Introduction
Numerous studies have reported that several chronic
diseases with a high incidence are associated with inflammation. As
documented, the progression of certain central neurodegenerative
diseases, including Alzheimer's disease, Parkinson's disease and
multiple sclerosis, involve neuroinflammation (1–3).
Inflammation is also important in the development of circulatory
diseases, including atherosclerosis, blood viscosity and primary
hypertension (4–6). Certain types of malignant tumors are
known to be closely associated with the inflammatory mechanism
(7–9). Therefore, the development of novel
anti-inflammatory drugs is of ongoing interest. Isaindigotone is an
alkaloid extracted from Radix isatidis (10). The parent compound of isaindigotone
is formed by the connection of pyrroquinolone with conjugated
benzylidene (Fig. 1) (10). Isaindigotone possesses extensive
pharmacological activities, including antibiotic, antiviral,
anti-endotoxin, anti-inflammatory and antitumor activities
(11–14). It has been previously shown that
isaindigotone is able to pass through the blood-brain barrier and
be transferred to the central nervous system (15). Therefore, understanding whether
isaindigotone can inhibit the inflammatory response of the central
nervous system is of significance for preventing and relieving
central nervous system diseases.
Microglia (MG) are resident immune cells in the
central nervous system. The continuous activation of MG is a key
factor in the induction and intensification of central
neuroinflammation (16,17). Therefore, in the present study, an
in vitro inflammation model of lipopolysaccharide
(LPS)-induced BV-2 cells (an MG line) was selected to examine the
effects of isaindigotone on LPS-induced inflammatory responses. The
conclusions provide a theoretical and experimental basis for
further investigation and clinical development.
Materials and methods
Materials
Isaindigotone was purchased from J&K Scientific,
Ltd. (Shanghai, China) and was dissolved in 100% dimethyl sulfoxide
(DMSO). A stock solution of 10 mmol/l isaindigotone was prepared
and stored as small aliquots (5 µl) at −20°C for future use. The
BV-2 cell line was obtained from the Animal Experimental Center of
Sun Yat-Sen University (Guangzhou, China). MTT and DMSO were
purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). The
tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) ELISA
kits were purchased from Cell Signaling Technology, Inc. (Danvers,
MA, USA). All antibodies were purchased from Cell Signaling
Technology, Inc.
MTT assay
The BV-2 cells were cultured in high-glucose
Dulbecco's modified Eagle's medium (DMEM; Husbio, Inc., Shanghai,
China) supplemented with 10% fetal calf serum at 37°C. The cells
were adjusted to 1×105 cells/ml and were inoculated onto
a 96-well culture plate with 100 µl of cell suspension in each
well. After 24 h, isaindigotone (5, 10, 20, 40 and 80 mg/l), LPS (1
mg/l), and LPS (1 mg/l) + isaindigotone (80 mg/l) were added into
each well respectively. The MTT (10 µl of 5% MTT) was added into
each well at 24, and 48 h during culture, following which the cells
were further incubated for 4 h at 37°C. Subsequently, the culture
solution was removed and 100 µl DMSO was added to each well for
dissolution. The optical density values were read at 490 nm
following vibration mixing.
Chemotaxis assay
BV-2 cells in the logarithmic phase were selected
and pre-incubated in a 35-mm culture dish for 24 h. The BV-2 cells
were then dislodged, centrifuged (300 × g for 5 min at room
temperature) and resuspended in fresh complete culture solution. A
blank group and LPS group were set; the LPS group was further
divided into five subgroups according to isaindigotone
concentrations (0, 10, 20, 40 and 80 mg/l). The cells were adjusted
to 1×105 cells/ml, and 200 µl of cells were collected
and placed in the upper chambers of a Transwell assay plate, and 20
nmol/l of amide compound MMK-1 (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) was placed in each of the lower chambers as a
chemoattractant. The BV-2 cells were cultured in a CO2
incubator for 24 h. The culture solution was then removed and BV-2
cells were rinsed three times with PBS, followed by 10 min of
fixation in methyl alcohol at room temperature. The BV-2 cells were
then incubated with 5 mg/l of 4,6-diamino-2-phenylindole for 20 min
at room temperature and rinsed three times with PBS. The cells were
observed and counted under a light microscope. The experimental
results are expressed as the chemotaxis index, which is the ratio
of cell numbers between the treatment group and the blank
group.
Evaluation of IL-1β and TNF-α levels
by ELISA
The BV-2 cells were cultured in high-glucose DMEM
(10% fetal calf serum) and were adjusted to 1×105
cells/ml. The BV-2 cells were inoculated onto a cell culture plate
with 96 wells. The samples were divided into a blank group,
isaindigotone group (80 mg/l), blank + LPS group (1 mg/l), and LPS
(1 mg/l) + isaindigotone groups (10, 20, 40 and 80 mg/l). Each
group comprised six complex wells, with 100 µl of sample per well.
