Introduction
Alzheimer's disease (AD) is the most frequently
occurring type of adult dementia with 46.0 million diagnosed
individuals globally, causing a loss of one billion dollars to the
healthcare industry each year (1).
Clinically, patients with AD often present with progressive
cognitive decline. Pathologically, AD is characterized by the
accumulation of β amyloid (Aβ) peptides in plaques and neuronal
loss in a range of brain regions (1,2).
Despite this understanding of AD, there are currently no effective
measures for treating AD, and the prognosis remains poor (2). Thus, further investigation into the
pathogenesis of AD is required for therapeutic exploitation.
Emerging evidence has revealed that cerebral blood
flow (CBF) alterations are involved in AD pathogenesis. Clinical
studies have demonstrated that CBF disturbance occurs in at-risk
elderly subjects, even prior to Aβ accumulation (3–5).
Mouse models implicate capillary disturbances as precursors for
AD-associated neurodegenerative changes (6). Disturbed CBF may cause a decline in
brain perfusion, breakdown of the brain-blood barrier and cerebral
clearance disorder, which consequently results in Aβ accumulation
(7,8). However, the pattern of AD-associated
CBF alterations and how these contribute to AD pathology remains
unclear.
Magnetic resonance imaging (MRI)-arterial spin
labeling (ASL) provides an in vivo technique for measuring
CBF by magnetically labeling arterial water as an endogenous tracer
(9). Studies using ASL in humans
indicate that brain perfusion changes are already present prior to
the onset of AD symptoms (9,10).
ASL-measured CBF has been demonstrated to distinguish adults with
probable AD from cognitively normal individuals (11). Furthermore, CBF may sensitively
predict cognitive decline and conversion to moderate cognitive
impairment or AD over time (4).
Thus, ASL imaging may serve as a promising tool for the measurement
of CBF in preclinical AD.
The hippocampus and entorhinal cortex are thought to
be the first regions affected in AD pathology (12). With the progression of AD, an
increasing number of regions, including the frontal cortex, are
affected (13). Numerous studies
have attempted to disclose the factors causing the heterogeneous
development of AD pathology (14,15),
but few have focused on the contribution of CBF alterations.
Advances in micro-MRI techniques have allowed the quantification of
CBF in small animals with ASL (9,12),
providing novel avenues to study CBF in AD. Detailed study of ASL
in AD animals may help to further elucidate AD pathology and
identify novel therapeutic strategies.
The aim of the present study was to determine the
CBF alterations in four mouse brain regions (the entorhinal cortex,
hippocampus, frontoparietal cortex and thalamus). Mice of four
varying age groups mimicking the respective stages of AD in humans
[2 months (pre-clinical AD), 3.5 months (sub-clinical AD), 5 months
(early-clinical AD) and 8 months (mid-clinical AD)] were used to
evaluate the age-associated changes in regional CBF in a transgenic
mouse model of AD (16), and to
subsequently investigate the underlying vascular pathogenesis. The
ultimate goal was to identify experimental cues for the detection
of AD in patients using ASL.
Materials and methods
Animals
The present study was ethically approved by the
Institutional Animal Care and Use Committee of the Medical College
of Zhengzhou University (Zhengzhou, China). The use of animals was
based on the guidelines published in the National Institutes of
Health Guide for the care and use of laboratory animals (16).
AβPPSWE/PS1ΔE9 (APP/PS1)
transgenic mice (C57BL/6J) evidently exhibit the pathological and
behavioral changes of AD and are widely used to mimic human AD in
research (17). All mice were
tested using polymerase chain reaction-genotyping of the tail
tissue to assess their genotype (16). In the present study, a total of 36
female APP/PS1 mice (age, 45–50 days; weight, 15.1–18.2 g) and 36
age-matched female wild-type (WT) littermates (age, 45–50 days;
weight, 14.9–18.5 g) were purchased from The Institute of
Experimental Animals of The Chinese Academy of Medical Science
(Beijing, China). The mice were randomly assigned to four groups.
Due to a similar evolution in pathology and behavior with human AD,
each group of mice was tested at ages 2, 3.5, 5 or 8 months old
(mo) to mimic the pre-, sub-, early- and mid-clinical stage of AD,
respectively (APP/PS1 mice, n=9 in each group; WT mice, n=9 in each
group) (18). All mice had free
access to food and water and were housed in cages in an
environmentally controlled room (temperature, 22±1°C; relative
humidity, 40–60%) under a 12-h light/dark cycle.
Morris water maze (MWM) test
The spatial memory learning and memory was assessed
using MWM test as previously described (16,19).
The test was performed in a white circular pool (diameter, 100 cm)
divided into four equal quadrants. The pool was filled with water
made opaque with non-fat milk at a temperature of 22±1°C. Mice were
allowed to swim for 60 sec in the pool with a visible platform.
Subsequently, an escape platform (diameter, 6 cm) was placed in the
center of quadrant II, 1 cm below the water surface. Each mouse
performed the test three times per day for 60 sec for 5 successive
days, and in each test the mice were allowed to swim freely and to
reach the escape platform. The swimming time (escape latency) was
recorded and the average time was calculated. At day 6 each mouse
performed a test without escape platform and the mouse was allowed
to swim freely for 1 min. The number of platform crossings was
recorded for each mouse.
MRI-ASL
All MR scans were conducted on a 7.0-T MRI scanner
(V.70/16; PharmaScan; Bruker Corporation, Ettlingen, Germany) with
a 16 cm bore size and a high-performance gradient system. The
signal was received using a 23 mm surface coil. Mice were
anesthetized by inhalation of 5% isoflurane, placed in a prone
position on the bed, and maintained under anesthesia with 2%
isoflurane. The animal's head was secured with a bite bar and two
ear bars. Body temperature was maintained at 37°C with a warm
waterbed. The respiratory and heart rates were continuously
monitored using a vital sign monitoring system. Anatomical
T2-weighted MR images containing the whole brain were acquired on
37 slices at thicknesses of 0.7-mm without any gap between them,
with the relaxation enhancement (RARE) sequence with the following
parameters: RARE factor 8, repletion time (TE) = 4000 msec, echo
time (TR) = 24 msec, field of interest (FOV) = 20 mm × 20 mm, and
matrix = 256×256. The T2-weighted images were used to align the
subsequent ASL location. ASL images were produced using the
Flow-sensitive Alternating Inversion Recovery technique (20,21).
