Introduction
Chronic sinusitis with nasal polyposis (CRSwNP) is
an inflammatory disease of the nasal cavity. Paranasal sinuses
develop grape-like structures, leading to nasal obstruction,
olfactory disturbance, headache, chronic inflammatory diseases and
postnasal drip, which severely affect the quality of life of
patients (1,2). CRS is usually caused by repeated
bouts of acute inflammation, which lead to pathological changes of
the nasal aperture and epithelial ciliary dysfunction (1). The nasal aperture becomes swollen,
hypertrophic and polypoid, thereby obstructing drainage of the
sinus orifices and leading to CRS (2). The characteristics of CRSwNP include
T helper (Th) 2 regulation and eosinophilic inflammation (3).
Interleukin-10 (IL-10) is a potent anti-inflammatory
cytokine, which protects the host from excessive tissue damage, and
serves a key role in the development and maintenance of immune
tolerance and homeostasis (4). The
levels of IL-10 are reduced in various chronic inflammatory
diseases, including inflammatory bowel disease (5) and spontaneous colitis (6). Imbalance in the levels of IL-10
amplifies the local inflammatory response, which aggravates
histopathological changes, and increases the risk of developing
autoimmune diseases (7).
Consequently, IL-10 is a potential therapeutic target for nasal
polyps (8,9).
Electroacupuncture (EA) is a non-drug, adjuvant
therapy for certain inflammatory diseases, such as peripheral
inflammation (10). It reportedly
prevents the nuclear transport of the nuclear factor (NF)-κB p65
subunit, and thereby inhibits the NF-κB signaling pathway and
reduces inflammatory damage (11).
Additionally, acupuncture has been used as a therapy for CRS
(12,13); however, the mechanisms via which
this is achieved have not been fully elucidated. The present study
evaluated the therapeutic effects of EA combined with IL-10
overexpression on CRS in mice and investigated the associated
mechanisms.
Materials and methods
Mouse model of CRS
A total of 35 male C57BL/6J mice (3 months of age;
body weight ~25 g) were obtained from Hunan Slake Jingda Laboratory
Co., Ltd (Hunan, China) and maintained in specific pathogen-free
conditions at a temperature of 23±2°C and a relative humidity of
45–65% under a controlled 12:12-h light/dark cycle, with access to
food and water ad libitum. On days 0 and 5, the mice were
injected intraperitoneally with 2 µg ovalbumin (OVA; cat. no.
S7951MSDS; Sigma-Aldrich; Merck KGaA) + 2 mg aluminum hydroxide gel
(cat. no. A8222; Sigma-Aldrich; Merck KGaA) to induce CRS as
previously described (14). On
days 12–19, the animals received 40 µl of 3% OVA nasally.
Subsequently, three drops (~3 µl) of 3% OVA were administered per
week to induce a long-term inflammatory response over 12 weeks. The
control group was treated with a similar volume of saline. All
protocols were supervised and approved by the Ethics Committee of
Jiangxi University of Chinese Medicine.
Reverse transcription-quantitative PCR
(RT-qPCR)
Virus-encoded IL-10 (pGreenPuro™ plasmid packaged in
serotype 5 adenovirus) was established by Shanghai GenePharma Co.,
Ltd. and confirmed in rat cardiac fibroblasts. Adenovirus
containing empty pGreenPuro plasmid was used as the control vector.
Rat cardiac fibroblasts were purchased from the Cell Bank of
Chinese Academy of Sciences, and cultured at 37°C with 5%
CO2 in Dulbecco's Modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% fetal calf serum
(HyClone; GE Healthcare Life Sciences), 100 U/ml penicillin and 100
mg/ml streptomycin. Cells at 70% confluence were transfected with
virus (1×105 IFU/ml) using Lipofectamine™ 2000
(Invitrogen; Thermo Fisher Scientific, Inc.). At 24 h later, RNA
was extracted from cardiac fibroblasts using TRIzol®
reagent (Baosheng Science & Technology Innovation Co., Ltd.)
and reverse transcribed into cDNA using an Ultrapure SMART MMLV RT
kit (cat. no. 639522, Takara Biotechnology Co., Ltd.) according to
the manufacturer's protocol. The cDNA was used as a template for
detection by fluorescence qPCR (SYBR Green Master Mix; cat. no.
HY-K0501; MedChemExpress LLC) according to the following protocol:
40 cycles of 95°C for 10 sec (denaturation), 53°C for 30 sec
(annealing) and 72°C for 30 sec (extension), and a final extension
of 72°C for 10 min. The relative expression of IL-10 mRNA was
calculated with GAPDH as the internal reference using the
2−∆∆Cq method as previously described (15). The primers are presented in
Table I.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene | Primer (5′-3′) | Primer length
(bp) | Product length
(bp) | Annealing temperature
(°C) |
|---|
| IL-10 | Forward,
CAACATACTGCTGACAGATTCCT | 23 | 236 | 58.4 |
|
| Reverse,
GCTCCACTGCCTTGCTTT | 18 |
|
|
| GAPDH | Forward,
GCAAGTTCAACGGCACAG | 18 | 141 | 58.6 |
|
| Reverse,
CGCCAGTAGACTCCACGAC | 19 |
|
|
Experimental groups
The animals were divided into seven groups (n=5 in
each group) as follows: i) Control group; ii) OVA group; iii) OVA +
EA group; iv) OVA + vector group; v) OVA + vector + EA group; vi)
OVA + IL-10 group; and vii) OVA + IL-10 + EA group. Mice in the
control, OVA and OVA + EA groups were injected with 0.5 ml saline
via the tail vein. On day 20 following first injection with OVA, a
volume of 0.5 ml of the virus encoding blank vector was injected in
the OVA + vector and OVA + vector + EA groups, whereas 0.5 ml
adenovirus encoding IL-10 was injected in the OVA + IL-10 and OVA +
IL-10 + EA groups. Following the virus injection, the animals in
the OVA + EA, OVA + vector + EA and OVA + IL-10 + EA groups
received the first round of electroacupuncture on day 20. Briefly,
the animals were anesthetized using isoflurane (1% in oxygen). EA
was conducted in the following corresponding acupoints of mice: i)
Zusanli (lateral knee joint, 3.5 mm below the capitulum
fibulae; a 3-mm straight puncture); ii) Feishu (between the
ribs below the third thoracic vertebra); iii) Yingxiang
(lateral upper end of the nostril, at the hairless junction; a 2–3
mm oblique puncture inward and upward); and iv) Hegu (1-mm
straight puncture between the first and second metacarpal bones of
the forelimb). Each mouse was stimulated by EA using a Huatuo
SDZ-II Electronic Acupuncture Therapy Instrument (Suzhou Medical
Supplies Factory Co., Ltd.) at the same acupoint for 30 min (once a
day for 10 days). Following treatment, the animals were
anesthetized by isoflurane (1% in oxygen) via inhalation and
decapitated. Nasal tissues were collected on ice using surgical
tools under a dissecting microscope (SZ61; Olympus Corporation).
