Introduction
Ischemic stroke is a common clinical disorder that
affects the global population with high incidence, mortality and
disability (1,2). Cerebral ischemic injury (CII) caused
by cerebral ischemia is the main pathological and physiological
basis of ischemic stroke (3).
Angiogenesis is an important compensatory mechanism following
ischemic stroke, which has been implicated in animal models and
human patients (4). However, this
mechanism is not enough to attenuate CII. Thus, understanding the
mechanism controlling angiogenesis is of significance for the
development of effective therapeutic strategies for ischemic
stroke.
Macrophage migration inhibitory factor (MIF), one of
the first functional cytokines identified, is an important
angiogenic regulator (5,6). Rassaf et al (7) demonstrated that MIF upregulation
improved angiogenesis in myocardial ischemia/reperfusion injury.
Liao et al (8) reported
that MIF contributed to angiogenesis by upregulating interleukin
(IL)-8 in primary nasopharyngeal carcinoma. Girard et al
(9) demonstrated that
overexpression of MIF is involved in angiogenesis in the B16-F10
melanoma model, and the absence of MIF resulted in slower tumor
growth, which was associated with reduced vascularity. An
accumulating body of evidence has indicated that MIF is
overexpressed during ischemic stroke in patients and a rat stroke
model, and was associated with the severity of the pathology
(10,11). However, how MIF works in CII
remains unknown.
MicroRNAs (miRNAs/miRs) are small endogenous
non-coding RNAs that negatively regulate gene expression by binding
to the 3′-untranslated region (UTR) of target mRNAs (12,13).
Several miRNAs have been identified to be involved in the
regulation of angiogenesis. For example, Liu et al (14) revealed that miR-106b and miR-15b
modulate angiogenesis in myocardial infarction. Downregulation of
miR-195 promoted angiogenesis induced by cerebral infarction by
targeting vascular endothelial growth factor A (VEGFA) (15). In addition, Li et al
(16) revealed that miR-493
inhibited tube formation and the migration of rat brain
microvascular endothelial cells by suppressing MIF. However,
limited studies have focused on the functions of miRNAs in the
regulation of angiogenesis following cerebral ischemia.
The present study performed a miRNA microarray to
investigate miRNA expression in the serum samples of cerebral
ischemic patients. Then, the roles and underlying mechanisms of the
candidate miRNA, miR-451, in the regulation of angiogenesis were
investigated using a cell model of CII. The present results
indicate that miR-451 may be a potential therapeutic option for
CII.
Materials and methods
Serum samples
Serum samples were obtained from 15 patients with
cerebral ischemia who were also diagnosed with ischemic stroke by
MRI, as well as 15 healthy participants at the Workers' Hospital of
Tangshan City (Hebei, China). All experimental protocols were
approved by the Ethics Committee of the Workers' Hospital of
Tangshan City. Written informed consent was obtained from all
patients. All samples were flash-frozen in liquid nitrogen, and
stored at −80°C until further molecular analysis. The demographics
and clinical characteristics of the 15 cerebral ischemic patients
and 15 healthy controls are provided in Table I.
 | Table I.Demographic and clinical
characteristics in patients with cerebral ischemia and normal
controls. |
Table I.
Demographic and clinical
characteristics in patients with cerebral ischemia and normal
controls.
| Clinical
parameters | Patients (n=15), n
(% or range) | Healthy controls
(n=15), n (%) |
|---|
| Sex |
|
|
|
Male | 8 (53.3) | 6 (40) |
|
Female | 7 (46.7) | 9 (60) |
| Age (years) | 64 (54–72) | 60 (52–70) |
| Cerebral ischemic
risk factors |
|
|
|
Hypertension | 8 (53.3) | – |
|
Diabetes mellitus | 4 (26.7) | – |
| Atrial
Fibrillation | 5 (33.3) | – |
|
Hyperlipidemia | 6 (40) | – |
|
Coronary heart disease | 3 (20) | – |
|
Baseline median NIHSS
score | 8 | 0 |
|
25th-75th percentile | 6–11 | – |
|
Baseline DWI median volume
(cm3) | 18.58 | 0 |
|
25th-75th percentile
(cm3) | 9.79–30.48 | – |
miRNA microarray
Total RNA was isolated from the sera of patients
with cerebral ischemia by a miRNAeasy mini kit (Qiagen, Inc.,
Valencia, CA, USA). The purity and quantity of total RNA were
evaluated by NanoDrop ND-1000 Spectrophotometry (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and Agilent's 2100 Bioanalyzer
(Agilent Technologies, Inc., Santa Clara, CA, USA). Total RNA (200
ng) was labeled and hybridized with the miRCURY™ LNA Array (version
16.0; Exiqon; Qiagen, Inc.). Following washing, Axon GenePix 4000B
microarray scanner (Axon Instruments; Molecular Devices, LLC,
Sunnyvale, CA, USA) was used to scan the fluorescence intensity of
the microarray. Scanned images were then imported into the GenePix
Pro6.0 program (Axon Instruments; Molecular Devices, LLC) for grid
alignment and data extraction. Finally, the heat map of the 57
miRNAs with the most evident differences was created using a method
of hierarchical clustering with GeneSpring GX, version 7.3 (Agilent
Technologies, Inc.).
Cell culture and hypoxia
HUVECs were obtained from the cell bank of the
Chinese Academy of Sciences (Shanghai, China), and maintained in
M199 medium supplemented with 20 mg/ml endothelial cell growth
supplement (Upstate Biotechnology, Inc., Lake Placid, NY, USA) and
10% fetal bovine serum (FBS; HyClone; GE Healthcare Life Sciences,
Logan, UT, USA) at 37°C in a humidified incubator with an
atmosphere of 95% air and 5% CO2 (normoxic conditions).
For hypoxia, HUVECs were cultured in a hypoxia incubator (Sanyo
Electric Co., Ltd., Osaka, Japan) under hypoxic conditions (5%
CO2, 94% N2 and 1% O2,) for 6 h at
37°C. Cells cultured under normoxic conditions were used as
controls.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from serum samples and HUVECs
cells using TRIzol reagents (Invitrogen; Thermo Fisher Scientific,
Inc.) following the manufacturer's instructions and reverse
transcription was performed using PrimeScript™ RT reagent kit
(Takara Biotechnology Co., Ltd., Dalian, China) at 42°C for 30 min
and 85°C for 5 sec. For the detection of miRNA, RT-qPCR assays were
performed using the TaqMan miRNA Assay (Thermo Fisher Scientific,
Inc.) following the manufacturer's instructions. For detection of
the mRNA levels, qPCR was performed on an ABI PRISM 7300 sequence
detection system in an SYBR Green I Real-Time PCR kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The RT-qPCR reaction
system (30 µl) contained 5 µl cDNA, 15 µl 2X qPCR mix, 1 µl
upstream primer, 1 µl downstream primer and 8 µl double distilled
H2O. The PCR protocol was: 95°C for 15 min, followed by
40 cycles of 94°C for 15 sec, 55°C for 30 sec and 70°C for 30 sec
and a final extension step at 72°C for 5 min. U6 and GAPDH
functioned as the normalization controls in the expression analysis
of miR-451 and MIF, respectively. The relative expression of RNAs
was calculated using the 2−ΔΔCq method (17). Each reaction was conducted in
triplicate. The primers utilized for RT-qPCR analysis were as
follows: miR-451 forward, 5′-AAAGTCGACAAGCTCTCTGCTCAGCCTGTC-3′ and
reverse, 5′-AAAATATCTCGAGCCCCCACCCCTGCCTTGT-3′; U6 forward,
5′-TGCGGGTGCTCGCTTCGCAGC-3′ and reverse, 5′-CCAGTGCAGGGTCCGAGGT-3′;
MIF forward, 5′-GGCCTCACTTACCTGCACC-3′ and reverse,
5′-AACCATTTATTTCTCCCGACC-3′; GAPDH forward,
5′-GCAACTCCCACTCTTCCACC-3′ and reverse,
5′-GTCATACCAGGAAATGAGCTTGACA-3′. The RT-qPCR assays were performed
in triplicate and the change in expression level was calculated
using the 2−ΔΔCq method.
