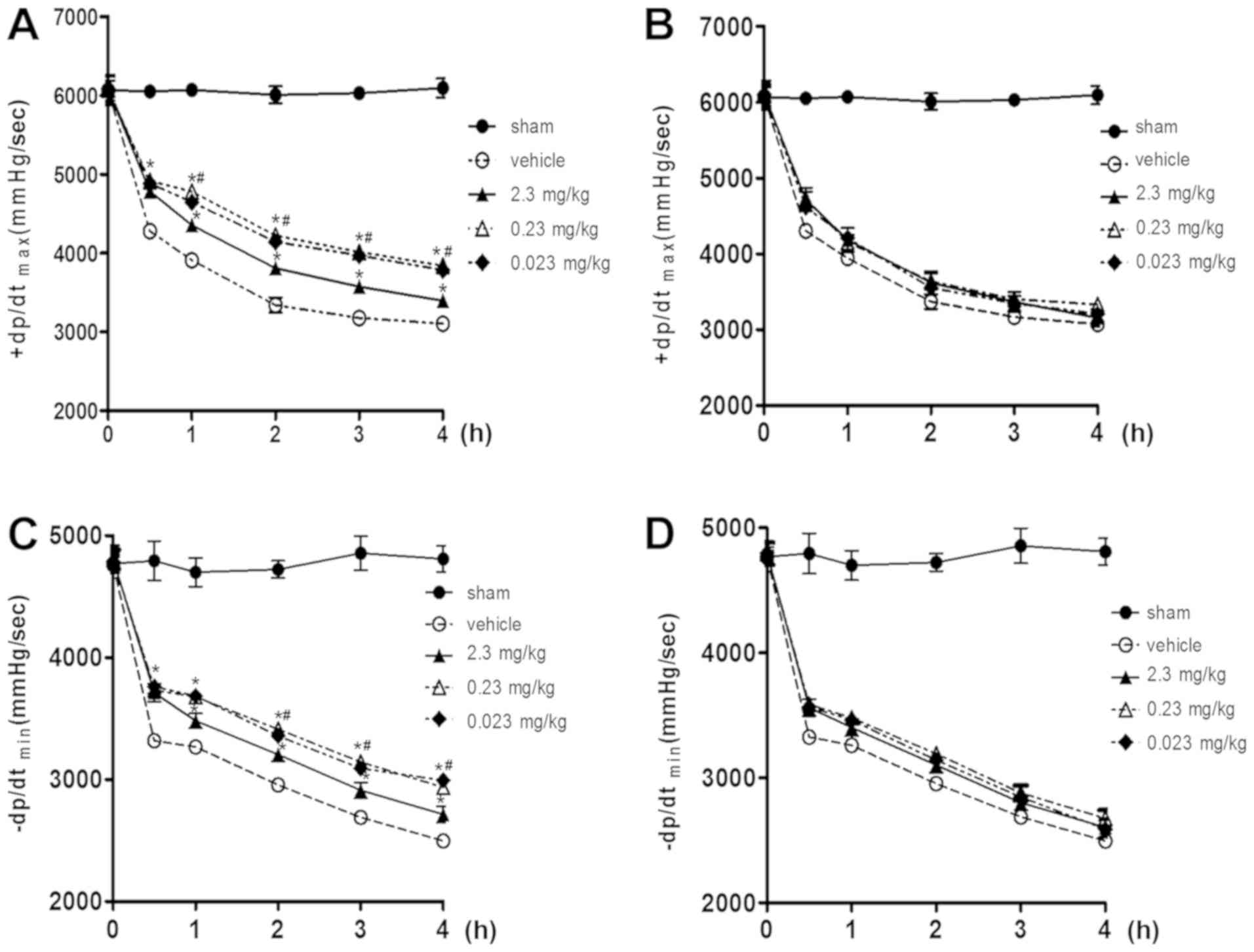
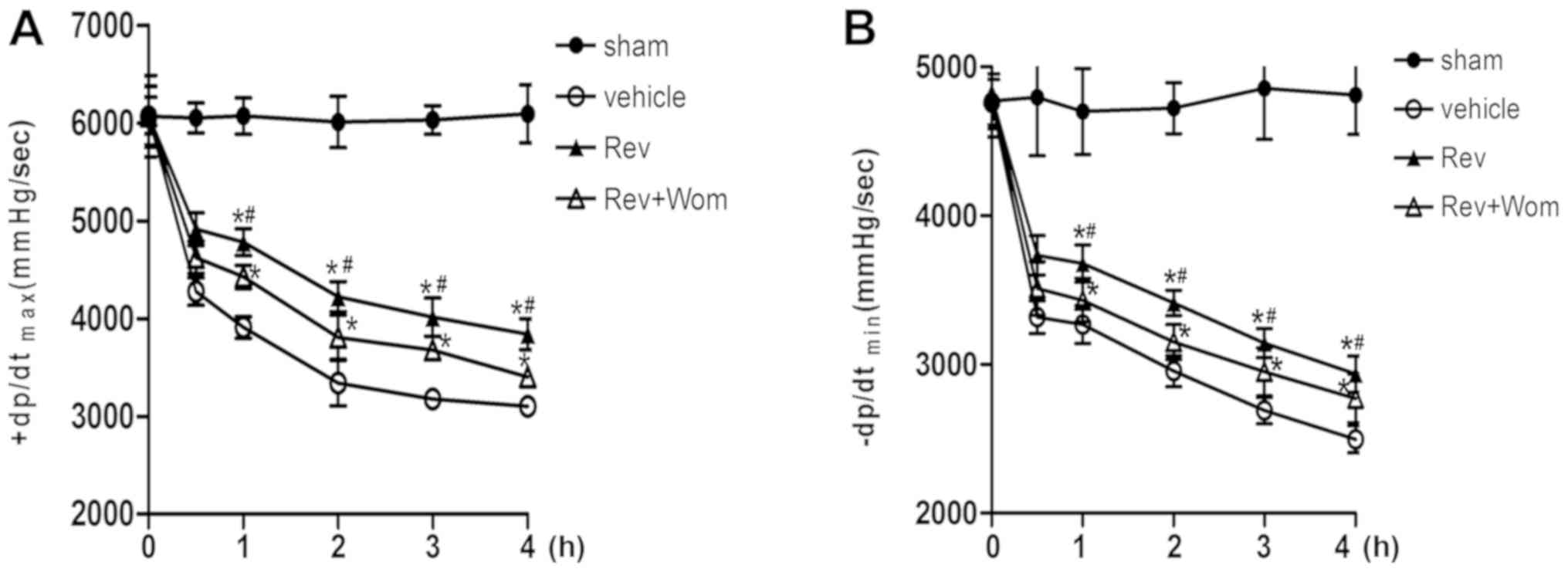
Left ventricular function
The left ventricular variables (+dp/dtmax
and -dp/dtmin) for all post-resuscitation animals were
reduced after ROSC until the end of the procedure. From 0.5 to 4 h
after ROSC, resveratrol preconditioning improved left ventricular
function significantly compared to vehicle (P<0.05). The effect
of resveratrol on left ventricular function was significant at
doses between 0.23 and 0.023 mg/kg, but not at 2.3 mg/kg
(P<0.05; Fig. 2A and C). During
the whole procedure, resveratrol improved +dp/dtmax and
-dp/dtmin in the postconditioning group compared to the
