Introduction
Cardiovascular diseases are one of the leading
causes of death worldwide, even though there has been great
improvement in medicine during the past few decades.
Atherosclerosis (AS) is a major cause of cardiovascular diseases.
Since it is a chronic inflammatory disease, multiple immune cell
types are involved in the pathogenesis of AS (1–3).
Much of the current evidence has indicated that macrophages
participate in the pathogenesis of AS. Macrophages could respond
rapidly to environmental signals through a multitude of receptors
and develop a specific and optimized activation state. Under some
conditions, monocytes form macrophage foam cells by taking up
low-density lipoprotein (LDL) into the vascular intima. These lipid
overloading foam cells are a sign of the initiation of AS. The
development of atheromatous plaques eventually leads to serious
cardiovascular diseases and the plaques are promoted through
accumulation and necrosis or apoptosis of foam cells (4).
The diffuse involvement of arteries throughout the
body and the appearance of multiple and simultaneous
atherosclerotic lesions are the characteristic feature of
atherosclerotic cardiovascular disease. Endovascular interventional
procedures that are ‘local’ treatments, such as stenting and
balloon angioplasty, of atherosclerotic arteries are the main
treatments, yet they are not effective for treating the underlying
cause of AS. Therefore, we need to find alternative therapies for
atherosclerotic cardiovascular disease. Mesenchymal stem cell
(MSCs) therapies have emerged as a promising tool for the treatment
of atherosclerotic cardiovascular disease (5,6).
MSCs, which mainly include adipose-derived stem cell (ADSCs) and
bone marrow-derived stem cells (BMSCs), could be isolated from
several tissues and expanded in vitro. MSCs could be
intravenously transfused and could migrate to atherosclerotic
arteries and differentiate into vascular smooth muscle cells, as
well as circulate in the blood system of the whole body. It has
been reported that intravenous infusion of MSCs for diffuse
multiple atherosclerotic lesions contributes to remodelling of the
vasculature (7,8). It is more important that
anti-inflammatory properties of MSCs be confirmed (9–11),
and it has also been reported that ADSCs and BMSCs could regulate
the activation state of macrophages, which possess potent
immunomodulatory abilities (12,13).
BMSCs are considered good candidates for stem cell
therapy due to their accessibility and non-tumourigenic activity.
However, their use has been limited by their low abundance,
especially in elderly people. ADSCs with similar features to BMSCs
can be obtained from subcutaneous adipose tissue from adult humans.
Hundreds of millions of ADSCs could be extracted from 1 to 2 litres
of adipose tissue. In addition, the separation process of ADSCs is
an efficient and safe procedure (14). Additionally, whether ADSCs are
better than BMSCs for altering macrophages has not been reported.
In the present report, the difference in metabolomics between ADSCs
and BMSCs from elderly people were explored, and conjugated
linoleic acid (CLA) from the metabolites of ADSCs and BMSCs have
marked anti-inflammatory effects through the regulation of
macrophages. Specifically, we show that ADSCs are better
suppressors of human macrophages compared to BMSCs when using
CLA.
Patients and methods
Participants
The present study was approved by the Ethics
Committee of The Second Affiliated Hospital of Harbin Medical
University (Harbin, China). Ten patients with coronary
atherosclerotic heart disease (CAD), aged 60–76 years old (age 67±7
years, 8 males, 2 females) were recruited into this research. The
baseline characteristics of the donors are shown in Table I. Before cardiac surgery, written
informed consent regarding this research were obtained from the
patients.
 | Table I.Clinical characteristics of
adipose-derived stem cells and bone marrow stem cells donors. |
Table I.
Clinical characteristics of
adipose-derived stem cells and bone marrow stem cells donors.
| Characteristic | Total n, range |
|---|
| Number of subjects,
n | 10 |
| Age, years (median,
range) | 67, 60–76 |
| Weight, kg (median,
range) | 65, 56–80 |
Cell cultures
ADSCs were derived from thoracic subcutaneous fat of
above-mentioned patients during operation under general
anaesthesia, as previously described (15). The adipose tissues were then washed
with phosphate-buffered saline (PBS) containing 1% penicillin and
streptomycin and subsequently digested with collagenase type I (1
mg/ml; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). The
conditions for digestion were maintained according to the
instructions for collagenase at 37°C for 60 min. Afterwards, we
filtered the suspension using a 200-µm nylon mesh, and the floating
adipocytes were removed after the suspension was centrifuged. The
remaining cells were harvested and cultured in Dulbecco's modified
Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% foetal bovine serum (FBS)
with 3.7 g/l sodium bicarbonate, and 1% penicillin and streptomycin
at 37°C with a 5% CO2 humidified atmosphere. At passage
3, 105 cells in 2 ml cell culture medium were plated in
6-well plates. The supernatants were collected and frozen at −80°C
for subsequent analyses after 3 days.
Bone marrow was aspirated from the sternum of each
participant and collected in heparinized tubes from upon cardiac
surgery (16). DMEM with 10% FBS,
3.7 g/l sodium bicarbonate and 1% penicillin and streptomycin was
used for culturing the isolated cells. Non-adherent cells were
removed by washing the cultures with the PBS solution after 3 days.
Again, 2 ml of cell culture medium of 105 cells were
plated in 6-well plates at passage 3, and the supernatants were
collected and frozen at −80°C for subsequent analyses after 3
days.
Peripheral blood monocytes were isolated from 40 ml
peripheral blood of the corresponding patient using human
lymphocyte separation solution and differential gradient
centrifugation with a Ficoll gradient (20 min, 800 × g) (17). Then, peripheral blood monocytes
were cultured in RPMI-1640 supplemented with 10% FBS at 37°C and a
5% CO2 humidified atmosphere. The cells were plated and
non-adherent cells were removed by gentle washing after 2 h. The
remaining adherent cells were harvested and the cell type was
confirmed by FSC-SSC parameters and surface expression of CD14 in
flow cytometry (FACS Calibur; BD Biosciences, San Jose, CA, USA)
(17,18). The identification results revealed
that the purity of monocytes was 72%. After collection, the
peripheral blood monocytes were cultured at 2×106 cells
in six-well plates and incubated with 100 µg/ml ox-LDL for 48 h in
serum-free RPMI-1640 to form foam cells.
Metabolomic profiling of supernatants
of ADSCs and BMSCs
Metabolomic analyses of the supernatant samples from
ADSCs and BMSCs were performed using the RRLC (6530 series; Agilent
Technologies, Inc., Santa Clara, CA, USA). In the present report, 6
major metabolic compounds from various pathways were selected for
metabolomics analysis.
Isolation and oxidation of LDL
Human LDL was isolated from the same participants as
above and oxidized (ox) as described by Boullier et al
(19). Briefly, LDL (density ¼
1.019–1.063 g/ml) was isolated from plasma by sequential
ultracentrifugation and incubated with 10 µmol/l CuSO4 for 18 h at
37°C. To prevent further oxidation, 0.1 mmol/l
ethylenediaminetetraacetic acid (EDTA) was added to collect the
ox-LDL at a concentration of 1 mg/ml. The extent of LDL oxidation
was assessed as described previously (20). In brief, ox-LDL preparations had
thiobarbituric acid-reactive substances of 0.30 mmol/g protein and
a relative mobility index on agarose gels of 2.0–2.5 compared to
native LDL.
M1 macrophages and foam cell
formation
Monocytes cultured in RPMI-1640 medium (Gibco;
Thermo Fisher Scientific, Inc.) with 10% FBS were differentiated
into M1 macrophages with 20 ng/ml GM-CSF and stimulated with 20
ng/ml IFN-γ and 1 ng/ml LPS for 24 h. Afterwards, the treated
macrophages were incubated with 100 µg/ml ox-LDL for another 24 h.
The cells were fixed in 4% formaldehyde and stained with Oil Red O,
and foam cells were counted by microscopy, as previously described
(21,22).
Transwell co-culture assay
Next, 2×105 macrophages or foam cells
were seeded in the lower compartment of 6-well Transwell
polyethylene terephthalate (PET) permeable supports in 24-mm
polycarbonate Transwell inserts with a pore size of 8 µm (Corning,
Inc., Corning, NY, USA) for 24 h. Serum-free medium was replaced 1
h before adding 2×106 ADSCs, BMSCs or BMSCs +cis-9,
trans-11 into the upper compartment of the Transwell inserts. In a
humidified chamber at 37°C, the co-cultures were incubated without
a medium change for 24 h, and the supernatants, as well as the
macrophages or foam cells at the bottom of the co-culture assay,
were collected for Oil Red O staining, flow cytometry analysis and
measurement of inflammatory factors in supernatants.
Measurement of intracellular lipid
droplets using oil red O staining
The macrophages and foam cells after they were
co-cultured with ADSCs, BMSCs or BMSCs +cis-9, trans-11 were washed
with PBS and fixed with 4% paraformaldehyde solution for 20 min.
Then, the cells were stained with 0.5% Oil Red O in isopropanol for
30 min and counterstained with haematoxylin for 5 min. The
macrophages were observed with an inverted fluorescence Microscope
(DMI4000B; Leica Microsystems GmbH, Wetzlar, Germany) and analysed
using Image-Pro Plus software 6.0. The number of lipid droplets was
presented as the mean value of integrated optical density
(IOD).
