Introduction
Uropathogenic Escherichia coli (UPEC) strains
are a subgroup of extra-intestinal pathogenic Escherichia
coli strains, which infect extra-intestinal sites, and urinary
tract infection (UTIs) are an example of the infection consequences
(1). A previous study demonstrated
that UPEC strains divide extraordinarily rapidly in human UTIs
(2). In recent years, the
association between bacterial prostatitis and UPEC has been
increasingly investigated. For example, UPEC infections primarily
contribute to co-resistance against fluroquinolone and β-lactams,
and cause treatment complications (3). A previous animal study demonstrated a
high prevalence of UPEC persistence in prostate tissue after 14
days of bacterial infection, suggesting that UPEC causes severe
prostatic diseases, including prostatitis (4).
Prostatic epithelial cells are the first layer
against pathogenic microorganism infection, and are exposed to
inflammatory mediators and infectious cells (5). Once the prostate is infected by
bacteria, including UPEC, prostate epithelial cells release
inflammatory factors, and the effector cells release tumor necrosis
factor, intercellular adhesion molecules and other cytokines
(6). Prostate epithelial cell
infection is associated with the development of prostatitis,
eventually contributing to fluid imbalance (5). Prostate epithelial cell growth is an
important consideration for the loss of defense function of cells
in prostatitis (7). For instance,
the c-Jun N-terminal kinase 2/signal transducer and activator of
transcription pathway is involved in cell apoptosis; the prostate
epithelial cells regulate the growth of the cells through this
pathway and subsequently secrete inflammatory factors to resist the
bacteria (8).
Cellular tumor antigen p53 (p53) is a
well-documented anti-tumor gene and is involved in multiple cell
life functions, including cell apoptosis (9,10).
Previous studies demonstrated that p53 alleviated mitochondrial
damage induced by inflammatory-associated neurodegenerative
diseases by activating the cellular inflammatory response (11,12).
A meta-analysis of the association between prostatitis and prostate
cancer identified that prostatitis increased the risk of prostate
cancer (13). In the medullary
system, mild p53 activation may decrease the expression of the
c-myc gene and induce mutation of the APC, WNT signaling pathway
regulator gene, thus reducing the inflammatory response in mice
(14). Therefore, the cells become
more resistant to the development and invasion of intestinal
tumors, and as a regulator of macrophage function, p53 serves a key
role in the protection against tumors. A previous study
additionally demonstrated that the p53 gene was involved in the
regulation of the tumor inflammatory microenvironment, inhibited
the development of inflammation and subsequently decreased the
invasion of tumor cells (15).
Therefore, it was hypothesized that p53 is closely associated with
the growth of prostate epithelial cells in the bacterial
prostatitis caused by UPEC. The potential use of p53 in the
treatment of bacterial prostatitis was additionally considered.
In the present study, prostate epithelial cells were
infected with UPEC to identify the effects of Escherichia
coli on cell apoptosis and progression. Alterations in cytokine
expression were additionally studied to determine the association
between cell growth and cytokine expression. Furthermore, the
mechanism underlying the effect of infection on apoptosis of
prostate epithelial cells was examined, and the expression of
apoptosis-associated proteins was assessed. The present results
provide a theoretical basis for future studies on the pathogenesis
of bacterial prostatitis, which may help to develop novel treatment
strategies.
Materials and methods
Cells and UPEC
The prostate epithelial RWPE-1 cells, obtained from
American Type Culture Collection (ATCC; Manassas, VA, USA), were
cultured in keratinocyte-serum free medium (K-SFM; Life
Technologies; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
with 0.05 mg/ml bovine pituitary extract (Life Technologies; Thermo
Fisher Scientific, Inc.) and 5 ng/ml human recombinant epidermal
growth factor (Life Technologies; Thermo Fisher Scientific, Inc.)
in a humidified incubator with 5% CO2 at 37°C. The UPEC
CFT073 strain was additionally obtained from ATCC (16). The CFT073 strain was cultured in
lysogeny broth (Beyotime Institute of Biotechnology, Haimen, China)
and ampicillin (100 µg/ml; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) and subsequently incubated at 37°C for 12 h.
Exposure of prostate epithelial cells
to UPEC and transfection
RWPE-1 cells were cultured in K-SFM and upon
reaching 60% confluence, cells were starved for 24 h (17) to ensure that cell apoptosis and its
signaling pathways were altered prior to the infection of UPEC or
transfection of small interfering RNA (siRNA). The cells were
transfected with Lipofectamine® 2000 (Life Technologies;
Thermo Fisher Scientific, Inc.). RWPE-1 cells were infected with
CFT073 (multiplicity of infection of 100) and incubated at 37°C
with 5% CO2 for 0, 12 and 24 h. The control group cells
were treated with sterile PBS. The siRNA against p53 (sense,
5′-CUACUUCCUGAAAACAAC-3′; antisense, 5′-CGUUGUUUUCAGGAAGUAG-3′) and
its negative control (NC; 5′-GGCTACGTCCAGGAGCGCACC-3′) siRNA were
purchased from Shanghai GenePharma Co., Ltd. (Shanghai, China).
Cells were transfected with 50 nM siRNA, and experiments were
performed 24 h following transfection. The transfected cells were
subsequently treated with UPEC for 12 h to examine the effect of
silencing p53.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) was used to obtain total RNA from the
cells, according to the manufacturer's protocol. The M-MLV Reverse
Transcriptase (Promega Corporation, Madison, WI, USA) was utilized
to synthesize cDNA from the RNA and random primers (Takara Bio,
Inc., Otsu, Japan), following a standard protocol (18). qPCR was performed using an
SYBR-Green kit (Takara Bio, Inc.) according to the manufacturer's
protocol in an ABI-7300 system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). qPCR was performed as follows: 95°C for 30 sec,
then 40 cycles of 95°C for 5 sec and 60°C for 30 sec, and finally
61°C for 1 min. The primers were purchased from Shanghai GenePharma
Co., Ltd. and were used to perform the RT-qPCR, according to the
manufacturer's protocol. Each PCR was replicated three times. The
mRNA relative expression was measured using the 2−∆∆Cq
method (19). The primer sequences
used in present study are presented in Table I. GAPDH was used as the internal
control.
 | Table I.Primers sequences used for polymerase
chain reaction. |
Table I.