A coronal slice at a thickness of 2 mm that simultaneously crossed
the frontal lobe and hippocampus was examined. Selective and
non-selective ASL images were alternatively acquired. The ASL
parameters were as follows: TR = 5000.0 msec; TE = 16.0 msec; FOV =
1.80 cm; slice thickness = 2 mm; matrix = 64×64; number of
excitations = 1. The total scan time was ~20 min and 18 sec.
ASL data analysis
The obtained ASL images were analyzed using the
workstation software ParaVision 6.0 (Bruker Corporation) to
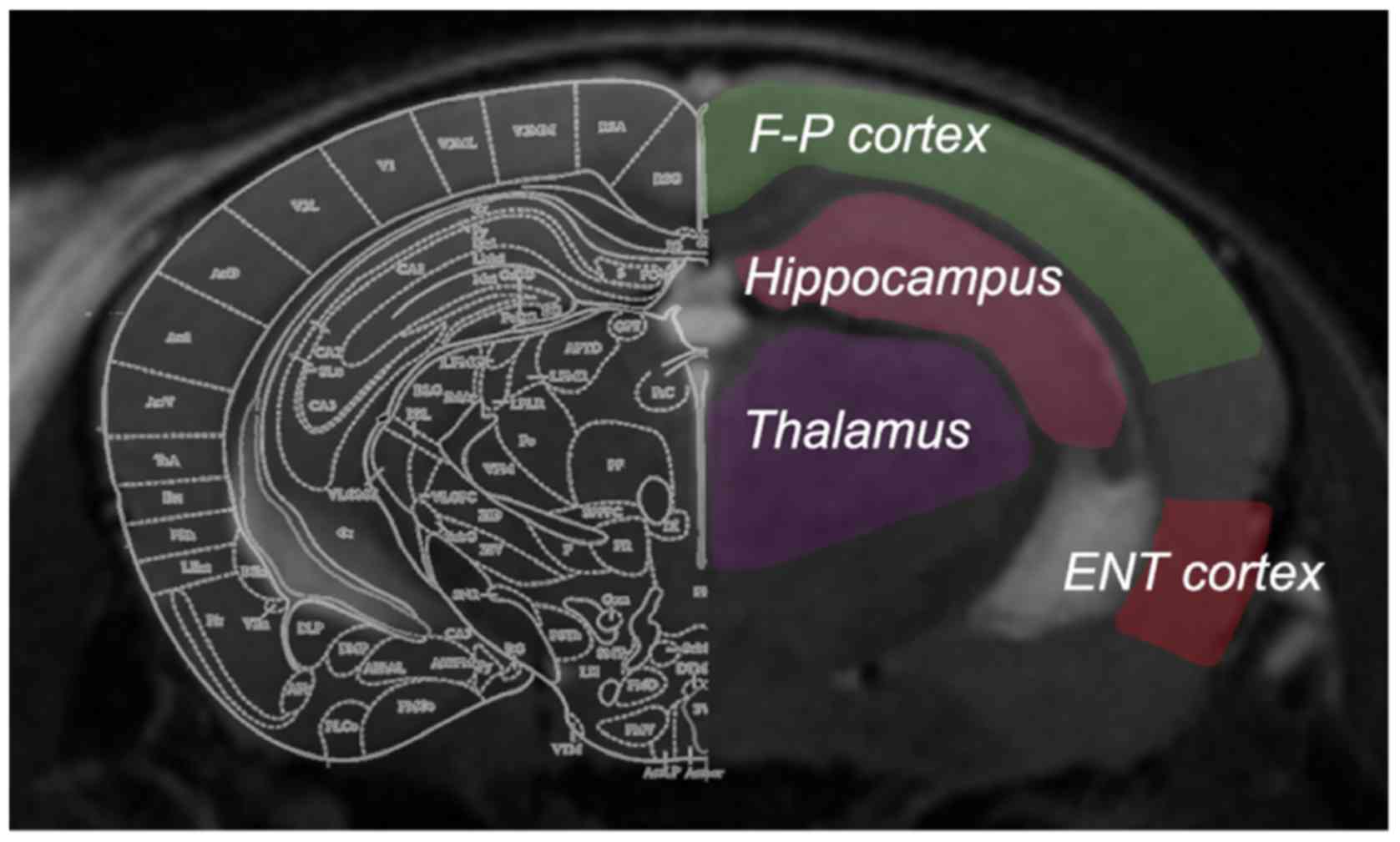
generate CBF images. To determine CBF values, four regions of
interest (ROIs) were manually drawn on the non-selective inversion
image (Fig. 1), according to the
mouse brain atlas by Paxinos and Watson (22). Anatomical T2-weighted images with
the same slice location were displayed concurrently for reference.
The ROIs were outlined carefully to include the hippocampus,
entorhinal cortex, frontoparietal cortex and thalamus, with the
involvement of major arteries excluded. The ROIs were then overlaid
onto the CBF map with an accurate boundary alignment. From the CBF
map, the mean blood flow within the ROIs of each brain was
calculated. Bilateral ROIs from the same mouse were analyzed
together.
Histological evaluation
Subsequent to MR examination, for histological
evaluation, the mice were subject to cardiac perfusion with 4%
paraformaldehyde at 4°C for >30 min while remaining deeply
anesthetized. Then, brains were collected, post-fixed at room
temperature for at least 24 h and sliced into 2-mm-thick coronal
blocks. The location of the examined brain blocks was selected
based on the distance from the bregma upon ASL imaging. The brain
blocks that involved the frontoparietal cortex and dorsal
hippocampus were embedded in paraffin and sliced into 6-µm-thick
sections for staining.
Immunohistochemical (IHC) staining was used to
reveal vascular histology, which was performed as described
previously (23). Brain sections
were dewaxed, dehydrated, and incubated in epitope retrieval
solution (OriGene Technologies, Inc., Beijing, China) at 100°C for
40 min. Endogenous peroxidase activity was blocked in 4%
H2O2 at room temperature for 30 min, followed
by three washes with PBS. Then, sections were stained with a
cluster of differentiation 31 (CD31) antibody (1:500; clone 1A10;
cat. no. PA0250; Novocastra; Leica Microsystems, Ltd., Milton
Keynes, UK) at 4°C overnight, followed by incubation with a
secondary antibody (1:50; horseradish peroxidase-labeled anti-mouse
immunoglobulin G; Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) at 37°C for 60 min and 3,3′-diaminobenzidine
(Beijing Zhongshang Jinqiao Biotechnology Co., Ltd., Beijing,
China). Counterstaining was performed using hematoxylin (at 100
mg/ml for 30 sec), and then sections were cover-slipped subsequent
to dehydration with a graded alcohol series. The obtained sections
were captured with a camera (TCS SP2 laser confocal microscope;
Leica Microsystems, Ltd., Heidelberg, Germany; magnification, ×200)
and analyzed using the ImageJ software (version 1.50b; National
Institutes of Health, Bethesda, MD, USA). The vessel density
(vessel number per mm2), vessel size (area per vessel)
and vessel area (vessel area per mm2) were calculated
based on the CD31-stained sections. Data from bilateral asymmetric
brain regions were pooled together for analysis.
