Introduction
Ethanol has a toxic effect on the heart, resulting
in cardiomyocyte damage. Long-term high intake of ethanol leads to
a non-ischemic dilated cardiomyopathy termed alcoholic
cardiomyopathy (ACM) (1), which is
characterized by cardiac hypertrophy and compensatory systolic
dysfunction (2). The apoptosis of
cardiomyocytes serves an important role in the pathogenesis of ACM.
X-linked inhibitor of apoptosis protein (XIAP) is a member of the
inhibitor of apoptosis proteins (IAP) family and is the most potent
known IAP protein in human tissues (3–5).
MicroRNAs (miRs) are small RNA molecules whose length is 21–23
nucleotides. It is suggested that abnormalities in miR levels serve
an important role in cardiac hypertrophy, pathological remodeling
and the occurrence of heart failure (6). In response to ethanol-induced
cardiomyopathy, another study revealed abnormalities in the
expression of multiple miRs in hypertrophic myocardial tissue,
including miR-133a, miR-125 and miR-195 (7), suggesting that ACM may be associated
with the abnormal expression of certain miRs.
A previous study assessed ACM-associated miRs that
are differentially expressed, which include miR-186-5p and
miR-488-3p among others. TargetScan bioinformatics software
predicted miR-186-5p-associated target genes (949 in total) and
demonstrated that XIAP is a target gene of miR-186-5p. The study
demonstrated that miR-186 binds to the XIAP 3′ untranslated region
(UTR) and decreases its expression, thus regulating the expression
levels of downstream target proteins including caspase 3,
BCL2-associated agonist of cell death, cyclin D1 and microtubule
affinity regulating kinase 2 (8).
XIAP may therefore be an important anti-apoptotic protein in human
tissues. However, no studies have reported on its function in
ethanol-induced cardiomyopathy, to the best of our knowledge.
In this experiment, the levels of apoptosis in
ethanol-treated cardiomyocytes were first analyzed using flow
cytometry. Modifications of the expression levels of miR-186-5p and
XIAP were detected in ethanol-treated human AC16 cardiomyocytes,
which were derived from left ventricular cells (9). These results elucidated the specific
molecular mechanisms of ethanol-induced cardiomyocyte
apoptosis.
Materials and methods
Materials
AC16 human cardiomyocytes, derived from left
ventricular cardiomyocytes, were supplied by Guangzhou Shenglei
Biological Technology Company (Guangzhou, China) and stored in the
central laboratory of the First Affiliated Hospital of China
Medical University (Liaoning, China). Anhydrous ethanol, which is
the highly purified ethanol-water solution with an ethanol
concentration of 99.5%, was obtained from Guoyao Group Industry
Co., Ltd. (Beijing, China). F12/Dulbecco's modified Eagle's medium
(DMEM) was purchased from Gibco (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). The MTT kit and RNA extraction reagent
TRIzol® were purchased from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). The reverse transcriptase reagent was obtained
from Takara (Takara Bio, Inc., Otsu, Japan), Dharma FECT
transfection reagent was obtained from Dharmacon (GE Healthcare
Dharmacon, Inc., Lafayette, CO, USA), and the luciferase reporter
gene plasmid vector (pmirGLO) was purchased from Shanghai gemma
pharmaceutical technology Co., Ltd. miR-186-5p mimic/inhibitor was
supplied by Guangzhou RiboBio Co., Ltd. (Guangzhou, China), miR-186
primer was supplied by Shanghai Bio-biology Company (Shanghai,
China). The XIAP plasmid, the quantitative polymerase chain
reaction (qPCR) reagent SYBR® Master Mix,
Lipofectamine® 3000, primary antibodies against Actin
(cat. no. 3700) and XIAP (cat. no. 2042), and enhanced
chemiluminescence (ECL) color kits were all obtained from Cell
Signaling Technology, Inc. (Danvers, MA, USA), the luciferase
reporter gene kit was supplied by Promega Corporation (Madison, WI,
USA), and the Annexin V- fluorescein isothiocyanate
(FITC)/Phycoerythrin (PE) Apoptosis Detection kit was supplied by
BD Pharmingen (BD Biosciences, San Jose, CA, USA).
AC16 myocardial cell culture and
maintenance
The AC16 myocardial cells were cultured at 37°C in
an incubator (Sanyo MCO-18AIC carbon dioxide incubator; Sanyo,
Osaka, Japanese) containing 5% CO2 to a density of ~90%,
and the cells were rinsed with PBS. A solution of 0.25% trypsin in
PBS was used to digest the cells and to examine the cell state.
When the cells became round and bright, the cells were detached
from the wall of the culture bottle using a dropper, and 5 ml DMEM
was added to terminate the reaction. The cell mixture was collected
in 15 ml sterile test tubes using a dropper. The solution was
centrifuged at 1,000 × g and room temperature for 5 min. The
supernatant was discarded, and the cells were counted. The cell
culture bottles were placed under the microscope to observe cell
morphology and density, and the best results were achieved when
cell density reached 80–90%.
Flow cytometry
Previous studies have demonstrated that abnormal
mitochondrial structures in cardiomyocytes may be observed in
patients or animal models with alcoholic cardiomyopathy; these
manifest as a reduction in the number of mitochondria, swelling of
the mitochondria, along with disorganization and reduction or
elimination of the cristae (10,11).
Based on our preliminary experiments, 200, 400 and 800 mmol/l
ethanol was applied to cardiomyocytes for different durations (24
or 48 h) in order to prepare the smears for electron microscopy.
The ultrastructures of AC16 cardiomyocytes were observed under
electron microscopy (Olympus CX41; Olympus Corporation, Tokyo,
Japan) and compared with the characteristics of human
cardiomyocytes of ACM from the literature, with the goal of
determining the ideal culture time required for model
establishment. The mitochondrial structures of cardiomyocytes from
patients with ACM and animal models were observed under the
electron microscope, and were revealed to be abnormal, which showed
that the number of mitochondria decreased markedly, the volume of
mitochondria increased, the structures of the mitochondrial ridge
were disordered, and sometimes the structure of mitochondrial ridge
disappeared along with vacuolar changes (12,13).
As a result, it was determined that the effects of 200 and 800
mmol/l on cardiomyocytes for 24 and 48 h were suitable for this
experiment.
During the logarithmic growth phase, the cells were
incubated in 6-well culture plates. When the cell density reached
60–70%, ethanol was added to the culture plates. The experiment was
divided into 6 groups: 0 mmol/l, 24 h group and 48 h group; 200
mmol/l, 24 h group and 48 h group; and 800 mmol/l, 24 h group and
48 h group. The cells were continuously maintained at 37°C in a 5%
CO2 incubator (Sanyo MCO-18AIC carbon dioxide incubator;
Sanyo). Following treatment, the incubated plates were removed from
the incubator, and cells extracted using 0.25% trypsin in PBS.
Following centrifugation (1,000 × g at room temperature for 5 min)
as aforementioned, the supernatant was removed. Following washing,
100 µl of 1X PLB binding buffer (Cell Signaling Technology, Inc.)
was added to each tube, and the cells were flicked gently.
Subsequently, 3 µl Annexin V-FITC/PI was added to each tube, which
where flicked gently again and allowed to stand for 15 min under
dark conditions. Approximately 200 µl of 1X binding buffer was
added to each tube, and flow cytometry (FlowJo 7.6.2 Software;
FlowJo LLC, Ashland, OR, USA) was used to detect and measure the
levels of apoptosis.
Fluorescence detection of target gene
by dual-luciferase reporter gene
Using bioinformatics software (TargetScan7.1;
www.targetscan.org/vert_71/docs/help.html; and
miRanda database; www.mirbase.org/), the gene binding sequence of
miR-186 was predicted to regulate XIAP, and the gene sequences of
the XIAP 3′-UTR and the mutated (mut) XIAP 3′-UTR were designed and
synthesized. The two types of synthetic gene fragment were cloned
into luciferase reporter carriers of the pmirGLO to construct the
wild-type carrier (pmirGLO-wt-XIAP-3′-UTR) and the mutant carrier
(pmirGLO-mut-XIAP-3′-UTR). The sequence information were as
follows: mut XIAP 3′-UTR,5′-UCAUCUGGAUUUUUUUAAGAAAU-3′,
pmirGLO-wt-XIAP-3′-UTR,5′-UCAUCUGGAUUUUUUAUUCUUUU-3′ and
pmirGLO-mut-XIAP-3′-UTR, 5′-UCAUCUGGAUUUUUUUAAGAAAU-3′.
