Introduction
Cadmium, an environmental pollutant, seriously
affects public health worldwide. A large number of studies have
shown that cadmium exerts multi-organ and multi-system toxicity,
and that it is able to produce carcinogenic, orthodontic and
mutagenic effects, for example, muscle wastage, hemolysis,
immunosuppression, and a decrease in fertility (1–4).
‘Itai-itai’ disease was first known to the world after
mining-related cadmium poisoning in Japan in 1955 (5,6).
Bone is a main target organ for cadmium toxicity. Previous studies
have indicated that cadmium may damage osteoblasts in culture by
decreasing bone calcium and prostaglandin 2 (PGE2) levels (7–9). It
has also been confirmed that cadmium affects bone metabolism both
directly and indirectly (10).
Researchers also demonstrated that the osteotoxicity of cadmium may
be caused by alterations to vitamin D metabolism and disruption of
the balance of calcium absorption and excretion (11,12).
Moreover, cadmium damage directly increases the risk of bone
fracture and osteoporosis, and cadmium can affect the activation of
osteoclasts and osteoblasts, leading to imbalance between bone
resorption and formation (13–18).
Under normal circumstances, the body or cells
constantly produce free radicals, while the antioxidant system
scavenges these free radicals. Such a dynamic balance maintains a
stable metabolism in contrast to the condition in which an
imbalance would cause free radical accumulation and lipid
peroxidation (19). Cadmium not
only induces the initiation of oxidative damage, produces lipid
peroxide, destroys the intracellular state of redox equilibrium,
but also interferes with the function of the antioxidant system.
Cadmium mainly mediates oxidative stress through an indirect
reaction pathway, largely by reducing the level of antioxidants in
cells and mediating mitochondrial functional damage, increasing the
production of reactive oxygen species (ROS) (20–23).
Therefore, the toxic effects of cadmium on osteoblasts may be the
result of oxidative stress and ROS levels.
Geniposide, a type of iridoid glycoside, is the main
active component of Gardenia jasminoides (Rubiaceae).
Geniposide is considered to have anti-inflammatory, antioxidant
activity as well as antitumor properties (24–28).
Researchers have reported that geniposide also exhibits effects on
brain by reducing inflammatory response of microglial cells and
protecting the neural tissue from cerebral ischemia (29,30);
and on digestive system diseases, namely by suppressing
helicobacter pylori infections (31). Geniposide activates osteoblasts to
facilitate osteogenesis, and suppresses osteoclast activity and
inhibits bone resorption (32). In
addition, geniposide may promote the growth of osteoblast MC3T3-E1
cells, and suppress H2O2-induced apoptosis
(33). To the best of our
knowledge, current investigations have focused heavily on the
antioxidative capacity of geniposide. Recent studies have shown
that geniposide protected PC12 cells from oxidative damage through
its radical scavenging activity (34,35).
Geniposide was also found to protect against oxygen and glucose
deprivation-induced neuronal cell death in rat hippocampal slice
cultures (36). Thus, it was
speculated that geniposide may protect osteoblasts from oxidative
stress induced by cadmium.
The present study aimed to determine the protective
effects of geniposide against cadmium-induced osteoblast
(MC-3T3-E1) injury, and to investigate its underlying protective
mechanisms with a focus on oxidative stress.
Materials and methods
Reagents
Geniposide (purity >98%) was purchased from
Pure-one Bio Technology, Co., Ltd (Shanghai, China). Geniposide was
dissolved in water, pH 7.4. Cadmium chloride (CdCl2) was
purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany).
Cell culture and morphological
observation
Rat MC-3T3-E1 cells (Riken Cell Bank, Tsukuba,
Ibaraki, Japan) were cultured in Dulbecco's modified Eagle's medium
(DMEM; Thermo Fisher Scientific, Inc., Waltham, MA, USA) with 10%
(v/v) fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.)
and 100 U/ml penicillin (or 100 µg/ml streptomycin) in a 37°C
incubator with 5% CO2 humidified atmosphere. The
morphology of primary cultured MC-3T3-E1 cells was observed using
an inverted microscope (×40).
Cell Counting Kit-8 (CCK-8) assay
The CCK-8 assay kit (Beyotime Institute of
Biotechnology, Haimen, China) was used to measure cell viability.
MC-3T3-E1 cells (5×103 cells/well) were cultured in
96-well plates and were treated with CdCl2 (0–20 µM).
Geniposide (100, 200 and 400 µg/ml) was used as previously
described (37) to treat the cells
in order to detect its effect on CdCl2-induced
injury.
For the cell viability assay, 10 µl CCK-8 solution
was added into each well, and the cells were incubated for another
3 h at 37°C. Cell viability was determined using a microplate
reader as previously described (38) by reading the optical density at a
wavelength of 450 nm, and at a reference wavelength of 630 nm.
Flow cytometry
Cell apoptosis was detected in MC-3T3-E1 cell
cultures using a flow cytometer. The cells were harvested and
re-suspended in Annexin binding buffer at 1×105
cells/ml. Then, the suspension was incubated with Annexin V-FITC
and propidium iodide (PI) [cat. no. 70-AP101-60; MultiSciences
(Lianke) Biotech Co., Ltd., Hangzhou, China] in the dark for 15 min
at 4°C. The apoptosis of the cell samples was analyzed by flow
cytometry with BD CellQuest Pro Software version 1.2 (BD
Biosciences, San Jose, CA, USA).
The ROS levels were measured using
2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) as previously
described (39). DCFH-DA
(Sigma-Aldrich; Merck KGaA), without fluorescence, can enter the
cell membrane and form DCFH in the cell. DCFH is then oxidized to
form a fluorescent substance DCF in the presence of ROS. MC-3T3-E1
cells were stained with DCFDA and held for 30 min at room
temperature. Finally, DCF fluorescence levels were measured by flow
cytometry and the data were analyzed using Summit Software (version
4.3; Dako; Agilent Technologies, Inc., Santa Clara, CA, USA).
Enzyme-linked immunosorbent assay
(ELISA)
Oxidative stress-related factors malondialdehyde
(MDA; cat. no. ml077384; Enzyme-linked Biotechnology Co., Ltd.,
Shanghai, China), lactate dehydrogenase (LDH; cat. no. ml076593;
Enzyme-linked Biotechnology Co., Ltd.) and superoxidase dismutase
(SOD; cat. no. ml077379; Enzyme-linked Biotechnology Co., Ltd.)
were measured using ELISA. MC-3T3-E1 cells were seeded on a 24-well
plate, and cell-free supernatants were harvested after 3 h. The
concentrations of MDA, LDH and SOD in the supernatants of MC-3T3-E1
cells were determined using ELISA kits following the manufacturer's
instructions.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was performed for the purpose of examining
gene expression profiles of Bax, Bcl-2, survivin, NF-κB ligand
(RANKL), osteoprotegerin (OPG), osterix, nuclear factor erythroid
2-related factor (Nrf2), heme oxygenase-1 (HO-1) and NAD(P)H
quinone dehydrogenase 1 (NQO1). Total RNA was extracted using
TRIzol® regent (Invitrogen; Thermo Fisher Scientific,
Inc.) following the manufacturer's instructions. RNA was reverse
transcribed into cDNA using GoScript™ RT kit (Promega Corporation,
Madison, WI, USA). The RT temperature protocol consisted of 37°C
for 15 min and at 85°C for 5 sec. RT-qPCR was conducted using SYBR
Fast qPCR Mix (Invitrogen; Thermo Fisher Scientific, Inc.) The
thermocycling conditions were: 94°C for 3 min for an initial
denaturation, followed by 30 denaturation cycles at 94°C for 5 sec,
annealing and elongation at 60°C for 30 sec; and final extension at
72°C for 10 min. The primer sequences are summarized in Table I. The quantity of RNA was
calculated using the 2−ΔΔCq method (40), and the level of expression of an
RNA was normalized to GAPDH (denoted ‘relative expression’).