Statistical analysis
All results were expressed as the mean ± standard
error of the mean. Statistical analyses were performed using
GraphPad Prism 6.0 (GraphPad Software, Inc., La Jolla, CA, USA). A
two-way analysis of variance (ANOVA) was used to analyze the MWM
and ASL data; one-way ANOVA was used to analyze the IHC data,
followed by Tukey's multiple comparison tests to assess
between-group differences. Pearson's correlation test was used to
assess the association between ASL data and vessel area. P<0.05
was considered to indicate a statistically significant
difference.
Results
Age-associated alterations of regional
CBF occur in APP/PS1 mice
In the present study, ASL was used to reveal CBF
changes in APP/PS1 mice of varying ages; representative CBF images
are presented in Fig. 2A. Compared
with WT controls, APP/PS1 mice had increased CBF in the
frontoparietal cortex and thalamus at age 2 mo; this increase was
more notable at age 3.5 mo. By contrast, CBF was only slightly
increased in the entorhinal cortex and hippocampus of APP/PS1 mice
at 2 and 3.5 mo compared with WT controls (Fig. 2B). In APP/PS1 mice at 5 mo,
increased CBF was reversed in the frontoparietal cortex and
thalamus but maintained a consistent level in the entorhinal cortex
and hippocampus. At age 8 mo, CBF was reduced in a number of brain
regions of APP/PS1 mice even in the WT controls (Fig. 2A). However, MR images revealed no
notable structural changes in the brain of APP/PS1 mice at any age
(data not shown).
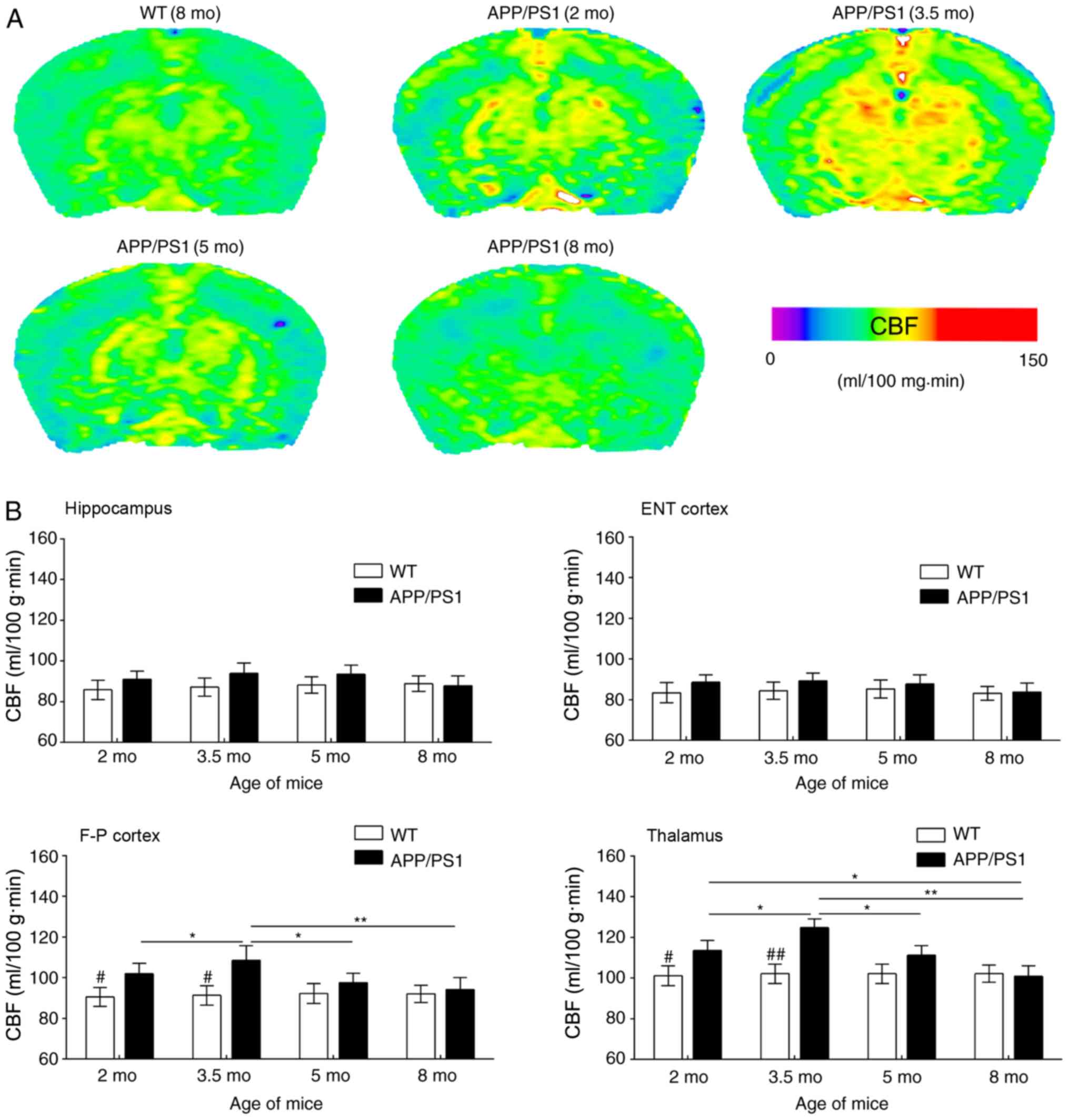 | Figure 2.Results of the ASL examination. (A)
Typical CBF maps, taken at the frontoparietal lobe and hippocampus
from WT (8 mo) and APP/PS1 mice (2, 3.5, 5 and 8 mo). A color map
was applied. (B) Age-associated changes in CBF in the ENT cortex,
hippocampus, F-P cortex and thalamus for WT and APP/PS1 mice of
varying ages. #P<0.05 and ##P<0.01 vs.
age-matched mice; *P<0.05 and **P<0.01 vs. APP/PS1 mice of
other age groups. ASL, arterial spin labeling; CBF, cerebral blood
flow; WT, wild-type; mo, months old; ENT, entorhinal; F-P,
frontoparietal. |
ASL images were quantitatively analyzed based on the
ROIs in Fig. 1 (Fig. 2B). Compared with the age-matched WT
controls, CBF values in the frontoparietal cortex and thalamus of
APP/PS1 mice were significantly increased at 2 mo; this CBF
increase was more evident at 3.5 mo, but appeared to decline at 5
mo and returned to basal levels at 8 mo. Despite a slight CBF
increase in the hippocampus and entorhinal cortex of APP/PS1 mice
at ages 2 and 3.5 mo, increased CBF was not statistically
significant at any age including 5 and 8 mo, compared with the
age-matched controls. Regional analysis revealed that the
frontoparietal cortex and thalamus exhibited transient CBF
increases in early AD, whereas the entorhinal cortex and
hippocampus exhibited no notable changes in CBF.
