Introduction
Glioma is considered the most common malignant tumor
affecting the central nervous system in adults. Glioma is
associated with a poor prognosis, an aggressive behavior, rapid
progression and frequent recurrence (1). In particular, the median survival
time of patients with glioblastoma (GBM) is usually <1 year
(2). Nowadays, there are various
treatment strategies for glioma, such as chemotherapy, radiotherapy
and surgery. Nonetheless, the prognosis of patients with glioma
remains (3). Thus, the
identification of the underlying molecular mechanisms of the
initiation and progression of glioma is of utmost importance.
It has been shown that one of the targets of c-Myc
is cell division cycle-associated 7-like protein (CDCA7L). CDCA7L
can interact with c-Myc (4).
Generally, the activation of the c-Myc oncogene is considered
critical for the pathogenesis of massive human malignant tumors,
which include glioma (4–7). It has been reported that CDCA7L is
dysregulated in a number of cancer types (4,8).
This is of utmost importance for the determination of the
epigenetic mechanisms of the development and progression of cancer.
Furthermore, it is also considered that CDCA7L plays a significant
role in cancer and therefore, in tumor progression. It may also be
a potential therapeutic target.
It has been shown in the existing research that
human glioma tissues highly express CDCA7L (9). However, to the best of our knowledge,
the explicit mechanism of CDCA7L in glioma remains largely unknown.
In this study, we aimed to explore the role of CDCA7L in glioma
progression both in vivo and in vitro and to
elucidate the underlying mechanisms.
Materials and methods
OncoLnc and Oncomine databases
The OncoLnc database (http://www.oncolnc.org/) was used to obtain prognostic
data of 152 patients with GBM and 510 patients with low-grade
glioma (LGG), while the Oncomine database was used to obtain the
CDCA7L expression data of 80 patients with GBM. Generally, each
case of CDCA7l expression was obtained based on the normalized
results of RSEM RNASeqV2 for all types of cancer, among which, the
overall survival (OS) has been calculated in the days beginning
from the date of diagnosis to death. At the same time, the mRNA
expression level of CDCA7L was identified as either decreased
(<0 in z-scores) or increased (>0 in z-scores). This was
applied to compare the survival of the patients.
Cell lines and cell culture
The Shanghai Cell Bank provided the U251 (cat. no.
TCHu58), A172 (cat. no. TCHu171), human glioma U87 (cat. no.
TCHu138; glioblastoma cells of unknown origin). ScienCel provided
the normal human astrocytes (HEB, cat. no. 1800). Dulbecco's
modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific) was
used to cultivate these cells; the medium was also supplemented
with 10% fetal bovine serum and the cells were cultured at 37°C
with 5% CO2.
Patients and tissue samples
Patients with GBM (n=22) undergoing an initial
glioma resection surgery at the First Affiliated Hospital of
Xinxiang Medical University between 2014 and 2017 were enrolled in
this study. Moreover, 6 normal white matter brain tissues (NBTs)
were obtained from temporal lobectomy from patients with temporal
lobe epilepsy. Informed consent was obtained from each patient
taking part in this research. The Medical Ethics Committee of the
First Affiliated Hospital of Xinxiang Medical University approved
the use of the samples for this research. Prior to the resection,
each patient was treatment naive.
U87 glioma cell transfection
Shanghai Genechem Biotechnology Co., Ltd. provided
the negative control siRNA (NC siRNA) and GFP-expressing CDCA7L
small interfering RNA (siRNA). The siRNA sequences used in this
study were: CDCA7L siRNA, 5′-GCCAGAUUUCUUCCCAGUAdTdT-3′ (sense) and
5′-UACUGGGAAGAAAUCUGGCdTdT-3′ (antisense); and NC siRNA,
5′-UUCUCCGAACGUGUCACGUdTdT-3′ (sense);
5′-ACGUGACACGUUCGGAGAAdTdT-3′ (antisense). In total
5×105 U87 cells at 60% confluence, were plated in each
well of a 6-well plate. The cells were then transfected NC siRNA
and CDCA7L siRNA using Lipofectamine 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.). After being transfected for a day, the
cells were observed under a fluorescence microscope (Leica
Microsystems, GmbH). The CDCA7L siRNA transfection efficiency was
examined by western blot analysis and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR).
RT-qPCR
RNA was extracted from the glioma tissues and cell
lines using TRIzol based on the protocol of the manufacturer
(Invitrogen; Thermo Fisher Scientific, Inc.). RT-qPCR was carried
out to detect the cyclin D1 (CCND1) and CDCA7L expression levels
using the one-step RT-PCR kit (Takara Bio, Inc.) based on the
protocol of the manufacturer. Genechem Co. Ltd. provided the
CDCA7L. β-actin was used as an internal control. The primer
sequences were as follows: CDCA7L forward,
5′-TTGGCGACTCGCTACCAGAT-3′ and reverse, 5′-AATGAAAGCGCACATCCTGC-3′;
CCND1 forward, 5′-CAGATCATCCGCAAACACGC-3′ and reverse,
5′-AAGTTGTTGGGGCTCCTCAG-3′; and β-actin forward,
5′-AGAGCCTCGCCTTTGCCGATCC-3′, and reverse,
5′-CTGGGCCTCGTCGCCCACATA-3′. The reaction conditions were as
follows: 95°C for 30 sec, followed by 40 amplification cycles of
95°C for 5 sec and 60°C for 34 sec. The 2−ΔΔCq method
(10) was used to quantify the
mRNA expression levels of CDCA7L and CCND1. The β-actin mRNA
expression level was used to normalize the mRNA expression
levels.
Western blot analysis
At 72 h following transfection, the cells were
harvested while the lysates of the cell were generated using RIPA
lysis buffer for approximately 30 min at 4°C. The BCA Protein Assay
kit (Pierce; Thermo Fisher Scientific) was used to determine the
protein concentration. Subsequently, 12.5% SDS-PAGE was used to
separate the total protein evenly (50 µg/lane) followed by transfer
to PVDF membranes. Membranes were blocked with 5% skim milk powder
in TBS-T for 2 h at room temperature and then incubated with
primary antibodies at 4°C overnight. The primary antibodies were
obtained from Abcam [anti-β-actin antibody (diluted at 1/5,000,
ab6276), anti-CCND1 (diluted at 1/200, ab16663) and rabbit
anti-CDCA7L (diluted at 1/2,000, ab70637)]. The secondary antibody
used was horseradish peroxidase (HRP)-conjugated goat anti-rabbit
IgG (1:10,000, ab6721; Abcam) at room temperature for 1 h. β-actin
was used as an internal control for the normalization of the
protein expression levels. The proteins bands were visualized using
an Enhanced Chemiluminescence Plus Western Blotting Detection
system (GE Healthcare). Band intensities were quantified by
densitometry using ImageJ Software version 1.6 (National Institutes
of Health).
