Introduction
Obstructive sleep apnea hypopnea syndrome (OSAHS)
can aggravate the nerve damage caused by brain ischemia, which is
an independent risk factor increasing the morbidity and mortality
rates of ischemic stroke (1,2). It
has been shown that cognitive dysfunction following brain ischemia
is closely associated with cellular metabolic changes, signal
transduction system disorder and nerve cell death following brain
tissue ischemia and hypoxia (3,4).
Autophagy, a cellular defense mechanism of eukaryotic cells, is
essential in maintaining intracellular homeostasis, and determines
the final outcome of cells: Recovery, repair, damage, aggravation
or death (5). The
phosphatidylinositol 3-kinase (PI3K)/protein kinase B
(Akt)/mammalian target of rapamycin (mTOR) classic cascade
signaling pathway effectively regulates multiple physiological and
pathological mechanisms, is involved in various eukaryotic
processes, including autophagy, proliferation and apoptosis, and is
important in diseases of the central nervous system (6,7).
Following OSAHS-complicated ischemic stroke, a variety of factors
are aggravated, including oxygen free radicals, oxidative stress
and inflammatory reactions. These factors can cause increased nerve
cell death, either directly or indirectly. Therefore, the present
study hypothesized that the PI3K-mTOR autophagy pathway is
important in the pathological progress of nerve damage following
intermittent hypoxia (IH)-aggravated brain ischemia. In the present
study, the whole brain ischemia/reperfusion (I/R) model was
prepared using rats exposed to intermittent hypoxia. Expression of
the PI3K-mTOR autophagy pathway and loss of nerve cells in the
hippocampus were observed. The role of the PI3K-mTOR autophagy
pathway in IH-aggravated brain I/R was investigated and discussed.
The results provided experimental evidence for the clinical
prevention and treatment of OSAHS complicated by hypoxic brain
vascular diseases.
Materials and methods
Materials
Male Wistar rats (n=80) were provided by Vital River
Laboratories Co., Ltd. (Beijing, China). The certificate number for
the laboratory animals was SCXK (Jing) 2012-013. Animals were kept
in colony cages; under the following laboratory conditions in a
ventilated room: 18–25°C, 35–50% humidity and a 12 h light dark
cycle, with free access to food and water. The present study was
performed with approval from the Animals Welfare Care Committee of
North China University of Science and Technology (Tangshan, China).
The hypoxia control program was provided by the Science and
Technology Center of Tsinghua University (Beijing, China). Oxygen
measurement equipment was purchased from Jiande Meicheng
Electrochemical Analytical Instrument Factory (Jiande, China). The
animal hypoxia chamber was purchased from China Huaibei Zhenghua
Science and Technology Co., Ltd. (Huaibei, China). Pure nitrogen
was purchased from Tangshan General Gas High-Tech Co., Ltd.
(Tangshan, China). The Morris water maze was provided by the
Pharmaceutical Research Institute of China Academy of Medical
Sciences (Beijing, China).
Groups of animals and model
preparation
The rats were divided by random number table into a
sham operation (SO) group (n=20), I/R group (n=20), IH for 7 days
(IH7)+I/R group (n=20) and IH for 21 days (IH21)+I/R group (n=20).
Each group was further divided into a 6 and 24 h subgroup, with 10
rats in each subgroup.
The blood vessels in the rats of the SO group were
separated and exposed. Electrocoagulation of the vertebral artery
and occlusion of the carotid artery were not performed. For the
rats in the I/R, IH7+I/R and IH21+I/R groups, the whole brain
ischemia model was prepared using the optimized Pulsinelli 4-vessel
occlusion method (8). Anesthesia
was induced by intraperitoneal injection of 10% chloral hydrate at
a dose of 300 mg/kg, (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany). An incision was made in the rear occipital and the holes
on the bilateral flank were carefully exposed. The pre-heated
electrocoagulation needle was inserted and electrocoagulation was
performed for 2–4 sec each time to occlude the bilateral vertebral
artery. The rats were fixed in a supine position on the surgical
bench and an incision was made in the middle of the neck to
separate the common carotid arteries on both sides. After 24 h, the
bilateral common carotid arteries were occluded at the same time
for 15 min to achieve complete whole brain ischemia.
The rats in the IH7+I/R and IH21+I/R groups were
placed in a hypoxia chamber every day from 8:00 a.m. to 15:00 p.m.
Nitrogen and air were introduced into the chamber recurrently. Each
cycle lasted for 120 sec and nitrogen was added continuously for 30
sec to maintain an oxygen level of 5–21% in the chamber. The oxygen
concentration was monitored using a digital oxygen analyzer for 7
and 21 days, respectively.
