Introduction
Nasopharyngeal carcinoma (NPC) is a common type of
epithelial squamous cell head and neck carcinoma, and is the most
common type of nasopharyngeal tumor (1). Despite recent advances in the
diagnosis and treatment of NPC, the 10-year survival rate of
patients with NPS remains poor, and the occurrence rate in
South-eastern Asia and North Africa increased over the past years
(2). Therefore, the development of
effective therapeutic strategies and novel prognostic molecular
markers is necessary to improve the survival rate of patients with
NPC.
A number of previous studies have demonstrated that
multiple microRNAs (miRNAs) may act as oncogenes or tumor
suppressor genes; therefore, the dysregulation of miRNAs was
identified to be involved in the process of cancer development and
progression (3,4). In addition, miRNAs may be used as
molecular biomarkers for cancer prognosis and targeted therapies
(5–8). miRNA-30a (miR-30a) was previously
demonstrated to have an important role in the proliferative,
metastatic and invasive potential of ovarian carcinoma (9), gallbladder cancer (10) and other types of cancer (11–13).
However, the molecular mechanism underlying miR-30a function in
human NPC remains unclear.
Epithelial-mesenchymal transition (EMT) is an
important process for tumor cell invasion of epithelial and
non-epithelial cancers, and zinc finger E-box binding homeobox 2
(ZEB2) was demonstrated to promote EMT (14). A previous study demonstrated that
ZEB2 served as a DNA-binding transcriptional repressor that may be
able to interact with activated SMAD family member 1, thus
regulating the bone morphogenetic protein signaling pathway
(15). Previous studies
investigating the role of ZEB2 in cancer identified that the
expression of ZEB2 is important for the development of cancer
(16), and the inhibition of ZEB2
may suppress cancer cell growth, migration and invasion (17). In addition, a previous studies
demonstrated that the association between ZEB2 and Sp1
transcription factor was able to promote cancer cell survival and
angiogenesis during metastasis via the upregulation of survivin and
vascular endothelial growth factor (18). Certain miRNAs, including miR-335
and miR-200c, were identified to bind to the 3′ untranslated region
(3′-UTR) of ZEB2, inhibiting cancer progression (17,19);
therefore, miRNAs may be used for the development of novel
therapeutic strategies to treat cancer.
In the present study, ZEB2 was identified as a
direct target gene of miR-30a in human NPC. miR-30a overexpression
was identified to induce apoptosis and suppress proliferation,
migration and invasion of NPC cells. The present data suggested
that miR-30a may possess the potential to be used as a novel
diagnostic marker and therapeutic target for the treatment of
patients with NPC.
Materials and methods
NPC samples
NPC and paracancerous tissues were collected from 4
patients at The People's Hospital of Longhua (Shenzhen, China) in
August 2017. All volunteers [Patient 1 (a 46-year-old male),
Patient 2 (a 59-year-old male), Patient 3 (a 53-year-old female)
and Patient 4 (a 58-year-old male)] provided written informed
consent. The consent procedure was approved by The Animal Care and
Use Committee of People's Hospital of Longhua (Shenzhen, China).
Inclusion criteria for the study were as follows: i) Having
received a clinical or referral record in the electronic medical
record of NPC during the period of the study; and ii) being ≥40
years of age at the time of diagnosis. Exclusion criteria for the
study were as follows: i) Having a secondary cancer; ii) not having
received primary care in the year prior to cancer diagnosis; and
iii) not having any matched controls.
Following resection, the collected tissues were
placed in a solution containing PBS, 0.1 g/ml streptomycin and 100
U/ml penicillin. The tissues were washed three times using PBS.
Subsequently, NPC tissues and paracancerous tissues were lysed, and
total RNA and protein were extracted for reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blot assay, respectively.
Cell culture
The human NPC cell line, C666-1, and 293 cells were
purchased from The Cell Bank of Type Culture Collection of Chinese
Academy of Science (Shanghai, China). The two cell lines were
cultured in Dulbecco's Modified Eagle's Medium (DMEM; HyClone; GE
Healthcare Life Sciences, Logan, UT, USA) supplemented with 10%
fetal bovine serum (HyClone; GE Healthcare Life Sciences), 0.1 g/ml
streptomycin and 100 U/ml penicillin (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) in a humidified incubator at 37°C with 5%
CO2. The culture medium was replaced every other day,
and the C666-1 cells were passaged (dilution, 1:4) every 5 or 6
days.
miR-30a preparation and
transfection
Potential target genes of miR-30a were first
analyzed in silico using TargetScan 7.2 (http://www.targetscan.org). miR-30a
(5′-UGUAAACAUCCUCGACUGGAAG-3′) was purchased from Sangon Biotech
Co., Ltd. (Shanghai, China). A non-specific miRNA
(5′-ACGUGACACGUUCGGAGAAUU-3′) was used as the negative control
(Ctrl miRNA). The reverse complementary sequence of miRNA-30a
(5′-CUUCCAGUCGAGGAUGUUUACA-3′) was used as the miR-30a inhibitor.
Ctrl miRNA and miR-30a inhibitor was purchased from Sangon Biotech
Co., Ltd. Cell transfection was performed using
Lipofectamine® 3000 transfection reagent (cat. no.
L3000008; Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) with 10 nM of miRNA, according to the manufacturer's protocol.
Briefly, 1×105 cells were transfected with miRNA
molecules. Following transfection for 24 h, the cells in each group
were harvested for subsequent experimentation.
Overexpression of ZEB2 in NPC cells
and grouping
Total RNA was isolated from 293 cells using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.) and
used as a template to obtain genomic cDNA using a PrimeScript
reverse transcription-polymerase chain reaction (RT-PCR) kit (cat.
no. RR014B; Takara Biotechnology Co., Ltd., Dalian, China)
according to the manufacturer's protocols, and the coding sequence
plus 3′-UTR of ZEB2 was amplified using PCR (Phusion®
High-Fidelity DNA Polymerase, M0530L, New England BioLabs, Inc.,
Ipswich, MA, USA) and subsequently cloned into a pCI vector
(Addgene, Inc., Cambridge, MA, USA) using NheI/SalI
restriction sites. The following primer sequences were used to
amplify ZEB2: Forward (F), 5′-ATGAAGCAGCCGATCATGGCG-3′ and
reverse®, 5′-CACACATCTTGGAGCAAAAGCATG-3′. PCR was
performed under the following conditions: Initial denaturation at
95°C for 5 min, 35 cycles of 95°C for 35 sec, 60°C for 35 sec and
72°C for 2.5 min, followed by a final extension at 72°C for 5
min.
To overexpress ZEB2 in NPC cells, C666-1 cells were
transfected with the pCI-ZEB2 vector (final concentration, 1 µg/ml)
using Lipofectamine 3000 (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. Following
transfection for 24 h, the cells in each group were harvested for
the subsequent experimentation. The overexpression efficiency was
examined by RT-qPCR. Cells transfected with Ctrl miRNA, pCI-empty
vector, pCI-ZEB2 vector (ZEB2 vector), miR-30a, miR-30a inhibitor,
pCI-empty vector + Ctrl miRNA (Ctrl), pCI-ZEB2 vector + Ctrl miRNA
(ZEB2 overexpression), pCI-empty vector + miR-30a (miR-30a
overexpression) and pCI-ZEB2 vector + miR-30a (ZEB2+miR-30a
overexpression) were used for further analysis.