In the LPS group, the BV-2 cells were incubated with 1 mg/l LPS for
24 h. In the isaindigotone group, the BV-2 cells were incubated
with isaindigotone (80 mg/l) for 24 h. In the LPS + isaindigotone
group, the BV-2 cells were incubated with different concentrations
of isaindigotone (10, 20, 40 and 80 mg/l) and 1 mg/l LPS for 24 h.
The secretory levels of IL-1β and TNF-α in the supernatant were
assessed by ELISA.
Observation of cell morphology
BV-2 cells in the logarithmic phase were selected
and incubated in a 6-well culture plate. The BV-2 cells were
divided into a blank group, isaindigotone group (40 mg/l), LPS
group, and LPS + isaindigotone group (40 mg/l) after 24 h. In the
LPS group, the BV-2 cells were co-incubated with 1 mg/l of LPS for
24 h. Subsequent morphological changes in the BV-2 cells were
observed using an inverted phase-contrast microscope.
Western blotting
BV-2 cells in the logarithmic phase were selected
and divided into blank, LPS, and LPS + isaindigotone groups. In the
LPS group, the BV-2 cells were incubated with 1 µg. L−1
LPS for 20 min. In the LPS + isaindigotone group, the BV-2 cells
were co-incubated with 20 mg/l isaindigotone and 1 mg/l LPS for 20
min. The BV-2 cells from all groups were collected, and total
proteins were extracted using RIPA lysis buffer. The protein
concentrations were determined using the bicinchoninic acid method.
Subsequently, the proteins were prepared with 5X loading buffer;
the loading quantity was 20–40 µg. A 10% SDS polyacrylamide gel
electrophoresis was performed, following which the proteins were
transferred onto a PVDF membrane through the semi-dry method. The
PVDF was blocked using 5% powdered skim-milk for 2 h, and then
incubated with the primary antibodies against NF-κB (cat. no. 8242;
1:1,000; Cell Signaling Technology, Inc.), phospho-NF-κB (cat. no.
3033; 1:1,000; Cell Signaling Technology, Inc.), β-actin (cat. no.
3700; 1:1,000; Cell Signaling Technology, Inc.) overnight at 4°C,
followed by incubation with HRP-linked anti-mouse (cat. no. 7076;
1:1,000; Cell Signaling Technology, Inc.) or HRP-linked anti-rabbit
(cat. no. 7074; 1:1,000; Cell Signaling Technology, Inc.) at room
temperature for 30 min. The signals were detected using an enhanced
chemiluminescence substrate (Bioss Biotechnology, Beijing, China),
and the optical density of the bands was measured by a BandScan
imaging analysis system.
Statistical analysis
The results are expressed as the mean ± standard
deviation. Statistical comparisons were performed using one-way
analysis of variance followed by the least significant difference
test the SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of isaindigotone on BV-2 cell
survival
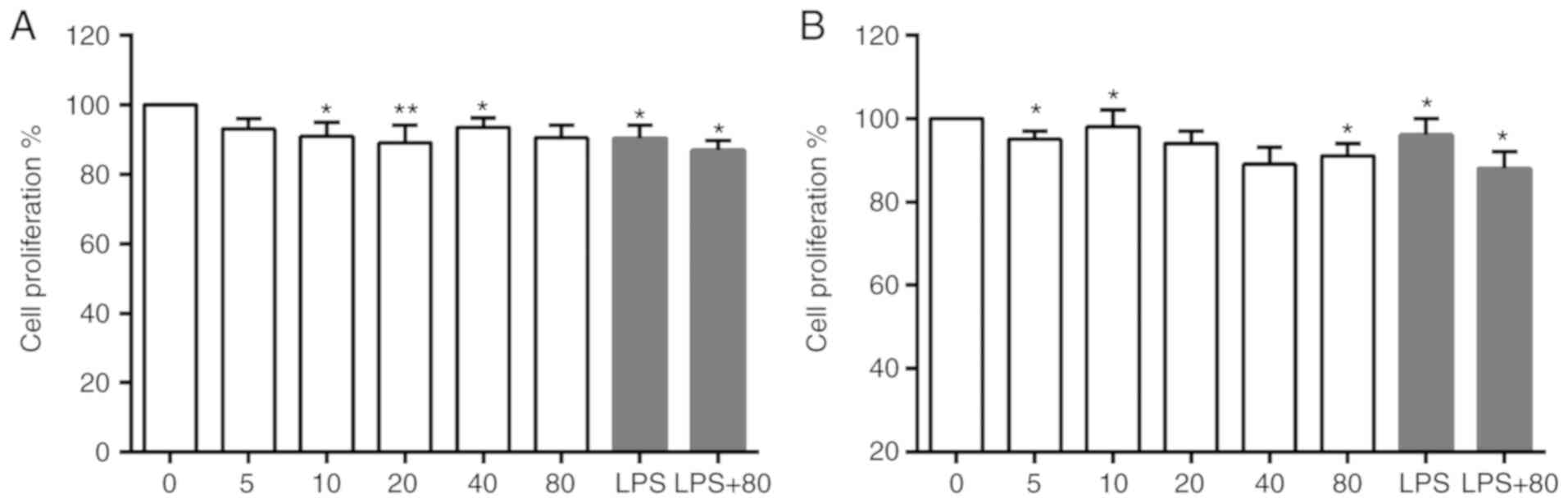
Different concentrations of isaindigotone with or
without LPS (1 mg/l) were applied to BV-2 cells for 24 and 48 h.