Cell transfection
HUVECs were seeded at a density of
6×104/cm2 in 6-well plates and allowed to
settle for 24 h to ensure that 40–50% confluence was achieved prior
to transfection. miR-451 mimic (5′-AAACCGUUACCAUUACUGAGUU-3′) and
its negative control (NC; 5′-UCGCUUGGUGCAGGUCGGGAA-3′), miR-451
inhibitor (5′-AACUCAGUAAUGGUAACGGUUU-3′) and negative control (NC;
5′-CAGUACUUUUGUGUAGUACAA-3′) were synthesized by Shanghai
GenePharma Co., Ltd. (Shanghai, China). The MIF small interfering
RNA (siRNA, (5′-ACACCAACGUGCCCCGCGCdTdT-3′) and NC siRNA
(5′-GCGCGGGGCACGUUGGUGUdTdT-3′) were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, CA, USA). In addition, to enhance the
expression of MIF, the coding domain sequences of MIF mRNA were
amplified by PCR, and inserted into pcDNA 3.0 vector (Invitrogen;
Thermo Fisher Scientific, Inc.), and named pcDNA-MIF. Cells were
cultured to 80% confluence, followed by transfection with miR-451
mimics (50 nM), control mimics, miR-451 inhibitor (50 nM) or
control inhibitor using Lipofectamine 2000® (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions. MIF expressing vector (2 µg) was transfected into
HUVECs with Lipofectamine® 2000 following manufacturer's
protocol. After 24 h, the transfected cells were subjected to
hypoxic conditions for 6 h. The transfection efficiency was
determined by RT-qPCR.
Tube formation
Confluent HUVEC monolayers were 0.25% trypsinized
and plated onto 24-well plates that were coated with Matrigel
(Becton, Dickinson and Company, Franklin, Lakes, NJ, USA) and
incubated in M199 medium for 24 h at 37°C. Then, the transfected
cells were subjected to hypoxic conditions for 6 h as
aforementioned and tube-like structures were observed under a
routine light microscope and images captured from five randomly
selected microscopic fields. Tube formations were calculated by
counting the number of branches, which was conducted using ImageJ
software (Version 1.46, National Institutes of Health, Bethesda,
MD, USA).
Transwell invasion assay
Invasion activities of HUVECs were analyzed using
Boyden chambers with 8-mm pore membranes coated with Matrigel
(Becton, Dickinson and Company) following manufacturer's protocol.
Briefly, following 24 h transfection, HUVECs (1×104 per
well) were seeded into the upper chambers in 200 µl serum-free M199
medium, and the lower chambers were filled with 500 µl of M199
medium containing 10% FBS. Following exposure to hypoxic or
normoxic conditions for 6 h, cells on the surface of the filter
were fixed with 4% formaldehyde, stained with 0.5% crystal violet
at 37°C for 30 min, and counted under a routine light microscope
(Phenix Optical Instrument Group Company, Jiangxi, China). Cells
were counted in five randomly chosen microscopic fields.
Wound healing assay
HUVECs (1×106 per well) were plated onto
6-well plates and incubated in M199 for 24 h at 37°C. Following 24
h transfection, the cells were scrapped with a 10 µl pipette tip,
fresh serum-free medium was added and then cells were exposed to
hypoxic or normoxic conditions for 6 h. Initial images were
acquired as a reference and, following 6 h secondary images were
taken corresponding to the photographed region capture initially.
Wound healing was evaluated by measuring the distance of the
wounded region with an absence of cells using ImageJ software
(Version 1.46, National Institutes of Health, Bethesda, MD,
USA).
Bioinformatics
TargetScan (version 7.0; www.targetscan.org/) and PicTar (version 2006;
http://pictar.mdc-berlin.de) target gene
prediction software were used to select MIF as a target gene of
miR-451.
Luciferase reporter assay
The 3′-untranslated region (UTR) of MIF and the
mutated sequence were inserted into the pGL3 control vector
(Promega Corporation, Madison, WI, USA) to construct the wild-type
(wt) MIF-3′-UTR vector and mutant MIF-3′-UTR vector, respectively.
For the luciferase reporter assay, HUVECs were transfected with the
corresponding vectors using Lipofectamine 2000®
(Invitrogen; Thermo Fisher Scientific, Inc.); a 48 h
post-transfection, the dual-luciferase reporter assay system
(Promega Corporation) was used to measure luciferase activity. To
correct for differences in transfection and harvesting
efficiencies, Renilla luciferase activity was used to
normalize the firefly luciferase activity. All experiments were
performed in triplicate.
Western blot analysis
Total protein was extracted from HUVECs cells using
radioimmunoprecipitation lysis buffer (Beyotime Institute of
Biotechnology, Shanghai, China). Concentrations of total cellular
protein were determined using a BCA assay kit (Pierce; Thermo
Fisher Scientific, Inc.). Total protein samples (40 µg) were
analyzed by 8% SDS-PAGE and transferred to polyvinylidene
difluoride membranes (GE Healthcare, Chicago, IL, USA) by
electroblotting. Membranes were blocked with 5% nonfat milk at room
temperature for 1 h, followed by incubation overnight at 4°C with
primary antibodies. Primary antibodies against MIF (cat. no.
sc-130329; Santa Cruz Biotechnology, Inc., Danvers, MA, USA;
1:1,000 dilution), phospho (p)-VEGF Receptor 2 (cat. no. 2478;
VEGFR2; Tyr1175; Cell Signaling Technology, Inc.; 1:1,000
dilution), VEGF (cat. no. 2463; Cell Signaling Technology, Inc.;
1:1,000 dilution) and total VEGFR2 (cat. no. 9698; Cell Signaling
Technology, Inc.; 1:1,000 dilution) and β-actin (cat. no. sc-58673;
Santa Cruz Biotechnology, Inc.; 1:2,000 dilution) were incubated
with the membrane at 4°C overnight. Following incubation with
anti-rabbit IgG (H+L; DyLight™ 680 Conjugate; cat. no. 5366; Cell
Signaling Technology, Inc.; 1:10,000 dilution), bands were detected
using an enhanced chemiluminescence kit (GE Healthcare). The
intensity of the bands of interest was analyzed by ImageJ software
(version 1.46; National Institutes of Health).