Vessel histology
CD31 staining was used to investigate the vascular
causes underlying CBF changes; representative images are presented
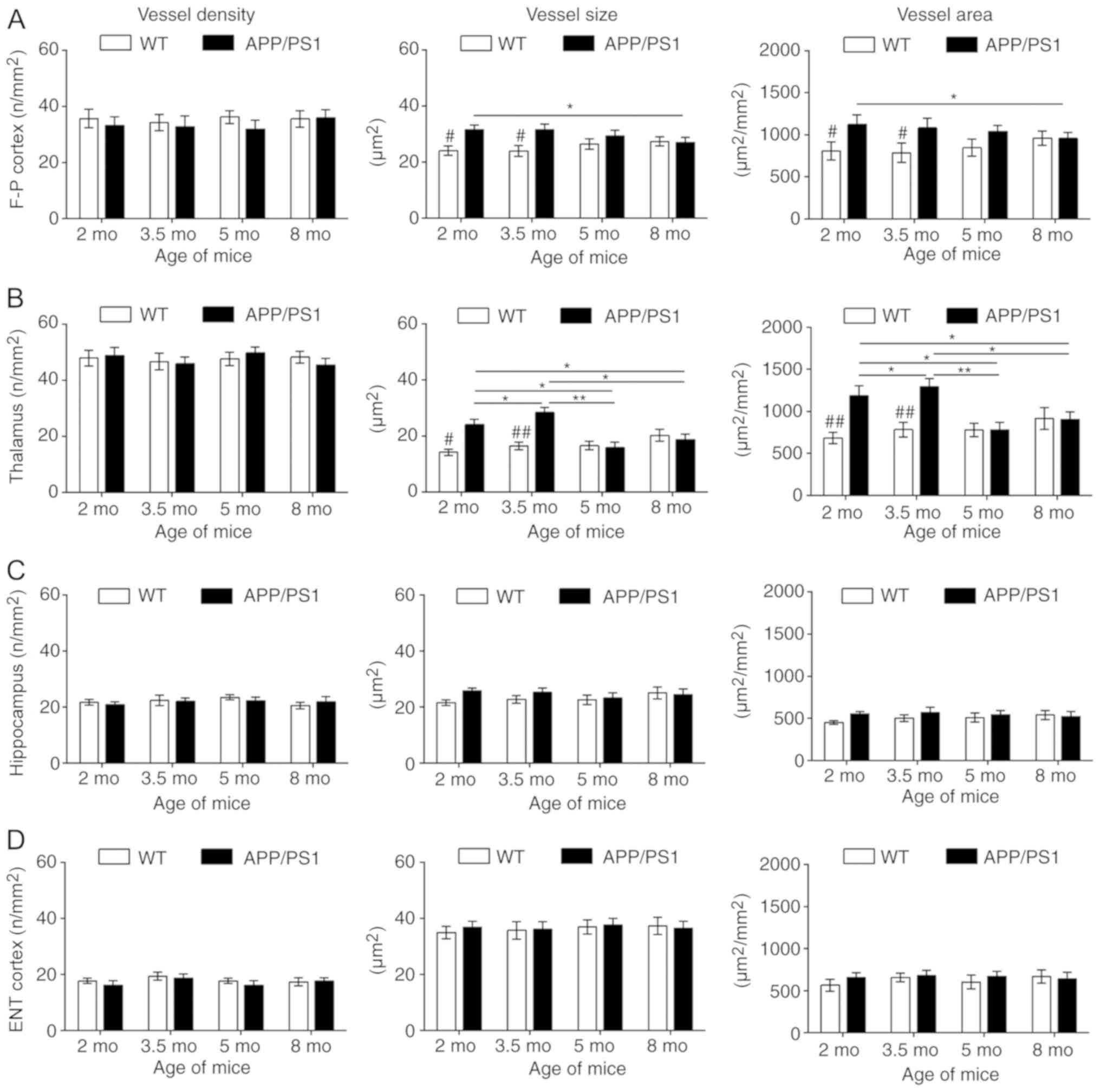
in Fig. 3. Compared with the
age-matched WT controls, the vessel size and vessel area were
significantly increased in the frontoparietal cortex (Fig. 4A) and thalamus (Fig. 4B) of APP/PS1 mice at 2 and 3.5 mo,
with the strongest increase at 3.5 mo. Although APP/PS1 mice also
demonstrated an increase in vessel size in the hippocampus
(Fig. 4C) and entorhinal cortex
(Fig. 4D), the increased size was
not significantly larger compared with that of control mice. In
APP/PS1 mice at 5 and 8 mo, the vessel size and vessel area in the
frontoparietal cortex and thalamus returned to levels equal to that
of the control mice, but the vessel size and vessel area vessel
decreased in the thalamus and cortex in APP/PS1 mice compared with
the age-matched controls. No difference in vessel density was
observed in all four ROIs between the APP/PS1 and control mice at
any age.