Primers sequences used for polymerase
chain reaction.
| Gene | Sense primer
(5′→3′) | Antisense primer
(5′→3′) |
|---|
| p53 |
TCGCTGCGAAGGACATTTGGG |
AGCGACGCTTCCTGTAAACCC |
| Bax |
GGGAGCCAAATGCTTTGCTAG |
CCCTCGGTTTACGAAACGATC |
| Caspase-9 |
AGGACTCAAATTCTGTTGCCACC |
AGGACTCAAATTCTGTTGCCACC |
| Caspase-3 |
TGGAACAAATGGACCTGTTGACC |
AGGACTCAAATTCTGTTGCCACC |
| GAPDH |
CGGAGTCAACGGATTTGGTCGTAT |
AGCCTTCTCCATGGTGGTGAAGAC |
Cell proliferation analysis
Cell proliferation was assessed by a Cell Counting
Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc., Kumamoto,
Japan) assay and performed according to the manufacturer's
protocol. A total of 5×103 cells were seeded in each
well of 96-well plates treated with UPEC. The cells were exposed to
UPEC for separate periods (0, 12 and 24 h). Finally, the absorbance
of the treated cells was detected at 450 nm.
Terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling
(TUNEL) assay
Cells (3×106) were fixed at room
temperature for 30 min with 4% paraformaldehyde. A TUNEL detection
kit (Beyotime Institute of Biotechnology) was used to analyze cell
apoptosis of the different treatment groups, according to the
manufacturer's protocol. Cells were incubated with TUNEL reagent
for 60 min at 37°C without light. DAPI (5 mg/ml) was used to stain
the nuclei of the treated cells for 20 min at 37°C without light.
Cells were mounted with Antifade Mounting Medium (Beyotime
Institute of Biotechnology) and subsequently observed in five
fields per view using a fluorescence microscope under 450–500 nm
light (magnification, ×40).
Flow cytometry analysis
Pre-treated cells (2×106) were enriched
from cell petri dishes and washed twice with cold PBS to remove
floating cells. An Annexin V-Fluorescein Isothiocyanate Apoptosis
Detection kit (Nanjing KeyGen Biotech Co., Ltd., Nanjing, China)
was used to detect the cell apoptosis rate. Apoptosis was measured
using a flow cytometer (BD Biosciences, San Jose, CA, USA) and BD
CellQuest™ version 2.0 software (BD Biosciences).
Western blot analysis
RWPE-1 cells treated with UPEC or p53 siRNA were
lysed for 30 min with lysis buffer (Beyotime Institute of
Biotechnology) on ice and subsequently centrifuged at 4°C and 100 ×
g for 15 min. A bicinchoninic acid protein assay kit (Beyotime
Institute of Biotechnology) was used to evaluate the concentration
of extracted protein. The different sized proteins (20 µl/lane)
were separated using 10% SDS-PAGE and the bands were transferred to
polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA,
USA). The membranes were blocked with 5% skimmed milk powder at
room temperature for 1 h and incubated with primary antibodies
overnight at 4°C. The following antibodies from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA) were used: Apoptosis