Fresh tissues were stored at −80°C or fixed in 4% paraformaldehyde
overnight at 4°C.
Hematoxylin and eosin staining
Nasal tissues were washed for 1 h with
phosphate-buffered saline (PBS), following which they were fixed
with 4% paraformaldehyde at 4°C overnight. The tissues were then
dehydrated by 70, 80 and 90% ethanol, and mixed with anhydrous
ethanol and xylene for 15 min, xylene I for 15 min and xylene II
for 15 min (until transparent). Tissues were embedded in paraffin
and sliced (10 µm). The paraffin slices were dewaxed and hydrated.
The sections were stained with hematoxylin for 3 min at room
temperature, differentiated by ethanol hydrochloride for 15 sec,
washed slightly and stained with eosin for 3 min at room
temperature. The sections were then mounted on slides and observed
under a light microscope (magnification, ×200).
Immunohistochemistry
Nasal tissues were fixed in 4% paraformaldehyde
overnight at 4°C. The tissues were subjected to dehydration,
embedding and slicing. The paraffin sections (10 µm) were then
dewaxed and hydrated. Sections were incubated with 3% (v/v)
hydrogen peroxide for 5 min at room temperature to block endogenous
peroxidase activity. Following blocking in 5% goat serum (cat. no.
G9023; Sigma-Aldrich; Merck KGaA) at room temperature for 2 h, the
sections were incubated with the following primary antibodies
overnight at 4°C: Rabbit polyclonal anti-IL-10 (1:300; cat. no.
bs-20373R; BIOSS) and rabbit polyclonal anti-interferon-γ (IFN-γ;
1:300; cat. no. bs-0480R; BIOSS). The slides were then washed with
PBS and incubated with the secondary antibody (horseradish
peroxidase-labeled goat anti-rabbit IgG; 1:10,000; cat. no.
A16104SAMPLE; Thermo Fisher Scientific, Inc.) for 30 min at room
temperature. Sections were subsequently stained with
3,3′-diaminobenzidine chromogen for 3 min and counterstained with
hematoxylin and eosin (3%, 3 min) at room temperature. Images of
four fields for each group were acquired under a light microscope
(BX51; Olympus Corporation), and expression density was analyzed by
ImageJ software version 1.48 (National Institutes of Health).
Western blotting
Protein was extracted from each group using a
protein isolation kit (ReadyPrep; cat. no. 28-9425-44; GE
Healthcare Life Sciences). The concentration of the proteins was
quantified using a bicinchoninic acid assay. Thereafter, 25 µg of
protein was separated via 12% SDS-PAGE. The proteins were then
transferred onto polyvinylidene difluoride membranes. The membranes
were blocked in 5% milk for 2 h at room temperature. The following
primary antibodies were incubated with tissues overnight at 4°C:
Rabbit polyclonal anti-IL-10 (1:300; cat. no. bs-20373R; BIOSS),
rabbit polyclonal anti-IFN-γ (1:300; cat. no. bs-0480R; BIOSS) and
mouse monoclonal anti-GAPDH (1:2,000; cat. no. TA-08; ZSBIO). The
membrane was incubated with peroxidase-conjugated anti-mouse
(1:2,000; cat. no. TA130003) or anti-rabbit (1:2,000; cat. no.
TA140003; OriGene Technologies, Inc.) secondary antibodies for 2 h
at room temperature. Protein bands were visualized using enhanced
chemiluminescence (SuperSignal™ West Pico Chemiluminescent
Substrate; cat. no. 34580; Thermo Fisher Scientific, Inc.). Five
repeats were performed, and expression density was analyzed using
ImageJ version 1.48.