Cell viability assay
Cell viability was measured using Cell Counting
Assay kit-8 (CCK-8; CK04; Dojindo, Molecular Technologies, Inc.,
Kumamoto, Japan) according to the manufacturer's protocol. Briefly,
following pre-incubation with ox-LDL (100 µg/ml) for 24 h, M1
macrophages pre-treated in HTS Transwell 96-well plates with or
without ADSCs, BMSCs or BMSCs+ cis-9, trans-11 at a density of
1×104 cells per well. Then, 10 µl CCK-8 reagent was
added to the medium for 2 h, and the absorbance was measured using
a microplate reader (Infinite M200 Pro; Tecan, Group, Ltd.,
Mannedorf, Switzerland) at 450 nm. Each experiment was performed in
triplicate and was repeated at least three times.
Flow cytometry analysis
Before the analysis with flow cytometry, macrophages
and foam cells were stained cells by using the Annexin V-FITC and
PI Detection kit (BD Pharmingen; BD Biosciences). In brief, the
cells were trypsinized and resuspended at a density of
106/ml. After centrifugation, the pellet was washed
twice with ice-cold PBS and was suspended in binding buffer. The
cells were maintained in the dark at room temperature for 15 min
after incubation with FITC-labelled Annexin V and PI (Molecular
Probes; Thermo Fisher Scientific, Inc.), and the cells were
analysed using flow cytometry. More than 1×104 cells per
group were counted and each assessment was repeated three times to
get a proper statistical analysis.
Measurement of inflammatory
factors
The protein levels of TNF-α, IL-6, IL-8 and IL-10 in
the culture supernatants of macrophages and foam cells were
measured with ELISA kits (Sigma-Aldrich; Merck KGaA) according to
the manufacturer's protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from macrophages and foam
cells using TRIzol Reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). First-strand complementary DNA (cDNA) was synthesized using
Golden 1st cDNA Synthesis kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.) from 1 mg of total RNA. Applied Biosystems
synthesized the following primers: Hum P65 forward primer,
5′-CTGTATGGCACTGGCATTGTC-3′ and reverse,
5′-GGCACTTGAGAAGAGGGAGAG-3′ (length of 196 bp); Hum IL-6 forward,
5′-AGCCACTCACCTCTTCAGAAC-3′ and reverse,
5′-GCAAGTCTCCTCATTGAATCCAG-3′ (length of 200 bp); Hum TNF-α
forward, 5′-GTCTGGGCAGGTCTACTTTGG-3′ and reverse,
5′-GAGGTTGAGGGTGTCTGAAGG-3′ (length of 119 bp); Hum GAPDH forward,
5′-CCACTCCTCCACCTTTGAC-3′ and reverse, 5′-ACCCTGTTGCTGTAGCCA-3′
(length of 102 bp). RT-qPCR was performed with a Bio-Rad
Min-Opticon2 (Bio-Rad, Inc., Hercules, CA, USA) using SYBR-Green
PCR master mix kits (Tian Gen Biotech, Beijing, China). Initial
denaturation was performed at 95°C for 15 min, followed by 40
cycles of denaturation at 95°C for 10 sec, then annealing at 60°C
for 30 sec and extension at 60°C for 30 sec. The data were analyzed
using the Rotor-Gene Q software (version 1.7; Qiagen AB,
Sollentuna, Sweden), and relative mRNA levels were calculated using
the 2−∆∆Cq method (23)
and normalized against GAPDH. Each sample was analyzed in
triplicate, and the experiment was repeated ten times.
Western blot analysis
After the treatment described above, the protein
expression levels of NF-κB p65 and phosphorylated NF-κB p65 in the
lysates of cells harvested in RIPA sample buffer were detected by
Western blot analysis (24). The
samples were subjected to Western blot analyses using NF-κB p65,
phosphorylated NF-κB p65 (both Boster Biological Technology,
Pleasanton, CA, USA) and β-actin as a loading control. The protein
content was quantified with a BCA kit (Genscript, Piscataway, NJ,
USA), separated by 10% SDS-PAGE, and then transferred to
polyvinylidene fluoride (PVDF) membranes (EMD Millipore, Billerica,
MA, USA). The membranes were blocked in 5% (w/v) non-fat milk
diluted with TBST (Tris-HCl 20 mM, NaCl 150 mM, 0.1% Tween-20, pH
7.5) at room temperature for 1 h, incubated with a primary antibody
for 1 h at room temperature or overnight at 4°C, and then the
membranes were incubated with the appropriate secondary horseradish
peroxidase-conjugated antibodies for 1 h at room temperature. The
blot results were detected using Pierce ECL Plus Substrate
(Genscript), as described by the manufacturer. The density of the
target bands was quantified with Image-Pro Plus 6.0 (Media
Cybernetics, Inc., Rockville, MD, USA) software.
Statistical analysis
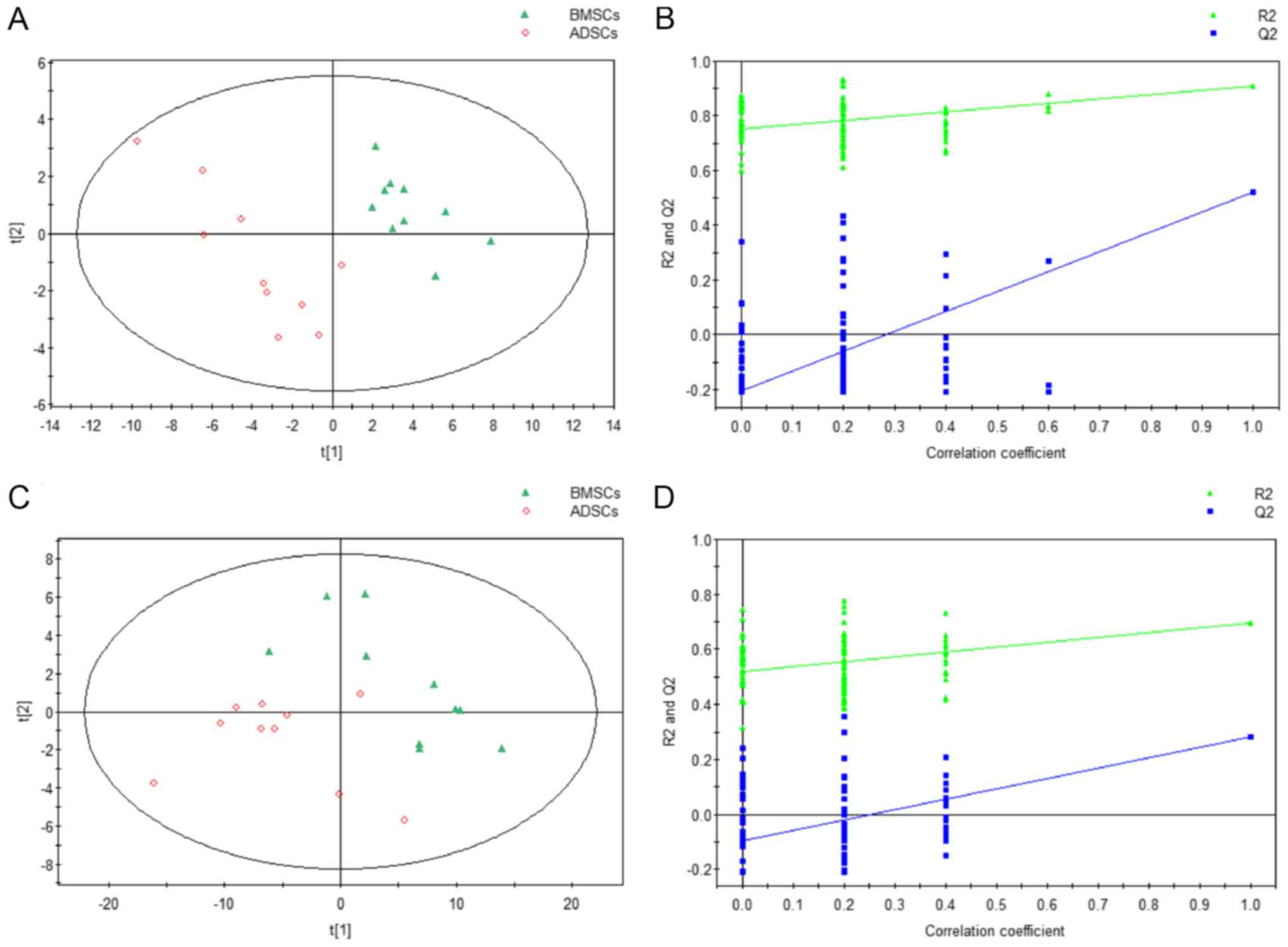
At first, the data on metabolic profiles of ADSCs or
BMSCs were used in principal component analysis (PCA) to detect the
group trends and outliers (25).
Then, we applied a Wilcoxon rank sum test to determine the
significance of each metabolite at P<0.05. To identify the
differences in metabolites between ADSCs and BMSCs, a partial least
squares discriminant analysis (PLS-DA) was used (25). We included permutation tests with
100 iterations to validate the supervised model and avoid
overfitting (26). Based on the
PLS-DA model, we calculated parameters that described the variable
importance in the projection (VIP) for each metabolite. With
thresholds of P-values and VIP values of 0.05 and 1, respectively,
the metabolic biomarkers were detected. The Wilcoxon rank sum test
was used with the R platform. The PCA and PLS-DA were performed
using SIMCA-P (version 11.5; Umetrics, Malmo, Sweden).
The other statistical analyses and graphing were
performed using GraphPad Prism software, version 5.00 (GraphPad
Software, Inc., La Jolla, CA, USA) by setting the statistical
significance at P<0.05. Two-group comparisons were analysed by
the unpaired two-tailed t-test. Multiple comparisons were evaluated
by analysis of variance with Tukey's or Dunnett's post hoc test, as
appropriate.