H&E staining
A total of five rats were randomly selected from
each group at 6 and 24 h post-I/R, respectively. Cardiac perfusion
was performed using 4% paraformaldehyde. The rats were sacrificed
and, following decapitation, brain tissue was removed. The tissue
between the optic chiasm plane and transverse section was
dehydrated, embedded in paraffin, sectioned, dewaxed, cleared in
xylene, stained with H&E and observed under a light microscope
(magnification, ×400).
Observation using transmission
electron microscopy
Five rats were randomly selected from each group at
6 and 24 h post-I/R, respectively. The hippocampal tissue from
these animals was removed, sectioned into 1 mm3 tissue
blocks, fixed in 40 ml/l glutaraldehyde, rinsed twice in 0.1 mol/l
cacodylate acid buffer, fixed in 10 g/l osmium tetroxide, rinsed
twice with buffer and placed at 4°C overnight. The tissues were
dehydrated using an acetone concentration gradient, embedded in
epoxy resin, sectioned into 40–50 nm ultra-thin slides, double
stained with uranyl acetate and lead citrate, and observed under an
electron microscope.
Immunohistochemical staining
The brain tissue slides were subjected to
conventional dewaxing to water, blocking in 0.3%
H2O2 for 10 min and heated antigen retrieval
for 90 sec. Antibodies against PI3-K, mTOR and Beclin-1 were added
onto the slides, respectively, and the slides were incubated in a
humid box at 37°C for 30 min. The secondary antibody was added and
the slides were incubated at 37°C for 40 min. Mouse monoclonal
anti-PI3-K (1:200, Bioss, Product batch number: bs-0128R); Mouse
monoclonal anti-mTOR (1:150, Bioss, Beijing, China, Product batch
number: bs-5331R); Mouse monoclonal anti-Beclin-1 (1:200,
Baomanbio, Shanghai, China, Product batch number: OSA00006W). The
slides were then subjected to DAB development, dehydration,
clearing, neutral resin-mounting and microscope observation.
Quantitative analysis of the positive rate was performed using the
following method. A total of five slides were selected from each
parameter, and five regions in the hippocampus were randomly
selected in each slide under a light microscope (magnification,
×400). The number of positive cells was counted using a FACS-like
tissue cytometry analysis system (Tissue FAXS plus, TissueGnostics
GmbH, Vienna, Austria).
RT-qPCR analysis
Five rats were randomly selected from each group at
6 and 24 h post-I/R, respectively. A 50–100 mg sample of tissue
from the CA1 region of rat the hippocampus was collected and
homogenized in TRIzol solution. Total RNA was extracted. cDNA
samples were blended with DEPC-treated Water and SYBR-Green Master
Mix in a total volume of 20 µl. The OD260/280 was measured using a
Roter-Gene 3000 Fluorescence quantitative PCR instrument. The RNA
concentrations were calculated based on the OD260 and the RNA
samples were stored at −80°C. The following primers were used:
PI3K, forward GAAACCCAGTCACCTAGGGC and reverse
5′-GGTGGGCAGTACGAACTCAA-3′; mTOR, forward
5′-GGTGGACGAGCTCTTTGTCA-3′ and reverse 5′-AGGAGCCCTAACACTCGGAT-3′;
Beclin-1, forward 5′-CTCTCGTCAAGGCGTCACTTC-3′ and reverse
5′-CCTTAGACCCCTCCATTCCTCA-3′; GAPDH forward
5′-CTCCCATTCCRCCACCTTTG-3′ and reverse 5′-CCACCACCCTGTTGCTGAG-3′.
The RT-qPCR procedure was performed as follows: Stage 1 and 2 (RT
reaction) comprising one cycle at 42°C for 50 min and 95°C for 10
sec. Stage 3 (qPCR reaction) involving repetition of 45 cycles at
95°C for 15 sec and 56°C for 20 sec. Stage 4 involved melting curve
analysis following the dissociation protocol.
Water maze test
The laboratory animals were subjected to a water
maze test at 48 h post-I/R. The escape platform was placed in the
second quadrant of the water maze. The water level was 2–3 cm above
the platform, and temperature was maintained at 25±1°C. The rats
were placed in a fixed position in the four quadrants,
respectively. The time limit for swimming was 90 sec. The length of
time a rat spent locating the platform was recorded. The length of
time was recorded as 90 sec if a rat failed to locate the platform
within 90 sec. A water maze video tracking system (MED-SYST-VWM,
MED Associates, Inc., Fairfax, VT, USA) were used to follow, image
and analyze the route of a rat from the four quadrants to the
platform, the latency and the number of times a rat crossed the
platform.
Statistical analysis
A database of experimental data was established
using Microsoft Excel 2003 (Microsoft Corporation, USA, Redmond,
WA, USA). All data are expressed as the mean ± standard error. SPSS
17.0 statistical analysis software (SPSS, Inc., Chicago, IL, USA)
was used for one-way analysis of variance. P<0.05 was considered
to indicate a statistically significant difference.