regulator BAX (Bax; 1:1,000; sc-20067); p53 (1:1,000; sc-47698);
protein kinase B (Akt; 1:1,000; sc-24500); phosphorylated (p)-Akt
(1:1,000; sc-33437) and β-actin (1:500; sc-517582). Subsequently,
membranes were incubated with horseradish peroxidase-conjugated
secondary antibodies (goat anti-rat immunoglobulin G, 1:1,000;
A0192, and goat anti-rabbit immunoglobulin G; 1:1,000; A0208;
Beyotime Institute of Biotechnology) for 1.5 h at room temperature.
Each protein of interest was detected using enhanced
chemiluminescence reagent (Beyotime Institute of
Biotechnology).
Cytokine assay
Following treatment with UPEC, cytokines secreted by
RWPE-1 cells into the culture medium were determined by ELISAs
(Human IL-4 Quantikine ELISA Kit, S4050; Human IL-6 Quantikine
ELISA Kit, SS600C; Human IL-8/CXCL8 Quantikine ELISA Kit, S8000C;
Human IL-10 Quantikine ELISA Kit, S1000B; all from R&D Systems,
Inc., Minneapolis, MN, USA) or MILLIPLEX MAP Human
Cytokine/Chemokine Magnetic Bead multiplex assay (EMD Millipore)
according to the manufacturer's protocol.
Statistical analysis
All assays were conducted at least three times
independently. The data are presented as the mean ± standard
deviation, and comparisons among different treated groups were
analyzed by one-way analysis of variance followed by
Student-Newman-Keuls test using SPSS 13.0 (SPSS, Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
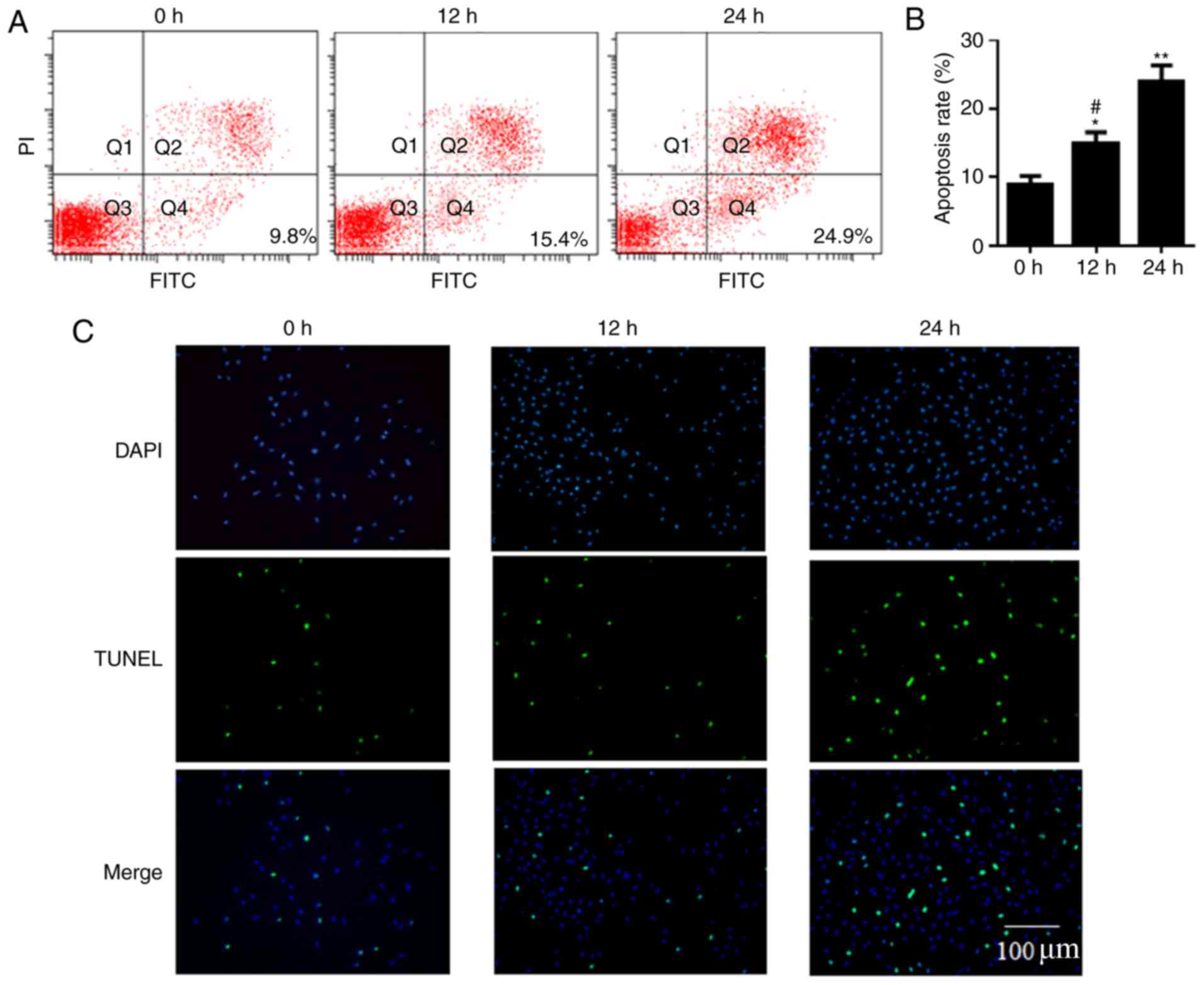
Apoptotic rate of RWPE-1 cells is
increased by infection with UPEC
The apoptosis rate of prostatic epithelial RWPE-1
cells treated with UPEC was analyzed by flow cytometry and TUNEL
assays. The cell apoptosis rates were 9.8, 15.4 and 24.9% at 0, 12
and 24 h, respectively, demonstrated by the flow cytometry assay.
The apoptosis rate at 12 h and 24 h was significantly higher
compared with at 0 h (P<0.05; Fig.
1A and B). The TUNEL assay confirmed that cell apoptosis at 12
h and 24 h increased compared with at 0 h following treatment with
UPEC (Fig. 1C).
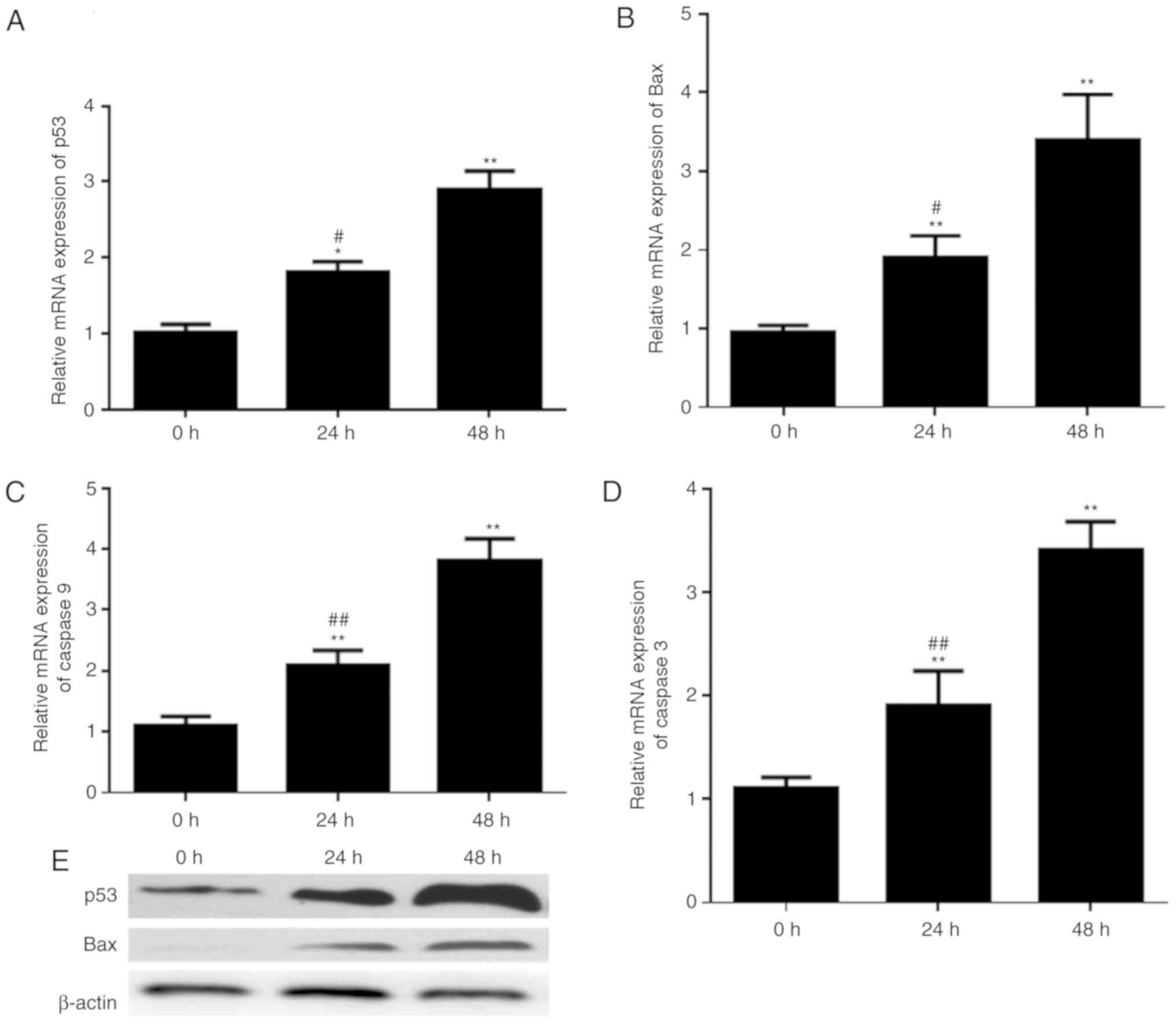
Alterations of p53, Bax, caspase-9 and
caspase-3 expression in prostatic epithelial cells infected with
UPEC
Alterations in the expression of
apoptosis-associated proteins, due to the effect of UPEC on
apoptosis rates, of RWPE-1 cells were measured. The expressions of
p53, Bax, caspase-9 and caspase-3 mRNA were examined by RT-qPCR. It
was identified that the mRNA expression levels of p53 and Bax were
increased upon exposure to UPEC in a time-dependent manner
(Fig. 2A and B). Additionally, the
transcriptional expression level of caspase-9 and caspase-3
demonstrated similar trends (Fig. 2C
and D). Furthermore, western blotting demonstrated that p53 and
Bax protein expressions were upregulated upon infection with UPEC
(Fig. 2E).