Results
Metabolomics of supernatants from
ADSCs and BMSCs
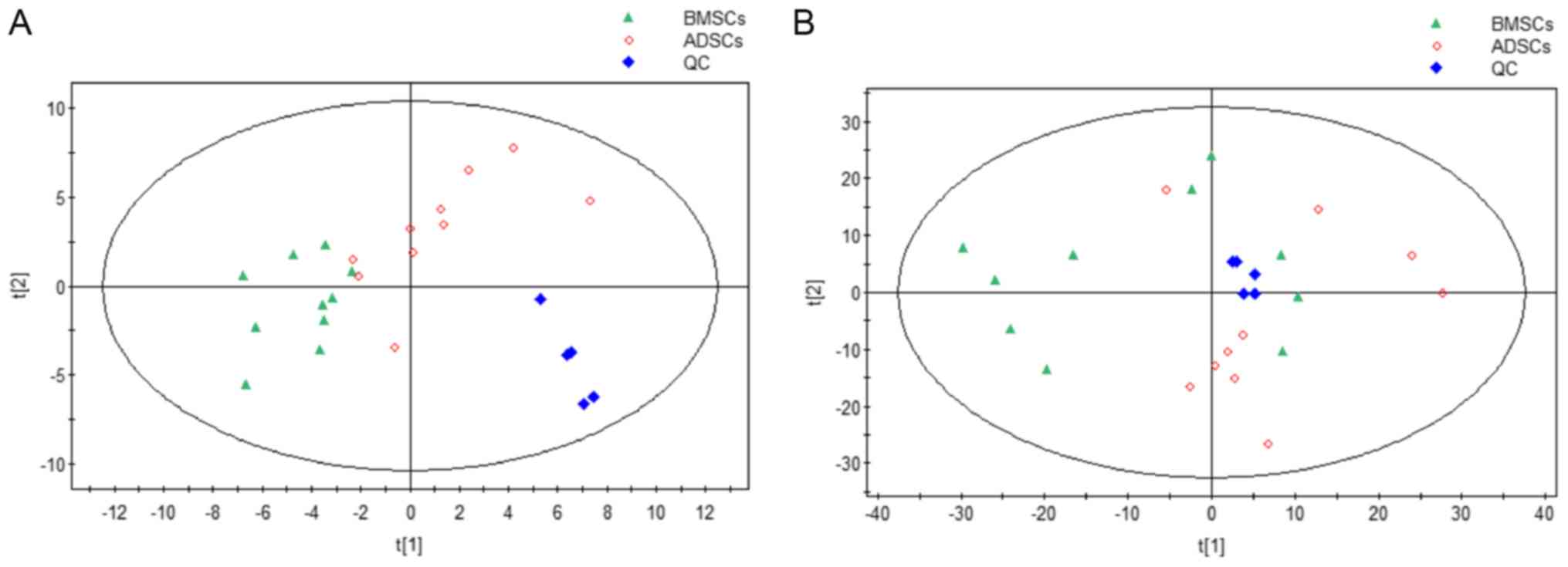
The results of the overall PCA based on all the
samples suggested that the QC (quality control) samples were
closely clustered in plots of PCA scores, which demonstrated that
the results of our metabolic profiling platform were robust.
Moreover, there were no outliers on the whole, and there were
separation trends between BMSCs and ADSCs (Fig. 1). Additionally, there were no
outliers overall, and there were separation trends between BMSCs
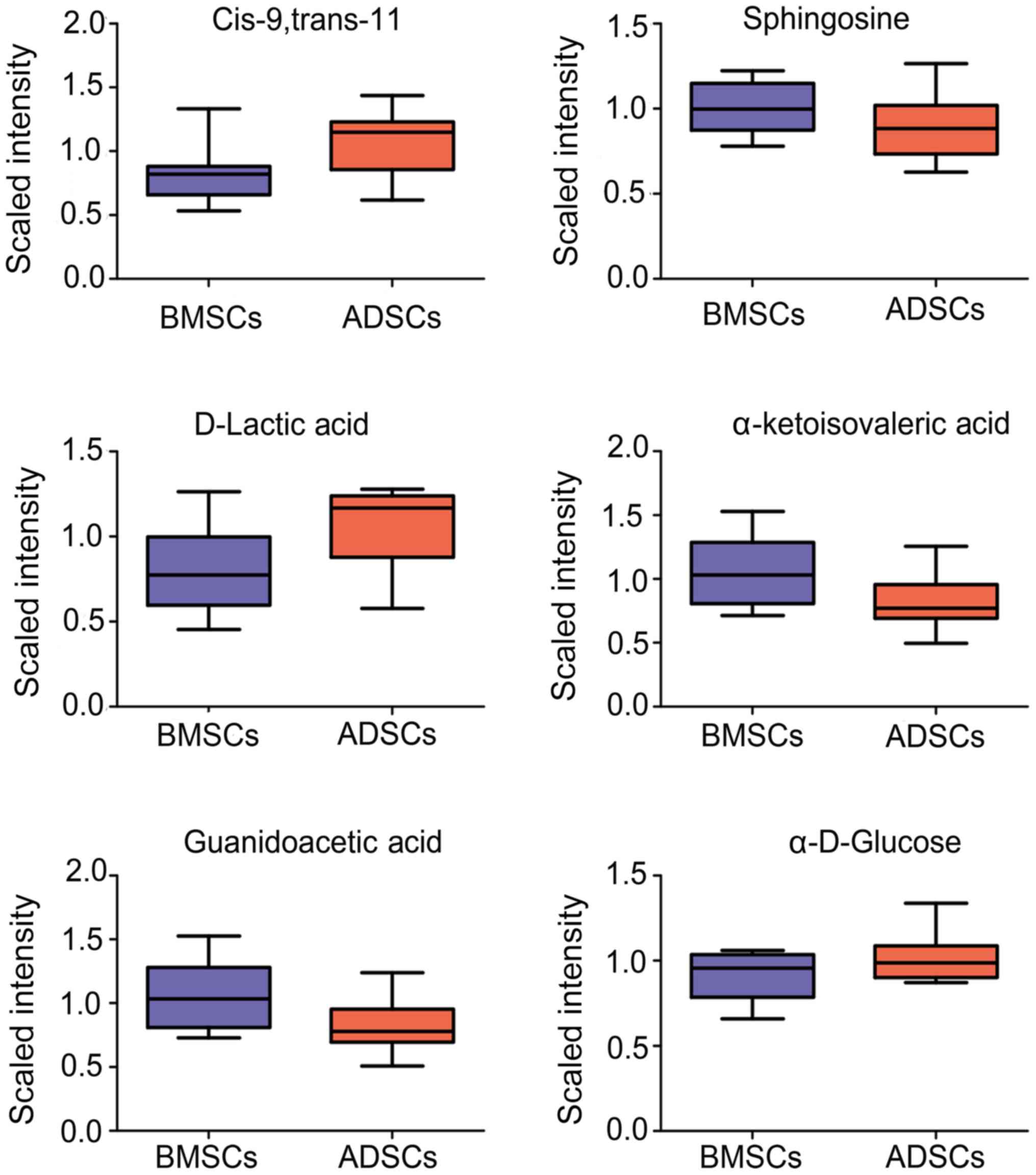
and ADSCs (Fig. 2). Cis-9,
trans-11, Sphingosine, D-Lactic acid, α-ketoisovaleric acid,
Guanidinoacetic acid, and α-D-Glucose ester were observed to be
elevated in the supernatant of ADSCs compared to BMSCs (Fig. 3).
