Introduction
Clinically, intervertebral disc (IVD) degeneration
(IVDD) causes lower back pain and disc herniation, which are some
of the most common disorders leading to morbidity and deterioration
in quality of adult life. A decrease in the number of living cells
is one of the initial inducers of IVDD in nucleus pulposus (NP)
cells (NPCs) (1,2). Notably, aberrant apoptosis and
accelerated aging of NPCs are considered the two major cellular
processes associated with IVDD (3,4). As
cellular loss due to excessive apoptosis may serve an important
role in IVDD (3), suppression of
apoptosis has been suggested as a potential strategy to alleviate
IVDD (5,6).
17β-Estradiol (E2) has been widely
studied for its role in suppressing apoptosis, mainly through the
involvement of integrins and extracellular matrix (ECM) receptors
(7–12). In our previous studies, it was
demonstrated that E2 protects against aberrant apoptosis
in rat NPCs by upregulating integrin α2β1 and type II collagen
(COL2), and downregulating matrix metalloproteinase (MMP)-3 and
MMP-13 (13,14). Previous studies have reported that
integrins, including β1, β4 and αvβ3, can promote cell
proliferation and inhibit cell apoptosis via the PI3K/Akt pathway
(15–18). Activation of the PI3K/Akt pathway
has been shown to promote cell survival and protect rat NPCs from
apoptosis (19–21). However, the pathway downstream of
PI3K/Akt that is associated with the anti-apoptosis process remains
unclear.
Glycogen synthase kinase-3β (GSK-3β), mTOR and NF-κB
are key proteins in the pathways downstream of PI3K/Akt (22–25);
these proteins are inhibited by SB216763, rapamycin and SC75741,
respectively. The present study hypothesized that these signaling
pathways may take part in retarding the progress of IVDD-associated
diseases. The present study aimed to explore the three downstream
signaling pathways of PI3K/Akt and determine which pathways were
involved in the effect of estrogen against apoptosis. Notably, this
work is expected to provide a novel target for a therapeutic
strategy to prevent and treat IVD degenerative diseases.
Materials and methods
Reagents and antibodies
The following reagents and antibodies were used in
this study: DMEM/F12 (HyClone; GE Healthcare), FBS (HyClone; GE
Healthcare), trypsin (Sigma-Aldrich; Merck KGaA), DMSO (Beijing
Solarbio Science & Technology Co., Ltd.), Cell Counting Kit-8
(CCK-8; Dojindo Molecular Technologies, Inc.), FITC Annexin V
Apoptosis Detection kit I (BD Pharmingen; BD Biosciences), IL-1β
(PeproTech, Inc.), E2 (Sigma-Aldrich; Merck KGaA) and
ICI182780 (Sigma-Aldrich; Merck KGaA). Secondary antibodies (cat.
no. 7074; Cell Signaling Technology, Inc.), and primary antibodies
against mTOR (cat. no. 2972), phosphorylated (p)-mTOR (cat. no.
2971), GSK-3β (cat. no. 9315), p-GSK-3β (cat. no. 9322), NF-κB
(cat. no. 4764), p-NF-κB (cat. no. 3031) and cleaved caspase-3
(cat. no. 9664) (all Cell Signaling Technology, Inc.), and β-actin
(Wuhan Sanying Biotechnology). Rapamycin, SC75741 and SB216763
(Selleck Chemicals). A protease inhibitor and phosphorylase
inhibitor (Beijing Solarbio Science & Technology Co., Ltd.),
PVDF membranes (EMD Millipore) and an enhanced chemiluminescence
reagent (Beijing Solarbio Science & Technology Co., Ltd.).
Ethics statement
The animal protocols were approved by the
Institutional Animal Care and Use Committee of The Third Hospital
of Hebei Medical University.
Cell culture
Briefly, three male Sprague-Dawley rats (weight, 200
g; age, 2 months) were purchased from the Animal Experimental
Center of Hebei Medical University, and were raised under standard
conditions: Temperature, 25±1°C; 12-h light/dark cycle; and
humidity, 60–70%. All rats were given free access to food and
water. Rats were sacrificed by intravenous administration of 150
mg/kg pentobarbital sodium. Lumbar spinal columns were removed en
bloc under aseptic conditions, and lumbar IVDs were collected. The
gel-like NP was separated from the annulus fibrosus under a
dissecting microscope and was sequentially treated with 0.25% type
II collagenase (Sigma-Aldrich; Merck KGaA) for 1 h and 0.2% trypsin
with EDTA (1 mmol/l) for 5 min. The solution containing partially
digested tissue was then transferred to a 50-ml culture flask
containing DMEM and 20% FBS and cultured at 37°C in a humidified
atmosphere containing 5% CO2. NPCs adhered to the bottom
of the culture flask after 3 days. When confluent (after 1 week),
the NPCs were dissociated using a 0.25% trypsin solution with EDTA
(1 mmol/l) and further subcultured. First-passage cells maintained
in monolayers were used for subsequent experiments. The cells were
serum starved for an additional 24 h with phenol red-free medium
and underwent cell apoptosis, binding and viability assays. Rat
NPCs were divided into seven groups as follows: Control (treated
with an equivalent volume of PBS), IL-1β, IL-1β + E2,
IL-1β + E2 + ICI182780, IL-1β + E2 +
rapamycin, IL-1β + E2 + SC75741 and IL-1β +
E2 + SB216763.
CCK-8 and MTS assays
Cell proliferation and viability were assessed using
a CCK-8 kit and the MTS method using CellTiter 96®
AQueous MTS Reagent Solution (Promega Corporation), respectively.
Both kits were conducted according to the manufacturers' protocols.
Briefly, 100 µl cells (2×103 cells/well) were
transferred into 96-well plates following trypsin digestion, and
six parallel wells were used for each treatment. The culture plate
was incubated at 37°C in an incubator containing 5% CO2
for 24 h. After attachment, the cells were cultured for 90 min in
serum-free, phenol red-free medium, after which, E2 (1
µM) (13) was added to the medium.
