Introduction
Heat shock (HS) is a life-threatening disorder
associated with excessive heat and characterized by a syndrome of
multiple organ dysfunction in which central nervous system
dysfunction dominates (1,2). The direct cytotoxicity of heat,
inflammation and endothelial cell (EC) injury is considered to
initiate multiple organ failure (3–5). ECs
have a variety of biological functions, including the regulation of
coagulation, cell adhesion, nutrient exchange and vascular tone;
ECs also serve an important role in the balance of local
pro-inflammatory and anti-inflammatory mediators (6–12).
Previous studies in cell lines and animal models have shown that
ECs are an early target of heat stress injury and are a prominent
feature of severe HS (5,13,14).
Thus, understanding the changes in ECs in response to heat stress
is important for identifying novel therapeutic approaches to HS
injury treatment.
MicroRNAs (miRNAs) are small RNAs that regulate gene
expression at the post-transcriptional level and modulate
biological responses and cell phenotypes through regulation of the
expression levels of key proteins in multiple biological pathways
(15). Certain miRNAs have been
demonstrated to affect the function of ECs. For example, miRNA
(miR)-155, miR-320, miR-222, miR-125b, miR-410 and miR-218 have
been reported to regulate adherens junction disassembly responses,
cell migration and cell morphology, contributing to changes in the
permeability and integrity of the vasculature (16–18).
As underlying regulatory mechanisms such as miRNA expression in
response to HS were unknown, miRNA expression was analyzed in our
previous study via miRNA microarrays; it was demonstrated that heat
stress altered miRNA expression in HUVECs, with 31 miRNAs differing
significantly in expression between HUVECs exposed to severe heat
treatment and untreated control cells (20 miRNAs were downregulated
and miRNAs were 11 upregulated) (19). Only 1% of the human miRNA
assemblage (31/3,100) exhibited differential expression following
heat treatment, indicating that the regulatory activity of heat
stress may be specific toward a subset of miRNAs in HUVECs.
Additionally, associations between miRNAs and genes have been
previously evaluated on the basis of their differential expression
levels, and according to interactions between miRNAs and genes
reported in the Sanger miRNA database; these associations were used
to build a miRNA-gene network that was employed to evaluate the
regulatory effects of miRNAs on genes (19). Using this network model,
miR-3613-3p was identified as a key miRNA that may serve a
prominent role in the response to heat stress (19).
In the present study, the biological functions and
underlying molecular mechanisms of miR-3613-3p in ECs during heat
stress were preliminarily investigated. The results may contribute
to a better understanding of the mechanisms underlying the
relationships between miRNAs and biological responses of ECs and
may facilitate new diagnostic and therapeutic strategies for the
treatment of heat stress.
Materials and methods
Bioinformatics analysis
Potential target genes of miR-3613-3p were predicted
using three databases: TargetScan 7.2 (http://www.targetscan.org/), miRanda-mirSVR 5.0
(http://www.microrna.org/microrna/home.do) and miRDB
5.0 (http://www.mirdb.org). Functional annotation
using Gene Ontology (GO) (20,21)
enrichment analysis was performed to determine the main functions
of putative target genes of miR-3613-3p. Additionally, Kyoto
Encyclopedia of Genes and Genomes (KEGG) (22–24)
database analysis was performed to identify the enriched pathways
associated with these target genes. Fisher's exact and
χ2 tests were applied to assess the significance of GO
terms and pathways, and the false discovery rate (FDR) was
calculated to correct the P-value. Only GO terms and pathways with
an adjusted P<0.05 and an FDR<0.05 were selected. Data
containing two groups were analyzed using Fisher's exact test, and
multiple comparisons between groups were assessed using the
χ2 test.
Cell culture and treatment
Human umbilical vein endothelial cells (HUVECs;
American Type Culture Collection) were cultured in RPMI 1640 medium
(Invitrogen; Thermo Fisher Scientific, Inc.) supplemented with 5%
FBS (Invitrogen; Thermo Fisher Scientific, Inc.), 1% EC growth
supplement (PromoCell GmbH), 100 U/ml penicillin and 100 µg/ml
streptomycin (Invitrogen; Thermo Fisher Scientific, Inc.) in a
humidified atmosphere with 5% CO2 at 37°C. All
experiments were performed using HUVECs at passage 3–6. To induce
heat stress, culture dishes containing cells in media were sealed
with Parafilm and immersed in a circulating water bath at 43±0.5°C
for 1 h; cells in culture dishes placed in a circulating water bath
at 37±0.5°C for 1 h were used as a control. After treatment, the
culture medium was replaced with fresh medium, and the cells were
incubated at 37°C for an additional 24 h.
RNA extraction
Total RNA from HUVECs (~5×106 cells) was
individually isolated using TRIzol® (Thermo Fisher
Scientific, Inc.) and an miRNeasy mini kit (Qiagen, Inc.) according
to the manufacturers’ protocols. RNA quantity and quality were
measured using a NanoDrop™ spectrophotometer (ND-1000; NanoDrop
Technologies; Thermo Fisher Scientific, Inc.) and RNA integrity was
detected by gel electrophoresis. In general, RNA concentrations of
8–15 ng/µl, and 10 ng/sample of total RNA were used for subsequent
reverse transcription-quantitative PCR (RT-qPCR) analysis.
RT-qPCR
The miRNA expression levels were determined using
RT-qPCR using primers which were synthesized and purchased from
Shanghai GenePharma Co., Ltd. The following primers were used:
miR-3613-3p, sense 5′-CGTCCCTTCCCAACCCGAAAAAAA-3′, antisense
5′-CGCAGGGTCCGAGGTATTC-3′; and U6, sense 5′-CTCGCTTCGGCAGCACA-3′
and antisense 5′-AACGCTTCACGAATTTGCGT-3′. Briefly, sample total RNA
(10 ng) was reverse transcribed into cDNA using specific stem-loop
primers and a TaqMan® MicroRNA Reverse Transcription kit
(Applied Biosystems; Thermo Fisher Scientific, Inc.). Mixtures were
incubated for 30 min at 16°C, 30 min at 42°C and 5 min at 85°C, and
held at 4°C in a 15-µl reaction volume. Following the reverse
transcription reaction, qPCR was performed using SYBR Green
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol and an ABI 7300 Real-Time PCR system
(Bio-Rad Laboratories, Inc.). qPCR conditions were 95°C for 10 min,
followed by 40 cycles of 95°C for 15 sec and 60°C for 60 sec in a
20 µl reaction volume (25,26).
Signals were normalized to U6 small nuclear RNA, which was analyzed
simultaneously. The relative expression of each miRNA was
calculated using the 2−ΔΔCq method (27). The experiments were performed at
least three times.
Transient transfection
The miR-3613-3p mimic (cat. no. miR10017991-1-5) and
negative control (miR-NC; cat. no. miR1N0000001-1-5) were purchased
from Guangzhou RiboBio Co., Ltd. Cells in the logarithmic growth
phase (5×105 cells/well) were inoculated into 24-well
plates and cultured to 80% confluence on the day prior to
transfection. Subsequently, miR-3613-3p mimic or miR-NC were
transfected into HUVECs at a final concentration of 50 nM using
Lipofectamine® 3000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) at room temperature for 24 h according to the
manufacturer's protocol. At 24 h post-transfection, the transfected
cells were harvested for miRNA and protein assays.
Flow cytometric analysis of
apoptosis
Apoptosis was analyzed with an Annexin V- FITC
Apoptosis kit (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. HUVECs (~1×106
cells) were collected, washed with ice-cold PBS and resuspended in
binding buffer containing 5 µl annexin V-FITC for 10 min in the
dark at room temperature. Subsequently, the cells were pelleted by
centrifugation at ~157 × g and 4°C for 10 min, the buffer was
removed and the cells were resuspended in reaction buffer
containing 10 µl propidium iodide (PI). Following incubation in the
dark at room temperature for 15 min, the stained cells were
analyzed for apoptosis using a FACSCanto™ II flow cytometer (BD
Biosciences). The early apoptotic rates were examined. FlowJo
version 7.6.1 software (FlowJo LLC) was used to analyze the data.
The experiment was performed at least three times.