Statistical analysis
Statistical analysis was performed using SPSS
software (version 18.0; IBM Corp., Chicago, IL, USA). Data were
presented as the mean ± standard deviation. Student's t-test or
one-way analysis of variance followed by Tukey post hoc test was
used to analyze the difference among/between sample groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
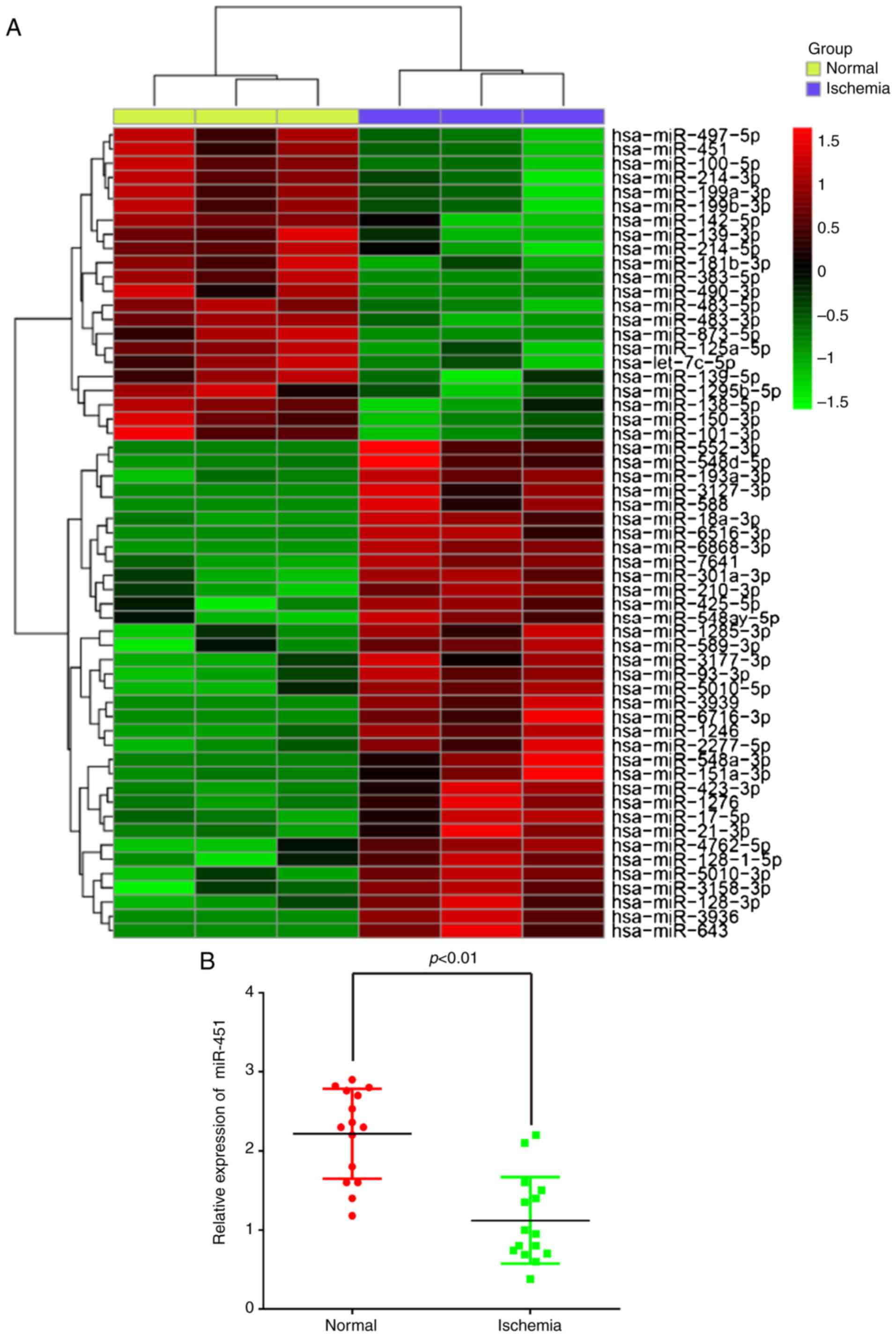
miR-451 is downregulated in the serum
samples of patients with cerebral ischemia
To explore the potential involvement of miRNAs in
CII, the present study performed miRNA microarray profiling in
serum samples from patients with cerebral ischemia. The miRNA
microarray identified 35 miRNAs that were upregulated and 22 miRNAs
that were downregulated in the ischemia group when compared with
the control group (Fig. 1A). Among
them, miR-451 was the most significantly downregulated, and
previous studies have revealed that miR-451 functions as a
suppressor of angiogenesis in hepatocellular carcinoma (18) and human osteosarcoma (19), but little is known regarding its
role in the regulation of angiogenesis in CII. Therefore, the
present study decided to focus on miR-451 in CII for further
study.
To confirm the microarray findings, the miR-451
expression levels were determined by RT-qPCR in all samples.
miR-451 was significantly downregulated in the serum samples from
patients with cerebral ischemic compared with the normal
participants (Fig. 1B), which is
consistent with the results observed following microarray analysis.
All of these results suggest that the alterations in miR-451
expression may serve important roles in CII.
Overexpression of miR-451 suppresses
the tube formation and migration of HUVECs under hypoxic
conditions
To assess the biological role of miR-451 in
angiogenesis, the present study applied HUVECs under hypoxic
conditions to mimic ischemia in vitro (20,21)
and the expression of miR-451 was determined by RT-qPCR. As shown
in Fig. 2A, hypoxia treatment
induced a significant decrease in the expression of miR-451 in
HUVECs, which reached a peak at 6 h and then raised to near basal
levels by 12 h. Therefore, the present study selected the 6 h time
point as the subsequent experimental condition to study the
influence of miR-451 on angiogenesis. As angiogenesis is defined as
new microvessel formation via branching off from pre-existing
vessels, which involves multi-step biological processes, including
proliferation, migration and the formation of tube-like vascular
structures (22–24), capillary-like tube formation and
migration assays are normally used to evaluate angiogenesis in
vitro (25). To further
investigate the role of miR-451 in tube formation and migration,
HUVECs were transfected with miR-451 mimics or mimics NC, followed
by normoxic or hypoxia treatment. It was observed that the
expression of miR-451 was enhanced following miR-451 mimics
transfection under normoxia or hypoxia conditions (Fig. 2B). Furthermore, the tube formation
assays demonstrated that overexpression of miR-451 significantly
reduced the ability of HUVECs to form tubular structures during
hypoxia (Fig. 2C). The Transwell
assay revealed that the number of invaded cells was significantly
reduced in miR-451 mimics group when compared with mimics NC group
(Fig. 2D). In addition, the
results also revealed that miR-451 mimics decreased the cell
migration distance when compared with the mimics NC group (Fig. 2E).
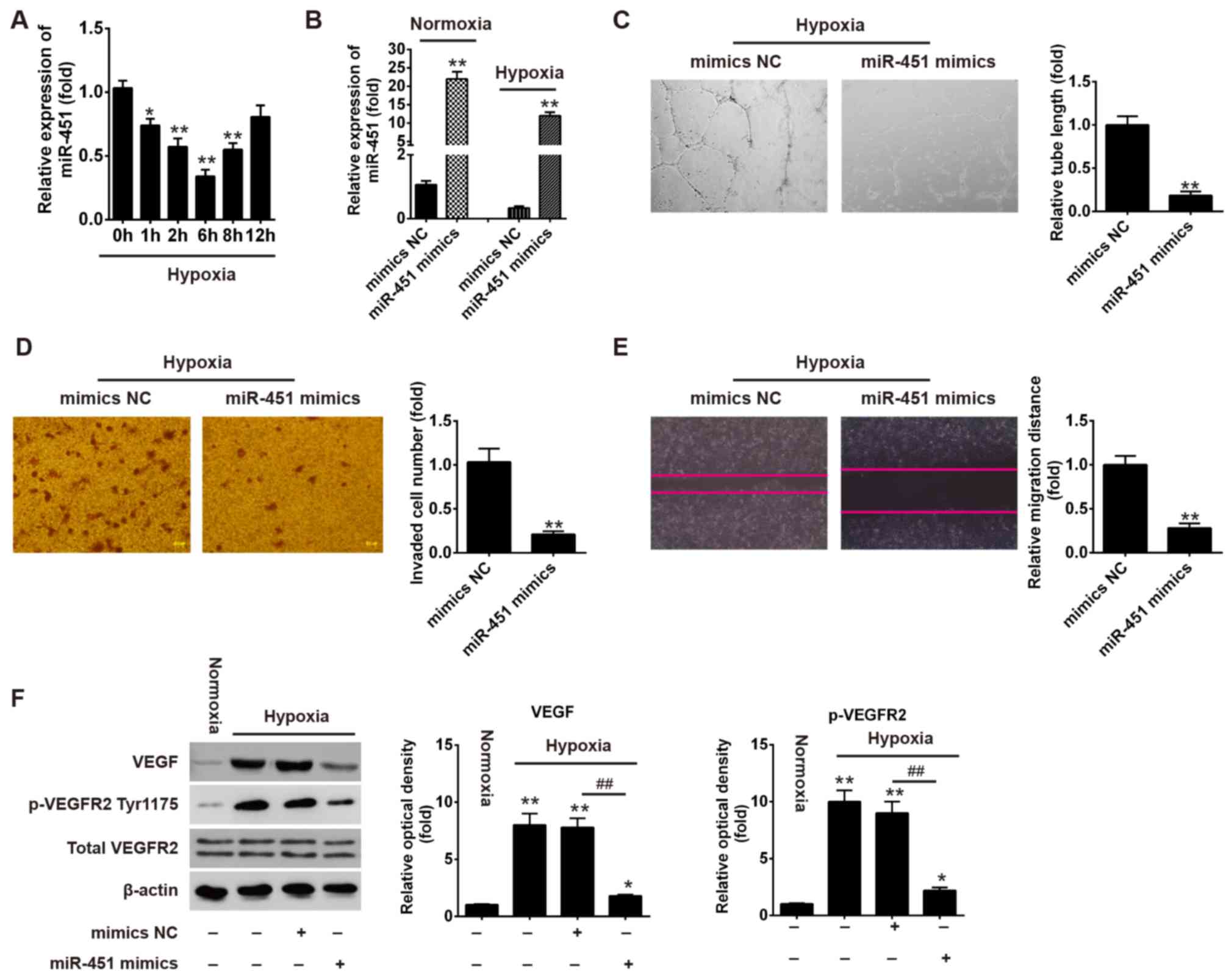 | Figure 2.Overexpression of miR-451 suppresses
the tube formation and migration of HUVECs under hypoxic
conditions. (A) HUVECs were treated with hypoxia for the indicated
time points. The relative expression level of miR-451 was detected
by RT-qPCR. *P<0.05 and **P<0.01 vs. control group (0 h).