vehicle, however, the observed differences were not significant
(P>0.05; Fig. 2B and D).
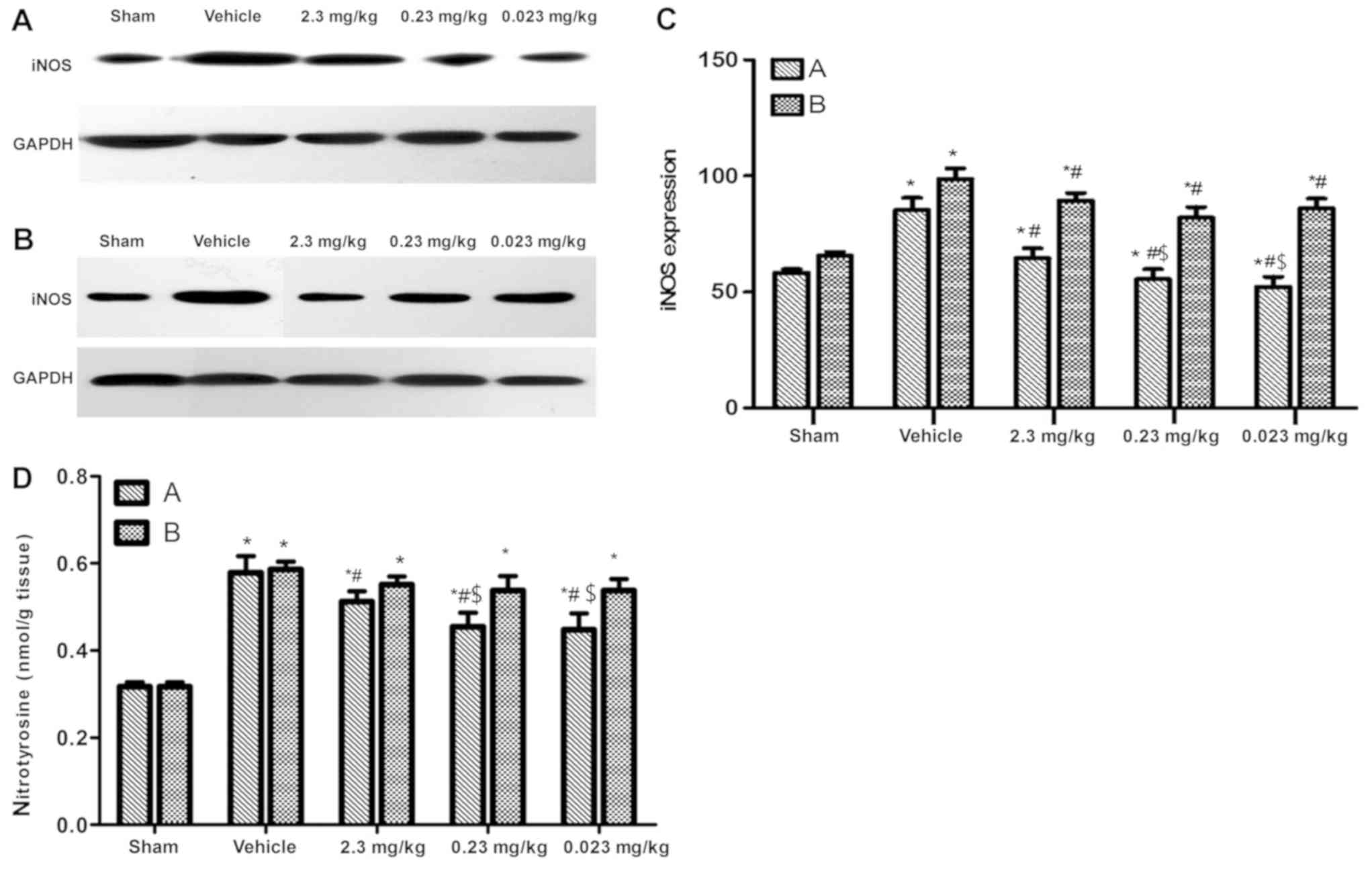
iNOS expression in heart tissue
Then, 4 h after ROSC, iNOS expression in the vehicle
groups was significantly increased compared to the sham groups
(P<0.05). However, the iNOS levels in the preconditioning groups
were significantly lower compared to the vehicle group (P<0.05),
while the 0.23 and 0.023 mg/kg administered groups were not
significantly different compared to the sham group (P>0.05). The
iNOS levels in the postconditioning groups were lower compared to
the vehicle group (P<0.05), but were higher compared to the sham
group (P<0.05; Fig. 3A-C).
