Introduction
Acute lung injury (ALI) and its severe form, acute
respiratory distress syndrome, are serious and potentially
life-threatening syndromes (1).
Despite the tremendous advancement in the prevention and treatment
of ALI, no effective therapeutic regimen is available, and the
mortality rate remains as high as 40% (2). Therefore, it is important to improve
understanding of the pathophysiology of ALI in order to develop
effective strategies for its prevention and treatment.
Long-noncoding RNAs (lncRNAs) provide new insights
into the pathogenesis of ALI, and may serve as novel therapeutic
agents. Recent studies have reported that the lncRNAs lincRNA-p21,
cancer susceptibility 2 and H19 imprinted maternally expressed
transcript regulate lipopolysaccharide (LPS)-induced ALI (3–5). The
lncRNA metastasis-associated lung adenocarcinoma transcript 1
(MALAT1) was first reported as a prognostic marker of metastasis in
lung cancer (6). MALAT1 was found
to be aberrantly expressed in high-glucose-induced podocyte injury
(7) and LPS-induced acute kidney
injury (8). In addition, MALAT1
was shown to suppress the inflammatory responses in LPS-induced ALI
by regulating microRNA-146 (miRNA/miR-146a) (9). However, the mechanisms underlying the
effects of MALAT1 on cell proliferation and apoptosis in
LPS-induced ALI remain unclear.
LncRNAs act as competing endogenous RNAs (ceRNAs)
and modulate the expression and biological functions of miRNAs
(10). miRNAs such as miR-126-5p
and miR-181a are central regulators involved in the pathogenesis of
ALI (11,12). The expression of miR-17-5p was
found to be inhibited in LPS-induced ALI, whereas miR-17-5p
overexpression was shown to counteract the effects of LPS through
the inhibition of forkhead box A1 (FOXA1) expression (13). FOXA1 is a member of the
winged-helix family of transcription factors and shares structural
similarities with FOXA2 and FOXA3. Song et al (14) reported that the overexpression of
FOXA1 promoted pulmonary epithelial cell apoptosis in ALI. Whether
MALAT1 acts as a ceRNA for the miR-17-5p/FOXA1 axis, consequently
regulating cell proliferation and apoptosis, in LPS-induced A549,
remains unclear.
In the present study, the expression of MALAT1 was
evaluated in LPS-induced A549 cells and its role in cell
proliferation, cell cycle progression and apoptosis was
investigated. The regulatory action of MALAT1 in LPS-induced ALI
was also explored.
Materials and methods
Cell culture and transfection
The A549 cell line was purchased from the Cell Bank
of Type Culture Collection of Chinese Academy of Sciences. Cells
were cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
and 1% penicillin-streptomycin. Cells were maintained at 37°C in a
5% CO2 atmosphere. The following small interfering RNAs
(siRNAs) and miRNA inhibitors were purchased from Guangzhou RiboBio
Co., Ltd.: si-MALAT1 (5′-CAAGCAGACAGCCCGTGCTGCTT-3′), si-negative
control (si-NC; 5′-TTCTCCGAACGTGTCACGTTT-3′), miR-17-5p inhibitor
(5′-GATGGACGTGACATTCGTGAAAC-3′) and negative control inhibitor (NC
inhibitor; 5′-TTCTCCGAACGTGTCACGTTT-3′). The FOXA1-coding sequence
including Xho1 and Xba 1 restriction sites was
chemically synthesized by Genewiz, Inc. and cloned into pcDNA 3.1
(ov-FOXA1; Promega Corporation). The empty pcDNA 3.1 plasmid served
as a negative control (ov-NC). A549 cell (2×105
cells/well) were transfected with 50 nM si-MALAT1, 50 nM si-NC, 50
nM miR-17-5p inhibitor, 50 nM NC inhibitor, 1 µg/µl ov-FOXA1 and/or
1 µg/µl ov-NC using 1 µl Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's instructions. At 48 h after transfection, cells were
treated with 1 µg/ml LPS for 24 h. The concentration of LPS used
was selected based on previous reports (13,15).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated from cells using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and subjected
to RT using an ImProm-II™ Reverse Transcription System (Promega
Corporation). The conditions of RT were 30°C for 10 min and 42°C
for 60 min, followed by 85°C for 10 min. qPCR was performed using
the SYBR® Premix ExTaq™ II kit (Takara Biotechnology
Co., Ltd.) on a 7500 Real-Time PCR System (Applied Biosystems;
Thermo Fisher Scientific, Inc.). qPCR thermocycling conditions were
as follows: Denaturation at 95°C for 10 min, followed by 40 cycles
at 95°C for 15 sec and 65°C for 32 sec. The relative expression
levels of mRNA/lncRNA and miRNA were calculated using the
2−ΔΔCq method (16).
GAPDH and U6 served as reference genes. The sequences of primers
were as follows: MALAT1, forward 5′-GATAAATTTAAACCTGAAAA-3′ and
reverse 5′-ATCTTGTTTCTATCTTCCAA-3′; miR-17-5p, forward
5′-ACACTCCAGCTGGGCAAAGTGCTTACAGTGC-3′ and reverse
5′-CTCAACTGGTGTCGTGGA-3′; FOXA1, forward 5′-CCTCTTCCCCTATTACCGGC-3′
and reverse 5′-GTCCGGGGAGCGTGCCACCT-3′; U6, forward
5′-CTCGCTTCGGCAGCACA-3′ and reverse 5′-AACGCTTCACGAATTTGCGT-;3
GAPDH, forward 5′-GCTCATTTGCAGGGGGGAG-3′ and reverse
5′-GTTGGTGGTGCAGGAGGCA-3′. All reactions were performed in
triplicate.
Cell proliferation assay
Cell proliferation was assessed using the CellTiter
96® AQueous One Solution Cell Proliferation Assay (MTS,
Promega). Briefly, transfected cells were seeded in three
duplicates at a density of 1×104 cells/well in a 96-well
plate in 100 µl culture medium containing 10% FBS and incubated for
various time points (0, 24, 48 and 72 h) following 1 µg/ml LPS
treatment. After incubation, 20 µl MTS was added to each well and
the cells were incubated for 2 h at 37°C and 5% CO2 in
the dark. The optical density at 490 nm was measured using a
microplate absorbance reader.
Cell cycle analysis
Cell cycle assays were performed using the Cell
Cycle Detection kit (Nanjing Keygen Biotech Co., Ltd.). Briefly,
1×106 cells were collected, rinsed twice with PBS and
collected by centrifugation at 800 × g for 5 min at 4°C. The cell
pellet was resuspended in ice-cold 70% ethanol and stored at 25°C
for 2 h. The cells were collected by centrifugation at 800 × g for
5 min at 4°C, washed twice with cold PBS, and 100 µl RNase A was
added to resuspend the cell pellet; the mixture was incubated at
37°C for 30 min. Subsequently, 400 µl propidium iodide (PI) was
added and incubated at 4°C for 30 min in the dark. The PI signal
was detected using a BD caliber flow cytometer (BD Biosciences).
The population of cells in G1, S and G2
phases is expressed as the percentage of total gated cells which
analyzed by FlowJo software (version 10.5.3, FlowJo LLC).