Statistical analysis
Data are presented as the mean ± standard error of
the mean (n=5/group for each experiment). SPSS software (version
17; SPSS, Inc.) was used to analyze the data. One-way analysis of
variance was applied to the data, followed by the Newman-Keuls
post-hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
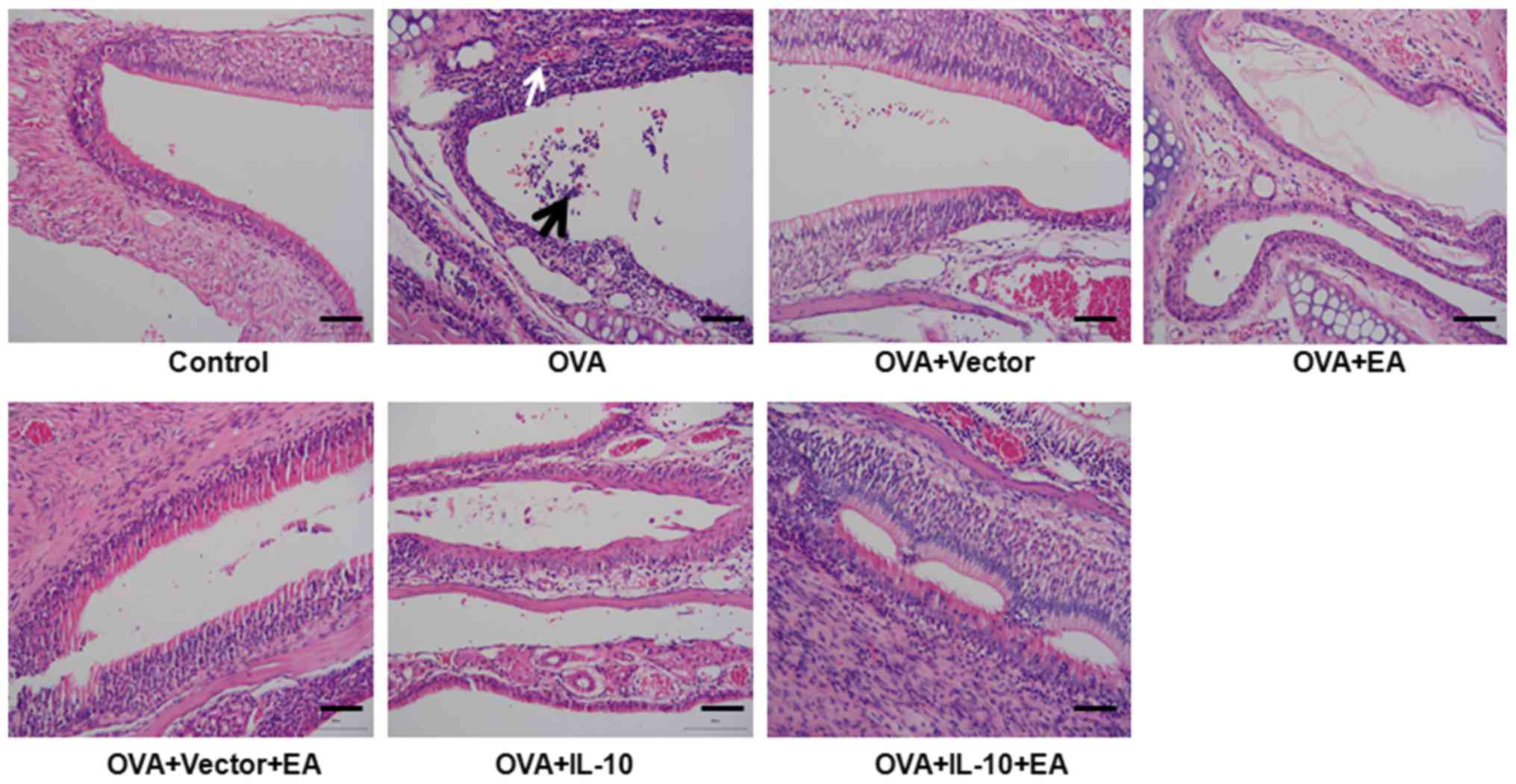
Morphological changes in the mucosa of
the nasal sinuses following establishment of the CRS model
As presented in Fig.
1, the pseudostratified epithelium of the mucosa of the nasal
sinuses in the control group exhibited a neatly arranged, complete
structure. No infiltration of inflammatory cells was observed in
the submucosa. By contrast, the OVA group showed severe
inflammation, a disordered arrangement of mucosal epithelium,
necrosis and exfoliation. These results indicated that injury of
pseudostratified epithelium was induced in the CRS model.
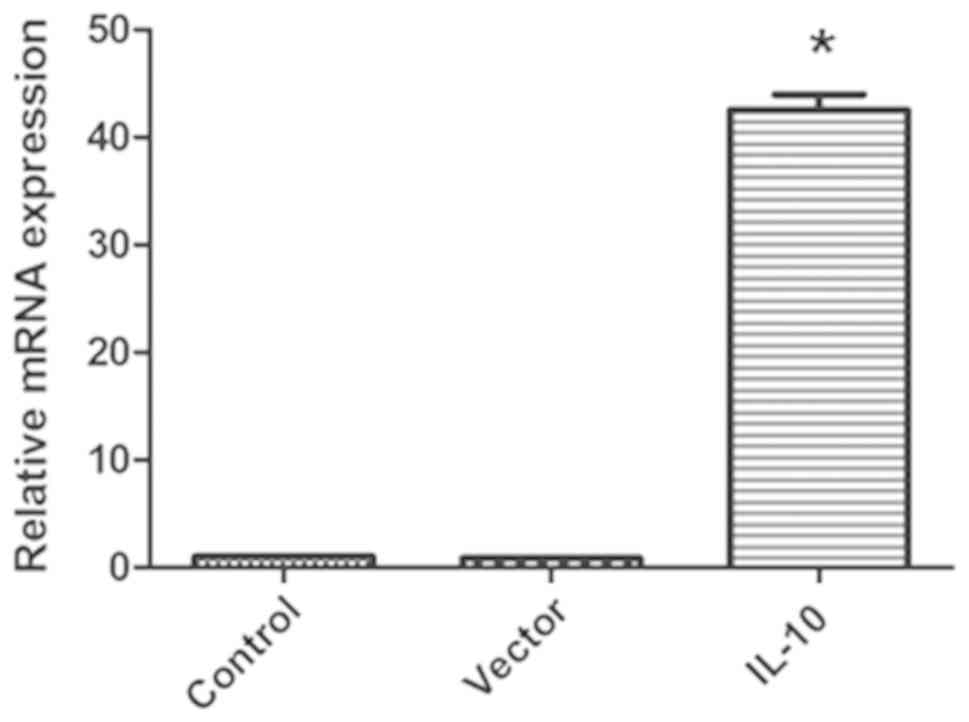
Virus-encoded IL-10 promotes IL-10
expression
As presented in Fig.
2, the expression of IL-10 in cardiac fibroblasts transduced
with virus-encoded IL-10 was significantly increased compared with
in the control group (P<0.05), whereas the empty vector virus
did not affect IL-10 expression. Therefore, the virus-encoded IL-10
was used in the subsequent experiments.