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene | Primer sequence
(5′-3′) |
|---|
| Bax | Forward:
TTCATCCAGGATCGAGCAGAG |
|
| Reverse:
TGAGGACTCCAGCCACAAAGAT |
| Bcl-2 | Forward:
CTGGTGGACAACATCGCTCTG |
|
| Reverse
GGTCTGCTGACCTCACTTGTG |
|
Survivin | Forward:
CCCTGCCTGGCAGCCCTTTC |
|
| Reverse:
CTGGCTCCCAGCCTTCCA |
| RANKL | Forward:
TCGGGTTCCCATAAAGTC |
|
| Reverse:
GAAGCAAATGTTGGCGTA |
| OPG | Forward:
GCAGCATCGCTCTGTTCCTGTA |
|
| Reverse:
ATGGTGGTGAAGACGCCAGTA |
| Osterix | Forward:
GCCTACTTACCCGTCTGACTTT |
|
| Reverse:
GCCCACTATTGCCAACTGC |
| Nrf2 | Forward:
GCCAGCTGAACTAATTAGAC |
|
| Reverse:
GATTCGTGCACAGCAGCA |
| HO-1 | Forward:
TTGTCTCTCTGGAATGGAAGG |
|
| Reverse:
CTCTACCGACCATTCTG |
| NQO1 | Forward:
CATTCTGAAAGGCTGGTTTGA |
|
| Reverse:
CTAGCTTTGATCTGGTTGTCAG |
| GAPDH | Forward:
GGCACAGTCAAGGCTGAGAATG |
|
| Reverse:
ATGGTGGTGAAGACGCCAGTA |
Western blot analysis
MC-3T3-E1 cells were washed three times with PBS,
and detached from the dishes by scraping. Cells were centrifuged at
12,000 × g for 5 min at 4°C and re-suspended in RIPA lysis buffer
(Thermo Fisher Scientific, Inc.) with phenylmethanesulfonyl
fluoride (PMSF) at 1:200 dilution. The homogenate was centrifuged
at 12,000 × g for 10 min at 4°C. Protein concentrations were
quantified using a bicinchoninic acid protein assay kit (Beyotime
Institute of Biotechnology). Equivalent amounts of total protein
(20 µg/lane) were loaded on a 10% Tris-glycine, 10% SDS-PAGE
(Beyotime Institute of Biotechnology) for separation. Proteins were
then transferred onto a polyvinylidene difluoride membrane, which
were blocked with 5% milk in TBS containing 0.2% Tween-20 (TBST) at
room temperature for 2 h, and incubated with primary antibodies as
follows: Rabbit anti-Bcl-2 (cat. no. ab32124, 1:1,000) anti-Bax
(cat. no. ab32503, 1:1,000), anti-survivin (cat. no. ab76424,
1:1,000); rabbit anti-RANKL (cat. no. ab9957, 1:1,000), anti-OPG
(cat. no. ab73400, 1:1,000), anti-osterix (cat. no. ab94744,
1:1,000); mouse anti- HO-1 antibody (ab13248, 1:1,000), NQO1
antibody (ab34173, 1:1,000), Nrf2 antibody (ab137550, 1:1,000) and
anti-GAPDH (ab9485, 1:1,000) overnight at 4°C, all purchased from
Abcam (Cambridge, UK). The membranes were washed with TBST, and
then incubated with horseradish peroxidase-conjugated secondary
antibodies goat anti-rabbit (cat. no. ab205718; 1:2,000; Abcam) and
goat anti-mouse (cat. no. ab205719; 1:5,000; Abcam) at 4°C for 1 h.
The blots were visualized using enhanced chemiluminescence (ECL;
Thermo Fisher Scientific, Inc.). An ECL system (Amersham; GE
Healthcare, Chicago, IL, USA) was used to detect the bands.
Quantity one software version 4.6.2 (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) was used for densitometry analysis.
Statistical analysis
GraphPad Prism version 6.0 software (GraphPad
Software, Inc., La Jolla, CA, USA) was used for conducting
statistical analysis. Data are presented as the mean ± standard
deviation. Statistical significance was analyzed using one-way
analysis of variance, followed by Turkey's multiple comparison
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
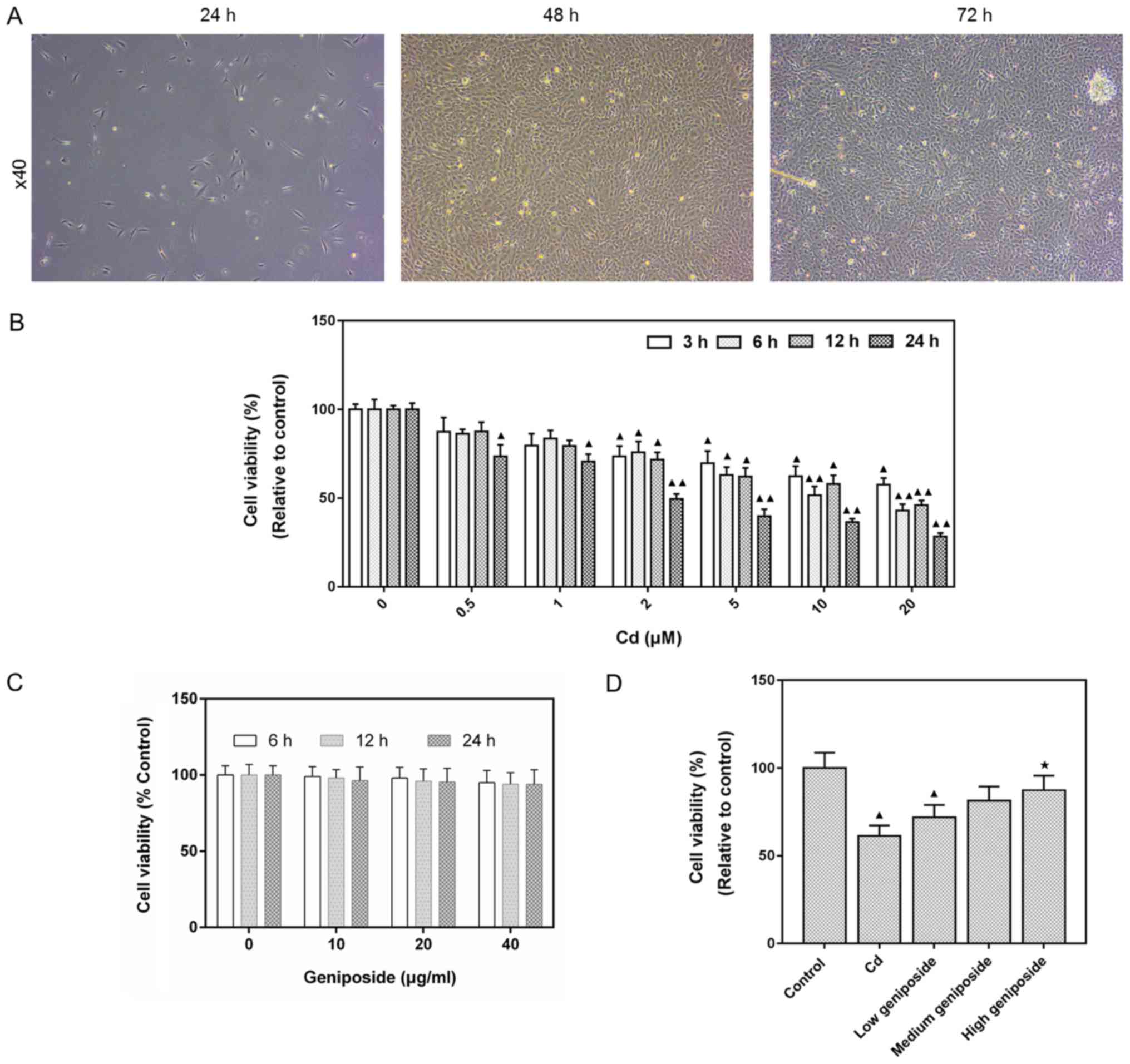
Morphological observation of MC-3T3-E1
osteoblasts
After MC-3T3-E1 cells were inoculated and cultured
for 24 h, the growth of adherent cells was observed using an
inverted microscope (Fig. 1A). The
cells showed a fibroblast-like appearance. To be more specific, the
cells appeared to be irregular fusi-formed, triangular, or
polygonal, and no fusion between cells was identified. As the
duration of the culture time prolonged, cell bodies grew larger and
the cell morphology was stretched. It appeared fiber bundles,
triangles and polygons with more protuberances. Some elongated
protuberances were often found to link cells with distant
protuberant cells and to increase cell-to-cell contact. Meanwhile,
the number of cells was found to increase, and cells were mostly
spindle-shaped or cubic.