After 5 min, the inhibitors rapamycin, SC75741 and SB216763 were
added. In the medium, the final concentrations of rapamycin,
SC75741 and SB216763 were 100 nM, 1 and 1 µM, respectively. After
30 min, IL-1β (75 ng/ml) was added, and the cells were cultured at
37°C in an incubator containing 5% CO2 for 1, 2, 4, 8,
12, 24 and 48 h (26).
Subsequently, 10 µl CCK-8 was added to each well, and the cells
were cultured for 1 h. The density of living cells was determined
by measuring absorbance with a microplate reader (Shimadzu) at 450
nm (CCK-8).
For the MTS assay, 12 h after the cells were
processed as for the CCK-8 analysis, 20 µl MTT solution was added
to each well and NPCs were incubated for 4 h at 37°C in an
incubator containing 5% CO2. Subsequently, 150 µl DMSO
was added to each well and the plate was oscillated for 10 min to
fully melt the crystals at room temperature. The density of living
cells was determined by measuring absorbance with a microplate
reader at 492 nm (MTS). Cell proliferation and viability were
normalized by calculating the relative value to the control. The
concentration of ICI182780 (1 µM) used was the same as that
reported in our previous study (13).
Cellular binding assay
Rat NPCs were treated as aforementioned and their
ability to bind COL2 α1 chain (COL2α1) was assessed. Briefly,
24-well plates were coated overnight at 4°C with COL2α1 (20 µg/ml;
Sigma-Aldrich; Merck KGaA). Nonspecific binding sites were blocked
by incubating the coated plates with 10 mg/ml bovine serum albumin
(Beijing Solarbio Science & Technology Co., Ltd.) for 60 min,
followed by two washes with ice-cold PBS. A total of
3×104 cells were placed in each well and allowed to
adhere at 37°C for 60 min. After adhesion, the cells were stained
with 0.5% toluidine blue for 60 min at 37°C, fixed with 4%
paraformaldehyde for 15 min and solubilized in 1% SDS for 60 min at
room temperature. Extracted dye was quantified by measuring the
absorbance value at 590 nm using a plate reader (Dynatech
MR5000).
Fluorescence-activated cell sorting
(FACS) analysis
Cell apoptosis was detected by FACS using the FITC
Annexin V Apoptosis Detection kit I according to the manufacturer's
protocol. Rat NPCs were washed twice with cold PBS and then
resuspended in 1X binding buffer at a concentration of
1×106 cells/ml. Subsequently, 1×105 cells
were transferred to a 5-ml culture tube, and 5 µl FITC-Annexin V
and 5 µl PI were added to the tube. The cells were vortexed and
incubated for 15 min at room temperature in the dark. Finally, 400
µl 1X binding buffer was added to each tube, and the cells were
analyzed by Cell Quest Pro 6.0 software (BD Biosciences) after 1
h.
Western blotting
The protein expression levels of p-mTOR, mTOR,
p-GSK-3β, GSK-3β, p-NF-κB, NF-κB and cleaved caspase-3 were
determined by western blotting, with β-actin used as the internal
reference protein. Rat NPCs were washed with ice-cold PBS and
harvested in 100 µl RIPA buffer (Beijing Solarbio Science &
Technology Co., Ltd.) containing 1% protease inhibitor and 0.5%
phosphorylase inhibitor (Beijing Solarbio Science & Technology
Co., Ltd.). The bicinchoninic acid method was used to determine
protein concentration. The lysates were centrifuged at 4°C for 15
min at 16,000 × g and equal amounts of protein (20 µg) were
electrophoresed. Specifically, for the detection of mTOR and
p-mTOR, proteins were separated by 10% SDS-PAGE, whereas for
GSK-3β, p-GSK-3β, NF-κB and cleaved caspase-3 detection, proteins
were separated by 12% SDS-PAGE. Proteins were transferred by
electroblotting to PVDF membranes. The membranes were blocked with
5% non-fat dry milk in TBS [50 mmol/l Tris (pH 7.6) and 150 mmol/l
NaCl (0.1%)] and incubated overnight at 4°C in 5% non-fat dry milk
in PBS-0.1% Tween (PBST) with the corresponding primary antibodies
(1:1,000). After three washes in PBST for 30 min, the membranes
were incubated with a secondary antibody (1:5,000) at room
temperature for 2 h. Immunolabeling was detected using an enhanced
chemiluminescence reagent. The relative intensity of each blot was
assessed and analyzed using the AlphaImager 2200 software package
(ProteinSimple), and the levels of target proteins were determined
as (p-protein/control)/(total protein/control).
Statistical analysis
Statistical analyses were performed using SPSS for
Windows, version 18.0 (SPSS, Inc.). All data are presented as the
means ± standard deviation of independent experiments (n=6). If the
data satisfied the criteria for normality and homogeneity of
variance, statistical analysis among multiple groups was performed
using one-way analysis of variance, which was accompanied by
pairwise comparison using the SNK-q test. P<0.05 was considered
to indicate a statistically significant difference.
Results
Apoptosis assay
Rat NPCs were divided into seven groups as follows:
Control (treated with an equivalent volume of PBS), IL-1β, IL-1β +
E2, IL-1β + E2 + ICI182780, IL-1β +
E2 + rapamycin, IL-1β + E2 + SC75741 and
IL-1β + E2 + SB216763. FACS analysis was then used to
determine the number of apoptotic cells. As shown in Fig. 1A and B, the percentage of apoptotic
cells increased following treatment with IL-1β alone (10.10%).
Cells pretreated with E2 (IL-1β + E2 group)
exhibited a reduced rate of apoptosis (7.43%). To further elucidate
the potential contribution of mTOR, the inhibitor rapamycin was
used. When cells were incubated with E2, IL-1β and
rapamycin, the protective effects of E2 were reduced
(9.85%); a similar result was revealed for cells pretreated with
ICI182780, an estrogen receptor (ER) antagonist. Statistical
analysis demonstrated that pretreatment with rapamycin promoted
apoptosis induced by IL-1β, which was confirmed by western blot
analysis of cleaved caspase-3 (Fig.