Western blot analysis
HUVECs (5×105 cells/well) were seeded in
24-well plates and incubated for 24 h prior to lysis. Cells were
lysed in 1X sample buffer (62.5 mM Tris, pH 6.8; 10% glycerol; 2%
SDS) and homogenized. The protein concentration was measured using
a Bicinchoninic Acid Protein assay kit (Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. Equal amounts of
protein (20 µg/well) were separated by 10% SDS-PAGE and transferred
onto polyvinylidene difluoride membranes (EMD Millipore). The
membranes were blocked with 5% non-fat milk powder in PBS + 0.1%
Tween-20 at room temperature for 2 h. The membranes were
subsequently incubated overnight at 4°C with mouse anti-human
mitogen-activated protein kinase kinase kinase 2 (MAP3K2; 1:1,000,
cat. no. SAB5300054, Sigma Aldrich; Merck KGaA) or mouse anti-human
actin (1:500, cat. no. A4700, Sigma Aldrich; Merck KGaA) primary
antibodies. A goat anti-mouse horseradish peroxidase-conjugated
immunoglobulin G secondary antibody (1:10,000; cat. no. SAB3701029;
Sigma Aldrich; Merck KGaA) was used, with incubation at room
temperature for 2 h. Protein bands were visualized with Enhanced
Chemiluminescence Western Blot Detection reagent (Thermo Fisher
Scientific, Inc.). The membranes were exposed to light-sensitive
film and densitometric analysis was conducted using ImageJ v1.50
software (National Institutes of Health). The expression levels of
protein were normalized to the actin endogenous control. The
western blotting experiments were performed at least three
times.
Luciferase gene reporter assay
HUVECs (1×105 cells/well) were seeded in
24-well plates and incubated for 24 h prior to transfection. Cells
were co-transfected with 100 nM luciferase vectors (pGL3-MAP3K2
3′UTR wild-type or mutant reporter plasmid; Guangzhou RiboBio Co.,
Ltd.) and either 50 nM miR-3613-3p mimic or miR-NC using
Lipofectamine® 3000 at room temperature for 24 h.
Firefly and Renilla luciferase activities were quantified
using the Dual-Luciferase reporter system (Promega Corporation)
according to the manufacturer's protocol. The firefly luciferase
activiy was normalized to Renilla luciferase activity. All
experiments were performed in triplicate; n=3–6 for each
experiment.
Statistical analysis
Quantitative variables are presented as the mean ±
standard deviation. Student's t-test was used to calculate
statistical significance between two groups. One-way ANOVA was used
for multiple group comparisons followed by Tukey post hoc test.
Statistical values were calculated using SPSS version 20.0 (IBM
Corp.). P<0.05 was considered to indicate a statistically
significant difference.
Results
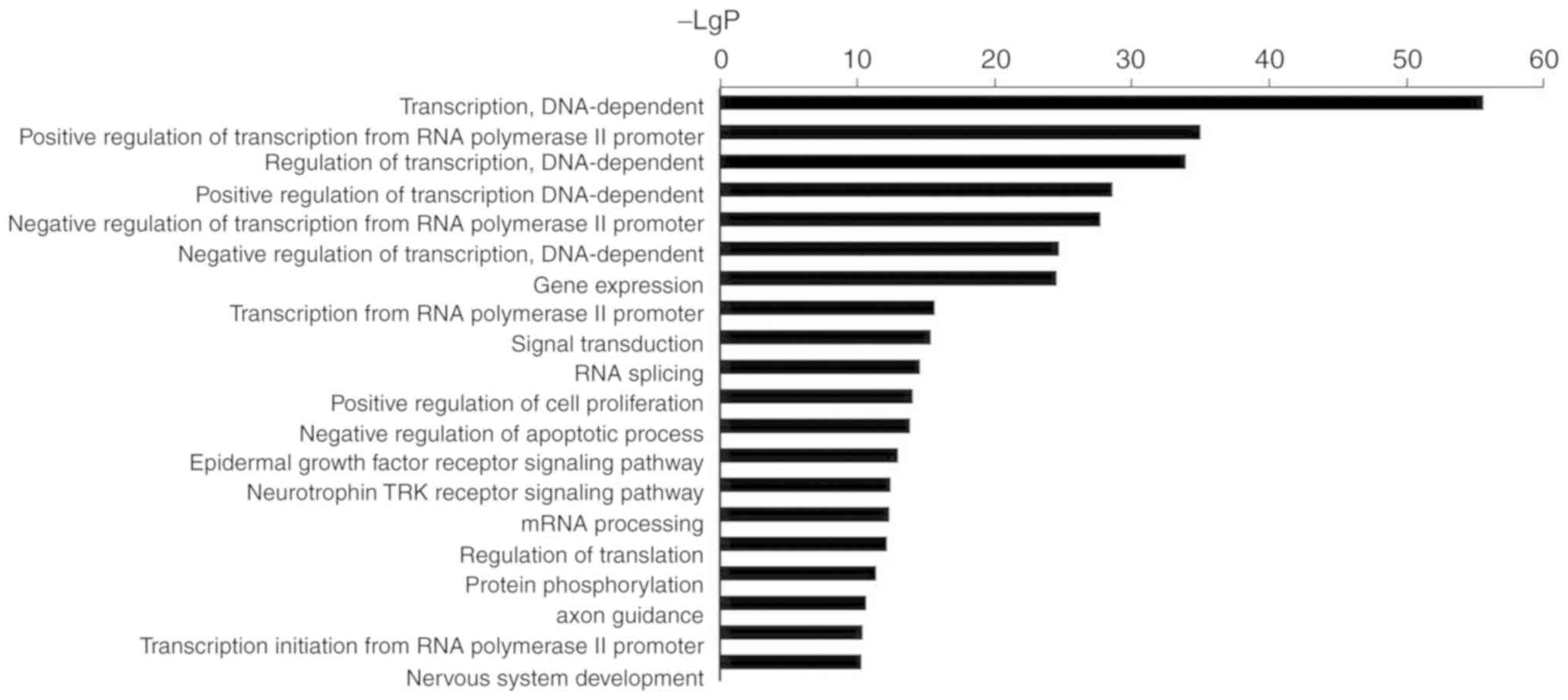
GO term analysis of miR-3613-3p
GO analysis results were presented in Fig. 1, and Tables I (data repository) and
II. According to GO analysis of
the 865 genes potentially regulated by miR-3613-3p, the top
biological processes associated with the genes regulated by
miR-3613-3p were associated with transcription. A number of the
genes were also involved in cell proliferation and apoptosis.
 | Table II.Top 20 significant GO biological
processes of microRNA-3613-3p target genes. |
Table II.
Top 20 significant GO biological
processes of microRNA-3613-3p target genes.