Apoptosis assay
Apoptotic cells were quantified using the Annexin
V-FITC/PI Apoptosis Detection kit (Nanjing Keygen Biotech Co.,
Ltd.). Briefly, cells were harvested, washed twice with ice-cold
PBS and resuspended in 500 µl binding buffer. The cells were then
incubated with 5 µl Annexin V-FITC and 5 µl PI, incubated for 15–20
min in the dark at 25°C, and analyzed with FlowJo software using a
BD FACSCalibur™ flow cytometer (BD Biosciences).
Western blot analysis
Cells were collected and lysed in RIPA buffer
(Thermo Fisher Scientific, Inc.) supplemented with a protease
inhibitor (Thermo Fisher Scientific, Inc.). The protein
concentration was measured using a bicinchoninic acid protein assay
kit (Thermo Fisher Scientific, Inc.). Equal amounts of total
protein (20 µg) were separated by 10% SDS-PAGE and transferred onto
a PVDF membrane. Membranes were blocked with PBS containing 10%
non-fat dry milk overnight at 4°C. After blocking, the membranes
were incubated with primary antibodies against FOXA1 (1:2,000; cat.
no. sc-101058; Santa Cruz Biotechnology, Inc.) or GAPDH (1:5,000;
cat. no. 97166; Cell Signaling Technology, Inc.) at 4°C overnight,
washed three times and incubated with a horseradish
peroxidase-conjugated secondary antibody (1:20,000; cat. no.
1036-05; SouthernBiotech) for 2 h at 25°C. Proteins were visualized
using an ECL substrate kit (GE Healthcare) and an ECL detection
system (GE Healthcare). Image-Pro Plus 6.0 software (Media
Cybernetics, Inc.) was used to quantify relative protein densities.
GAPDH was used as a loading control.
Luciferase reporter assay
miRNAs that interacted with the MALAT1 sequence were
predicted by LncBase Predicted v.2 software (http://carolina.imis.athena-innovation.gr/diana_tools/web/index.php?r=lncbasev2%2Findex-predicted)
(17) and miRcode version 11
(http://www.mircode.org/). The wild-type (WT) or
mutant (MUT) MALAT1 containing the putative target site for
miR-17-5p were chemically synthesized by Genewiz, Inc. and
subcloned into the psiCHECK2 vector (Promega Corporation) to
analyze the interaction between MALAT1 and miR-17-5p. miR-17-5p
mimics or inhibitors were co-transfected with the
psiCHECK2-WT-MALAT1 or MUT-MALAT1 vector. Luciferase activity was
measured 48 h after transfection using the Dual-Luciferase Reporter
Assay System (Promega Corporation), according to the manufacturer's
instructions. The ratio of firefly luciferase to Renilla
luciferase activity was calculated.
Statistical analysis
Statistical analyses were conducted using the SPSS
version 19.0 (IBM Corp). Each experiment was replicated three
times. Data are presented as the mean ± SD. Differences between two
groups were assessed using the independent sample t-test. Multiple
groups were compared using one-way ANOVA followed by Tukey's
post-hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
LPS promotes MALAT1 expression and
apoptosis, and inhibits the proliferation of A549 cells
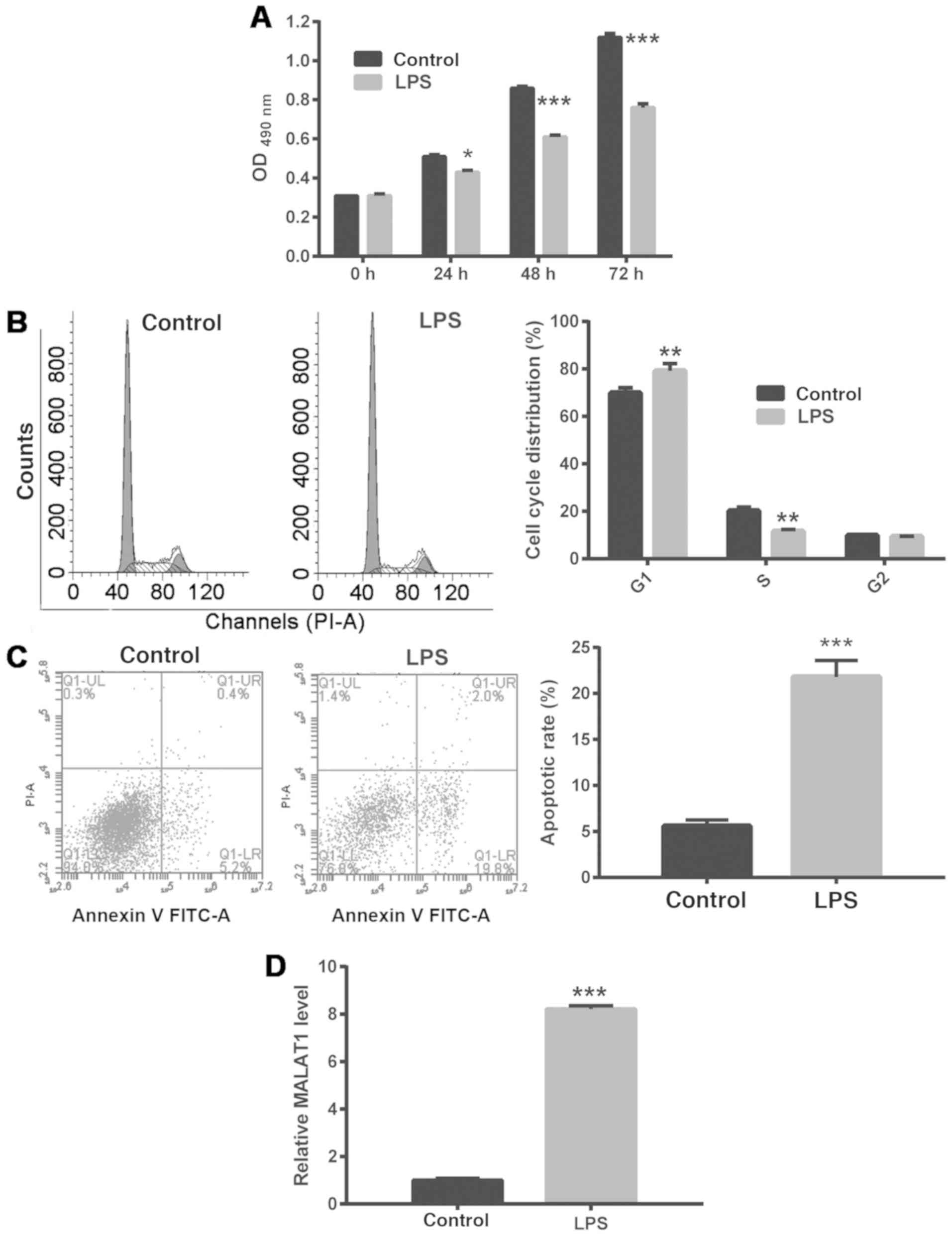
To explore the molecular mechanism underlying ALI,
A549 cells were treated with 1 µg/ml LPS to simulate ALI. It was
found that LPS treatment of A549 cells significantly inhibited
their proliferation, arrested them at the G1/S phase
checkpoint and promoted apoptosis (Fig. 1A-C). In addition, treatment with 1
µg/ml LPS significantly enhanced MALAT1 expression in A549 cells
(Fig. 1D).