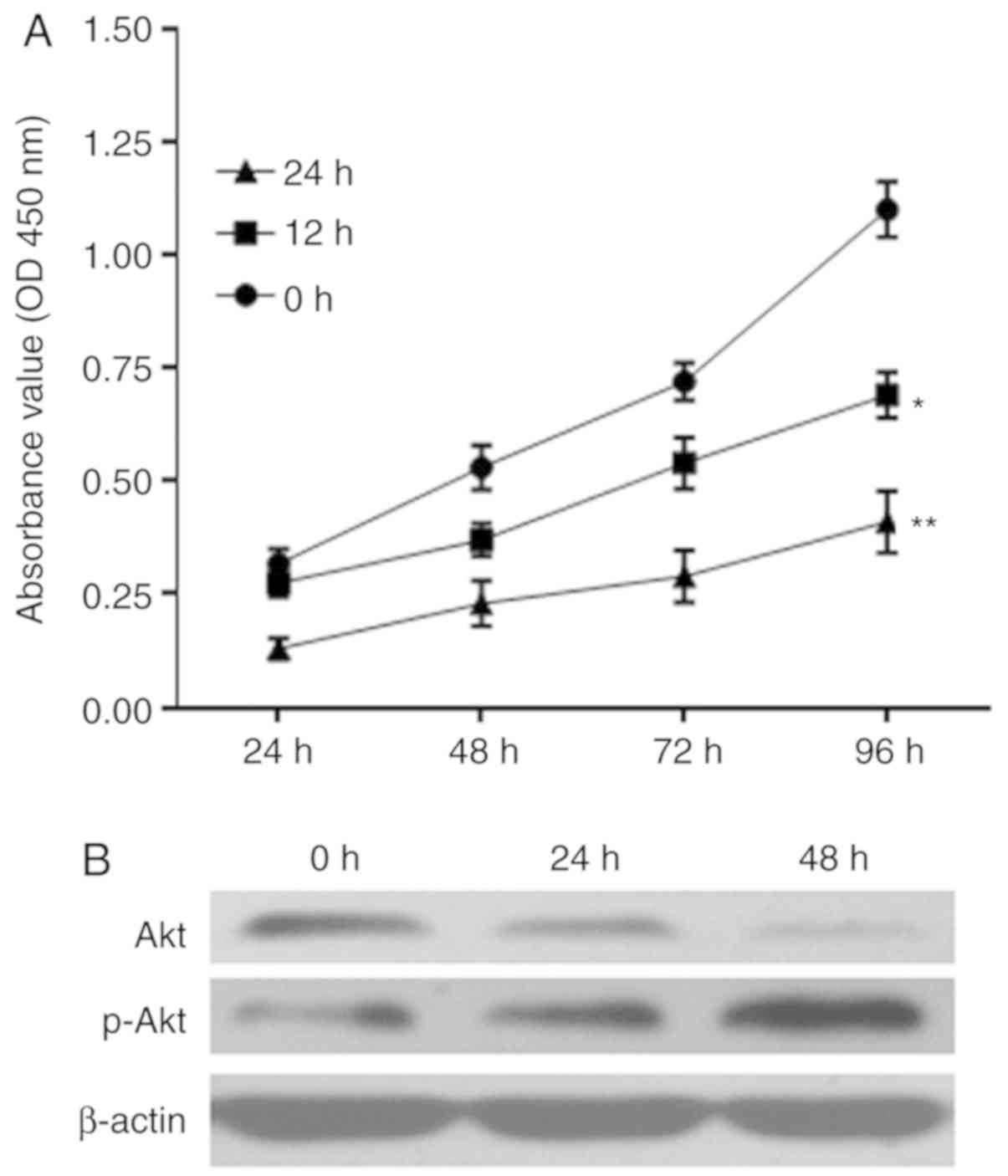
Proliferation of RWPE-1 cells is
inhibited by infection with UPEC
The proliferation of RWPE-1 cells infected with UPEC
was assessed by a CCK-8 assay. Optical density values at 450 nm
demonstrated differences in the proliferation of prostate
epithelial RWPE-1 cells treated with UPEC for 12 and 24 h compared
with 0 h in the CCK-8 assay; as the treatment time increased, the
proliferative ability of RWPE-1 cells at 96 h significantly
decreased (P<0.05; Fig. 3A).
Infection of UPEC additionally led to a downregulation of Akt
protein expression and promotion of p-Akt expression (Fig. 3B).