| GO ID | GO term | Enrichment | P-value | FDR |
|---|
| GO:0006351 | Transcription,
DNA-dependent | 4.429852641 |
3.20039×10−56 |
8.225×10−53 |
| GO:0045944 | Positive regulation
of transcription from RNA polymerase II promoter | 5.752041467 |
1.42286×10−35 |
1.82837×10−32 |
| GO:0006355 | Regulation of
transcription, DNA-dependent | 4.150877171 |
1.52829×10−34 |
1.30924×10−31 |
| GO:0045893 | Positive regulation
of transcription, DNA-dependent | 6.299381822 |
3.20544×10−29 |
2.0595×10−26 |
| GO:0000122 | Negative regulation
of transcription from RNA polymerase II promoter | 6.076613899 |
2.3074×10−28 |
1.186×10−25 |
| GO:0045892 | Negative regulation
of transcription, DNA-dependent | 6.125889879 |
2.8019×10−25 |
1.20015×10−22 |
| GO:0010467 | Gene
expression | 4.938916527 |
4.02629×10−25 |
1.47822×10−22 |
| GO:0006366 | Transcription from
RNA polymerase II promoter | 5.640723054 |
3.17439×10−16 | 1.01977
×10−13 |
| GO:0007165 | Signal
transduction | 3.253151632 |
5.30103×10−16 |
1.51374×10−13 |
| GO:0008380 | RNA splicing | 6.443742656 |
3.21943×10−15 |
8.27393×10−13 |
| GO:0008284 | Positive regulation
of cell proliferation | 4.766174616 |
1.15335×10−14 |
2.69465×10−12 |
| GO:0043066 | Negative regulation
of apoptotic process | 4.366833866 |
1.86122×10−14 |
3.98612×10−12 |
| GO:0007173 | Epidermal growth
factor receptor signaling pathway | 6.966208276 |
1.33741×10−13 |
2.64395×10−11 |
| GO:0048011 | Neurotrophin TRK
receptor signaling pathway | 5.55742861 |
4.69776×10−13 |
8.62374×10−11 |
| GO:0006397 | mRNA
processing | 6.873325499 |
5.87456×10−13 |
1.00651×10−10 |
| GO:0006417 | Regulation of
translation | 12.12939794 |
8.03991×10−13 |
1.29141×10−10 |
| GO:0006468 | Protein
phosphorylation | 4.727953191 |
5.1723×10−12 |
7.8193×10−10 |
| GO:0007411 | Axon guidance | 4.715925224 |
2.91722×10−11 |
4.16514×10−9 |
| GO:0006367 | Transcription
initiation from RNA poly merase II promoter | 6.163579932 |
5.51489×10−11 |
7.45962×10−9 |
| GO:0007399 | Nervous system
development | 4.918192274 |
5.84827×10−11 |
7.51503×10−9 |
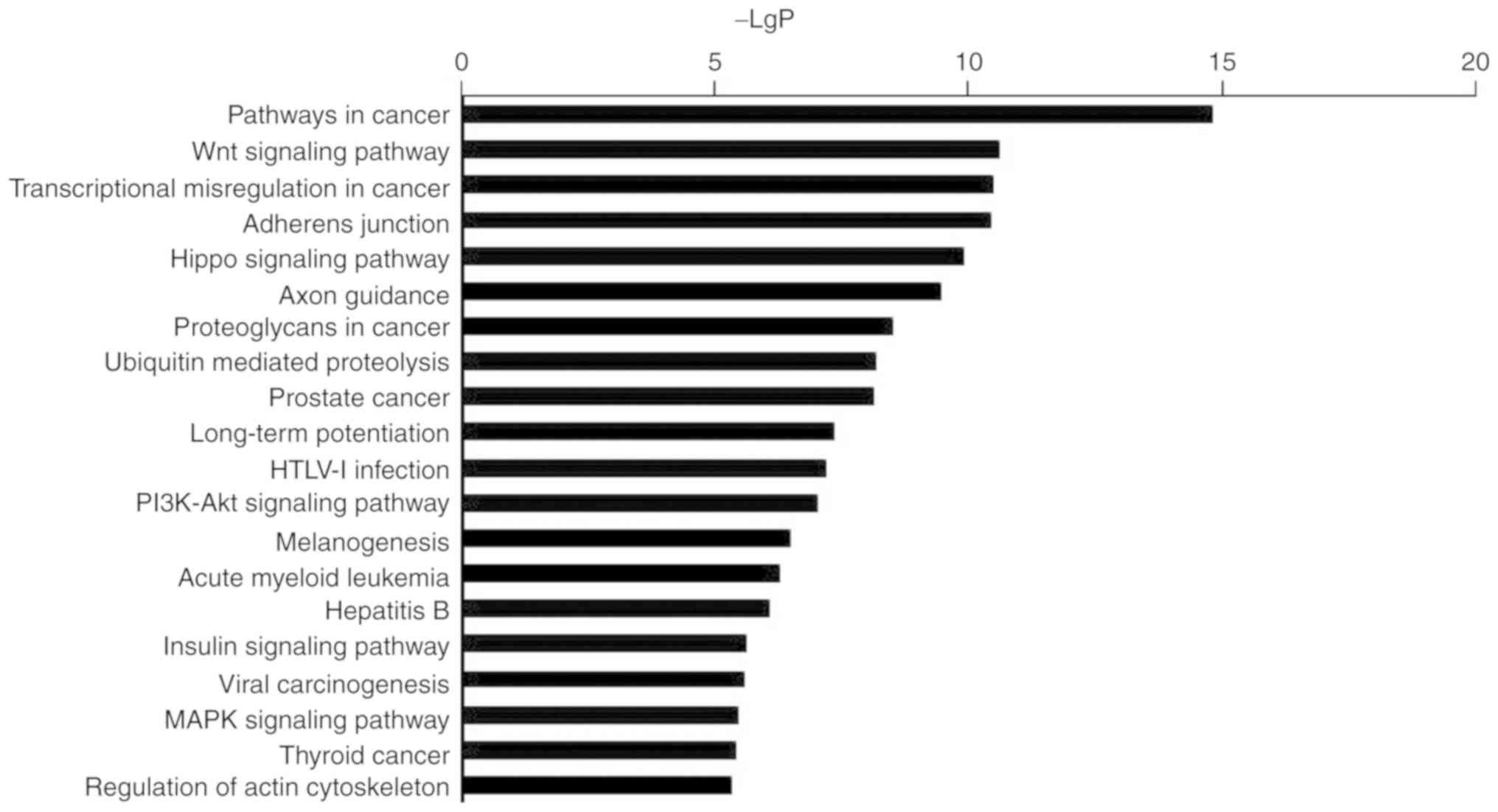
KEGG pathway analysis of
miR-3613-3p
Pathway analysis by KEGG revealed the signaling
pathways affected by miR-3613-3p in heat-treated cells. The most
significantly enriched pathways regulated by miR-3613-3p analyzed
by KEGG analysis were presented in Fig. 2 and Table III. Enriched KEGG pathway
analyses revealed that the top signaling pathways regulated by
miR-3613-3p were centralized in cancer-associated terms, which
participated in the regulation of apoptosis, proliferation and cell
cycle. Additional identified signaling pathways were also
associated with transcriptional misregulation in cancer and
adherens junctions.
 | Table III.Top 20 significant KEGG pathways of
microRNA-3613-3p target genes. |
Table III.
Top 20 significant KEGG pathways of
microRNA-3613-3p target genes.