RT-qPCR
In total, 50 mg of homogenized NPC and paracancerous
tissues were lysed using 1 ml Trizol® (Invitrogen;
Thermo Fisher Scientific Inc., Waltham, MA, USA). A total of
5×106 C666-1 cells from each group were lysed using 1 ml
Trizol®. Total RNA was extracted using Trizol according
to the manufacturer's protocol, and cDNA was synthesized using 1 µg
RNA from each sample. RT was performed using a Transcriptor
first-strand cDNA synthesis kit (Promega Corporation, Madison, WI,
USA) under the following conditions: 20°C for 10 min, 42°C for 60
min and 95°C for 5 min. qPCR was conducted using an
SYBR® Fast qPCR Mix kit (Takara Biotechnology Co.,
Ltd.). Following an initial polymerase activation and denaturation
step at 50°C for 2 min and 95°C for 5 min, respectively, the
samples in each group underwent 40 amplification cycles of 95°C for
20 sec, 65°C for 10 sec and 72°C for 30 sec in the LightCycler 480
instrument (Roche Diagnostics, Basel, Switzerland). RT-qPCR results
were quantified using the 2−ΔΔCq method as previously
described (20–22). In the present study, GAPDH and U6
were used as reference genes for the normalization of ZEB2 and
miR-30a, respectively. The expression levels of the genes analyzed
were normalized to their expression levels in the corresponding
control group. The primer sequences used were the following:
miR-30a F, 5′-ACACTCCAGCTGGGTTGCATAGTCACAAAAGT-3 and R,
5′-ACACTCCAGCTGGGTGTAAACATCCTACACTCT-3′; U6 F,
5′-CTCGCTTCGGCAGCACA-3′ and R, 5′-AACGCTTCACGAATTTGCGT-3′; ZEB2 F,
5′-CTCTTCCCACACGCTTAGTT-3′ and R, 5′-GGCCTAAGCTTACAGTGTCATG-3′;
GAPDH F, 5′-GGGAAACTGTGGCGTGAT-3′ and R,
5′-GAGTGGGTGTCGCTGTTGA-3′.
Western blot analysis
Radioimmunoprecipitation assay lysis buffer (RIPA;
cat. no. 89900; Thermo Fisher Scientific, Inc.) was used to extract
the total protein. The protein concentration was measured using a
bicinchoninic acid assay kit (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany). Homogenized tissue (50 mg) was lysed using 0.3 ml RIPA
lysis buffer. For the in vitro experiments, 1×106
cells were lysed using 0.1 ml RIPA lysis buffer. Western blot assay
was performed as previously described (23–25).
In brief, protein (15 µg/lane) was separated via 10% SDS-PAGE and
then transferred to nitrocellulose membranes. Membranes were
blocked with 5% bovine serum albumin (Thermo Fisher Scientific,
Inc.) for 2 h at room temperature, then incubated with primary
antibodies overnight at 4°C. In the present study, the primary
antibodies used were: Anti-ZEB2 (1:1,000; Abcam, Cambridge, UK;
cat. no. ab223688) and anti-GAPDH (1:5,000; Abcam; cat. no.
ab8245). The secondary antibodies used were: Anti-mouse IgG
[horseradish peroxidase (HRP)-conjugated; 1:5,000; Sigma-Aldrich;
Merck KGaA; cat. no. A-9044] and anti-rabbit IgG (HRP-conjugated;
1:5,000; Sigma-Aldrich; Merck KGaA; cat. no. A-0545). Protein bands
were visualized using an enhanced chemiluminescence kit (Thermo
Fisher Scientific, Inc.) and ChemiDoc Imagers (ChemiDoc™ XRS +
System; Bio-Rad Laboratories, Inc., Hercules, CA, USA). Protein
expression was quantified using ImageJ 1.x software (National
Institutes of Health, Bethesda, MD, USA).
Dual-luciferase reporter assay
The 3′-UTR sequence of human ZEB2 gene was amplified
using PCR and cloned into a psiCHECK-1-based luciferase plasmid
(Addgene, Inc., Cambridge, MA, USA) in which the Renilla
luciferase sequence was replaced with a firefly luciferase sequence
(restriction enzyme sites: NheI/SgfI) from pGL-4.22
vector (Addgene, Inc.). pRL Renilla luciferase control
reporter vectors (Promega Corporation) was co-transfected as an
internal reference. The construction of the psiCHECK plasmid
containing the mutated 3′-UTR of ZEB2 was performed as previously
described (26–28). In brief, cell transfection was
performed using Lipofectamine 3000 transfection reagent according
to the manufacturer's protocol. Cells (3×105) were
co-transfected with 1 µg plasmids, and miR-30a mimics, inhibitor or
Ctrl miRNA for 24 h, then the dual luciferase assay was performed
using a Dual Luciferase Assay Kit according to the manufacturer's
instructions (Promega Corporation). In the present study, firefly
and Renilla luciferase values were detected; for the
evaluation of relative luciferase activity, the firefly luciferase
activity was normalized to the Renilla luciferase value.
Colony-formation assay, cell
proliferation and cell cycle analysis
To investigate the colony-forming ability of cancer
cells, 100 cells were seeded into 12-well plates and incubated for
7 days in an incubator at 37°C with 5% CO2. The cells
were subsequently fixed with 75% ethanol for 20 min at room
temperature and stained using crystal violet (5 g/l) for 20 min at
room temperature. Cell colonies in each groups were imaged using an
Epson Perfection V600 scanner (Seiko Epson Corporation, Suwa,
Japan) and the results were analyzed using BioSpot®
version 5.0 software (Cellular Technology Limited, Cleveland, OH,
USA).
To examine cell proliferation, the proliferation
index of each group was assessed using a CCK-8 assay (Dojindo
Molecular Technologies, Inc., Kumamoto, Japan) as previously
described (29). The proliferation
index was calculated as the absorbance detected in the experimental
group-the absorbance detected in the blank group.
To analyze the cell cycle, 5×106 cells
were fixed with 70% ethanol for 30 min at 4°C. The cell samples
were stained with 200 µl propidium iodide (PI; Beyotime Institute
of Biotechnology, Haimen, China) in a solution containing
ribonuclease A (Beyotime Institute of Biotechnology) for 10 min at
room temperature. Subsequently, the samples were analyzed using a
FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA) and
FlowJo 7.6.1 (FlowJo LLC, Ashland, OR, USA).
Cell apoptosis assay
Cell apoptosis assay was performed using a Cell
Apoptosis Assay kit (Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. NPC cells in each group were
dissociated into single cells with trypsin, washed with PBS, and
incubated with a solution containing Annexin V-fluorescein
isothiocyanate and PI. Cells were analyzed using a FACSCalibur flow
cytometer and FlowJo 7.6.1.
Cell migration and invasion
assays
The migration and invasion of cancer cells were
measured using Transwell plates (pore filter size, 8 µm; Corning
Inc., Corning, NY, USA). NPC cells were seeded in the upper chamber
at a concentration of 1×105 cells/well in serum-free
DMEM. Inserts covered with Matrigel were used for invasion assays,
whereas normal inserts were used for migration assays. High glucose
DMEM (HyClone; GE Healthcare Life Sciences) containing 10% FBS was
plated in the lower chamber. Subsequently, cells were incubated for
48 h at 37°C. Cells on the upper chambers were removed, and
migrating or invading cancer cells were fixed in 10% neutral
buffered formalin for 15 min at room temperature, and stained with
crystal violet (5 g/l) for 20 min at room temperature. The number
of cancer cells in four randomly-selected fields of view were
counted under a light microscrope (magnification, ×100) for each
group.
Tumor formation assay
ZEB2 cDNA was obtained as described above. In
addition, the miR-30a sequence was purchased from Sangon Biotech
Co., Ltd., and the two sequences were separately cloned into
pMXs-based retroviral plasmids (Addgene, Inc.). In total, 40,000
cancer cells were transduced in 6-well culture dishes using
pMX-based retroviruses (5×105 PFU) as previously
described (20). To obtain a
stable overexpression, cells were infected using retroviral
particles containing pMXs-ZEB2 (ZEB2 retrovirus), pMXs-empty vector
(Ctrl retrovirus), pMXs-miR-30a (miR-30a retrovirus) and pMXs-ZEB2
+ pMXs-miR-30a (ZEB2 retrovirus + miR-30a retrovirus).