The two recombinant carrier plasmids were
co-transfected into AC16 cardiomyocytes with miR-186 mimic (1.2 µg)
or miR-186-negative control (mimic control; 1.2 µg) using a
transfection reagent Lipofectamine® 3000, respectively.
The sequence information for the miR-186-5p mimics was as follows:
Forward, 5′-GCGCTAAGGCACGCGGT-3′ and reverse,
5′-CAGTGCAGGGTCCGAGGT-3′.
The transfections of the miR and pmirGlo into the
cardiomyocyte culture was processed in groups of four as follows:
Co-transfection of miR-186-5p mimic control and
pmirGLO-wt-XIAP-3′-UTR; co-transfection of miR-186-5p mimic and
pmirGLO-wt-XIAP-3′-UTR; co-transfection of miR-186-5p mimic control
and pmirGLO-mut-XIAP-3′-UTR; co-transfection of miR-186-5p mimic
and pmirGLO-mut-XIAP-3′-UTR. Luciferase activity was measured 48 h
post-transfection using a luciferase assay kit (Shanghai Gemma
Pharmaceutical Technology Co., Ltd., Shanghai, China) and
normalized to Renilla luciferase activity.
Cell transfection
When the cells had been digested, they were evenly
inoculated into 6-well culture plates and continuously cultivated.
When the cultures grew to a unilaminar density of 70%, they were
starved for 2 h with serum-free medium. The transfection mixture
was composed of ~100 µl serum-free medium, 4.5 µl transfection
reagent (Lipofectamine® 3000) and 1.2 µg plasmid, which
were incubated for 15 min. This mixture was added to the wells by
dripping with a micropipette, and the plates were incubated (37°C,
5% CO2) for 5 h. The transfection medium was removed,
and the complete medium containing 10% fetal bovine serum (Cell
Signaling Technology, Inc.) was added. Cells were maintained in
37°C constant temperature incubator (5% CO2 for 24 h)
for the follow-up tests.
Western blotting
Protein lysis was used to extract proteins from
cells using the radioimmunoprecipitation assay buffer with 1 mmol/l
phenylmethylsulfonyl fluoride (Cell Signaling Technology, Inc.) on
ice and quantified using the Bradford method. Protein samples were
prepared using SDS-PAGE protein loading buffer, and 20 µg protein
was added to each gel lane. The gel concentrations used in this
experiment were 5% for the concentrated gel, and 10% for the
separation gel. The loading volume of the sample fluid in each well
was 15 µl. Finally, 5 µl protein standard was added to each of the
left- and right-side sample wells. The electrodes were connected,
and the voltage was adjusted to 80 V. When the leading edge of the
protein marker was transferred from the concentrated gel to the
separating gel, the voltage was adjusted to 120 V. Electrophoresis
was performed with constant voltage. The segregated condition of
the protein marker was examined, and electrophoresis was stopped;
the sample was placed on ice and 100 V was applied, and it was
transferred a polyvinylidene fluoride membrane (EMD Millipore,
Billerica, MA, USA) for 90 min. The membrane was blocked with 1.5%
bovine serum albumin (BSA; Cell Signaling Technology, Inc.) at room
temperature for 2 h. Primary anti-XIAP (1:1,000) and anti-Actin
(1:400) were diluted with 1.5% BSA and incubated overnight at 4°C.
The membrane was incubated with the a horseradish
peroxidase-conjugated secondary antibody (cat. no. 4410; Cell
Signaling Technology, Inc.; 1:10,000) at 37°C for 2 h and then
washed with Tris Buffered Saline-Tween (0.05%; Cell Signaling
Technology, Inc.) using a shaking bed 3 times (5 min/wash). The ECL
kit was used to develop the membrane, and the results were analyzed
using a BioImaging System (ChemiDoc™; Bio-Rad, Laboratories, Inc.,
Hercules, CA, USA) to display fluorescence imaging of antigen and
antibody responses.
Reverse transcription-qPCR
(RT-qPCR)
PCR was performed using the TRIzol®
one-step method to extract total RNA from cardiomyocytes. In brief,
1 µg total RNA was used to synthesize cDNA in 20 µl of RT system;
0.5 µl cDNA and the target gene upstream and downstream primers
were added. PCR amplification was performed in a 20 µl reaction
volume. The thermocycling conditions used were 45 cycles of 37°C
for 30 min and 85°C for 5 min. The primer sequences used were as
follows: miR-186 forward, 5′-GCGCTAAGGCACGCGGT-3′, and reverse,
5′-CAGTGCAGGGTCCGAGGT-3′; XIAP forward, 5′-GGCACGAGCAGGGTTTCTT-3′
and reverse, 5′-TCCAACTGCTGAGTCTCCATATTG-3′; β-actin forward,
5′-ATAGCACAGCCTGGATAGCAACGTAC-3′ and reverse,
5′-CACCTTCTACAATGAGCTGCGTGTG-3′. The amplification and dissolution
curve were confirmed. Relative expression of genes was measured
using the 2−ΔΔcq method (14).
Statistical analysis
The software used for statistical analysis of the
data was SPSS 16.0 (SPSS Inc., Chicago, IL, USA). Data were
presented as the mean ± standard deviation of 3 repeated
experiments and analyzed using one-way analysis of variance method,
which was used for the comparison of multiple groups. Dunnett's
test was used for the post hoc comparisons between groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
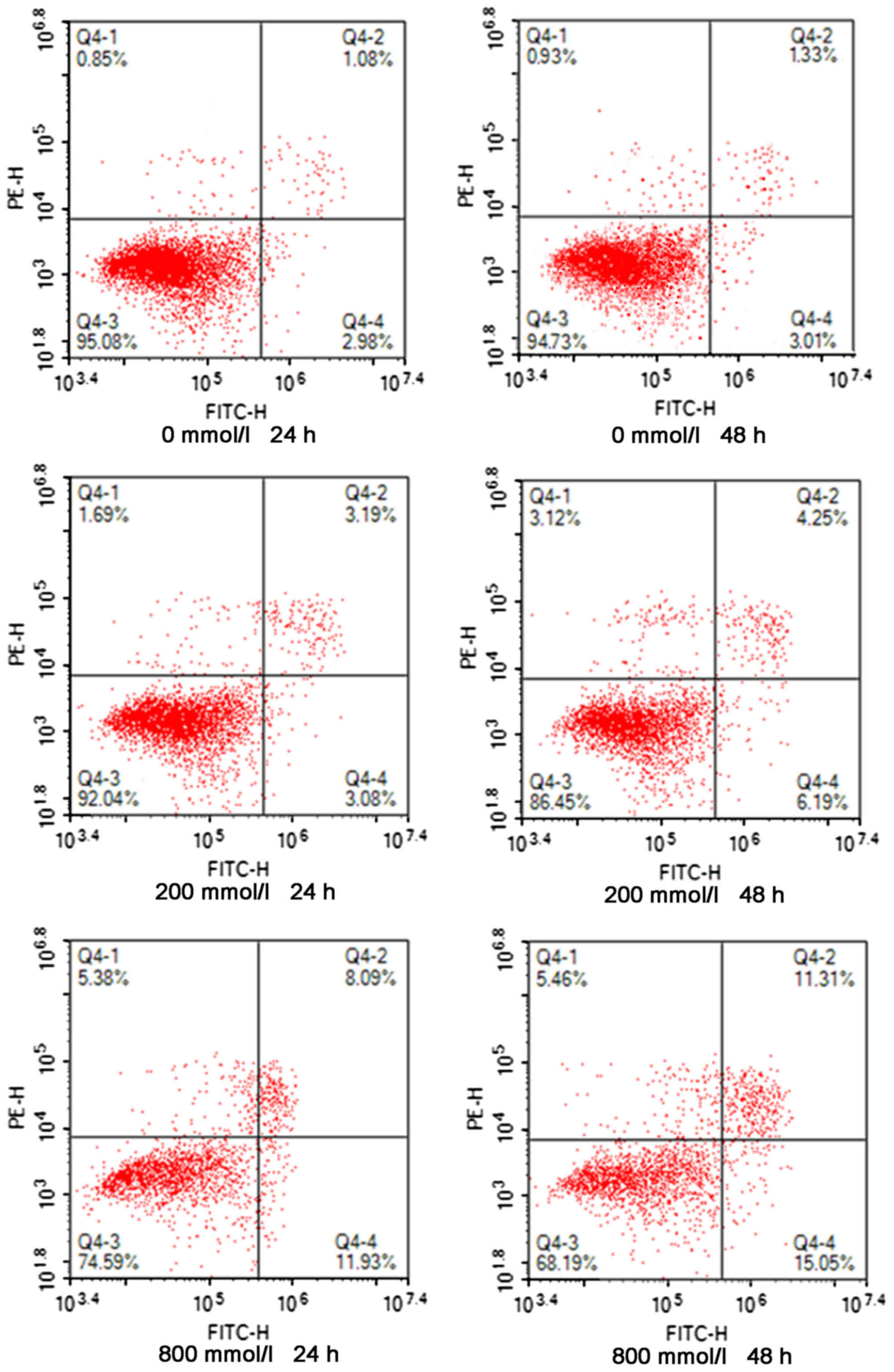
Ethanol-induced apoptosis in AC16
cardiomyocytes
In the present study, the mitochondrial structures
of ethanol-treated cardiomyocytes under electron microscopy
revealed that the number of mitochondria decreased markedly, the
volume of mitochondria increased, the structures of the
mitochondrial ridge were disordered, and sometimes the structure of
mitochondrial ridge disappeared along with vacuolar changes (data
not shown); these results were consistent with those of previous
studies (12,13). AC16 cardiomyocytes were exposed to
different ethanol concentrations (0, 200 and 800 mmol/l) for
different durations (24 and 48 h), and analyzed the changes in the
levels of apoptosis for each ethanol concentration and duration of
time using flow cytometry. As presented in Fig. 1, following ethanol treatment, the
levels of apoptosis in AC16 cardiomyocytes increased compared with
the control group, which was dependent on ethanol concentration and
duration. The levels of apoptosis increased with the increase in
ethanol concentration and duration of action.