Results
H&E staining
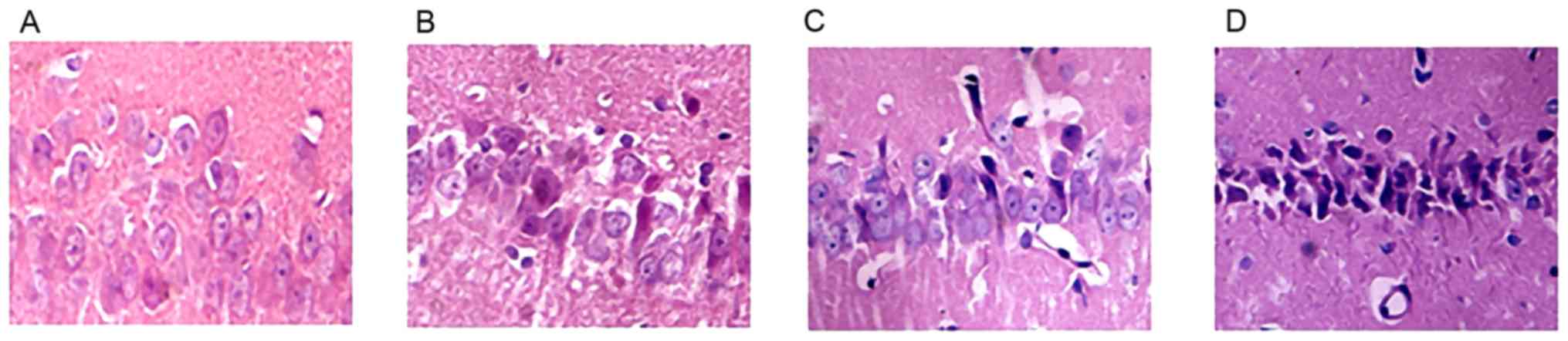
The nerve cells in the hippocampus of the SO group
rats were neatly arranged and exhibited normal structures; nuclei
were clear and the nucleoli were visible. Swelling of neurons was
observed in the majority of the I/R group rats, with loose
structures. The nuclei of these neurons were shrunken and darkly
stained, with complete disappearance of nuclei in certain neurons
and the formation of vacuole-like structures. In the OSAHS hypoxia
groups, the morphology and structure of the nerve cells showed
marked damage. A large number of nerve cells underwent necrosis,
exhibiting loose structures. The nuclei of these neurons were
shrunken and darkly stained, with complete disappearance of nuclei
in certain neurons and the formation of vacuole-like structures.
The damage to nerve cell morphology and structure was more severe
in the IH21+I/R group. Imaging analysis showed that, compared with
the SO group, the survival rates of nerve cells were reduced in all
I/R groups (P<0.05). Compared with the I/R group, the survival
rates of nerve cells at each time point were reduced in both IH
groups (P<0.05), however, these changes were more marked in the
IH21+I/R group. The results are shown in Table I and Fig. 1A-D.
 | Table I.Comparison of survival rates of rat
hippocampal nerve cells among treatment groups. |
Table I.
Comparison of survival rates of rat
hippocampal nerve cells among treatment groups.
|
|
| Survival rate of
nerve cells (%) |
|---|
|
|
|
|
|---|
| Group | Animals (n) | 6 h | 24 h |
|---|
| SO | 5 | 99.08±0.84 | 98.65±0.75 |
| I/R | 5 |
78.65±1.43a |
71.71±1.39a |
| IH7+I/R | 5 |
65.742±1.20a,b |
60.75±1.39a,b |
| IH21+I/R | 5 |
49.06±1.70a–c |
45.12±1.51a–c |
Observation using transmission
electron microscopy
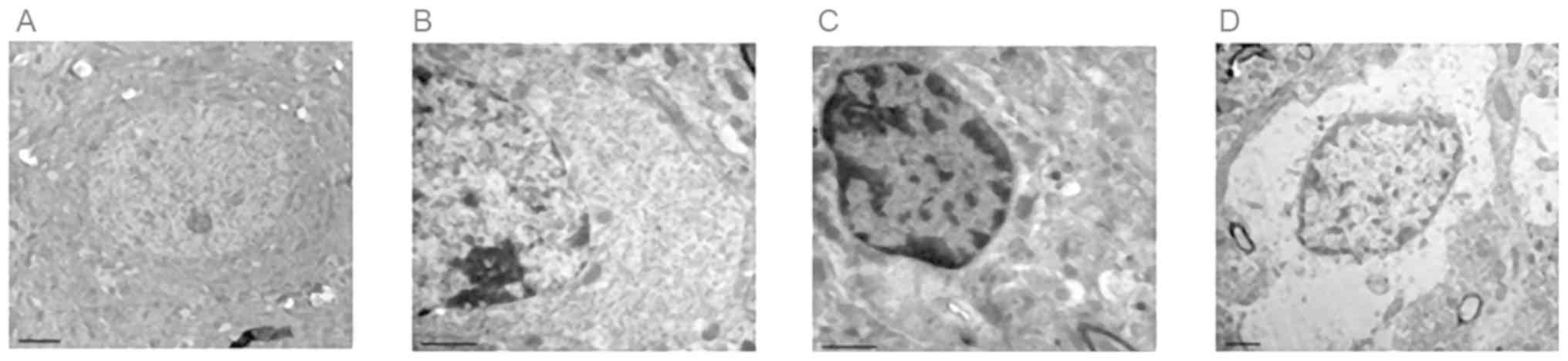
In the SO group, the rat hippocampus nerve cells
were in ordered arrangement with a smooth nuclear membrane.