Athymic nude mice were purchased from Charles River
Laboratories, Inc. (Wilmington, MA, USA). Male mice in each group
(n=3/group, 12 mice in total; 22–25 g, 6–8 weeks old; n=3/group)
were injected intrahepatically with infected cancer cells
(5×106 cells/mouse). Mice were maintained in a fully
controlled animal facility (12:12-h light:dark cycle at 22±2°C with
50% humidity), and were housed in standard clear plastic cages with
access to food and water ad libitum. Following 6 weeks, all
animals were sacrificed using isoflurane gas, and tumor weight and
volume were measured in each group. Tumor volume was calculated
using the following formula: (length × width2)/2. During
the 6 weeks, animal health and behavior were monitored every other
day. In case of body weight loss >20% within 3 weeks, mice were
immediately sacrificed. The animal experiments were approved by The
Animal Care and Use Committee of People's Hospital of Longhua
(Shenzhen, China).
Statistical analysis
Data are presented as the mean ± standard error of
the mean. Statistical analysis was performed using SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA). The detection of different
samples form each patients or different cell groups was repeated
for 3 times. Unpaired Student's t-test was used to compare two
groups. One-way analysis of variance followed by Bonferroni's post
hoc test was used to compare three or more groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
ZEB2 is a direct target of miR-30a in
human NPC cells
Human NPC tissues and paracancerous tissues were
collected from four patients to investigate the association between
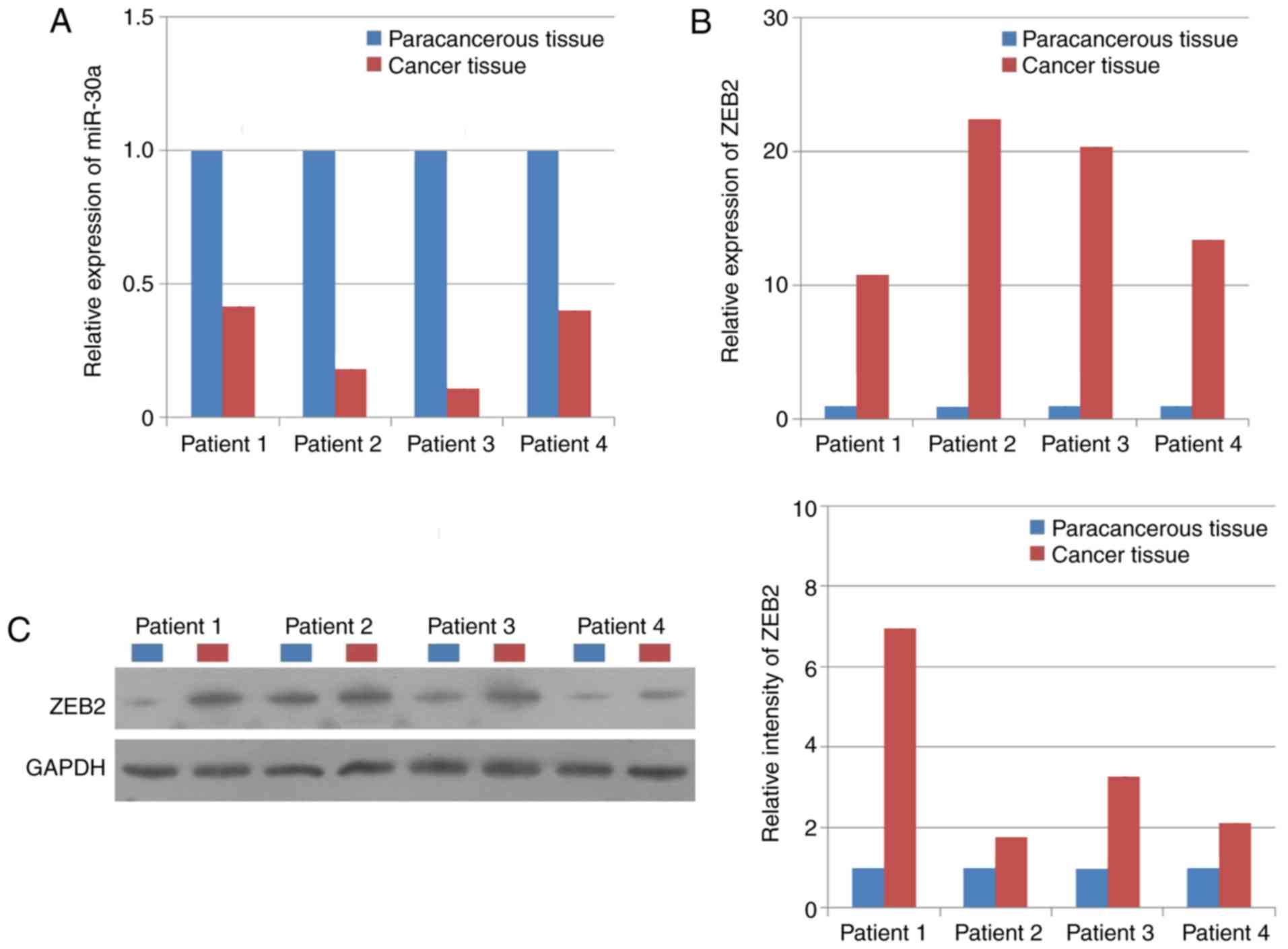
the expression levels of miR-30a and ZEB2. The result suggested
that the expression level of miR-30a was significantly increased in
human paracancerous tissues compared with NPC tissues (Fig. 1A); whereas the mRNA and protein
expression levels of ZEB2 exhibited the opposite trend (Fig. 1B and C), suggesting that there was
a negative association between the expression levels of miR-30a and
ZEB2 in NPC tissues.
In addition, a human NPC cell line, C666-1, was used
to analyze the negative association between miR-30a and ZEB2 in NPC
cells. miR-30a inhibitor or miR-30a were transfected in the C666-1
cells. RT-qPCR results suggested that miR-30a inhibitor decreased
miR-30a expression level by >70%, and the expression level of
miR-30a following overexpression increased by ~5-fold compared with
the control group (Fig. 2A). In
addition, the mRNA and protein expression levels of ZEB2 were
decreased following overexpression of miR-30a in human NPC cells.
Conversely, C666-1 cells transfected with miR-30a inhibitor
exhibited an increased expression level of ZEB2 compared with cells
transfected with the control miRNA (Fig. 2B and C).