Cell viability analysis
After 48 h following transfection with NC siRNA or
CDCA7L, the U87 cells at the exponential phase were seeded into
96-well plates at a density of 4×103 cells/well. Cell
proliferation was examined using the Cell-Counting kit-8 (CCK-8)
based on the instructions of the manufacturer (Beijing TransGen
Biotech Co., Ltd.).
Cell proliferation assay
The U87 glioma cells, which had been plated into
6-well cell culture plates were then counted; the corresponding
density was 300 cells/well for colony formation. The cells were
then incubated for 20 days under 37°C. Subsequently, 4%
paraformaldehyde was used to fix the visible colonies.
Subsequently, 0.1% crystal violet was used to stain the cells for a
further 30 min at room temperature. The colonies were viewed and
counted under a light microscope (Nikon Corporation, Tokyo, Japan)
with at least five fields randomly. The number of colonies was
calculated as the colony-forming efficiency.
Following transfection with NC siRNA or CDCA7L
siRNA, the glioma U87 cells were counted by cell counting
instrument (Countess, Invitrogen; Thermo Fisher Scientific, Inc.)
and then seeded into 24-well plates at a density of
1×104 cells/well. After incubation at 37°C for 48 h, the
cells were exposed to 50 µM 5-ethynyl-2′-deoxyuridine (EdU;
Invitrogen; Thermo Fisher Scientific) for 2.5 h. The reaction
cocktail (EdU Imaging Kits, Invitrogen; Thermo Fisher Scientific),
Inc. was used to stain at room temperature for 30 min and
permeabilize the U87 cell nuclei. The samples were then visualized
under a fluorescence microscope (Leica Microsystems, GmbH).
Flow cytometry
The U87 cells were infected with CDCA7L siRNA or NC
siRNA and incubated at 37°C. The transfected glioma cells were then
harvested, washed twice with phosphate-buffered saline (PBS), and
fixed with 75% ice-cold ethanol. Subsequently, the cells were
examined using the Cell Cycle Staining kit (Multi Sciences) and
incubated for 30 min in dark accord 37°C according to the
manufacturer's instructions. In addition, to detect the effect of
CDCA7L siRNA on cell apoptosis, the U87 cells were harvested by
trypsinization and incubated with FITC-conjugated Annexin V and PI
following the manufacturer's instructions (Keygen Biotech).
Finally, the cells were analyzed by flow cytometry and BD
CellQuest™ software version 5.1 (BD Biosciences).
Transwell invasion assay
The invasion assay was performed using a Transwell
chamber (Millipore). In brief, the transfected cells were seeded in
the upper chamber containing the serum-free medium
(1×105 cells), while the lower chamber contained DMEM
supplemented with 10% FBS and coated with Matrigel (20%; Corning
Incorporated). Then cells were incubated for 6 h at 37°C.
Subsequently, the cells were fixed with pre-cooled 4% methanol for
5 min at room temperature and stained with hematoxylin and eosin
(H&E; Beyotime Institute of Biotechnology) for 20 min at room
temperature following transfection with CDCA7L siRNA or NC siRNA,
and the invading cells were photographed under a light microscope
(Leica Microsystems, GmbH); the number of cells was counted.
Glioma xenografts in animals and
immunohistochemistry (IHC)
The animal experiments were approved by the Animal
Care and Use Committee of the First Affiliated Hospital of Xinxiang
Medical and were carried out in strict accordance with the
experimental protocol. Specifically, the glioma U87 cells were
transfected with CDCA7L siRNA or NC siRNA, and a total of 12 male
BALB/c-A nude mice (4 weeks old) were randomly divided into the
CDCA7L downregulated group or the control group. The mice were kept
in an air laminar flow chamber with a temperature of 26–28°C and a
humidity of 40–60%. Food and water were sterilized under high
pressure and were freely available. After being anesthetized with
chloral hydrate (350 mg/kg; intraperitoneally), a total of
4×106 transfected U87 cells were implanted into each
nude mouse to detect the glioma xenograft formation. The survival
time of the mice was observed until 1 month, and intracranial tumor
formation was assessed by bioluminescence imaging. At the end of
the experiment, the mice were exposed to CO2 to achieve
euthanasia [CO2 (10 l/min); chamber volume: 0.15
m3 (height × width × length, 60×50×50 cm) and the brain
glioma tissue sections were incubated with anti-Ki-67 (ab16667;
Abcam). Subsequently, 3 fields were selected to examine the
percentage of positive tumors and staining intensities, and the
CCND1 expression levels in the glioma xenografts were detected
through western blot analysis.
Statistical analysis
The statistical analyses applied the SPSS 21.0
statistical software package. All data are expressed as the means ±
standard deviations (SD), and all experiments were repeated 3
times. Differences between 2 groups were analyzed using a Student's
t-test (two-tailed). Furthermore, Kaplan-Meier analysis was used to
assess the survival rate of both glioma patients and mice, while
the log-rank test was used to assess the statistical significance.
A P-value <0.05 was considered to indicate a statistically
significant difference.