Chromatin was evenly scattered in the nuclei. There were abundant
organelles in the nerve cell cytoplasm, exhibiting normal
structures. In the I/R groups, the nuclear membrane of the rat
hippocampus nerve cells was dissolved. Chromatin had shrunk in the
nuclei. The number of nerve cell cytoplasmic organelles was reduced
but with integrated structure. In the IH7+I/R group, the nuclei
were stained on the edge and cytoplasmic organelles disappeared in
neurons. In the IH21+I/R group, nuclear shrinkage was observed and
nuclear chromatin was dissolved. Cytoplasmic organelles were
reduced in the nerve cells, with obscure structures, as shown in
Fig. 2A-D.
Water maze test
Compared with the rats in the SO group, the rats in
the I/R group exhibited increased escape latency and decreased
platform crossing (P<0.05). Compared with the I/R group, the
rats in the OSAHS group exhibited increased escape latency and
decreased platform crossing (P<0.05). Compared with the IH7+I/R
group, the rats from the IH21+I/R group exhibited increased escape
latency and decreased platform crossing (P<0.05). The results
are shown in Table II.
 | Table II.Comparisons of latency and times
platform crossed among rats from each treatment group in the water
maze test. |
Table II.
Comparisons of latency and times
platform crossed among rats from each treatment group in the water
maze test.
| Group | Animals (n) | Latency period | Times platform
crossed (n) |
|---|
| SO | 10 | 7.20±1.45 | 12.47±0.92 |
| I/R | 10 |
14.04±0.62a |
8.53±0.60a |
| IH7+I/R | 10 |
17.23±0.89a,b |
6.57±0.65a,b |
| IH21+I/R | 10 |
29.79±1.96a–c |
1.15±0.33a–c |
Immunohistochemistry
PI3K
The immunohistochemical staining showed that PI3K
(brown) was located in the cytoplasm and predominantly expressed in
neurons. Positively stained cells were occasionally observed in the
SO group. At each time point (6 and 24 h), the numbers of
PI3K-positive cells were increased in the I/R groups, compared with
that in the SO group (P<0.05). At each time point (6 and 24 h),
the numbers of PI3K-positive cells were increased in the IH groups,
compared with that in the I/R group (P<0.05). These changes were
more marked in the IH21+I/R group (P<0.05), as shown in Table III and Fig. 3A1-D1.