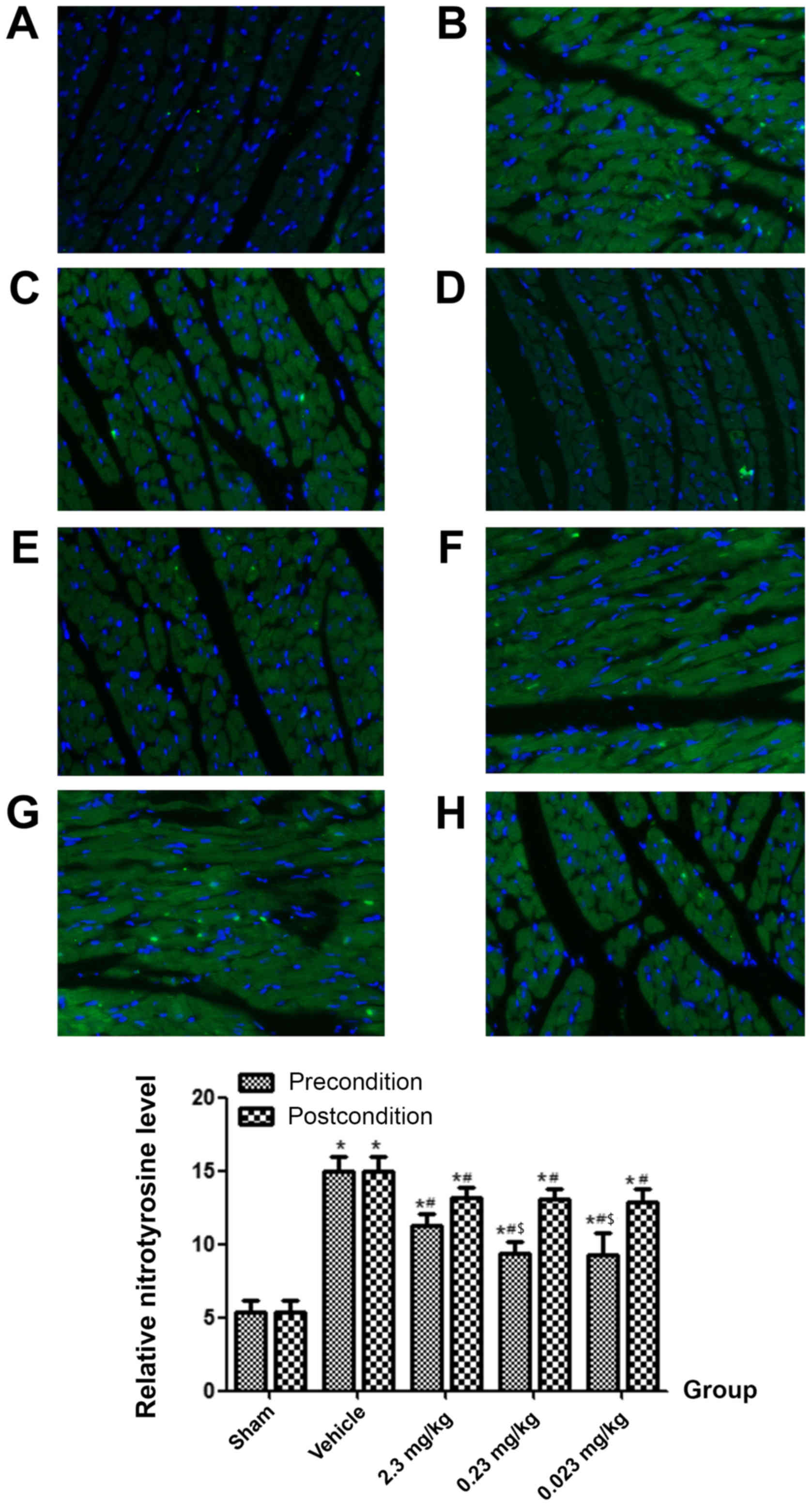
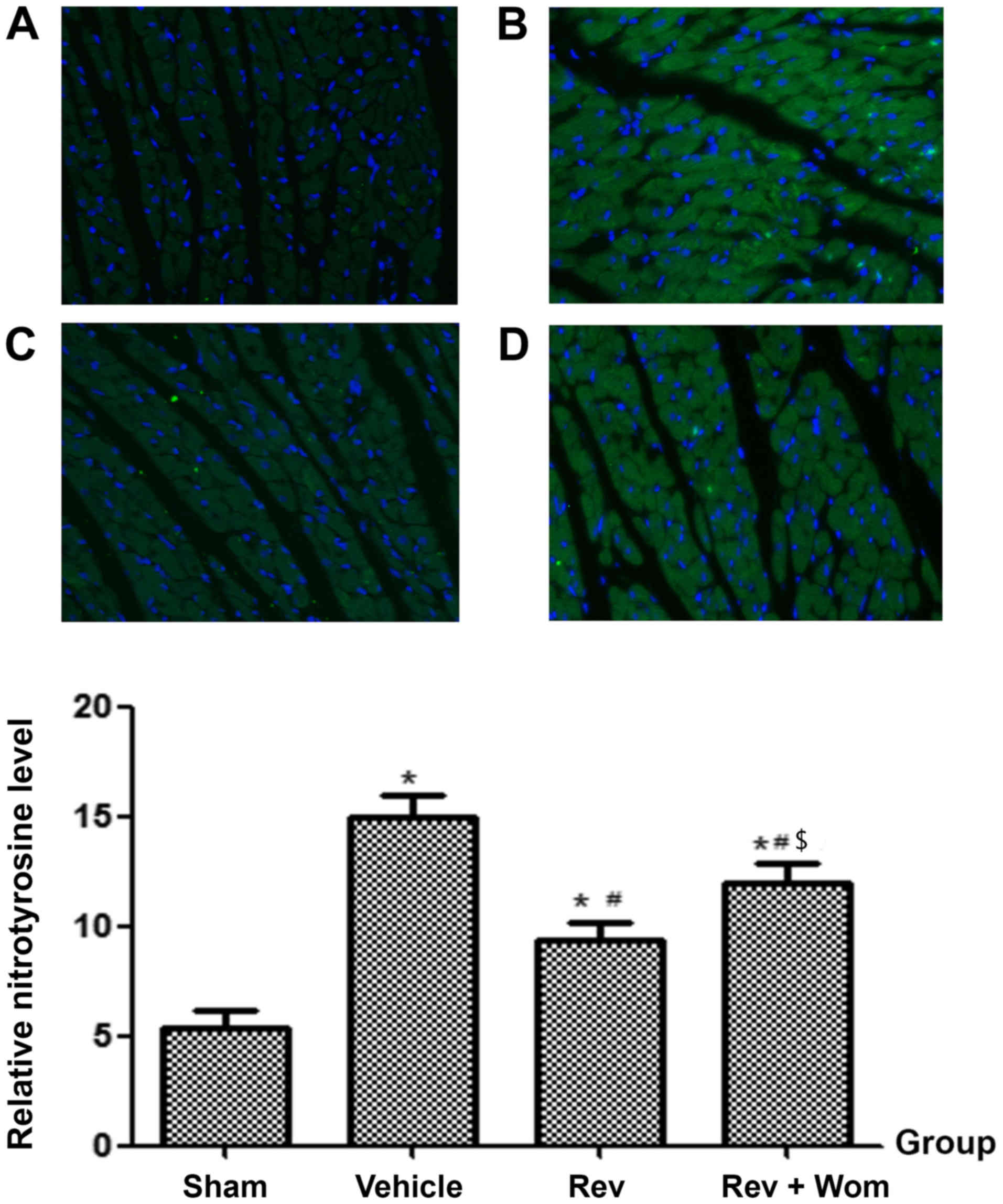
Nitrotyrosine expression in heart
tissue
Four hours after ROSC, the expression of
nitrotyrosine in the vehicle group was significantly higher
compared to the sham group (P<0.05). Nitrotyrosine levels in the
preconditioning groups were significantly lower compared to the
vehicle group (P<0.05), especially in the 0.23 and 0.023 mg/kg
administered groups. However, resveratrol postconditioning did not
significantly decrease the nitrotyrosine expression levels compared
to the vehicle (P>0.05; Figs.
3D and 4).
Role of the PI3K/Akt signalling
pathway in the myocardial protective effect of resveratrol;
wortmannin prevents the protective effects of resveratrol in
PRMD
From 0.5 h after ROSC until the end of the
procedure, the left ventricular function variables
(+dp/dtmax and -dp/dtmin) in the resveratrol
(0.23 mg/kg)+wortmannin group were lower compared to the
resveratrol (0.23 mg/kg) group (P<0.05), but higher compared
with the vehicle group (P<0.05). Wortmannin partially inhibited
the beneficial effects of resveratrol on left ventricular function
(Fig. 5).