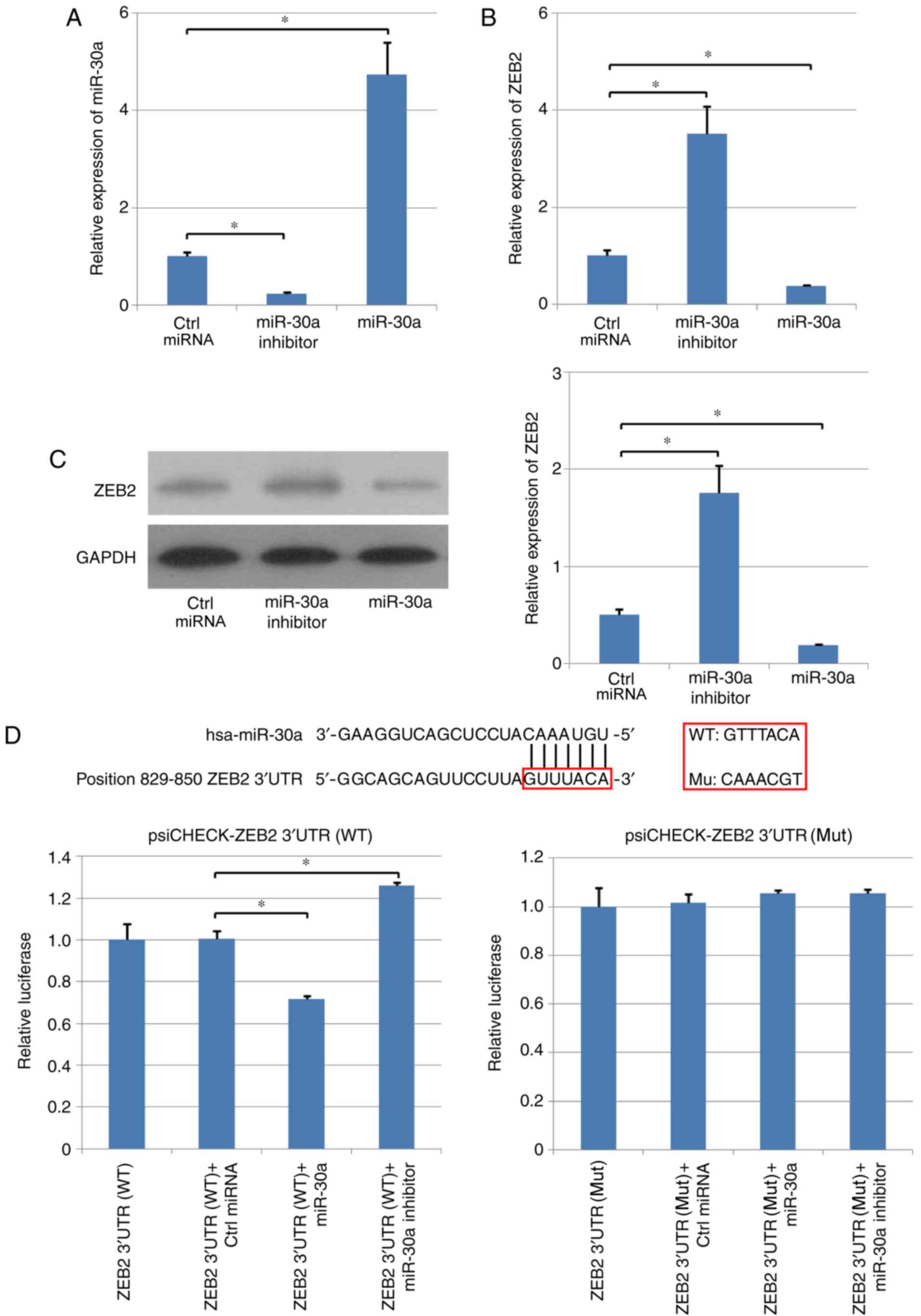 | Figure 2.Expression level of ZEB2 is directly
regulated by miR-30a. Effects of overexpression and inhibition of
miR-30a on the expression level of ZEB2 in C666-1 human
nasopharyngeal carcinoma cells. (A) Expression level of miR-30a in
various experimental groups, as assessed by RT-qPCR. (B) mRNA and
(C) protein expression levels of ZEB2 were determined using RT-qPCR
and western blotting, respectively. (D) Direct interaction between
miR-30a and ZEB2. Dual-luciferase reporter assay was used to
examine the direct interaction between ZEB2 and miR-30a.
*P<0.05. RT-qPCR, reverse transcription-quantitative polymerase
chain reaction; miR-30a, microRNA-30a; Ctrl, control; miRNA,
microRNA; ZEB2, zinc finger E-box binding homeobox 2; WT,
wild-type; UTR, untranslated region; Mut, mutant. |
The potential target genes of miR-30a were analyzed
using TargetScan (Fig. 2D),
suggesting that miR-30a may target the 3′-UTR of ZEB2 mRNA,
regulating the expression level of this coding gene. Therefore,
wild-type (WT) and mutant (Mut) 3′-UTRs of ZEB2 were cloned into
psi-CHECK vectors, C666-1 cells were transfected, and
dual-luciferase assay was performed. miR-30a repressed the
luciferase activity of WT ZEB2-3′-UTR plasmid; however,
transfection with miR-30a did not affect the luciferase activity of
Mut ZEB2-3′-UTR plasmid (Fig. 2D).
Furthermore, the inhibitory effects of miR-30a were suppressed by
miR-30a inhibitor in the WT ZEB2-3′-UTR group; however, the
luciferase activity in the Mut ZEB2-3′-UTR group was not altered
(Fig. 2D). Collectively, the
present results suggested that ZEB2 was a direct target of
miR-30a.
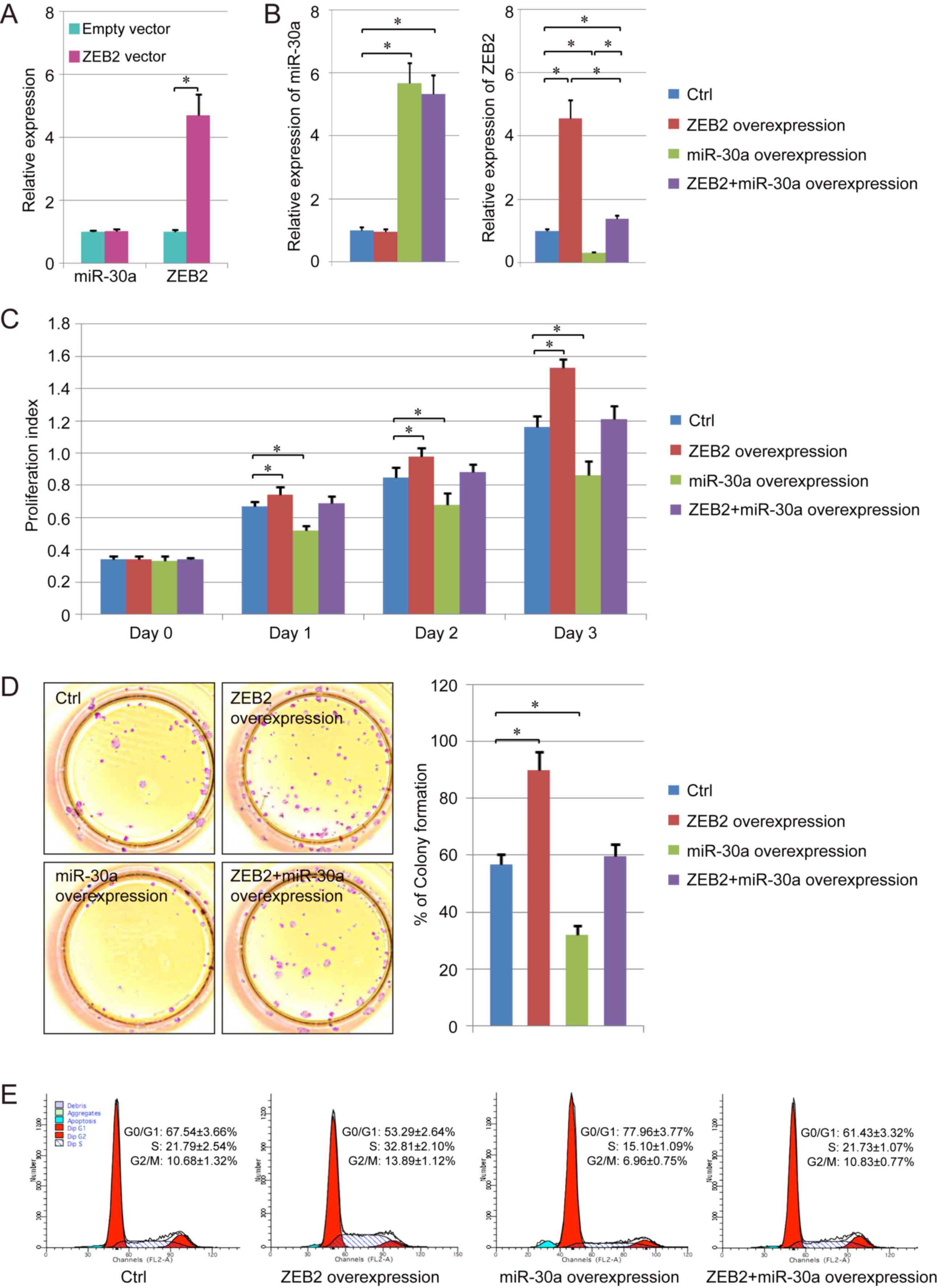
Effects of miR-30a on the
proliferative ability of NPC cells
ZEB2 overexpression did not affect the expression
level of miR-30a; however, the expression level of ZEB2 increased.