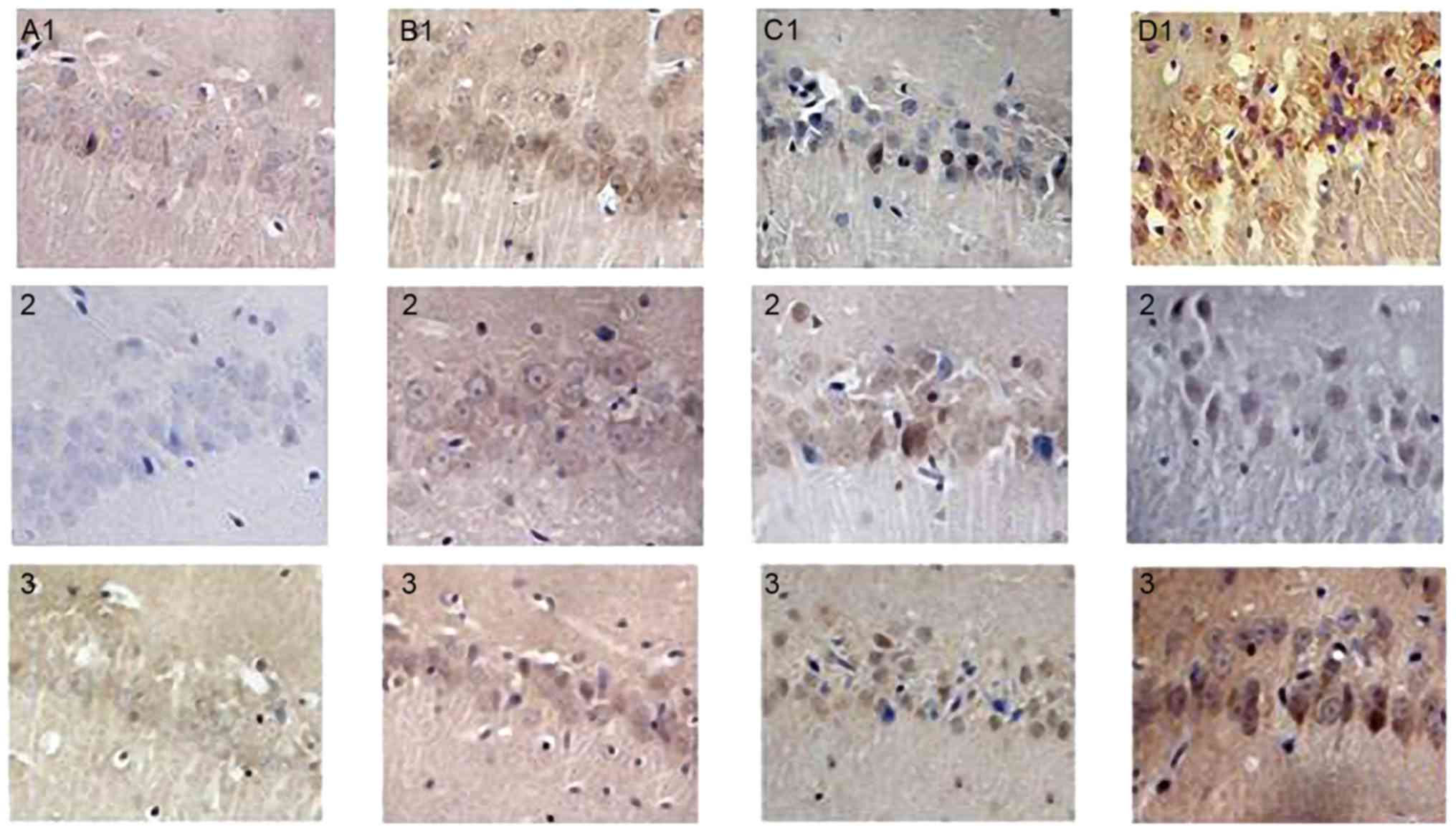 | Figure 3.Positive expression of PI3K, mTOR and
Beclin-1 24 h following I/R in the rat hippocampus of each group
(immunohistochemistry; magnification, ×400). (A1-D1) PI3K
immunohistochemistry result in hippocampal tissues of the SO, I/R,
IH7+I/R and IH21+I/R groups. (A2-D2) mTOR immunohistochemistry
result in hippocampal tissues of the SO, I/R, IH7+I/R and IH21+I/R
groups. (A3-D3) Beclin-1 immunohistochemistry result in hippocampal
tissues of the SO, I/R, IH7+I/R and IH21+I/R groups. PI3K,
phosphatidylinositol 3-kinase; mTOR, mammalian target of rapamycin;
SO, sham operation; I/R, ischemia/reperfusion; IH7 intermittent
hypoxia for 7 days; IH21, intermittent hypoxia for 21 days. |
 | Table III.PI3K-, mTOR- and Beclin-1-positive
cells in the rat hippocampal tissues of each treatment group mean ±
SE, Cell/High Magnification Sight (×400). |
Table III.
PI3K-, mTOR- and Beclin-1-positive
cells in the rat hippocampal tissues of each treatment group mean ±
SE, Cell/High Magnification Sight (×400).
|
|
| PI3K | mTOR | Beclin-1 |
|---|
|
|
|
|
|
|
|---|
| Group | n | 6 h | 24 h | 6 h | 24 h | 6 h | 24 h |
|---|
| SO | 5 | 7.04±0.47 | 10.79±0.70 | 14.65±0.48 | 15.40±0.58 | 2.06±0.23 | 2.10±0.30 |
| I/R | 5 |
19.56±0.41a |
20.80±0.60a |
22.38±0.46a |
24.16±0.60a |
8.58±0.58a |
10.58±0.49a |
| IH7+I/R | 5 |
23.05±0.58a,b |
23.93±0.32a,b |
25.31±0.39a,b |
27.46±0.74a,b |
11.12±0.35a,b |
13.49±0.38a,b |
| IH21+I/R | 5 |
25.62±0.41a–c |
26.07±0.64a–c |
30.40±0.43a–c |
32.86±0.50a–c |
15.57±0.57a–c |
18.78±0.43a–c |
mTOR
The imunohistochemical staining showed that mTOR
(brown) was located in the cytoplasm and predominantly expressed in
neurons. Positively stained cells were occasionally observed in the
SO group. At each time point (6 and 24 h), the numbers of
mTOR-positive cells were increased in the I/R groups, compared with
that in the SO group (P<0.05). At each time point (6 and 24 h),
the numbers of mTOR-positive cells were increased in the IH groups,
compared with that in the I/R group (P<0.05). These changes were
more marked in the IH21+I/R group (P<0.05), as shown in Table III and Fig. 3A2-D2.