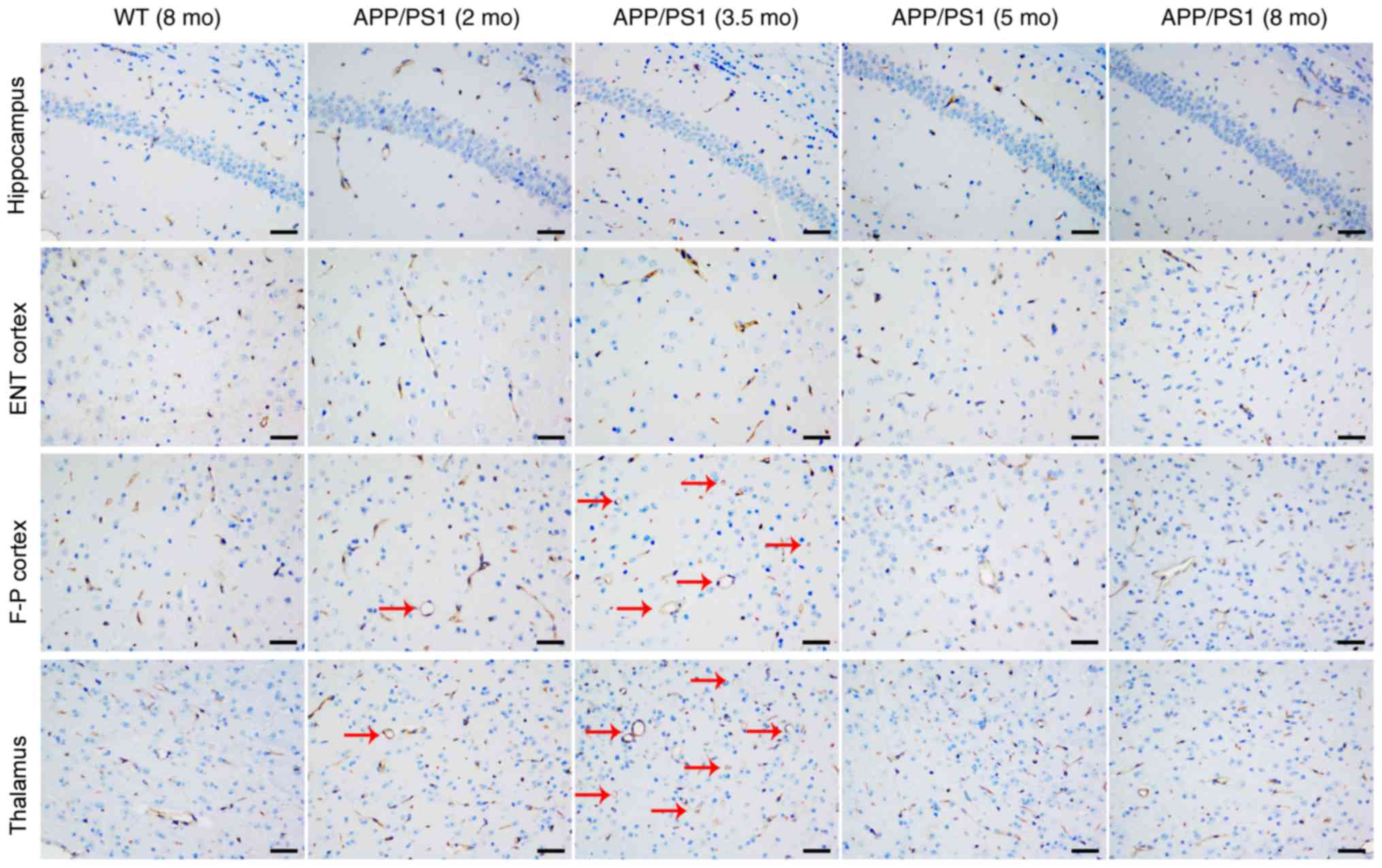 | Figure 3.Representative cluster of
differentiation 31 staining images of the hippocampus, ENT cortex,
F-P cortex and thalamus from WT (8 mo) and APP/PS1 mice (2, 3.5, 5
and 8 mo). Vessels appeared extended (as indicated by the red
arrows) in the F-P cortex and thalamus of APP/PS1 mice at ages 2
and 3.5 mo, but not at 5 and 8 mo. No other abnormalities were
observed in all brain regions of APP/PS1 mice at any age. Scale
bar, 50 µm. WT, wild-type; mo, months old; ENT, entorhinal; F-P,
frontoparietal. |
Correlation between ASL data and
vessel histology
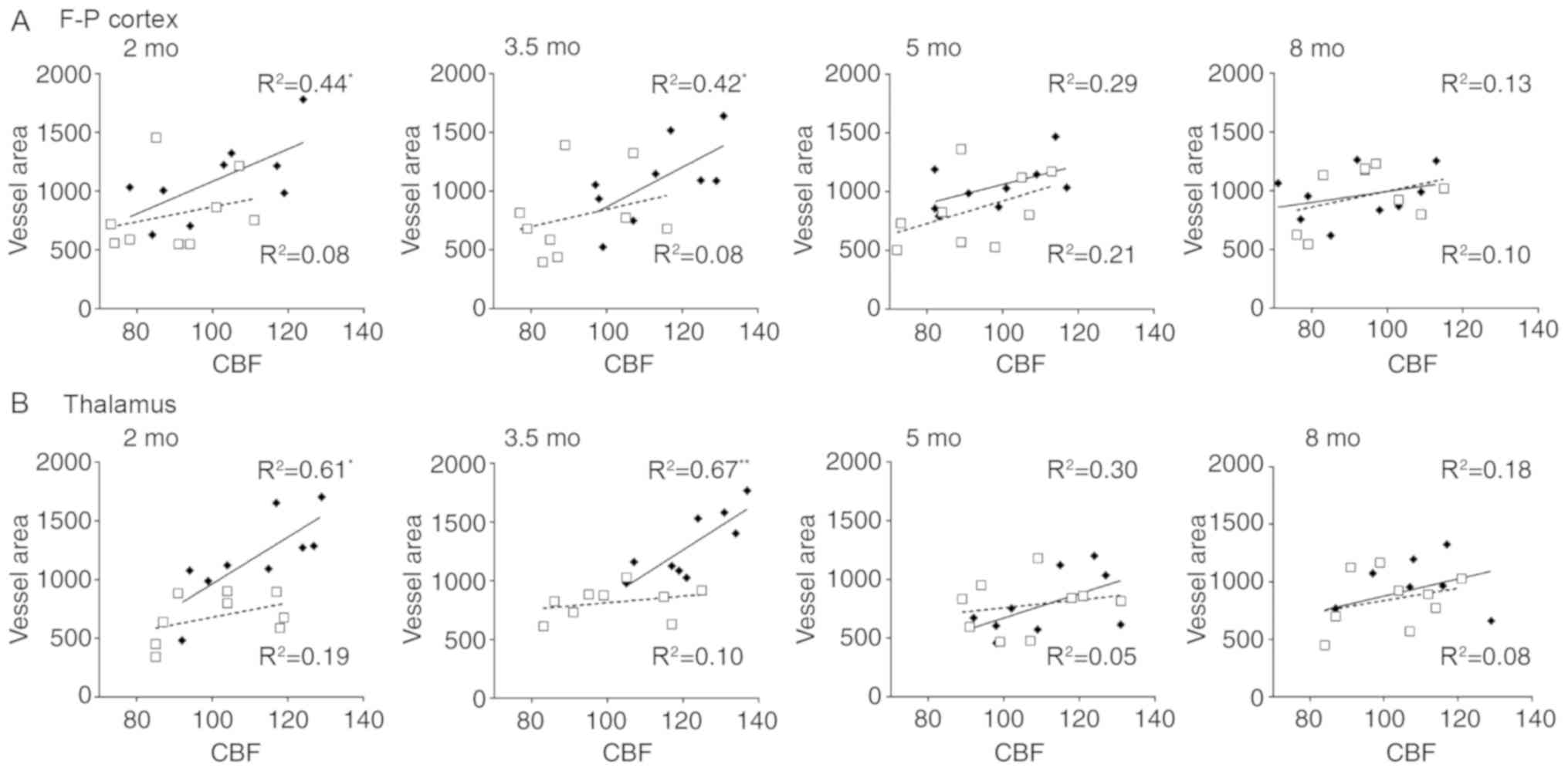
To investigate potential vascular involvement in CBF
alterations, correlation analysis was performed between ASL data
and vascular histology. Significant correlations were identified
between ASL values and vessel area in the frontoparietal cortex and
thalamus of APP/PS1 mice at 2 and 3.5 mo, the results of which are
summarized in Fig. 5. Results
revealed that CBF in the frontoparietal cortex was significantly
correlated with vessel area in mice aged 2 (R2=0.44;
P<0.001) and 3.5 mo (R2=0.42; P<0.001). Further
significant correlations were identified between thalamic CBF and
vessel area in mice aged 2 (R2=0.61; P<0.05) and 3.5
mo (R2=0.67; P<0.001). However, no significant
correlations were observed between the CBF and vessel area in mice
at 5 and 8 mo. No significant correlations were identified between
CBF and vessel density and vessel size in the entorhinal cortex and
thalamus in mice of any age (data not shown). These results suggest
that regional CBF increase may be associated with vessel extension
rather than proliferation.
Discussion
To investigate the role of CBF changes in AD
pathogenesis, the present study examined the age- and brain
region-associated alterations in CBF in APP/PS1 transgenic mice of
varying ages using ASL. A significant increase in CBF in the
frontoparietal cortex and thalamus of APP/PS1 mice was observed
prior to cognitive decline, which was then decreased following
cognitive decline. By contrast, no notable CBF changes were
observed in the entorhinal cortex and hippocampus of APP/PS1 mice.
By comparing the CBF in the frontoparietal cortex and thalamus,
APP/PS1 mice were distinguished from the controls prior to
cognitive decline. In addition, the vessel area, but not vessel
density, was notably increased in the frontoparietal cortex and
thalamus of APP/PS1 mice, and was significantly correlated with CBF
increases in these regions. To the best of our knowledge, the
present study is the first to detail the longitudinal changes of
regional CBF in early AD in addition to the underlying
mechanisms.
Although CBF disorder has been suggested to be
involved in AD pathogenesis several times (6,7), the
pattern of CBF changes in AD remain unclear, particularly for those
with early-stage AD. As AD often has a lengthy period of disease
progression (>10 years) prior to the onset of clinical signs
(24), the majority of patients
are already in the mid- to late-stage of AD when consenting to MRI
examination. Thus, it is difficult to observe early CBF changes in
patients with AD (24). Model