Differential effects of inhibiting
ox-LDL-induced lipid accumulation in macrophage foam cells
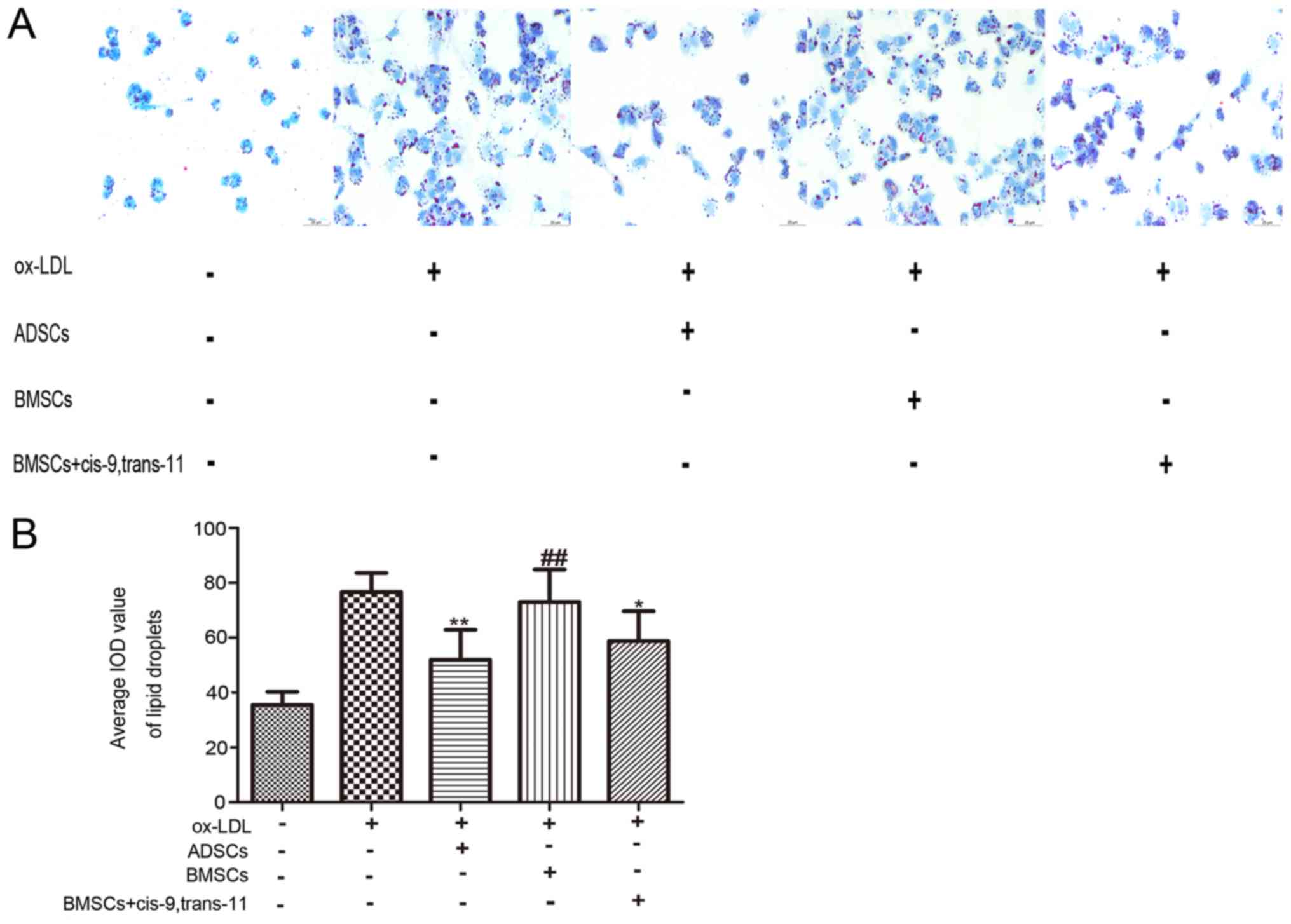
Many lipid droplets were present in macrophage foam
cells after treatment with ox-LDL (100 mg/l) for 24 h through Oil
Red O staining. However, the number of lipid droplets decreased
after pre-incubation of cells with ADSCs, BMSCs or BMSCs+cis-9,
trans-11 (Fig. 4A and B). The
number of lipid droplets from pre-incubation of ADSCs and
BMSCs+cis-9, trans-11 was less than those from pre-incubation with
BMSCs. In addition, the number of lipid droplets from
pre-incubation with ADSCs was less than those from pre-incubation
with BMSCs+ cis-9, trans-11.
Differential effects decreased the
secretion of inflammatory cytokines (TNF-α, IL-6, IL-8 and IL-10)
in ox-LDL-Stimulated macrophages
Lesional macrophages could secrete high amounts of
cytokines and chemokines, which further promoted the development of
AS by recruiting other immune cells into the vessel wall. TNF-α,
IL-6, IL-8 and IL-10 are essential for initiation and progression
of AS. As described in Fig. 5,
TNF-α, IL-6 and IL-8 protein levels were markedly increased, and
IL-10 had a marked decline in the medium of macrophages followed by
pre-incubation with 100 g/ml ox-LDL for 24 h. These changes of the
above inflammatory cytokines have decreased after treatment with
ADSCs, BMSCs and BMSCs+cis-9, trans-11. Nevertheless, the
inflammatory cytokines from pre-incubation with BMSCs showed
smaller changes compared to ADSCs and BMSCs+cis-9, trans-11. In
addition, pre-incubation with ADSCs significantly inhibited TNF-α,
IL-6 and IL-8 protein expression and promoted IL-10 expression.
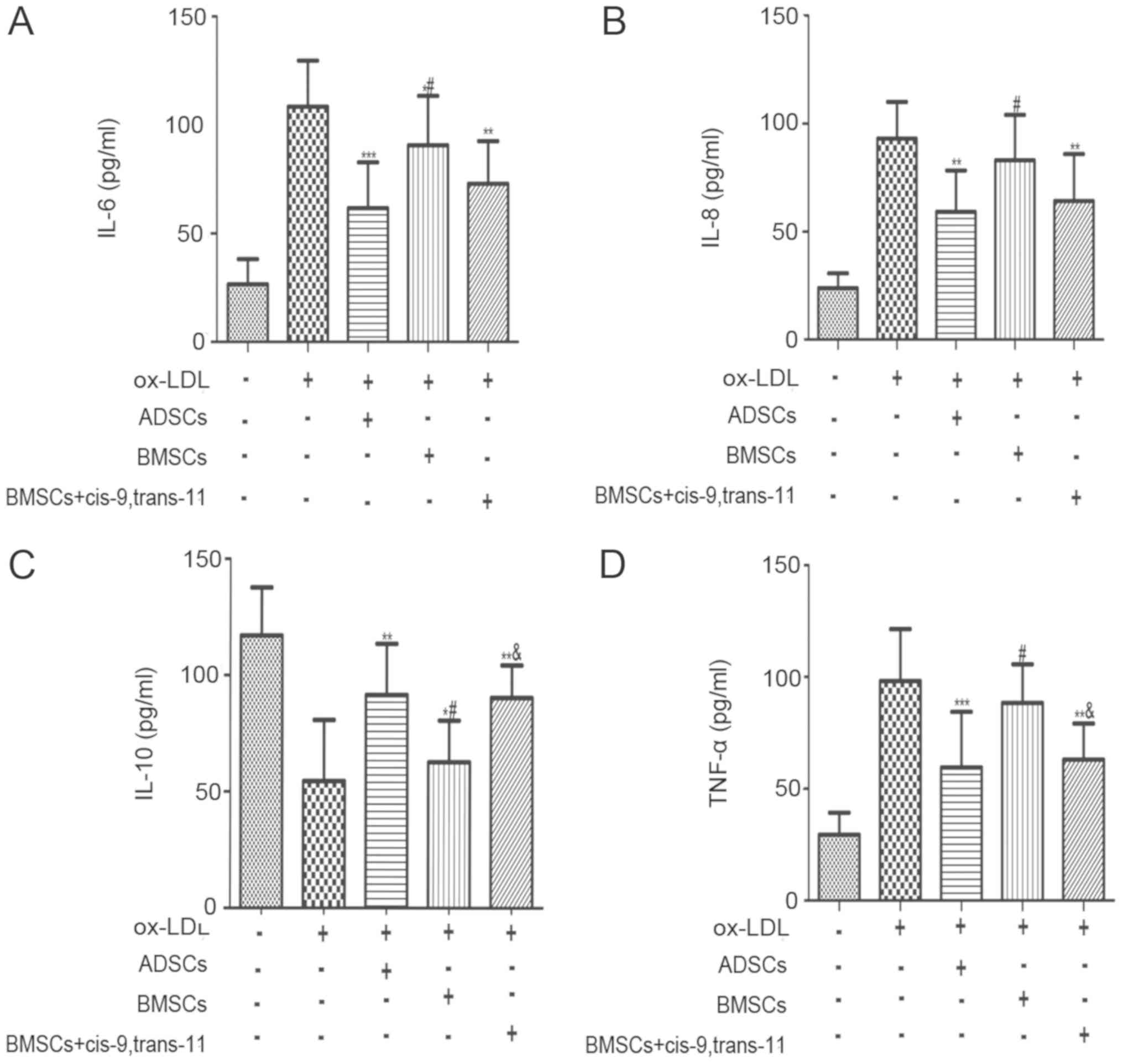 | Figure 5.ADSCs, BMSCs and BMSCs+cis-9,
trans-11 decreased the secretion of inflammatory factors (TNF-α and
IL-6) and increased the secretion of inflammatory factors (IL-10)
in ox-LDL-stimulated macrophages. Cells were pre-incubated with
ADSCs, BMSCs and BMSCs+cis-9, trans-11 following treatment with 100
g/ml ox-LDL for 24 h. The concentrations of (A) IL-6, (B) IL-8, (C)
IL-10 and (D) TNF-α secretion from the medium of macrophages were
measured using an ELISA kit. The results are expressed as a
percentage of the results obtained with a blank. Data are presented
as the mean ± standard deviation (n=10). *P<0.05, **P<0.01
and ***P<0.001 vs. the ox-LDL group; #P<0.05 vs.
the ADSCs group; &P<0.05 vs. the BMSCs group.