| KEGG ID | Pathway name | Enrichment | P-value | FDR |
|---|
| 05200 | Pathways in
cancer | 5.517577809 |
1.69483×10−15 |
3.25407×10−13 |
| 04310 | Wnt signaling
pathway | 7.209781992 |
2.54375×10−11 |
1.8035×10−9 |
| 05202 | Transcriptional
misregulation in cancer | 6.300548374 |
3.54805×10−11 |
1.8035×10−9 |
| 04520 | Adherens
junction | 10.59245368 |
3.75729×10−11 |
1.8035×10−9 |
| 04390 | Hippo signaling
pathway | 6.608966826 |
1.29264×10−10 |
4.96376×10−9 |
| 04360 | Axon guidance | 7.083198034 |
3.87582×10−10 |
1.24026×10−8 |
| 05205 | Proteoglycans in
cancer | 4.996029548 |
3.30873×10−9 |
9.07539×10−8 |
| 04120 | Ubiquitin mediated
proteolysis | 6.350355081 |
7.1811×10−9 |
1.63216×10−7 |
| 05215 | Prostate
cancer | 8.108979522 |
7.65076×10−9 |
1.63216×10−7 |
| 04720 | Long-term
potentiation | 8.712666126 |
4.7221×10−8 |
9.06642×10−7 |
| 05166 | HTLV–I
infection | 4.231711595 |
7.15157×10−8 |
1.24827×10−6 |
| 04151 | PI3K-Akt signaling
pathway | 3.713972712 |
1.06017×10−7 |
1.69628×10−6 |
| 04916 | Melanogenesis | 6.635140952 |
3.41775×10−7 |
5.04776×10−6 |
| 05221 | Acute myeloid
leukemia | 9.043849341 |
5.42797×10−7 |
7.44407×10−6 |
| 05161 | Hepatitis B | 5.224656207 |
8.84732×10−7 |
1.13246×10−5 |
| 04910 | Insulin signaling
pathway | 5.154994125 |
2.62577×10−6 |
3.15092×10−5 |
| 05203 | Viral
carcinogenesis | 4.233570054 |
2.90592×10−6 |
3.28198×10−5 |
| 04010 | MAPK signaling
pathway | 3.767111091 |
3.92363×10−6 |
4.1852×10−5 |
| 05216 | Thyroid cancer | 12.44308927 |
4.33983×10−6 |
4.38551×10−5 |
| 04810 | Regulation of actin
cytoskeleton | 4.076041866 |
4.92093×10−6 |
4.7241×10−5 |
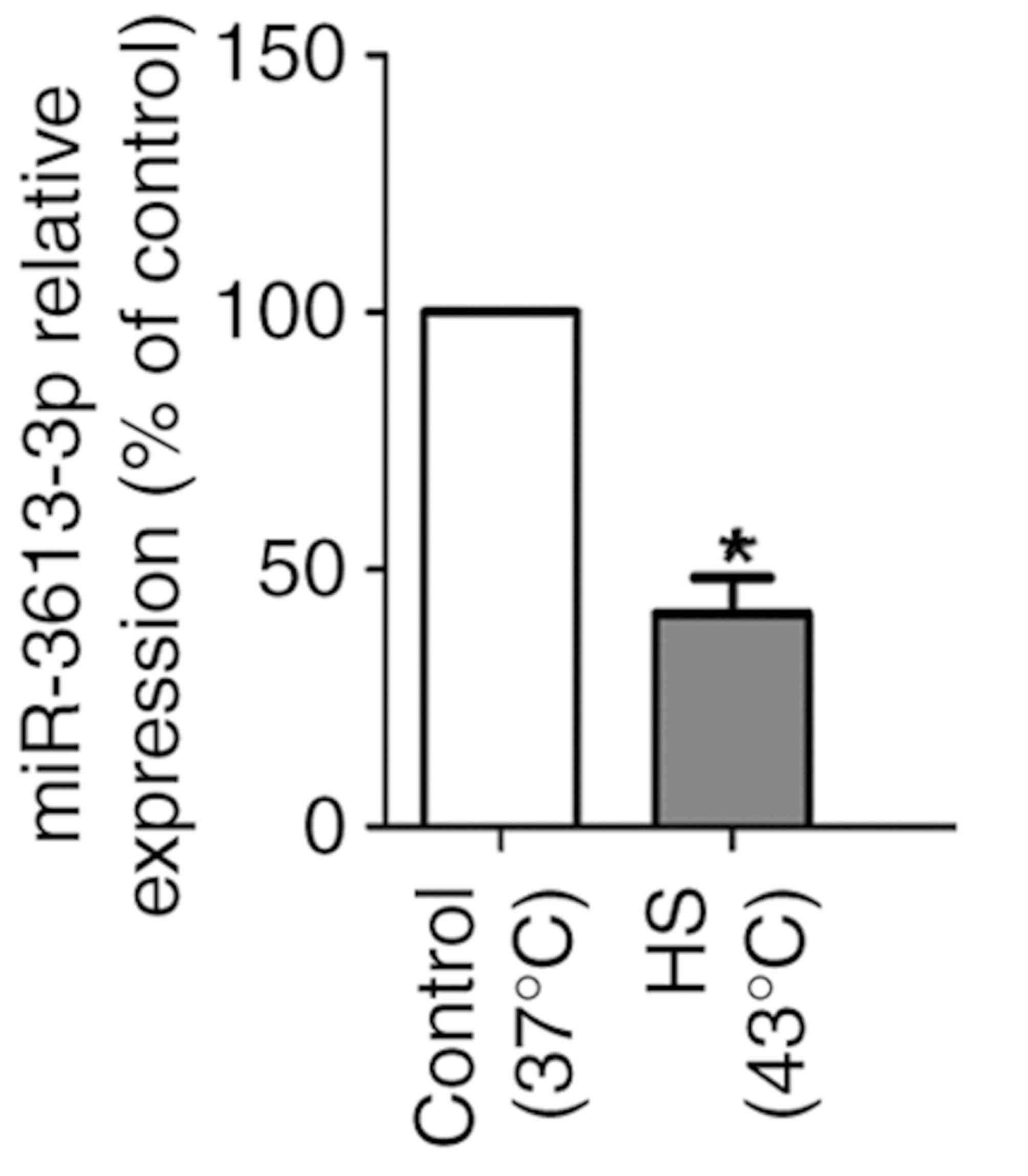
miRNA expression levels are
dysregulated in HUVECs during heat stress
Based on previous miRNA microarray analysis,
miR-3613-3p expression levels were decreased in HUVECs under heat
stress (19). To confirm the
downregulation of miR-3613-3p in heat-treated cells, miR-3613-3p
expression was analyzed by RT-qPCR, and the results verified that
miR-3613-3p expression was reduced in HS-treated HUVECs compared
with control cells (P<0.05; Fig.
3).
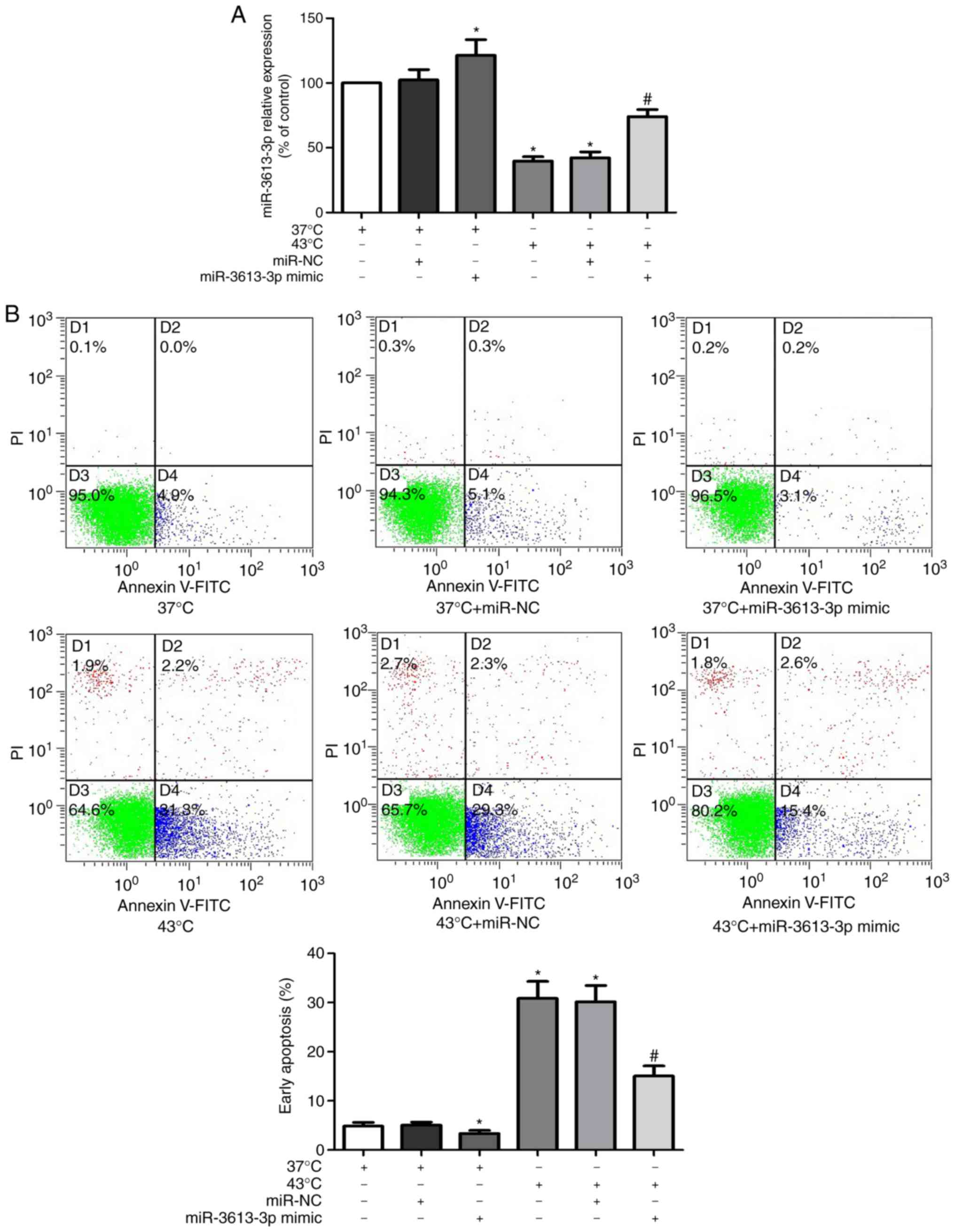
miR-3613-3p affects apoptosis in
HUVECs during heat stress
Previous bioinformatics analysis demonstrated that
miR-3613-3p negatively regulates apoptosis (19). To confirm this, miR-3613-3p mimic
was transfected into HUVECs, which increased the expression levels
of microRNAs-3613-3p (Fig. 4A).