In addition, the RT-qPCR results suggested that the expression
levels of miR-30a and ZEB2 increased following cotransfection of
miR-30a and ZEB2 in human NPC cells (Fig. 3A and B).
Furthermore, the proliferative abilities of cells in
various groups were assessed using a CCK-8 assay. The present
results suggested that the number of proliferating cells was
increased following ZEB2 overexpression. miR-30a overexpression
decreased the proliferation index of normal NPC cells and cells
overexpressing ZEB2 (Fig. 3C),
consistently with the colony formation assay results (Fig. 3D).
The effects of miR-30a and ZEB2 on the cell cycle
were further assessed using flow cytometry. miR-30a overexpression
was identified to increase the percentage of cells in
G0/G1 phase and decreased the percentage of
cells in S phase and G2/M phase in NPC cells. The
opposite effect was observed in cells overexpressing ZEB2, and the
cell cycle in ZEB2-overexpressing cells was partially reversed by
overexpression of miR-30a (Fig.
3E). Collectively, miR-30a suppressed NPC cell proliferation by
targeting ZEB2, downregulating its expression level.
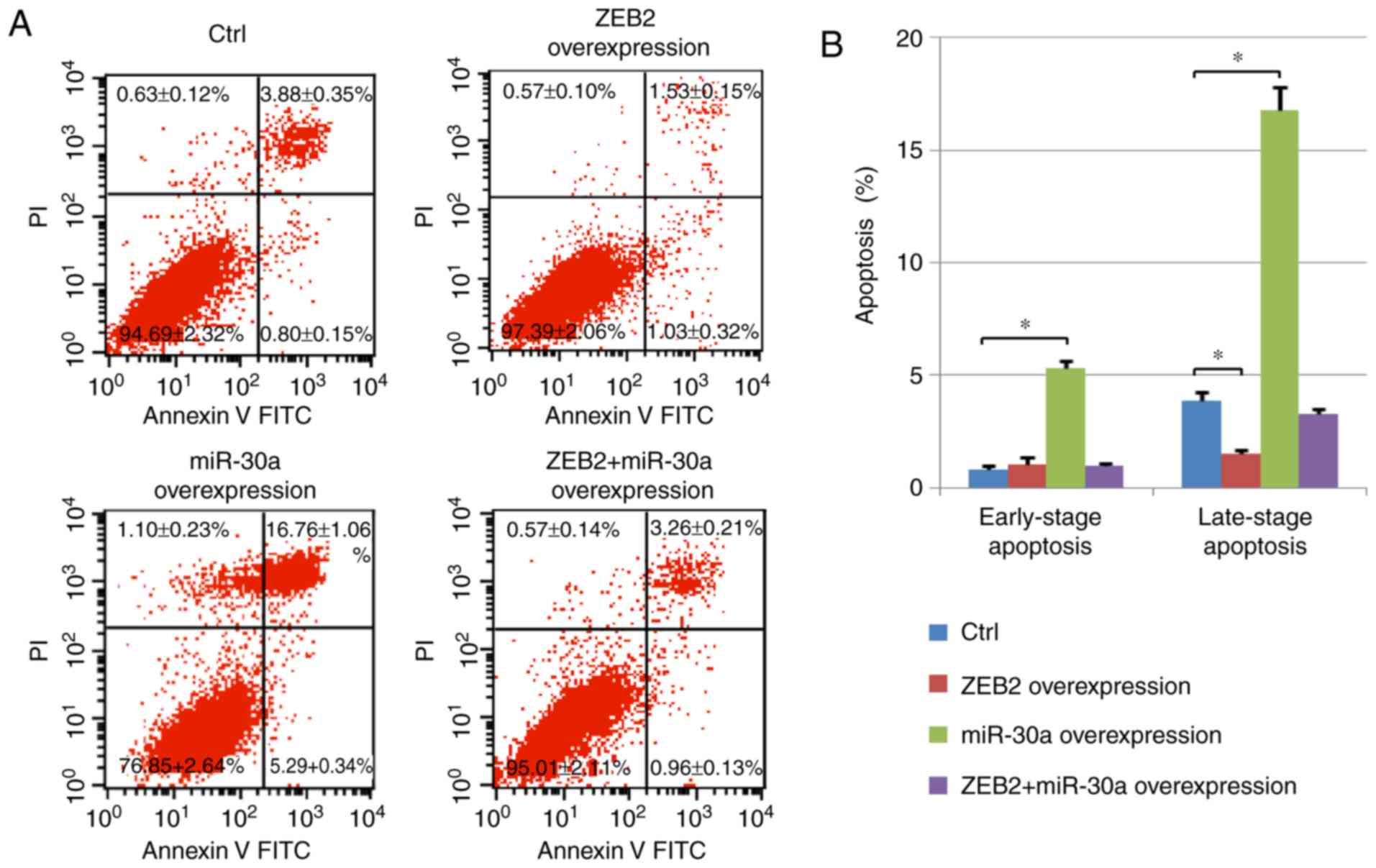
Effect of miR-30a on NPC cell
apoptosis
Cell apoptosis was measured using Annexin V/PI
staining. The number of early-stage apoptotic cells (Annexin
V-positive and PI-negative) and late-stage apoptotic cells (Annexin
V-positive and PI-positive) was assessed by flow cytometry. The
percentage of late-stage apoptotic cells increased following
miR-30a overexpression. The increased apoptosis observed following
miR30a overexpression was reversed by ZEB2 cotransfection.
Conversely, ZEB2 transfection decreased the percentage of
early-stage apoptotic cells in cells overexpressing miR-30a.
Notably, ZEB2 overexpression decreased the number of late-stage
apoptotic cells compared with the control group, and ZEB2 + miR-30a
group exhibited an increased number of apoptotic cells compared
with cells overexpressing ZEB2 alone (Fig. 4). Collectively, the present data
suggested that the effect of miR-30a on cell apoptosis was
dependent on ZEB2.