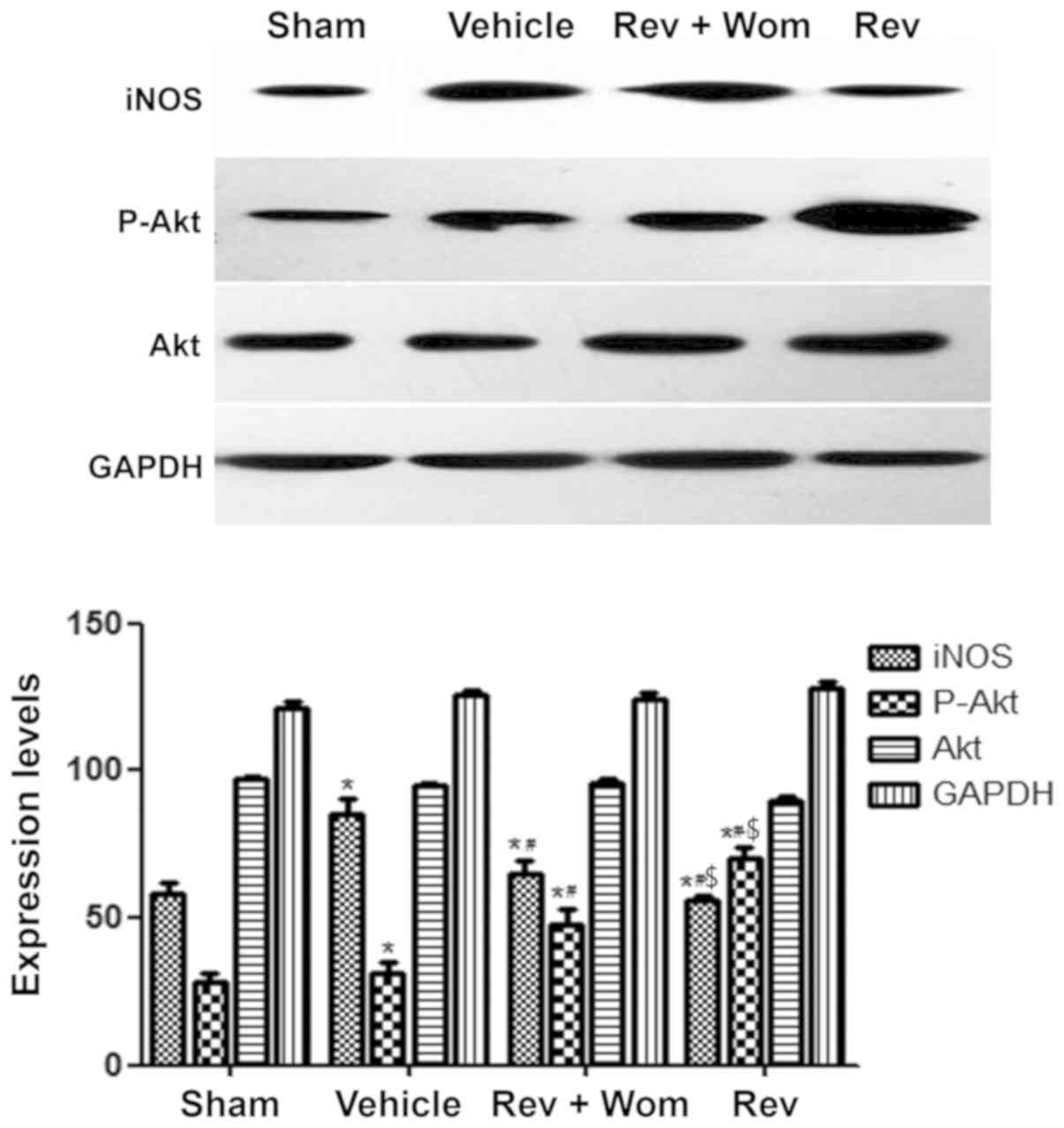
Preliminary results revealed that resveratrol
preconditioning ameliorated myocardial nitrative stress after ROSC.
Administration of resveratrol+wortmannin had higher iNOS expression
levels compared to resveratrol alone (P<0.05); however, iNOS
expression levels in the resveratrol+wortmannin and resveratrol
groups were lower compared to the vehicle group (P<0.05;
Figs. 6 and 7). These results indicated that
wortmannin partially reversed the inhibitory effect of resveratrol
on nitrative stress. p-Akt levels in the resveratrol group were
higher compared to the sham group and the vehicle group, while
wortmannin decreased p-Akt levels. These differences were
statistically significant (P<0.05). No significant differences
in Akt levels were observed for the four groups (Fig. 6).
Discussion
In the present study, resveratrol had diverse
effects on myocardial function after cardiac arrest. This effect
was dose- and time-dependent. Resveratrol preconditioning improved
myocardial function after CA, but not when administered post CA.
Lower doses (0.23, 0.023 mg/kg) had superior efficacy compared to
higher doses of resveratrol.
As the results revealed, resveratrol preconditioning
improved left ventricular function after CA, which was similar to
certain previous studies (16,23).
Given the sudden onset and unpredictability of CA, the protocol in
the present study appears infeasible in clinical practice, however,
oral resveratrol for a short period of time maybe practicable, as a
previous study reported, this however requires validation in the
future (24). In the present study
resveratrol, postconditioning did not significantly ameliorate
myocardial dysfunction after CA which was not similar to previous
research. In an in vitro ischemia-reperfusion rat model, 1
µM or 10 µM resveratrol administered during the reoxygenation
period after recovery from ischemia improved left ventricular
contractile function and attenuated myocardial injury (16). In another local myocardial
ischemia-reperfusion rat study, 10 µM resveratrol administered
during the ischemic period and before perfusion improved myocardial
infarct size and mitochondrial injury (15). The present study did not
demonstrate a significant postconditioning effect of resveratrol
after CA, which may be due to the following reasons. First, the
effect of resveratrol on the induction of mRNA transcript levels
may take a longer time resulting in delayed protein expression.
This delayed effect may be too long to reverse or inhibit
post-cardiac arrest syndrome (14,25).