Knockdown of MALAT1 reverses the
LPS-induced effects on proliferation and apoptosis in A549
cells
To investigate the biological role of MALAT1 on
proliferation and apoptosis in LPS-treated cells, A549 cells were
transfected with si-MALAT1 for 48 h and treated with or without 1
µg/ml LPS for 24 h. RT-qPCR results revealed that MALAT1 expression
was significant reduced in A549 cells transfected with si-MALAT1
compared with control cells, with or without 1 µg/ml LPS treatment
(Fig. 2A and B). Knockdown of
MALAT1 promoted the proliferation, G1/S phase transition
and suppressed apoptosis in LPS-treated A549 cells (Fig. 2C-E). Thus, MALAT1 knockdown
reversed the effects of LPS.
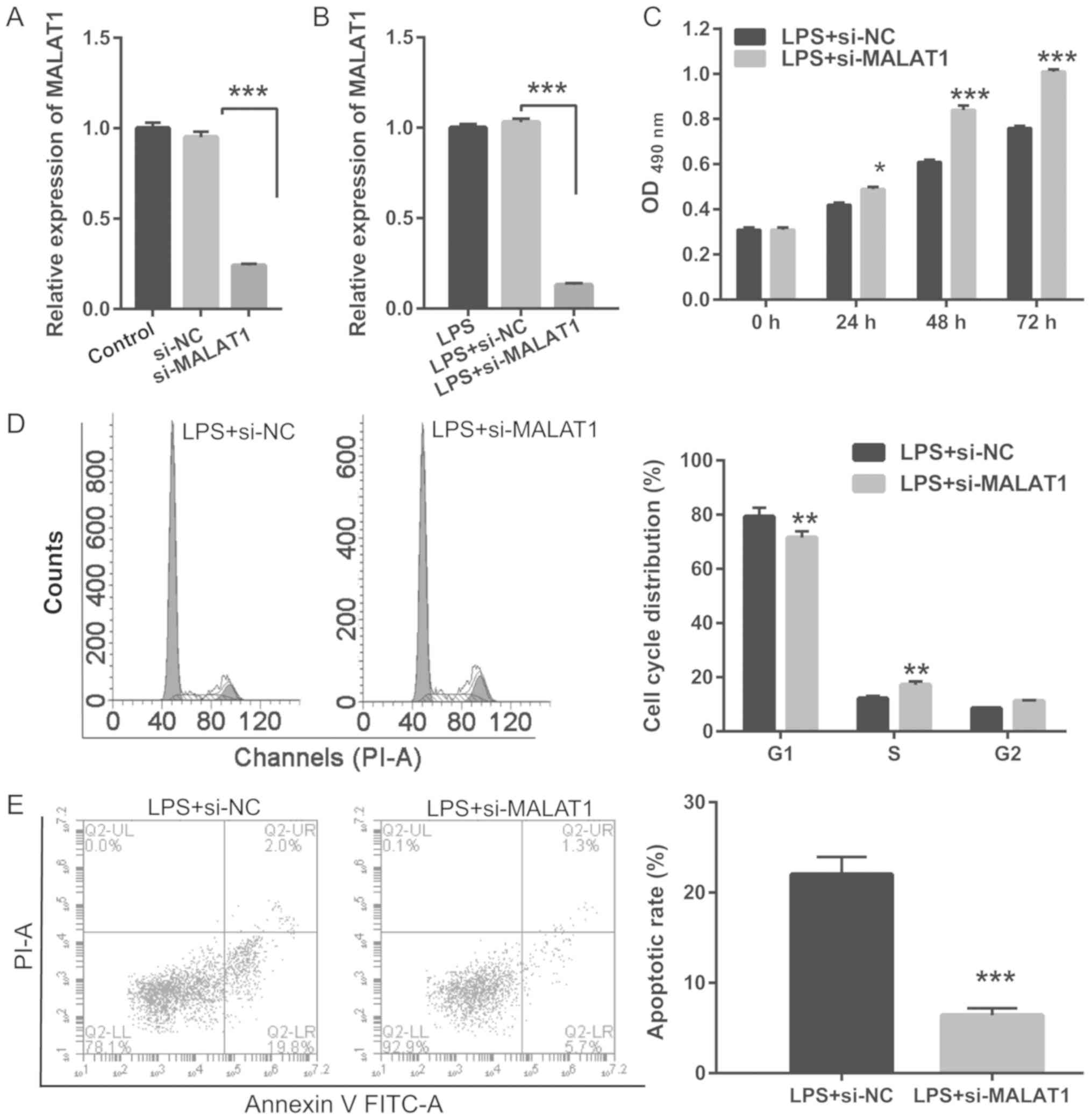 | Figure 2.MALAT1 knockdown reverses LPS-induced
A549 cell proliferation, inhibition of G1/S phase
transition and apoptosis. A549 cells transfected with si-MALAT1 or
si-NC at 48 h were induced with 1 µg/ml LPS for 24 h. (A) RT-qPCR
analysis of MALAT1 expression in A549 cells after si-MALAT1 or
si-NC transfection at 24 h. (B) RT-qPCR analysis of MALAT1
expression in 1 µg/ml LPS-treated A549 cells after si-MALAT1 or
si-NC transfection at 24 h. (C) Proliferation was assessed in A549
cells treated with 1 µg/ml LPS and transfected with si-MALAT1 or
si-NC at 24, 48 and 72 h. (D) Cell cycle analysis of A549 cells
treated with 1 µg/ml LPS and transfected with si-MALAT1 or si-NC
using flow cytometry at 24 h. (E) Apoptosis in A549 cells treated
with 1 µg/ml LPS and transfected with si-MALAT1 or si-NC using flow
cytometry at 24 h. *P<0.05, **P<0.01, ***P<0.001 vs.
respective controls. RT-qPCR, reverse transcription-quantitative
PCR; MALAT1, metastasis-associated lung adenocarcinoma transcript
1; si, small interfering RNA; NC, negative control; LPS,
lipopolysaccharide; OD, optical density; PI, propidium iodide. |
MALAT1 directly targets miR-17-5p to
regulate FOXA1 expression in LPS-treated A549 cells
LncBase Predicted v.2 and miRcode analysis allowed
for the identification of a putative miR-17-5p-binding site in the
MALAT1 sequence (Fig. 3A). To
determine if miR-17-5p is a direct target of MALAT1, a
dual-luciferase reporter assay was performed. miR-17-5p
overexpression significantly decreased the WT-MALAT1-associated
luciferase activity, whereas miR-17-5p inhibition significantly
increased the WT-MALAT1-associated luciferase activity. However,
miR-17-5p overexpression or inhibition had no effect on the
luciferase activity of MUT-MALAT1 (Fig. 3B). Furthermore, 1 µg/ml LPS
treatment for 24 h significantly inhibited the expression of
miR-17-5p (Fig. 3C) and promoted
the expression of FOXA1 (Fig. 3D and
E). MALAT1 knockdown significantly increased the expression
levels of miR-17-5p (Fig. 3F) and
decreased FOXA1 (Fig. 3G and H) in
A549 cells after 24 h exposure to LPS. These findings indicated
that MALAT1 knockdown reversed the LPS-induced effects on miR-17-5p
and FOXA1 expression in A549 cells.