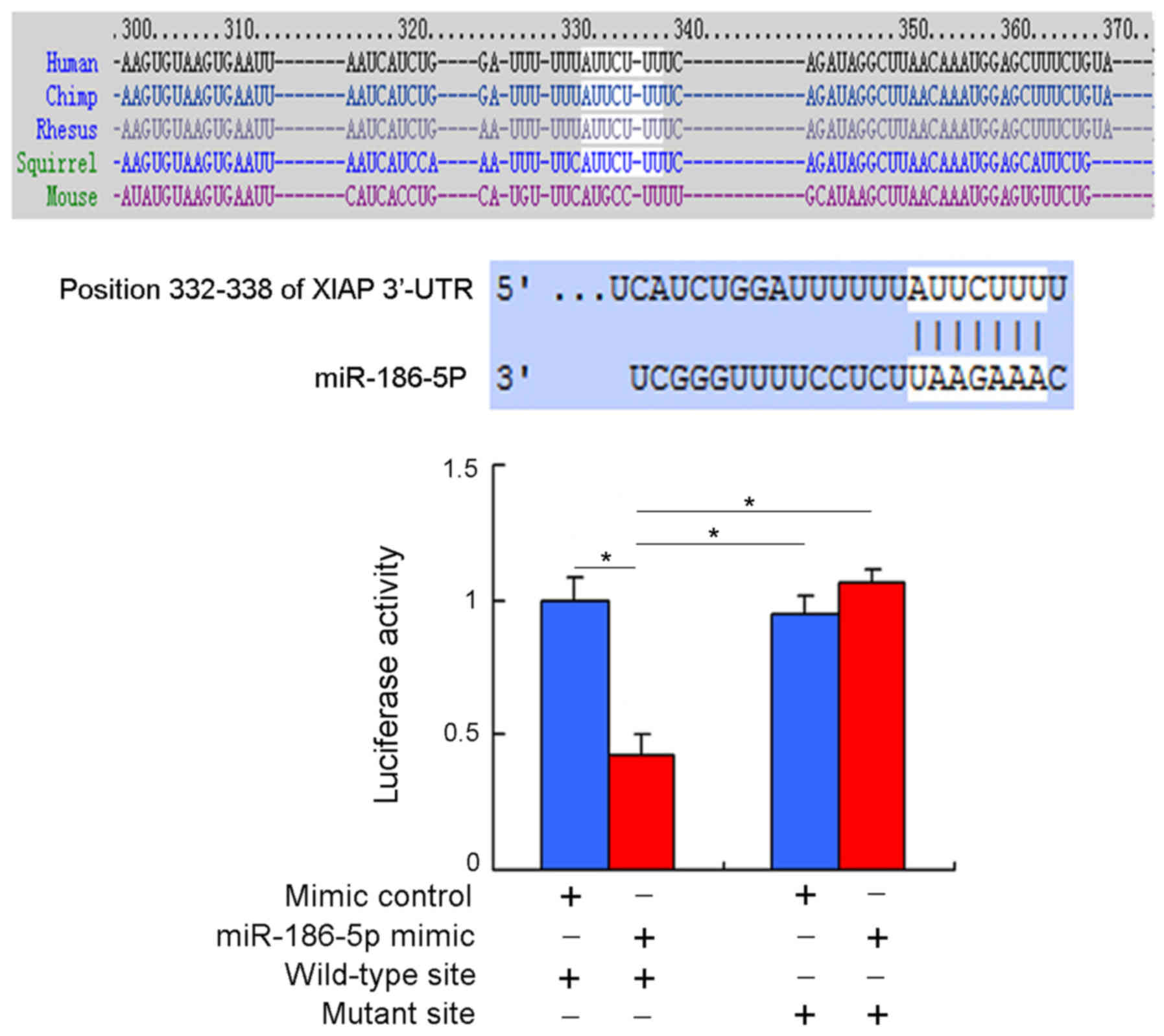
XIAP is a direct target gene of
miR-186-5p
The bioinformatics software Target Scan7.1 database
predicted that the 332–338 base position of the 3′-UTR of the XIAP
gene mRNA is a possible binding site for miR-186-5p. Moreover, the
complementary binding sequence between miR-186-5p and the 3′-UTR of
the XIAP gene mRNA was also identified in the miRanda database.
Compared with the miR-186 complementary binding sequence, the
binding stability between miR-186-5p and XIAP is better, thus
miR-186-5p may downregulate the expression of XIAP.
To further confirm whether XIAP is a direct target
gene for miR-186-5p, the dual-luciferase reporter gene assay and
RT-qPCR for target gene verification were performed. Firstly, a
wild-type pmirGLO-wt-XIAP-3′-UTR and a mutant
pmirGLO-wt-XIAP-3′-UTR were constructed and transfected into AC16
cardiomyocytes along with either a miR-186-5p mimic control or
miR-186-5p mimic (Fig. 2). The
results indicated that fluorescence intensity in the group with the
wild-type pmirGLO-wt-XIAP-3′-UTR combined with miR-186-5p mimic
significantly decreased (P<0.05) compared with the other three
groups, and no significant differences were observed in
fluorescence intensity between the other three groups (P>0.05).
Therefore there may be a direct binding site of miR-186 on the
3′-UTR of XIAP and miR-186 may inhibit the expression of XIAP.
Therefore, XIAP is a direct target of miR-186.