Second, a synergistic effect of heart injury and haemodynamic
instability may reduce the tolerance of internal organs to ischemia
(26,27). Third, different times of drug
administration may result in different effects. During CPR, chest
compressions only provide blood volumes of ~25–40% compared to
normal stroke volume which may not attain effective drug
concentration (28). In a rat CA
model, administration of sevoflurane at the onset of ROSC rather
than during resuscitation improved myocardial function (21). Finally, resveratrol was
administered via bolus injection in the present study. Given the
short half-life of resveratrol (~8–14 min), whether continuous
intravenous administration could improve myocardial function is yet
to be demonstrated.
Considering that the blood volume in rats is ~100
ml/kg of its body weight, the calculated blood concentrations
corresponding to the infused dose of resveratrol (MW:228.25) in the
present study would be 100, 10 and 1 µM. As previous studies
revealed, resveratrol is characterized by hormesis, which means a
stimulating effect at a lower level and an inhibitory effect at a
higher level (17,18). In our research, a lower level of
resveratrol in fact revealed improved myocardial function than a
higher level. The results were similar to a myocardial
ischemia-reperfusion injury study involving resveratrol (29). However, we did not investigate
whether lower doses would produce improved efficacy. A study by
Hung et al (12),
demonstrated that 2.3×10−4 mg/kg resveratrol improved
myocardial function in a rat myocardial infarction model. We
hypothesize that resveratrol has selective distribution in
vivo and hence plasma concentration does not represent
concentration in the myocardium.
Myocardial nitrative stress after ROSC was similar
to that reported previously in a mouse model (5). The authors observed that
mitochondrial tyrosine nitration by peroxynitrite was significantly
increased at 60 min after ROSC. In addition, it has been
demonstrated that the extent of nitrative stress is proportional to
the severity of myocardial dysfunction, with higher nitrative
stress resulting from worse myocardial function (30). The results from our study indicated
that myocardial nitrative stress was involved in the pathogenesis
of myocardial dysfunction after ROSC.
The present results revealed that nitrative stress
was involved in PCMD and resveratrol preconditioning ameliorated
myocardial nitrative stress and decreased the expression of iNOS
and nitrotyrosine. These results were similar to previous studies
(31,32) and suggested that resveratrol
inhibits nitrative stress via a bidirectional regulation of the
iNOS and eNOS expression levels. Resveratrol had a protective
effect by downregulating excessive iNOS expression and upregulating
endothelial NOS (eNOS) expression, with a concomitant minor
increase in NO (31). However,
there are several inconsistencies with previous studies.
Resveratrol was revealed to induce iNOS expression in porcine
pulmonary artery endothelial cells (33). The differences may be due to how
the models were produced, experimental protocols, or which
laboratory animals were used. It appears that lower expression of
iNOS, rather than higher expression is important for resveratrol to
exhibit its myocardial protective effect.
In the present study, wortmannin, a PI3K inhibitor,
partially inhibited the myocardial protective effects of
resveratrol by reducing p-Akt expression levels and increasing iNOS
and nitrotyrosine levels. This suggests the PI3K/Akt signalling
pathway was involved in the mechanism by which resveratrol inhibits
nitrative stress. Our results were similar to previous studies.
Wang et al (34)
demonstrated that resveratrol exerts an anti-inflammatory effect by
increasing the activity of adiponectin. It has been revealed that
adiponectin enhanced eNOS phosphorylation and decreased ROS
production, with a concomitant inhibition of nitrative stress via
the PI3K/Akt signalling pathway in a mouse model of myocardial
ischemia-reperfusion (35,36). Thus, it was surmised that
resveratrol can inhibit nitrative stress via adiponectin/PI3K/Akt,
but this hypothesis requires validation in a further study. In a
study by Huang et al (37),
resveratrol inhibited oxygen-glucose deprivation-induced cell
apoptosis via the NF-κB/iNOS/NO signalling pathway. In an animal
model of diabetic heart disease, resveratrol inhibited TNF-α and
NF-κB expression, and thereby inhibited iNOS and ROS expression,
which ameliorated nitrative stress and myocardial injury (38). From these studies, it is inferred
that TNF-α and NF-κB are also involved in the mechanism by which
resveratrol regulates iNOS expression via the PI3K/Akt signalling
pathway in a cardiac arrest model. However, this needs to be
established by further study.
A limitation of the present study is that healthy SD
rats were used, which is not consistent with the clinical reality
in which numerous CA victims have heart disease or are at a high
risk for heart disease. SD rats are different from humans with
regard to pathophysiological characteristics; hence, larger animals
such as swine should be considered for future studies.
In addition, the study was conducted for only 4 h
after ROSC, which is an appropriate early stage model for
myocardial dysfunction after ROSC; however, going beyond 4 h after
ROSC is necessary for investigating the effects of resveratrol on
intermediate- and late-stage myocardial dysfunction.
The molecular mechanism of resveratrol as
hypothesized in the present study is insufficient. The role of
inflammatory factors in the inhibitory effect of resveratrol on
nitrative stress should be investigated. In addition, as mentioned
in several studies, mitochondria are critical for myocardial
function after CA, and should be studied with regard to
resveratrol.
Resveratrol preconditioning could alleviate cardiac
dysfunction after resuscitation. This effect of resveratrol was
associated with its inhibitory effect on nitrative stress and may
partially involve the PI3K/Akt signalling pathway, however
postconditioning with resveratrol was not more effective compared
to the vehicle control.
Acknowledgements
The authors are grateful to Dr Si Rong He, Dr Hai Hu
and Dr Ya Rong He from West China hospital of Sichuan University
for their assistance.