Annexin V-FITC/PI flow cytometry was then used to evaluate the
effects of different expression levels of miR-3613-3p on the
apoptotic rates of HUVECs. The results revealed that miR-3613-3p
expression decreased in HUVECs following heat stress and that the
miR-3613-3p mimic increased the level of miR-3613-3p in
heat-treated and unheated cells (P<0.05; Fig. 4A). In addition, heat stress induced
apoptosis in HUVECs, whereas the miR-3613-3p mimic transfection
significantly decreased the apoptotic rate under heat stress
(P<0.05; Fig. 4B). These
results suggested that the level of miR-3613-3p may affect HUVEC
apoptosis during heat stress.
miR-3613-3p affects the expression of
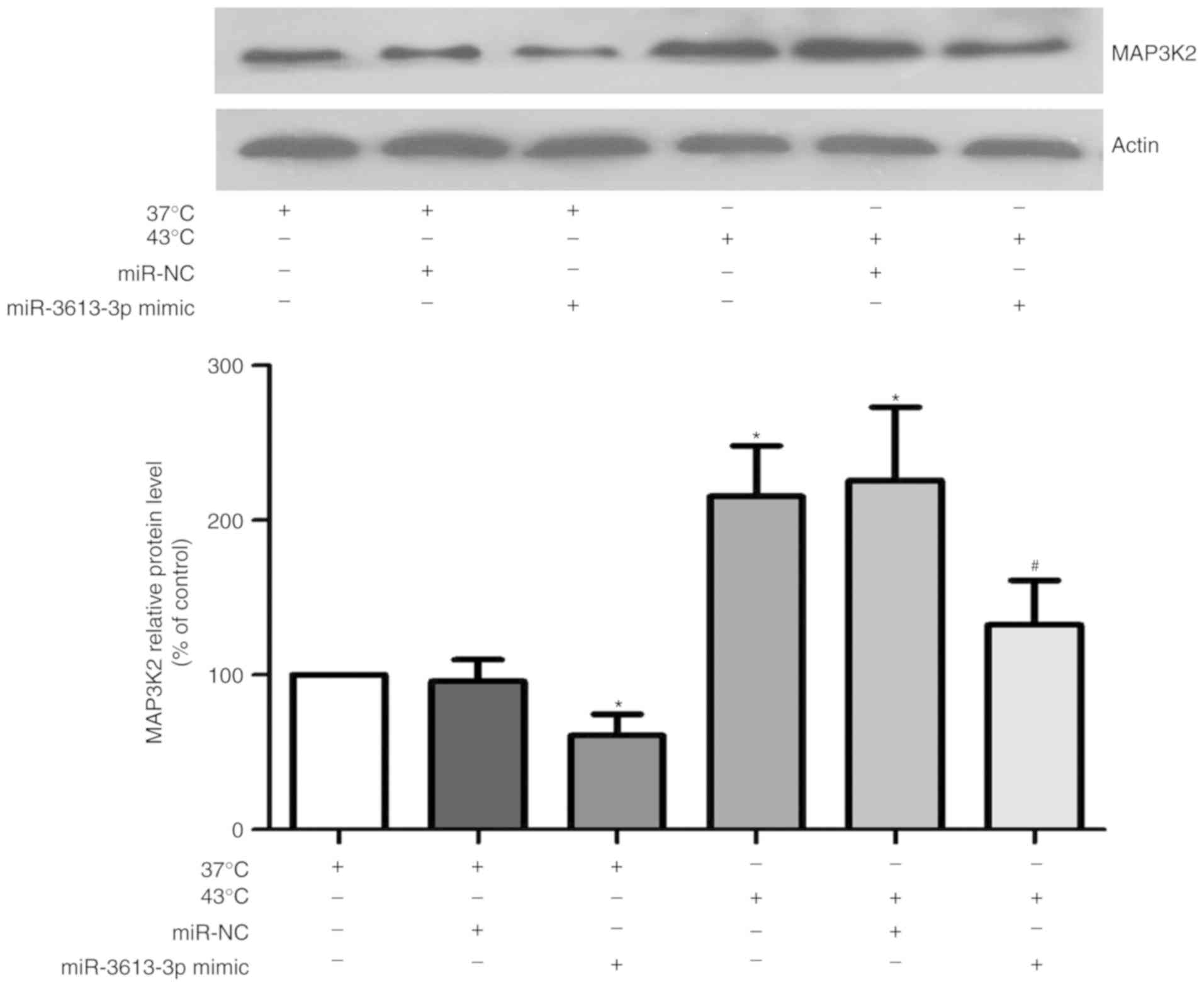
MAP3K2 in HUVECs during heat stress
Previous bioinformatics analysis indicated that
MAP3K2 may be a target gene of miR-3613-3p (19). To investigate the association
between miR-3613-3p and MAP3K2, HUVECs were transfected with
miR-3613-3p mimic for 24 h, and western blotting was performed. As
presented in Fig. 5, the protein
level of MAP3K2 was significantly reduced in the miR-3613-3p
mimic-transfected cells under heat treatment, compared with cells
only treated with heat stress (P<0.05). These results indicated
that miR-3613-3p overexpression may downregulate MAP3K2
expression.
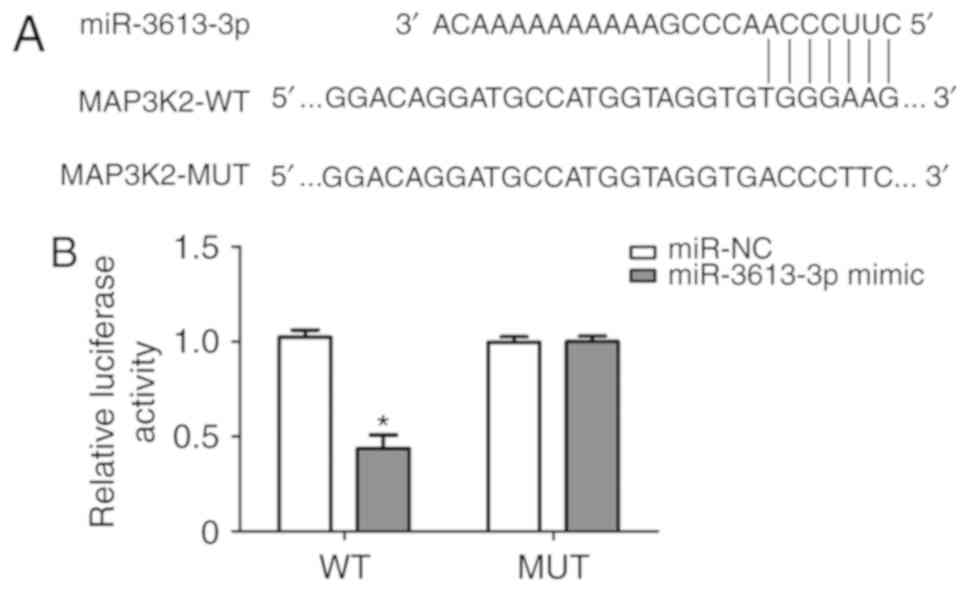
MAP3K2 is a direct target of
miR-3613-3p in HUVECs
To further demonstrate whether MAP3K2 was a direct
target of miR-3613-3p, MAP3K2 wild-type and mutant 3′UTR were
cloned into a luciferase reporter vector and transfected into
HUVECs (Fig. 6A). The effects of
miR-3613-3p were determined using the luciferase reporter assay.