animals provide valuable tools for investigating CBF changes in
early AD (25). The present study
revealed that APP/PS1 mice exhibited Aβ accumulation but no
behavioral impairments at age 2 mo, exhibited regional plaque
formation and partial cognitive deficits at 3.5 mo, complete
Alzheimer's behaviors and pathology at 5 mo and progressive
cognitive decline at age 8 mo, which corresponds with the pre-,
sub-, early- and mid-clinical stages of AD, respectively (16). Thus, APP/PS1 mice are ideal
subjects for assessing CBF changes associated with early AD.
ASL is a recently-developed technique for
determining CBF changes in a non-invasive manner (9). Advances in scanner resolution have
allowed for the measurement of regional CBF changes in mice brains
using micro-MRI (26,27). In the present study, a ROI-based
ASL analysis was performed to assess the age-associated changes of
CBF in APP/PS1 mice of varying ages. A significantly higher CBF was
observed in the frontoparietal cortex and thalamus of APP/PS1 mice
at age 2 mo (prior to cognitive decline) compared with the
controls, suggesting that ASL imaging may sensitively reveal CBF
changes in early AD. The CBF increase tended to be more prominent
at 3.5 mo, but was reduced at 5 and 8 mo. By comparing the CBF at 2
mo, it was demonstrated that APP/PS1 mice were notably different to
the controls, suggesting that an ASL-measured CBF increase may be
an early biomarker for AD diagnosis. These results are consistent
with earlier studies using ASL in humans, demonstrating that CBF
changes are present several years prior to the onset of clinical AD
symptoms (3). Furthermore,
ASL-measured CBF has been revealed to be able to distinguish
between cognitively normal individuals, adults at risk for AD and
individuals diagnosed with AD (4).
Vascular pathology has been suggested to be involved
in AD pathogenesis, which may involve impairment in the structure
and function of cerebral blood vessels (28). Accumulating evidence indicates a
synergistic association between vascular dysfunction and the
accumulation of Aβ and neurofibrillary tangles (28,29).
However, poor cerebral perfusion results in Aβ accumulation and
neurofibrillary tangles, and the neurotoxic effects of Aβ in turn
impair vascular function, including endothelial function and
neurovascular coupling, which consequently causes brain
hypoperfusion (28,30,31).
In the present study, an extensive reduction of CBF and
accumulation of Aβs in the brains of APP/PS1 mice at ages 5 and 8
mo was revealed, supporting the hypothesis of the synergy of
vascular dysfunction with Aβ pathology. Despite these results, the
cause-effect association between cerebral perfusion and Aβ
pathology, particularly in terms of which was first initiated,
remains unclear. Encouragingly, regional CBF increases and
intracellular Aβ staining but no cognitive decline and visible Aβ
plaques in APP/PS1 mice at 2 mo were observed in the present study,
suggesting that CBF changes may be initiated subsequent to Aβ
overproduction, but precede Aβ plaque formation. Such a result
provides the pathological evidence for CBF alterations as an early
biomarker for AD, even prior to Aβ plaque formation.