Isaindigotone (<80 mg/l) was observed to have little toxic
effect on BV-2 cells in vitro in the presence or absence of
LPS (Fig. 2A and B). This provided
a reference for determining drug concentration in the follow-up
experiments.
Effects of isaindigotone on the
chemotaxis of BV-2 cells
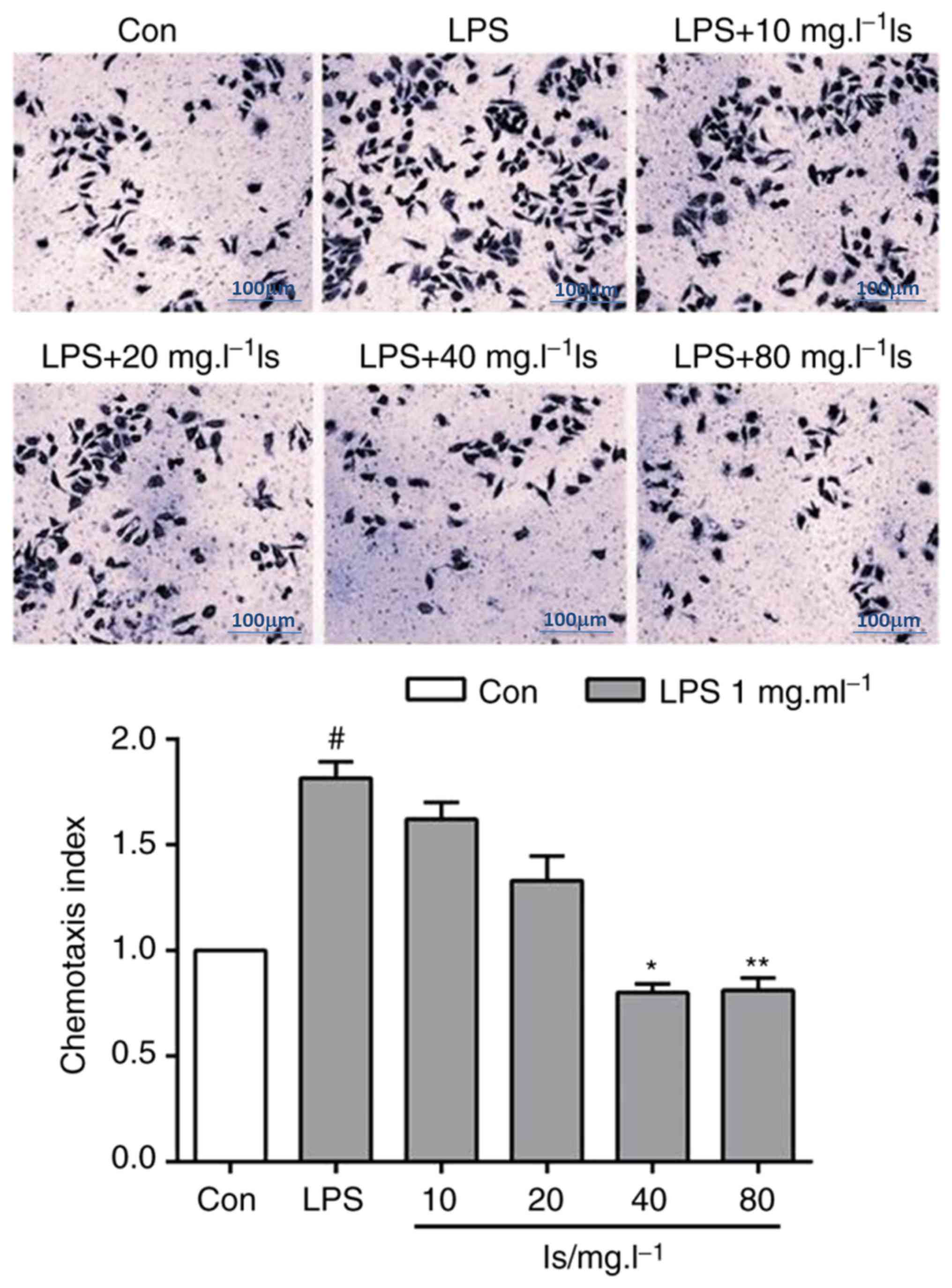
Following the abnormal activation of MG, the density
of surface receptors increased markedly after 24 h, mediating the
secretion of inflammatory factors and cell chemotaxis. The agonist
of formylpeptide receptor-2 (FPR2), MMK-1, induced chemotaxis of
the LPS-stimulated BV-2 cells (Fig.