The results demonstrated that upregulation of miR-3613-3p
significantly reduced the luciferase activity of pGL3-MAP3K2 3′UTR
WT (P<0.05), but the effects of miR-3613-3p was not observed
with the mutation of the miR-3613-3p target site in the MAP3K2
3′UTR (Fig. 6B). These results
suggested that MAP3K2 may be directly and negatively regulated by
miR-3613-3p.
Discussion
Among the miRNAs identified and target genes
predicted in our previous study, miR-3613-3p was identified as an
miRNA downregulated in heat-treated cells compared with non-treated
control (19); it was also
demonstrated to potentially regulate 865 genes. Similar results
were observed in a previous bioinformatics prediction, which
suggested that miRNAs regulate >30% of protein-coding genes and
that one miRNA may have hundreds or even thousands of potential
gene targets (15). Indeed, the
wide target range of miRNAs appears to be an important biological
mechanism that enables them to mediate different cellular responses
to environmental changes and is likely to significantly advance our
knowledge of miRNA biological functions. For example, several
studies have demonstrated that miR-3613-3p is upregulated in lung
adenocarcinoma (28) and in
gastric (29), colon (30), prostate (31) and thyroid papillary (32) cancers. In addition, the high level
of miR-3613-3p expression in gastric cancer cells was reduced by
cinobufagin (33), which is used
clinically to treat patients with solid malignant tumors. Thus,
miR-3613-3p might serve an important role in cancer by acting as an
oncogene. In addition, by targeting pain-associated genes,
including γ-aminobutyric acid receptor type A subunit β3,
N-methyl-D-aspartate 3A, transient receptor potential vanilloid-1,
neuropeptide Y receptor Y1, and sodium channel protein type 9
subunit α, miR-3613-3p may be involved in severe axial pain after
motor vehicle collision in African Americans (34). Upregulation of miR-3613-3p was
observed in the left atrial appendage in rheumatic mitral valve
disease with atrial fibrillation (35). Another report indicated that
decreased expression of miR-3613-3p may regulate B cell activation
through the Wnt pathway and participate in the pathogenesis of
immunoglobulin A nephropathy (36). Therefore, it was speculated that
miR-3613-3p may have different functions and regulate different
target genes in various tissues under different conditions. This
may also explain the large number of target genes of miR-3613-3p.
Overall, the effects of miR-3613-3p in ECs under heat stress need
to be clarified.
According to GO analysis, there was potential
significant enrichment of miR-3613-3p in transcription-related
functions (8 of 10 terms, 80%), and RNA polymerase II may be a key
regulatory point. This finding was expected as the transcriptional
status of cells changes markedly in response to heat stress.
Transcriptional activity is commonly considered to decrease
globally with transcriptional activation of certain genes that are
necessary for heat stress-related cellular responses (37). However, there is no clear
understanding of the exact mechanism of transcriptional repression.
RNA polymerase II activity reduction is mediated by Alu RNAs, which
are non-coding RNAs representing transcripts of short interspersed
elements (SINE) in humans (38).
Under heat stress, Alu RNAs are rapidly upregulated (39,40)
and bound directly to RNA polymerase II to form kinetically stable
complexes, thereby interfering with the binding of RNA polymerase
to DNA and subsequent formation of the closed complex (41). To mediate repression, small
repressing RNAs bind to RNA polymerase prior to formation of the
closed complex (42), which
prevents the mechanism from interfering with the transcription of
heat shock protein (HSP) genes that are associated with RNA
polymerase II molecules under non-heat shock conditions (43,44);
this results in HS-induced increase in HSP expression despite a
global decline in cell transcription. However, it is unclear
whether SINE-dependent transcriptional repression is universal in
cells under heat stress. The data from the present study suggested
that miR-3613-3p may perform a complex regulatory function in
transcription during heat stress. miR-3613-3p may be involved in
regulating a variety of transcription-related processes and appears
to simultaneously regulate opposing functions. For example,
miR-3613-3p was found to be involved not only in ‘positive
regulation of transcription’ but also in ‘negative regulation of
transcription’. It is not clear how miR-3613-3p participates in the
regulation of transcription and whether it depresses transcription
via SINE-dependent mechanisms. Thus, miR-3613-3p may serve a
complex role in the regulation of transcription through unknown and
complicated mechanisms.
KEGG pathway analysis provided a more detailed view
of the integrated relationship between the regulation of
miR-3613-3p and target genes. The pathways identified by KEGG
analysis serve crucial roles in regulating diverse processes of
ECs, including proliferation, apoptosis, survival, migration and
polarity, tight junction integrity and monocyte-EC adhesion
enhancement, mostly through a series of downstream transcription
factors (45–53). These data suggested that
miR-3613-3p may facilitate individual biological reactions not by
targeting an isolated pathway, but rather by targeting a complete
network.
Through the bioinformatics analysis in the present
study, ‘negative regulation of apoptotic process’ was identified as
a potential function of miR-3613-3p. Apoptosis is implicated in the
physiological and pathological processes of ECs under heat stress
(54–56). In vitro experiments on the
inhibitory effect of miR-3613-3p on apoptosis indicated that heat
stress induced miR-3613-3p repression and promoted apoptosis. In
addition, reintroduction of miR-3613-3p partly reversed the
promoting effect of heat stress on apoptosis, which suggested that
miR-3613-3p may be involved in regulating the apoptotic process in
ECs during heat stress; these results may provide new insight into
the treatment of HS.
Our previous miRNA-gene network analysis revealed a
series of hub target genes, and MAP3K2 appeared in the central
position of the network regulated by many miRNAs (19). MAP3K2 is a component of the
mitogen-activated protein kinase (MAPK) signaling pathway, which
preferentially regulates JNK and ERK5 pathways by phosphorylating
and activating MAP2K5 and MAP2K7 (57,58).
In addition, MAP3K2 participates in the regulation of apoptosis
through a number of downstream targets (59,60).
In the present study, bioinformatics analysis indicated that MAP3K2
was a target gene of miR-3613-3p. Therefore, miR-3613-3p may be
involved in EC apoptosis by targeting the MAP3K2 gene during heat
stress. In the current study, increased expression of MAP3K2 was
observed in ECs under heat stress accompanied by reduced expression
of miR-3613-3p compared with non-treated cells, whereas
overexpression of miR-3613-3p partly reversed MAP3K2 augmentation,
which suggested that MAP3K2 may be partially regulated by
miR-3613-3p. Additionally, miR-3613-3p directly inhibited the
expression of MAP3K2 by binding to its 3′UTR. It is noteworthy that
MAK3P2 may induce distinct effects on apoptosis under varying
external conditions (61–63), which may be due to distinct
downstream molecules. The MAP2K5/ERK5 pathway is required for
normal cardiovascular development and vascular integrity, and it
improves EC viability and reduces apoptosis (64,65);
conversely, JNK MAPKs promote apoptosis in ECs under most
conditions (66). MAK3P2
coordinately activates signaling through MEK5/ERK5 and MEK7/JNK
protein kinases to respond to different stimuli. Therefore, the
effects of MAP3K2 on EC apoptosis during heat stress may be induced
by JNK MAPKs. The present study demonstrated that heat stress may
decrease the activation of miR-3613-3p and promote the apoptotic
effect on HUVECs by MAP3K2 through targeted binding to 3′-UTR,
which leads to the inhibition of MAP3K2 expression.
There were certain limitations to the present study.
First, HUVECs cannot completely reflect the biological
characteristics of vascular ECs as venous endothelial function may
differ substantially from arterial or capillary endothelial
function. It remains to be determined whether similar regulatory
mechanisms are involved in vivo and in other cellular
systems. Second, the heat stress cell model used in the present
study did not ideally simulate the clinical course of HS, and
interactions between ECs and other cells were ignored. Third, some
of the bioinformatics results may have been affected by technical
deficiencies, such as technical artifacts introduced by the smaller
scale of experimentally validated data and other associated
biological data, deficiencies in effective integration of diverse
datasets or the different computational models (67,68).
In conclusion, bioinformatics analysis revealed a
range of target genes of miR-3613-3p, and a number of functions and
pathways involved. Additionally, ‘negative regulation of apoptotic
process’ was predicted to be a potential function of miR-3613-3;
in vitro analysis revealed that miR-3613-3p may suppress
apoptosis in ECs under heat stress, potentially by directly
targeting MAP3K2. These results shed new light on the underlying
mechanisms of heat stress in ECs, although further studies are
needed to explore the roles of miR-3613-3p in the pathogenesis of
heat stress in ECs.