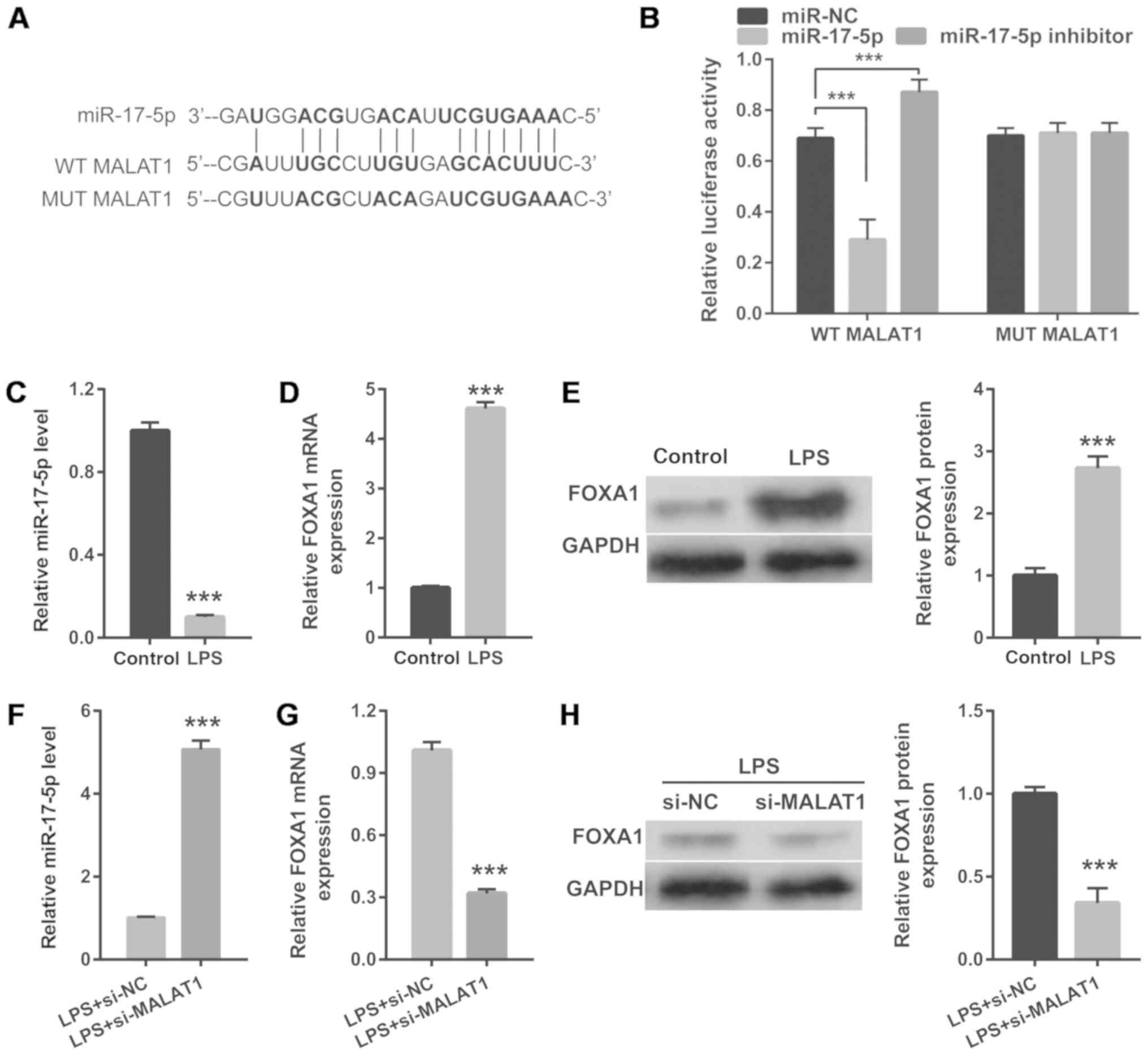 | Figure 3.MALAT1 directly targets miR-17-5p to
regulate miR-17-5p and FOXA1 expression. (A) The predicted binding
sites between MALAT1 and miR-17-5p. (B) A luciferase reporter assay
was conducted to detect luciferase activity after co-transfection
of A549 cells with WT-MALAT1 or MUT-MALAT1 and miR-NC, miR-17-5p
mimic or miR-17-5p inhibitor. (C) miR-17-5p expression in 1 µg/ml
LPS-treated A549 cells was measured using RT-qPCR analysis at 24 h.
(D) mRNA and (E) protein levels of FOXA1 in 1 µg/ml LPS-treated
A549 cells were measured using RT-qPCR and western blotting,
respectively, at 48 h. (F) miR-17-5p expression in 1 µg/ml
LPS-treated A549 cells transfected with si-MALAT1 was measured
using RT-qPCR at 24 h. FOXA1 (G) mRNA and (H) protein levels in 1
µg/ml LPS-treated A549 cells transfected with si-MALAT1 were
measured using RT-qPCR and western blotting, respectively, at 24 h.
***P<0.001. RT-qPCR, reverse transcription-quantitative PCR;
miR, microRNA; WT, wild-type; MALAT1, metastasis-associated lung
adenocarcinoma transcript 1; MUT, mutant; LPS, lipopolysaccharide;
FOXA1, forkhead box A1; si, small interfering RNA; NC, negative
control. |
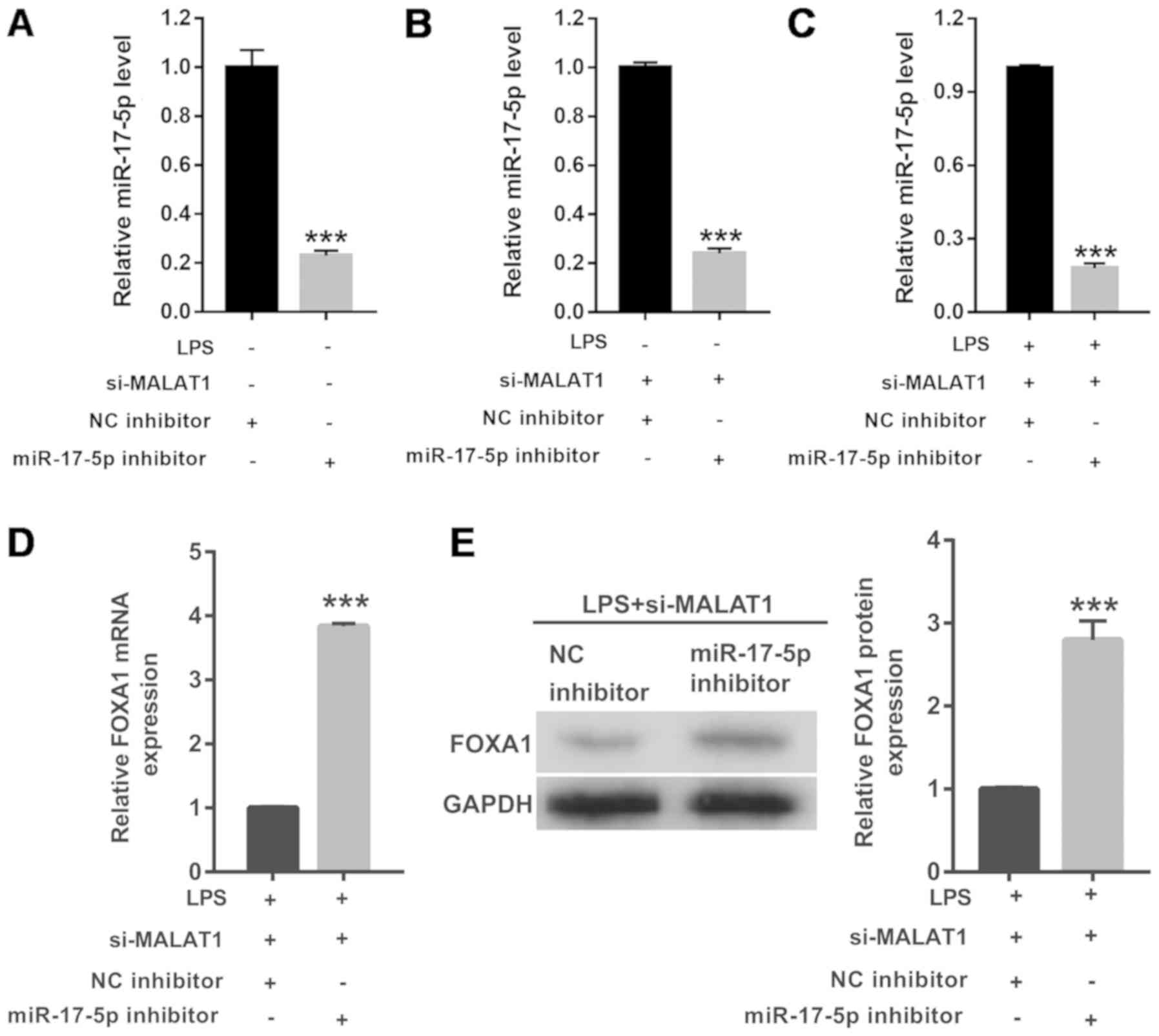
miR-17-5p inhibition reverses the
effects of MALAT1 knockdown on proliferation, cell cycle
progression and apoptosis in LPS-treated A549 cells
To further investigate the effects of MALAT1 on
LPS-treated A549 cells, the action of miR-17-5p expression was
suppressed using a specific inhibitor. RT-qPCR results revealed