Results
CDCA7L is overexpressed in human
glioma tissues and predicts a poor prognosis of patients with
glioma
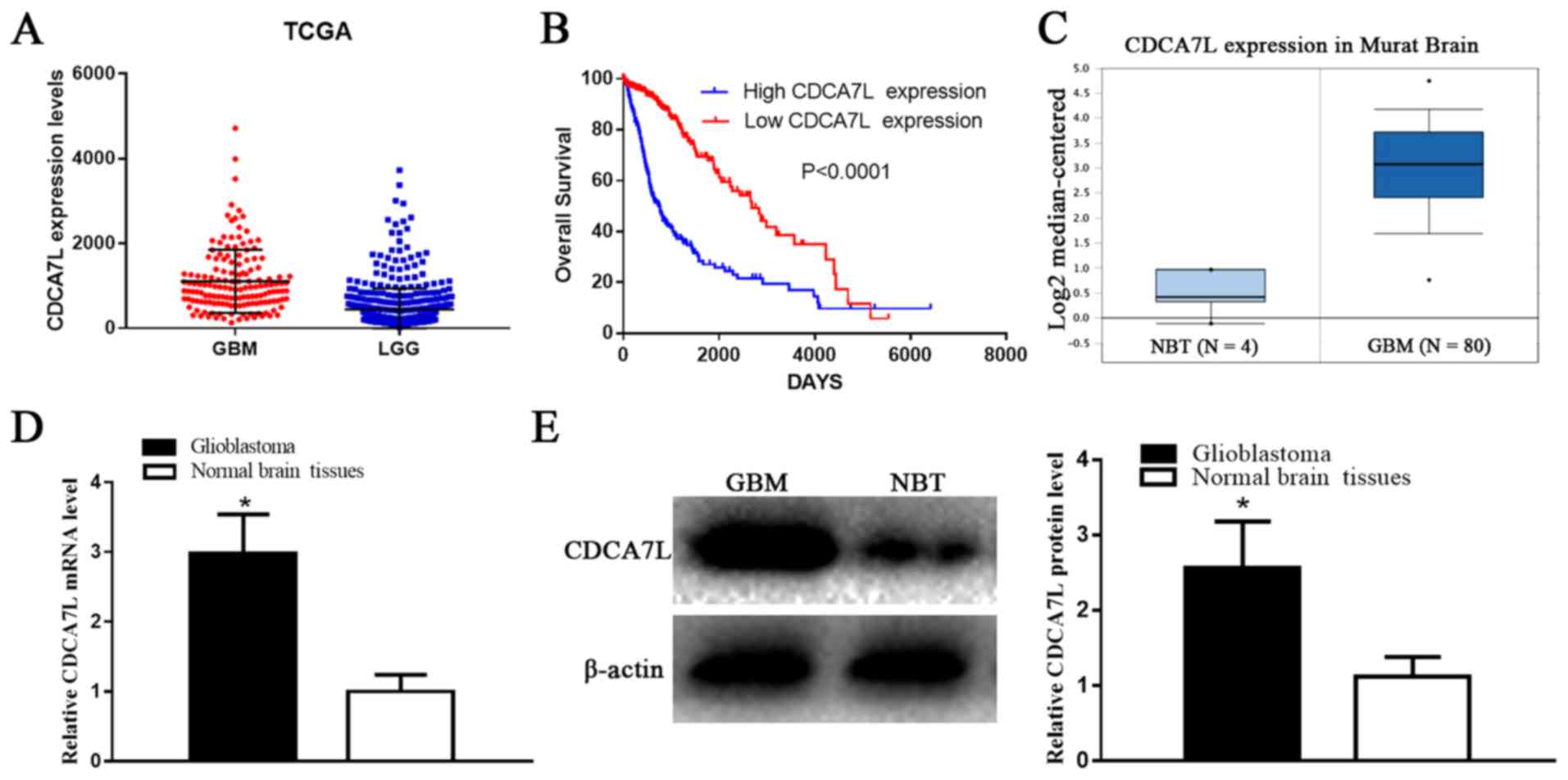
According to the OncoLnc database dataset, the
CDCA7L expression levels were markedly higher in the GBM tissues
compared with those in the LGG tissues (P<0.001) (Fig. 1A). Moreover, the clinical
information and gene expression profiles of the 510 LGG and 152 GBM
patients were matched in the current study, which further
determined whether CDCA7L expression levels were associated with
patient survival. Additionally, both Kaplan-Maier analysis and
log-rank comparisons were performed. The results presented in
Fig. 1B demonstrated that a higher
CDCA7L expression was associated with a shorter overall survival
(OS) of the glioma patients (P<0.0001). In addition, according
to CDCA7L expression in ‘Murat Brain’ (a glioma-related dataset in
the Oncomine database), the CDCA7L expression levels were markedly
higher in the GBM tissues compared to those in NBTs (P<0.0001)
(Fig. 1C). These findings indicate
that CDCA7L may play a vital role in glioma development, and may
thus potentially serve as a prognostic marker.
Subsequently, the CDCA7L expression levels in 6 NBTs
and 22 GBM samples were detected by RT-qPCR assay and western blot
analysis, respectively. The results indicated that the CDCA7L mRNA
(Fig. 1D) levels in the GBM
tissues were notably higher than those in the NBTs, and the average
CDCA7L protein expression level in the GBM tissues was higher than
that in the NBTs (P<0.01; Fig.
1E).
CDCA7L expression is inhibited by
CDCA7L siRNA in U87 human glioma cells
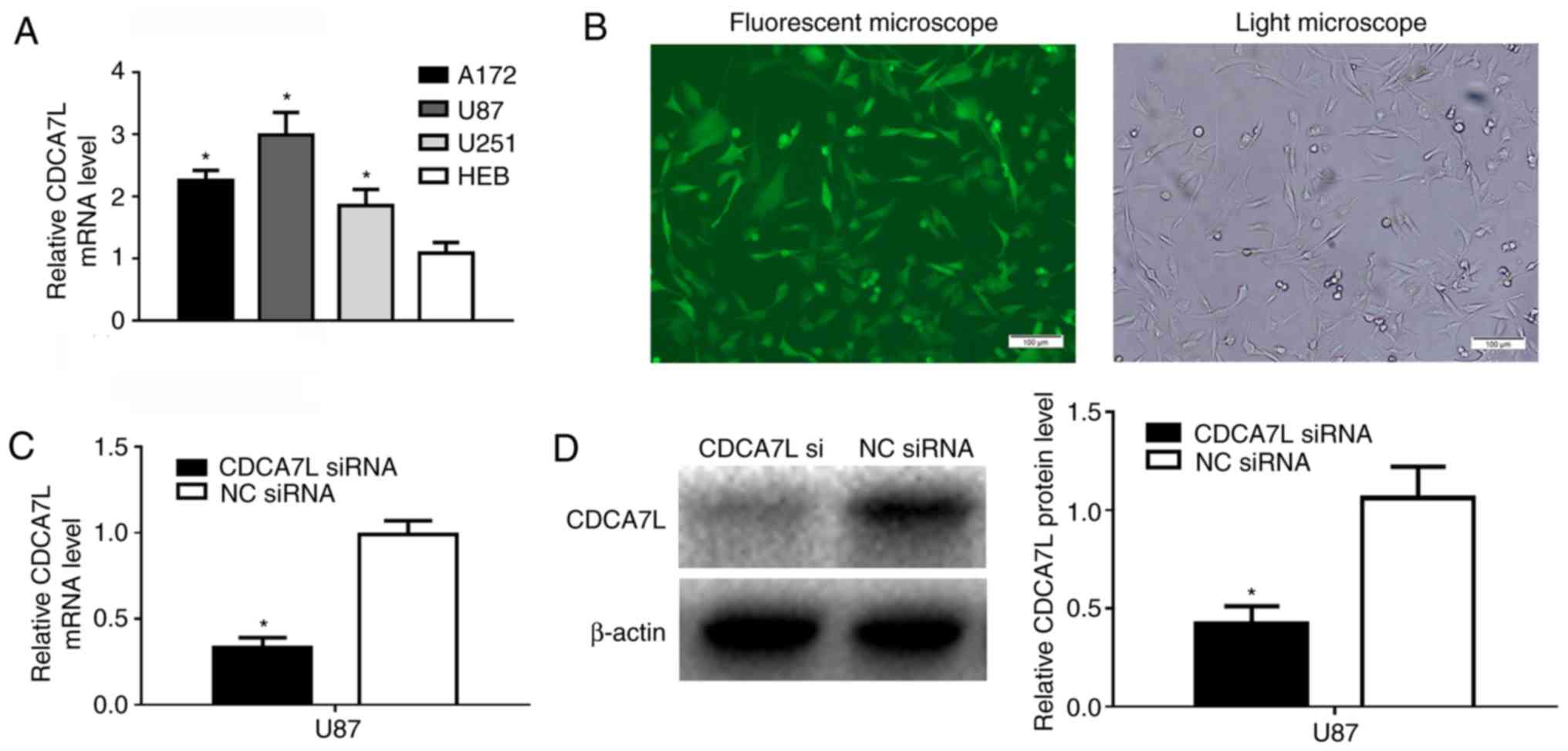
The CDCA7L expression levels in the U87, A172 and
U251 glioma cell lines were detected by RT-qPCR, and the results
revealed that CDCA7L mRNA epxression in the 3 glioma cell lines was
higher than that in the HEB cells (Fig. 2A). To explore the role of CDCA7L in
glioma, GFP-expressing CDCA7L siRNA and NC siRNA were transfected
into human glioma U87 cells, and >85% of the cells exhibited
positive green fluorescence following transfection (Fig. 2B), and the knockdown efficiency was
analyzed by RT-qPCR and western blot analysis. Following 72 h of
transfection, the CDCA7L mRNA (Fig.