BMSCs, bone marrow-derived stem cells; ADSCs, adipose-derived stem
cells; TNF-α, tumour necrosis factor-α; IL-, interleukin-; ox-LDL,
oxidized low-density lipoprotein. |
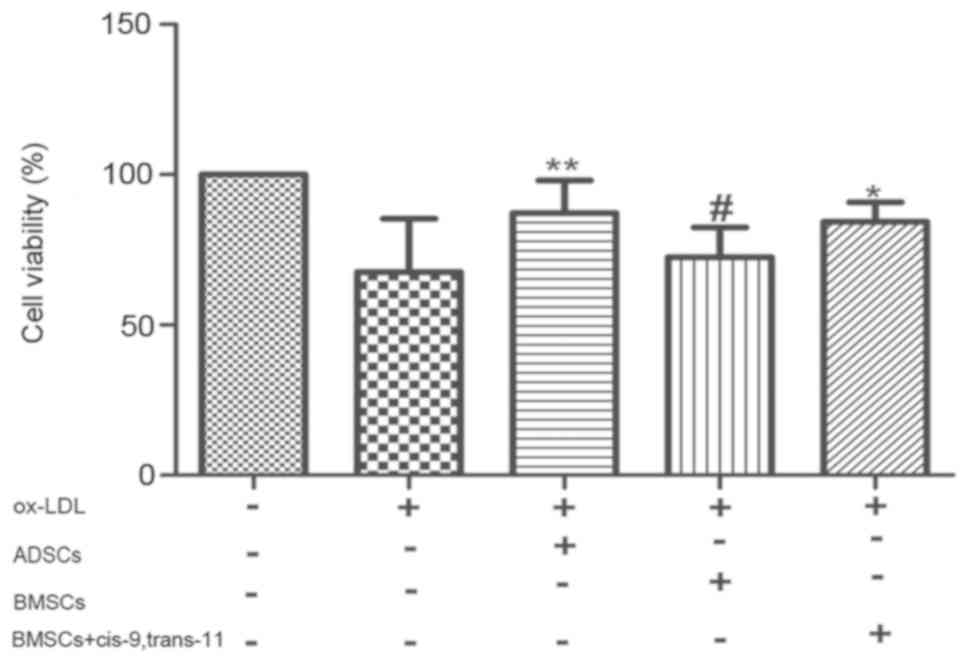
Effects of ADSCs, BMSCs and BMSCs+
cis-9, trans-11 on the viability of ox-LDL-stimulated
macrophages
Regarding the viability of the macrophages followed
100 g/ml ox-LDL for 24 h, ADSCs, BMSCs or BMSCs+cis-9, trans-11
exerted an obvious positive effect on cell viability. Here, we
showed that ADSCs significantly enhanced the viability of
Macrophages stimulated by ox-LDL (Fig.
6). We also found that cis-9, trans-11 enhanced the
proliferation of Macrophages stimulated by ox-LDL.
Different influence of attenuating
ox-LDL-induced apoptosis of macrophages
Apoptosis of macrophages plays a key role in AS
development. To determine the differences in ADSCs and BMSCs to
protect against ox-LDL-induced apoptosis, the treated cells were
subjected to Annexin V-FITC/PI double staining and analysed by flow
cytometry. As shown in Fig. 7A and
B, ox-LDL (100 g/ml) markedly increased apoptosis through the
results of flow cytometry analysis. Following pre-incubation with
ADSCs, BMSCs or BMSCs+cis-9, trans-11, the results of flow
cytometry analysis improved. Meanwhile, the effects of inhibited
macrophage apoptosis from co-cultures with ADSCs were better than
those with BMSCs and BMSCs+cis-9, trans-11, and BMSCs+cis-9,
trans-11 had a better effect than BMSCs. These observations
indicated that cis-9, trans-11 plays an important role in the
macrophage apoptotic pathway.
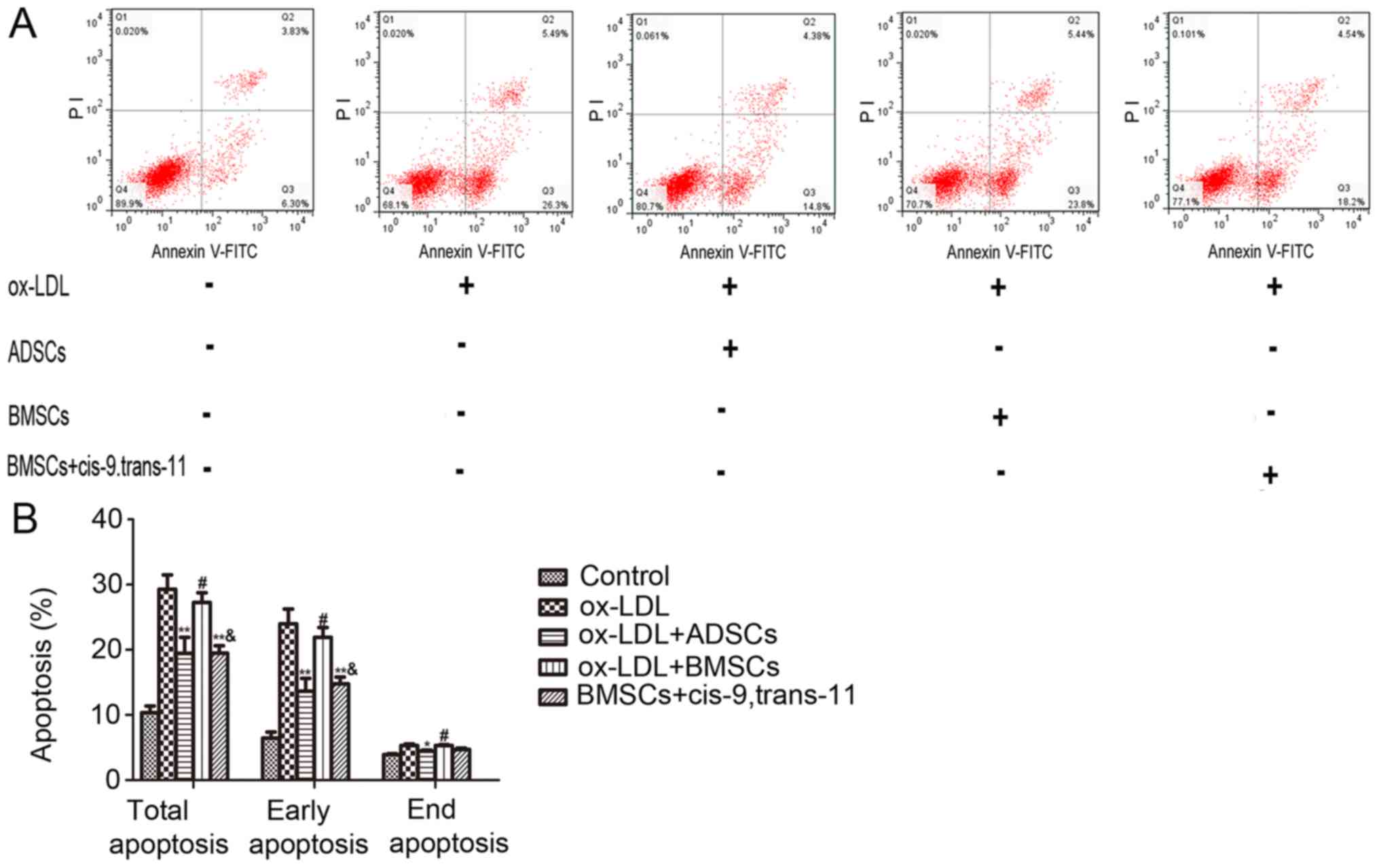 | Figure 7.ADSCs, BMSCs and BMSCs+cis-9,
trans-11 attenuated ox-LDL-induced apoptosis of M1 macrophages.
Cells were pre-incubated with cystathionine-γ-lyase (ADSCs, BMSCs
and BMSCs+cis-9, trans-11), followed by treatment with 100 g/ml
ox-LDL for 24 h. (A) The representative data of apoptotic cells
stained with Annexin V-FITC/PI which was detected by flow cytometry
analysis. (B) The percentage of apoptotic cells under different
treatments was measured and expressed as mean ± standard deviation
of at least three independent experiments (n=10). *P<0.05 and
**P<0.01 vs. the ox-LDL group; #P<0.05 vs. the
ADSCs group; &P<0.05 vs. the BMSCs group. BMSCs,
bone marrow-derived stem cells; ADSCs, adipose-derived stem cells;
ox-LDL, oxidized low-density lipoprotein; FITC, fluorescein
isothiocyanate; PI, propidium iodide. |
Effects of ADSCs, BMSCs or
BMSCs+cis-9, trans-11 expression on anti-inflammatory Gene mRNA
expression
The mRNA levels of the genes related to
pro-inflammatory factors were determined by RT-qPCR after
macrophage pre-incubation with 100 g/ml ox-LDL for 24 h and after
co-culture with ADSCs, BMSCs or BMSCs+cis-9, trans-11. As shown in
Fig. 8, the pro-inflammatory genes
TNF-α, CD36 and NF-κBp65 were increased after pre-incubation with
100 g/ml ox-LDL for 24 h, but ADSCs, BMSCs or cis-9, trans-11 all
reduced the expression of inflammatory gene significantly.
Comparatively, the decrease of expression of pro-inflammatory genes
was most after co-culture with ADSCs, and BMSCs+cis-9, trans-11
also significantly inhibited these gene expression than BMSCs alone
in this respect.