Treatment with EA and IL-10
ameliorates injury of the pseudostratified epithelium
The OVA group and OVA + vector group exhibited
severe inflammation, disordered arrangement of the mucosal
epithelium, necrosis and exfoliation. Treatment with EA (OVA + EA
group) or IL-10 (OVA + IL-10 group) resulted in attenuation of the
injury induced by the CRS model (Fig.
1). Notably, combination therapy with EA and IL-10 was more
effective than treatment with EA or IL-10 alone. In the OVA + IL-10
+ EA group, cells were neatly arranged and few inflammatory cells
were observed (Fig. 1).
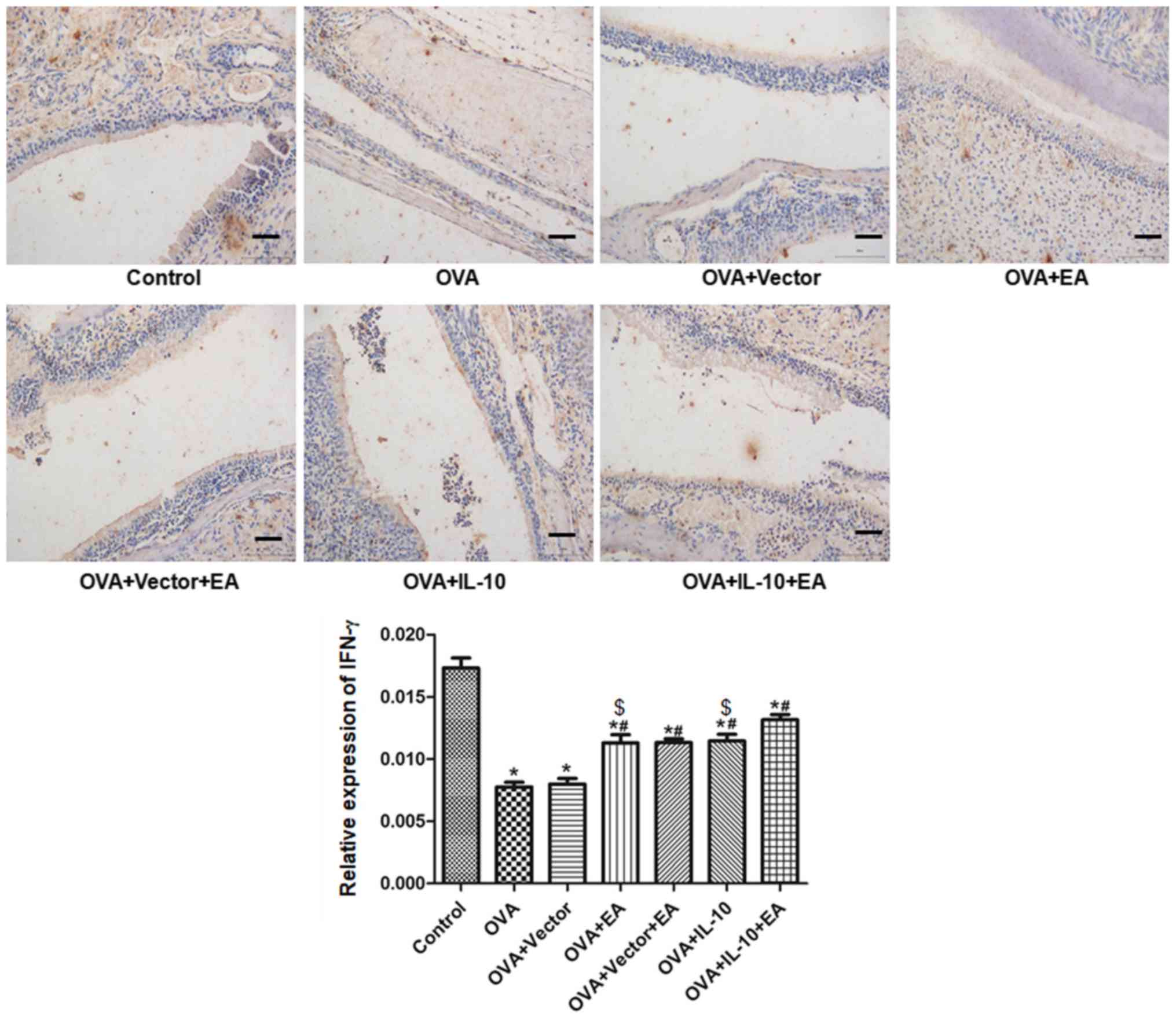
Treatment with EA and IL-10 increases
IFN-γ and IL-10 expression
The results of immunohistochemistry are presented in
Fig. 3. Compared with the control
group, the expression of IFN-γ in the OVA group was significantly
decreased (P<0.05). Compared with the OVA group, the expression
of IFN-γ was promoted by EA (OVA + EA group) and virus-encoded
IL-10 (OVA + IL-10 group; P<0.05), but not by the virus-encoding
vector (OVA + vector group). The combined effect of EA and IL-10
(OVA + IL-10 + EA group) was significantly increased compared with
EA or IL-10 alone (P<0.05).