Protective effect of geniposide on
CdCl2-injured MC-3T3-E1 cells
The cytotoxic effects of different CdCl2
concentrations (0–20 µM) at different time points (3, 6, 12 and 24
h) in MC-3T3-E1 cells were determined using CCK-8 assay. The
results showed that CdCl2 decreased MC-3T3-E1 cell
viability in time- and dose-dependent manners (20 µM, 3 h,
P<0.05; 20 µM, 24 h, P<0.01; Fig. 1B). Based on this findings, 20 µM of
CdCl2 was employed in all subsequent experiments.
Moreover, the cytotoxic effects of geniposide were also detected,
and that geniposide had no cytotoxic effects at a concentration of
100–400 µg/ml (Fig. 1C). As showed
in Fig. 1D, geniposide was able to
ameliorate the CdCl2 injury in cells and increase
viability in a dose-dependent manner, and treatment using 400 µg/ml
geniposide significantly increased cell viability (P<0.05).
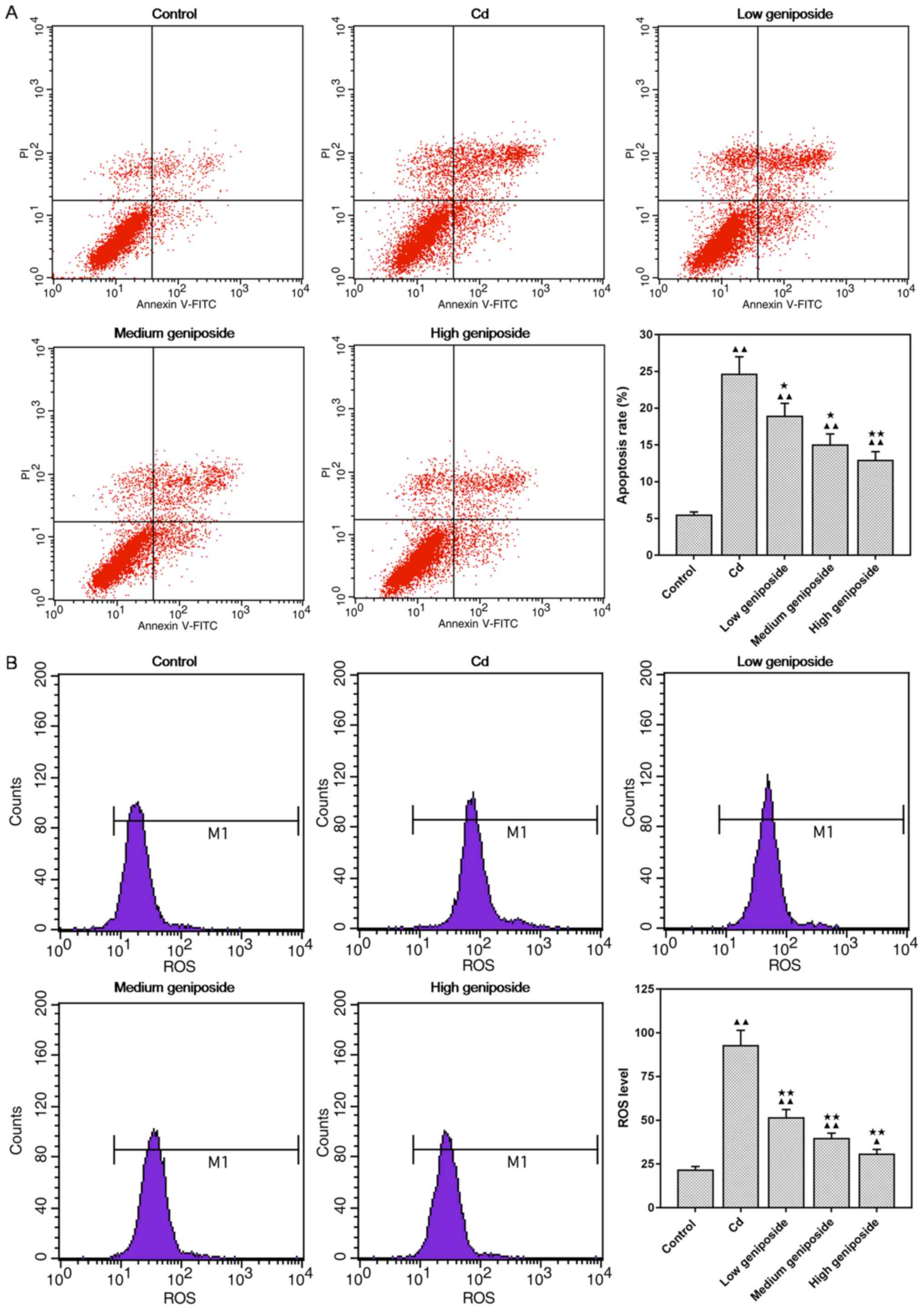
Geniposide decreases the apoptosis
induced by CdCl2 in MC-3T3-E1
As showed in Fig.
2A, the effect of geniposide on CdCl2-induced
apoptosis was investigated using flow cytometric analysis. We found
that the apoptosis induced by CdCl2 was significantly
decreased in a concentration-dependent manner after being
pretreated with geniposide (100 and 200 µg/ml: P<0.05, 400
µg/ml: P<0.01).
Geniposide decreases the ROS level in
CdCl2-injured MC-3T3-E1 cells
As showed in Fig.