2C) and protein (Fig. 2D)
expression levels in the U87 cells of CDCA7L siRNA group were
markedly lower than those in the NC siRNA group.
Downregulation of CDCA7L expression
markedly suppresses the growth of U87 glioma cells
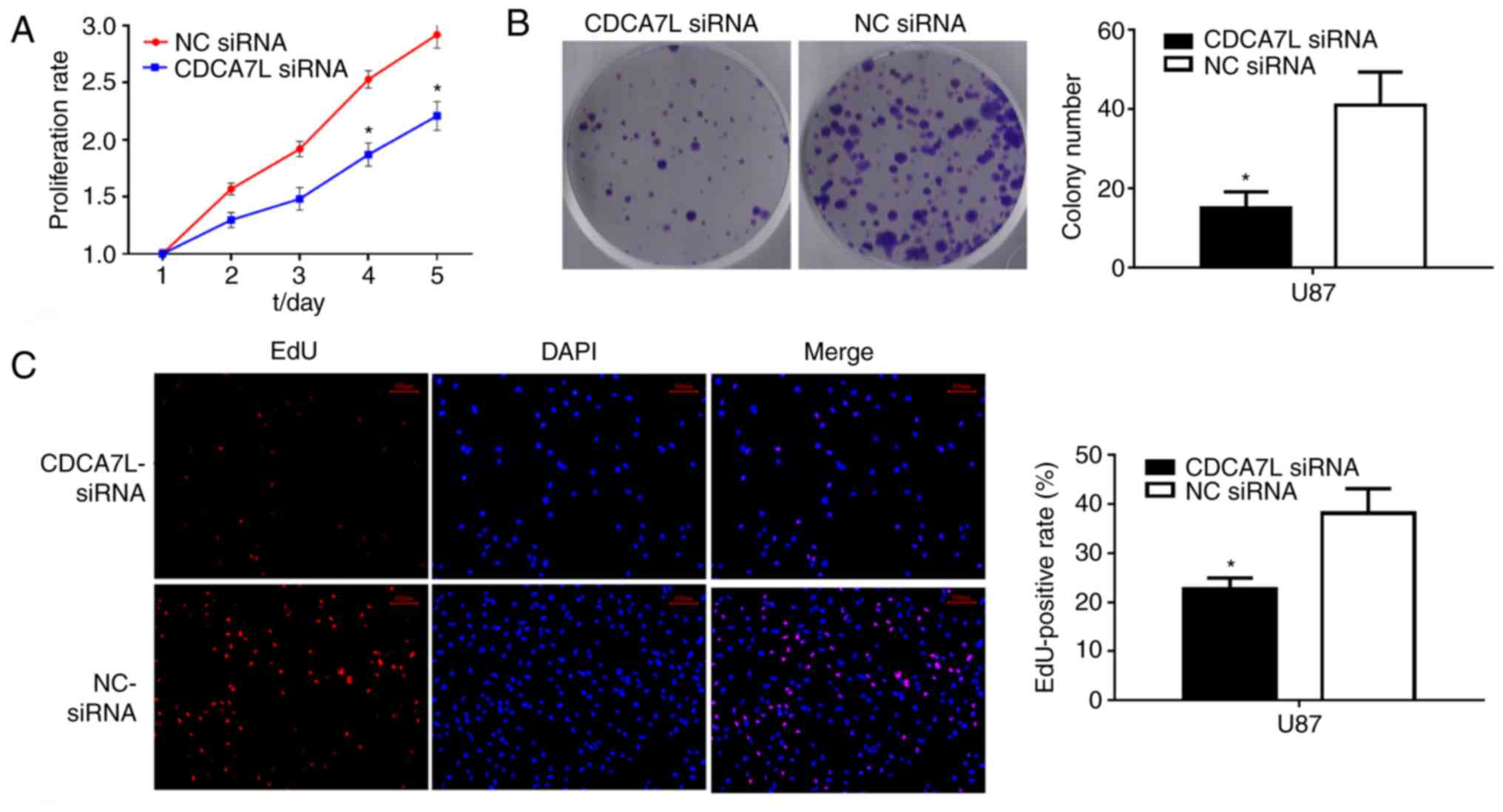
To examine the effects of CDCA7L on the growth of
glioma cells, CCK-8 and colony formation assays were performed
after the glioma U87 cells were transfected with CDCA7L siRNA or NC
siRNA. As shown in Fig. 3, the
knockdown of CDCA7L expression markedly reduced the proliferation
potential and evidently suppressed the growth of U87 cells
transfected with CDCA7L siRNA compared with those in the NC siRNA
group. Moreover, the growth of the CDCA7L siRNA-transfected cells
was also notably reduced in vitro on days 4 and 5 (Fig. 3A), and colony formation was also
evidently reduced following the silencing of CDCA7L (Fig. 3B).