1C).
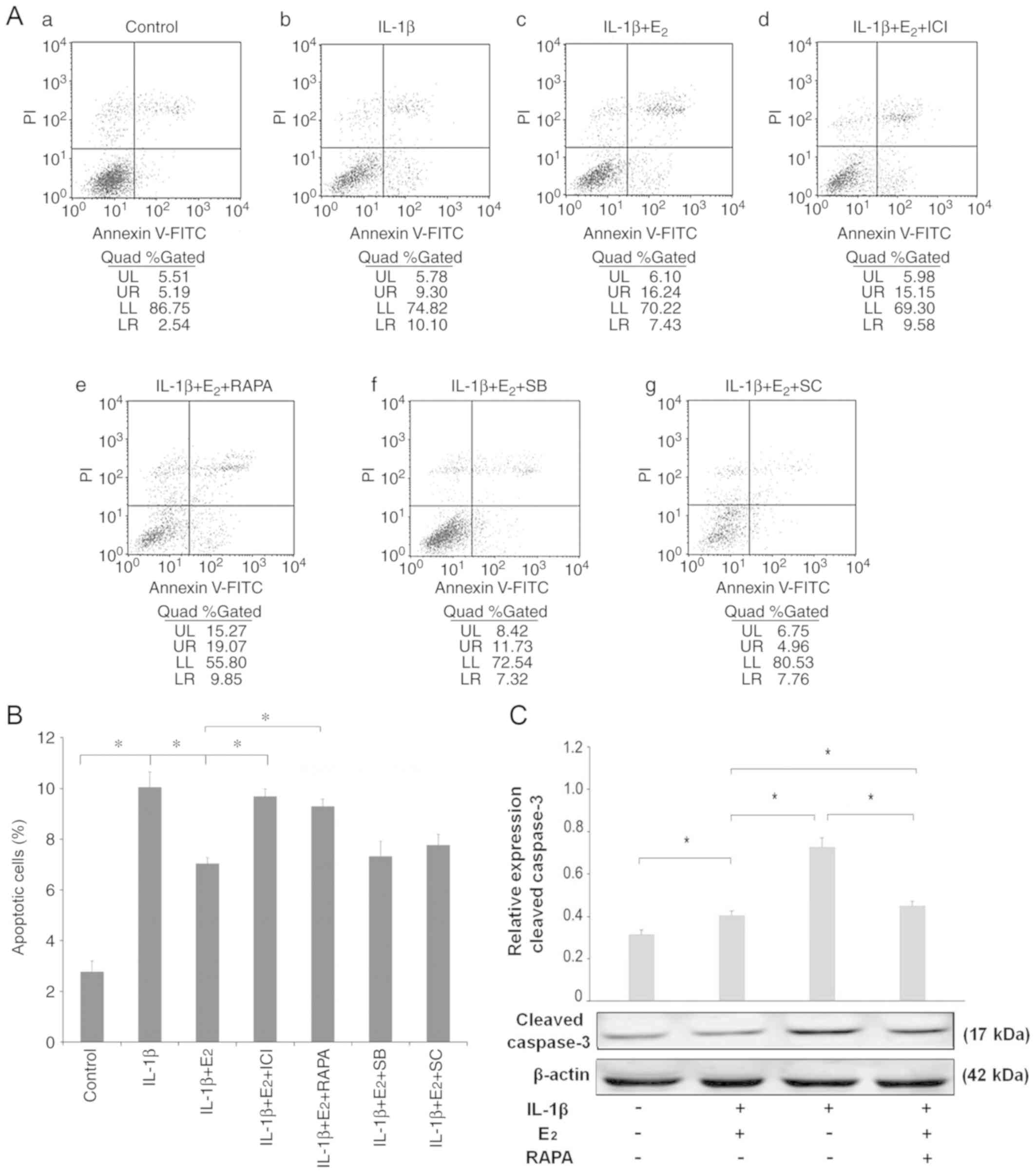 | Figure 1.Fluorescence-activated cell sorting
analysis of apoptosis. (A) Apoptotic nucleus pulposus cells were
detected using an Annexin V-FITC/PI kit. Apoptotic cells, which
were stained positive for Annexin V-FITC and negative for PI, were
counted. (a) Control, (b) IL-1β, (c) IL-1β + E2, (d)
IL-1β + E2 + ICI, (e) IL-1β + E2 + RAPA, (f)
IL-1β + E2 + SB, (g) IL-1β + E2 + SC. (B)
Data are presented as a percentage of the total cell count. Data
analysis was determined by one-way analysis of variance accompanied
by pairwise comparison using the SNK-q test. (C) Western blot
analysis of cleaved caspase-3. Data are presented as the means ±
standard deviation, n=6. *P<0.05. E2, 17β-estradiol;
ICI, ICI182780; IL-1β, interleukin-1β; PI, propidium iodide; RAPA,
rapamycin; SB, SB216763; SC, SC75741. |
Cell viability and proliferation
CCK-8 and MTS assays were used to measure cell
proliferation and viability, respectively. As shown in Fig. 2A, compared with the control group
at each point, the group treated with IL-1β alone had a lower
optical density (OD) value at 1–48 h (P<0.05), and the OD value
in the IL-1β + E2 group was significantly decreased at
4–48 h (P<0.05). In the rapamycin group, the OD value was
significantly reduced at 1–48 h compared with the control group
(P<0.05). At 12 h, the proliferation rate of NPCs in the IL-1β +
E2 group was significantly greater than in the IL-1β and
IL-1β + E2 + RAPA groups (P<0.05; Fig. 2B). Furthermore, the viability of
cells in the IL-1β + E2 group was significantly
increased compared with in the IL-1β + E2 + RAPA group
(P<0.05; Fig. 2C).