that miR-17-5p expression was significantly reduced in cells
transfected with the miR-17-5p inhibitor compared with the NC
inhibitor (Fig. 4A). RT-qPCR
results also revealed that miR-17-5p expression was significantly
reduced in cells co-transfected with si-MALAT1 and miR-17-5p
inhibitor, compared with cells co-transfected with si-MALAT1 and NC
inhibitor, in A549 cells treated with or without 1 µg/ml LPS
(Fig. 4B and C). Both the mRNA and
protein levels of FOXA1 were significantly increased by miR-17-5p
inhibitor (Fig. 4D and E).
miR-17-5p inhibition also significantly inhibited cell
proliferation (Fig. 5A) and
G1/S phase transition (Fig.
5B and C), and promoted apoptosis (Fig. 5D and E), thus reversing the effects
of si-MALAT1 on the LPS-treated A549 cells.
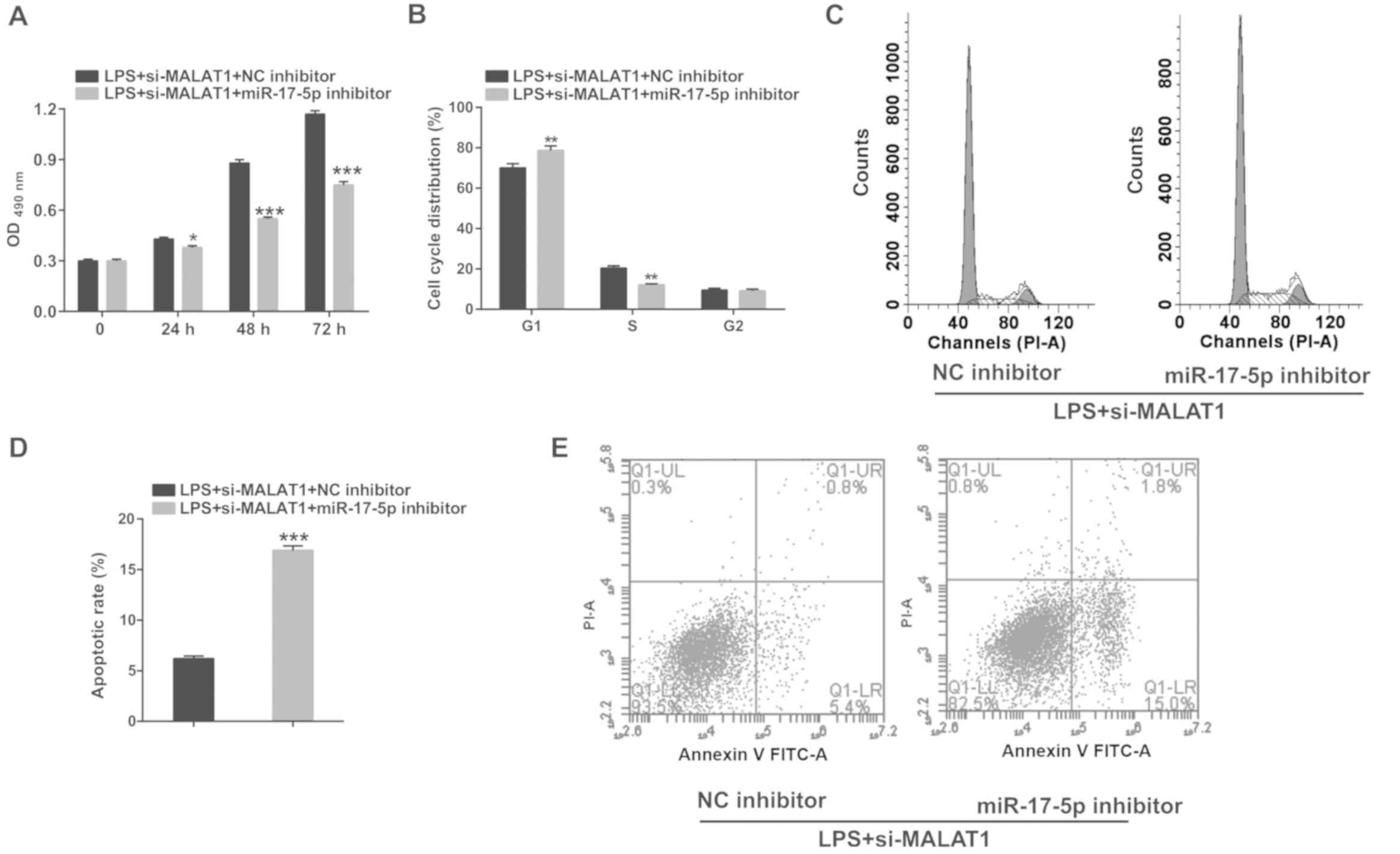 | Figure 5.Knockdown of miR-17-5p significantly
reverses the inhibitory effects of si-MALAT1 on proliferation,
G1/S phase transition and apoptosis in A549 cells
treated with 1 µg/ml LPS. A549 cells co-transfected with si-MALAT1
and miR-17-5p inhibitor (or NC inhibitor) were induced with 1 µg/ml
LPS for 24 h. (A) The effects of miR-17-5p inhibition on cell
proliferation were analyzed at 24, 48 and 72 h. The effects of
miR-17-5p knockdown on cell cycle progression were analyzed using
flow cytometry at 24 h. (B) Quantification of cell cycle analysis
and (C) a representative cell cycle plot. The effects of miR-17-5p
knockdown on apoptosis were analyzed using flow cytometry at 24 h.