HUVECs were transfected with miR-451 mimics or mimics NC. Following
24 h, cells were treated with normoxia or hypoxia for 6 h. (B) The
relative expression level of miR-451 was determined by RT-qPCR
under normoxia or hypoxia conditions. All data are expressed as the
mean ± standard deviation, **P<0.01 vs. mimics NC group. (C) The
tube formation of HUVECs was measured by Matrigel assays
(magnification, ×200). All data are expressed as the mean ±
standard deviation. **P<0.01 vs. mimics NC group. (D) The
invasive ability of HUVECs was measured by a Transwell assay
(magnification, ×200). All data are expressed as the mean ±
standard deviation. **P<0.01 vs. mimics NC group. (E) Cell
migration distance was detected by the wound healing assay
(magnification, ×200). All data are expressed as the mean ±
standard deviation. **P<0.01 vs. mimics NC group. (F) The
expressions of p-VEGFR2 (Tyr1175), VEGF and total VEGFR2 proteins
were measured by western blotting. All data are expressed as the
mean ± standard deviation. *P<0.05 and **P<0.01 vs. Normoxia
group; ##P<0.01, as indicated. RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; miR,
microRNA; HUVECs, human umbilical vein endothelial cells; VEGFR,
vascular endothelial growth factor receptor; VEGF, vascular
endothelial growth factor; p-, phosphorylated; NC, negative
control. |
Given the importance of the VEGF/VEGFR axis in
angiogenesis following CII (26–29),
the present study sought to determine whether miR-451 affects this
axis in HUVECs. The results of western blotting revealed that the
expressions of VEGF and p-VEGFR2 were markedly increased in HUVECs
under hypoxic conditions, whereas this promotional effect was
attenuated following treatment with miR-451 mimics (Fig. 2F), which indicated that the
overexpression of miR-451 could inhibit the VEGF/VEGFR2
pathway.
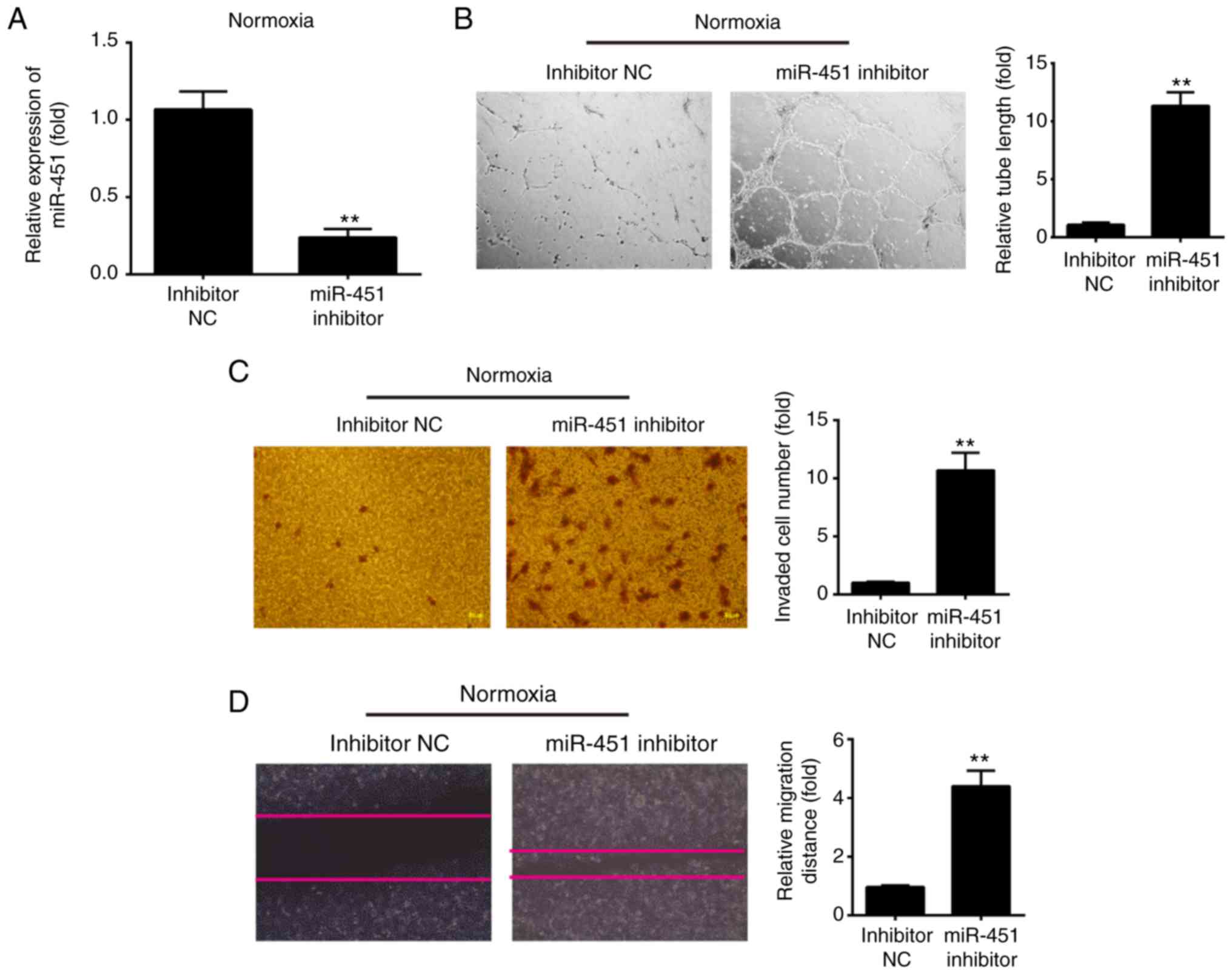
Knockdown of miR-451 promotes the tube
formation and migration of HUVECs under normoxic conditions
Next, to assess the effect of miR-145 inhibition on
HUVECs migration and tubulogenesis under normoxic conditions,
HUVECs were transfected with miR-451 inhibitor to decrease miR-451
expression and then under normoxic conditions for 6 h. Following
miR-451 inhibitor transfection, miR-451 expression was markedly
decreased when compared with inhibitor NC (Fig. 3A). Then, the angiogenic properties
of HUVECs were detected using tube formation, Transwell and wound
healing assays. The tube formation assays revealed that knockdown
of miR-451 promoted the ability of HUVECs to form tubular
structures during normoxia (Fig.