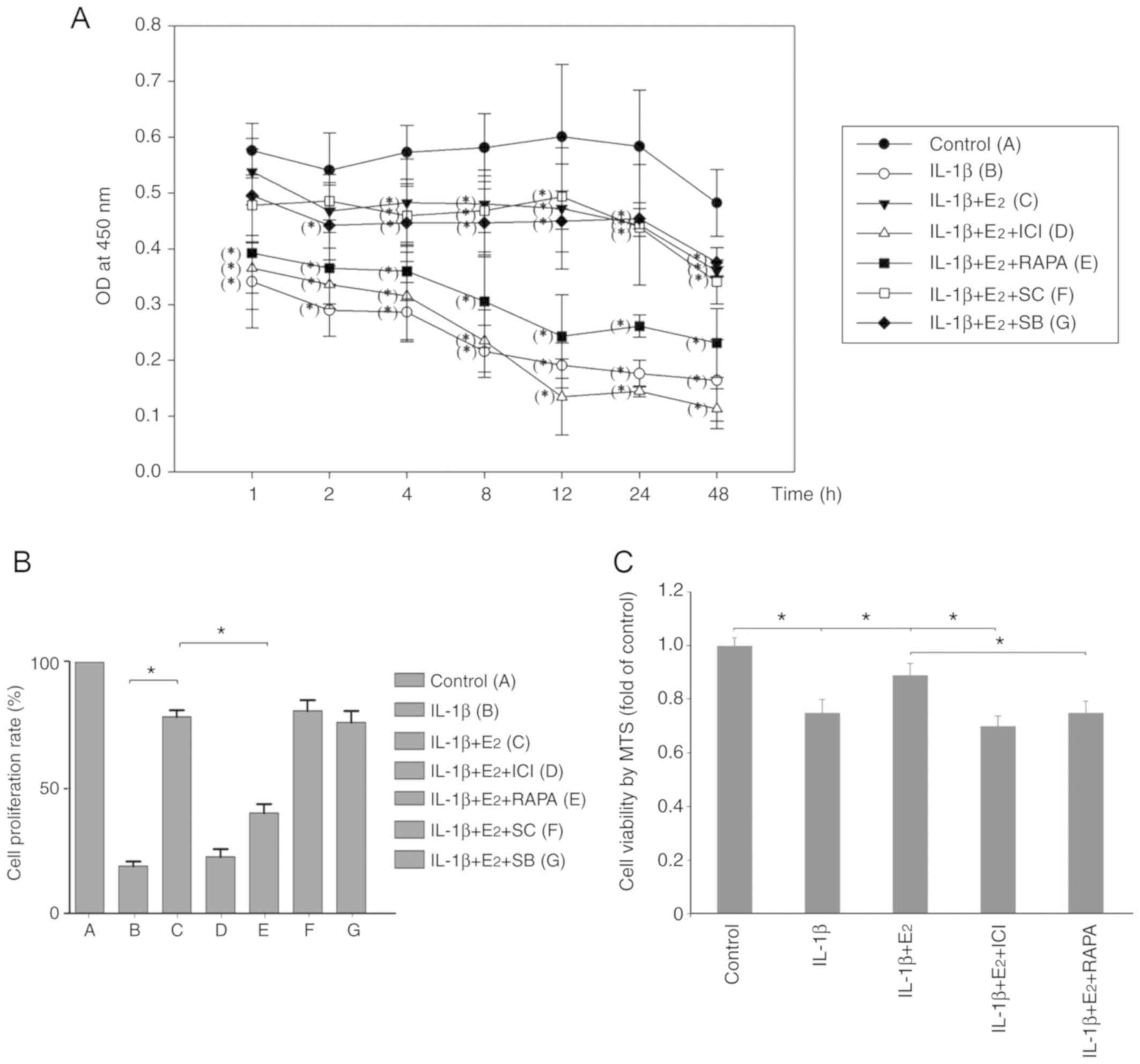 | Figure 2.(A and B) CCK-8 and (C) MTS assays of
cell proliferation and viability. (A) Optical density was measured
after incubating the nucleus pulposus cells with different
treatments for 1, 2, 4, 8, 12, 24 and 48 h. *P<0.05 vs. the
control at each point. (B) Cell proliferation and (C) cell
viability at 12 h was normalized relative to the control. Data are
presented as the means ± standard deviation, n=6. *P<0.05.
E2, 17β-estradiol; ICI, ICI182780; IL-1β,
interleukin-1β; RAPA, rapamycin; SB, SB216763; SC, SC75741. |
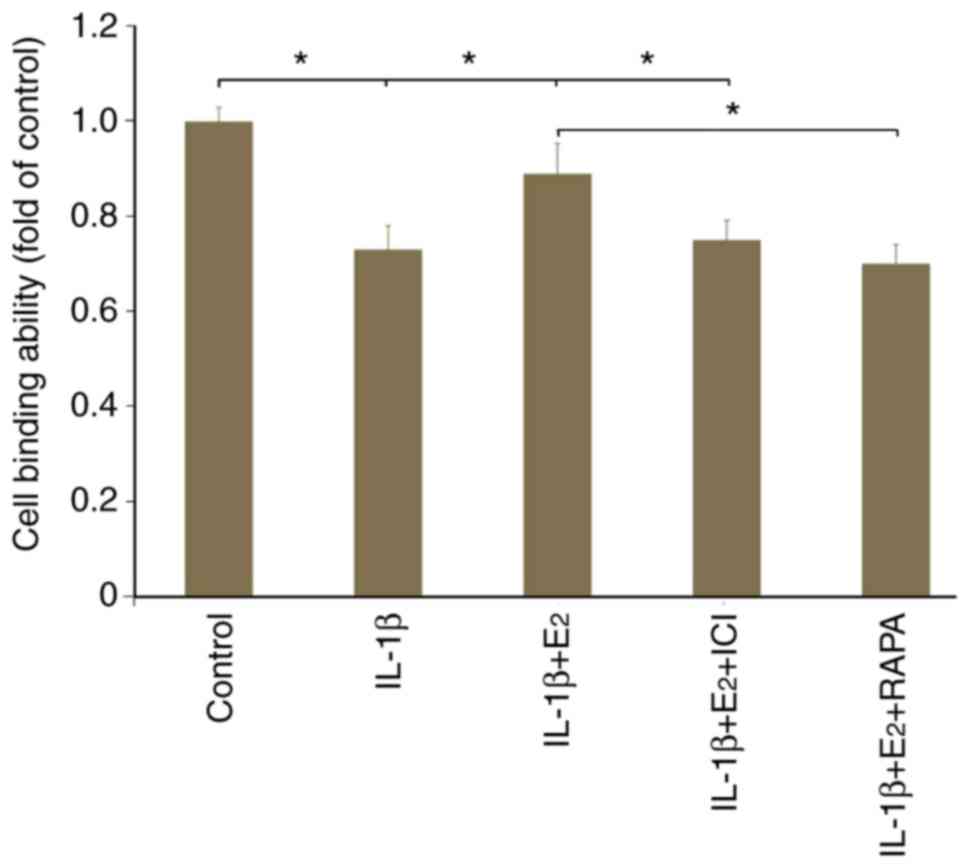
Cellular binding
As shown in Fig. 3,
IL-1β resulted in a decrease of ~30% in cell binding ability
compared with in the control group (P<0.05). The cytotoxic
effects of IL-1β were partly abolished by the addition of
E2; however, this was reversed by the use of ICI182780
or RAPA (P<0.05).