2B, CdCl2 exposure increased the ROS generation in
MC-3T3-E1 cells (P<0.01). However, pretreatment of MC-3T3-E1
cells with geniposide significantly decreased the generation of ROS
in a dose-dependent manner (P<0.01).
Geniposide affects MDA, LDH and
antioxidant enzyme SOD activities in CdCl2-injured
MC-3T3-E1 cells
As shown in Fig. 3,
the effects of geniposide on CdCl2-induced oxidative
stress-related factors were assessed using ELISA assay. We found
that the level of MDA was not significantly increased by exposure
of the cells to CdCl2, and that pretreatments at
different concentrations of geniposide also showed no significant
effect on the level of MDA (P>0.05, Fig. 3A). However, LDH was significantly
increased following exposure of the cells with CdCl2. By
contrast, a medium concentration of geniposide pretreatment
significantly decreased the level of LDH, compared to the cells
incubated with CdCl2 (P<0.05, Fig. 3B). Our results also showed that
antioxidant key enzyme SOD was decreased following CdCl2
exposure, but SOD was significantly higher following pretreatment
with a high concentration of geniposide (P<0.05, Fig. 3C).
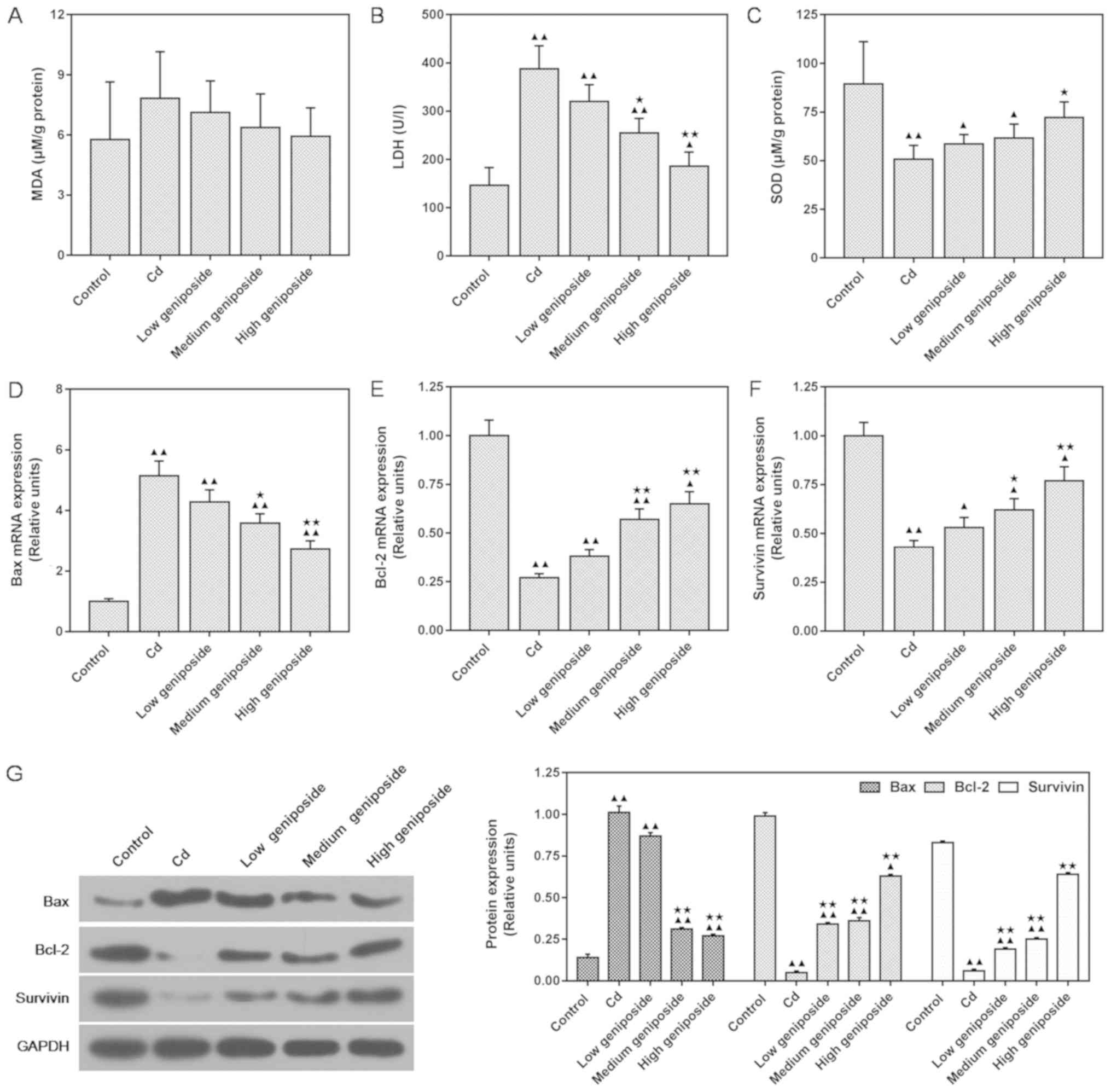 | Figure 3.Effects of different concentrations
of geniposide on CdCl2 (Cd)-induced expression of MDA,
LDH and SOD and the mRNA and protein levels of Bax, Bcl-2 and
survivin. Cells were pretreated with geniposide [100 (low), 200
(medium), 400 µg/ml (high)] for 24 h, followed by exposure to
CdCl2 (20 µM) for 3 h. (A-C) Oxidative stress-related
factors (MDA, LDH, SOD) were assessed by ELISA assay. (D-F) Reverse
transcription-quantitative PCR was used to determine the mRNA
expression of Bax, Bcl-2 and survivin. (G) Western blotting results
and relative units of protein levels. Expression of each protein in
the control or geniposide-pretreated MC-3T3-E1 cells following
normalization with a loading control GAPDH. Data are expressed as
the mean ± standard deviation from three independent experiments.
▲P<0.05 and ▲▲P<0.01, compared with the
control; *P<0.05 and **P<0.01, compared with CdCl2
alone. MDA, malondialdehyde; LDH, lactate dehydrogenase; SOD,
superoxidase dismutase. |
Geniposide regulates the expression of
Bax, Bcl-2 and survivin at the mRNA and protein levels of
CdCl2-injured MC-3T3-E1 cells
We determined the expression levels of Bax, Bcl-2
and survivin in MC-3T3-E1 cells using both western blotting and
qPCR analyses. As shown in Fig.
3D-G, compared with levels in cells exposed to
CdCl2, pretreatment with geniposide increased the
expression of Bcl-2 and survivin both at mRNA and protein levels in
a concentration-dependent manner (survivin, 200 µg/ml P<0.05;
400 µg/ml P<0.01) and reduced the expression of Bax at the mRNA
and protein levels (400 µg/ml, P<0.01). Both western blot and
qPCR analysis showed that medium and high concentrations of
geniposide could inhibit Bax, and increase Bcl-2 and survivin.
These results showed that geniposide strongly antagonized the
apoptotic process of CdCl2-induced MC-3T3-E1 cells.