Beclin-1
The immunohistochemical staining showed that
Beclin-1 (brown) was located in the cytoplasm and predominantly
expressed in neurons. Positively stained cells were occasionally
observed in the SO group. At each time point (6 and 24 h), the
numbers of Beclin-1-positive cells were increased in the I/R
groups, compared with that in the SO group (P<0.05). At each
time point (6 and 24 h), the numbers of Beclin-1-positive cells
were increased in the IH groups, compared with that in the I/R
group (P<0.05). These changes were more marked in the IH21+I/R
group (P<0.05), as shown in Table
III and Fig. 3A3-D3.
RT-qPCR analysis
PI3K
At each time point (6 and 24 h), the expression of
PI3K was significantly increased in the I/R group, compared with
that in the SO group (P<0.05). At each time point (6 and 24 h),
the expression of PI3K was significantly increased in the IH
groups, compared with that in the I/R group (P<0.05). These
changes were more marked in the IH21+I/R group, as shown in
Table IV.
 | Table IV.Changes in the relative mRNA
expression of PI3Ka and mTOR in rat hippocampal tissues of
treatment groups. |
Table IV.
Changes in the relative mRNA
expression of PI3Ka and mTOR in rat hippocampal tissues of
treatment groups.
|
|
| PI3K | mTOR | Beclin-1 |
|---|
|
|
|
|
|
|
|---|
| Group | n | 6 h | 24 h | 6 h | 24 h | 6 h | 24 h |
|---|
| SO | 5 | 1.00±0.00 | 1.00±0.00 | 1.00±0.00 | 1.00±0.00 | 1.00±0.00 | 1.00±0.00 |
| I/R | 5 |
1.38±0.06a |
1.43±0.07a |
1.22±0.10a |
1.27±0.08a |
1.57±0.11a |
1.79±0.09a |
| IH7+I/R | 5 |
1.63±0.09a,b |
1.71±0.09a,b |
1.53±0.06a,b |
1.56±0.10a,b |
1.98±0.15a,b |
2.10±0.08a,b |
| IH21+I/R | 5 |
2.18±0.07a–c |
2.29±0.06a–c |
1.65±0.06a–c |
1.73±0.07a–c |
2.37±0.14a–c |
2.54±0.24a–c |
mTOR
At each time point (6 and 24 h), the expression of
mTOR was significantly increased in the I/R group, compared with
that in the SO group (P<0.05). At each time point (6 and 24 h),
the expression of mTOR was significantly increased in the IH
groups, compared with that in the I/R group (P<0.05). These
changes were more marked in the IH21+I/R group, as shown in
Table IV.
Discussion
In addition to being a respiratory disease, OSAHS is
able to damage and involve multiple systems, leading to damage in
the brain, heart and other organs, and to the neurological system
in particular (9). Studies have
shown that, due to repeated hypoxia and hypercapnia, OSAHS can
cause cerebral blood rheology, cerebral metabolic change,
dysfunction of neurohumoral regulation, oxidative stress and
inflammation, initiating and aggravating the pathophysiological
progress of cerebral I/R (10).
This leads to neuron loss, and damage to the morphology and
structure of nerve cells in multiple cerebral regions, thereby
increasing nerve damage following cerebral I/R (11). The neurological dysfunction
following brain ischemia is directly associated with the loss of
nerve cells. For example, the results of a study by Sun et
al (12) showed that the
number of viable nerve cells was reduced and learning ability was
decreased following brain I/R. Running exercise increased the
survival rate of nerve cells and improved learning ability in rats.
The results of the present study showed that, compared with the I/R
group, rats in each OSAHS hypoxia group exhibited reduced nerve
cell survival in the hippocampus, severely damaged nerve cell
structure, and apparent dysfunction in learning and memory ability.
This indicated that OSAHS hypoxia aggravated rat neurological
damage following brain I/R, and these results were consistent with
those of clinical studies (13,14).
Autophagy is a process, which is extensively present
in eukaryotic organisms and functions through the lysosomal
pathway, which involves the identification, degradation and
reversal of functionally damaged proteins and organelles with
physiological and pathological factors. It is a third mechanism of
cellular death in addition to apoptosis and necrosis (15). Wang et al (16) established rat whole brain ischemia
models and observed nerve cells in the CA1 hippocampal region. It
was found that damage to neurons induced by whole brain ischemia
was significantly reduced following the addition of autophagy
inhibitor 3-MA at 1 h or 30 min prior to ischemia. This indicated
that ischemia-induced autophagy aggravated neuron damage led by
brain ischemia. The results of the present study showed that,
compared with the I/R group, the rats in the IH groups exhibited
aggravated damage in neuron structure, reduced neuron survival
rates and increased expression of Beclin-1. These changes were more
marked in the IH21+I/R group, indicating that autophagy in the
process of I/R alone was further activated by IH to promote neuron
loss following brain ischemia. IH can facilitate the activation of
autophagy. For example, Liu et al (17) established mouse IH models and
observed autophagy-associated protein expression in nerve cells of
the mouse hippocampal CA1 region following IH. The results showed
that the protein levels of LC3II/LC3 were increased and damage to
the microstructure of nerve cells was aggravated, indicating that
IH induced autophagy in the rat hippocampal nerve cells.