Furthermore, the effects of CDCA7L on cell
proliferation following the downregulation of CDCA7L were evaluated
through an EdU cell-image assay, and the results were consistent
with those of the CCK-8 and colony formation assays, indicating
that the EdU-positive rates of U87 cells were lower in the CDCA7L
siRNA group compared with those in the NC siRNA group and that the
number of EdU-positive cells was decreased by approximately 16.6%
(Fig. 3C). Thus, on the whole,
these data indicate that the knockdown of CDCA7L effectively
prevents the development of U87 glioma cells.
Inhibition of CDCA7L induces cell
arrest at the G0/G1 phase, and the apoptosis
and invasion of U87 glioma cells
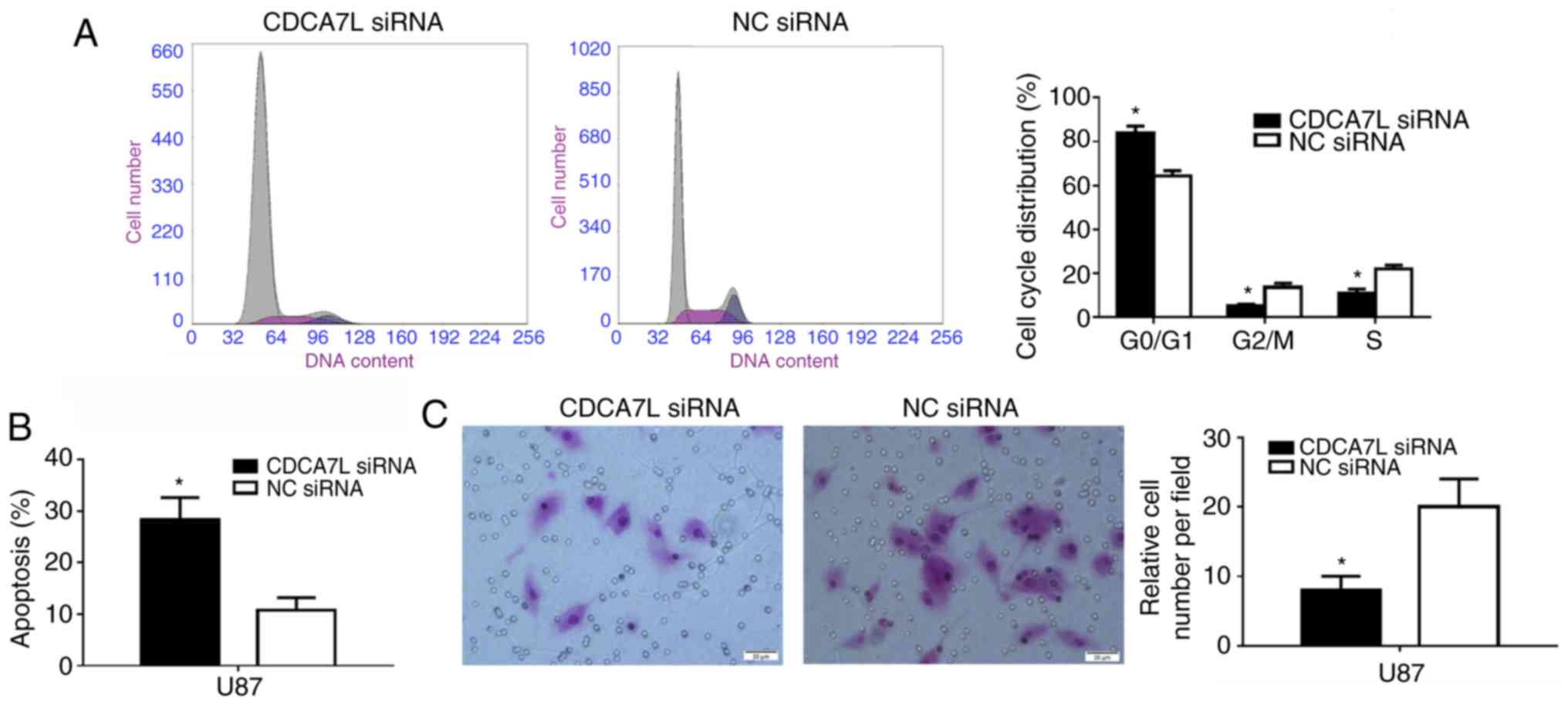
The cell cycle distribution of the U87 cells
following the downregulation of CDCA7L was subsequently examined
since proliferation is directly connected to cell cycle
distribution, and the cell cycle phases of the U87 cells were
measured by flow cytometry. As shown in Fig. 4A, the number of cells entering the
G0/G1 phase was increased by 19.30%
(P<0.01) and that of cells entering the S phase was decreased by
10.98% in the absence of CDCA7L (P<0.01).
Moreover, the effects of CDCA7L on U87 glioma cell
apoptosis were also examined. The results suggested that, the
percentage of apoptotic U87 cells in the CDCA7L siRNA group was
markedly higher than that of those in the NC siRNA group
(P<0.01; Fig. 4B). These
results indicated that the knockdown of CDCA7L expression evidently
inhibited the proliferation of U87 cells by increasing the
percentage of cells at the G0/G1 phase, while
decreasing the percentages of cells at the S and G2/M phases, and
inducing apoptosis.
Following transfection with CDCA7L siRNA or NC
siRNA, the invasive abilities of the transfected cells were
compared. As shown in Fig. 4C, the
downregulation of CDCA7L expression reduced the number of invading
cells by approximately 60% compared with the NC siRNA group, as
shown by Transwell assays.