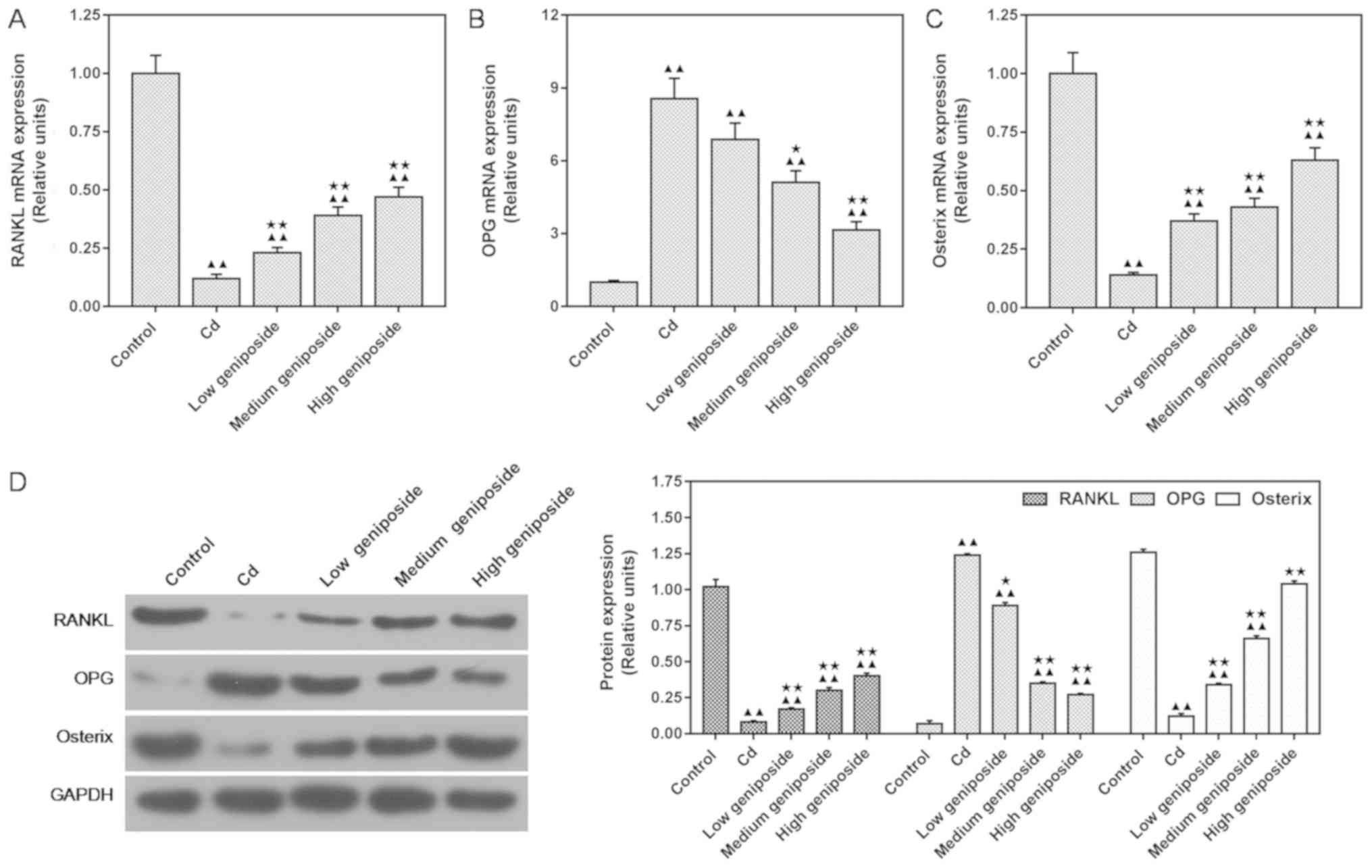
Geniposide regulates the expression of
RANKL, OPG and osterix at both the mRNA and protein levels in
CdCl2-injured MC-3T3-E1 cells
In order to investigate whether geniposide could
reverse the inhibition of CdCl2 on osteoblast formation,
we assessed the expression of osteoblast-related factors, RANKL,
OPG and osterix, by carrying out western blot and qPCR analyses in
MC-3T3-E1 cells. The results showed that exposure to
CdCl2 significantly inhibited osteoblast formation by
increasing the expression of OPG and by decreasing the expression
of RANKL and osterix both at the mRNA and protein levels. Medium
concentration of geniposide significantly reversed of the
inhibition mediated by CdCl2 on osteoblast formation
through upregulating the expression of RANKL and osterix
(P<0.01) and downregulating the expression of OPG (P<0.05).
The protein levels (Fig. 4A-C)
were consistent with the expression of mRNA (Fig. 4D).
Geniposide regulates the downstream
target genes of Nrf2 at both the mRNA and protein levels in
CdCl2-injured MC-3T3-E1 cells
In order to understand the mechanism of geniposide
in Cd-induced osteoblast injury, the Nrf2 signaling pathway was
evaluated in MC-3T3-E1 cells. As shown in Fig. 5, both qPCR and western blot
analysis identified an increase in Nrf2, HO-1 and NQO1 expression
in a dose-dependent manner following pretreatment with geniposide
in the CdCl2-injured MC-3T3-E1 cells. We found that a
low concentration of geniposide significantly increased the mRNA
expression of Nrf2 in comparison to that in cells exposed to
CdCl2 (P<0.05, Fig.
5A). However, both the increase of HO-1 and NQO1 mRNA
expression required treatment with a medium concentration of
geniposide (P<0.05, Fig. 5B and
C). Western blot analyses also showed that a low concentration
of geniposide increased the protein expression of Nrf2, HO-1 and
NQO1 compared to that in cells exposed only to CdCl2
(P<0.01, Fig. 5D).