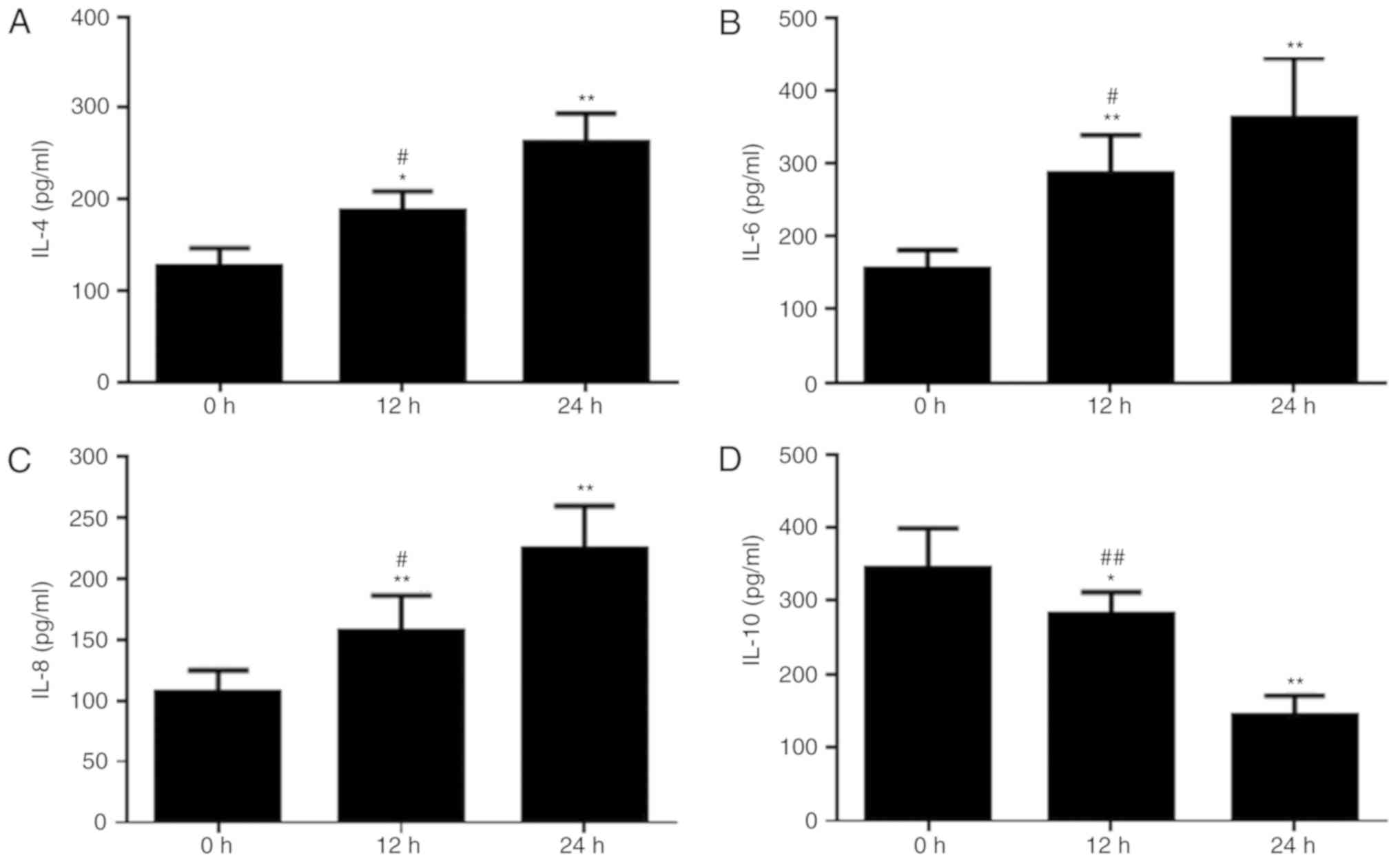
UPEC infection promotes secretion of
IL-4, IL-6 and IL-8, and inhibits IL-10 secretion
Cell growth and apoptosis are closely associated
with the secretion of ILs (20).
Therefore, alterations in the expression levels of ILs secreted by
the cells were studied, as an increase in the rate of apoptosis was
observed in prostatic epithelial cells infected with UPEC. The
concentrations of IL-4, IL-6, IL-8 and IL-10 in the supernatant of
cultured prostatic epithelial cells were examined. As the
incubation time with UPEC increased, the concentrations of IL-4,
IL-6 and IL-8 increased. The concentrations of IL-4, IL-6 and IL-8
were significantly increased at 12 and 24 h, compared with at 0 h
(P<0.05; Fig. 4A-C). However,
the concentration of IL-10 decreased as the UPEC stimulation time
increased. The concentration of IL-10 was significantly decreased
at 12 and 24 h, compared with at 0 h (P<0.05; Fig. 4D).
Inhibition of p53 alleviates prostatic
epithelial cell apoptosis induced by UPEC
siRNA of p53 was used to suppress the expression
level of p53 in prostatic epithelial cells and to examine the
mechanism of UPEC-induced apoptosis in prostatic epithelial cells.
The expression of p53 in the siRNA group was decreased compared
with the siRNA negative group following treatment with p53 siRNA
(Fig. 5A). The apoptosis rates of
the UPEC+siRNA group and PBS+siRNA group demonstrated significant
decreases compared with the UPEC+NC group, following treatment with
UPEC for 12 h (P<0.01; Fig. 5B and
C). The TUNEL assay additionally demonstrated that transfection
with p53 siRNA alleviated RWPE-1 apoptosis induced by infection
with UPEC (Fig. 5D). It was
demonstrated that the expression levels of IL-4 and IL-6 were
modulated by p53 inhibition when the RWPE-1 cells were infected
with UPEC (Fig. 5E and F). In
addition, when the expression of p53 was inhibited, the expression
of apoptosis-associated proteins was downregulated compared with
the UPEC+NC group (Fig. 5G).