However, CBF increases were heterogeneously
distributed in the AD brain. In APP/PS1 mice at 2 and 3.5 mo,
increased CBF in the frontoparietal cortex and thalamus, but not in
the entorhinal cortex and hippocampus, was observed. The absence of
CBF increase in the entorhinal cortex and hippocampus may indicate
that these regions are relatively hypoperfused. Furthermore, the
higher glucose metabolism that occurs prior to cognitive decline
may further exacerbate the hypoperfusion in the hippocampus and
entorhinal cortex of APP/PS1 mice, which causes the regions to be
vulnerable to Aβ accumulation (32). By contrast, increased CBF in the
frontoparietal cortex and thalamus may provide a greater energy
supply to enhance regional neuronal activity and Aβ product
clearance, thereby delaying Aβ accumulation in plaques (30,33).
However, CBF increase was only present for a very short period in
the current study, followed by an immediate reduction in CBF. These
results were consistent with early reports, and provide basic
evidence suggesting that early high brain perfusion occurs prior to
cognitive decline in AD (34). The
regional heterogeneity of CBF changes, as were revealed in the
present study, may be associated with the complicated distribution
of AD pathology; but relevant evidence is lacking and requires
further investigation.
In addition, the histological basis underlying the
alterations in CBF was investigated. The results revealed that
increased CBF was associated with a greater vessel area, but not
vessel density, in the thalamus and frontoparietal cortex of
APP/PS1 mice aged 2 and 3.5 mo, suggesting that vessel extension
rather than proliferation results in a CBF increase. This result
was supported by the histological analysis, which revealed no
increase in vessel density in any brain regions of the APP/PS1
mice. However, no correlations were observed between the changes in
CBF and vascular staining in the entorhinal cortex and hippocampus
of AD mice, indicative of regionally-absent vessel extension, which
may be associated with the vulnerability to AD pathology in these
two regions.
There were a number of limitations to the present
study. First, the present study does not use one cohort of mice to
examine age-associated CBF changes, which may cause within-animal
variations. Thus, animal selection and testing time were strictly
controlled. Despite this, within-subject changes cannot be avoided,
which should be considered when extrapolating the results. Next,
the ASL technique has relatively low spatial resolution, low
sensitivity to white matter CBF and limited brain coverage. Thus, a
brain atlas-guided T2-weighed image was used to outline ROIs, and
only larger-sized ROIs majorly involving gray matter were used to
enhance sensitivity of ASL. Third, there is a limited specificity
of ASL-measured CBF, and it is difficult to distinguish AD
pathology from other dementia types, since reduced CBF has been
reported in vascular dementia (34). Finally, ASL provides promise but it
has not been routinely used in clinical practice. For example,
certain methodological issues, including the lack of
standardization for ASL in multi-center studies, limit its
widespread use. Therefore, the results of the present study
represent a conservative revelation of CBF abnormalities occurring
in early AD.
In summary, the present study suggests that regional
CBF disorder transiently occurs in the brains of APP/PS1 mice with
early AD, that a CBF increase in the frontoparietal cortex and
thalamus may be an early biomarker for AD diagnosis and that fewer
brain vessel extensions in the hippocampus and entorhinal cortex
may be associated with their vulnerability to AD pathology. The
present study provided evidence that early CBF changes were
involved in AD pathology and ASL may be a promising tool for
measuring brain perfusion in early AD. However, future research is
required to examine whether or to what extent the results may be
applied to patients with AD.
Acknowledgements
Not applicable.
Funding
The present study was supported by The National High
Technology Research and Development Program of China (grant nos.
2014CB541603 and 2013AA020106) and The National Natural Science
Foundation of China (grant nos. 815711137 and 81771247).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YG and HL designed the study. YG, XL, NC, SW, JL and
ZW performed the experiments and collected data. MZ, RW and JW
analyzed and interpreted the data. XL and HL wrote the manuscript.
YG, XL and HL revised the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was ethically approved by The
Institutional Animal Care and Use Committee of The Medical College
of Zhengzhou University (Zhengzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Prince M, Wimo A, Guerchet M, Ali GC, Wu
YT and Prina M: An analysis of prevalence, incidence, cost and
trends. World Alzheimer Report 2015. The Global Impact of Dementia.
Alzheimer's Disease International (ADI). (London). 2015.https://www.alz.co.uk/research/WorldAlzheimerReport2015.pdf
|
|
2
|
Li XY, Bao XJ and Wang RZ: Potential of
neural stem cell-based therapies for Alzheimer's disease. J
Neurosci Res. 93:1313–1324. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Beason-Held LL, Goh JO, An Y, Kraut MA,
O'Brien RJ, Ferrucci L and Resnick SM: Changes in brain function
occur years before the onset of cognitive impairment. J Neurosci.
33:18008–18014. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chao LL, Buckley ST, Kornak J, Schuff N,
Madison C, Yaffe K, Miller BL, Kramer JH and Weiner MW: ASL
perfusion MRI predicts cognitive decline and conversion from MCI to
dementia. Alzheimer Dis Assoc Disord. 24:19–27. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim SM, Kim MJ, Rhee HY, Ryu CW, Kim EJ,
Petersen ET and Jahng GH: Regional cerebral perfusion in patients
with Alzheimer's disease and mild cognitive impairment: Effect of
APOE Epsilon4 allele. Neuroradiology. 55:25–34. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nicolakakis N and Hamel E: Neurovascular
function in Alzheimer's disease patients and experimental models. J
Cereb Blood Flow Metab. 31:1354–1370. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bell RD and Zlokovic BV: Neurovascular
mechanisms and blood-brain barrier disorder in Alzheimer's disease.
Acta Neuropathol. 118:103–113. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zlokovic BV: Role of the brain vascular
system and blood-brain barrier transport in clearance of
alzheimer's amyloid-beta peptide from the central nervous system.
US Patent 20,050,239,062, Filed. May 23–2001.issued October 27
2005.
|
|
9
|
Inoue Y, Tanaka Y, Hata H and Hara T:
Arterial spin-labeling evaluation of cerebrovascular reactivity to
acetazolamide in healthy subjects. AJNR Am J Neuroradiol.
35:1111–1116. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Verclytte S, Lopes R, Lenfant P, Rollin A,
Semah F, Leclerc X, Pasquier F and Delmaire C: Cerebral
hypoperfusion and hypometabolism detected by arterial spin labeling
MRI and FDG-PET in early-onset Alzheimer's disease. J Neuroimaging.