Funding
The present study was partly supported by grants
from the Support Project of Sichuan Science and Technology
Department (2013SZ0061).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
ZZ conceived and designed the study. HZ and QW
performed the experiments. ZW and YC conducted the analysis of
data. HZ wrote the manuscript and ZZ and YC revised the manuscript.
All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study was performed in strict accordance
to the recommendations by the Guide for the Care and Use of
Laboratory Animals, National Institutes of Health. The
Institutional Animal Care and Use Committee of West China Hospital
(Sichuan University; Approval no. 2016045A) approved the study
protocol.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hua W, Zhang LF, Wu YF, Liu XQ, Guo DS,
Zhou HL, Gou ZP, Zhao LC, Niu HX, Chen KP, et al: Incidence of
sudden cardiac death in China: Analysis of 4 regional populations.
J Am Coll Cardiol. 54:1110–1118. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Langhelle A, Tyvold SS, Lexow K, Hapnes
SA, Sunde K and Steen PA: In-hospital factors associated with
improved outcome after out-of-hospital cardiac arrest. A comparison
between four regions in Norway. Resuscitation. 56:247–263. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Granfeldt A: Organ dysfunction following
regional and global ischemia/reperfusion. Intervention with
postconditioning and adenocaine. Dan Med J. 59:B44962012.PubMed/NCBI
|
|
4
|
Adrie C, Adib-Conquy M, Laurent I, Monchi
M, Vinsonneau C, Fitting C, Fraisse F, Dinh-Xuan AT, Carli P,
Spaulding C, et al: Successful cardiopulmonary resuscitation after
cardiac arrest as a ‘sepsis-like’ syndrome. Circulation.
106:562–568. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Han F, Da T, Riobo NA and Becker LB: Early
mitochondrial dysfunction in electron transfer activity and
reactive oxygen species generation after cardiac arrest. Crit Care
Med 36 (11 Suppl). S447–S453. 2008. View Article : Google Scholar
|
|
6
|
Brookes P and Darley-Usmar VM: Hypothesis:
The mitochondrial NO (*) signaling pathway, and the transduction of
nitrosative to oxidative cell signals: An alternative function for
cytochrome C oxidase. Free Radic Biol Med. 32:370–374. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ischiropoulos H and Beckman JS: Oxidative
stress and nitration in neurodegeneration: Cause, effect, or
association? J Clin Invest. 111:163–169. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rossig L, Haendeler J, Hermann C, Malchow
P, Urbich C, Zeiher AM and Dimmeler S: Nitric oxide down-regulates
MKP-3 mRNA levels: Involvement in endothelial cell protection from
apoptosis. J Biol Chem. 275:25502–25507. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim YM, Bombeck CA and Billiar TR: Nitric
oxide as a bifunctional regulator of apoptosis. Circ Res.
84:253–256. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiao XY, Gao E, Yuan Y, Wang Y, Lau WB,
Koch W, Ma XL and Tao L: INO-4885 [5,10,15,20-tetra[N-(benzyl-4′-
carboxylate)- 2-pyridinium]-21H,23H-porphine iron(III) chloride], a
peroxynitrite decomposition catalyst, protects the heart against
reperfusion injury in mice. J Pharmacol Exp Ther. 328:777–784.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Arstall MA, Sawyer DB, Fukazawa R and
Kelly RA: Cytokine-mediated apoptosis in cardiac myocytes: The role
of inducible nitric oxide synthase induction and peroxynitrite
generation. Circ Res. 85:829–840. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hung LM, Chen JK, Huang SS, Lee RS and Su
MJ: Cardioprotective effect of resveratrol, a natural antioxidant
derived from grapes. Cardiovasc Res. 47:549–555. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li D, Qu Y, Tao L, Liu H, Hu A, Gao F,
Sharifi-Azad S, Grunwald Z, Ma XL and Sun JZ: Inhibition of iNOS
protects the aging heart against beta-adrenergic receptor
stimulation-induced cardiac dysfunction and myocardial ischemic
injury. J Surg Res. 131:64–72. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mokni M, Limam F, Elkahoui S, Amri M and
Aouani E: Strong cardioprotective effect of resveratrol, a red wine
polyphenol, on isolated rat hearts after ischemia/reperfusion
injury. Arch Biochem Biophys. 457:1–6. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shen M, Jia GL, Wang YM and Ma H:
Cardioprotective effect of resvaratrol pretreatment on myocardial
ischemia-reperfusion induced injury in rats. Vascul Pharmacol.
45:122–126. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Goh SS, Woodman OL, Pepe S, Cao AH, Qin C
and Ritchie RH: The red wine antioxidant resveratrol prevents
cardiomyocyte injury following ischemia-reperfusion via multiple
sites and mechanisms. Antioxid Redox Signal. 9:101–113. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hassan-Khabbar S, Cottart CH, Wendum D,
Vibert F, Clot JP, Savouret JF, Conti M and Nivet-Antoine V:
Postischemic treatment by trans-resveratrol in rat liver
ischemia-reperfusion: A possible strategy in liver surgery. Liver
Transpl. 14:451–459. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gurusamy N, Lekli I, Mukherjee S, Ray D,
Ahsan MK, Gherghiceanu M, Popescu LM and Das DK: Cardioprotection
by resveratrol: A novel mechanism via autophagy involving the
mTORC2 pathway. Cardiovasc Res. 86:103–112. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen MH, Liu TW, Xie L, Song FQ, He T, Mo
SR and Zeng ZY: A simpler cardiac arrest model in the mouse.