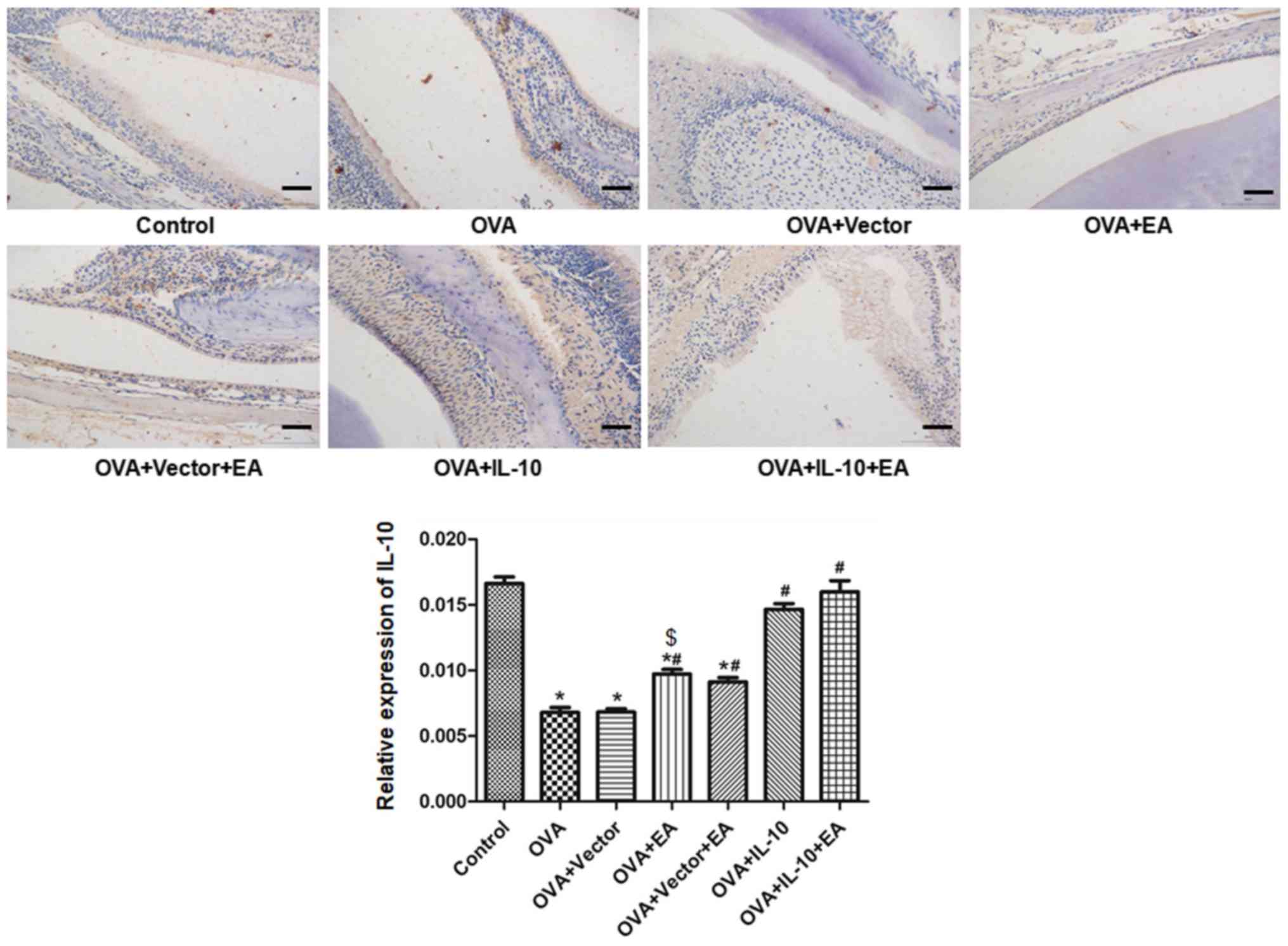
The results of IL-10 expression are shown in
Fig. 4. Compared with the control
group, the expression of IL-10 in the OVA group was significantly
decreased (P<0.05). Compared with the OVA group, the expression
of IL-10 was promoted by EA (OVA + EA group) and virus-encoded
IL-10 (OVA + IL-10 group; P<0.05), but not by the virus-encoding
vector (OVA + vector group). Combined treatment with EA did not
further promote IL-10 expression compared with IL-10 overexpression
alone (P>0.05).
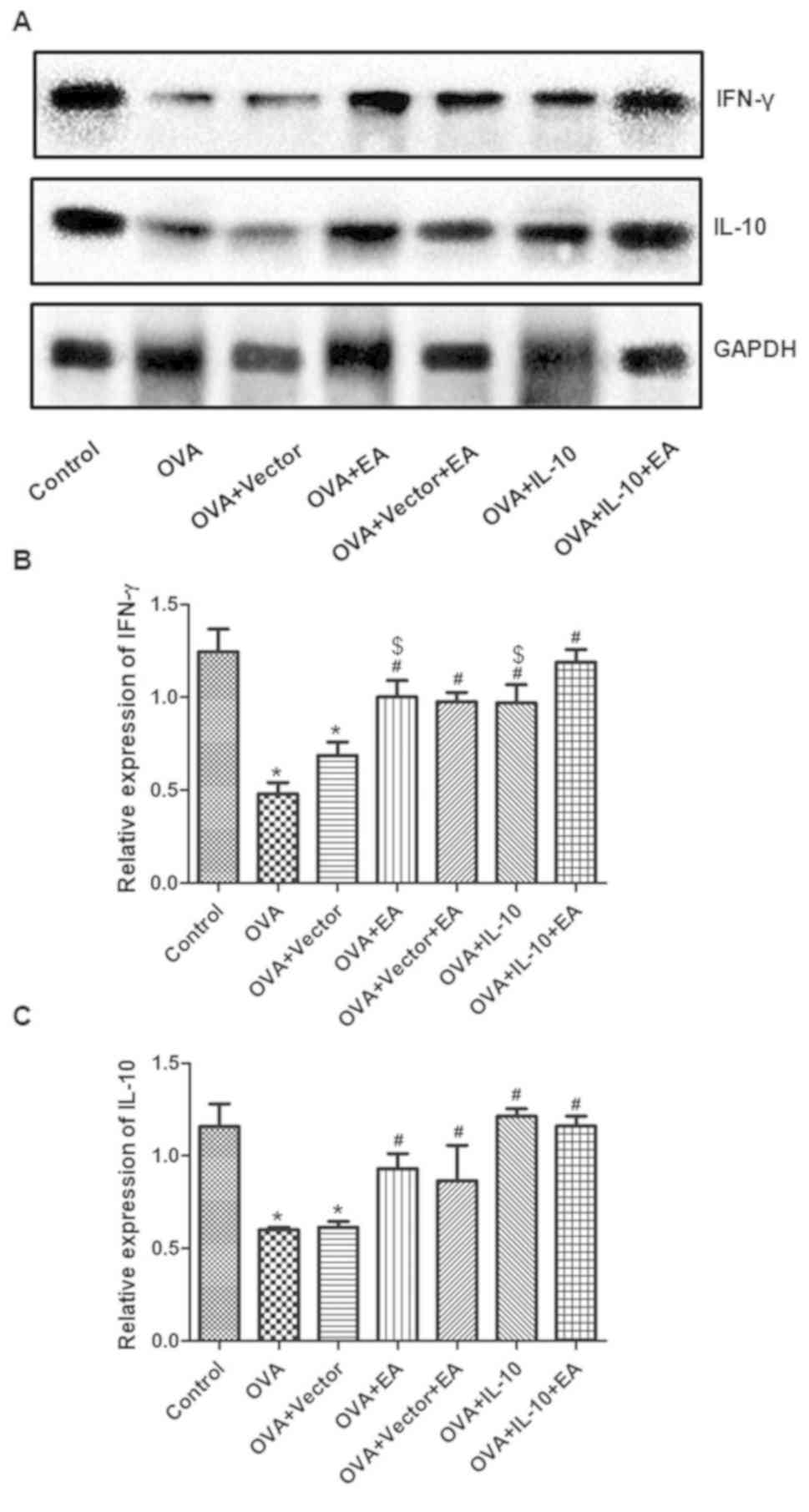
The expression of IFN-γ and IL-10 was also
quantified by western blotting. Consistently, OVA treatment reduced
IFN-γ and IL-10 expression, whereas EA or IL-10 overexpression
promoted the expression of IL-10 and IFN-γ in OVA-treated mice. The
combination of EA and overexpression of IL-10 further promoted
IFN-γ, but not IL-10 levels, compared with the individual
treatments (Fig. 5).
Discussion
The current study provided novel data demonstrating
the protective effects of EA and IL-10 on CRS in mice. Based on the
morphological changes observed, EA and virus-encoded IL-10
ameliorated the injured pseudostratified epithelium. Notably, the
combined application of EA and IL-10 expression induced additive
effects, likely via the upregulation of IFN-γ.
An animal model of sinusitis is important for the
basic and clinical research of this type of disease. The stability
and repeatability of the model also guarantee progress of the
research. From the early 20th century, animal models have been used
in sinusitis research, and OVA has been used as an agent to induce
CRS (16). In the current study,
OVA was used to induce CRS, whereas aluminum hydroxide gel was used
to protect against gastric injury induced by OVA, and morphological
changes of the mucosa of the nasal sinus were observed (14). The pseudostratified epithelium of
the mucosa of the nasal sinus was impaired in the model group, and
was characterized by severe inflammation, disordered arrangement of
the mucosal epithelium, necrosis and exfoliation, all of which are
symptoms of sinusitis (14).
Furthermore, EA treatment ameliorated the abnormal changes of the
mucosa. The results obtained in the current study suggested that
the mouse model of sinusitis was successfully induced, and that EA
exerted protective effects in this model.
As a major anti-inflammatory cytokine, IL-10 serves
important roles in tumors, infections, organ transplantation, and
the hematopoietic and cardiovascular systems (17,18).
Furthermore, IL-10 is closely associated with the blood, digestion