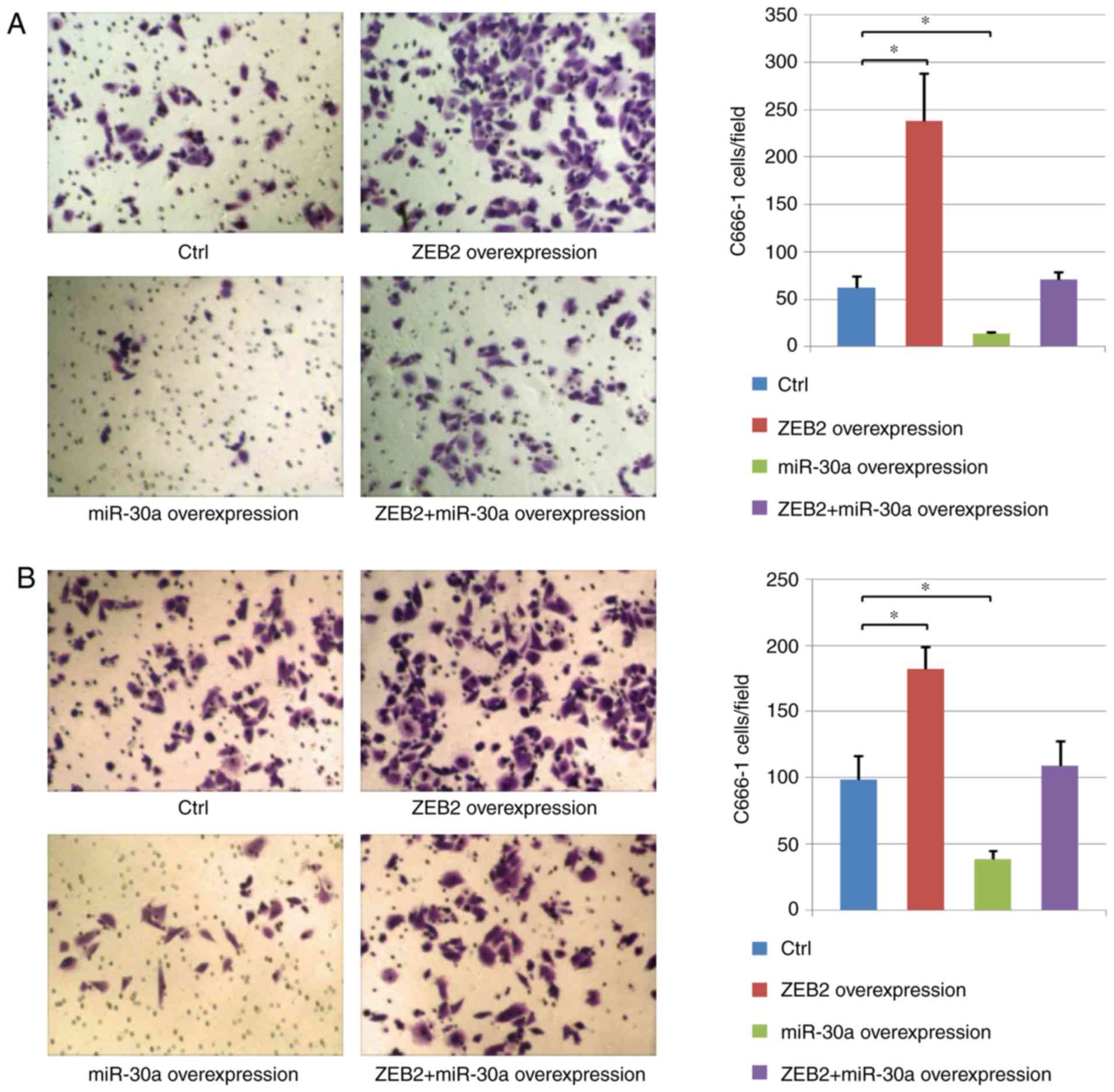
miR-30a inhibits NPC cell migration
and invasion
The effects of ZEB2 and miR-30a on the migratory and
invasive ability of cancer cells were analyzed using Transwell and
Matrigel assays, respectively. The present results suggested that
cell migration and invasion increased following ZEB2
overexpression. Furthermore, compared with the control group, human
NPC cells exhibited decreased migration and invasion following
miR-30a overexpression. In addition, miR-30a overexpression
inhibited the effects of ZEB2 overexpression, suggesting a role for
miR-30a in regulating the migration and invasion of human NPC cells
via ZEB2 (Fig. 5).
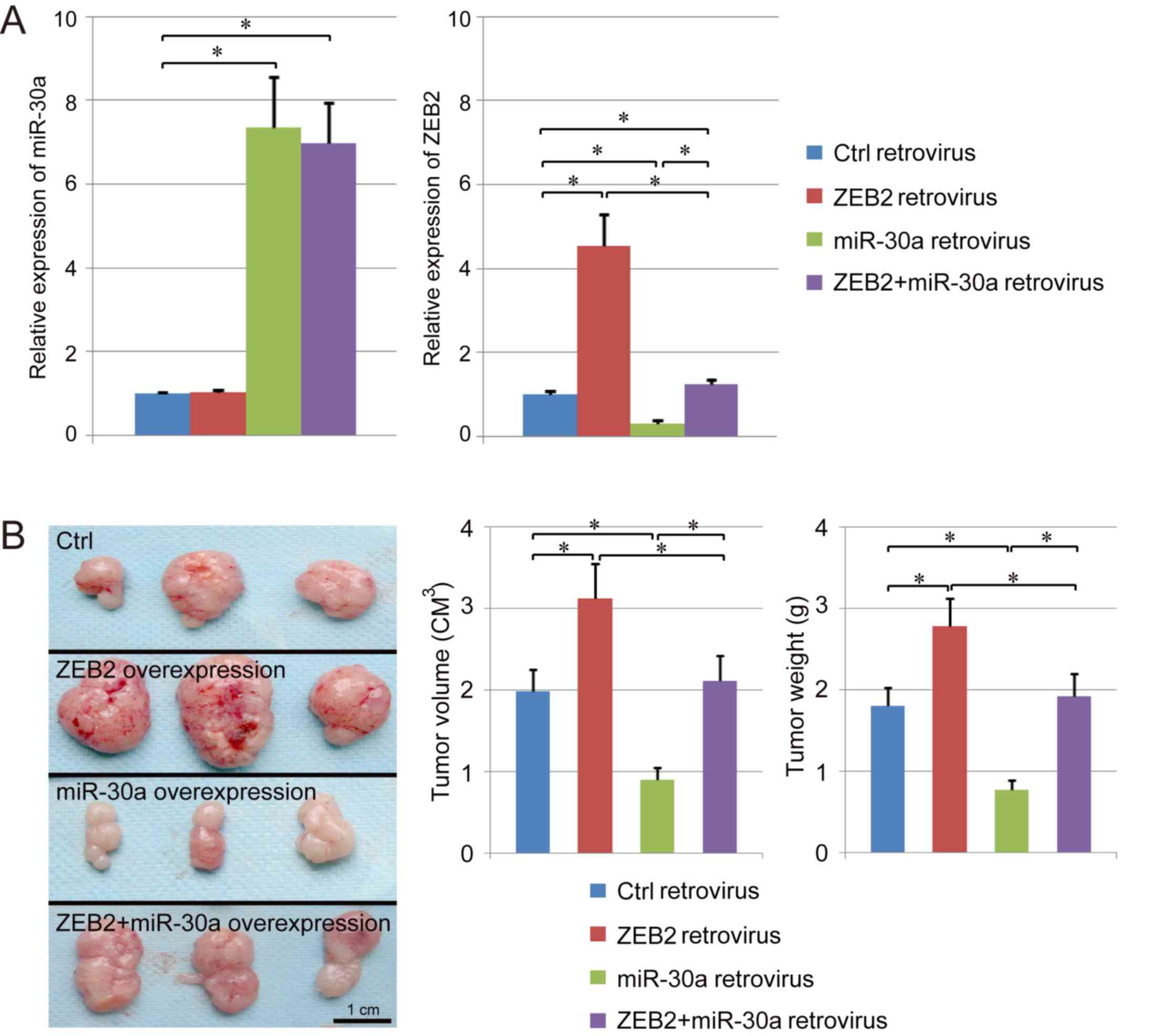
Effects of miR-30a on NPC growth in
vivo
A ZEB2 overexpressing stable cell line, miR-30a
overexpressing stable cell line and ZEB2 + miR-30a overexpressing
stable cell line were established using retroviral infection. The
expression levels of ZEB2 and miR-30a were assessed by RT-qPCR
(Fig. 6A). The expression level of
ZEB2 was suppressed by retroviral-mediated miR-30a overexpression
compared with the control group. Following co-infection with
miR-30a and ZEB2, the expression level of ZEB2 decreased
significantly compared with cells infected with ZEB2 alone
(Fig. 6A). In addition, the growth
of cancer cells infected with various vectors was assessed in
vivo using a xenograft model. The present results suggested
that ZEB2 overexpression increased the weight and the volume of NPC
and promoted tumor growth in vivo. Notably, tumor growth was
suppressed by miR-30a overexpression, and growth of tumors
overexpressing miR-30a was restored following concomitant
overexpression of ZEB2 (Fig. 6B).