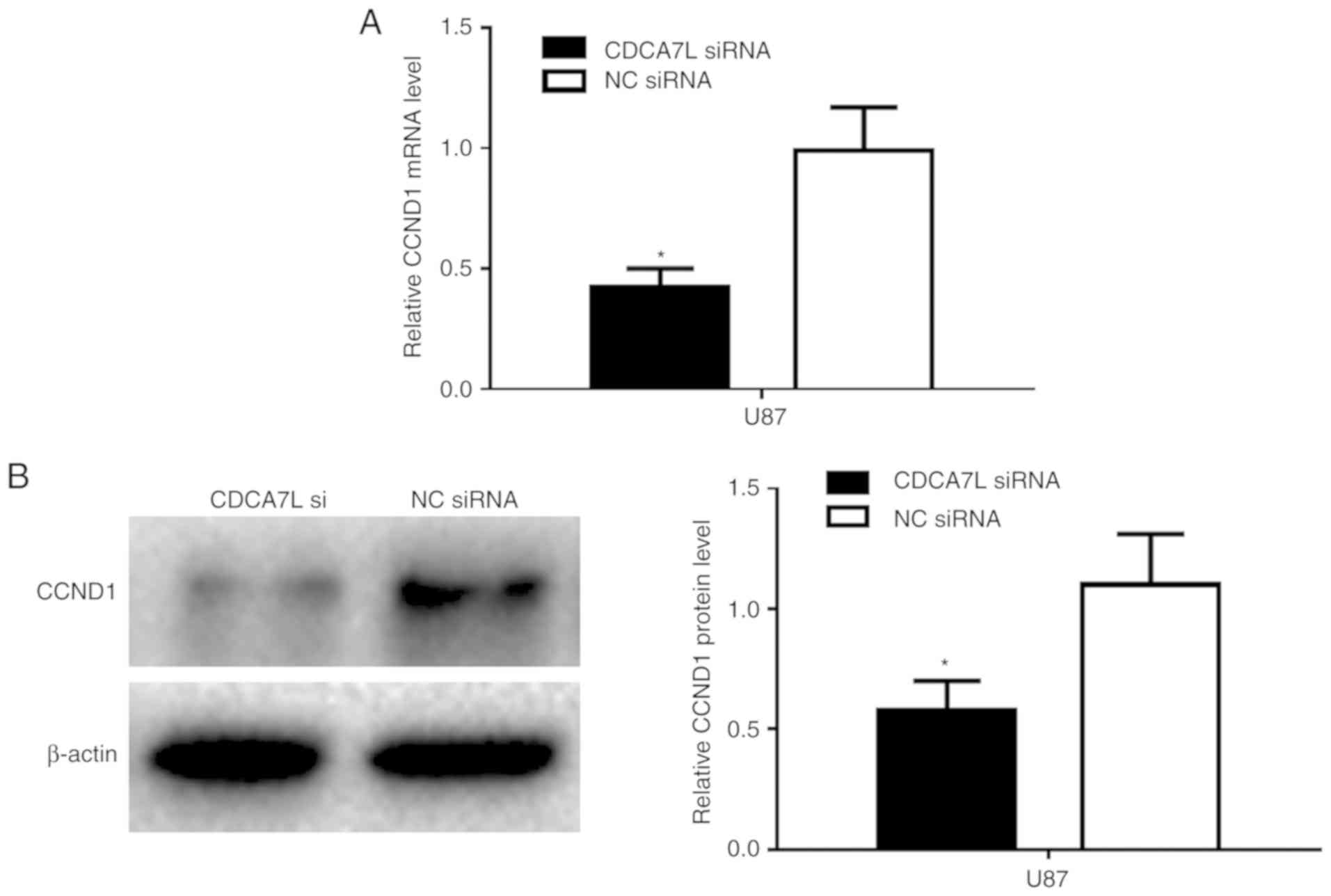
Downregulation of CDCA7L inhibits cell
proliferation by targeting CCND1
Furthermore, to investigate the target of CDCA7L in
GBM cells in vitro, the U87 cells were transfected with
CDCA7L siRNA or NC siRNA, and the CCND1 mRNA and protein expression
levels were determined by RT-qPCR and western blot analysis,
respectively. The results revealed that the CCND1 mRNA expression
level was markedly reduced following transfection with CDCA7L siRNA
compared with NC siRNA (Fig. 5A).
In addition, the CCND1 protein expression level was markedly lower
in the CDCA7L siRNA-transfected group (Fig. 5B).
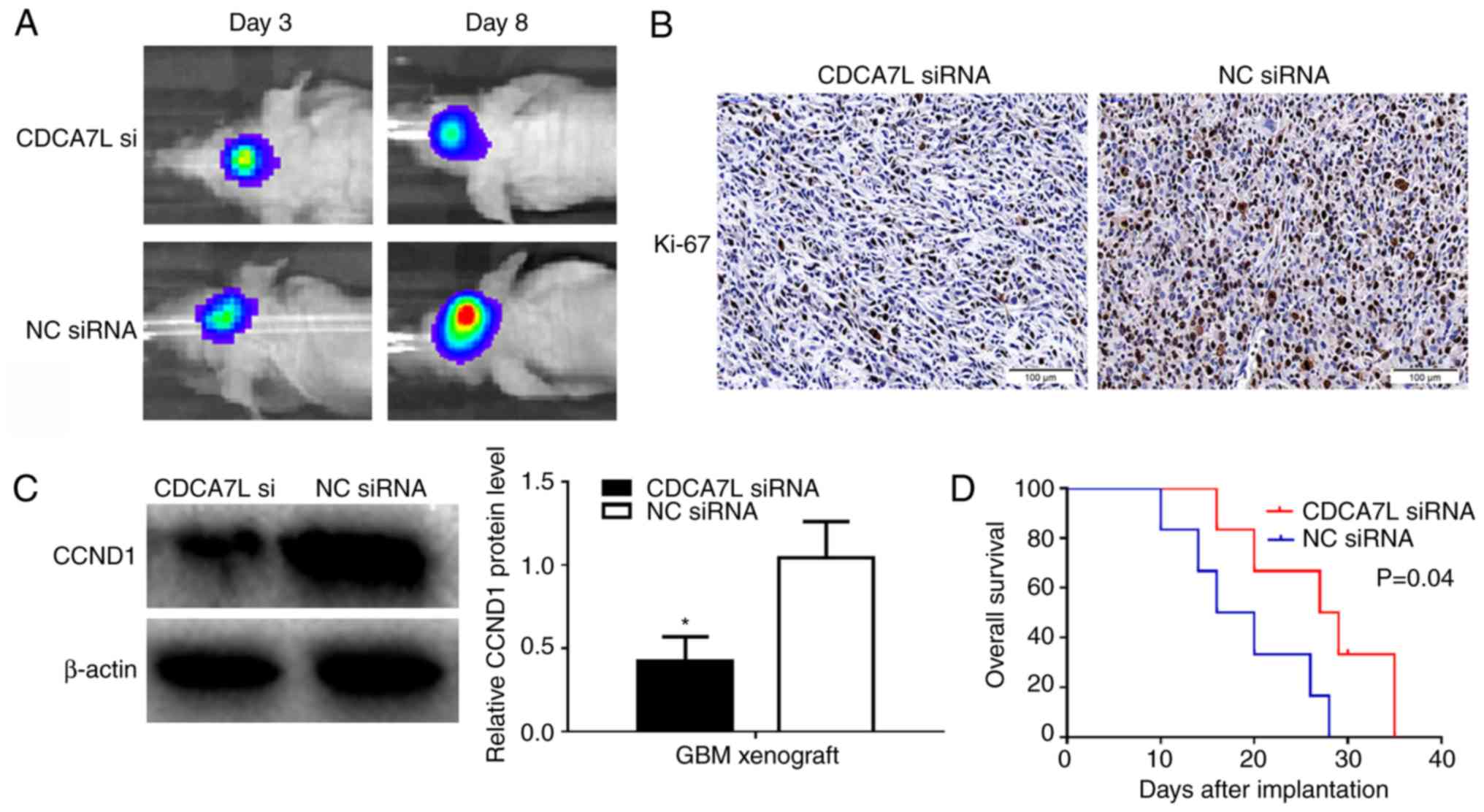
Downregulation of CDCA7L suppresses
the glioma xenograft growth in vivo
To assess whether CDCA7L downregulation can could
suppress glioma growth in vivo, U87 human glioma cells
transfected with CDCA7L siRNA or NC siRNA were injected into the
brains of nude mice to form intracranial xenografts.