3B). The Transwell assay revealed that the number of invading
cells was significantly increased in the miR-451 inhibitor group
when compared with the inhibitor NC group (Fig. 3C). In addition, miR-451 inhibitor
also increased the cell migration distance when compared with the
inhibitor NC group (Fig. 3D). All
of these results indicated that low level miR-451 is beneficial to
angiogenesis in HUVECs.
MIF is a direct target of miR-451
To explore the molecular mechanisms by which miR-451
regulates the tube formation, invasion and migration of HUVECs,
candidate target genes of miR-451 were computationally screened
using TargetScan and PicTar algorithms. Among several predicted
target genes, MIF, which has been reported to improve angiogenesis,
was of interest due to its high scores in the two algorithms. As
shown in Fig. 4A, miR-451
contained a sequence that was complementary to MIF. In addition,
previous research has shown that MIF was a direct target of miR-451
in human osteosarcoma (30).
However, the association between miR-451 and MIF in CII remains
unclear. Firstly, the present study determined the expression of
MIF in HUVECs under hypoxic conditions by RT-qPCR. As shown in
Fig. 4B, hypoxia treatment caused
a significant increase in the expression of MIF in HUVECs, which
reached a peak at 6 h and declined to near basal levels by 12 h.
The expression levels of MIF in all serum samples were also
determined and it was revealed that the expression of MIF was
significantly increased in the ischemia group when compared with
the normal group (Fig. 4C). To
further confirm that MIF was negatively regulated by miR-451, the
present study performed western blot analysis to determine the
protein level of MIF. The expression of MIF at the protein level
was significantly downregulated following the overexpression of
miR-451 in HUVECs cells under hypoxic conditions, but upregulated
following knockdown of miR-451 in HUVECs cells under normoxic
conditions (Fig. 4D and E). To
verify whether MIF is a direct target of miR-451, the 3′-UTR of MIF
containing the WT or Mut miR-451 target sequences was cloned into
the pmirGLO vector. Co-transfection was conducted with these
reporter plasmids and miR-451 mimics, mimics NC, miR-451 inhibitor
or inhibitor-NC into HUVECs, then luciferase activities were
analyzed 48 h post-transfection. Luciferase reporter gene assays
demonstrated that overexpression of miR-451 markedly repressed,
while knockdown of miR-451 increased, the relative luciferase
activity of constructs containing the WT MIF 3′-UTR. However, the
luciferase activity of the reporter containing the mutant binding
site was not altered (Fig. 4F).
These data indicated that miR-451 regulates the expression of MIF
in HUVECs.
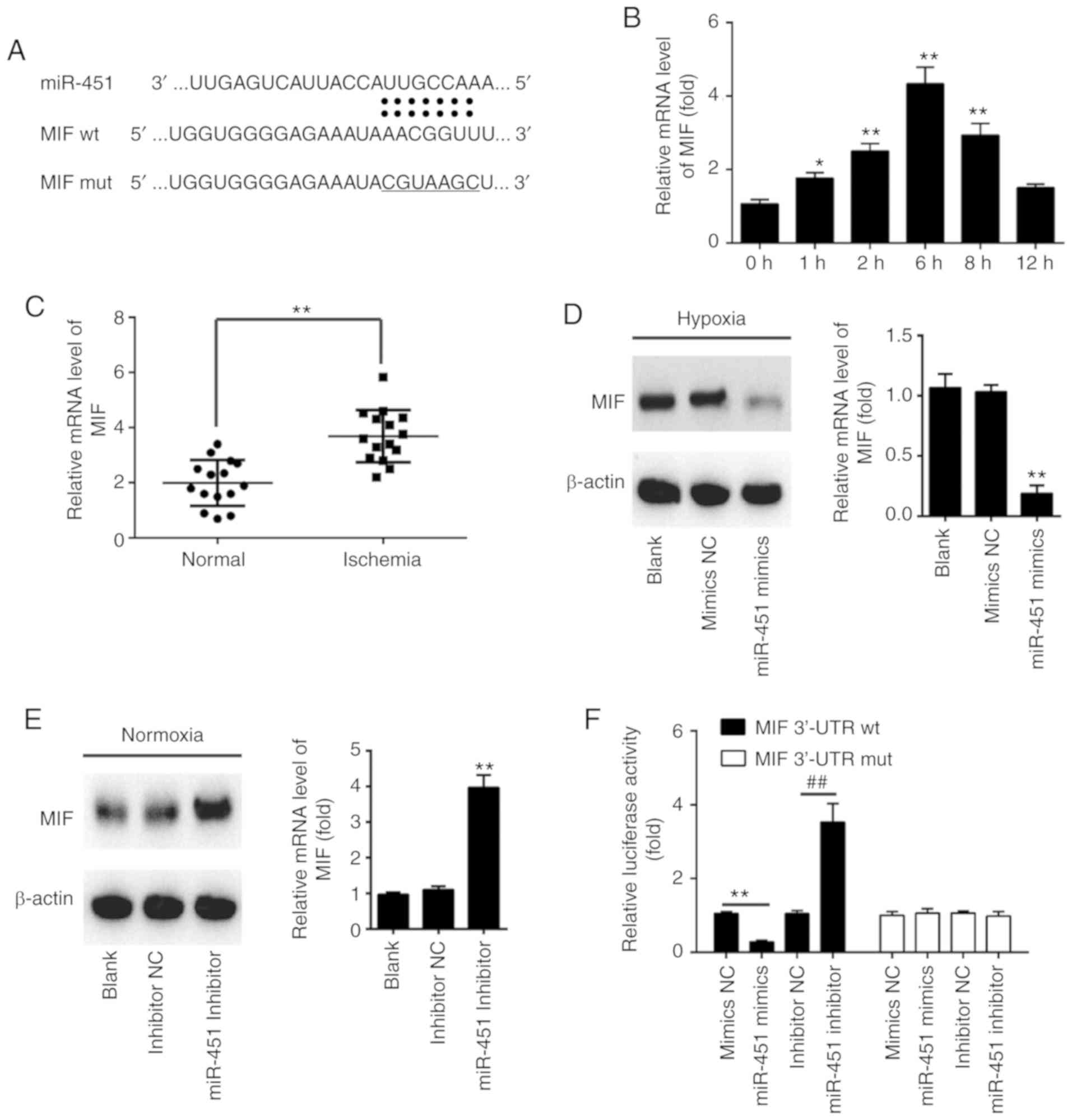 | Figure 4.MIF is a direct target of miR-451.
(A) Schematic of the MIF 3′UTR containing the miR-451 binding
sites. (B) HUVECs were treated with hypoxia for the indicated time
points. The relative expression level of MIF was detected by
RT-qPCR. *P<0.05 and **P<0.01 vs. control group (0 h). (C)
miR-451 level was measured in serum samples from cerebral ischemic
patients (n=15) and normal participants (n=15) by RT-qPCR.
**P<0.01, as indicated. (D) HUVECs were transfected with miR-451
mimics or mimics NC. Following 24 h, cells were treated with
hypoxia for 6 h. The expression levels of MIF protein were
determined by western blotting. **P<0.01 vs. mimics NC group.
(E) HUVECs were transfected with miR-451 inhibitor or inhibitor NC.