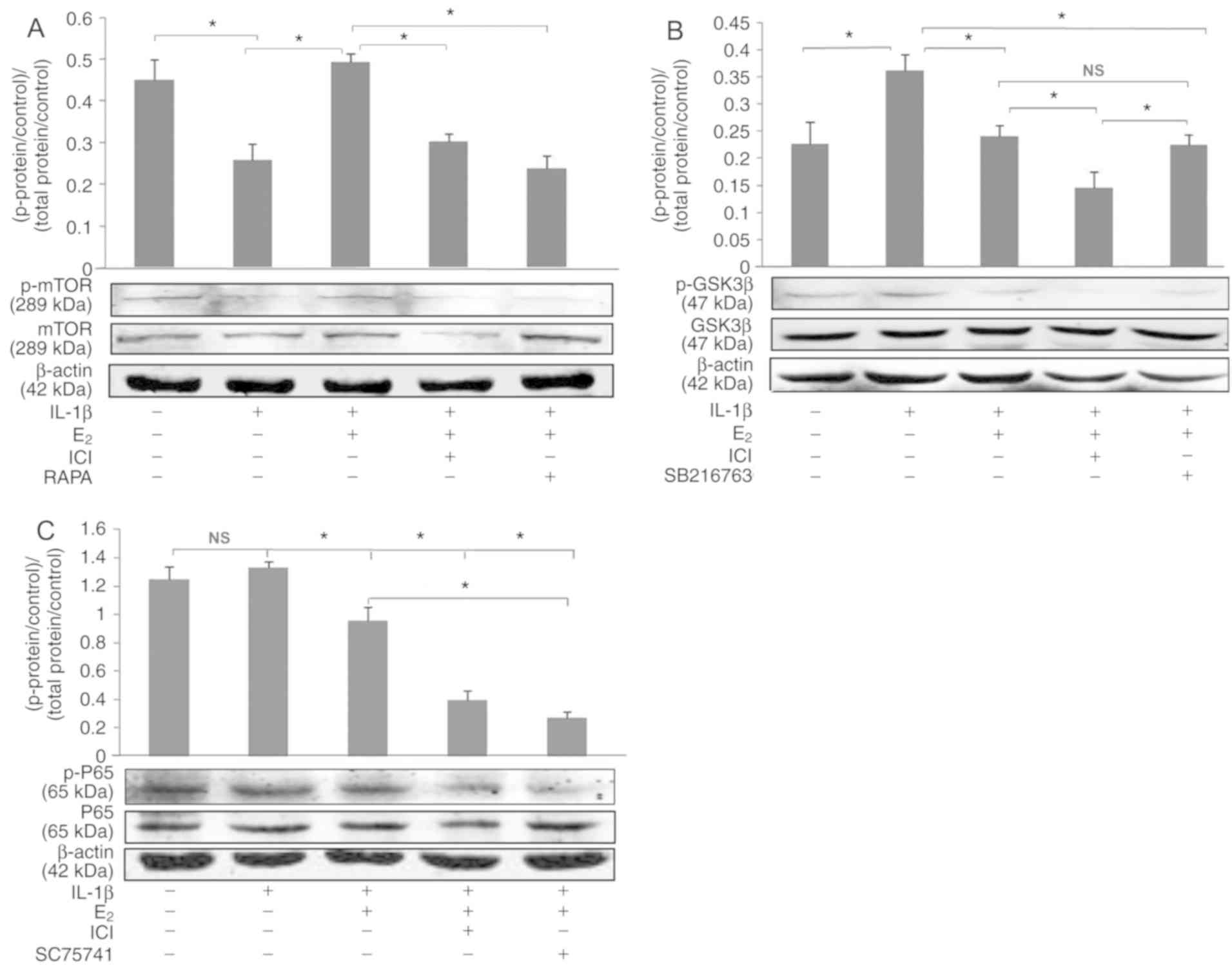
Western blot analysis
Rat NPCs were treated as aforementioned. As shown in
Fig. 4, IL-1β significantly
decreased the expression ratio of p-mTOR/mTOR, which was reversed
by E2 (P<0.05). However, treatment with the ER
antagonist (ICI182780) or the mTOR signal pathway inhibitor
(rapamycin) decreased the expression ratio of p-mTOR/mTOR compared
with in the IL-1β + E2 group (P<0.05; Fig. 4A). As shown in Fig. 4B, E2 reversed
IL-1β-induced expression of p-GSK3β/GSK3β (P<0.05); however,
ICI182780 did not reverse the effects of E2 on
p-GSK3β/GSK3β. Furthermore, no significant difference in
p-NF-κB/NF-κB was observed between the control and IL-1β groups
(P>0.05; Fig. 4C).