3). Following co-incubation of the BV-2 cells with different
concentrations (40 and 80 mg/l) of isaindigotone, the BV-2 cell
chemotaxis induced by MMK-1 was reduced significantly compared with
that in the LPS group.
Effects of isaindigotone on the
secretion of inflammatory factors from BV-2 cells
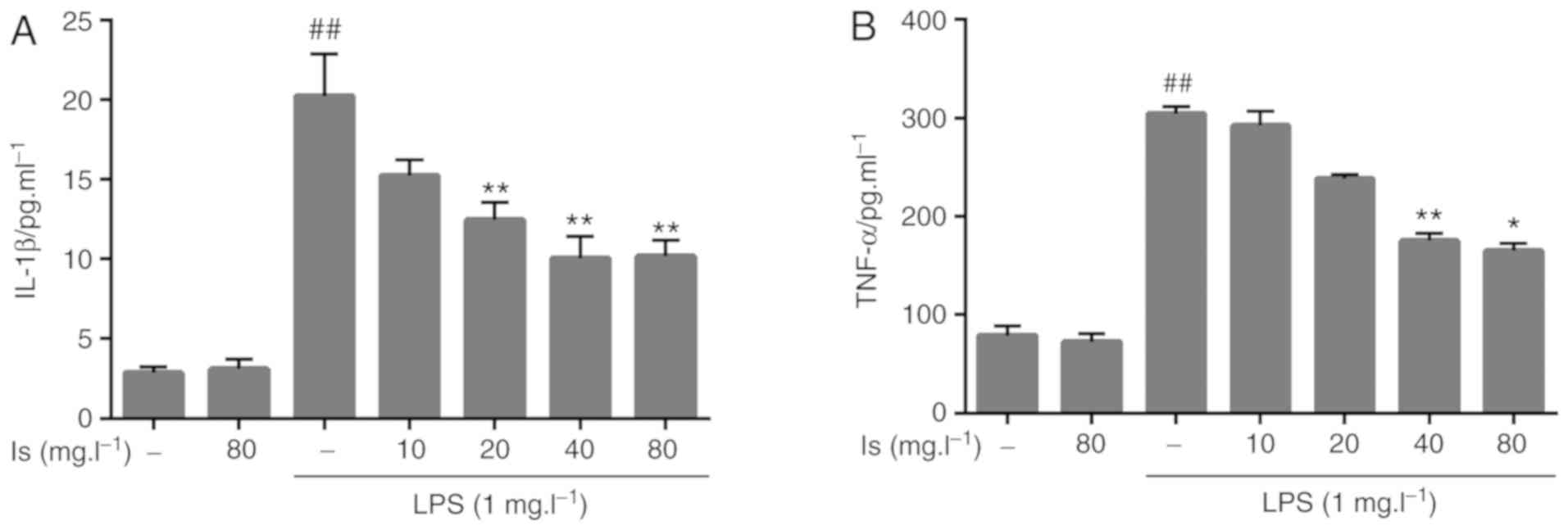
Compared with the blank group, incubation with LPS
significantly upregulated the secretion of IL-1β and TNF-α in the
cell supernatants. Following incubation of the BV-2 cells with
different concentrations of isaindigotone (40 and 80 mg/l), the
levels of IL-1β and TNF-α secretion were significantly inhibited
compared with those in the LPS group (Fig. 4).