Following 24 h, cells were treated with normoxia for 6 h. The
expression levels of MIF protein was determined by western
blotting. **P<0.01 vs. inhibitor NC group. (F) Relative
luciferase activity in HUVECs co-transfected with wild-type or
mutant-type 3′UTR MIF reporter plasmids and miR-451 or miR-NC. All
data are expressed as the mean ± standard deviation. **P<0.01,
as indicated; ##P<0.01, as indicated. RT-qPCR,
reverse transcription-quantitative polymerase chain reaction; MIF,
migration inhibitory factor; HUVECs, human umbilical vein
endothelial cells; miR, microRNA; NC, negative control; UTR,
untranslated region; wt, wild-type; mut, mutant. |
MIF reverses the effects of miR-451 on
the regulation of angiogenesis in vitro
To investigate whether MIF was involved in the
anti-angiogenesis effects of miR-451, the present study transfected
pcDNA-MIF plasmids into HUVECs cells, followed by exposure to
hypoxic conditions. Western blotting was conducted to examine
whether MIF protein expression was effectively enhanced. As shown
in Fig. 5A, pcDNA-MIF transfection
significantly overexpressed MIF protein expression in HUVECs. In
addition, miR-451 mimics inhibited tube formation capacity,
invasive ability and migration distance, and its inhibitory effect
was attenuated when MIF was overexpressed (Fig. 5B-D). To inhibit MIF protein
expression, the present study transfected MIF siRNA into HUVECs,
followed by exposure to normoxic conditions. Western blotting was
also performed to examine whether MIF protein expression was
effectively altered. As shown in Fig.
5E, MIF siRNA transfection significantly inhibited MIF protein
expression in HUVECs. Furthermore, the miR-451 inhibitor promoted
tube formation capacity, invasive ability and migration distance,
and its promotional effect was reduced when MIF was knocked down
(Fig. 5F-H). These data suggested
that the miR-451/MIF axis may serve an important role in the
regulation of angiogenesis in HUVECs.
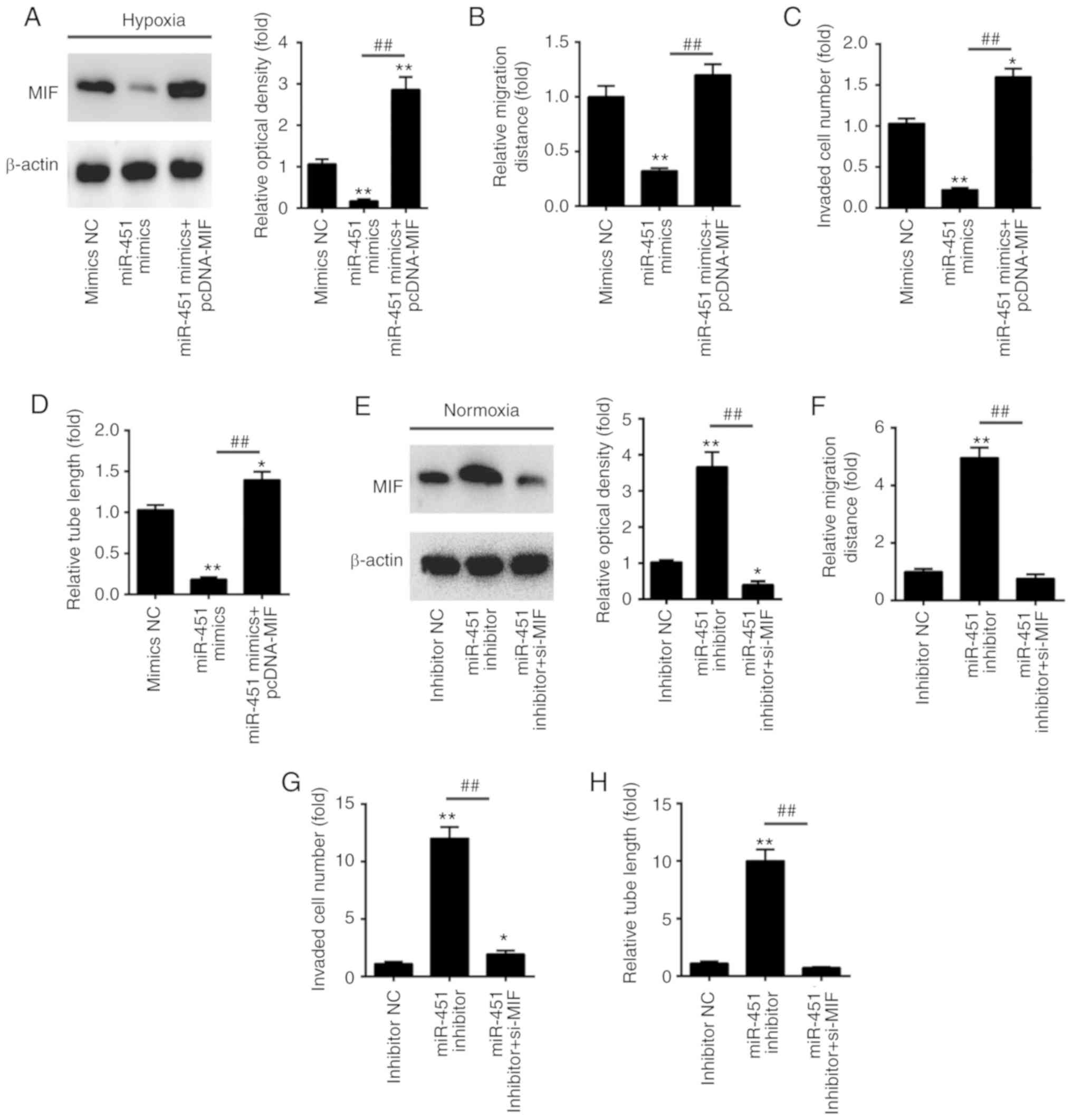 | Figure 5.miR-451 inhibits angiogenesis by
targeting MIF. HUVECs were co-transfected with miR-451 mimics or
pcDNA-MIF. Following 24 h, cells were treated with hypoxia for 6 h.
(A) The expression levels of MIF protein were determined by western
blotting. (B) The cell migration distance was detected by the wound
healing assay, (C) the penetrating ability of HUVECs was measured
by the Transwell assay, and (D) the tube formation of HUVECs was
measured by Matrigel assays. *P<0.05 and **P<0.01 vs. mimics
NC group; ##P<0.01 vs miR-451 mimics group. (E-H)
HUVECs were co-transfected with the miR-451 inhibitor or si-MIF.
Following 24 h, cells were treated with hypoxia for 6 h. (E) The
expression levels of MIF protein were determined by western
blotting. (F) The cell migration distance was detected by the wound
healing assay, (G) the penetrating ability of HUVECs was measured
by the Transwell assay and (H) the tube formation of HUVECs was
measured by Matrigel assays. *P<0.05 and **P<0.01 vs.
inhibitor NC group; ##P<0.01, as indicated. All data
are expressed as the mean ± standard deviation. miR, microRNA; NC,
negative control; MIF, migration inhibitory factor; HUVECs, human
umbilical vein endothelial cells; si-, small interfering RNA. |
Discussion
In the present study, miR-451 was downregulated in
serum samples from patients with cerebral ischemic. In an in
vitro model of CII, hypoxia treatment promoted the tube
formation and migration of HUVECs, while this enhancement was
attenuated when miR-451 was overexpressed. Similar to the hypoxia
condition, knockdown of miR-451 promoted the angiogenesis of HUVECs
under normoxic condition. The present study further identified that
MIF was a novel target of miR-451 and MIF mediated the effect of
miR-451 on angiogenesis in HUVECs.