The activation of autophagy is dependent on the
PI3K/Akt/mTOR signaling pathway (18). It has been shown that the
activities of PI3K, Akt and mTOR are significantly increased
following brain ischemia. Ishrat et al (19) prepared rat focal brain ischemia
models by cerebral artery occlusion and observed significantly
elevated phosphorylation levels of PI3K and Akt in addition to
nerve cell apoptosis, indicating that brain ischemia activated the
PI3K/Akt signaling pathway. Zhao et al (20) exposed rats with chronic brain
ischemia to flavonoids from hawthorn leaves, and detected the
protein expression levels of PI3K and Akt using
immunohistochemistry. The results showed that flavonoids from
hawthorn leaves increased the protein expression of PI3K and Akt,
and reduced brain tissue damage. It has been reported that
(21) under ischemia and hypoxic
conditions, the cellular PI3K/Akt signaling pathway is activated
and autophagy can be induced by regulating the activity of mTOR.
The results from a study by Liu and Xu (22) demonstrated that sevoflurane
pre-treatment reduced the autophagy of cardiac muscle cells during
I/R through activating the PI3K/Akt signaling pathway and enhancing
downstream mTOR activity, which had a protective effect on cardiac
muscles. Gong et al (23)
established focal brain ischemia models in Sprague-Dawley rats and
found that the PI3K inhibitor, LY294002, inhibited the PI3K-mTOR
signaling pathway and subsequently inhibited autophagy, increasing
the area of infarction in rats. The results of the present study
showed that the expression levels of PI3K and mTOR were elevated in
all the IH groups, and this was associated with the degree of
hypoxia. This indicated that IH increased autophagy and aggravated
neurological dysfunction following cerebral ischemia by activating
the PI3K/Akt/mTOR signaling pathway. At present, the mechanisms
underlying the regulation of PI3K/Akt/mTOR signaling by IH remain
to be fully elucidated. However, it has been shown that the
characteristic chronic IH hypoxia in OSAHS, similar to damage from
I/R, aggravates hypoxia, and induces oxygen free radical
production, ATP depletion and inflammation (24), which may be one of the reasons why
the PI3K/Akt signaling pathway was activated by IH following
cerebral ischemia.
In conclusion, the present study demonstrated that
IH promoted autophagy and aggravated neurological dysfunction
following brain ischemia through regulating the PI3K/Akt/mTOR
cascade signaling pathway. These results provided experimental
evidence for the prevention and treatment of OSAHS-complicated
brain ischemia.
Acknowledgements
Not applicable.
Funding
This study was supported by the Health Department of
Hebei Province Key Project and Leading Talent Project (grant no.
zd2013087) and the Tangshan City Science and Technology Project
(grant no. 14130220B).
Availability of data and materials
All data generated and/or analyzed during the
present study are included in this published article.
Authors' contributions
XG conceived the present study. YNZ made substantial
contributions in data analysis and wrote the manuscript. YL and RF
performed the experiments. YLB, XFG, JML and CXC designed the
study. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The Animal Experimentation Ethics Committee of North
China University of Science and Technology (Hebei, China) approved
the experimental animal protocol of the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Deng XZ, Liu B and Li Y: Research progress
of obstructive sleep apnea hyponea syndrome and hypertension. Adv
Cardiovasc Dis. 35:230–233. 2014.(In Chinese).
|
|
2
|
Cho ER, Kim H, Seo HS, Suh S, Lee SK and
Shin C: Obstructive sleep apnea as a risk factor for silent
cerebral infarction. J Sleep Res. 22:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang Z, Zhao YN and Li JM: Effects of
grape seed proanthocyanidin extract on peroxidation and ability of
learning and memory after cerebral ischemia/re-perfusion injury in
rats. Chin J Rehabil Theory Pract. 20:827–830. 2014.(In
Chinese).
|
|
4
|
Tang YA: Study on cognitive impairment and
mechanism causing by chronic cerebral hypoperfusion in rats.
Sichuan Med J. 31:1226–1228. 2010.(In Chinese).
|
|
5
|
Wang WY, Cui ZR and Jiang W: Autophagy in
research progress in the role of brain ischemia-reperfusion injury.