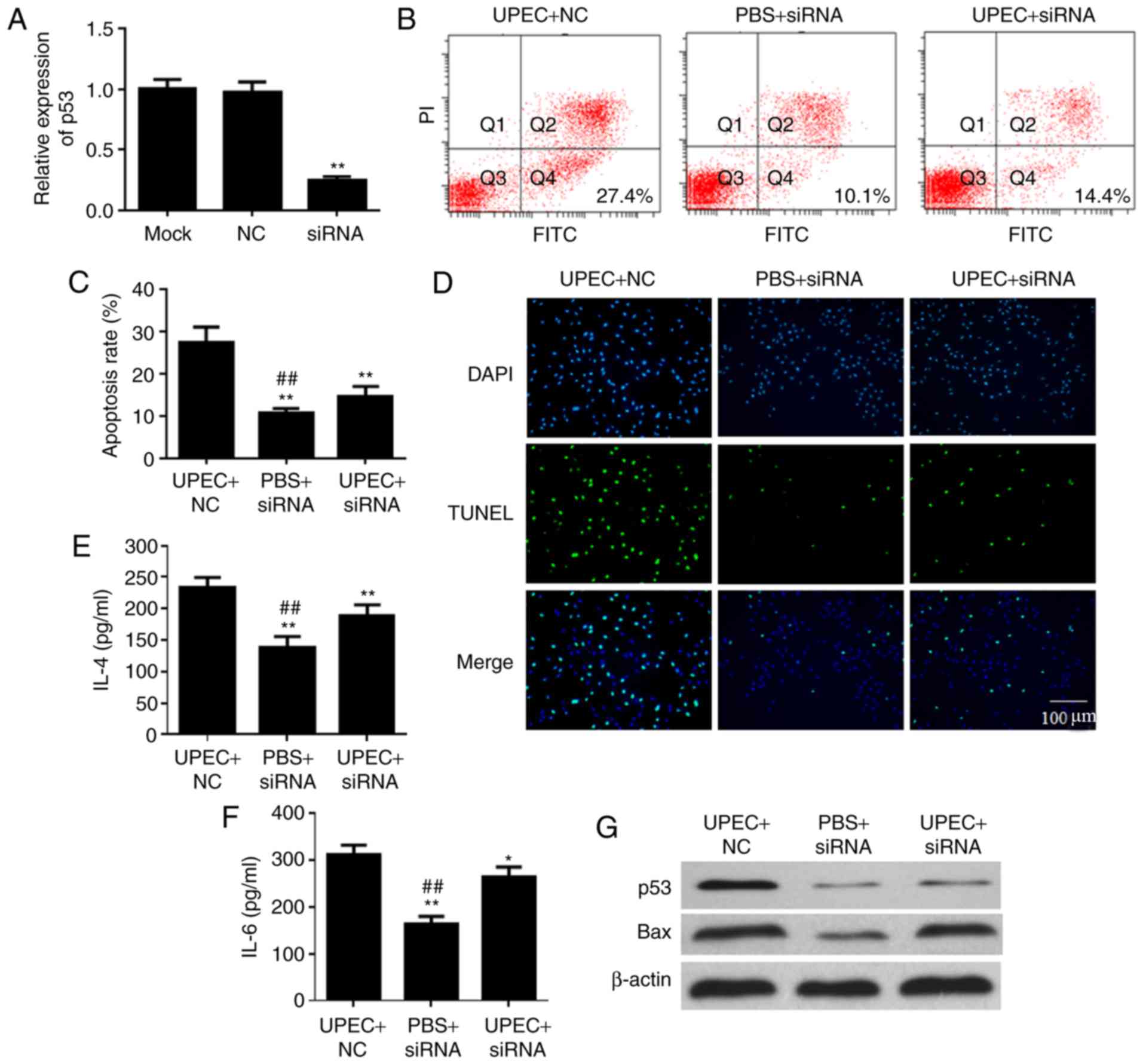 | Figure 5.Inhibition of p53 gene expression
alleviates the apoptosis of prostatic epithelial cells caused by
Escherichia coli infection. (A) siRNA inhibits p53 gene
expression. **P<0.01 vs. NC. Apoptosis rate of RWPE-1 cells was
determined by (B) flow cytometric plots and (C) analysis. (D) TUNEL
assay detected the cell apoptosis of RWPE-1 cells. Expression
levels of (E) IL-4 and (F) IL-6 were affected by UPEC and p53 siRNA
detected by ELISA. *P<0.05, **P<0.01 vs. UPEC+NC;
##P<0.01 vs. UPEC+siRNA. Data are presented as the
mean ± standard deviation. (G) Protein expression of p53 and Bax
under treatment with UPEC and transfection of p53 siRNA were
detected by western blotting. siRNA, small interfering RNA; p53,
cellular tumor antigen p53; NC, negative control; UPEC,
uropathogenic Escherichia coli; TUNEL, terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling; IL,
interleukin; Bax, apoptosis regulator BAX; PI, propidium iodide;
FITC, fluorescein isothiocyante; Q, quadrant. |
Discussion
Escherichia coli has been identified as a
part of the normal intestinal flora; however, alterations in its
location and number of bacteria are likely to cause disease,
particularly inflammation. Escherichia coli infection causes
acute lung injury (21), diarrhea
(22), pelvic inflammatory
(23) and other diseases. UTIs are
the second most common infectious diseases following respiratory
infection (24), and is
additionally closely associated with Escherichia coli. A
previous study observed that >95% of UTIs were caused by single
bacteria, of which 90% were UTIs caused by Escherichia coli
(25). UPEC is able to produce a
number of different virulence factors, including toxins, adhesins
and siderophore receptors (26).
Following contact with the host organism, these virulence factors
serve a critical role by coordinating with each other to avoid and
antagonize the immune attacks of the host, and to invade,
proliferate and infringe on the host organism (27). In addition, the effect of
Escherichia coli infection on the infected cells is to
enhance the apoptosis of cells (28). Therefore, the aim of the present
study was to investigate the effect of Escherichia coli on
the growth of prostate epithelial cells.
p53 is a tumor suppressor gene that has been widely
studied and has numerous functions. It is involved in the
regulation of various cell activities, including the occurrence
(29), development (30), apoptosis and metastasis (31) of cancer cells. A previous study
demonstrated that the p53 gene in prostate cancer cells was
activated, and patients with prostate cancer demonstrated a better
response to treatment (32). In
addition, p53 serves a significant role in cellular inflammation.
In the process of Mycobacterium tuberculosis infection, p53
may serve as a vector for microRNA to regulate the apoptosis of
infected cells and serve an important regulatory role (33). A previous study additionally
identified that p53 gene expression increased in prostatitis,
particularly in acute prostatitis (34). Due to the increased expression of
p53 in inflammation and prostatitis caused by bacterial infection,
the function of p53 in the process of inflammation was examined in
the present study.
The regulation between pro-inflammatory factors,
including tumor necrosis factor, IL-4, IL-6 and IL-8, and
anti-inflammatory factors, including IL-10, is necessary in order
to balance the immune response (35). In addition, cytokines serve an
important role in cell signaling transmission, including
inflammatory reactions and apoptosis (36). A previous study identified that
infection with Mycoplasma pneumonia resulted in an imbalance
in the inflammatory response, leading to the conversion of
apoptosis to cell death, and ultimately resulting in more severe
pulmonary inflammation (37). The
high expression level of IL-6 demonstrates a close association
between inflammation and cell survival, and IL-6 inhibits apoptosis
by regulating hallmarks in inflammation and multiple signaling
pathways (18,38). At the prostatitis stage, cytokines
associated with inflammation, including the expression level of
IL-10, may be indicators for the treatment course of patients
(39). It was identified that ILs
are involved in the apoptosis pathway. For example, IL-10 gene
silencing caused downregulation of phosphoinositide 3-kinase/Akt
and B cell lymphoma-2 (Bcl-2) gene expression, and increases the
Bcl-2-binding component 3, Bax, caspase-3 and caspase-3 cleavage
expression levels, to regulate the apoptosis of breast cancer cells
(40). Therefore, it was
hypothesized that the secretion of ILs by the prostate epithelial
cells may alter subsequent to transfection with siRNA against p53,
alleviating the apoptosis of prostate epithelial cells induced by
UPEC.