and diseases of the cardiovascular system (19). Impaired IL-10 production, in
response to Staphylococcus aureus enterotoxin B in nasal
polyps, likely exacerbates the pathophysiological mechanisms of
eosinophilic chronic rhinosinusitis, including eosinophilia and
obstruction of the lower airway (20). In the present study, IL-10 levels
were reduced in the OVA group compared with the control group. By
contrast, EA treatment promoted IL-10 expression. These data
suggested that EA likely exerted protective effects in CRS by
increasing IL-10 levels. IL-10 upregulation has a protective effect
in CRSwNP (8,9). In allergic fungal rhinosinusitis,
IL-10 levels were reportedly reduced (21), indicating that IL-10 may be a
potential therapeutic target for the treatment of CRSwNP caused by
various pathologic factors (22).
In the present study, EA alone improved IL-10 expression in the CRS
model, but in combination with IL-10 overexpression, did not
further promote IL-10 expression. IL-10 acts by blocking the
metabolism of macrophages as part of this inflammatory response
(CD4+ T-cell responses) (23).
Specifically, IL-10 is able to inhibit lipopolysaccharide-induced
glucose uptake and glycolysis, and promote oxidative
phosphorylation (24). In
addition, a reduction in IL-10 levels damaged mitochondria by
promoting mitochondrial autophagy, and subsequently eliciting
inflammation (25).
The IFN-γ cytokine has antiviral, immunomodulatory
and antitumor properties (26,27).
In addition to inducing the differentiation and maturation of Th1
cells and increasing IFN-γ levels in the serum, EA inhibits the
differentiation and maturation of Th2 cells and the production of
cytokines (28). Thus, EA inhibits
the transformation of Thl cells to Th2 cells, and thereby corrects
any imbalance in the number of Thl/2 cells (28,29)
and enhances the cellular immune response. In the present study,
IL-10 overexpression attenuated the reduction in IFN-γ levels
observed in the OVA model. Furthermore, EA alone promoted IFN-γ
expression. These findings suggested that IL-10 may act upstream of
IFN-γ in immunotherapy. Indeed, IL-10 controlled IFN-γ and the
production of tumor necrosis factor during experimental endotoxemia
(30). However, IFN-γ could also
reprogram IL-10 activity in macrophages (31). Nevertheless, the cooperative
actions of IFN-γ and IL-10 in the regulation of cell functions
cannot be ignored (32). In the
current study, EA did not further promote IL-10 levels but
increased IFN-γ when applied in combination with IL-10
overexpression. These findings suggested that EA may function via
various mechanisms to promote IFN-γ expression.