26:207–212. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Maier FC, Wehrl HF, Schmid AM, Mannheim
JG, Wiehr S, Lerdkrai C, Calaminus C, Stahlschmidt A, Ye L, Burnet
M, et al: Longitudinal PET-MRI reveals β-amyloid deposition and
rCBF dynamics and connects vascular amyloidosis to quantitative
loss of perfusion. Nat Med. 20:1485–1492. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Z: Characterizing early Alzheimer's
disease and disease progression using hippocampal volume and
arterial spin labeling perfusion MRI. J Alzheimers Dis. 42 (Suppl
4):S495–S502. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kerbler GM, Fripp J, Rowe CC, Villemagne
VL, Salvado O, Rose S and Coulson EJ; Alzheimer's Disease
Neuroimaging Initiative, : Basal forebrain atrophy correlates with
amyloid β burden in Alzheimer's disease. Neuroimage Clin.
7:105–113. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Badea A, Johnson GA and Jankowsky JL:
Remote sites of structural atrophy predict later amyloid formation
in a mouse model of Alzheimer's disease. Neuroimage. 50:416–427.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Burns JM, Donnelly JE, Anderson HS, Mayo
MS, Spencer-Gardner L, Thomas G, Cronk BB, Haddad Z, Klima D,
Hansen D and Brooks WM: Peripheral insulin and brain structure in
early Alzheimer disease. Neurology. 69:1094–1104. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li XY, Men WW, Zhu H, Lei JF, Zuo FX, Wang
ZJ, Zhu ZH, Bao XJ and Wang RZ: Age- and brain region-specific
changes of glucose metabolic disorder, learning, and memory
dysfunction in early Alzheimer's disease assessed in APP/PS1
transgenic mice using 18F-FDG-PET. Int J Mol Sci.
17:E17072016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang H, Liu J, Zong Y, Xu Y, Deng W, Zhu
H, Liu Y, Ma C, Huang L, Zhang L and Qin C: miR-106b aberrantly
expressed in a double transgenic mouse model for Alzheimer's
disease targets TGF-β type II receptor. Brain Res. 1357:166–174.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zong YY, Wang XY, Wang HL, Liu YL, Huang
L, Ma CM, Zhang LF and Qin C: Continuous analysis of senile plaque
and behaviour in APPswe/PSΔE9 double-transgenic gene mouse model of
Alzheimer disease. Chin J Comp Med. 2008.http://en.cnki.com.cn/Article_en/CJFDTOTAL-ZGDX200809003.htm
|
|
19
|
Li X, Zhu H, Sun X, Zuo F, Lei J, Wang Z,
Bao X and Wang R: Human neural stem cell transplantation rescues
cognitive defects in APP/PS1 model of Alzheimer's disease by
enhancing neuronal connectivity and metabolic activity. Front Aging
Neurosci. 8:2822016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nasrallah FA, Lee EL and Chuang KH:
Optimization of flow-sensitive alternating inversion recovery
(FAIR) for perfusion functional MRI of rodent brain. NMR Biomed.
25:1209–1216. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bitner BR, Brink DC, Mathew LC, Pautler RG
and Robertson CS: Impact of arginase II on CBF in experimental
cortical impact injury in mice using MRI. J Cereb Blood Flow Metab.
30:1105–1109. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Paxinos G and Franklin KBJ: The Mouse
Brain in Stereotaxic Coordinates. Second edition. Academic Press.
(San Diego). 2001.
|
|
23
|
Zhang L, Liu C, Wu J, Tao JJ, Sui XL, Yao
ZG, Xu YF, Huang L, Zhu H, Sheng SL and Qin C: Tubastatin
A/ACY-1215 improves cognition in Alzheimer's disease transgenic
mice. J Alzheimers Dis. 41:1193–1205. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hampel H, Prvulovic D, Teipel S, Jessen F,
Luckhaus C, Frölich L, Riepe MW, Dodel R, Leyhe T, Bertram L, et
al: The future of Alzheimer's disease: The next 10 years. Prog
Neurobiol. 95:718–728. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li X, Bao X and Wang R: Experimental
models of Alzheimer's disease for deciphering the pathogenesis and
therapeutic screening (Review). Int J Mol Med. 37:271–283. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Abrahamson EE, Foley LM, Dekosky ST,
Hitchens TK, Ho C, Kochanek PM and Ikonomovic MD: Cerebral blood
flow changes after brain injury in human amyloid-beta knock-in
mice. J Cereb Blood Flow Metab. 33:826–833. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hébert F, Grand'maison M, Ho MK, Lerch JP,
Hamel E and Bedell BJ: Cortical atrophy and hypoperfusion in a
transgenic mouse model of Alzheimer's disease. Neurobiol Aging.
34:1644–1652. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dickstein DL, Walsh J, Brautigam H,
Stockton SD Jr, Gandy S and Hof PR: Role of vascular risk factors
and vascular dysfunction in Alzheimer's disease. Mt Sinai J Med.
77:82–102. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Beach TG, Wilson JR, Sue LI, Newell A,
Poston M, Cisneros R, Pandya Y, Esh C, Connor DJ, Sabbagh M, et al:
Circle of Willis atherosclerosis: Association with Alzheimer's
disease, neuritic plaques and neurofibrillary tangles. Acta
Neuropathol. 113:13–21. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mattsson N, Tosun D, Insel PS, Simonson A,
Jack CR Jr, Beckett LA, Donohue M, Jagust W, Schuff N and Weiner
MW; Alzheimer's Disease Neuroimaging Initiative, : Association of
brain amyloid-β with cerebral perfusion and structure in
Alzheimer's disease and mild cognitive impairment. Brain.
137:1550–1561. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Platt B, Welch A and Riedel G: FDG-PET
imaging, EEG and sleep phenotypes as translational biomarkers for
research in Alzheimer's disease. Biochem Soc Trans. 39:874–880.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jupp B, Williams J, Binns D, Hicks RJ,
Cardamone L, Jones N, Rees S and O'Brien TJ: Hypometabolism
precedes limbic atrophy and spontaneous recurrent seizures in a rat
model of TLE. Epilepsia. 53:1233–1244. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Michels L, Warnock G, Buck A, Macauda G,
Leh SE, Kaelin AM, Riese F, Meyer R, O'Gorman R, Hock C, et al:
Arterial spin labeling imaging reveals widespread and
Aβ-independent reductions in cerebral blood flow in elderly
apolipoprotein epsilon-4 carriers. J Cereb Blood Flow Metab.
36:581–595. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wierenga CE, Hays CC and Zlatar ZZ:
Cerebral blood flow measured by arterial spin labeling MRI as a
preclinical marker of Alzheimer's disease. J Alzheimers Dis. 42
(Suppl 4):S411–S419. 2014. View Article : Google Scholar : PubMed/NCBI
|