Effects of isaindigotone on the
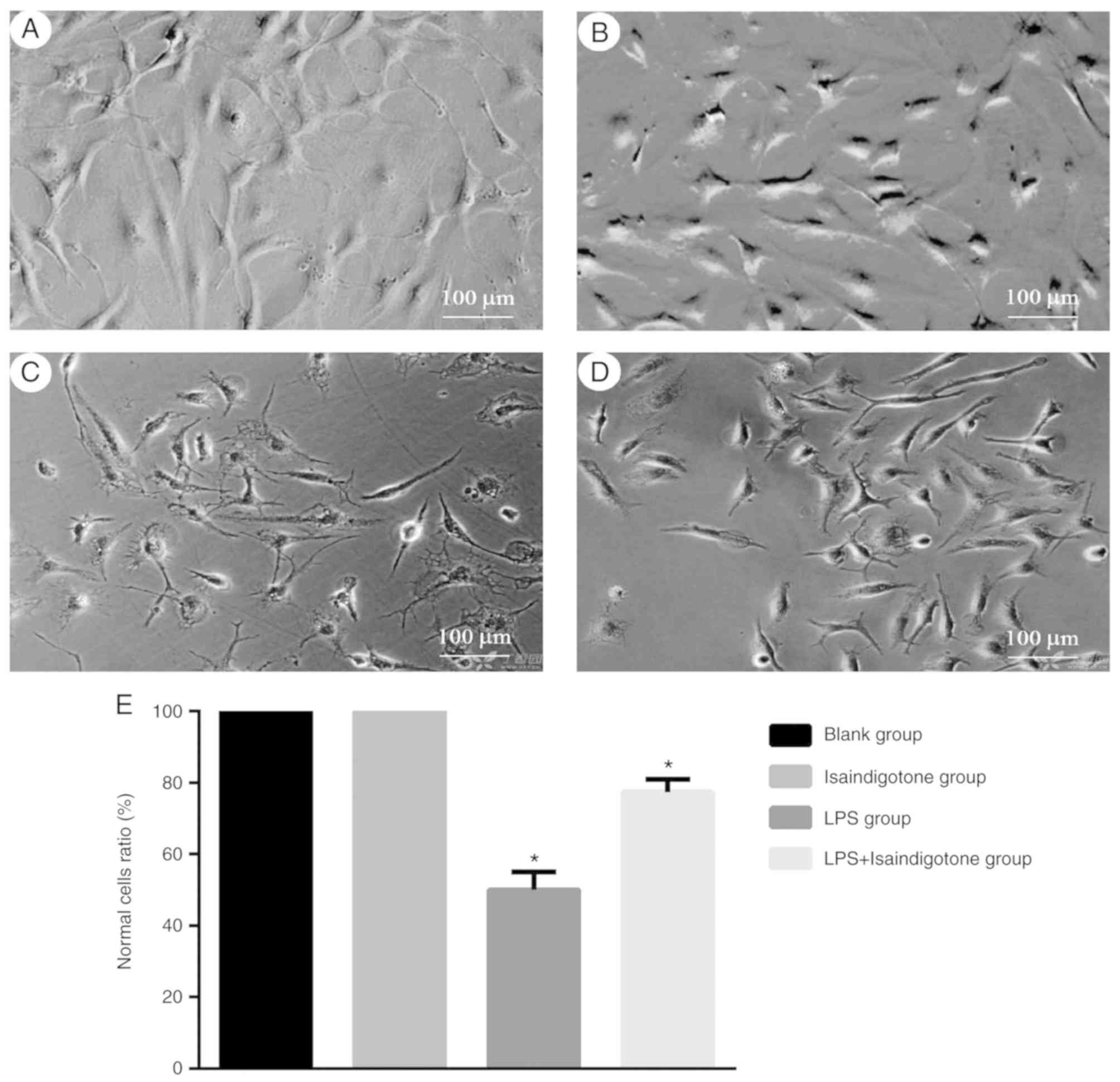
morphology of BV-2 cells
BV-2 cell morphology altered significantly following
treatment with 1 mg/l of LPS. Prior to treatment, the MG were in a
relative static state, accompanied by small thin and long soma.
Following treatment, morphological changes of the BV-2 cells
included protuberance, shrinkage and coarsening into an amoebic
appearance (Fig. 5A-D). The BV-2
cells were treated simultaneously with 40 mg/l of isaindigotone and
LPS after 24 h, which revealed that isaindigotone reversed the
LPS-induced morphological changes in BV-2 cells (Fig. 5E).
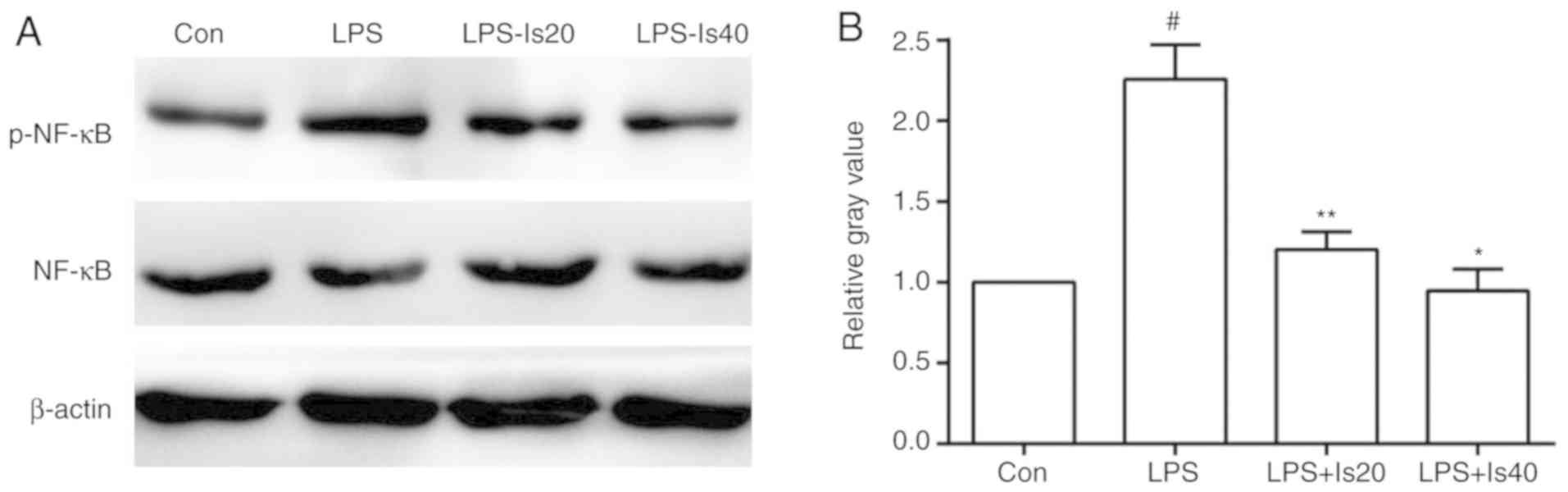
Effects of isaindigotone on the
phosphorylation of NF-κB in BV-2 cells
The phosphorylation of NF-κB in BV-2 cells
intensified following stimulation with 1 mg/l of LPS, but was
significantly downregulated following treatment with 20 and 40 mg/l
isaindigotone (Fig. 6A and B).