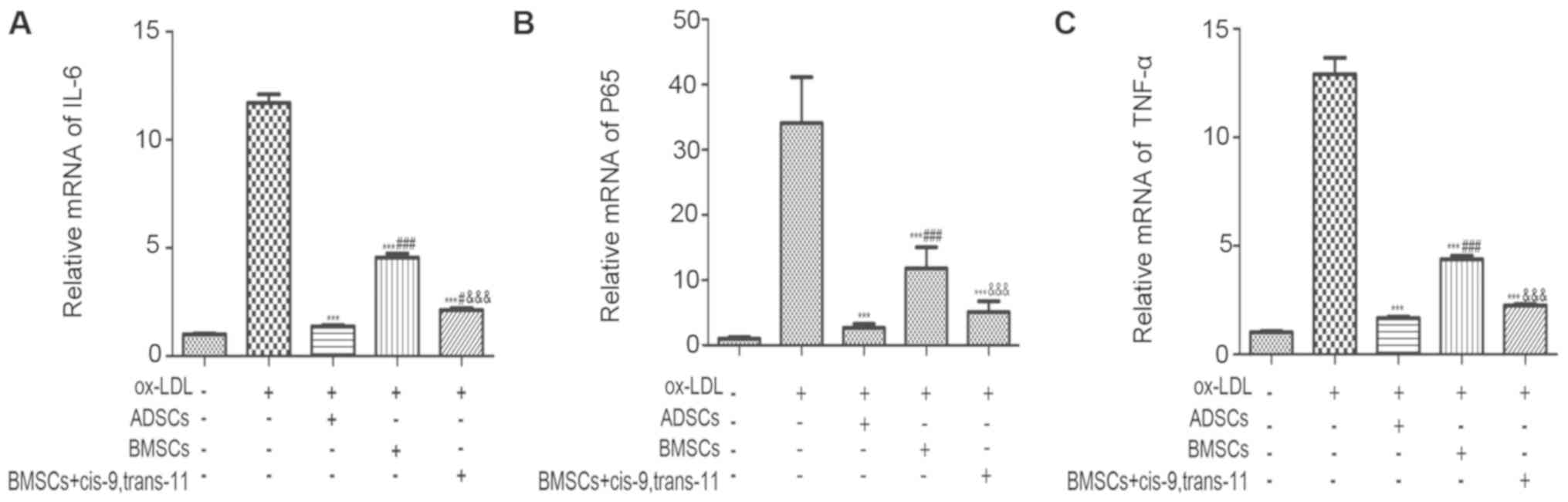 | Figure 8.Effects of the inhibition of
proinflammatory factors on oxLDL-induced NFκBp65, TNF-α and IL-6
upregulation. Reverse transcription-quantitative polymerase chain
reaction analysis of (A) IL-6, (B) NFκBp65 and (C) TNF-α mRNA level
in macrophages co-cultured with ADSCs, BMSCs or BMSCs+cis-9,
trans-11. Data are expressed as the mean ± standard deviation
(n=10). ***P<0.001 vs. the ox-LDL group; #P<0.05
and ###P<0.001 vs. the ADSCs group;
&&&P<0.001 vs. the BMSCs group. NFκBp65,
nuclear factor-κBp65; TNF-α, tumour necrosis factor-α; IL-,
interleukin-; BMSCs, bone marrow-derived stem cells; ADSCs,
adipose-derived stem cells; ox-LDL, oxidized low-density
lipoprotein. |
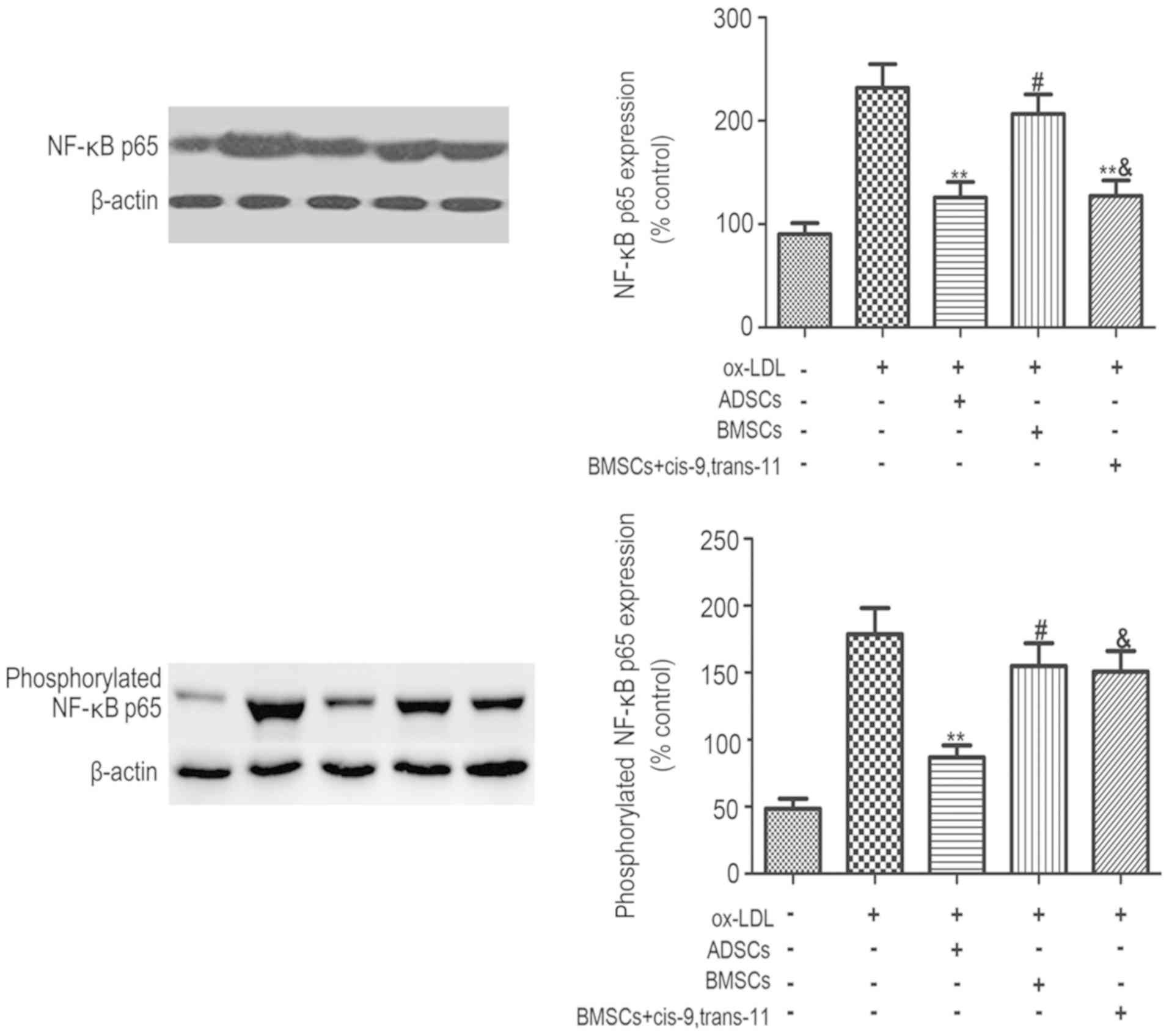
Effects of ADSCs, BMSCs and
BMSCs+cis-9, trans-11 on inflammatory protein expression
The protein levels of NF-κBp65 (Fig. 9) and phosphorylated NF-κBp65
(Fig. 9) following macrophage
co-culture with ADSCs, BMSCs and BMSCs+cis-9, trans-11 were
detected using Western blot analysis as shown in Fig. 8A. The inflammatory protein NF-κBp65
and phosphorylated NF-κBp65 were decreased consistently by 0.6-
(Non), 0.5- (Ox-LDL), and 0.7- (Non), 0.6- (Ox-LDL) fold.
Discussion
Macrophages are the key factor in the initiation and
progression of AS. Macrophages demonstrate versatility and
plasticity accompanied by environmental signals. Foam cells are a
hallmark feature of atherosclerotic plaques that follow exposure to
ox-LDL (27,28). As inflammation develops, necrotic
lipid-filled cores are formed after the death of macrophages and
foam cells. Substances that are toxic to cells are released from
these cells and are thought to contribute to plaque
destabilization. A clinical event, such as a heart attack or
stroke, could be caused by this destabilization. Accordingly,
targeting that improves the anti-inflammatory ability of
macrophages may provide a therapeutic strategy for treating AS
(27).
The immunomodulatory properties of MSCs are one
factor that could lead to new strategies, but the mechanisms of
action for these cells are complicated and not fully understood.
The major finding of the metabolomics part of this study was that
supernatant levels of Cis-9, trans-11 (Cis-9, trans-11) in ADSCs
were significantly higher compared to BMSCs. Additionally, cis-9,
trans-11 (Cis-9, trans-11) is one isomer of CLA that is present in
human adipose tissue. CLA has anti-atherogenic properties that have
been confirmed in animal models (29–31).
Cis-9, trans-11 has been reported as the key isomer involved in the
obstruction of AS development (32). Many studies have demonstrated that
CLA dienes could modulate the function of immune system cells
through some inflammatory pathways, such as the PPARγ pathway,
which is an important member of this nuclear receptor family
(33–35).
A few recent reports have shown that MSCs may
regulate the immunogenicity of macrophages (36–38).
Indeed, it has been reported that the immunogenicity and phagocytic
capacity of macrophages could be regulated through the release of
cytokines by ADSCs (39). However,
the effects of reducing the inflammatory reaction of macrophage by
ADSCs was more obviously as compared to BMSCs, and whether they
work through secretion of cis-9, trans-11 has yet to be
investigated. Unfortunately, the exact quantity of the secretion of
cis-9, trans-11 by ADSCs and BMSCs via metabolomics has not been
measured. Because whether slightly increased CLA is harmful to the
human body has not been reported, 30 µM CLAs exogenous cis-9,
trans-11 was added to the medium of BMSCs in our study (40).
The new findings of our present study suggest human
MSCs, and particularly ADSCs, regulate immunogenicity of
ox-LDL-stimulated M1 macrophages by secretion of cis-9, trans-11
(cis-9, trans-11). In this experiment, the culture of peripheral
blood monocytes from the same donors in the presence of GM-CSF,
IFN-γ and LPS triggered their differentiation and maturation led to
the development of M1 macrophages, which was confirmed by FSC-SSC
parameters and surface expression of CD14 by flow cytometry.