Discussion
In a previous study, E2 was reported to
protect IVD cells against aberrant apoptosis caused by IL-1β
cytotoxicity, which was attributed to upregulation of integrin α2β1
(13). Upon ligand binding,
integrins act not only as structural links between the ECM and the
actin cytoskeleton, but also as sites of signal transduction from
the ECM to intracellular signaling pathways. PI3K and Akt
constitute an important signaling pathway of integrin, which has
been reported to serve a central role in the effect of estrogen
against IL-1β-induced apoptosis of NPCs (19–21).
However, the downstream signaling pathway remains unclear. The
results of the present study confirmed that E2 inhibited
IL-1β-induced apoptosis of NPCs via mTOR, the downstream signaling
pathway of PI3K/Akt.
In our preliminary experiments, the NP of female
rats was significantly smaller than those of male rats; therefore,
separation of the NP from female rat annulus fibrosus was
difficult, and a large number of annulus fibrosus cells were able
to mix into NPCs during the subsequent cell culture process.
Therefore, male rats were used for tissue isolation and cell
culture in the present study, in accordance with our previous
report (14). In the present
study, a CCK-8 assay confirmed that the proliferation of NPCs was
suppressed by the mTOR antagonist rapamycin compared with that in
the IL-1β + E2 group. Cell binding assay, western
blotting and FACS analysis confirmed that E2 antagonized
IL-1β-induced NPC apoptosis, which in accordance with a previous
study (19).
It has long been recognized that there is a
continuous increase in cell death during human IVDD. Notably,
abnormal apoptosis is considered the main cellular process
associated with IVDD (4,27). The Akt signaling pathway is known
to have a central role in cell survival, and a role for Akt in the
regulation of growth, survival and inhibition of apoptosis of NPCs
is well established (28–30). mTOR is downstream of PI3K/Akt and
acts as a major sensor of cell growth. This serine/threonine
protein kinase is involved in protein translation, proliferation
and the anti-apoptosis response (31,32).
It was therefore hypothesized that mTOR may participate in
PI3K/Akt-induced inhibition of NPC apoptosis. In present study,
treatment with the mTOR inhibitor, rapamycin, effectively decreased
cellular adhesion and p-mTOR/mTOR expression, and increased
IL-1β-induced NPC apoptosis, thus indicating that mTOR is a
downstream protein of the PI3K signaling pathway that may regulate
cell apoptosis and adhesion.
In the present study, E2 pretreatment
resulted in an increase in the phosphorylation ratio of mTOR,
whereas without this pretreatment, IL-1β inhibited activation of
mTOR. A previous study (33)
suggested that estrogen can serve an anti-apoptotic role by uniting
with the ER. ICI182780 is an ER antagonist that inhibits the
binding of ER to target estrogen response elements and abolishes
transcriptional activity in cells. Rapamycin is an inhibitor of
mTOR. Both ICI182780 and rapamycin significantly downregulated the
p-mTOR/mTOR expression ratio compared with in the IL-1β +
E2 group, which suggested that activation of the mTOR
pathway in NPCs may have a role in E2-induced
suppression of IL-1β cytotoxicity. It was hypothesized that
activation of the Akt/mTOR pathway may be involved in the
protection against apoptosis in rat NPCs. This hypothesis is
consistent with the previously described effects in other cell
types (24,34,35).
Previous reports have revealed that the mTOR pathway
serves an important role in osteoclast and microglia cell apoptosis
(36–38). However, to the best of our
knowledge, no studies have reported whether mTOR participates in
the effects of E2 against IL-1β-induced apoptosis of
NPCs. mTOR controls apoptotic cell death through eukaryotic
translation initiation factor 4E-binding protein 1 and p70S6K.
Activation of p70S6K by mTOR blocks apoptosis through pathways that
can increase expression of the anti-apoptotic proteins Bcl-2/Bcl-xL
and inactivate the pro-apoptotic protein BAD (39). Bcl-2 blocks the release of
cytochrome c through the mitochondrial outer membrane and
inhibits cytochrome c-mediated caspase activation (40). Cleaved caspase-3 is associated with
the initiation of apoptosis via the mitochondrial (intrinsic)
pathway. In this study, activated caspase-3 was suppressed by
E2, which was reversed by rapamycin, as measured by
western blotting, thus indicating that mTOR may participate in
E2-induced inhibition of NPC apoptosis.
GSK-3β and NF-κB are also key proteins in the
pathways downstream of PI3K/Akt. In the present study, FACS results
exhibited no significant differences in the apoptotic ratio of NPCs
in the IL-1β + E2 group treated with or without GSK-3β
and NF-κB inhibitors. The GSK-3β signaling pathway serves an
important role in inducing cellular apoptosis via mediating
mitochondrial functions, which can active caspase-8 and caspase-2,
induce the cleavage of Bid and release cytochrome c, thus
leading to apoptosis and mitochondrial dysfunction (41,42).
The expression ratio of p-GSK-3β/GSK-3β was significantly increased
in the IL-1β group, as determined by western blot analysis.
Therefore, this study concluded that the GSK-3β signaling pathway
may be involved in the apoptosis of NPCs induced by IL-1β.
Furthermore, NF-κB regulates the expression of >150 genes
involved in inflammation, cell proliferation, differentiation and
survival. In the current study, the NF-κB inhibitor, SC75741, did
not decrease NPC apoptosis compared with in the IL-1β +
E2 group, indicating that NF-κB did not serve a role in
E2 and IL-1β-regulated NPC apoptosis.
In conclusion, the present study suggested that the
PI3K/Akt/mTOR/caspase-3 signaling pathway may be involved in
protection against IL-1β-induced NPC apoptosis. These findings may
provide a novel therapeutic target for the prevention and treatment
of IVD degenerative diseases.