Discussion
Isaindigotone, found widely in R. isatidis,
is a natural antioxidant (14,15).
This compound has multiple functions, including cancer
preventative, anticancer and neuroprotective activities, and is
suitable for the treatment of cardiovascular diseases. Methods for
the extraction and the assessment of content and purity of
isaindigotone have been well developed and quality-controlled
(11–14). The in vivo reaction mode,
mechanism of action and metabolism of isaindigotone have been
investigated extensively. The long-term and in-depth chemical and
clinical data also reflect the lack of toxic side effects of
isaindigotone and its preparation, its high level of safety, low
accumulation potential and minimal residues (15). These findings improve current
understanding of isaindigotone, facilitating the development and
utilization of this compound. In the present study, the effects of
isaindigotone on the LPS-induced activation of BV-2 cells were
preliminarily examined from the perspective of inflammation. The
results provide a basis for further in vivo experiments and
provide a foundation for the development of isaindigotone-based
drugs, indicated for the prevention and treatment of central
nervous system degradation diseases caused by
neuroinflammation.
MG are regarded as ‘inspectors’ of the central
nervous system (16). They are
rapidly activated by foreign matter or detrimental stimuli. As a
result, MG release abundant cytokines for immunity and inflammation
regulation, thereby enabling the elimination of inflammatory agents
in nervous tissue or cells with metabolic disorders (17). However, continuously activated MG
release excessive inflammatory factors that aggravate
neuroinflammation and damage normal nervous tissue (18). LPS can induce MG to generate a
series of inflammatory responses, which significantly increase the
expression of inflammation-associated receptors located on MG
surface membranes. The secretion of inflammatory factors from MG is
also activated to facilitate neuroinflammation following
stimulation by LPS (19,20). Therefore, inhibiting the abnormal
activation and inflammatory responses of MG bears clinical
significance in terms of improving inflammation in the
microenvironment and protecting neurons from damage. The present
study showed that isaindigotone significantly inhibited BV-2 MG
cells from releasing inflammatory factors, and influenced the
functions of inflammatory receptors and phosphorylation of NF-κB.
This indicates that isaindigotone can inhibit the abnormal
activation of MG, preventing and relieving inflammatory damage to
the central nervous system.
Following abnormal activation, MG secrete
inflammatory cytokines which include TNF-α and IL-1β (21). These cytokines are key factors that
promote neuroinflammation. TNF-α not only induces the secretion of
multiple proinflammatory factors, but also induces cell apoptosis
and inflammatory reaction cascades (22). The upregulated expression of TNF-α
in the brain can directly reflect the severity of
neuroinflammation. IL-1β is one of the important proinflammatory
factors in the IL-1 family (23).
As a regulatory protein, IL-1β can stimulate the secretion of
TNF-α, IL-6, interferon and chemotactic factors through the
phosphorylation of NF-κB in MG, thus inducing continuous
deterioration through neuroinflammation. In the present study,
isaindigotone was shown to inhibit the LPS-induced secretion of
TNF-α and IL-1β by BV-2 cells, thus relieving the vascular
deterioration caused by persistent neuroinflammation.
FPR2, which is a member of the chemokine receptor
family, is a G protein-coupled receptor with seven transmembrane
helices. FPR2 possesses a comprehensive set of functions and can
mediate inflammatory and immune responses by combining with
specific ligands, all of which feature different sources and high
structural diversity (24). MMK-1,
an agonist with specificity for FPR2, can promote the secretion of
IL-1β, IL-6 and TNF-α, and cell chemotaxis, and can activate
neutrophil granulocytes, monocytes and T-cells, thus exerting
proinflammatory effects (25). In
the present study, isaindigotone showed a capacity for inhibiting
MMK-1-induced BV-2 cell chemotaxis, indicating that this compound
can inhibit the functions of FPR2 and influence inflammation.