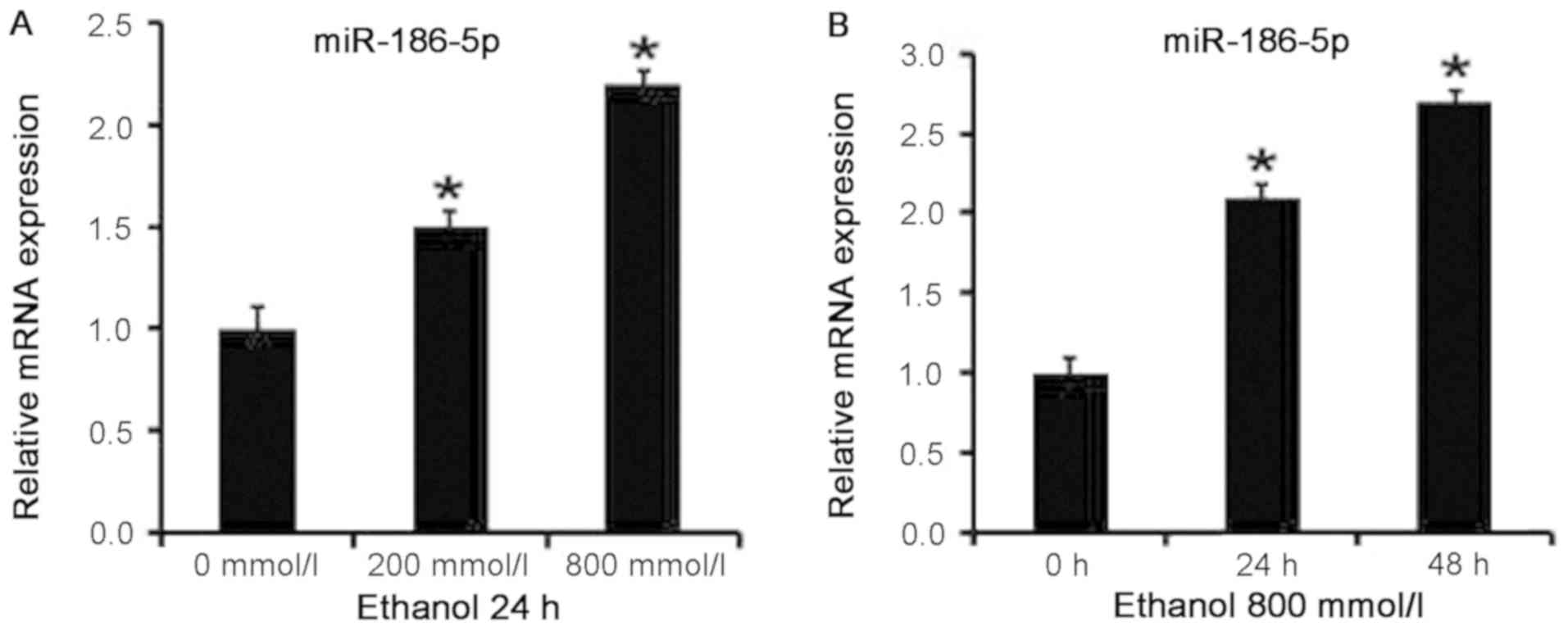
Ethanol intake upregulates the
expression of miR-186-5p in AC16 cardiomyocytes
AC16 cardiomyocytes were treated with ethanol, and
the expression levels of miR-186-5p in the cells were detected by
RT-qPCR. Expression levels of miR-186-5p differed between the
ethanol treatment and control groups (P<0.05), increasing with
the higher ethanol concentrations (Fig. 3A) and with longer treatment
durations (Fig. 3B). Therefore,
increases in ethanol concentration and length of exposure led to
the upregulation of miR-186 expression in AC16 cardiomyocytes.
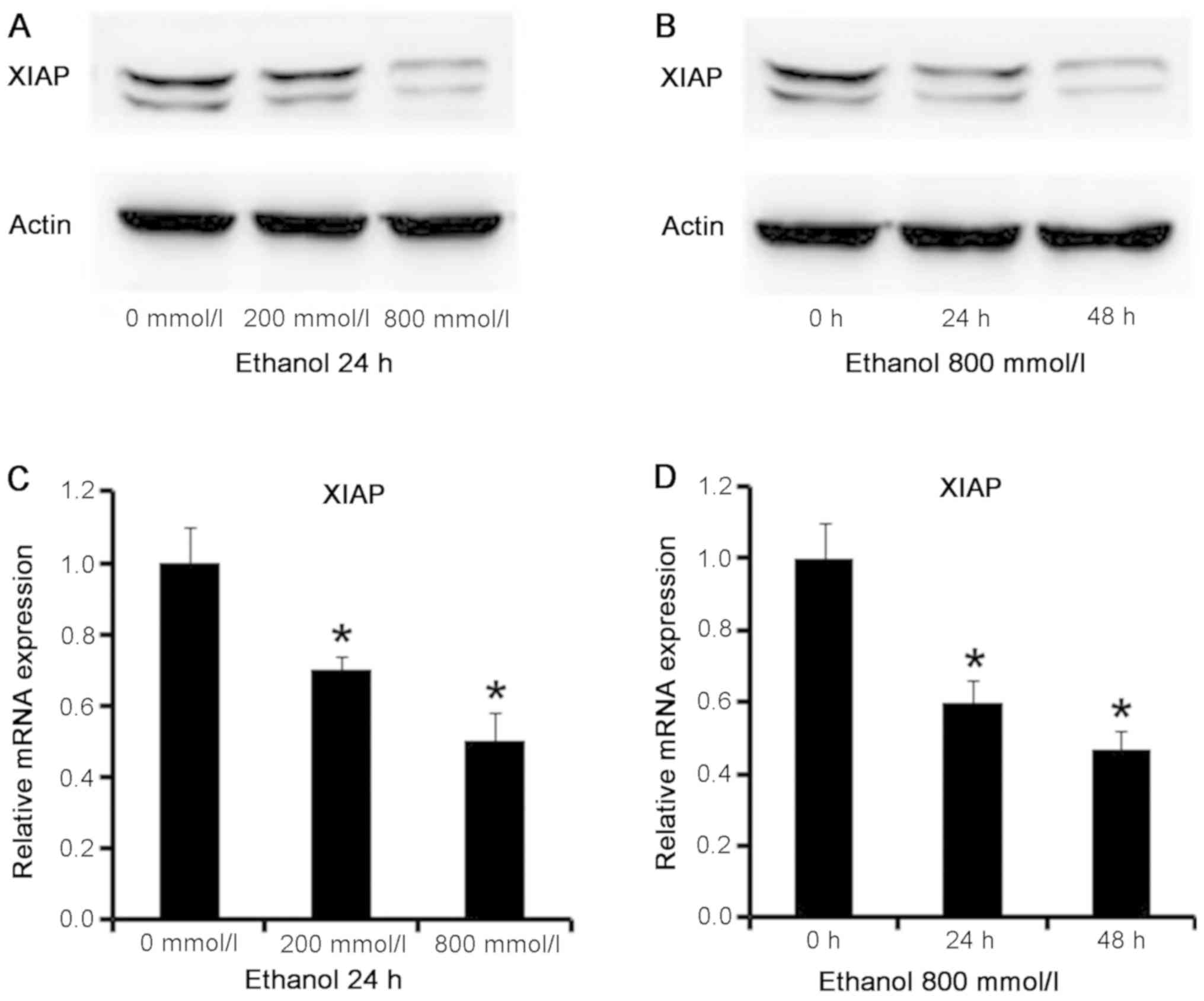
Ethanol intake downregulates the
expression levels of the apoptosis-associated protein XIAP in AC16
cardiomyocytes
In order to ascertain the specific mechanism of
ethanol-induced apoptosis in cardiomyocytes, the levels of
apoptosis-associated markers were detected in AC16 cardiomyocytes
treated with ethanol by western blotting. The results (Fig. 4A and B) demonstrated that ethanol
treatment reduced the protein levels of XIAP in AC16
cardiomyocytes, and the decrease was dependent on ethanol
concentration and duration of exposure.
Similarly, the mRNA level of XIAP in ethanol-treated
AC16 cardiomyocytes was detected by RT-qPCR. The results (Fig. 4C and D) confirmed that the
expression levels of XIAP significantly decreased between ethanol
treatment groups and control group (P<0.05), and that XIAP
levels further reduced with the increase in ethanol concentration
and duration of treatment. It is suggested that ethanol may
downregulate the mRNA expression levels of XIAP in AC16
cardiomyocytes, and this downregulation is dependent on ethanol
concentration and length of ethanol exposure.