Resuscitation. 75:372–379. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yin XL, Zhang W, Yang Y and Shen H:
Increasing expression of (CCAAT enhancer binding protein)
homologous protein induced by endoplasmic reticulum stress in
myocardium after cardiac arrest and resuscitation in rat.
Resuscitation. 83:378–385. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Knapp J, Bergmann G, Bruckner T, Russ N,
Bottiger BW and Popp E: Pre- and postconditioning effect of
sevoflurane on myocardial dysfunction after cardiopulmonary
resuscitation in rats. Resuscitation. 84:1450–1455. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mukherjee S, Dudley JI and Das DK:
Dose-dependency of resveratrol in providing health benefits. Dose
Response. 8:478–500. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shen M, Wu RX, Zhao L, Li J, Guo HT, Fan
R, Cui Y, Wang YM, Yue SQ and Pei JM: Resveratrol attenuates
ischemia/reperfusion injury in neonatal cardiomyocytes and its
underlying mechanism. PLoS One. 7:e512232012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Boocock DJ, Faust GE, Patel KR, Schinas
AM, Brown VA, Ducharme MP, Booth TD, Crowell JA, Perloff M, Gescher
AJ, et al: Phase I dose escalation pharmacokinetic study in healthy
volunteers of resveratrol, a potential cancer chemopreventive
agent. Cancer Epidemiol Biomarkers Prev. 16:1246–1252. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Juan SH, Cheng TH, Lin HC, Chu YL and Lee
WS: Mechanism of concentration-dependent induction of heme
oxygenase-1 by resveratrol in human aortic smooth muscle cells.
Biochem Pharmacol. 69:41–48. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Safar P: Effects of the postresuscitation
syndrome on cerebral recovery from cardiac arrest. Crit Care Med.
13:932–935. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Senthil M, Brown M, Xu DZ, Lu Q, Feketeova
E and Deitch EA: Gut-lymph hypothesis of systemic inflammatory
response syndrome/multiple-organ dysfunction syndrome: Validating
studies in a porcine model. J Trauma. 60:958–967. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rubertsson S, Grenvik A and Wiklund L:
Blood flow and perfusion pressure during open-chest versus
closed-chest cardiopulmonary resuscitation in pigs. Crit Care Med.
23:715–725. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bradamante S, Barenghi L, Piccinini F,
Bertelli AA, De Jonge R, Beemster P and De Jong JW: Resveratrol
provides late-phase cardioprotection by means of a nitric oxide-
and adenosine- mediated mechanism. Eur J Pharmacol. 465:115–123.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu D, Bassuk J, Arias J, Kurlansky P,
Lozano H, Lamas G and Adams JA: Different roles of nitric oxide
synthase isoforms in cardiopulmonary resuscitation in pigs.
Resuscitation. 73:144–153. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hung LM, Su MJ and Chen JK: Resveratrol
protects myocardial ischemia-reperfusion injury through both
NO-dependent and NO-independent mechanisms. Free Radic Biol Med.
36:774–781. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang HX, Duan GL, Wang CN, Zhang YQ, Zhu
XY and Liu YJ: Protective effect of resveratrol against
endotoxemia-induced lung injury involves the reduction of
oxidative/nitrative stress. Pulm Pharmacol Ther. 27:150–155. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hsieh TC, Juan G, Darzynkiewicz Z and Wu
JM: Resveratrol increases nitric oxide synthase, induces
accumulation of p53 and p21 (WAF1/CIP1), and suppresses cultured
bovine pulmonary artery endothelial cell proliferation by
perturbing progression through S and G2. Cancer Res. 59:2596–2601.
1999.PubMed/NCBI
|
|
34
|
Wang A, Liu M, Liu X, Dong LQ, Glickman
RD, Slaga TJ, Zhou Z and Liu F: Up-regulation of adiponectin by
resveratrol: The essential roles of the Akt/FOXO1 and AMP-activated
protein kinase signaling pathways and DsbA-L. J Biol Chem.
286:60–66. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ji L, Fu F, Zhang L, Liu W, Cai X, Zhang
L, Zheng Q, Zhang H and Gao F: Insulin attenuates myocardial
ischemia/reperfusion injury via reducing oxidative/nitrative
stress. Am J Physiol Endocrinol Metab. 298:E871–E880. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang WQ, Zhang HF, Gao GX, Bai QX, Li R
and Wang XM: Adiponectin inhibits hyperlipidemia-induced platelet
aggregation via attenuating oxidative/nitrative stress. Physiol
Res. 60:347–354. 2011.PubMed/NCBI
|
|
37
|
Huang T, Gao D, Jiang X, Hu S, Zhang L and
Fei Z: Resveratrol inhibits oxygen-glucose deprivation-induced
MMP-3 expression and cell apoptosis in primary cortical cells via
the NF-kappaB pathway. Mol Med Rep. 10:1065–1071. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang H, Morgan B, Potter BJ, Ma L,
Dellsperger KC, Ungvari Z and Zhang C: Resveratrol improves left
ventricular diastolic relaxation in type 2 diabetes by inhibiting
oxidative/nitrative stress: In vivo demonstration with magnetic
resonance imaging. Am J Physiol Heart Circ Physiol. 299:H985–H994.
2010. View Article : Google Scholar : PubMed/NCBI
|