Bioluminescence imaging revealed that tumor growth in the CDCA7L
siRNA group (maximum diameter, 0.5 cm) was inhibited compared with
that in NC siRNA group (maximum diameter, 1.0 cm) (Fig. 6A). Moreover, IHC experiments were
performed to examine the Ki-67 levels, which are commonly used to
detect tumor proliferation. The results suggested that the Ki-67
expression levels were markedly reduced in the CDCA7L siRNA group
(Fig. 6B). In addition, western
blot analysis also revealed the downregulation of CCND1 expression
in the CDCA7L siRNA group, which was consistent with the results
in vitro (Fig. 6C).
Furthermore, to analyze survival in the different treatment groups,
a Kaplan-Meier survival curve was plotted, which revealed that the
survival of the mice in the CDCA7L siRNA groups was evidently
prolonged compared with that of mice in the NC siRNA groups
(Fig. 6D). Thus, these data
suggested that the knockdown of CDCA7L expression suppresses glioma
growth by downregulating CCND1 expression in vivo.
Discussion
As a transcriptional regulator, c-Myc can promote
tumor development by regulating a variety of target genes; however,
the biological roles of these target genes in the development of
GBM remains unclear. CDCA7L belongs to the JPO protein family,
which has been recently identified as a target gene of c-Myc
(11,12). Additionally, CDCA7L can also
complement the c-Myc transformation-defective mutant W135E and
potentiate the Myc-mediated transformation (13).
A high CDCA7L expression has been reported in
several cancer types, and CDCA7L may be critical for cancer
progression, and may serve as a potential treatment target for
cancer. Moreover, CDCA7L has been shown to induce colony formation
and contribute to the MYC-mediated transformation of
medulloblastoma cells, suggesting that CDCA7L plays a critical role
in the development of medulloblastoma (4). Tian et al (8) reported that CDCA7L activated the
extracellular signal-regulated kinase 1/2 signaling pathway and
controlled the cell cycle, thereby promoting the progression of
hepatic carcinoma.
In the current study, the public expression profiles
and clinical data of glioma patients were collected from the
OncoLnc and Oncomine databases. The analysis of the databases
suggested that the CDCA7L expression level was markedly upregulated
in GBM compared with that in LGG tissues, and a high CDCA7L
expression was associated with a poor prognosis of glioma patients.
Secondly, CDCA7L was proven to be highly expressed in GBM tissues
compared with that in NBTs. Thirdly, to explore the role of CDCA7L
in glioma, CDCA7L siRNA was constructed and transfected into U87
glioma cells, which downregulated CDCA7L expression in the U87
cells in vitro. Taken together, the results of this study
demonstrated that the downregulation of CDCA7L expression evidently
suppressed the proliferation of U87 cells through increasing the
percentage of cells at the G0/G1 phase, while
decreasing the percentage of cells in the S and G2/M phases, and
inducing apoptosis. In addition, the inhibition of CDCA7L
expression markedly suppressed the invasive ability of U87 glioma
cells.
As a target gene of c-Myc, CDCA7L can interact with
c-Myc, and c-Myc has been found in multiple studies to be closely
related to cyclin D1 in a variety of tumors (14–16).
Moreover, previous studies have proven that CDCA7L can regulate the
cell cycle, and that CCND1 may be an important target gene of
CDCA7L in hepatocellular carcinoma (HCC) (8). However, the mechanisms of action of
CDCA7L in glioma remains to be further elucidated. It was found in
the current study that the mRNA and protein expression levels of
CCND1 were markedly downregulated following transfection with
CDCA7L siRNA compared with NC siRNA in vitro. Consistent
with the results of the assays in vitro, the xenograft
assay, IHC assay and western blot analysis also demonstrated that
tumor growth was inhibited in response to CDCA7L inhibition, and
that the Ki-67 and CCND1 expression levels were decreased in
vivo. Moreover, recent studies (17–19)
have suggested that CCND1 is a proto-oncogene located on human
chromosome 11q13, which is highly expressed in multiple types of
cancer, such as glioma, colorectal cancer (CRC) and ovarian cancer.
Typically, CCND1 can encode cyclin D1, while the latter can bind
and activate CDK6 and CDK4, which can lead to the phosphorylation
of pRb, thus driving cell cycle progression from the G1 phase to
the S phase (20). In addition,
CCND1 overexpression can also result in a number of potentially
oncogenic effects, which have been shown to be associated with poor
patient outcomes (21).
Therefore, these results suggest that CDCA7L
promotes the growth of U87 glioma cells through targeting CCND1.
Nevertheless, the role of CDCA7L in other glioma cell lines and the
explicit mechanisms underlying such an association warrant further
assessment and validation.
In conclusion, the findings of this study indicate
that CDCA7L plays a vital role in the progression and prognosis of
human glioma. Moreover, CDCA7L is highly expressed in human glioma
tissues, and a high CDCA7L expression predicts a poor prognosis for
glioma patients. In addition, the downregulation of CDCA7L
expression by CDCA7L siRNA inhibits the proliferation of U87 glioma
cells by downregulating CCND1 both in vitro and in
vivo. These findings indicate that the downregulation of CDCA7L
supresses the growth of glioma U87 cells by inhibiting CCND1, and
that CDCA7L may serve as a novel target in the clinical treatment
for gliomas.
Acknowledgements
Not applicable.
Funding
This article was supported by the Youth Fund Project
of the First Affiliated Hospital of Xinxiang Medical University
(grant no. QN-2017-B009).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QKJ, XSL and FZS performed the cell culture,
transfection, the cell proliferation assay and glioma xenografts.
JWM performed the bioluminescence imaging in vivo. LYH and
LH performed the IHC, western blot analysis and RT-qPCR analysis.