(D) Quantification of apoptosis and (E) a representative flow
cytometry plot. *P<0.05, **P<0.01, ***P<0.001 vs.
respective control. LPS, lipopolysaccharide; MALAT1,
metastasis-associated lung adenocarcinoma transcript 1; miR,
microRNA; si, small interfering RNA; NC, negative control; PI,
propidium iodide. |
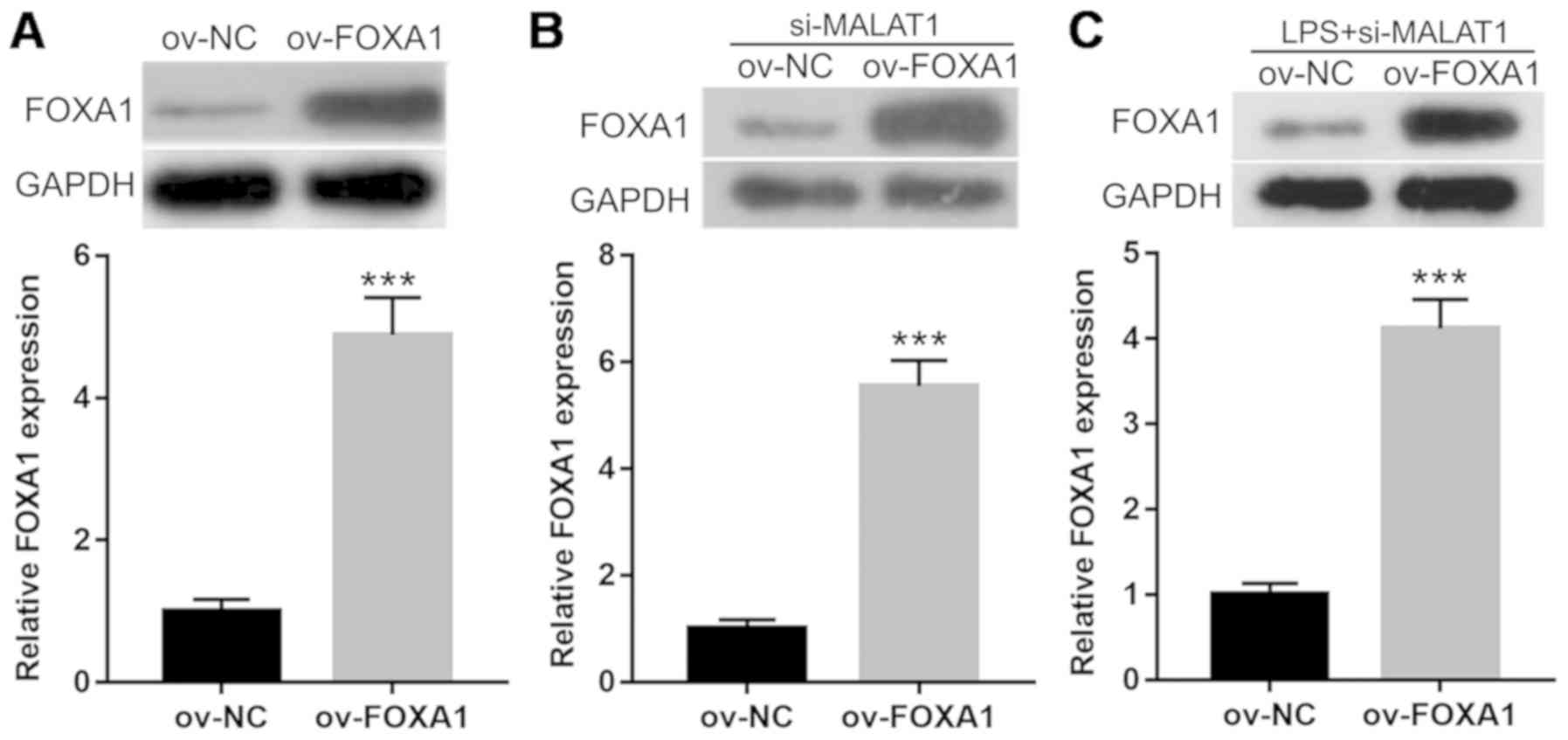
FOXA1 overexpression reverses the
effects of MALAT1 knockdown on proliferation, cell cycle
progression and apoptosis in LPS-treated A549 cells
A549 cells were co-transfected with si-MALAT1 and
ov-FOXA1 for 48 h, and then treated with 1 µg/ml LPS for 24 h to
investigate the effect of FOXA1 on MALAT1 expression. Western blot
analysis showed that the protein expression levels of FOXA1 were
significantly increased in cells transfected with ov-FOXA1 compared
with those transfected with ov-NC (Fig. 6A). Results from western blotting
analysis also showed that the protein expression levels of FOXA1
significantly increased in A549 cells co-transfected with si-MALAT1
and ov-FOXA1, compared with those co-transfected with si-MALAT1 and
ov-NC, in cells with or without 1 µg/ml LPS treatment (Fig. 6B and C). Furthermore, FOXA1
overexpression significantly inhibited cell proliferation (Fig. 7A) and G1/S phase transition
(Fig. 7B and C), and promoted
apoptosis (Fig. 7D and E), thus
reversing the effects of si-MALAT1 on LPS-treated A549 cells.