However, co-culture of ox-LDL-stimulated M1 macrophages with ADSCs,
BMSCs and BMSCs+cis-9, trans-11 caused significant changes. The
findings demonstrated that ADSCs resulted in a greater increase in
the secretion of anti-inflammatory cytokines, such as IL-10 and a
greater decrease in the secretion of pro-inflammatory cytokines,
such as TNF-, IL-6 and IL-8 of ox-LDL-stimulated M1 macrophages
compared to BMSCs and BMSCs+bovinic acid. A similar effect was
observed with mRNA expression of TNF-α, CD36 and NF-κBp65. Protein
expression of NF-κBp65 and phosphorylated NF-κBp65 in ADSCs
co-cultured with M1 macrophage foam cells were also significantly
diminished. Additionally, we found that ADSCs increased macrophage
foam cell phagocytosis and cell viability, as well as decreased
macrophage foam cell apoptosis, compared to BMSCs. However, the
anti-inflammatory effects, phagocytosis, cell viability and
anti-apoptotic effects in BMSCs+cis-9, trans-11 co-cultured with M1
macrophage foam cells were more markedly enhanced compared to
BMSCs, and the results were still weaker than ADSCs co-cultured
with M1 macrophage foam cells. Overall, our data indicate that
protection against AS of ADSCs acts on M1 macrophage foam cells and
that a major mechanism involves activation of an NF-κBp65-TNF-α
pathway. These findings could provide further insight into a
potential mechanism for ADSCs in AS.
MSCs have emerged as a promising tool for the
treatment of AS due to their anti-inflammatory properties. As
previously suggested for BMSCs, cyclo-oxygenase-2 (COX-2)
activation seems to play an important role in anti-inflammatory
properties (38). Although some
previous studies have shown that ADSCs have the capacity to inhibit
TNF-α-dependent inflammation and induce angiogenesis as well as
enhance overall tissue repair (41), there are few studies that explain
the underlying mechanism of inhibiting TNF-α by ADSCs. The results
of our present study indicated decreases in TNF-α, IL-6 and IL-8
and an increase in IL-10 production following treatment with ADSCs,
BMSCs and BMSCs+ cis-9, trans-11 in M1 macrophages, which coincides
with previous studies exhibiting potent anti-inflammatory
properties of cis-9, trans-11 in RAW macrophages (42,43).
The decrease in TNF-α and IL-6 could impede the expression of
NF-κBp65 and phosphorylated NF-κBp65, which are involved in
systemic inflammation in the atherosclerotic process (44). Both RT-qPCR and Western blot
analysis in this study have indicated that expression of NF-κB p65
and phosphorylated NF-κBp65 in nuclei of ADSCs had the strongest
inhibitory effect on M1 macrophages. This outcome suggests the
secretion of cis-9, trans-11 by ADSCs plays a major role in an
anti-inflammatory capability that was better than the capability of
BMSCs. However, anti-inflammatory capability, phagocytic activity,
anti-apoptotic capability and the cell viability assay in M1
macrophage foam cells of BMSCs+ cis-9, trans-11 was still weaker
than ADSCs, which suggests that the mechanisms of cis-9,
trans-11-TNF-α-dependent inflammation do not have a role in the
anti-inflammatory processes of M1 macrophage foam cells. As shown
in previous studies for ADSCs, soluble LIGHT (a lymphotoxin-related
inducible ligand that competes for glycoprotein D binding to herpes
virus entry mediator on T cells) also has a role in the immune
reaction (45). Zhou et al
(46) reported that the activated
microglia/macrophage anti-inflammatory responses were more robust
in Sprague-Dawley rats that underwent a transplant with ADSCs
compared to Sprague-Dawley rats that underwent a transplant with
BMSCs from the decreased numbers of ED1-positive macrophages
(46).
At the same time, our experiments need to be
improved. We considered that cis-9, trans-11 was the most abundant
CLA isomer (over 75–80% of total CLA) suppressing inflammatory
response, apoptosis and functioning at cholesterol homeostasis in
macrophages, when we designed this experiment. Therefore, we have
not examined the role of cis-9, trans-11 in ox-LDL-stimulated M1
macrophage without BMSCs. In the next experiment, we will design
the experiment more reasonably.
In summary, our present study unmasked cis-9,
trans-11 from ADSCs as an important mechanism to regulate
macrophage immune function. The application of ADSC therapy can be
further developed by studies of their specific mechanisms.
Therefore, based on these results, ADSCs may be more suitable than
BMSCs for stem cell therapy in AS, especially for older patients.
The specific molecular mechanisms will be investigated more
thoroughly in our future work.
Acknowledgements
The authors would like to thank Miss Li Rui-Ting
(China Pharmaceutical University, Jiangsu, China) and Miss Sun Meng
(Division of Cardiovascular Surgery, Second Affiliated Hospital of
Harbin Medical University, Key Laboratory of Education Ministry for
Myocardial Ischemia, Heilongjiang, China) for their technical and
scientific advice.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81471805,
81871501); Heilongjiang Provincial Science and Technology Research
Project (grant no. H2018021); Postdoctoral Science Foundation of
Heilongjiang Province (grant no. LBH- Q17094); Harbin Municipal
Science and Technology Research Fund of Innovative Talents Project
(grant no. RC2016QN004036) and “Yu Weihan” Outstanding Young
Investigator Award (2014) to Kai Kang; Key Laboratory of Education
Ministry for Myocardial Ischemia Open Research Foundation(grant no.
KF201711) was given to Jian-zhong Li; National Natural Science
Foundation of China (grant no. 81571681, 81701707, 81871886) were
given to X-PL, S-QJ and X-LY respectively.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
J-ZL, B-DX, HQ, J-CH, X-PL, KK and S-LJ were
involved in the study design. J-ZL, T-HC, HQ, X-PL, X-LY, FZ, X-LL,
HW and J-CH performed the experiments, data analysis and manuscript
writing. J-ZL, KK, S-LJ and S-QJ were involved in the study design
and revised the manuscript. All authors read and approved the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethical
Committee of the Second Affiliated Hospital of Harbin Medical
University (approval no. KY-2017-076). All included patients were
informed about the nature of the study and gave their written
informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AS
|
atherosclerosis
|
|
LDL
|
low-density lipoprotein
|
|
MSCs
|
Mesenchymal stem cell
|
|
ADSCs
|
adipose-tissue-derived stromal
cells
|
|
BMSCs
|
bone marrow mesenchymal stem cells
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
FBS
|
foetal bovine serum
|
|
PBS
|
phosphate-buffered saline
|
|
EDTA
|
ethylenediaminetetraacetic acid
|
|
ox-LDL
|
oxidized LDL
|
|
RPMI
|
Roswell Park Memorial Institute
|
|
PET
|
polyethylene terephthalate
|
|
IOD
|
integrated optical density
|
|
CCK-8
|
Cell Counting Assay kit-8
|
|
PCA
|
principal component analysis
|
|
PLS-DA
|
partial least squares discriminant
analysis
|
|
VIP
|
variable importance in the
projection
|
|
QC
|
quality control
|
|
CLA
|
conjugated linoleic acid
|
|
CLAs
|
conjugated linoleic acid dienes
|
|
COX-2
|
cyclo-oxygenase-2
|
|
PVDF
|
polyvinylidene fluoride
|
References
|
1
|
Cheong C and Choi JH: Dendritic cells and
regulatory T cells in atherosclerosis. Mol Cells. 34:341–347. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Frostegård J: Immunity, atherosclerosis
and cardiovascular disease. BMC Med. 11:1172013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gerry AB and Leake DS: Effect of low
extracellular pH on NF-κB activation in macrophages.
Atherosclerosis. 233:537–544. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wigren M, Nilsson J and Kolbus D:
Lymphocytes in atherosclerosis. Clin Chim Acta. 413:1562–1568.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Callegari A, Coons ML, Ricks JL, Yang HL,
Gross TS, Huber P, Rosenfeld ME and Scatena M: Bone marrow- or
vessel wall-derived osteoprotegerin is sufficient to reduce
atherosclerotic lesion size and vascular calcification.
Arterioscler Thromb Vasc Biol. 33:2491–2500. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eirin A, Zhu XY, Krier JD, Tang H, Jordan
KL, Grande JP, Lerman A, Textor SC and Lerman LO: Adipose
tissue-derived mesenchymal stem cells improve revascularization
outcomes to restore renal function in swine atherosclerotic renal
artery stenosis. Stem Cells. 30:1030–1041. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yoon YS, Wecker A, Heyd L, Park JS,
Tkebuchava T, Kusano K, Hanley A, Scadova H, Qin G, Cha DH, et al:
Clonally expanded novel multipotent stem cells from human bone
marrow regenerate myocardium after myocardial infarction. J Clin
Invest. 115:326–338. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gojo S, Gojo N, Takeda Y, Mori T, Abe H,
Kyo S, Hata J and Umezawa A: In vivo cardiovasculogenesis by direct
injection of isolated adult mesenchymal stem cells. Exp Cell Res.