Acknowledgements
The authors would like to thank Dr Cuini Wang
(Department of Immunology, Shanghai Medical College, Institute for
Immunobiology of Fudan University) for her advice and our medical
writer for their help in preparing the manuscript.
Funding
No funding was received.
Availability of data and material
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request. Table I is published in the following repository:
https://figshare.com/s/81268025c7dac8b67bd2
(DOI:10.6084/m9.figshare.7435958).
Authors' contributions
QL and ZT conceived and designed all the
experiments, performed data analysis for the study, and contributed
towards overall supervision of the project, and the drafting and
editing the manuscript. JL was involved in the design of the study,
performed experiments and data analysis, and was a major
contributor to the writing of the manuscript. SL and GZ analyzed
and interpreted the data regarding miR-3613-3p. XH performed
reverse transcription-quantitative PCR for miR-3613-3p. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Robine JM, Cheung SL, Le Roy S, Van Oyen
H, Griffiths C, Michel JP and Herrmann FR: Death toll exceeded
70,000 in Europe during the summer of 2003. C R Biol. 331:171–178.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bouchama A and Knochel JP: Heat stroke. N
Engl J Med. 346:1978–1988. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lu KC, Wang JY, Lin SH, Chu P and Lin YF:
Role of circulating cytokines and chemokines in exertional
heatstroke. Crit Care Med. 32:399–403. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bouchama A, Ollivier V, Roberts G, Al
Mohanna F, de Prost D, Eldali A, Saussereau E, El-Sayed R and
Chollet-Martin S: Experimental heatstroke in baboon: Analysis of
the systemic inflammatory response. Shock. 24:332–335. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roberts GT, Ghebeh H, Chishti MA,
Al-Mohanna F, El-Sayed R, Al-Mohanna F and Bouchama A:
Microvascular injury, thrombosis, inflammation, and apoptosis in
the pathogenesis of heatstroke: A study in baboon model.
Arterioscler Thromb Vasc Biol. 28:1130–1136. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bazzoni G and Dejana E: Endothelial
cell-to-cell junctions: Molecular organization and role in vascular
homeostasis. Physiol Rev. 84:869–901. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Busse R and Fleming I: Vascular
endothelium and blood flow. Handb Exp Pharmacol. 43–78. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Toda N, Nakanishi S and Tanabe S:
Aldosterone affects blood flow and vascular tone regulated by
endothelium-derived NO: Therapeutic implications. Br J Pharmacol.
168:519–533. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cines DB, Pollak ES, Buck CA, Loscalzo J,
Zimmerman GA, McEver RP, Pober JS, Wick TM, Konkle BA, Schwartz BS,
et al: Endothelial cells in physiology and in the pathophysiology
of vascular disorders. Blood. 91:3527–3561. 1998.PubMed/NCBI
|
|
10
|
Michiels C: Endothelial cell functions. J
Cell Physiol. 196:430–443. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Minshall RD and Malik AB: Transport across
the endothelium: Regulation of endothelial permeability. Handb Exp
Pharmacol. 107–144. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pober JS and Sessa WC: Evolving functions
of endothelial cells in inflammation. Nat Rev Immunol. 7:803–815.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu Y, Zhou G, Wang Z, Guo X, Xu Q, Huang
Q and Su L: NF-κB signaling is essential for resistance to heat
stress-induced early stage apoptosis in human umbilical vein
endothelial cells. Sci Rep. 5:135472015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bouchama A, Hammami MM, Haq A, Jackson J
and al-Sedairy S: Evidence for endothelial cell activation/injury
in heatstroke. Crit Care Med. 24:1173–1178. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pepini T, Gorbunova EE, Gavrilovskaya IN,
Mackow JE and Mackow ER: Andes virus regulation of cellular
microRNAs contributes to hantavirus-induced endothelial cell
permeability. J Virol. 84:11929–11936. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nicoloso MS, Spizzo R, Shimizu M, Rossi S
and Calin GA: MicroRNAs-the micro steering wheel of tumour
metastases. Nat Rev Cancer. 9:293–302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun HX, Zeng DY, Li RT, Pang RP, Yang H,
Hu YL, Zhang Q, Jiang Y, Huang LY, Tang YB, et al: Essential role
of microRNA-155 in regulating endothelium-dependent vasorelaxation
by targeting endothelial nitric oxide synthase. Hypertension.
60:1407–1414. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu J, Zhu G, Xu S, Liu S, Lu Q and Tang
Z: Analysis of miRNA expression profiling in human umbilical vein
endothelial cells affected by heat stress. Int J Mol Med.
40:1719–1730. 2017.PubMed/NCBI
|
|
20
|
Ashburner M, Ball CA, Blake JA, Botstein
D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwiqht SS, Eppiq JT,
et al: Gene ontology: Tool for the unification of biology. Nat
Genet. 25:25–29. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
The Gene Ontology Consortium: The Gene
Ontology resource: 20 years and still GOing strong. Nucleic Acids
Res 47(D1). D330–D338. 2019.
|
|
22
|
Kanehisa M, Sato Y, Furumichi M, Morishima
K and Tanabe M: New approach for understanding genome variations in
KEGG. Nucleic Acids Res 47(D1). D590–D595. 2019. View Article : Google Scholar
|
|
23
|
Kanehisa M, Furumichi M, Tanabe M, Sato Y
and Morishima K: KEGG: New perspectives on genomes, pathways,
diseases and drugs. Nucleic Acids Res 45(D1). D353–D361. 2017.
View Article : Google Scholar
|
|
24
|
Kanehisa M and Goto S: KEGG: Kyoto
encyclopedia of genes and genomes. Nucleic Acids Res. 28:27–30.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tang S, Allagadda V, Chibli H, Nadeau JL
and Mayer GD: Comparison of cytotoxicity and expression of metal
regulatory genes in zebrafish (Danio rerio) liver cells exposed to
cadmium sulfate, zinc sulfate and quantum dots. Metallomics.
5:1411–1422. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tang S, Cai Q, Chibli H, Allaqadda V,
Nadeau JL and Mayer GD: Cadmium sulfate and CdTe-quantum dots alter
DNA repair in zebrafish (Danio rerio) liver cells. Toxicol Appl
Pharmacol. 272:443–452. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pu Q, Huang Y, Lu Y, Peng Y, Zhang J, Feng
G, Wang C, Liu L and Dai Y: Tissue-specific and plasma microRNA
profiles could be promising biomarkers of histological
classification and TNM stage in non-small cell lung cancer. Thorac
Cancer. 7:348–354. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bibi F, Naseer MI, Alvi SA, Yasir M,
Jiman-Fatani AA, Sawan A, Abuzenadah AM, Al-Qahtani MH and Azhar
EI: microRNA analysis of gastric cancer patients from Saudi Arabian
population. BMC Genomics. 17 (Suppl 9):S7512016. View Article : Google Scholar
|
|
30
|
Ji H, Chen M, Greening DW, He W, Rai A,
Zhang W and Simpson RJ: Deep sequencing of RNA from three different
extracellular vesicle (EV) subtypes released from the human LIM1863
colon cancer cell line uncovers distinct miRNA-enrichment
signatures. PLoS One. 9:e1103142014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sohn EJ, Won G, Lee J, Lee S and Kim SH:
Upregulation of miRNA3195 and miRNA374b mediates the
anti-angiogenic properties of melatonin in hypoxic PC-3 prostate
cancer cells. J Cancer. 6:19–28. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Suresh R, Sethi S, Ali S, Giorgadze T and
Sarkar FH: Differential expression of MicroRNAs in papillary
thyroid carcinoma and their role in racial disparity. J Cancer Sci
Ther. 7:145–154. 2015.PubMed/NCBI
|
|
33
|
Zhou RP, Chen G, Shen ZL and Pan LQ:
Cinobufacin suppresses cell proliferation via miR-494 in BGC- 823
gastric cancer cells. Asian Pac J Cancer Prev. 15:1241–1245. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Linnstaedt SD, Walker MG, Parker JS, Yeh
E, Sons RL, Zimny E, Lewandowski C, Hendry PL, Damiron K, Pearson
C, et al: MicroRNA circulating in the early aftermath of motor
vehicle collision predict persistent pain development and suggest a
role for microRNA in sex-specific pain differences. Mol Pain.