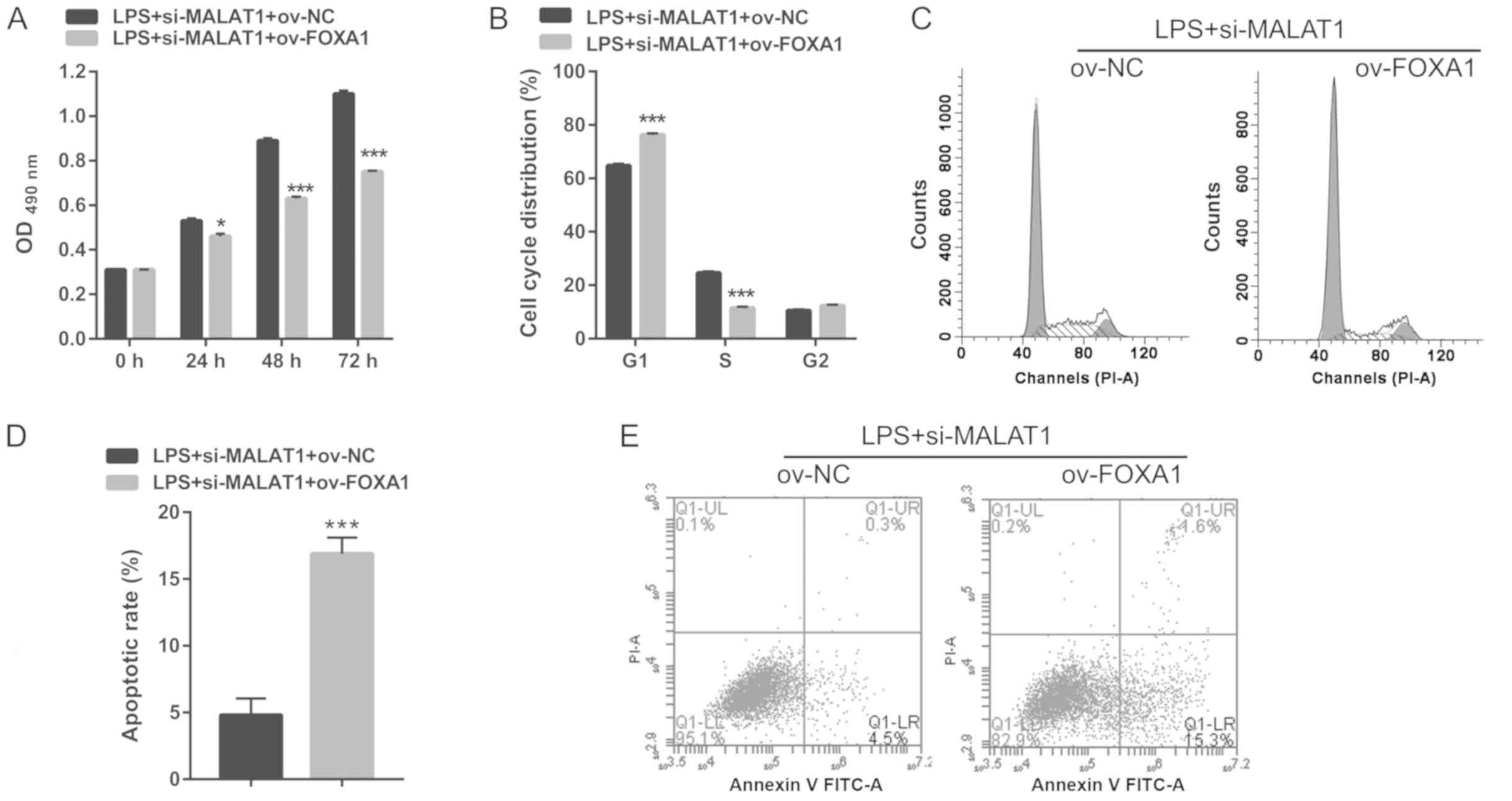 | Figure 7.FOXA1 overexpression significantly
reverses the inhibitory effects of si-MALAT1 on proliferation,
G1/S phase transition and apoptosis in A549 cells
treated with 1 µg/ml LPS. A549 cells were co-transfected with
si-MALAT1 and ov-FOXA1 (or ov-NC) then treated with 1 µg/ml LPS for
24 h. (A) The effects of FOXA1 overexpression on cell proliferation
were analyzed at 24, 48 and 72 h. B and D: The effects of FOXA1
overexpression on cell cycle were analyzed with flow cytometry at
24 h. (B) Quantification of cell cycle analysis and (C) a
representative cell cycle plot. C and E: The effects of FOXA1
overexpression on apoptosis were analyzed with flow cytometry at 24
h. (D) Quantification of apoptosis and (E) a representative flow
cytometry plot. *P<0.05, ***P<0.001 vs. respective control.
OD, optical density; LPS, lipopolysaccharide; si, small interfering
RNA; MALAT1, metastasis-associated lung adenocarcinoma transcript
1; ov, overexpression plasmid; NC, negative control; FOXA1,
forkhead box A1; PI, propidium iodide. |
Discussion
LncRNAs have an important role in the
pathophysiology of various human diseases, including ALI (18). MALAT1 is known to modulate
inflammatory responses via miR-146a in LPS-induced ALI (9). However, the molecular mechanism
underlying the MALAT1-mediated regulation of cell proliferation and
apoptosis in this disease remains unclear. In the present study, it
was found that LPS treatment markedly upregulated the expression of
MALAT1, suppressed proliferation, and promoted cell cycle arrest
and apoptosis in A549 cells. MALAT1 knockdown reversed these
effects. In addition, miR-17-5p as identified as a direct target of
MALAT1. The knockdown of miR-17-5p reversed the effects of
MALAT1-mediated suppression on proliferation, cell cycle
progression and apoptosis in LPS-treated A549 cells. These data
revealed that the MALAT1/miR-17-5p/FOXA1 axis underlies LPS-induced
A549 cell injury.
MALAT1 has an important role in cell proliferation
and apoptosis. Previous studies have revealed that the upregulation
of MALAT1 in LPS-treated cells inhibits cell proliferation, and
promotes apoptosis and proinflammatory cytokine expression, in
acute kidney injury and neonatal respiratory distress syndrome
(8,19). Dai et al (9) revealed that MALAT1 expression was
upregulated in the lung tissues of LPS-induced ALI rats and that
the knockdown of MALAT1 attenuated this inflammatory response. Li
et al (20) reported that
suppression of MALAT1 expression alleviates ALI. Consistent with
these findings, the present study observed that LPS treatment
upregulated MALAT1 expression, suppressed proliferation and
promoted apoptosis in A549 cells. Moreover, MALAT1 knockdown
promoted cell proliferation and G1/S phase transition,
and inhibited apoptosis of LPS-treated A549 cells. These results
showed that the knockdown of MALAT1 alleviated LPS-induced A549
cell injury through the restoration of cell proliferation and
prevention of apoptosis. These findings, along with those reported
by Dai et al (9),
demonstrate the regulatory function of MALAT1 in multiple processes
of ALI, including proliferation, cell cycle progression, apoptosis
and inflammatory responses.
MALAT1 serves as an endogenous miRNA sponge and
negatively regulates miRNA expression. Previous studies have
demonstrated that MALAT1 functions as a ceRNA by sequestering
miR-19b, miR-199b and miR-146a to regulate LPS-induced murine
chondrogenic cell inflammatory injury, acute spinal cord injury,
and LPS-induced acute kidney injury, respectively (8,21,22).
Dai et al (9) found that
MALAT1 knockdown attenuated inflammatory responses by sequestering
miR-146a in ALI. In the present study, miR-17-5p was identified as
a novel potential target of MALAT1 by bioinformatic analyses. The
results of a luciferase reporter assay demonstrated the direct
interaction between MALAT1 and miR-17-5p, indicating that miR-17-5p
is a direct target of MALAT1. A previous study reported a marked
increase in the expression of miR-17-5p in LPS-induced nasal
epithelial cells, which was related to the SMAD7-dependent increase
in the severity of LPS-induced nasal epithelial cell injury
(23). miR-17-5p downregulation
causes FOXA1 overexpression and promoted alveolar type II
epithelial cell apoptosis in LPS-induced ALI (13). Song et al (14) found an increase in the expression
of FOXA1 in lung tissues, wherein it serves as a proapoptotic
transcription factor. Furthermore, miR-17-5p could also target
FOXA1 to inhibit apoptosis in LPS-induced ALI. In the present
study, it was found that miR-17-5p expression was downregulated and
FOXA1 expression was upregulated in LPS-treated A549 cells,
consistent with the results of a previous report (13). Moreover, the knockdown of MALAT1
increased the expression level of miR-17-5p and inhibited the
expression of FOXA1, whereas the simultaneous suppression of MALAT1
and miR-17-5p promoted FOXA1 expression. These results indicated
that MALAT1 functions as a sponge for miR-17-5p and regulates FOXA1
expression. miR-17-5p inhibitor or FOXA1 overexpression reversed
the effects of si-MALAT1 on proliferation, cell cycle progression
and apoptosis of LPS-treated A549 cells. Thus, the knockdown of
MALAT1 promoted cell proliferation and inhibited apoptosis through
the upregulation of miR-17-5p, and downregulation of FOXA1, in
LPS-induced ALI. The results of the present study and those of Dai
et al (9) showed that
MALAT1 regulates cell proliferation, cell cycle progression,
apoptosis and inflammation based on its miRNA sequestering
activity.