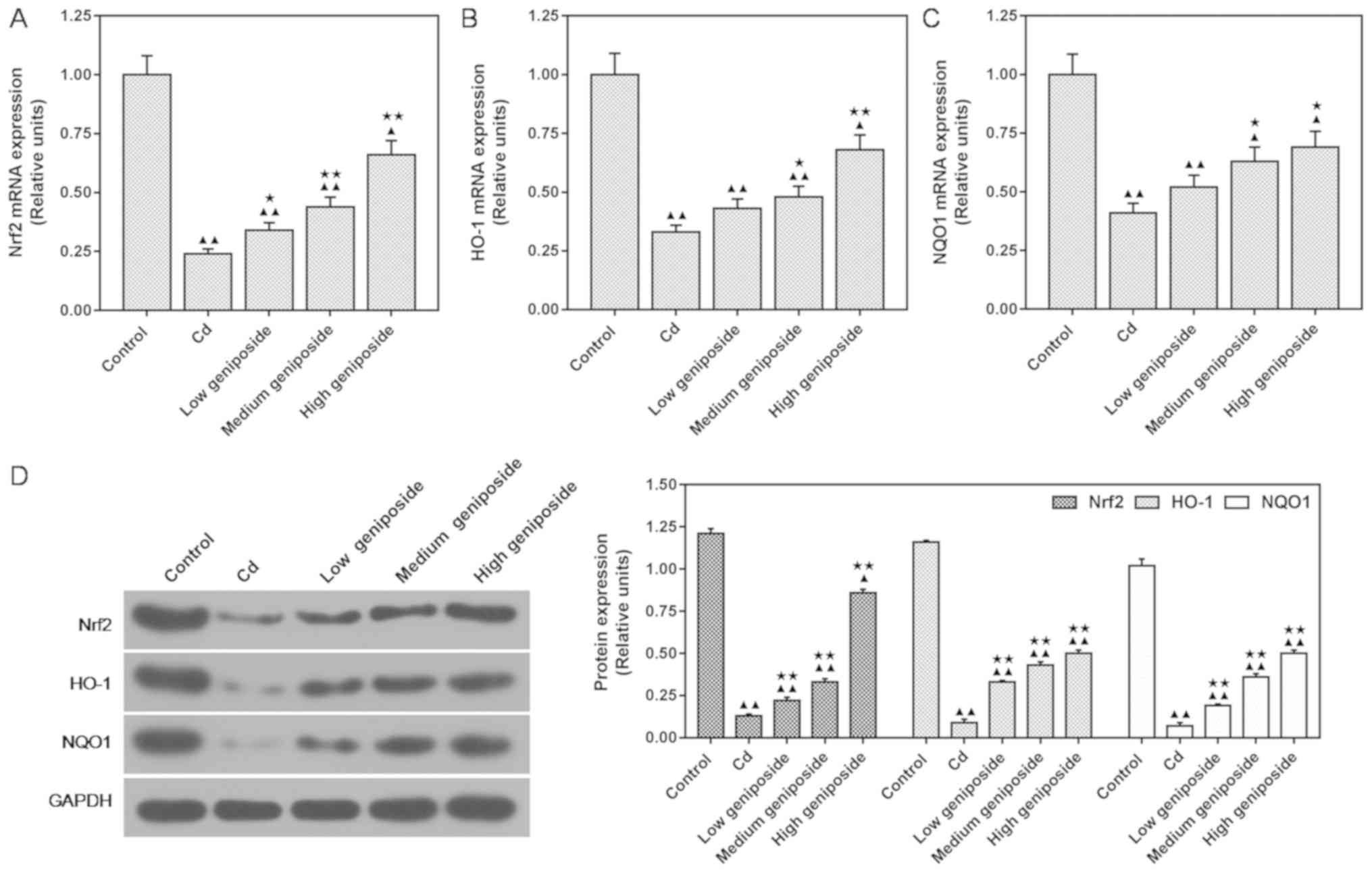 | Figure 5.Effects of different concentrations
of geniposide on CdCl2 (Cd)-induced mRNA and protein
levels of Nrf2, HO-1 and NQO1. Cells were pretreated with
geniposide [100 (low), 200 (medium), 400 µg/ml (high)] for 24 h,
followed by exposure to CdCl2 (20 µM) for 3 h. (A-C)
qPCR was used to determine the mRNA expression of Nrf2, HO-1 and
NQO1. (D) Western blotting results and relative units of protein
levels. Expression of each protein in control or geniposide
pretreated MC-3T3-E1 cells following normalization with a loading
control GAPDH. Data are expressed as the mean ± standard deviation
from three independent experiments. ▲P<0.05 and
▲▲P<0.01, compared with the control; *P<0.05 and
**P<0.01, compared with CdCl2 alone. Nrf2, nuclear
factor erythroid 2-related factor; HO-1, heme oxygenase-1; NQO1,
NAD(P)H quinone dehydrogenase 1. |
Discussion
In the present study, osteoblast MC-3T3-E1 cells
were pretreated with three different concentrations (100, 200 and
400 µg/ml) of geniposide for 24 h, and exposed to 20 µM
CdCl2 for additional 3 h. Furthermore, qPCR and western
blot analysis showed that geniposide at a high concentration was
able to significantly enhance the cell viability, while a low
concentration of geniposide could antagonize apoptosis by
downregulating Bax and upregulating both Bcl-2 and survivin.
Furthermore, we found that geniposide could reverse
the injury of CdCl2 on osteoblast formation, which is
consistent with another study in which geniposide promoted
osteoblast formation (33). As
previously reported, the RANKL/RANK/OPG system is an important
signal transduction pathway in the process of bone metabolism. The
receptor activator of RANKL with its cognate receptor (RANK)
promotes differentiation and bone resorption activity of
osteoclasts. OPG can also combine RANK, disrupting the balance of
bone metabolism (17,41,42).
It has been suggested that cadmium can accumulate in human
osteoblast-like MG-63 cells and affect their viability, and that
high concentrations of cadmium could inhibit bone formation via the
OPG/RANKL pathway (17,43). However, a limited number of studies
have focused on geniposide in relation to RANKL and OPG in
osteoblast cells. In the present study, we demonstrated that
low-dose geniposide obviously increased expression of RANKL, and
that a medium-dose could decrease expression of OPG. Osterix is a
novel transcription factor in the differentiation of osteoblasts,
and it is specifically expressed in all developing bones (44). Geniposide promotes osteogenic
activity of osteoblasts by increasing the expression of osterix in
a dose-dependent manner. It was indicated that geniposide promoted
the balance of bone metabolism.
Occupational cadmium exposure and domestic cadmium
pollution seriously affect the health of individuals worldwide,
causing neuronal damage, cardiovascular effects, reproductive
toxicity and osteoblast injury (4,7,45,46).
A large amount of evidence has confirmed that reducing internal
oxidative stress and increasing endogenous antioxidant proteins are
vital in avoiding cell injury (26,34,39,47).
Pan et al (48) highlighted
the importance of oxidative stress in cadmium exposure disorder,
and many compounds produce protective effects against
cadmium-induced oxidative injury, for example, quercetin, catechin
and nobiletin (49–51). Geniposide had been reported to
protect against cadmium-induced toxic oxidative stress in rat
kidney tissue (25). Thus, we
concluded that geniposide may prevented cadmium-induced injury.
Reactive oxygen species (ROS) are generally produced
in the mitochondria. Excessive exogenous oxidants and certain
extreme environments including heavy metal, chemotherapeutic drugs,
sodium fluoride lead to the overproduction of ROS (52–54).
Over-generated ROS damage proteins, lipids and DNA, ultimately
causing cell death or apoptosis. CdCl2 exposure was
found to significantly increase ROS generation (55,56).
Geniposide was found to noticeably decrease ROS levels, to
downregulate LDH and to upregulate antioxidase SOD. In order to
understand the protective mechanism of geniposide against oxidative
stress injury, we detected the downstream target genes of Nrf2,
HO-1 and NQO1. Nrf2, a basic leucine-zipper transcription factor,
plays an important role in preventing the development of oxidative
stress and is also one of the essential regulators of antioxidative
stress genes. The role of Nrf2 has been confirmed using Nrf2
knock-out mice in vivo, and it binds to antioxidant response
element (ARE) sites in the promoter of cytoprotective phase II
genes to regulate their expression (57–61).
Our study showed that geniposide not only completely increased the
mRNA and protein expression of Nrf2, but also increased antioxidant
protein HO-1 and phase II detoxifying enzyme NQO1. Thus, we
inferred that the induction of Nrf2 could promote the downstream
genes HO-1 and NQO1 so as to attenuate the oxidative stress
reaction. Taken together, our study indicated that geniposide could
induce Nrf2, suggesting that the Nrf2 pathway may take part in the
progressive effects of geniposide on antioxidative stress.
In conclusion, our finding suggests that geniposide
could antagonize oxidative stress caused by CdCl2.
Activation of Nrf2, HO-1 and NQO1 may be associated with the effect
of geniposide on MC-3T3-E1 cells. Our study identifies a potential
agent for the treatment of cadmium-induced osteoblast injury.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
TH and HS made substantial contributions to the
conception and design of the study. JZ, YZ, HL and YH performed
data acquisition, data analysis and interpretation. TH and HS
drafted the article, critically revised its intellectual content.