Treatment with EA may promote the secretion of
adrenocortical hormones, thereby enhancing the function of the
pituitary-adrenal cortex and sympathetic-adrenal systems to improve
resistance to certain diseases, such as myocardial ischemia injury
(33). In addition, EA therapy
enhanced immunity by improving the phagocytic ability of the
mononuclear macrophage system (34) and function of T helper cells
(35) in rats. Moreover, EA
increased microvascular perfusion, improved blood circulation,
supported the lymphatic and nervous systems and regulated cellular
and humoral immunity in humans (36). Thus, the exact mechanisms of EA in
the treatment of CRSwNP require further investigation.
The present study possessed to certain limitations.
EA promoted IL-10 expression and IL-10 overexpression ameliorated
the pathological changes of CRSwNP; however, the underlying
mechanisms were not elucidated. The manner via which IL-10
regulates IFN-γ requires clarification. An increased number of
quantified parameters to evaluate pathological changes should be
detected to confirm the effectiveness and uniformity of the
treatment administered by nasal drops. Finally, a transgenic model,
such as IL-10 knockout mice, may be more effective in determining
the underlying mechanisms.
In conclusion, EA and IL-10 effectively inhibit
inflammation and increase the expression of IFN-γ, and thereby
exert various specific effects in the treatment of CRS in mice.
These data may provide novel insight for the future treatment of
CRS.
Acknowledgements
Not applicable.
Funding
The current study was supported by grants from the
Jiangxi Education Department, the Jiangxi Provincial Health
Planning Commission (grant no. 2016A102) and the State
Administration of Chinese Medicine (grant no. 201507006).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LZ and YS conceived and designed the experiments.
JH, ZW and XL performed the experiments and analyzed the data. LZ
and YS wrote the manuscript. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The experimental protocols were approved by the
Ethics Committee of Jiangxi University of Chinese Medicine.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li XZ, Zhao SC, Cai XL, Wang YF, Chen J,
Ma XF and Zhang H: Differences in expression of YKL-40 and TLR4 in
nasal sinus mucosa of chronic sinusitis patients with and without
nasal polyps. J Biol Regul Homeost Agents. 32:537–543.
2018.PubMed/NCBI
|
|
2
|
Fokkens WJ, Lund VJ, Mullol J, Bachert C,
Alobid I, Baroody F, Cohen N, Cervin A, Douglas R, Gevaert P, et
al: European position paper on rhinosinusitis and nasal polyps
2012. Rhinol Suppl. 23:3 p preceding table of contents, 1–298.
2012.PubMed/NCBI
|
|
3
|
Bachert C, Zhang L and Gevaert P: Current
and future treatment options for adult chronic rhinosinusitis:
Focus on nasal polyposis. J Allergy Clin Immunol. 136:1431–1440.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schmetterer KG and Pickl WF: The
IL-10/STAT3 axis: Contributions to immune tolerance by thymus and
peripherally derived regulatory T-cells. Eur J Immunol.
47:1256–1265. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shah N, Kammermeier J, Elawad M and
Glocker EO: Interleukin-10 and interleukin-10-receptor defects in
inflammatory bowel disease. Curr Allergy Asthma Rep. 12:373–379.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lennon EM, Borst LB, Edwards LL and Moeser
AJ: Mast cells exert anti-inflammatory effects in an
IL10−/− model of spontaneous colitis. Mediators Inflamm.
2018:78173602018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Iyer SS and Cheng G: Role of interleukin
10 transcriptional regulation in inflammation and autoimmune
disease. Crit Rev Immunol. 32:23–63. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu J, Han R, Kim DW, Mo JH, Jin Y, Rha KS
and Kim YM: Role of interleukin-10 on nasal polypogenesis in
patients with chronic rhinosinusitis with nasal polyps. PLoS One.
11:e01610132016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang C, Lou H, Wang X, Wang Y, Fan E, Li
Y, Wang H, Bachert C and Zhang L: Effect of budesonide transnasal
nebulization in patients with eosinophilic chronic rhinosinusitis
with nasal polyps. J Allergy Clin Immunol. 135:922–929.e6. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim HW, Kang SY, Yoon SY, Roh DH, Kwon YB,
Han HJ, Lee HJ, Beitz AJ and Lee JH: Low-frequency
electroacupuncture suppresses zymosan-induced peripheral
inflammation via activation of sympathetic post-ganglionic neurons.
Brain Res. 1148:69–75. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen X, Tang C, Xie H, Tang N, Gao J and
Liu R: Effect of electro-acupuncture on hepatic Toll-like receptor
4 and nuclear factor κB expressions in rats with non-alcoholic
fatty liver disease. Nan Fang Yi Ke Da Xue Xue Bao. 34:1584–1588.
2014.(In Chinese). PubMed/NCBI
|
|
12
|
Rössberg E, Larsson PG, Birkeflet O,
Söholt LE and Stavem K: Comparison of traditional Chinese
acupuncture, minimal acupuncture at non-acupoints and conventional
treatment for chronic sinusitis. Complement Ther Med. 13:4–10.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim JI, Choi JY, Lee MS, Kim TH, Kim AR,
Jung SY, Shin MS and Kim KH: Acupuncture for improving chronic
rhinosinusitis complicated with persistent allergic rhinitis. A
prospective observational study. Forsch Komplementmed. 17:333–335.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim DY, Lee SH, Carter RG, Kato A,
Schleimer RP and Cho SH: A recently established murine model of
nasal polyps demonstrates activation of B cells, as occurs in human
nasal polyps. Am J Respir Cell Mol Biol. 55:170–175. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mendiola M, Tharakan A, Chen M, Asempa T,
Lane AP and Ramanathan M Jr: Characterization of a novel high-dose
ovalbumin-induced murine model of allergic sinonasal inflammation.