Acknowledgements
The authors would like to acknowledge the support of
Hebei Medical University Affiliated Animal Experimental Center for
supplying the experimental equipment.
Funding
The present study was supported by the Natural
Science Foundation of China (grant nos. 81572166 and 81601917) and
the Natural Science Foundation of Hebei Province (grant nos.
H2016206073 and H2018206313).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
WD conceived and designed the experiments. HG
conducted the experiments. FZ and SL acquired the data and provided
reagents. SY analyzed the data. HG wrote the manuscript. DY and LM
contributed to interpretation of the data and critical revision of
the manuscript. HW contributed to interpretation of the data and
revision of the manuscript, particularly regarding the FACS
section. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The animal protocols were approved by the
Institutional Animal Care and Use Committee of The Third Hospital
of Hebei Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
IVD
|
intervertebral disc
|
|
IVDD
|
IVD degeneration
|
|
ECM
|
extracellular matrix
|
|
NP
|
nucleus pulposus
|
|
NPCs
|
NP cells
|
|
IL-1β
|
interleukin-1β
|
|
E2
|
17β-estradiol
|
|
ER
|
estrogen receptor
|
|
COL2
|
type II collagen
|
|
COL2α1
|
COL2 α1 chain
|
|
S6K
|
S6 kinase
|
References
|
1
|
Antoniou J, Steffen T, Nelson F,
Winterbottom N, Hollander AP, Poole RA, Aebi M and Alini M: The
human lumbar intervertebral disc: Evidence for changes in the
biosynthesis and denaturation of the extracellular matrix with
growth, maturation, ageing, and degeneration. J Clin Invest.
98:996–1003. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Buckwalter JA: Aging and degeneration of
the human intervertebral disc. Spine (Phila Pa 1976). 20:1307–1314.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao CQ, Jiang LS and Dai LY: Programmed
cell death in intervertebral disc degeneration. Apoptosis.
11:2079–2088. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Le Maitre CL, Freemont AJ and Hoyland JA:
Accelerated cellular senescence in degenerate intervertebral discs:
A possible role in the pathogenesis of intervertebral disc
degeneration. Arthritis Res Ther. 9:R452007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Park JB, Park IC, Park SJ, Jin HO, Lee JK
and Riew KD: Anti-apoptotic effects of caspase inhibitors on rat
intervertebral disc cells. J Bone Joint Surg Am. 88:771–779. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Portt L, Norman G, Clapp C, Greenwood M
and Greenwood MT: Anti-apoptosis and cell survival: A review.
Biochim Biophys Acta. 1813:238–259. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bozzo C, Graziola F, Chiocchetti A and
Canonico PL: Estrogen and beta-amyloid toxicity: Role of integrin
and PI3-K. Mol Cell Neurosci. 45:85–91. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nelson K, Helmstaedter V, Moreau C and
Lage H: Estradiol, tamoxifen and ICI 182,780 alter alpha3 and beta1
integrin expression and laminin-1 adhesion in oral squamous cell
carcinoma cell cultures. Oral Oncol. 44:94–99. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zandi PP, Carlson MC, Plassman BL,
Welsh-Bohmer KA, Mayer LS, Steffens DC and Breitner JC: Hormone
replacement therapy and incidence of Alzheimer disease in older
women: The Cache County Study. JAMA. 288:2123–2129. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Craig MC, Maki PM and Murphy DG: The
Women's Health Initiative Memory Study: Findings and implications
for treatment. Lancet Neurol. 4:190–194. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Honda K, Sawada H, Kihara T, Urushitani M,
Nakamizo T, Akaike A and Shimohama S: Phosphatidylinositol 3-kinase
mediates neuroprotection by estrogen in cultured cortical neurons.
J Neurosci Res. 60:321–327. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Turner CE: Paxillin and focal adhesion
signalling. Nat Cell Biol. 2:E231–E236. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang SD, Ma L, Gu TX, Ding WY, Zhang F,
Shen Y, Zhang YZ, Yang DL, Zhang D, Sun YP and Song YL:
17β-Estradiol protects against apoptosis induced by levofloxacin in
rat nucleus pulposus cells by upregulating integrin α2β1.
Apoptosis. 19:789–800. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang SD, Yang DL, Sun YP, Wang BL, Ma L,
Feng SQ and Ding WY: 17β-estradiol protects against apoptosis
induced by interleukin-1beta in rat nucleus pulposus cells by
down-regulating MMP-3 and MMP-13. Apoptosis. 20:348–357. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Su CC, Lin YP, Cheng YJ, Huang JY, Chuang
WJ, Shan YS and Yang BC: Phosphatidylinositol 3-kinase/Akt
activation by integrin-tumor matrix interaction suppresses
Fas-mediated apoptosis in T cells. J Immunol. 179:4589–4597. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu ZZ, Xiu P, Lv JW, Wang FH, Dong XF, Liu
F, Li T and Li J: Integrin αvβ3 is required for cathepsin B-induced
hepatocellular carcinoma progression. Mol Med Rep. 11:3499–3504.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huveneers S, Truong H and Danen HJ:
Integrins: Signaling, disease, and therapy. Int J Radiat Biol.
83:743–751. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tang K, Nie D, Cai Y and Honn KV: The
beta4 integrin subunit rescues A431 cells from apoptosis through a
PI3K/Akt kinase signaling pathway. Biochem Biophys Res Commun.
264:127–132. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang SD, Ma L, Yang DL and Ding WY:
Combined effect of 17β-estradiol and resveratrol against apoptosis
induced by interleukin-1β in rat nucleus pulposus cells via
PI3K/Akt/caspase-3 pathway. PeerJ. 4:e16402016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Deng X, Chen S, Zheng D, Shao Z, Liang H
and Hu H: Icariin prevents H2O2-induced
apoptosis via the PI3K/Akt pathway in rat nucleus pulposus
intervertebral disc cells. Evid Based Complement Alternat Med.