In conclusion, the present study demonstrated that
the knockdown of MALAT1 alleviates LPS-induced injury in A549 cells
by targeting the miR-17-5p/FOXA1 axis. These findings suggested
that MALAT1 plays an important role in LPS-induced injury in A549
cells and may serve as a novel therapeutic target. Further studies
are warranted to evaluate the role of MALAT1 in vivo and
verify the impact of MALAT1 suppression on ALI pathogenesis.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Medical
Scientific Research Foundation of Guangdong Province, China (grant
no. A2015385).
Availability of data and materials
Datasets analyzed during the present study are
available from the corresponding author on reasonable request.
Authors' contributions
SW and ZiZ conceived and designed the present study,
and developed the methodology. SW, KW and XH performed the
experiments and collected the data. SW, WT and ZhZ analyzed and
interpreted the data. SW and ZiZ drafted the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mokra D and Kosutova P: Biomarkers in
acute lung injury. Respir Physiol Neurobiol. 209:52–58. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Villar J, Sulemanji D and Kacmarek RM: The
acute respiratory distress syndrome: Incidence and mortality, has
it changed? Curr Opin Crit Care. 20:3–9. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li H, Shi H, Gao M, Ma N and Sun R: Long
non-coding RNA CASC2 improved acute lung injury by regulating
miR-144-3p/AQP1 axis to reduce lung epithelial cell apoptosis. Cell
Biosci. 8:152018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu L, Sun L, Hua Y, Yang C and Teng Y:
Overexpression of long non-coding RNA H19 protects lung fibroblasts
from LPS-induced injury by targeting miR-181a and Runx2 via
activation of Notch and JNK pathways. J Cell Biochem. Jan
8–2018.(Epub ahead of print).
|
|
5
|
Zhou WQ, Wang P, Shao QP and Wang J:
Lipopolysaccharide promotes pulmonary fibrosis in acute respiratory
distress syndrome (ARDS) via lincRNA-p21 induced inhibition of
Thy-1 expression. Mol Cell Biochem. 419:19–28. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gutschner T, Hämmerle M, Eissmann M, Hsu
J, Kim Y, Hung G, Revenko A, Arun G, Stentrup M, Gross M, et al:
The noncoding RNA MALAT1 is a critical regulator of the metastasis
phenotype of lung cancer cells. Cancer Res. 73:1180–1189. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hu M, Wang R, Li X, Fan M, Lin J, Zhen J,
Chen L and Lv Z: LncRNA MALAT1 is dysregulated in diabetic
nephropathy and involved in high glucose-induced podocyte injury
via its interplay with β-catenin. J Cell Mol Med. 21:2732–2747.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ding Y, Guo F, Zhu T, Li J, Gu D, Jiang W,
Lu Y and Zhou D: Mechanism of long non-coding RNA MALAT1 in
lipopolysaccharide-induced acute kidney injury is mediated by the
miR-146a/NF-κB signaling pathway. Int J Mol Med. 41:446–454.
2018.PubMed/NCBI
|
|
9
|
Dai L, Zhang G, Cheng Z, Wang X, Jia L,
Jing X, Wang H, Zhang R, Liu M, Jiang T, et al: Knockdown of LncRNA
MALAT1 contributes to the suppression of inflammatory responses by
up-regulating miR-146a in LPS-induced acute lung injury. Connect
Tissue Res. 59:581–592. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Denzler R, Agarwal V, Stefano J, Bartel DP
and Stoffel M: Assessing the ceRNA hypothesis with quantitative
measurements of miRNA and target abundance. Mol Cell. 54:766–776.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tang R, Pei L, Bai T and Wang J:
Down-regulation of microRNA-126-5p contributes to overexpression of
VEGFA in lipopolysaccharide-induced acute lung injury. Biotechnol
Lett. 38:1277–1284. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li W, Qiu X, Jiang H, Han Y, Wei D and Liu
J: Downregulation of miR-181a protects mice from LPS-induced acute
lung injury by targeting Bcl-2. Biomed Pharmacother. 84:1375–1382.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu Z, Zhang C, Cheng L, Hu M, Tao H and
Song L: The microRNA miR-17 regulates lung FoxA1 expression during
lipopolysaccharide-induced acute lung injury. Biochem Biophys Res
Commun. 445:48–53. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song L, Zhang B, Feng Y, Luo X, Wei X and
Xiao X: A role for forkhead box A1 in acute lung injury.
Inflammation. 32:322–332. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Luo Y, Che W and Zhao M: Ulinastatin
post-treatment attenuates lipopolysaccharide-induced acute lung
injury in rats and human alveolar epithelial cells. Int J Mol Med.
39:297–306. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Paraskevopoulou MD, Vlachos IS, Karagkouni
D, Georgakilas G, Kanellos I, Vergoulis T, Zagganas K, Tsanakas P,
Floros E, Dalamagas T and Hatzigeorgiou AG: DIANA-LncBase v2:
Indexing microRNA targets on non-coding transcripts. Nucleic Acids
Res. 44(D1): D231–D238. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bolha L, Ravnik-Glavač M and Glavač D:
Long noncoding RNAs as biomarkers in cancer. Dis Markers.
2017:72439682017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Juan C, Wang Q, Mao Y, Cao Q, Li S, Qiao
C, Zhang D and Zhou G: Knockdown of LncRNA MALAT1 contributes to
cell apoptosis via regulating NF-κB/CD80 axis in neonatal
respiratory distress syndrome. Int J Biochem Cell Biol.
104:138–148. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li H, Shi H, Ma N, Zi P, Liu Q and Sun R:
BML-111 alleviates acute lung injury through regulating the
expression of lncRNA MALAT1. Arch Biochem Biophys. 649:15–21. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pan L, Liu D, Zhao L, Wang L, Xin M and Li
X: Long noncoding RNA MALAT1 alleviates lipopolysaccharide-induced
inflammatory injury by upregulating microRNA-19b in murine
chondrogenic ATDC5 cells. J Cell Biochem. 119:10165–10175. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou HJ, Wang LQ, Wang DB, Yu JB, Zhu Y,
Xu QS, Zheng XJ and Zhan RY: Long noncoding RNA MALAT1 contributes
to inflammatory response of microglia following spinal cord injury
via the modulation of a miR-199b/IKKβ/NF-κB signaling pathway. Am J
Physiol Cell Physiol. 315:C52–C61. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang N, Li W, Wang X and Qi S:
MicroRNA-17-5p aggravates lipopolysaccharide-induced injury in
nasal epithelial cells by targeting Smad7. BMC Cell Biol. 19:12018.
View Article : Google Scholar : PubMed/NCBI
|