Recently, accumulating studies have revealed the
important roles of miRNAs in regulating angiogenesis in cancer and
other diseases (31–33). For example, Shi et al
(34) revealed that inhibition of
miR-103 could promote ischemic stroke angiogenesis and reduce
infarction volume by enhancing VEGF in rats subjected to middle
cerebral artery occlusion. Lou et al (20) demonstrated that upregulation of
miR-210 can activate the Notch signaling pathway, which may
contribute to angiogenesis following cerebral ischemia. Li et
al (16) revealed that
downregulation of miR-493 promoted angiogenesis in a rat model of
ischemic stroke by targeting MIF. In another study, Yi et al
(35) reported that miR-193-5p
modulated angiogenesis through insulin-like growth factor 2 in type
2 diabetic cardiomyopathy. These results led to the conclusion that
miRNAs may also serve an important role in the regulation of
angiogenesis in CII. In the present study, a miRNA microarray
screen was conducted, resulting in a set of differentially
regulated miRNAs, including miR-451, which was downregulated in
serum samples from cerebral ischemic patients. These results
suggest that miR-451 may be involved in this pathological
condition.
In recent years, emerging evidence has revealed that
miR-451 serves important roles in the regulation of angiogenesis
via effects on its target mRNA. For example, miR-451 inhibited cell
migration and angiogenesis in human osteosarcoma by downregulating
IL 6R (19). Liu et al
(18) revealed that miR-451
inhibited angiogenesis in hepatocellular carcinoma by targeting the
IL-6R-signal transducer and activator of transcription-3 signaling
pathway. However, whether miR-451 has a similar mechanism in the
regulation of angiogenesis following CII remains unknown. To
further verify miR-451's role in angiogenesis following CII, the
present study performed experiments with hypoxic HUVECs cells,
which are always used to mimic CII. Subsequently, hypoxia treatment
was revealed to reduce the expression of miR-451 in HUVECs and
enhanced miR-451 expression attenuated the tube formation and
migration of HUVECs under hypoxic conditions. Notably, knockdown of
miR-451 promoted the angiogenesis of HUVECs under normoxic
conditions, which is consistent with the results of hypoxia
treatment. Previous studies have reported the involvement of the
VEGF/VEGFR axis in angiogenesis following CII. For example, For
example, Marti et al (29)
indicated that VEGF and the VEGF receptors (VEGFR-1 and VEGFR-2)
are upregulated by hypoxia in the brain following cerebral
ischemia, which mediated the angiogenic response in the ischemic
border zone and extended towards the core region of the infarcted
area. In the present study, overexpression of miR-451 could inhibit
the expressions of VEGF and VEGFR2 via hypoxia, which indicated
that miR-451 may regulate angiogenesis through the VEGF/VEGFR2
pathway.
Many studies have indicated that several angiogenic
factors are significantly induced following CII, such as VEGF and
angiopoietin-2 (36,37). To determine the mechanism by which
miR-451 functions in angiogenesis following CII, the present study
predicted its target genes through bioinformatics analysis. MIF, a
well-known angiogenic regulator, has been implicated in
angiogenesis (9,38). For example, Liao et al
(8) demonstrated that MIF
contributed to lymph node metastasis by inducing angiogenesis via
the upregulation of IL-8 expression in head and neck squamous cell
carcinoma. Notably, a recent study indicated that MIF enhanced
microvessel-like tube formation by promoting the expression of
angiogenesis associated genes in endothelial cells (39). MIF was also involved in CII
(11). Loss of MIF exacerbated
injury in the female brain following experimental stroke, which was
independent of changes in pro-inflammatory cytokine levels
(40). In the present study, MIF
expression was increased in serum samples and HUVECs exposed to
hypoxia, which led to the hypothesis that miR-451 targets MIF to
impair angiogenesis following CII. To test this hypothesis, the
present study performed rescue experiments by ectopically
expressing a miR-resistant variant of MIF. It was observed that
enforced expression of MIF restored the tube formation and
migration of HUVECs reduced by miR-451 overexpression under hypoxic
conditions, while silencing MIF reversed the promotional effects,
triggered by miR-451 knockdown, on the angiogenesis of HUVECs under
normoxic conditions. Collectively, miR-451 acts as a negative
regulator of angiogenesis in the HUVEC model largely through
downregulation of MIF.
Rapid and accurate diagnosis of CII is critical to
enable the development of appropriate treatment. Although the
medical advancements are made frequently, effective diagnosis of
acute CII is still lacking (41).
Therefore, there is a requirement for a more rapid and simple tool
for CII diagnosis. Circulating miRNAs can serve as indicators for
the diagnosis, progression and prognosis of various diseases
(42–44). Thus, miRNA levels in peripheral
blood may be closely associated when under conditions of CII. In
the present study, differential expression of several miRNAs in the
serum samples of cerebral ischemic patients were observed when
compared with healthy subjects; in particular, the expression of
miR-451 was downregulated. Through further study, it was verified
that the level of miR-541 was decreased in HUVECs under hypoxic
conditions when compared with those under normoxic conditions.
Notably, the present results revealed that miR-451 served an
important role in angiogenesis following CII. However, miR-451 was
identified as the most significantly downregulated miRNA in serum
samples, but it was not detected in endothelial tissue samples.
Therefore, it is difficult to distinguish whether the
downregulation of miR-451 in serum was derived from it in
endothelial cells in the same lesion or not. In the future, the
group will perform the relevant research in endothelial tissue
samples.
In conclusion, the present study has provided
evidence that miR-451 expression was downregulated following
cerebral ischemia. Downregulation of miR-451 could be beneficial
for angiogenesis by increasing MIF expression in hypoxic HUVECs.
These results may highlight the importance of miR-451 in treating
CII. Further study of these mechanisms has the potential to lead to
targeted clinical therapy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
QL, YL, DZ, HG and XG performed the experiments,
contributed to data analysis and wrote the paper. YL, DZ, HG and XG
analyzed the data. QL conceived and designed the study, and
contributed to data analysis and the experimental materials. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All individuals provided written informed consent
for the use of human specimens for clinical research. The present
study was approved by Workers' Hospital of Tangshan City (Hebei,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Johnston SC, Mendis S and Mathers CD:
Global variation in stroke burden and mortality: Estimates from
monitoring, surveillance, and modelling. Lancet Neurol. 8:345–354.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu AD, Wang YJ and Wang DZ; Chinese Stroke
Therapy Expert Panel for Intravenous Recombinant Tissue Plasminogen
Activator, : Consensus statement on the use of intravenous
recombinant tissue plasminogen activator to treat acute ischemic
stroke by the chinese stroke therapy expert panel. CNS Neurosci
Ther. 19:543–548. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lo EH, Dalkara T and Moskowitz MA:
Mechanisms, challenges and opportunities in stroke. Nat Rev
Neurosci. 4:399–415. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Semenza GL: Vasculogenesis, angiogenesis,
and arteriogenesis: Mechanisms of blood vessel formation and
remodeling. J Cell Biochem. 102:840–847. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Choudhary S, Hegde P, Pruitt JR, Sielecki
TM, Choudhary D, Scarpato K, Degraff DJ, Pilbeam CC and Taylor JA
3rd: Macrophage migratory inhibitory factor promotes bladder cancer
progression via increasing proliferation and angiogenesis.
Carcinogenesis. 34:2891–2899. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Asare Y, Schmitt M and Bernhagen J: The
vascular biology of macrophage migration inhibitory factor (MIF).
Expression and effects in inflammation, atherogenesis and
angiogenesis. Thromb Haemost. 109:391–398. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rassaf T, Weber C and Bernhagen J:
Macrophage migration inhibitory factor in myocardial
ischaemia/reperfusion injury. Cardiovasc Res. 102:321–328. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liao B, Zhong BL, Li Z, Tian XY, Li Y and
Li B: Macrophage migration inhibitory factor contributes
angiogenesis by up-regulating IL-8 and correlates with poor
prognosis of patients with primary nasopharyngeal carcinoma. J Surg
Oncol. 102:844–851. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Girard E, Strathdee C, Trueblood E and
Queva C: Macrophage migration inhibitory factor produced by the
tumour stroma but not by tumour cells regulates angiogenesis in the
B16-F10 melanoma model. Br J Cancer. 107:1498–1505. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang L, Zis O, Ma G, Shan Z, Zhang X, Wang
S, Dai C, Zhao J, Lin Q, Lin S and Song W: Upregulation of
macrophage migration inhibitory factor gene expression in stroke.