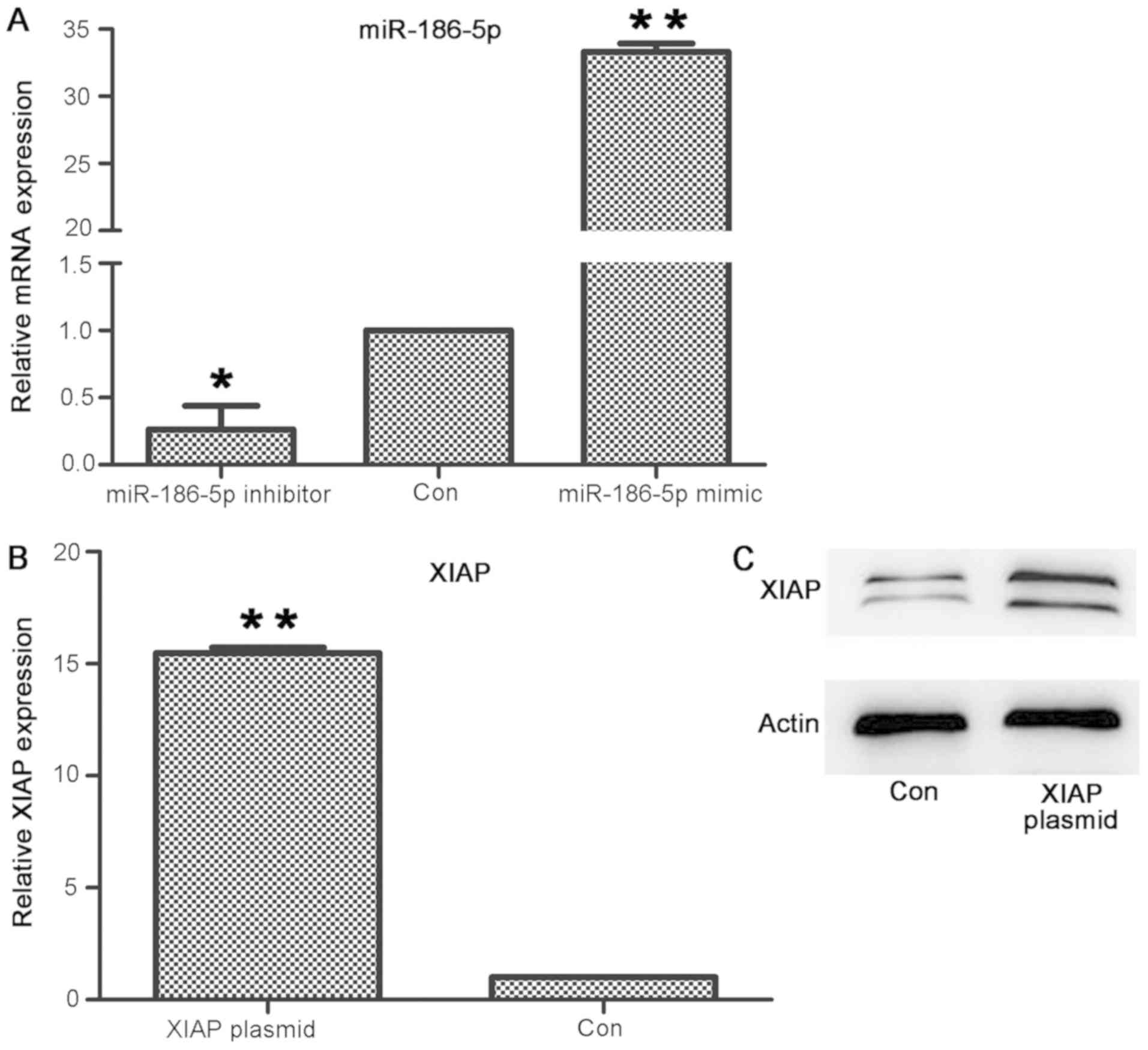
Transfection of miR-186
mimic/inhibitor and XIAP upregulates the expression levels of
miR-186 mimic and XIAP, and downregulates the expression levels of
miR-186 inhibitor in AC16 cardiomyocytes
miR-186-5p mimic/inhibitor and XIAP vectors were
transfected into AC16 cardiomyocytes through transient
transfection. RT-qPCR and western blotting were used to detect the
expression levels of miR-186-5p and XIAP in the cells. The results
(Fig. 5) were as follows: The mRNA
expression levels of miR-186-5p increased in cells following
transfection with miR-186-5p mimic, and decreased in cells
following transfection with miR-186-5p inhibitor (Fig. 5A). The differences were significant
compared with the control group (where the miR mimic control served
as the control group; P<0.05); the mRNA expression levels
(Fig. 5B) and protein levels
(Fig. 5C) of XIAP increased in the
cells after transfection with the XIAP vector. The differences in
mRNA expression were significant compared with the control group
(P<0.05). Thus, transfections with either the miR-186
mimic/inhibitor or XIAP in AC16 cardiomyocytes were successful.
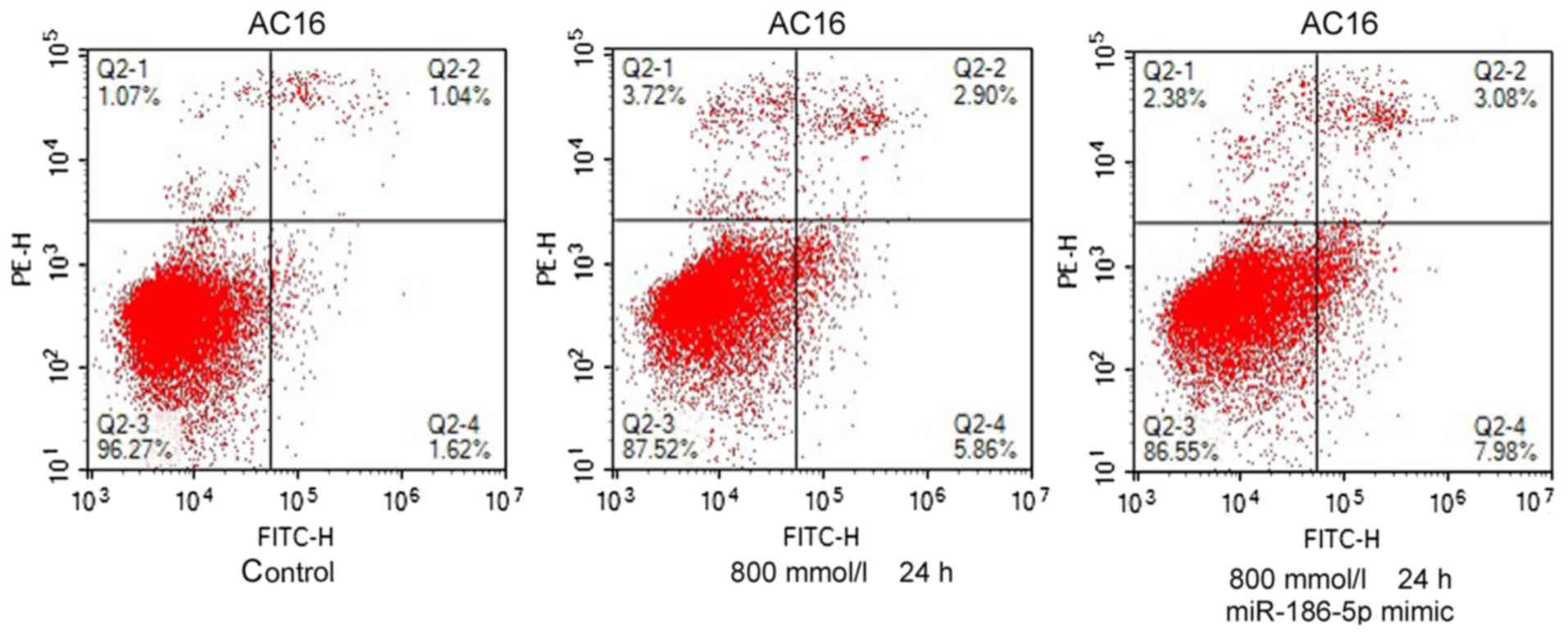
miR-186-5p may be involved in the
ethanol-induced apoptosis of AC16 cardiomyocytes
In previous sections, it was demonstrated that
ethanol treatment increased the apoptosis levels of AC16
cardiomyocytes and upregulated the expression levels of miR-186-5p.
In order to investigate the role of miR-186-5p in the process of
ethanol-induced apoptosis of cardiomyocytes, AC16 cardiomyocytes
were treated with ethanol (800 mmol/l), and alterations in
apoptosis levels were examined using flow cytometry. While
miR-186-5p mimic was transfected into AC16 cardiomyocytes prior to
ethanol treatment (800 mmol/l for 24 h), and the levels of
apoptosis further increased. The results demonstrate that ethanol
induced cardiomyocyte apoptosis and the levels of apoptosis further
increased following transfection of miR-186-5p mimic (Fig. 6). Therefore, ethanol may induce
cardiomyocyte apoptosis and miR-186-5p may further promote
ethanol-induced cardiomyocyte apoptosis.