QKJ and RHL performed the flow cytometric analysis. YJM collected
and analyzed the patient data and performed the statistical
analysis. BZJ contributed to the study conception and revised the
study critically for important intellectual content. XSL and RHL
were the major contributors to the writing of the manuscript. QKJ
and JWM were the major contributors to modifying the manuscript.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
According to the Declaration of Helsinki of 1964 and
all subsequent revisions, and with approval of the Medical Ethics
Committee of The First Affiliated Hospital of Xinxiang Medical
University, the present study was accomplished with informed
consent obtained from all patients. The animal experiments were
approved by the Animal Care and Use Committee of the First
Affiliated Hospital of Xinxiang Medical and were carried out in
strict accordance with the experimental protocol.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CDCA7L
|
cell division cycle-associated 7
like
|
|
GBM
|
glioblastoma
|
|
LGG
|
low-grade glioma
|
|
NBTs
|
normal brain tissues
|
|
siRNA
|
small interfering RNA
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
CCND1
|
cyclin D1
|
|
CCK8
|
Cell-Counting kit-8
|
|
PBS
|
phosphate-buffered saline
|
|
IHC
|
immunohistochemistry
|
References
|
1
|
Fan YH, Xiao B, Lv SG, Ye MH, Zhu XG and
Wu MJ: Lentivirus-mediated knockdown of chondroitin polymerizing
factor inhibits glioma cell growth in vitro. Oncol Rep.
38:1149–1155. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xiao B, Fan Y, Ye M, Lv S, Xu B, Chai Y,
Wu M and Zhu X: Downregulation of COUP-TFII inhibits glioblastoma
growth via targeting MPC1. Oncol Lett. 15:9697–9702.
2018.PubMed/NCBI
|
|
3
|
Ji CX, Fan YH, Xu F, Lv SG, Ye MH, Wu MJ,
Zhu XG and Wu L: MicroRNA-375 inhibits glioma cell proliferation
and migration by downregulating RWDD3 in vitro. Oncol Rep.
39:1825–1834. 2018.PubMed/NCBI
|
|
4
|
Huang A, Ho CS, Ponzielli R,
Barsyte-Lovejoy D, Bouffet E, Picard D, Hawkins CE and Penn LZ:
Identification of a novel c-Myc protein interactor, JPO2, with
transforming activity in medulloblastoma cells. Cancer Res.
65:5607–5619. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schmidt EV: The role of c-myc in cellular
growth control. Oncogene. 18:2988–2996. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oster SK, Ho CS, Soucie EL and Penn LZ:
The myc oncogene: Marvelously Complex. Adv Cancer Res. 84:81–154.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yi R, Feng J, Yang S, Huang X, Liao Y, Hu
Z and Luo M: miR-484/MAP2/c-Myc-positive regulatory loop in glioma
promotes tumor-initiating properties through ERK1/2 signaling. J
Mol Histol. 49:209–218. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tian Y, Huang C, Zhang H, Ni Q, Han S,
Wang D, Han Z and Li X: CDCA7L promotes hepatocellular carcinoma
progression by regulating the cell cycle. Int J Oncol.
43:2082–2090. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shen FZ, Li XS, Ma JW, Wang XY, Zhao SP,
Meng L, Liang SF and Zhao XL: Cell division cycle associated 7 like
predicts unfavorable prognosis and promotes invasion in glioma.
Pathol Res Pract. 215:50–56. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Prescott JE, Osthus RC, Lee LA, Lewis BC,
Shim H, Barrett JF, Guo Q, Hawkins AL, Griffin CA and Dang CV: A
novel c-Myc-responsive gene, JPO1, participates in neoplastic
transformation. J Biol Chem. 276:48276–48284. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Goto Y, Hayashi R, Muramatsu T, Ogawa H,
Eguchi I, Oshida Y, Ohtani K and Yoshida K: JPO1/CDCA7, a novel
transcription factor E2F1-induced protein, possesses intrinsic
transcriptional regulator activity. Biochim Biophys Acta.
1759:60–68. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ou XM, Chen K and Shih JC: Monoamine
oxidase A and repressor R1 are involved in apoptotic signaling
pathway. Proc Natl Acad Sci USA. 103:10923–10928. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li X, Tian Z, Jin H, Xu J, Hua X, Yan H,
Liufu H, Wang J, Li J, Zhu J, et al: Decreased c-Myc mRNA stability
via the MicroRNA 141-3p/AUF1 axis is crucial for p63alpha
inhibition of cyclin D1 gene transcription and bladder cancer cell
tumorigenicity. Mol Cell Biol. 38:2018. View Article : Google Scholar
|
|
15
|
Ma J, Ren Y, Zhang L, Kong X, Wang T, Shi
Y and Bu R: Knocking-down of CREPT prohibits the progression of
oral squamous cell carcinoma and suppresses cyclin D1 and c-Myc
expression. PLoS One. 12:e01743092017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vadde R, Radhakrishnan S, Reddivari L and
Vanamala JK: Triphala extract suppresses proliferation and induces
apoptosis in human colon cancer stem cells via suppressing
c-Myc/Cyclin D1 and elevation of Bax/Bcl-2 ratio. Biomed Res Int.
2015:6492632015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu Z, Zeng X, Tian D, Xu H, Cai Q, Wang J
and Chen Q: MicroRNA-383 inhibits anchorage-independent growth and
induces cell cycle arrest of glioma cells by targeting CCND1.
Biochem Biophys Res Commun. 453:833–838. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim MK, Park GH, Eo HJ, Song HM, Lee JW,
Kwon MJ, Koo JS and Jeong JB: Tanshinone I induces cyclin D1
proteasomal degradation in an ERK1/2 dependent way in human
colorectal cancer cells. Fitoterapia. 101:162–168. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xia B, Yang S, Liu T and Lou G: miR-211
suppresses epithelial ovarian cancer proliferation and cell-cycle
progression by targeting Cyclin D1 and CDK6. Mol Cancer. 14:572015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Monteiro LS, Diniz-Freitas M,
Warnakulasuriya S, Garcia- Caballero T, Forteza-Vila J and Fraga M:
Prognostic significance of cyclins A2, B1, D1, and E1 and CCND1
numerical aberrations in oral squamous cell carcinomas. Anal Cell
Pathol (Amst). 2018:72535102018.PubMed/NCBI
|
|
21
|
Katunaric M, Jurisic D, Petkovic M,
Grahovac M, Grahovac B and Zamolo G: EGFR and cyclin D1 in nodular
melanoma: Correlation with pathohistological parameters and overall
survival. Melanoma Res. 24:584–591. 2014. View Article : Google Scholar : PubMed/NCBI
|