J Shanghai Jiaotong Univ. 34:248–253. 2014.(In Chinese).
|
|
6
|
Sami A and Karsy M: Targeting the
PI3K/AKT/mTOR signaling pathway in glioblastoma: Novel therapeutic
agents and advances in understanding. Tumour Biol. 34:1991–2002.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jeong EH, Choi HS, Lee TG, Kim HR and Kim
CH: Dual inhibition of PI3K/Akt/mTOR pathway and role of autophagy
in non-small cell lung cancer cells. Tuberc Respir Dis (Seoul).
72:343–351. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bin Y, Yu LJ, Wang J, Zhao L, Hu XY, Ji
CN, Wei YL, Yang QF, Jiang QS, Yang JQ, et al: Time course change
of PGI2 and TA2 levels in rat cortex following global cerebral
ischemia reperfusion. Chin Pharmacol Bull. 29:1667–1671. 2013.(In
Chinese).
|
|
9
|
Capampangan DJ, Wellik KE, Parish JM,
Aguilar MI, Snyder CR, Wingerchuk D and Demaerschalk BM: Is
obstructive sleep apnea an independent risk factor for stroke? A
critically appraised topic. Neurologist. 16:269–273. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guo X, Zhao Y, Li J, Liu W and Chen C:
Activation of autophagy pathway in hippocampus and deterioration of
learning and memory ability by intermittent hypoxia in rats after
cerebral ischemia. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi.
32:1212–1216. 2016.(In Chinese). PubMed/NCBI
|
|
11
|
Yang D and Liu ZH: Exploring the
relationship and pathogenesis between obstructive sleep apnea and
cardiovascular diseases. Adv Cardiovasc Dis. 32:645–648. 2011.(In
Chinese).
|
|
12
|
Sun ZM, Zhao YN, Li JM, Chen CX, Zhao X
and Chen NL: Effect of intensity of exercise on learning ability
and oxygen free radical metabolism in rats after cerebral
ischemia-reperfu-sion. Chin J Rehabil Theory Pract. 21:26–30.
2015.(In Chinese).
|
|
13
|
Lu WL, Guo Y, Li DX and Zhang S: Effects
of obstructive sleep apnea syndrome on cognitive function in
ischemic stroke patients. Chinese Community Doctors,. 30:26–27.
2014.(In Chinese).
|
|
14
|
Mansukhani MP, Bellolio MF, Kolla BP,
Enduri S, Somers VK and Stead LG: Worse outcome after stroke in
patients with obstructive sleep apnea: An observational cohort
study. J Stroke Cerebrovasc Dis. 20:401–405. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ding H, Tang YH and Huang XP: Effects of
autophagy in cerebral ischemic injury. Chin Pharmacol Bull.
31:1048–1052. 2015.(In Chinese).
|
|
16
|
Wang JY, Xia Q, Chu KT, Pan J, Sun LN,
Zeng B, Zhu YJ, Wang Q, Wang K and Luo BY: Severe global cerebral
ischemia-induced programmed necrosis of hippocampal CA1 neurons in
mt is prevented by 3-Methyladenine: A widely used inhibitor of
autophagy. J Neuropathol Exp Neurol. 70:314–322. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu HY, Chen R, Li YN, Zhang YL and Liu
CF: Chronic intermittent hypoxia area in mice hippocampal CAl
neurons autophagy. Chin J Tuberc Respir Dis. 34:467–469. 2011.(In
Chinese).
|
|
18
|
Wu MM, Wan RH and Chen NH: Research
progress on the mTOR signaling pathway and neurodegenerative
disease. Chin Pharmacol Bull. 27:1481–1483. 2011.(In Chinese).
|
|
19
|
Ishrat T, Sayeed I, Atif F, Hua F and
Stein DG: Progesterone is neuroprotective against ischemic brain
injury through its effects on the phosphoinositide 3-kinase/protein
kinase B signaling pathway. Neuroscience. 210:442–450. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao L, Zhang XH, Wu BC, et al: Hawthorn
leaves flavonoids on cerebral ischemia rats organization PI3K/Akt
signal pathway. Guangdong Med J. 35:982–984. 2014.(In Chinese).
|
|
21
|
Sengupta S, Peterson TR and Sabatini DM:
Regulation of the mTOR complex 1 pathway by nutrients, growth
factors, and stress. Mol Cell. 40:310–322. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu L and Xu PC: Sevoflurane pretreatment
of rats in vitro cardiac ischemia-reperfusion injury when autophagy
and the effect of PI3K/Akt signaling pathway in the role. Chin J
Anesthesiol. 34:492–496. 2014.
|
|
23
|
Gong L, Wang Z, Xing YG, et al: Effects of
ischemic postconditioning on apoptosis in cerebral
ischemia-reperfusion injury in rats and its mechanisms. J Int
Neurol Neurosurg. 39:229–234. 2012.
|
|
24
|
Yang ML and Cai RW: Inflammatory reaction
in obstructive sleep apnea syndrome and ischemic strok. Med
Recapitul. 16:919–921. 2010.
|