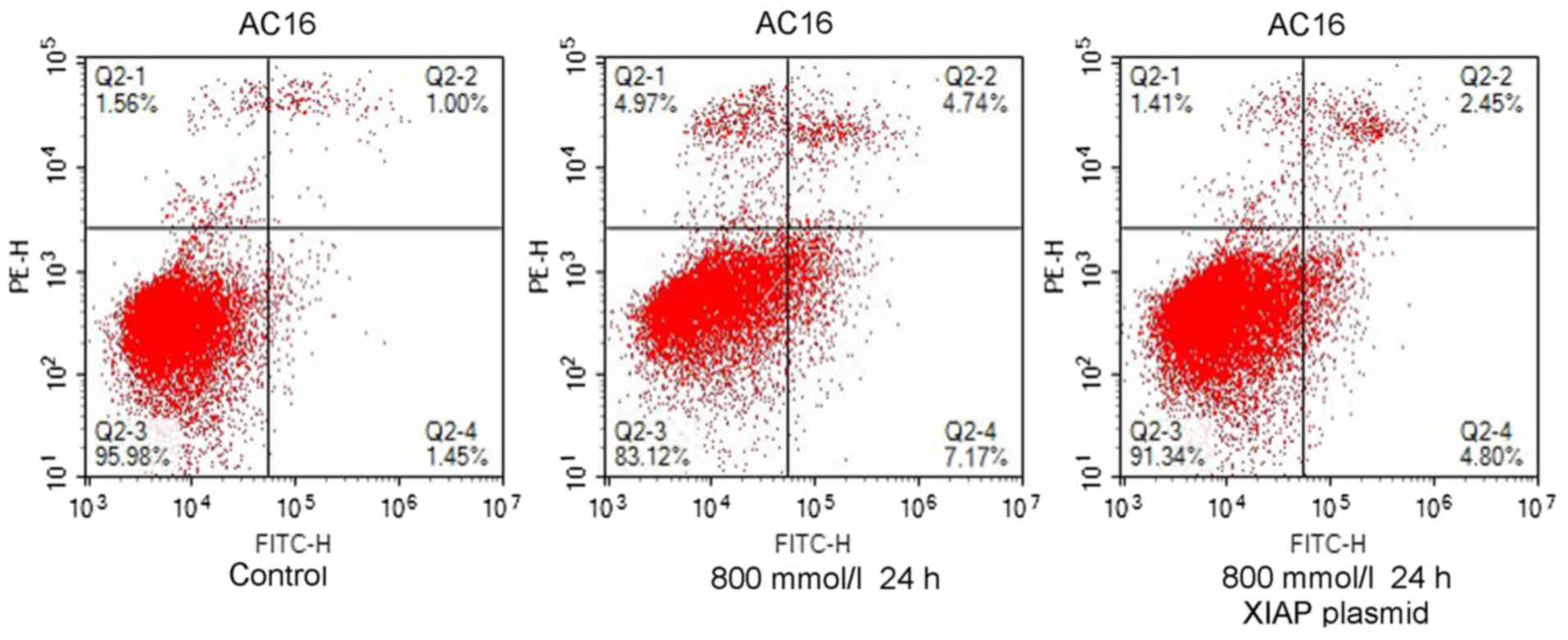
Upregulation of XIAP inhibits the
ethanol-induced apoptosis of AC16 cardiomyocytes
In order to further examine the role of XIAP in
ethanol-induced apoptosis in the heart tissue, AC16 cardiomyocytes
were treated with ethanol (800 mmol/l), and the levels of apoptosis
were detected via flow cytometry. Meanwhile, the XIAP plasmid was
transfected into AC16 cardiomyocytes prior to ethanol treatment
(800 mmol/l), and the alterations in apoptosis levels were detected
via flow cytometry. The results were as follows: The apoptosis
levels of ethanol-treated AC16 cardiomyocytes increased, but the
apoptosis levels decreased following transfection with the XIAP
plasmid (Fig. 7). Thus, ethanol
may induce cardiomyocyte apoptosis and XIAP may partially reverse
the ethanol-induced apoptosis of cardiomyocytes.
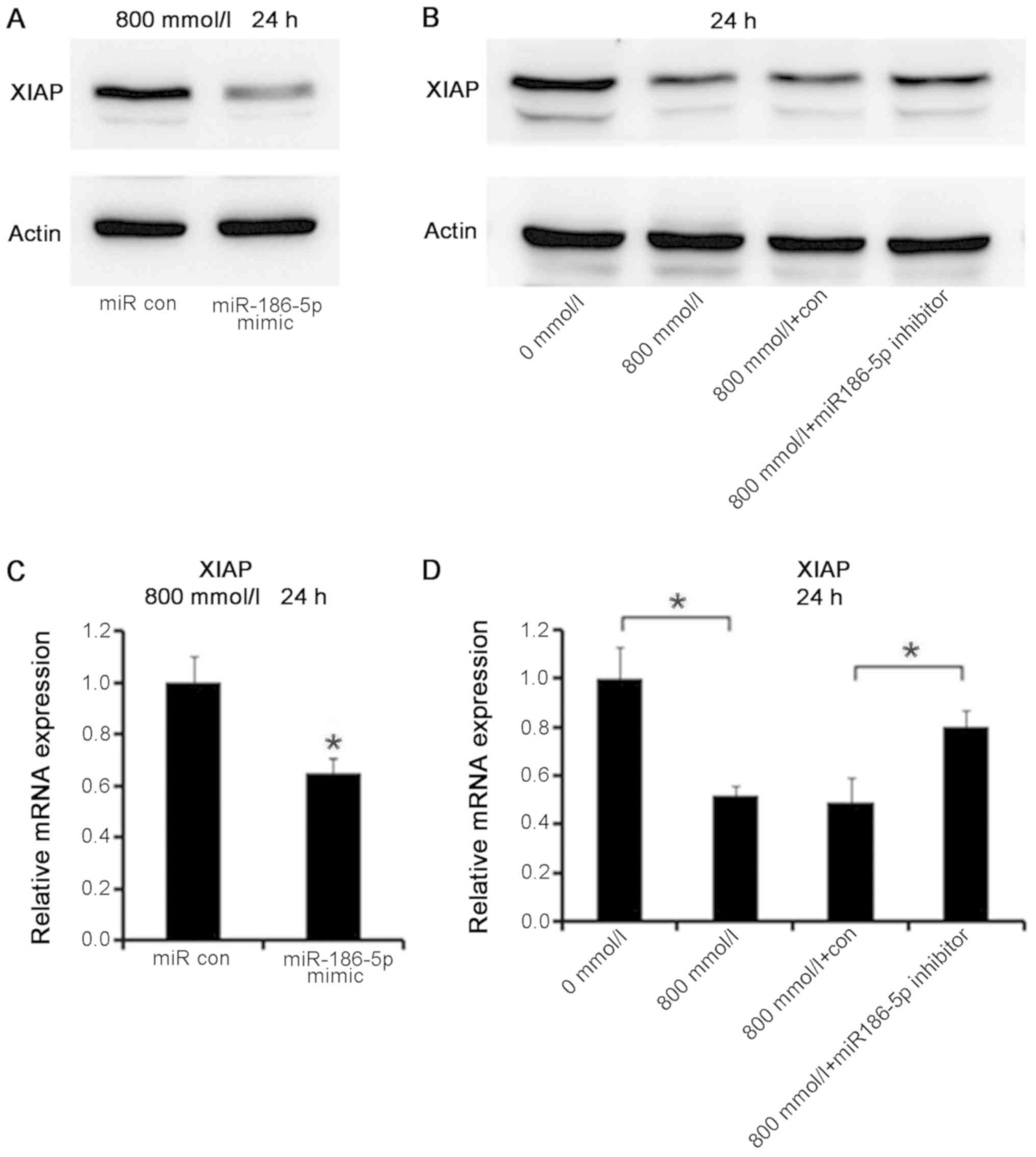
miR-186-5p regulates the expression of
XIAP in AC16 cardiomyocytes
According to the aforementioned experiments,
miR-186-5p and XIAP may serve a role in the apoptosis of
ethanol-treated AC16 cardiomyocytes. The dual-luciferase reporter
gene experiment detected that miR-186-5p may be directly combined
with the 3′-UTR (332–338) of the XIAP mRNA and further inhibit the
expression of XIAP directly. Therefore, it was hypothesized that
XIAP may be a target gene for miR-186-5p and thus regulate
apoptosis in AC16 cardiomyocytes. To confirm this hypothesis,
miR-186-5p mimic was transfected into AC16 cardiomyocytes to
upregulated its expression, and the protein and mRNA expression
levels of XIAP in these cells was detected using western blotting
and RT-qPCR, respectively.