All authors read and approved the final version of the manuscript.
TH, HS, ZL and HL agreed to be accountable for all aspects of the
work and ensuring that questions related to the accuracy or
integrity of the work were appropriately investigated and
resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sato K, Iwamasa T, Tsuru T and Takeuchi T:
An ultrastructural study of chronic cadmium chloride-induced
neuropathy. Acta Neuropathol. 41:185–190. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sakata S, Iwami K, Enoki Y, Kohzuki H,
Shimizu S, Matsuda M and Moriyama T: Effects of cadmium on in vitro
and in vivo erythropoiesis: Erythroid progenitor cells (CFU-E),
iron and erythropoietin in cadmium-induced iron deficiency anemia.
Exp Hematol. 16:581–587. 1988.PubMed/NCBI
|
|
3
|
Blakley BR: Humoral immunity in aged mice
exposed to cadmium. Can J Vet Res. 52:291–292. 1988.PubMed/NCBI
|
|
4
|
Saygi S, Deniz G, Kutsal O and Vural N:
Chronic effects of cadmium on kidney, liver, testis and fertility
of male rats. Biol Trace Elem Res. 31:209–214. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sakamoto M, Itai T and Murata K: Effects
of prenatal methylmercury exposure: From minamata disease to
environmental health studies. Nihon Eiseigaku Zasshi. 72:140–148.
2017.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Morikawa Y, Nakagawa H, Tabata M, Nishijo
M, Senma M, Kitagawa Y, Kawano S, Teranishi H and Kido T: Study of
an outbreak of itai-itai disease. Nihon Eiseigaku Zasshi.
46:1057–1062. 1992.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kong YY, Yoshida H, Sarosi I, Tan HL,
Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G,
Itie A, et al: OPGL is a key regulator of osteoclastogenesis,
lymphocyte development and lymph-node organogenesis. Nature.
397:315–323. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bhattacharyya MH, Whelton BD, Stern PH and
Peterson DP: Cadmium accelerates bone loss in ovariectomized mice
and fetal rat limb bones in culture. Proc Natl Acad Sci USA.
85:8761–8765. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
WHO, . Environmental Health Criteria.
Cadmium-Environmental Aspects. 135:134–136. 1992.
|
|
10
|
Martineau C, Abed E, Medina G, Jomphe LA,
Mantha M, Jumarie C and Moreau R: Involvement of transient receptor
potential melastatin-related 7 (TRPM7) channels in cadmium uptake
and cytotoxicity in MC3T3-E1 osteoblasts. Toxicol Lett.
199:357–363. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kawamura J, Yoshida O, Nishino K and
Itokawa Y: Disturbances in kidney functions and calcium and
phosphate metabolism in cadmium-poisoned rats. Nephron. 20:101–110.
1978. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shinichi S and Kiyoshi N: Effects of
vitamin D on calcium and bone metabolism. Clin Calcium. 13:863–868.
2003.(In Japanese). PubMed/NCBI
|
|
13
|
Wang C and Bhattacharyya MH: Effect of
cadmium on bone calcium and 45Ca in nonpregnant mice on a
calcium-deficient diet: Evidence of direct effect of cadmium on
bone. Toxicol Appl Pharmacol. 120:228–239. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brama M, Politi L, Santini P, Migliaccio S
and Scandurra R: Cadmium-induced apoptosis and necrosis in human
osteoblasts: Role of caspases and mitogen-activated protein kinases
pathways. J Endocrinol Invest. 35:198–208. 2012.PubMed/NCBI
|
|
15
|
Wallin M, Barregard L, Sallsten G, Lundh
T, Karlsson MK, Lorentzon M, Ohlsson C and Mellström D: Low-level
cadmium exposure is associated with decreased bone mineral density
and increased risk of incident fractures in elderly men: The MrOS
Sweden study. J Bone Miner Res. 31:732–741. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wilson AK, Cerny EA, Smith BD, Wagh A and
Bhattacharyya MH: Effects of cadmium on osteoclast formation and
activity in vitro. Toxicol Appl Pharmacol. 140:451–460. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen X, Zhu G, Gu S, Jin T and Shao C:
Effects of cadmium on osteoblasts and osteoclasts in vitro. Environ
Toxicol Pharmacol. 28:232–236. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Coonse KG, Coonts AJ, Morrison EV and
Heggland SJ: Cadmium induces apoptosis in the human osteoblast-like
cell line Saos-2. J Toxicol Environ Health A. 70:575–581. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang CF, Shen HM, Shen Y, Zhuang ZX and
Ong CN: Cadmium-induced oxidative cellular damage in human fetal
lung fibroblasts (MRC-5 cells). Environ Health Perspect.
105:712–716. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Thompson J and Bannigan J: Cadmium: Toxic
effects on the reproductive system and the embryo. Reprod Toxicol.
25:304–315. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Joseph P: Mechanisms of cadmium
carcinogenesis. Toxicol Appl Pharmacol. 238:272–279. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bertin G and Averbeck D: Cadmium: Cellular
effects, modifications of biomolecules, modulation of DNA repair
and genotoxic consequences (a review). Biochimie. 88:1549–1559.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
López E, Arce C, Oset-Gasque MJ, Cañadas S
and Gonzalez MP: Cadmium induces reactive oxygen species generation
and lipid peroxidation in cortical neurons in culture. Free Radic
Biol Med. 40:940–951. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Koo HJ, Lim KH, Jung HJ and Park EH:
Anti-inflammatory evaluation of gardenia extract, geniposide and
genipin. J Ethnopharmacol. 103:496–500. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu E, Han L, Wang J, He W, Shang H, Gao X
and Wang T: Eucommia ulmoides bark protects against renal injury in
cadmium-challenged rats. J Med Food. 15:307–314. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yin F, Liu J, Zheng X, Guo L and Xiao H:
Geniposide induces the expression of heme oxygenase-1 via
PI3K/Nrf2-signaling to enhance the antioxidant capacity in primary
hippocampal neurons. Biol Pharm Bull. 33:1841–1846. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang SW, Lai CY and Wang CJ: Inhibitory
effect of geniposide on aflatoxin B1-induced DNA repair synthesis
in primary cultured rat hepatocytes. Cancer Lett. 65:133–137. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Koo HJ, Lee S, Shin KH, Kim BC, Lim CJ and
Park EH: Geniposide, an anti-angiogenic compound from the fruits of
Gardenia jasminoides. Planta Med. 70:467–469. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang J, Hou J, Zhang P, Li D, Zhang C and
Liu J: Geniposide reduces inflammatory responses of oxygen-glucose
deprived rat microglial cells via inhibition of the TLR4 signaling
pathway. Neurochem Res. 37:2235–2248. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang J, Li D, Hou J and Lei H: Protective
effects of geniposide and ginsenoside Rg1 combination treatment on
rats following cerebral ischemia are mediated via microglial
microRNA-155-5p inhibition. Mol Med Rep. 17:3186–3193.
2018.PubMed/NCBI
|
|
31
|
Chang CH, Wu JB, Yang JS, Lai YJ, Su CH,
Lu CC and Hsu YM: The suppressive effects of geniposide and genipin
on helicobacter pylori infections in vitro and in vivo. J Food Sci.