Int Forum Allergy Rhinol. 6:964–972. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ip WKE, Hoshi N, Shouval DS, Snapper S and
Medzhitov R: Anti-inflammatory effect of IL-10 mediated by
metabolic reprogramming of macrophages. Science. 356:513–519. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rossi O, van Berkel LA, Chain F, Tanweer
Khan M, Taverne N, Sokol H, Duncan SH, Flint HJ, Harmsen HJ,
Langella P, et al: Faecalibacterium prausnitzii A2-165 has a high
capacity to induce IL-10 in human and murine dendritic cells and
modulates T cell responses. Sci Rep. 6:185072016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jung M, Ma Y, Iyer RP, DeLeon-Pennell KY,
Yabluchanskiy A, Garrett MR and Lindsey ML: IL-10 improves cardiac
remodeling after myocardial infarction by stimulating M2 macrophage
polarization and fibroblast activation. Basic Res Cardiol.
112:332017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Haruna T, Kariya S, Fujiwara T, Higaki T,
Makihara S, Kanai K, Fujiwara R, Iwasaki S, Noguchi Y, Nishizaki K
and Okano M: Association between impaired IL-10 production
following exposure to Staphylococcus aureus enterotoxin B and
disease severity in eosinophilic chronic rhinosinusitis. Allergol
Int. 67:392–398. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rai G, Das S, Ansari MA, Singh PK, Gupta
N, Sharma S, Akhter N, Ramachandran VG, Haque S and Dar SA:
Phenotypic and functional profile of Th17 and Treg cells in
allergic fungal sinusitis. Int Immunopharmacol. 57:55–61. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang YX, Gu ZW, Cao ZW and Hao LY:
Nonylphenol can aggravate allergic rhinitis in a murine model by
regulating important Th cell subtypes and their associated
cytokines. Int Immunopharmacol. 70:260–267. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nova-Lamperti E, Fanelli G, Becker PD,
Chana P, Elgueta R, Dodd PC, Lord GM, Lombardi G and
Hernandez-Fuentes MP: IL-10-produced by human transitional B-cells
down-regulates CD86 expression on B-cells leading to inhibition of
CD4+ T-cell responses. Sci Rep. 6:200442016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Y, Yi J, Chen X, Zhang Y, Xu M and
Yang Z: The regulation of cancer cell migration by lung cancer
cell-derived exosomes through TGF-β and IL-10. Oncol Lett.
11:1527–1530. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hayashi M, Yanaba K, Umezawa Y, Yoshihara
Y, Kikuchi S, Ishiuji Y, Saeki H and Nakagawa H: IL-10-producing
regulatory B cells are decreased in patients with psoriasis. J
Dermatol Sci. 81:93–100. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kawamoto S, Oritani K, Asakura E, Ishikawa
J, Koyama M, Miyano K, Iwamoto M, Yasuda S, Nakakubo H, Hirayama F,
et al: A new interferon, limitin, displays equivalent
immunomodulatory and antitumor activities without myelosuppressive
properties as compared with interferon-alpha. Exp Hematol.
32:797–805. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sakai S, Kauffman KD, Sallin MA, Sharpe
AH, Young HA, Ganusov VV and Barber DL: CD4 T cell-derived IFN-γ
plays a minimal role in control of pulmonary mycobacterium
tuberculosis infection and must be actively repressed by PD-1 to
prevent lethal disease. PLoS Pathog. 12:e10056672016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yuliatun L, Amalia SH, Rahma AA and Yaumi
LA: Electro-acupuncture therapy increases serum interferon-γ levels
in rats with 7, 12 Dimethylbenz(α)anthracene (DMBA)-induced breast
tumors. Asian Pac J Cancer Prev. 18:1323–1328. 2017.PubMed/NCBI
|
|
29
|
Carneiro ER, Xavier RA, De Castro MA, Do
Nascimento CM and Silveira VL: Electroacupuncture promotes a
decrease in inflammatory response associated with Th1/Th2
cytokines, nitric oxide and leukotriene B4 modulation in
experimental asthma. Cytokine. 50:335–340. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Marchant A, Bruyns C, Vandenabeele P,
Ducarme M, Gérard C, Delvaux A, De Groote D, Abramowicz D, Velu T
and Goldman M: Interleukin-10 controls interferon-gamma and tumor
necrosis factor production during experimental endotoxemia. Eur J
Immunol. 24:1167–1171. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Herrero C, Hu X, Li WP, Samuels S, Sharif
MN, Kotenko S and Ivashkiv LB: Reprogramming of IL-10 activity and
signaling by IFN-gamma. J Immunol. 171:5034–5041. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yanagawa Y, Iwabuchi K and Onoe K:
Co-operative action of interleukin-10 and interferon-gamma to
regulate dendritic cell functions. Immunology. 127:345–353. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cui S, Zhou Y, Wu S, Cao J, Zhu G and Zhou
M: Electroacupuncture improved the function of myocardial ischemia
involved in the hippocampus-paraventricular nucleus-sympathetic
nerve pathway. Evid Based Complement Alternat Med.
2018:28706762018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zeng Q, He H, Wang XB, Zhou YQ, Lin HX,
Tan ZP, He SF and Huang GZ: Electroacupuncture preconditioning
improves myocardial infarction injury via enhancing AMPK-dependent
autophagy in rats. Biomed Res Int. 2018:12381752018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhu J, Chen XY, Li LB, Yu XT, Zhou Y, Yang
WJ, Liu Z, Zhao N, Fu C, Zhang SH and Chen YF: Electroacupuncture
attenuates collagen-induced arthritis in rats through vasoactive
intestinal peptide signalling-dependent re-establishment of the
regulatory T cell/T-helper 17 cell balance. Acupunct Med.
33:305–311. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tan CF, Yan J, Wang C, Chang XR, Xie WJ,
Yang JJ, Liu M, Lin HB and He XC: Effects of electroacupuncture and
moxibustion pretreatment on expressions of HSP 27, HSP 70, HSP 90
at different time-points in rabbits with myocardial
ischemia-reperfusion injury. Zhen Ci Yan Jiu. 42:31–38. 2017.(In
Chinese). PubMed/NCBI
|