The effect of UPEC on prostatic epithelial cell
apoptosis was investigated. Flow cytometry and a TUNEL assay
demonstrated that UPEC infection promoted the apoptosis of prostate
epithelial RWPE-1 cells. The western blotting and RT-qPCR assay
determined the effect of UPEC infection of RWPE-1 cell
apoptosis-associated proteins, and demonstrated that the mRNA and
protein of p53 and Bax expression were increased. In addition, the
mRNA expression levels of caspase-9 and caspase-3 were upregulated
under treatment with UPEC. The proliferation of prostate epithelial
cells decreased as a result, and the progression of bacterial
prostatitis was promoted by UPEC infection. The progression of
prostatitis inflammation was additionally regulated by altering the
secretion of IL-4 IL-6, IL-8 and IL-10 following UPEC infection;
however, due to experimental limitations, the effects of p53
downregulation on the expression of all ILs were not fully
investigated. Therefore, additional experiments are required to
provide further insight into the roles of p53 and IL signaling in
prostatic epithelial cells following UPEC infection.
When siRNA against p53 gene was transfected, the ILs
secreted by prostatitis cells altered, suggesting that siRNA of p53
alleviated the damage of UPEC on the prostate cells. Furthermore,
when the p53 gene, which is closely associated with the apoptotic
process, was inhibited by siRNA, the pro-apoptotic effect of UPEC
on prostate RWPE-1 cells was alleviated. Following infection with
UPEC, the apoptosis of prostate epithelial cells was increased.
However, the apoptosis of prostate epithelial cells was decreased
upon p53 gene silencing. Therefore, it was hypothesized that the
p53 gene is one of the key genes in UPEC infection of prostate
epithelial cells and the formation of bacterial prostatitis, which
additionally promoted the apoptosis of RWPE-1 cells. All the
present results demonstrated that inhibition of p53 gene expression
alleviated the apoptosis of bacterial prostatitis cells, which
additionally provides novel insight for the development of
treatments for bacterial prostatitis caused by UPEC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Basic
Research Projects of Beijing (grant no. 2016B032; China).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZJ designed the study, and HW performed the
experiments and statistical analysis. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ali I, Shabbir M and Noor Ul I:
Antibiotics susceptibility patterns of uropathogenic E. coli with
special reference to fluoroquinolones in different age and gender
groups. J Pak Med Assoc. 67:1161–1165. 2017.PubMed/NCBI
|
|
2
|
Forsyth VS, Armbruster CE, Smith SN,
Pirani A, Springman AC, Walters MS, Nielubowicz GR, Himpsl SD,
Snitkin ES and Mobley HL: Rapid growth of uropathogenic Escherichia
coli during human urinary tract infection. MBio. 9(pii): e00186–18.
2018.PubMed/NCBI
|
|
3
|
Basu S and Mukherjee M: Incidence and risk
of co-transmission of plasmid-mediated quinolone resistance and
extended-spectrum β-lactamase genes in fluoroquinolone-resistant
uropathogenic Escherichia coli: A first study from Kolkata, India.
J Glob Antimicrob Resist. 14:217–223. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boehm BJ, Colopy SA, Jerde TJ, Loftus CJ
and Bushman W: Acute bacterial inflammation of the mouse prostate.
Prostate. 72:307–317. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Seo MY, Im SJ, Gu NY, Kim JH, Chung YH,
Ahn MH and Ryu JS: Inflammatory response of prostate epithelial
cells to stimulation by Trichomonas vaginalis. Prostate.
74:441–449. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Longhi C, Comanducci A, Riccioli A, Ziparo
E, Marazzato M, Aleandri M, Conte AL, Lepanto MS, Goldoni P and
Conte MP: Features of uropathogenic Escherichia coli able to invade
a prostate cell line. New Microbiol. 39:146–149. 2016.PubMed/NCBI
|
|
7
|
Wagenlehner FM, Pilatz A, Bschleipfer T,
Diemer T, Linn T, Meinhardt A, Schagdarsurengin U, Dansranjavin T,
Schuppe HC and Weidner W: Bacterial prostatitis. World J Urol.
31:711–716. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ho CH, Fan CK, Yu HJ, Wu CC, Chen KC, Liu
SP and Cheng PC: Testosterone suppresses uropathogenic Escherichia
coli invasion and colonization within prostate cells and inhibits
inflammatory responses through JAK/STAT-1 signaling pathway. PLoS
One. 12:e01802442017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bilancio A, Bontempo P, Di Donato M, Conte
M, Giovannelli P, Altucci L, Migliaccio A and Castoria G: Bisphenol
A induces cell cycle arrest in primary and prostate cancer cells
through EGFR/ERK/p53 signaling pathway activation. Oncotarget.
8:115620–115631. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hong SH, Lee DH, Lee YS, Jo MJ, Jeong YA,
Kwon WT, Choudry HA, Bartlett DL and Lee YJ: Molecular crosstalk
between ferroptosis and apoptosis: Emerging role of ER
stress-induced p53-independent PUMA expression. Oncotarget.
8:115164–115178. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Choi SE, Park YS and Koh HC:
NF-κB/p53-activated inflammatory response involves in
diquat-induced mitochondrial dysfunction and apoptosis. Environ
Toxicol. 33:1005–1018. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Siegl C and Rudel T: Modulation of p53
during bacterial infections. Nat Rev Microbiol. 13:741–748. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Perletti G, Monti E, Magri V, Cai T,
Cleves A, Trinchieri A and Montanari E: The association between
prostatitis and prostate cancer. Systematic review and
meta-analysis. Arch Ital Urol Androl. 89:259–265. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He XY, Xiang C, Zhang CX, Xie YY, Chen L,
Zhang GX, Lu Y and Liu G: p53 in the myeloid lineage modulates an
inflammatory microenvironment limiting initiation and invasion of
intestinal tumors. Cell Rep. 13:888–897. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Uehara I and Tanaka N: Role of p53 in the
regulation of the inflammatory tumor microenvironment and tumor
suppression. Cancers (Basel). 10:E2192018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saldaña Z, De la Cruz MA, Carrillo-Casas
EM, Durán L, Zhang Y, Hernández-Castro R, Puente JL, Daaka Y and
Girón JA: Production of the Escherichia coli common pilus by
uropathogenic E. coli is associated with adherence to HeLa and
HTB-4 cells and invasion of mouse bladder urothelium. PLoS One.