2017:26942612017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang T, Yang SD, Liu S, Wang H, Liu H and
Ding WY: 17β-estradiol inhibites tumor necrosis factor-α induced
apoptosis of human nucleus pulposus cells via the pi3k/akt pathway.
Med Sci Monit. 22:4312–4322. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin CY, Chen JH, Fu RH and Tsai CW:
Induction of Pi form of glutathione S-transferase by carnosic acid
is mediated through PI3K/Akt/NF-κB pathway and protects against
neurotoxicity. Chem Res Toxicol. 27:1958–1966. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang H, Zhong R, Xia Z, Song J and Feng
L: Neuroprotective effects of rhynchophylline against ischemic
brain injury via regulation of the Akt/mTOR and TLRs signaling
pathways. Molecules. 19:11196–11210. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang J, Wang C, Yu S, Luo Z, Chen Y, Liu
Q, Hua F, Xu G and Yu P: Sevoflurane postconditioning protects rat
hearts against ischemia-reperfusion injury via the activation of
PI3K/AKT/mTOR signaling. Sci Rep. 4:73172014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim DE, Kim B, Shin HS, Kwon HJ and Park
ES: The protective effect of hispidin against hydrogen
peroxide-induced apoptosis in H9c2 cardiomyoblast cells through
Akt/GSK-3β and ERK1/2 signaling pathway. Exp Cell Res. 327:264–275.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang H, Ding W, Yang D, Gu T, Yang S and
Bai Z: Different concentrations of 17β-estradiol modulates
apoptosis induced by interleukin-1β in rat annulus fibrosus cells.
Mol Med Rep. 10:2745–2751. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Boos N, Weissbach S, Rohrbach H, Weiler C,
Spratt KF and Nerlich AG: Classification of age-related changes in
lumbar intervertebral discs: 2002 Volvo Award in basic science.
Spine (Phila Pa 1976). 27:2631–2644. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tuttle RL, Gill NS, Pugh W, Lee JP,
Koeberlein B, Furth EE, Polonsky KS, Naji A and Birnbaum MJ:
Regulation of pancreatic beta-cell growth and survival by the
serine/threonine protein kinase Akt1/PKBalpha. Nat Med.
7:1133–1137. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dickson LM and Rhodes CJ: Pancreatic
beta-cell growth and survival in the onset of type 2 diabetes: A
role for protein kinase B in the Akt? Am J Physiol Endocrinol
Metab. 287:E192–E198. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Elghazi L, Balcazar N and Bernal-Mizrachi
E: Emerging role of protein kinase B/Akt signaling in pancreatic
beta-cell mass and function. Int J Biochem Cell Biol. 38:157–163.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Avruch J, Hara K, Lin Y, Liu M, Long X,
Ortiz-Vega S and Yonezawa K: Insulin and amino-acid regulation of
mTOR signaling and kinase activity through the Rheb GTPase.
Oncogene. 25:6361–6372. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Um SH, D'Alessio D and Thomas G: Nutrient
overload, insulin resistance, and ribosomal protein S6 kinase 1,
S6K1. Cell Metab. 3:393–402. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Doublier S, Lupia E, Catanuto P,
Periera-Simon S, Xia X, Korach K, Berho M, Elliot SJ and Karl M:
Testosterone and 17β-estradiol have opposite effects on podocyte
apoptosis that precedes glomerulosclerosis in female estrogen
receptor knockout mice. Kidney Int. 79:404–413. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen L, Xu B, Liu L, Luo Y, Yin J, Zhou H,
Chen W, Shen T, Han X and Huang S: Hydrogen peroxide inhibits mTOR
signaling by activation of AMPKalpha leading to apoptosis of
neuronal cells. Lab Invest. 90:762–773. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhou J, Wu J, Zheng F, Jin M and Li H:
Glucagon-like peptide-1 analog-mediated protection against
cholesterol-induced apoptosis via mammalian target of rapamycin
activation in pancreatic βTC-6 cells-1mTORβTC-6. J Diabetes.
7:231–239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shang YC, Chong ZZ, Wang S and Maiese K:
Erythropoietin and Wnt1 govern pathways of mTOR, Apaf-1, and XIAP
in inflammatory microglia. Curr Neurovasc Res. 8:270–285. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shang YC, Chong ZZ, Wang S and Maiese K:
Prevention of β-amyloid degeneration of microglia by erythropoietin
depends on Wnt1, the PI 3-K/mTOR pathway, Bad, and Bcl-xL. Aging
(Albany NY). 4:187–201. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Glantschnig H, Fisher JE, Wesolowski G,
Rodan GA and Reszka AA: M-CSF, TNFalpha and RANK ligand promote
osteoclast survival by signaling through mTOR/S6 kinase. Cell Death
Differ. 10:1165–1177. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pastor MD, García-Yébenes I, Fradejas N,
Pérez-Ortiz JM, Mora-Lee S, Tranque P, Moro MA, Pende M and Calvo
S: mTOR/S6 kinase pathway contributes to astrocyte survival during
ischemia. J Biol Chem. 284:22067–22078. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Konstantinidis K, Whelan RS and Kitsis RN:
Mechanisms of cell death in heart disease. Arterioscler Thromb Vasc
Biol. 32:1552–1562. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lin CF, Chen CL, Chiang CW, Jan MS, Huang
WC and Lin YS: GSK-3beta acts downstream of PP2A and the PI
3-kinase-Akt pathway and upstream of caspase-2 in ceramide-induced
mitochondrial apoptosis. J Cell Sci. 120:2935–2943. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lin CF, Tsai CC, Huang WC, Wang YC, Tseng
PC, Tsai TT and Chen CL: Glycogen synthase kinase-3β and caspase-2
mediate ceramide- and etoposide-induced apoptosis by regulating the
lysosomal-mitochondrial axis. PLoS One. 11:e01454602016. View Article : Google Scholar : PubMed/NCBI
|