288:51–59. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ichim TE, Alexandrescu DT, Solano F, Lara
F, Campion Rde N, Paris E, Woods EJ, Murphy MP, Dasanu CA, Patel
AN, et al: Mesenchymal stem cells as anti-inflammatories:
Implications for treatment of Duchenne muscular dystrophy. Cell
Immunol. 260:75–82. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sharma AK, Bury MI, Fuller NJ, Marks AJ,
Kollhoff DM, Rao MV, Hota PV, Matoka DJ, Edassery SL, Thaker H, et
al: Cotransplantation with specific populations of spina bifida
bone marrow stem/progenitor cells enhances urinary bladder
regeneration. Proc Natl Acad Sci USA. 110:4003–4008. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sharma AK, Hota PV, Matoka DJ, Fuller NJ,
Jandali D, Thaker H, Ameer GA and Cheng EY: Urinary bladder smooth
muscle regeneration utilizing bone marrow derived mesenchymal stem
cell seeded elastomeric poly(1,8-octanediol-co-citrate) based thin
films. Biomaterials. 31:6207–6217. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Anderson P, Souza-Moreira L, Morell M,
Caro M, O'Valle F, Gonzalez-Rey E and Delgado M: Adipose-derived
mesenchymal stromal cells induce immunomodulatory macrophages which
protect from experimental colitis and sepsis. Gut. 62:1131–1141.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wise AF, Williams TM, Kiewiet MB, Payne
NL, Siatskas C, Samuel CS and Ricardo SD: Human mesenchymal stem
cells alter macrophage phenotype and promote regeneration via
homing to the kidney following ischemia-reperfusion injury. Am J
Physiol Renal Physiol. 306:F1222–F1235. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Heneidi S, Simerman AA, Keller E, Singh P,
Li X, Dumesic DA and Chazenbalk G: Awakened by cellular stress:
Isolation and characterization of a novel population of pluripotent
stem cells derived from human adipose tissue. PLoS One.
8:e647522013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Russo V, Yu C, Belliveau P, Hamilton A and
Flynn LE: Comparison of human adipose-derived stem cells isolated
from subcutaneous, omental, and intrathoracic adipose tissue depots
for regenerative applications. Stem Cells Transl Med. 3:206–217.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kang K, Sun L, Xiao Y, Li SH, Wu J, Guo J,
Jiang SL, Yang L, Yau TM, Weisel RD, et al: Aged human cells
rejuvenated by cytokine enhancement of biomaterials for surgical
ventricular restoration. J Am Coll Cardiol. 60:2237–2249. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Müller I, Schönberger T, Schneider M,
Borst O, Ziegler M, Seizer P, Leder C, Müller K, Lang M,
Appenzeller F, et al: Gremlin-1 is an inhibitor of macrophage
migration inhibitory factor and attenuates atherosclerotic plaque
growth in ApoE-/-mice. J Biol Chem. 288:31635–31645. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Seizer P, Schönberger T, Schött M, Lang
MR, Langer HF, Bigalke B, Krämer BF, Borst O, Daub K, Heidenreich
O, et al: EMMPRIN and its ligand cyclophilin A regulate MT1-MMP,
MMP-9 and M-CSF during foam cell formation. Atherosclerosis.
209:51–57. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Boullier A, Li Y, Quehenberger O, Palinski
W, Tabas I, Witztum JL and Miller YI: Minimally oxidized LDL
offsets the apoptotic effects of extensively oxidized LDL and free
cholesterol in macrophages. Arterioscler Thromb Vasc Biol.
26:1169–1176. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yao S, Sang H, Song G, Yang N, Liu Q,
Zhang Y, Jiao P, Zong C and Qin S: Quercetin protects macrophages
from oxidized low-density lipoprotein-induced apoptosis by
inhibiting the endoplasmic reticulum stress-C/EBP homologous
protein pathway. Exp Biol Med (Maywood). 237:822–831. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hong L, Xie ZZ, Du YH, Tang YB, Tao J, Lv
XF, Zhou JG and Guan YY: Alteration of volume-regulated chloride
channel during macrophage-derived foam cell formation in
atherosclerosis. Atherosclerosis. 216:59–66. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ricci R, Sumara G, Sumara I, Rozenberg I,
Kurrer M, Akhmedov A, Hersberger M, Eriksson U, Eberli FR, Becher
B, et al: Requirement of JNK2 for scavenger receptor A-mediated
foam cell formation in atherogenesis. Science. 306:1558–1561. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ayaori M, Sawada S, Yonemura A, Iwamoto N,
Ogura M, Tanaka N, Nakaya K, Kusuhara M, Nakamura H and Ohsuzu F:
Glucocorticoid receptor regulates ATP-binding cassette
transporter-A1 expression and apolipoprotein-mediated cholesterol
efflux from macrophages. Arterioscler Thromb Vasc Biol. 26:163–168.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Trygg J, Holmes E and Lundstedt T:
Chemometrics in metabonomics. J Proteome Res. 6:469–479. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
van Velzen EJ, Westerhuis JA, van
Duynhoven JP, van Dorsten FA, Hoefsloot HC, Jacobs DM, Smit S,
Draijer R, Kroner CI and Smilde AK: Multilevel data analysis of a
crossover designed human nutritional intervention study. J Proteome
Res. 7:4483–4491. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ross R: Rous-whipple award lecture.
Atherosclerosis: A defense mechanism gone awry. Am J Pathol.
143:987–1002. 1993.PubMed/NCBI
|
|
28
|
Pennings M, Meurs I, Ye D, Out R, Hoekstra
M, Van Berkel TJ and Van Eck M: Regulation of cholesterol
homeostasis in macrophages and consequences for atherosclerotic
lesion development. FEBS Lett. 580:5588–5596. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kritchevsky D, Tepper SA, Wright S,
Czarnecki SK, Wilson TA and Nicolosi RJ: Conjugated linoleic acid
isomer effects in atherosclerosis: Growth and regression of
lesions. Lipids. 39:611–616. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee KN, Kritchevsky D and Pariza MW:
Conjugated linoleic acid and atherosclerosis in rabbits.
Atherosclerosis. 108:19–25. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Toomey S, Harhen B, Roche HM, Fitzgerald D
and Belton O: Profound resolution of early atherosclerosis with
conjugated linoleic acid. Atherosclerosis. 187:40–49. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Arbonés-Mainar JM, Navarro MA, Guzmán MA,
Arnal C, Surra JC, Acín S, Carnicer R, Osada J and Roche HM:
Selective effect of conjugated linoleic acid isomers on
atherosclerotic lesion development in apolipoprotein E knockout
mice. Atherosclerosis. 189:318–327. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ramakers JD, Plat J, Sébédio JL and
Mensink RP: Effects of the individual isomers cis-9,trans-11 vs.
trans-10,cis-12 of conjugated linoleic acid (CLA) on inflammation
parameters in moderately overweight subjects with LDL-phenotype B.
Lipids. 40:909–918. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schleser S, Ringseis R and Eder K:
Conjugated linoleic acids have no effect on TNF alpha-induced
adhesion molecule expression, U937 monocyte adhesion, and chemokine
release in human aortic endothelial cells. Atherosclerosis.
186:337–344. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang H, Guo Y and Yuan J: Conjugated
linoleic acid enhanced the immune function in broiler chicks. Br J
Nutr. 94:746–752. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dayan V, Yannarelli G, Billia F, Filomeno
P, Wang XH, Davies JE and Keating A: Mesenchymal stromal cells
mediate a switch to alternatively activated monocytes/macrophages
after acute myocardial infarction. Basic Res Cardiol.
106:1299–1310. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim J and Hematti P: Mesenchymal stem
cell-educated macrophages: A novel type of alternatively activated
macrophages. Exp Hematol. 37:1445–1453. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Németh K, Leelahavanichkul A, Yuen PS,
Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller
BH, Brown JM, et al: Bone marrow stromal cells attenuate sepsis via
prostaglandin E(2)-dependent reprogramming of host macrophages to
increase their interleukin-10 production. Nat Med. 15:42–49. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Adutler-Lieber S, Ben-Mordechai T,
Naftali-Shani N, Asher E, Loberman D, Raanani E and Leor J: Human
macrophage regulation via interaction with cardiac adipose
tissue-derived mesenchymal stromal cells. J Cardiovasc Pharmacol
Ther. 18:78–86. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Stachowska E, Baśkiewicz-Masiuk M,
Dziedziejko V, Adler G, Bober J, Machaliński B and Chlubek D:
Conjugated linoleic acids can change phagocytosis of human
monocytes/macrophages by reduction in Cox-2 expression. Lipids.
42:707–716. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jiang D, Qi Y, Walker NG, Sindrilaru A,
Hainzl A, Wlaschek M, MacNeil S and Scharffetter-Kochanek K: The
effect of adipose tissue derived MSCs delivered by a chemically
defined carrier on full-thickness cutaneous wound healing.
Biomaterials. 34:2501–2515. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lee Y, Thompson JT and Vanden Heuvel JP:
9E,11E-conjugated linoleic acid increases expression of the
endogenous antiinflammatory factor, interleukin-1 receptor
antagonist, in RAW 264.7 cells. J Nutr. 139:1861–1866. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yu Y, Correll PH and Vanden Heuvel JP:
Conjugated linoleic acid decreases production of pro-inflammatory
products in macrophages: Evidence for a PPAR gamma-dependent
mechanism. Biochim Biophys Acta. 1581:89–99. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang Y, Wang GZ, Rabinovitch PS and Tabas
I: Macrophage mitochondrial oxidative stress promotes
atherosclerosis and nuclear factor-κB-mediated inflammation in
macrophages. Circ Res. 114:421–433. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kim HM, Jeong CS, Choi HS, Kawada T and Yu
R: LIGHT/TNFSF14 enhances adipose tissue inflammatory responses
through its interaction with HVEM. FEBS Lett. 585:579–584. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhou Z, Chen Y, Zhang H, Min S, Yu B, He B
and Jin A: Comparison of mesenchymal stromal cells from human bone
marrow and adipose tissue for the treatment of spinal cord injury.
Cytotherapy. 15:434–448. 2013. View Article : Google Scholar : PubMed/NCBI
|