9:e1012002014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shen XF, Ren LB, Teng Y, Zheng S, Yang XL,
Guo XJ, Wang XY, Sha KH, Li N, Xu GY, et al: Luteolin decreases the
attachment, invasion and cytotoxicity of UPEC in bladder epithelial
cells and inhibits UPEC biofilm formation. Food Chem Toxicol.
72:204–211. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu SY, Huang X and Cheong KL: Recent
advances in marine algae polysaccharides: Isolation, structure and
activities. Mar Drugs. 15:E3882017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nicholl MB, Ledgewood CL, Chen X, Bai Q,
Qin C, Cook KM, Herrick EJ, Diaz-Arias A, Moore BJ and Fang Y:
IL-35 promotes pancreas cancer growth through enhancement of
proliferation and inhibition of apoptosis: Evidence for a role as
an autocrine growth factor. Cytokine. 70:126–133. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Curley GF, Jerkic M, Dixon S, Hogan G,
Masterson C, O'Toole D, Devaney J and Laffey JG: Cryopreserved,
xeno-free human umbilical cord mesenchymal stromal cells reduce
lung injury severity and bacterial burden in rodent Escherichia
coli-induced acute respiratory distress syndrome. Crit Care Med.
45:e202–e212. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ercumen A, Arnold BF, Naser AM, Unicomb L,
Colford JM Jr and Luby SP: Potential sources of bias in the use of
Escherichia coli to measure waterborne diarrhoea risk in low-income
settings. Trop Med Int Health. 22:2–11. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kow N, Holthaus E and Barber MD: Bacterial
uropathogens and antibiotic susceptibility of positive urine
cultures in women with pelvic organ prolapse and urinary
incontinence. Neurourol Urodyn. 35:69–73. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Magistro G, Marcon J, Schubert S, Gratzke
C and Stief CG: Pathogenesis of urinary tract infections: An
update. Urologe A. 56:720–727. 2017.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tarchouna M, Ferjani A, Ben-Selma W and
Boukadida J: Distribution of uropathogenic virulence genes in
Escherichia coli isolated from patients with urinary tract
infection. Int J Infect Dis. 17:e450–e453. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Terlizzi ME, Gribaudo G and Maffei ME:
UroPathogenic Escherichia coli (UPEC) infections: Virulence
factors, bladder responses, antibiotic, and non-antibiotic
antimicrobial strategies. Front Microbiol. 8:15662017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Johnson JR, Jelacic S, Schoening LM,
Clabots C, Shaikh N, Mobley HL and Tarr PI: The IrgA homologue
adhesin Iha is an Escherichia coli virulence factor in murine
urinary tract infection. Infect Immun. 73:965–971. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Eskandari M, Jani S, Kazemi M, Zeighami H,
Yazdinezhad A, Mazloomi S and Shokri S: Ameliorating effect of
ginseng on epididymo-orchitis inducing alterations in sperm quality
and spermatogenic cells apoptosis following infection by
uropathogenic Escherichia coli in rats. Cell J. 18:446–457.
2016.PubMed/NCBI
|
|
29
|
Semczuk A, Ignatov A, Obrzut B, Reventos J
and Rechberger T: Role of p53 pathway alterations in uterine
carcinosarcomas (malignant mixed Müllerian tumors). Oncology.
87:193–204. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xie H and Wang H: PRL-3 promotes breast
cancer progression by downregulating p14ARF-mediated p53
expression. Oncol Lett. 15:2795–2800. 2018.PubMed/NCBI
|
|
31
|
Ali AS, Grönberg M, Federspiel B, Scoazec
JY, Hjortland GO, Grønbæk H, Ladekarl M, Langer SW, Welin S,
Vestermark LW, et al: Expression of p53 protein in high-grade
gastroenteropancreatic neuroendocrine carcinoma. PLoS One.
12:e01876672017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chopra H, Khan Z, Contreras J, Wang H,
Sedrak A and Zhu Y: Activation of p53 and destabilization of
androgen receptor by combinatorial inhibition of MDM2 and MDMX in
prostate cancer cells. Oncotarget. 9:6270–6281. 2017.PubMed/NCBI
|
|
33
|
Liang S, Song Z, Wu Y, Gao Y, Gao M, Liu
F, Wang F and Zhang Y: MicroRNA-27b modulates inflammatory response
and apoptosis during Mycobacterium tuberculosis infection. J
Immunol. 200:3506–3518. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang W, Bergh A and Damber JE: Increased
p53 immunoreactivity in proliferative inflammatory atrophy of
prostate is related to focal acute inflammation. APMIS.
117:185–195. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu Y, Shi B, Li Y and Zhang H: Protective
effect of luteolin against renal ischemia/reperfusion injury via
modulation of pro-inflammatory cytokines, oxidative stress and
apoptosis for possible benefit in kidney transplant. Med Sci Monit.
23:5720–5727. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li L, Wang B, Yu P, Wen X, Gong D and Zeng
Z: Medium and long chain fatty acids differentially modulate
apoptosis and release of inflammatory cytokines in human liver
cells. J Food Sci. 81:H1546–H1552. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bai F, Ni B, Liu M, Feng Z, Xiong Q and
Shao G: Mycoplasma hyopneumoniae-derived lipid-associated membrane
proteins induce inflammation and apoptosis in porcine peripheral
blood mononuclear cells in vitro. Vet Microbiol. 175:58–67. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kumari N, Dwarakanath BS, Das A and Bhatt
AN: Role of interleukin-6 in cancer progression and therapeutic
resistance. Tumour Biol. 37:11553–11572. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
La Vignera S, Calogero AE, Castiglione R,
D'Agata R, Giammusso B, Condorelli R and Vicari E: IL-6, TNFalfa,
IL-10 in the seminal plasma of patients with bacterial male
accessory gland infections after sequential therapy. Minerva Urol
Nefrol. 60:141–145. 2008.PubMed/NCBI
|
|
40
|
Alotaibi MR, Hassan ZK, Al-Rejaie SS,
Alshammari MA, Almutairi MM, Alhoshani AR, Alanazi WA, Hafez MM and
Al-Shabanah OA: Characterization of apoptosis in a breast cancer
cell line after IL-10 silencing. Asian Pac J Cancer Prev.
19:777–783. 2018.PubMed/NCBI
|