Over recent decades, toll-like receptor 4 (TLR4),
which is involved in inflammation, immunity adjustment, cell
adhesion and chemotaxis, has increased in interest worldwide. The
signal transduction pathway of TLR4 can be activated by oxidative
stress, LPS and cytokines (particularly IL-1β) to regulate the
secretion and activation of cell proinflammatory factors, adhesion
molecules and other transcription factors, thus mediating cell
behavior. The activation of TLR4 initiates a pro-inflammatory
response, which depends on the activation of mitogen-activated
protein kinases and NF-κB (26).
Subsequently, NF-κB is phosphorylated and the activated NF-κB
translocates from cytoplasm to nucleus to promote the transcription
of various inflammatory marker genes, including those of
interleukins, cytokines, chemokines, inducible nitric oxide
synthase and cyclooxygenase-2 (27). As a result, inhibiting TLR4-NF-κB
signaling pathway activation can prevent the cell inflammatory
responses mediated by NF-κB and reduce inflammation-induced damages
in affected nervous cells and tissues (28). Experiments investigating the effect
of isaindigotone on the activation of NF-κB signaling, which is the
downstream signaling of the TLR4 pathway, showed that isaindigotone
pretreatment significantly inhibited the LPS-induced activation of
NF-κB in BV-2 cells, indicating that this natural compound can
affect cell behavior. An increasing body of data suggests that
inflammation, and in particular neuroinflammation, is involved in
the pathophysiology of certain types of epilepsy and convulsive
disorders. In an epileptic animal model, the TLR4-NF-κB signaling
pathway was shown to be activated in separated microglial cells
(29). However, the in-depth
mechanism and anti-inflammatory effect of isaindigotone in
vivo require further investigation.
In conclusion, isaindigotone was observed to inhibit
the LPS-induced inflammatory reactions of BV-2 cells. The
anti-inflammatory action of this molecule was associated with the
inhibited release of immune cell inflammatory factors, attenuation
of inflammatory cell chemotaxis and decreased phosphorylation of
NF-κB. These data provide a theoretical and experimental basis for
examining the mechanism of isaindigotone with respect to inhibiting
neuroinflammation.
Acknowledgements
Not applicable.
Funding
This study design was supported by a grant from the
National Science Foundation of China (grant no. 81302701).
Availability of data and materials
The datasets used or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors contributions
JX conceived the study and the experiments,
interpreted the results and prepared the manuscript. HX performed
the experiments and helped prepare the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Savitz J and Harrison NA: Interoception
and inflammation in psychiatric disorders. Biol Psychiatry Cogn
Neurosci Neuroimaging. 3:514–524. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Branchford BR and Carpenter SL: The role
of inflammation in venous thromboembolism. Front Pediatr.
6:1422018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gelders G, Baekelandt V and Van der Perren
A: Linking neuroinflammation and neurodegeneration in parkinson's
disease. J Immunol Res. 2018:47842682018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Katsuki S, Matoba T, Koga JI, Nakano K and
Egashira K: Anti-inflammatory nanomedicine for cardiovascular
disease. Front Cardiovasc Med. 4:872017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Escárcega RO, Lipinski MJ, García-Carrasco
M, Mendoza-Pinto C, Galvez-Romero JL and Cervera R: Inflammation
and atherosclerosis: Cardiovascular evaluation in patients with
autoimmune diseases. Autoimmun Rev. 17:703–708. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang C, Syed TW, Liu R and Yu J: Role of
endoplasmic reticulum stress, autophagy, and inflammation in
cardiovascular disease. Front Cardiovasc Med. 4:292017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bhatelia K, Singh K and Singh R: TLRs:
Linking inflammation and breast cancer. Cell Signal. 26:2350–2357.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Valdés-Rives SA and González-Arenas A:
Autotaxin-lysophosphatidic acid: From inflammation to cancer
development. Mediators Inflamm. 2017:91730902017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qu X, Tang Y and Hua S: Immunological
approaches towards cancer and inflammation: A cross talk. Front
Immunol. 9:5632018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Molina P, Tárraga A, Gonzalez-Tejero A,
Rioja I, Ubeda A, Terencio MC and Alcaraz MJ: Inhibition of
leukocyte functions by the alkaloid isaindigotone from Isatis
indigotica and some new synthetic derivatives. J Nat Prod.
64:1297–1300. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yan JW, Li YP, Ye WJ, Chen SB, Hou JQ, Tan
JH, Ou TM, Li D, Gu LQ and Huang ZS: Design, synthesis and
evaluation of isaindigotone derivatives as dual inhibitors for
acetylcholinesterase and amyloid beta aggregation. Bioorg Med Chem.