82:3021–3028. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ha H, Ho J, Shin S, Kim H, Koo S, Kim IH
and Kim C: Effects of eucommiae cortex on osteoblast-like cell
proliferation and osteoclast inhibition. Arch Pharm Res.
26:929–936. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lin J, Fan YJ, Mehl C, Zhu JJ, Chen H, Jin
LY, Xu JH and Wang HM: Eucommia ulmoides Oliv. antagonizes
H2O2-induced rat osteoblastic MC3T3-E1
apoptosis by inhibiting expressions of caspases 3, 6, 7 and 9. J
Zhejiang Univ Sci B. 12:47–54. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu J, Yin F, Zheng X, Jing J and Hu Y:
Geniposide, a novel agonist for GLP-1 receptor, prevents PC12 cells
from oxidative damage via MAP kinase pathway. Neurochem Int.
51:361–369. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen Y, Zhang H, Li YX, Cai L, Huang J,
Zhao C, Jia L, Buchanan R, Yang T and Jiang LJ: Crocin and
geniposide profiles and radical scavenging activity of gardenia
fruits (Gardenia jasminoides Ellis) from different cultivars and at
the various stages of maturation. Fitoterapia. 81:269–273. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee P, Lee J, Choi SY, Lee SE, Lee S and
Son D: Geniposide from Gardenia jasminoides attenuates neuronal
cell death in oxygen and glucose deprivation-exposed rat
hippocampal slice culture. Biol Pharm Bull. 29:174–176. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cheng F, Ma C, Sun L, Zhang X, Zhai C, Li
C, Zhang S, Ren B, Liu S, Liu S, et al: Synergistic neuroprotective
effects of Geniposide and ursodeoxycholic acid in
hypoxia-reoxygenation injury in SH-SY5Y cells. Exp Ther Med.
15:320–326. 2018.PubMed/NCBI
|
|
38
|
Hu KH, Li WX, Sun MY, Zhang SB, Fan CX, Wu
Q, Zhu W and Xu X: Cadmium induced apoptosis in MG63 cells by
increasing ROS, activation of p38 MAPK and inhibition of ERK 1/2
Pathways. Cell Physiol Biochem. 36:642–654. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pathak N and Khandelwal S: Influence of
cadmium on murine thymocytes: Potentiation of apoptosis and
oxidative stress. Toxicol Lett. 165:121–132. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Theoleyre S, Wittrant Y, Tat SK, Fortun Y,
Redini F and Heymann D: The molecular triad OPG/RANK/RANKL:
Involvement in the orchestration of pathophysiological bone
remodeling. Cytokine Growth Factor Rev. 15:457–475. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Roodman GD: Treatment strategies for bone
disease. Bone Marrow Transplant. 40:1139–1146. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Levesque M, Martineau C, Jumarie C and
Moreau R: Characterization of cadmium uptake and cytotoxicity in
human osteoblast-like MG-63 cells. Toxicol Appl Pharmacol.
231:308–317. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nakashima K, Zhou X, Kunkel G, Zhang Z,
Deng JM, Behringer RR and de Crombrugghe B: The novel zinc
finger-containing transcription factor osterix is required for
osteoblast differentiation and bone formation. Cell. 108:17–29.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Valois AA and Webster WS: The choroid
plexus as a target site for cadmium toxicity following chronic
exposure in the adult mouse: An ultrastructural study. Toxicology.
55:193–205. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jamall IS, Naik M, Sprowls JJ and
Trombetta LD: A comparison of the effects of dietary cadmium on
heart and kidney antioxidant enzymes: evidence for the greater
vulnerability of the heart to cadmium toxicity. J Appl Toxicol.
9:339–345. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu E, Han L, Wang J, He W Shang H, Gao X
and Wang T: Eucommia ulmoides bark protects against renal injury in
cadmium-challenged rats. J Med Food. 15:307–314. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Pan YX, Luo Z, Zhuo MQ, Wei CC, Chen GH
and Song YF: Oxidative stress and mitochondrial dysfunction
mediated Cd-induced hepatic lipid accumulation in zebrafish Danio
rerio. Aquat Toxicol. 199:12–20. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Mao T, Han C, Wei B, Zhao L, Zhang Q, Deng
R, Liu J, Luo Y and Zhang Y: Protective effects of quercetin
against cadmium chloride-induced oxidative injury in goat sperm and
zygotes. Biol Trace Elem Res. 185:344–355. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wongmekiat O, Peerapanyasut W and Kobroob
A: Catechin supplementation prevents kidney damage in rats
repeatedly exposed to cadmium through mitochondrial protection.
Naunyn Schmiedebergs Arch Pharmacol. 391:385–394. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Qu Y, Liu Y, Chen L and Zhu Y, Xiao X,
Wang D and Zhu Y: Nobiletin prevents cadmium-induced neuronal
apoptosis by inhibiting reactive oxygen species and modulating
JNK/ERK1/2 and Akt/mTOR networks in rats. Neurol Res. 40:211–220.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kováčik J, Dresler S, Peterková V and
Babula P: Metal-induced oxidative stress in terrestrial
macrolichens. Chemosphere. 203:402–409. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Varricchi G, Ameri P, Cadeddu C, Ghigo A,
Madonna R, Marone G, Mercurio V, Monte I, Novo G, Parrella P, et
al: Antineoplastic drug-induced cardiotoxicity: A redox
perspective. Front Physiol. 9:1672018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kim J, Kwon WS, Rahman MS, Lee JS, Yoon
SJ, Park YJ, You YA and Pang MG: Effect of sodium fluoride on male
mouse fertility. Andrology. 3:544–551. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chatterjee S, Kundu S, Sengupta S and
Bhattacharyya A: Divergence to apoptosis from ROS induced cell
cycle arrest: Effect of cadmium. Mutat Res. 663:22–31. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wang SH, Shih YL, Kuo TC, Ko WC and Shih
CM: Cadmium toxicity toward autophagy through ROS-activated
GSK-3beta in mesangial cells. Toxicol Sci. 108:124–131. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Sporn MB and Liby KT: NRF2 and cancer: The
good, the bad and the importance of context. Nat Rev Cancer.
12:564–571. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ishii T, Itoh K, Takahashi S, Sato H,
Yanagawa T, Katoh Y, Bannai S and Yamamoto M: Transcription factor
Nrf2 coordinately regulates a group of oxidative stress-inducible
genes in macrophages. J Biol Chem. 275:16023–16029. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lee IT, Wang SW, Lee CW, Chang CC, Lin CC,
Luo SF and Yang CM: Lipoteichoic acid induces HO-1 expression via
the TLR2/MyD88/c-Src/NADPH oxidase pathway and Nrf2 in human
tracheal smooth muscle cells. J Immunol. 181:5098–5110. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Nguyen T, Sherratt PJ and Pickett CB:
Regulatory mechanisms controlling gene expression mediated by the
antioxidant response element. Annu Rev Pharmacol Toxicol.
43:233–260. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Khor TO, Huang MT, Prawan A, Liu Y, Hao X,
Yu S, Cheung WK, Chan JY, Reddy BS, Yang CS and Kong AN: Increased
susceptibility of Nrf2 knockout mice to colitis-associated
colorectal cancer. Cancer Prev Res (Phila). 1:187–191. 2008.
View Article : Google Scholar : PubMed/NCBI
|