Collectively, the present results suggested that miR-30a may
exhibit the potential to suppress the growth of NPC in vivo
by inhibiting the expression level of ZEB2.
Discussion
Previous studies reported that miR-30a may have a
role in cancer development and progression (9,13,30).
A recent study suggested that miR-30a may modulate clear cell renal
cell carcinoma (ccRCC) aggressiveness via repression of the
expression level of ZEB2 (31).
Chen et al (31),
identified that, in ccRCC cells and tissues, the expression level
of miR-30a was decreased, and decreased expression levels of
miR-30a were associated with a poor prognosis in patients with
ccRCC. In addition, overexpression of miR-30a in ccRCC cells was
previously identified to suppress cellular proliferation, invasion
and EMT by regulating ZEB2 in vitro and in vivo,
indicating that the expression level of ZEB2 was negatively
associated with miR-30a. The present results are in line with a
previous study that demonstrated the direct association between
miR-30a and ZEB2 in human breast cancer (32). Collectively, these previous studies
suggested that miR-30a may serve a role in the regulation of cancer
progression (31,32). Wang et al (33) identified an association between the
expression level of miR-30a and the survival rate of patients with
NPC. However, the results of Wang et al (33) were in contrast with previous
studies (31,32). Wang et al (33) observed that the expression level of
miR-30a in NPC primary tumors was decreased compared with
metastatic tumors, and overexpression of miR-30a increased cell
metastasis and invasion in vitro and in vivo.
Mechanistically, this previous study identified that miR-30a
interacted with the 3′-UTR of E-cadherin, decreasing its expression
level and promoting epithelial-mesenchymal transition, thus
decreasing the survival rate of patients with NPC (33). The present study aimed to
investigate the functional association between miR-30a and ZEB2 in
NPC. In the present study, miR-30a was identified to target the
3′-UTR of ZEB2, negatively regulating the expression level of ZEB2
in human NPC tissues and cells. miR-30a overexpression promoted
cell apoptosis and inhibited the proliferative, migratory and
invasive abilities of NPC cell, suggesting that miR-30a may serve a
role in the development and progression of human NPC. Using the
dual-luciferase reporter assay, the direct interaction between
miR-30a and ZEB2 was observed, and the expression level of ZEB2 was
identified to be suppressed by miR-30a. Furthermore, functional
experiments were performed in the present study to investigate the
effects of miR-30a on cell viability. Therefore, the effects of
miR-30a, ZEB2 and miR-30a + ZEB2 overexpression on cell viability
and proliferation were examined.
Although the role of miR-30a was previously
investigated, the molecular mechanism underlying the function of
miR-30a in human NPC remains unclear. In particular, the
association between the downregulation of the expression level of
miR-30a and the development of NPC required further investigation.
A previous study observed that the level of hypermethylation in the
promoter of certain miRNAs increased in cancer cells, resulting in
the downregulation of these miRNAs (34). Therefore, the hypermethylation of
miRNA promoters may serve an important role in the regulation of
miRNAs, thus modulating the development and progression of cancer.
Further investigation is required to examine the association
between hypermethylation and the regulation of miR-30a expression.
Previous studies demonstrated that miR-30a may be downregulated by
the long non-coding RNA deleted in lymphocytic leukemia 2, which
exhibited an increased expression level in cancer cells, suggesting
a possible mechanism underlying the regulation of miR-30a in NPC
(31).
Previous studies observed that miR-30a was able to
directly target multiple genes. For example, miR-30a was
significantly downregulated in human gallbladder cancer, and E2F
transcription factor 7 (E2F7) was identified to be a target of
miR-30a (35). Overexpression of
miR-30a inhibited the expression level of E2F7 and the
re-establishment of the expression level of E2F7 reversed the
inhibitory effects of miR-30a on cancer cell proliferation and
metastasis (35). Notably, miR-30a
may regulate the viability of cancer cells via multiple pathways,
and the expression levels and roles of various target genes of
miR-30a may be cancer type-specific. The present results suggested
that, in human NPC, ZEB2 and ZEB2 downstream genes may serve an
important role compared with other genes targeted by miR-30a.
Nevertheless, further studies are required to investigate multiple
signaling pathways associated with miR-30a. Understanding the
important pathways and genes regulated by miR-30a in various types
of cancers may aid the development of novel strategies to treat
patients with cancer. Notably, in the present study, the number of
patients was not sufficient to perform linear regression analysis
of the expression levels of ZEB2 and miR-30a, and an increased
number of patients is required in order to investigate the role of
these two genes in further clinical studies.
The present results suggested that ZEB2 was a target
of miR-30a in human NPC, and miR-30a negatively regulated the
expression level of ZEB2 by directly binding to its 3′-UTR. miR-30a
overexpression increased the level of apoptosis in human NPC cells
and inhibited cell proliferation, migration and invasion in C666-1
cells by suppressing the expression level of ZEB2. Collectively,
the present results suggested that miR-30a may have the potential
to become a novel diagnostic biomarker in human NPC, and may
facilitate the development of gene therapy strategies to treat
NPC.
Acknowledgements
Not applicable.
Funding
The present study was supported by The Shenzhen
Science and Technology Research and Development Fund (Shenzhen,
China; grant no. JCYJ 20170307141944428), and The Social
Development Project of Dongguan (Dongguan, China; grant no.
2016108101025).
Availability of data and materials
The analyzed datasets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
WL, CX, MZ and SL conceived, designed and supervised
the experiments. XC, JL, DS and SZ performed the experiments. XC,
WL, WX, CX, MZ and SL analyzed the data. WX and DS contributed
reagents, materials and analysis tools. MZ, SL, XC and JL drafted
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study (animal and human experiments) was
approved by The Animal Care and Use Committee of People's Hospital
of Longhua (Shenzhen, China). All patients provided written
informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yee-Lin V, Pooi-Fong W and Soo-Beng AK:
Nutlin-3, A p53-Mdm2 antagonist for nasopharyngeal carcinoma
treatment. Mini Rev Med Chem. 18:173–183. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu J and Hann SS: Functions and roles of
long-non-coding RNAs in human nasopharyngeal carcinoma. Cell
Physiol Biochem. 45:1191–1204. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rupaimoole R and Slack FJ: MicroRNA
therapeutics: Towards a new era for the management of cancer and
other diseases. Nat Rev Drug Discov. 16:203–222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tutar L, Özgür A and Tutar Y: Involvement
of miRNAs and pseudogenes in cancer. Methods Mol Biol. 1699:45–66.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gabra MM and Salmena L: MicroRNAs and
acute myeloid leukemia chemoresistance: A mechanistic overview.
Front Oncol. 7:2552017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cheng L, Zhan B, Luo P and Wang B:
miRNA375 regulates the cell survival and apoptosis of human
nonsmall cell carcinoma by targeting HER2. Mol Med Rep.