20:2527–2534. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shakhidoyatov KM and Elmuradov BZ:
Tricyclic Quinazoline Alkaloids: Isolation, synthesis, chemical
modification, and biological activity. Chem Nat Comp. 50:781–800.
2014. View Article : Google Scholar
|
|
13
|
Lei LM and Pang QP: Recent progress in the
studies of chemistry, pharmacology, quality control and extraction
methods on the Radix isatidis. Shi Zhen Guo Yi Guo Yao.
18:2578–2580. 2007.(In Chinese).
|
|
14
|
He LW, Liu HQ, Chen YQ, Yang JY, Wang TL
and Li W: Total synthesis and anti-viral activities of an extract
of Radix isatidis. Molecules. 19:20906–20912. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou W and Zhang XY: Research progress of
Chinese herbal medicine Radix isatidis (banlangen). Am J
Chin Med. 41:743–764. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pósfai B, Cserép C, Orsolits B and Dénes
Á: New insights into microglia-neuron interactions: A neuron's
perspective. Neuroscience. May 19–2018.(Epub ahead of print).
View Article : Google Scholar
|
|
17
|
Sevenich L: Brain-resident microglia and
blood-borne macrophages orchestrate central nervous system
inflammation in neurodegenerative disorders and brain cancer. Front
Immunol. 9:6972018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Altmann C and Schmidt MHH: The role of
microglia in diabetic retinopathy: Inflammation, microvasculature
defects and neurodegeneration. Int J Mol Sci. 19:E1102018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Olajide OA, Kumar A, Velagapudi R, Okorji
UP and Fiebich BL: Punicalagin inhibits neuroinflammation in
LPS-activated rat primary microglia. Mol Nutr Food Res Sep.
58:1843–1851. 2014. View Article : Google Scholar
|
|
20
|
Li Y, Lv O, Zhou F, Li Q, Wu Z and Zheng
Y: Linalool inhibits LPS-induced inflammation in BV2 microglia
cells by activating Nrf2. Neurochem Res. 40:1520–1525. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang WY, Tan MS, Yu JT and Tan L: Role of
pro-inflammatory cytokines released from microglia in Alzheimer's
disease. Ann Transl Med. 3:1362015.PubMed/NCBI
|
|
22
|
Jiang Y, An Y, Jiang D, Wu B, Yang Y and
Sun D: TNF-α regulating interleukin-33 induces acute pancreatic
inflammation in rats. Ann Clin Lab Sci. 46:54–59. 2016.PubMed/NCBI
|
|
23
|
Bucher H, Mang S, Keck M, Przibilla M,
Lamb DJ, Schiele F, Wittenbrink M, Fuchs K, Jung B, Erb KJ and
Peter D: Neutralization of both IL-1α/IL-1β plays a major role in
suppressing combined cigarette smoke/virus-induced pulmonary
inflammation in mice. Pulm Pharmacol Ther. 44:96–105. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li Y, Cai L, Wang H, Wu P, Gu W, Chen Y,
Hao H, Tang K, Yi P, Liu M, et al: Pleiotropic regulation of
macrophage polarization and tumorigenesis by formyl peptide
receptor-2. Oncogene. 30:3887–3899. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee HY, Kim H, Lee SY, Jung YS, Kim SD and
Bae YS: A membrane-tethering pepducin that inhibits formyl peptide
receptor 2-induced signaling. Pharmazie. 69:293–296.
2014.PubMed/NCBI
|
|
26
|
Pei Z, Li H, Guo Y, Jin Y and Lin D:
Sodium selenite inhibits the expression of VEGF, TGFbeta(1) and
IL-6 induced by LPS in human PC3 cells via TLR4-NF-(K)B signaling
blockage. Int Immunopharmacol. 10:50–56. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang ZS, Chen LZ, Zhou HP, Liu XH and Chen
FH: Diarylpentadienone derivatives (curcumin analogues): Synthesis
and anti-inflammatory activity. Bioorg Med Chem Lett. 27:1803–1807.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang YW, Zhang HH, Wang YL, Guo SS, Li T,
Chen L, Zhuang SX, Zhou ZM and Yang WP: Effect of huangqin tang on
the regulatory NF-κB p65 signal pathway in rats with ulcerative
colitis. Yao Xue Xue Bao. 50:21–27. 2015.(In Chinese). PubMed/NCBI
|
|
29
|
Vitaliti G, Pavone P, Mahmood F, Nunnari G
and Falsaperla R: Targeting inflammation as a therapeutic strategy
for drug-resistant epilepsies: An update of new immune-modulating
approaches. Hum Vaccin Immunother. 10:868–875. 2014. View Article : Google Scholar : PubMed/NCBI
|