The western blotting data demonstrated that AC16
cardiomyocyte transfection with miR186-5p mimic followed by
treatment with ethanol (800 mmol/l, 24 h) upregulated the
expression levels of miR-186-5p, causing a decrease in the protein
levels of XIAP (Fig. 8A). In
addition, AC16 cardiomyocytes were transfected with miR-186-5p
inhibitor and treated with ethanol (800 mmol/l, 24 h), thereby
downregulating the expression levels of miR-186-5p, and causing the
protein expression levels of XIAP to increase (Fig. 8B).
Similarly, RT-qPCR was used to detect the mRNA
expression levels of XIAP in the cells. The results were as
follows: AC16 cardiomyocytes transfected with miR186-5p mimic were
treated with ethanol (800 mmol/l, 24 h), which upregulated the
expression levels of miR-186-5p, causing a significant decrease in
the mRNA expression levels of XIAP (P<0.05; Fig. 8C); AC16 cardiomyocytes transfected
with miR-186-5p inhibitor were treated with ethanol (800 mmol/l, 24
h), downregulating the expression levels of miR-186-5p, but causing
a significant increase in the mRNA expression levels of XIAP
(P<0.05; Fig. 8D). Therefore,
miR-186-5p may decrease the expression levels of XIAP in
ethanol-treated AC16 cardiomyocytes, and the introduction of the
miR-186-5p inhibitor partially reversed this phenotype, resulting
in higher expression levels of XIAP in ethanol-treated
cardiomyocytes.
Discussion
ACM refers to myocardial lesions characterized by
hypertrophy of the heart, arrhythmia, and congestive heart failure
caused by long-term ingestion of large amounts of ethanol. A study
demonstrated that drinking >90–120 g per day for 5–15 years may
increase the risk of cardiac structural and functional
modifications (15). Data have
demonstrated that the number of people who succumb to excessive
drinking is rising year by year (16). Therefore, more research is required
to elucidate the pathogenesis of alcoholic cardiomyopathy to allow
for better early diagnosis and treatment. However, the pathogenesis
of ACM remained to be clarified.
Various studies have analyzed the pathogenesis of
ACM, which includes cardiac toxicity induced by the principal
metabolites of ethanol (17,18),
apoptosis (19), mitochondrial
dysfunction (20) and abnormal
energy metabolism of cardiomyocytes, nutrient imbalance,
ventricular remodeling (21,22),
disorders of the neurohumoral system (23,24),
abnormal cell signal transduction and immunological and
inflammatory reactions (25).
Studies have reported that ethanol-induced apoptosis serves a key
role in the pathogenesis of ACM (26–29).
A study on the mechanism of ethanol-induced cardiomyocyte apoptosis
demonstrated that the levels of tumor necrosis factor-α, the
apoptotic protein Bcl-2-associated X and caspase-3 are
significantly increased in ACM (30). In the present study, AC16
cardiomyocytes were treated with ethanol and its effects on
apoptosis analyzed. The results demonstrated that the levels of
apoptosis increased following treatment with ethanol. With the
prolongation of ethanol treatment time, the cardiomyocyte apoptosis
increased. These results are consistent with previous studies
(31–34).
In recent years, studies on miRs have focused on
disease processes, including pathogenesis, development and
prognosis. By the end of 2006, scientists were realizing that miRs
serve important roles in the pathological development of various
heart diseases, and important progress on myocardial hypertrophy,
arrhythmia, heart failure and other diseases was achieved (35). This caused miRs to become an
important research focus in the field of cardiovascular disease, at
home and abroad. Notably, Zhang et al (36) investigated the effects of miR-186
overexpression or inhibition on the apoptosis of A549 cells, and
demonstrated that the significant downregulation of miR-186
expression was associated with curcumin-induced apoptosis. Based on
these research findings, it may be determined that miR-186 has a
complex association with apoptosis, and its varying roles in
apoptosis may be associated with the regulation of different
downstream signaling pathways or with the existence of different
subtypes of miR-186.
The goal of this study was to investigate whether
the expression of miR-186-5p is associated with alcohol-induced
apoptosis of AC16 cardiomyocytes. In our previous experiment (Liu
et al, unpublished data), the expression levels of partial
miRs, such as miR-133a, miR-125, miR-195, miR-186-5p and
miR-488-3p, in ethanol-treated cardiomyocytes were screened, and it
was observed that the expression levels of miR-186-5p increased
significantly. In the present study, transfecting miR-186-5p mimic
into the ethanol-treated cardiomyocytes led to the upregulation of
miR-186-5p, causing the level of apoptosis to further increase.
Thus, it may be that miR-186-5p serves an important role in
promoting the ethanol-induced apoptosis of cardiomyocytes.
XIAP is a member of the IAP family, and the most
potent known IAP protein in human tissues (3–6).
XIAP is able to inhibit apoptosis induced by viral infection
(37) or by the overexpression of
caspase family proteins (38). In
the present study, the expression levels of XIAP in ethanol-treated
AC16 cardiomyocytes were observed to be decreased. In order to
further confirm whether XIAP serves a role in the process of
ethanol-induced cardiomyocyte apoptosis, the expression levels of
XIAP were upregulated via plasmid transfection. As a result,
cardiomyocyte apoptosis was reduced following ethanol treatment and
compared with untransfected ethanol-treated cardiomyocytes.
Therefore, XIAP may inhibit alcohol-induced cardiomyocyte
apoptosis.
Abnormal expression levels of miR-186-5p and XIAP
were simultaneously detected in ethanol-treated cardiomyocytes.
Moreover, through the dual-luciferase reporter gene experiment, the
fluorescence intensity of the group of wild-type pmirGLO-wt-XIAP-3
′UTR in combination with miR-186-5p mimic was observed to be
decreased compared with the other three groups, a finding which was
substantiated by RT-qPCR. Thus, there may be a direct site on the
3′-UTR region of XIAP, which may allow interactions with
miR-186-5p, and miR-186-5p may target XIAP to inhibit its
expression. To verify this hypothesis, AC16 cardiomyocytes
transfected with miR186-5p mimic were treated with ethanol (800
mmol/l, 24 h), which upregulated the expression levels of
miR-186-5p, and observed that XIAP was downregulated at the protein
and mRNA levels. However, XIAP was upregulated at the protein and
gene levels in cardiomyocytes following transfection of miR-186-5p
inhibitor and then treatment with ethanol (800 mmol/l, 24 h).
Therefore, XIAP is a direct target gene of miR-186-5p, and
miR-186-5p along with XIAP may control the process of
ethanol-induced apoptosis in cardiomyocytes. The present study
further elucidated the pathogenesis of ACM. A limitation of the
present study is that a control experiment in non-myocardial cells
is lacking. As this research work focused on the study of ACM, no
non-myocardial cell lines were available. This will be improved
upon in future experiments.
In conclusion, ethanol intake may have caused the
apoptosis of AC16 cardiomyocytes, and the apoptosis levels were
dependent on ethanol dose and duration of action. In terms of the
specific mechanism of action, it was indicated that the expression
levels of miR-186-5p were upregulated, and the expression levels of
XIAP were downregulated in cardiomyocytes following ethanol
treatment. XIAP may be a target gene of miR-186, and together may
regulate the process of ethanol-induced apoptosis in
cardiomyocytes. This study provides a novel therapeutic target for
the prevention and treatment of ACM.
Acknowledgements
Not applicable.
Funding
This research was supported by The Natural Science
Fund Guidance Plan of Liaoning Province (grant no.
20180550419).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YL performed the experiments, organized the data and
wrote the article. BY conceived and designed the study. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Naimi TS, Nelson DE and Brewer RD: The
intensity of binge alcohol consumption Among U.S. adults. Am J Prev
Med. 38:201–207. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Steiner JL and Lang CH: Etiology of
alcoholic cardiomyopathy: Mitochondria, oxidative stress and
apoptosis. Int J Biochem Cell Biol. 89:125–135. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Edison N, Curtz Y, Paland N, Mamriev D,
Chorubczyk N, Haviv-Reingewertz T, Kfir N, Morgenstern D,
Kupervaser M, Kagan J, et al: Degradation of Bcl-2 by XIAP and ARTS
promotes apoptosis. Cell Rep. 21:442–454. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cesa LC, Shao H, Srinivasan SR, Tse E,
Jain C, Zuidereg ERP, Southworth DR, Mapp AK and Gestwicki JE:
X-linked inhibitor of apoptosis protein (XIAP) is a client of heat
shock protein 70 (Hsp70) and a biomarker of its inhibition. J Biol
Chem. 293:2370–2380. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang WZ, Zhou H and Yan Y: XIAP underlies
apoptosis resistance of renal cell carcinoma cells. Mol Med Rep.