15:1387–1392. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Poltronieri P: Editorial: Overview on
microRNAs in cancer development and virus infection. Microrna.
5:80–82. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tutar L, Tutar E and Tutar Y: MicroRNAs
and cancer; an overview. Curr Pharm Biotechnol. 15:430–437. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Y, Qiu C, Lu N, Liu Z, Jin C, Sun C,
Bu H, Yu H, Dongol S and Kong B: FOXD1 is targeted by miR-30a-5p
and miR-200a-5p and suppresses the proliferation of human ovarian
carcinoma cells by promoting p21 expression in a p53-independent
manner. Int J Oncol. 52:2130–2142. 2018.PubMed/NCBI
|
|
10
|
Ye YY, Mei JW, Xiang SS, Li HF, Ma Q, Song
XL, Wang Z, Zhang YC, Liu YC, Jin YP, et al: MicroRNA-30a-5p
inhibits gallbladder cancer cell proliferation, migration and
metastasis by targeting E2F7. Cell Death Dis. 9:4102018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guan Y, Rao Z and Chen C: miR-30a
suppresses lung cancer progression by targeting SIRT1. Oncotarget.
9:4924–4934. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang C, Ma X, Du J, Yao Z, Shi T, Ai Q,
Chen X, Zhang Z, Zhang X and Yao X: MicroRNA-30a as a prognostic
factor in urothelial carcinoma of bladder inhibits cellular
malignancy by antagonising Notch1. BJU Int. 118:578–589. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang S, Liu Q, Zhang Q and Liu L:
MicroRNA-30a-5p suppresses proliferation, invasion and tumor growth
of hepatocellular cancer cells via targeting FOXA1. Oncol Lett.
14:5018–5026. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen P, Liu H, Hou A, Sun X, Li B, Niu J
and Hu L: Prognostic significance of zinc finger E-box-binding
homeobox family in glioblastoma. Med Sci Monit. 24:1145–1151. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Verschueren K, Remacle JE, Collart C,
Kraft H, Baker BS, Tylzanowski P, Nelles L, Wuytens G, Su MT,
Bodmer R, et al: SIP1, a novel zinc finger/homeodomain repressor,
interacts with Smad proteins and binds to 5′-CACCT sequences in
candidate target genes. J Biol Chem. 274:20489–20498. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
He L, Yu K, Lu F, Wang J, Wu LN, Zhao C,
Li Q, Zhou X, Liu H, Mu D, et al: Transcriptional regulator ZEB2 is
essential for bergmann glia development. J Neurosci. 38:1575–1587.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kan Q, Su Y and Yang H: MicroRNA-335 is
downregulated in papillary thyroid cancer and suppresses cancer
cell growth, migration and invasion by directly targeting ZEB2.
Oncol Lett. 14:7622–7628. 2017.PubMed/NCBI
|
|
18
|
Ko D and Kim S: Cooperation between ZEB2
and Sp1 promotes cancer cell survival and angiogenesis during
metastasis through induction of survivin and VEGF. Oncotarget.
9:726–742. 2017.PubMed/NCBI
|
|
19
|
Zhou X, Men X, Zhao R, Han J, Fan Z, Wang
Y, Lv Y, Zuo J, Zhao L, Sang M, et al: miR-200c inhibits
TGF-β-induced-EMT to restore trastuzumab sensitivity by targeting
ZEB1 and ZEB2 in gastric cancer. Cancer Gene Ther. 25:68–76. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu P, Feng Y, Dong D, Liu X, Chen Y, Wang
Y and Zhou Y: Enhanced renoprotective effect of IGF-1 modifed human
umbilical cord-derived mesenchymal stem cells on gentamicin-induced
acute kidney injury. Sci Rep. 6:202872016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu P, Cai J, Dong D, Chen Y, Liu X, Wang
Y and Zhou Y: Effects of SOX2 on proliferation, migration and
adhesion of human dental pulp stem cells. PLoS One.
10:e01413462015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu P, Chen S, Li X, Qin L, Huang K, Wang
L, Huang W, Li S, Jia B, Zhong M, et al: Low immunogenicity of
neural progenitor cells differentiated from induced pluripotent
stem cells derived from less immunogenic somatic cells. PLoS One.
8:e696172013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tao S, Liu P, Luo G, Rojo de la Vega M,
Chen H, Wu T, Tillotson J, Chapman E and Zhang DD: p97 negatively
regulates NRF2 by extracting ubiquitylated NRF2 from the KEAP1-CUL3
E3 complex. Mol Cell Biol. 37:e00660–16. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu P, Feng Y, Dong C, Yang D, Li B, Chen
X, Zhang Z, Wang Y, Zhou Y and Zhao L: Administration of BMSCs with
muscone in rats with gentamicin-induced AKI improves their
therapeutic efficacy. PLoS One. 9:e971232014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang K, Liu P, Li X, Chen S, Wang L, Qin
L, Su Z, Huang W, Liu J, Jia B, et al: Neural progenitor cells from
human induced pluripotent stem cells generated less autogenous
immune response. Sci China Life Sci. 57:162–170. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yao Q, Pei Y, Zhang X and Xie B:
microRNA-96 acts as a tumor suppressor gene in human osteosarcoma
via target regulation of EZRIN. Life Sci. 203:1–11. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu Y, Wu T, Song J, Chen X, Zhang Y and
Wan Y: A mutant screening method by critical annealing
temperature-PCR for site-directed mutagenesis. BMC Biotechnol.
13:212013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu P, Zhang Y, Chen S, Cai J and Pei D:
Application of iPS cells in dental bioengineering and beyond. Stem
Cell Rev. 10:663–670. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao L, Feng Y, Chen X, Yuan J, Liu X,
Chen Y, Zhao Y, Liu P and Li Y: Effects of IGF-1 on neural
differentiation of human umbilical cord derived mesenchymal stem
cells. Life Sci. 151:93–101. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li Y, Zhang J, Liu Y, Zhang B, Zhong F,
Wang S and Fang Z: MiR-30a-5p confers cisplatin resistance by
regulating IGF1R expression in melanoma cells. BMC Cancer.
18:4042018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen Z, Zhang J, Zhang Z, Feng Z, Wei J,
Lu J, Fang Y, Liang Y, Cen J, Pan Y, et al: The putative tumor
suppressor microRNA-30a-5p modulates clear cell renal cell
carcinoma aggressiveness through repression of ZEB2. Cell Death
Dis. 8:e28592017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
di Gennaro A, Damiano V, Brisotto G,
Armellin M, Perin T, Zucchetto A, Guardascione M, Spaink HP,
Doglioni C, Snaar-Jagalska BE, et al: A p53/miR-30a/ZEB2 axis
controls triple negative breast cancer aggressiveness. Cell Death
Differ. 25:2165–2180. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang HY, Li YY, Fu S, Wang XP, Huang MY,
Zhang X, Shao Q, Deng L, Zeng MS, Zeng YX and Shao JY: MicroRNA-30a
promotes invasiveness and metastasis in vitro and in vivo through
epithelial-mesenchymal transition and results in poor survival of
nasopharyngeal carcinoma patients. Exp Biol Med (Maywood).
239:891–898. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lü L, Liu T, Gao J, Zeng H, Chen J, Gu X
and Mei Z: Aberrant methylation of microRNA-193b in human Barrett's
esophagus and esophageal adenocarcinoma. Mol Med Rep. 14:283–288.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen LL, Zhang ZJ, Yi ZB and Li JJ:
MicroRNA-211-5p suppresses tumour cell proliferation, invasion,
migration and metastasis in triple-negative breast cancer by
directly targeting SETBP1. Br J Cancer. 117:78–88. 2017. View Article : Google Scholar : PubMed/NCBI
|