Stroke. 40:973–976. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zis O, Zhang S, Dorovini-Zis K, Wang L and
Song W: Hypoxia signaling regulates macrophage migration inhibitory
factor (MIF) expression in stroke. Mol Neurobiol. 51:155–167. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Z, Yang D, Xie P, Ren G, Sun G, Zeng X
and Sun X: MiR-106b and MiR-15b modulate apoptosis and angiogenesis
in myocardial infarction. Cell Physiol Biochem. 29:851–862. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao WJ, Zhang HF and Su JY:
Downregulation of microRNA-195 promotes angiogenesis induced by
cerebral infarction via targeting VEGFA. Mol Med Rep. 16:5434–5440.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Q, He Q, Baral S, Mao L, Li Y, Jin H,
Chen S, An T, Xia Y and Hu B: MicroRNA-493 regulates angiogenesis
in a rat model of ischemic stroke by targeting MIF. FEBS J.
283:1720–1733. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu X, Zhang A, Xiang J, Lv Y and Zhang X:
miR-451 acts as a suppressor of angiogenesis in hepatocellular
carcinoma by targeting the IL-6R-STAT3 pathway. Oncol Rep.
36:1385–1392. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu SY, Deng SY, He YB and Ni GX: miR-451
inhibits cell growth, migration and angiogenesis in human
osteosarcoma via down-regulating IL 6R. Biochem Biophys Res Commun.
482:987–993. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lou YL, Guo F, Liu F, Gao FL, Zhang PQ,
Niu X, Guo SC, Yin JH, Wang Y and Deng ZF: miR-210 activates notch
signaling pathway in angiogenesis induced by cerebral ischemia. Mol
Cell Biochem. 370:45–51. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li L, Wang M, Mei Z, Cao W, Yang Y, Wang Y
and Wen A: lncRNAs HIF1A-AS2 facilitates the up-regulation of
HIF-1α by sponging to miR-153-3p, whereby promoting angiogenesis in
HUVECs in hypoxia. Biomed Pharmacother. 96:165–172. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ding G, Jiang Q, Li L, Zhang L, Zhang ZG,
Ledbetter KA, Gollapalli L, Panda S, Li Q, Ewing JR and Chopp M:
Angiogenesis detected after embolic stroke in rat brain using
magnetic resonance T2*WI. Stroke. 39:1563–1568. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ruan L, Wang B, ZhuGe Q and Jin K:
Coupling of neurogenesis and angiogenesis after ischemic stroke.
Brain Res. 1623:166–173. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Venna VR, Li J, Hammond MD, Mancini NS and
McCullough LD: Chronic metformin treatment improves post-stroke
angiogenesis and recovery after experimental stroke. Eur J
Neurosci. 39:2129–2138. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
He QW, Xia YP, Chen SC, Wang Y, Huang M,
Huang Y, Li JY, Li YN, Gao Y, Mao L, et al: Astrocyte-derived sonic
hedgehog contributes to angiogenesis in brain microvascular
endothelial cells via RhoA/ROCK pathway after oxygen-glucose
deprivation. Mol Neurobiol. 47:976–987. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun Y, Jin K, Xie L, Childs J, Mao XO,
Logvinova A and Greenberg DA: VEGF-induced neuroprotection,
neurogenesis, and angiogenesis after focal cerebral ischemia. J
Clin Invest. 111:1843–1851. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kaya D, Gursoy-Ozdemir Y, Yemisci M,
Tuncer N, Aktan S and Dalkara T: VEGF protects brain against focal
ischemia without increasing blood--brain permeability when
administered intracerebroventricularly. J Cereb Blood Flow Metab.
25:1111–1118. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Feng Y, Rhodes PG and Bhatt AJ:
Neuroprotective effects of vascular endothelial growth factor
following hypoxic ischemic brain injury in neonatal rats. Pediatr
Res. 64:370–374. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Marti HJ, Bernaudin M, Bellail A, Schoch
H, Euler M, Petit E and Risau W: Hypoxia-induced vascular
endothelial growth factor expression precedes neovascularization
after cerebral ischemia. Am J Pathol. 156:965–976. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu W, Liu SY, He YB, Huang RL, Deng SY,
Ni GX and Yu B: MiR-451 suppresses proliferation, migration and
promotes apoptosis of the human osteosarcoma by targeting
macrophage migration inhibitory factor. Biomed Pharmacother.
87:621–627. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang S and Olson EN: AngiomiRs--key
regulators of angiogenesis. Curr Opin Genet Dev. 19:205–211. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Suarez Y and Sessa WC: MicroRNAs as novel
regulators of angiogenesis. Circ Res. 104:442–454. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kuehbacher A, Urbich C and Dimmeler S:
Targeting microRNA expression to regulate angiogenesis. Trends
Pharmacol Sci. 29:12–15. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shi FP, Wang XH, Zhang HX, Shang MM, Liu
XX, Sun HM and Song YP: MiR-103 regulates the angiogenesis of
ischemic stroke rats by targeting vascular endothelial growth
factor (VEGF). Iran J Basic Med Sci. 21:318–324. 2018.PubMed/NCBI
|
|
35
|
Yi F, Shang Y, Li B, Dai S, Wu W, Cheng L
and Wang X: MicroRNA-193-5p modulates angiogenesis through IGF2 in
type 2 diabetic cardiomyopathy. Biochem Biophys Res Commun.
491:876–882. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen J, Zhang C, Jiang H, Li Y, Zhang L,
Robin A, Katakowski M, Lu M and Chopp M: Atorvastatin induction of
VEGF and BDNF promotes brain plasticity after stroke in mice. J
Cereb Blood Flow Metab. 25:281–290. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gui C, Li SK, Nong QL, Du F, Zhu LG and
Zeng ZY: Changes of serum angiogenic factors concentrations in
patients with diabetes and unstable angina pectoris. Cardiovasc
Diabetol. 12:342013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kanzler I, Tuchscheerer N, Steffens G,
Simsekyilmaz S, Konschalla S, Kroh A, Simons D, Asare Y, Schober A,
Bucala R, et al: Differential roles of angiogenic chemokines in
endothelial progenitor cell-induced angiogenesis. Basic Res
Cardiol. 108:3102013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shan ZX, Lin QX, Yang M, Zhang B, Zhu JN,
Mai LP, Deng CY, Liu JL, Zhang YY, Lin SG and Yu XY: Transcription
factor Ap-1 mediates proangiogenic MIF expression in human
endothelial cells exposed to Angiotensin II. Cytokine. 53:35–41.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Turtzo LC, Li J, Persky R, Benashski S,
Weston G, Bucala R, Venna VR and McCullough LD: Deletion of
macrophage migration inhibitory factor worsens stroke outcome in
female mice. Neurobiol Dis. 54:421–431. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhou X, Su S, Li S, Pang X, Chen C, Li J
and Liu J: MicroRNA-146a down-regulation correlates with
neuroprotection and targets pro-apoptotic genes in cerebral
ischemic injury in vitro. Brain Res. 1648:136–143. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shalaby T and Grotzer MA: Tumor-associated
CSF MicroRNAs for the prediction and evaluation of CNS
malignancies. Int J Mol Sci. 16:29103–29119. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yu X and Li Z: Serum microRNAs as
potential noninvasive biomarkers for glioma. Tumour Biol.
37:1407–1410. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yue X, Lan F, Hu M, Pan Q, Wang Q and Wang
J: Downregulation of serum microRNA-205 as a potential diagnostic
and prognostic biomarker for human glioma. J Neurosurg.
124:122–128. 2016. View Article : Google Scholar : PubMed/NCBI
|