11:662015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu H, Qin H, Chen GX, Liang MY, Rong J,
Yao JP and Wu ZK: Comparative expression profiles of microRNA in
left and right atrial appendages from patients with rheumatic
mitral valve disease exhibiting sinus rhythm or atrial
fibrillation. J Transl Med. 12:902014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang N, Bu R, Duan Z, Zhang X, Chen P, Li
Z, Wu J, Cai G and Chen X: Profiling and initial validation of
urinary microRNAs as biomarkers in IgA nephropathy. PeerJ.
3:e9902015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kantidze OL, Velichko AK and Razin SV:
Heat stress-induced transcriptional repression. Biochemistry
(Mosc). 80:990–993. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kramerov DA and Vassetzky NS: SINEs. Wiley
Interdiscip Rev RNA. 2:772–786. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu WM, Chu WM, Choudary PV and Schmid CW:
Cell stress and translational inhibitors transiently increase the
abundance of mammalian SINE transcripts. Nucleic Acids Res.
23:1758–1765. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Caizergues-Ferrer M, Bouche G, Banville D
and Amalric F: Effect of heat shock on RNA polymerase activities in
Chinese hamster ovary cells. Biochem Biophys Res Commun.
97:538–545. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mariner PD, Walters RD, Espinoza CA,
Drullinger LF, Wagner SD, Kugel JF and Goodrich JA: Human Alu RNA
is a modular transacting repressor of mRNA transcription during
heat shock. Mol Cell. 29:499–509. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yakovchuk P, Goodrich JA and Kugel JF: B2
RNA and Alu RNA repress transcription by disrupting contacts
between RNA polymerase II and promoter DNA within assembled
complexes. Proc Natl Acad Sci USA. 106:5569–5574. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rougvie AE and Lis JT: The RNA polymerase
II molecule at the 5′end of the uninduced hsp70 gene of D.
melanogaster is transcriptionally engaged. Cell. 54:795–804. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gilmour DS and Lis JT: RNA polymerase II
interacts with the promoter region of the noninduced hsp70 gene in
Drosophila melanogaster cells. Mol Cell Biol. 6:3984–3989. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yang CC, Ornatsky OI, McDermott JC, Cruz
TF and Prody CA: Interaction of myocyte enhancer factor 2 (MEF2)
with a mitogen-activated protein kinase, ERK5/BMK1. Nucleic Acids
Res. 26:4771–4777. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kato Y, Zhao M, Morikawa A, Sugiyama T,
Chakravortty D, Koide N, Yoshida T, Tapping RI, Yang Y, Yokochi T
and Lee JD: Big mitogen-activated kinase regulates multiple members
of the MEF2 protein family. J Biol Chem. 275:18534–18540. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kamakura S, Moriguchi T and Nishida E:
Activation of the protein kinase ERK5/BMK1 by receptor tyrosine
kinases. Identification and characterization of a signaling pathway
to the nucleus. J Biol Chem. 274:26563–26571. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
English JM, Pearson G, Baer R and Cobb MH:
Identification of substrates and regulators of the
mitogen-activated protein kinase ERK5 using chimeric protein
kinases. J Biol Chem. 273:3854–3860. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Watson FL, Heerssen HM, Bhattacharyya A,
Klesse L, Lin MZ and Segal RA: Neurotrophins use the Erk5 pathway
to mediate a retrograde survival response. Nat Neurosci. 4:981–988.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Nithianandarajah-Jones GN, Wilm B,
Goldring CE, Müller J and Cross MJ: ERK5: Structure, regulation and
function. Cell Signal. 24:2187–2196. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Oleinik NV, Krupenko NI and Krupenko SA:
Cooperation between JNK1 and JNK2 in activation of p53 apoptotic
pathway. Oncogene. 26:7222–7230. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhong S, Fromm J and Johnson DL: TBP is
differentially regulated by c-Jun N-terminal kinase 1 (JNK1) and
JNK2 through Elk-1, controlling c-Jun expression and cell
proliferation. Mol Cell Biol. 27:54–64. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chow CW, Dong C, Flavell RA and Davis RJ:
c-Jun NH(2)-terminal kinase inhibits targeting of the protein
phosphatase calcineurin to NFATc1. Mol Cell Biol. 20:5227–5234.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Li L, Tan H, Yang H, Li F, He X, Gu Z,
Zhao M and Su L: Reactive oxygen species mediate heat
stress-induced apoptosis via ERK dephosphorylation and Bcl-2
ubiquitination in human umbilical vein endothelial cells.
Oncotarget. 8:12902–12916. 2017.PubMed/NCBI
|
|
55
|
Gu ZT, Wang H, Li L, Liu YS, Deng XB, Huo
SF, Yuan FF, Liu ZF, Tong HS and Su L: Heat stress induces
apoptosis through transcription-independent p53-mediated
mitochondrial pathways in human umbilical vein endothelial cell.
Sci Rep. 4:44692014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhang S, Liu Y, Wang Z, Liu J, Gu Z, Xu Q
and Su L: PAR1-mediated c-Jun activation promotes heat
stress-induced early stage apoptosis of human umbilical vein
endothelial cells. Mol Med Rep. 15:2595–2603. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Dermott JM, Ha JH, Lee CH and Dhanasekaran
N: Differential regulation of Jun N-terminal kinase and p38MAP
kinase by Galpha12. Oncogene. 23:226–232. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Raviv Z, Kalie E and Seger R: MEK5 and
ERK5 are localized in the nuclei of resting as well as stimulated
cells, while MEKK2 translocates from the cytosol to the nucleus
upon stimulation. J Cell Sci. 117:1773–1784. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Schmidt C, Peng B, Li Z, Sclabas GM,
Fujioka S, Niu J, Schmidt-Supprian M, Evans DB, Abbruzzese JL and
Chiao PJ: Mechanisms of proinflammatory cytokine-induced biphasic
NF-kappaB activation. Mol Cell. 12:1287–1300. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Sun W, Vincent S, Settleman J and Johnson
GL: MEK kinase 2 binds and activates protein kinase C-related
kinase 2. Bifurcation of kinase regulatory pathways at the level of
an MAPK kinase kinase. J Biol Chem. 275:24421–24428. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Sui X, Kong N, Ye L, Han W, Zhou J, Zhang
Q, He C and Pan H: p38 and JNK MAPK pathways control the balance of
apoptosis and autophagy in response to chemotherapeutic agents.
Cancer Lett. 344:174–179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Xia Z, Dickens M, Raingeaud J, Davis RJ
and Greenberg ME: Opposing effects of ERK and JNK-p38 MAP kinases
on apoptosis. Science. 270:1326–1331. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kesavan K, Lobel-Rice K, Sun W, Lapadat R,
Webb S, Johnson GL and Garrington TP: MEKK2 regulates the
coordinate activation of ERK5 and JNK in response to FGF-2 in
fibroblasts. J Cell Physiol. 199:140–148. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Nithianandarajah-Jones GN, Wilm B,
Goldring CE, Müller J and Cross MJ: The role of ERK5 in endothelial
cell function. Biochem Soc Trans. 42:1584–1589. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wu Y and Chakrabarti S: ERK5 mediated
signalling in diabetic retinopathy. Med Hypothesis Discov Innov
Ophthalmol. 4:17–26. 2015.PubMed/NCBI
|
|
66
|
Weston CR and Davis RJ: The JNK signal
transduction pathway. Curr Opin Genet Dev. 19:142–149. 2007.
|
|
67
|
Xuan P, Han K, Guo Y, Li J, Li X, Zhong Y,
Zhang Z and Ding J: Prediction of potential disease-associated
microRNAs based on random walk. Bioinformatics. 31:1805–1815. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Chen X, Yin J, Qu J and Huang L: MDHGI:
Matrix decomposition and heterogeneous graph inference for
miRNA-disease association prediction. PLoS Comput Biol.
14:e10064182018. View Article : Google Scholar : PubMed/NCBI
|