17:125–130. 2018.PubMed/NCBI
|
|
6
|
Wang Z, Song J, Zhang L, Huang S, Bao L,
Chen F and Zhao X: Increased expression of microRNA-378a-5p in
acute ethanol exposure of rat cardiomyocytes. Cell Stress
Chaperones. 22:245–252. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jing L, Lin C, Lu Y, Huo P, Zhou L, Wang Y
and Tian Y: Investigation of microRNA expression profiles
associated with human alcoholic cardiomyopathy. Cardiology.
30:223–233. 2015. View Article : Google Scholar
|
|
8
|
Zheng J, Li XD, Wang P, Liu XB, Xue YX, Hu
Y, Li Z, Li ZQ, Wang ZH and Liu YH: CRNDE affects the malignant
biological characteristics of human glioma stem cells by negatively
regulating miR-186. Oncotarget. 6:25339–25355. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang J, Mo H, Liu C, Wu B, Wu Z, Li X, Li
T, He S, Li S, You Q, et al: Inhibition of miR-186-5p contributes
to high glucose-induced injury in AC16 cardiomyocytes. Exp Ther
Med. 15:627–632. 2018.PubMed/NCBI
|
|
10
|
Karnewar S, Vasamsetti SB, Gopoju R,
Kanugula AK, Ganji SK, Prabhakar S, Rangaraj N, Tupperwar N, Kumar
JM and Kotamraju S: Mitochondria-targeted esculetin alleviates
mitochondrial dysfunction by AMPK-mediated nitric oxide and SIRT3
regulation in endothelial cells: Potential implications in
atherosclerosis. Sci Rep. 6:241082016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Perez Pinzon MA, Stetler RA and Fiskum G:
Novel mitochondrial targets for neuroprotection. J Cereb Blood Flow
Metab. 32:1362–1376. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Karnewar S, Vasamsetti SB, Gopoju R,
Kanugula AK, Ganji SK, Prabhakar S, Rangaraj N, Tupperwar N, Kumar
JM and Kotamraju S: Mitochondria-targeted esculetin alleviates
mitochondrial dysfunction by AMPK-mediated nitric oxide and SIRT3
regulation in endothelial cells: Potential implications in
atherosclerosis. Sci Rep. 6:241082016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Perez-Pinzon MA, Stetler RA and Fiskum G:
Novel mitochondrial targets for neuroprotection. J Cereb Blood Flow
Metab. 32:1362–1376. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McNair P, Jones E, Truong Q and Singh H:
Incidental finding of single coronary artery in a patient with
alcoholic cardiomyopathy presenting as acute heart failure. Clin
Imaging. 42:224–227. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
George A and Figueredo VM: Alcoholic
cardiomyopathy: A review. J Card Fail. 17:844–849. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guo R and Ren J: Alcohol dehydrogenase
accentuates ethanol-induced myocardial dysfunction and
mitochondrial damage in mice: Role of mitochondrial death pathway.
PLoS One. 5:e87572010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ge W and Ren J: mTOR-STAT3-notch
signalling contributes to ALDH2-induced protection against cardiac
contractile dysfunction and autophagy under alcoholism. J Cell Mol
Med. 16:616–626. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guo R, Hu N, Kandadi MR and Ren J:
Facilitated ethanol metabolism promotes cardiomyocyte contractile
dysfunction through autophagy in murine hearts. Autophagy.
8:593–608. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang B, Turdi S, Li Q, Lopez FL, Eason
AR, Anversa P and Ren J: Cardiac overexpression of insulin-like
growth factor 1 attenuates chronic alcohol intake-induced
myocardial contractile dysfunction but not hypertrophy: Roles of
Akt, mTOR, GSK3beta, and PTEN. Free Radic Biol Med. 49:1238–1253.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu C, Ge F, Hyodo E, Arai K, Iwata S,
Lobdell H IV, Walewski JL, Zhou S, Clugston RD, Jiang H, et al:
Chronic ethanol consumption increases cardiomyocyte fatty acid
uptake and decreases ventricular contractile function in C57BL/6J
mice. J Mol Cell Cardiol. 59:30–40. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Piano MR and Phillips SA: Alcoholic
cardiomyopathy: Pathophysiologic insights. Cardiovasc Toxicol.
14:291–308. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Piano M: Alcoholic cardiomyopathy:
Incidence, clinical characteristics, and pathophysiology. Chest.
121:1638–1650. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cheng CP, Cheng HJ, Cunningham C, Shihabi
ZK, Sane DC, Wannenburg T and Little WC: Angiotensin II type 1
receptor blockade prevents alcoholic cardiomyopathy. Circulation.
114:226–236. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jing L, Jin CM, Li SS, Zhang FM, Yuan L,
Li WM, Sang Y, Li S and Zhou LJ: Chronic alcohol intake-induced
oxidative stress and apoptosis: Role of CYP2E1 and calpain-1 in
alcoholic cardiomyopathy. Mol Cell Biochem. 359:283–292. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lai Y, Guo H, Li J, Dai J, Ren C and Wang
Y: Comparison of surgical results in patients with hypertrophic
obstructive cardiomyopathy after classic or modified morrow septal
myectomy. Medicine (Baltimore). 96:e93712017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ji F, Liu Q, Feng Z, Han X and Li Z:
Genetic association between 1425G/A SNP in PRKCH and hypertrophic
cardiomyopathy in a Chinese population. Oncotarget.
8:114839–114844. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mu J, Zhang G, Xue D, Xi M, Qi J and Dong
H: Sudden cardiac death owing to arrhythmogenic right ventricular
cardiamyopathy: Two case reports and systematic literature review.
Medicine (Baltimore). 96:88082017. View Article : Google Scholar
|
|
29
|
Dahraoui S, Uwingabiye J, Belarj B, Biaz
A, Rachid A, Dami A, Bouhsain S, Ouzzif Z and Elmachatni Idrissi S:
Unexpected discovery of multiple myeloma following cardiomyopathy.
Clin Case Rep. 6:86–90. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fu KY, Zamudio R, Henderson-Frost J,
Almuedo A, Steinberg H, Clipman SJ, Duran G, Marcus R, Crawford T,
Alyesh D, et al: Association of caspase-1 polymorphisms with Chagas
cardiomyopathy among individuals in Santa Cruz, Bolivia. Rev Soc
Bras Med Trop. 50:516–523. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Adachi K, Hashiguchi S, Saito M, Kashiwagi
S, Miyazaki T, Kawai H, Yamada H, Iwase T, Akaike M, Takao S, et
al: Detection and management of cardiomyopathy in female
dystrophinopathy carriers. J Neurol Sci. 386:74–80. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Baltrūnienė V, Bironaitė D, Kažukauskienė
I, Bogomolovas J, Vitkus D, Ručinskas K, Žurauskas E, Augulis R and
Grabauskienė V: The role of serum adiponectin for outcome
prediction in patients with dilated cardiomyopathy and advanced
heart failure. Biomed Res Int. 2017:38182922017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bollen IAE, van der Meulen M, de Goede K,
Kuster DWD, Dalinghaus M and van der Velden J: cardiomyocyte
hypocontractility and reduced myofibril density in end-stage
pediatric cardiomyopathy. Front Physiol. 8:11032017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Buccheri D and Zambelli G: The link
between spontaneous coronary artery dissection and takotsubo
cardiomyopathy: Analysis of the published cases. J Thorac Dis.
9:5489–5492. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Van Rooij E, Sutherland LB, Liu N,
Williams AH, McAnally J, Gerard RD, Richardson JA and Olson EN: A
signature pattern of stress-responsive microRNAs that can evoke
cardiac hypertrophy and heart failure. Proc Naatl Acad Sci USA.
103:18255–18260. 2006. View Article : Google Scholar
|
|
36
|
Zhang J, Du Y, Wu C, Ren X, Ti X, Shi J,
Zhao F and Yin H: Curcumin promotes apoptosis in human lung
adenocarcinoma cells through miR-186* signaling pathway. Oncol Rep.
24:1217–1223. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Crook NE, Clem RJ and Miller LK: An
apoptosis-inhibiting baculovirus gene with a zinc finge-like motif.
J viorl. 67:2168–2174. 1993.
|
|
38
|
LaCasse EG: Pulling the plug on a cancer
cell by eliminating XIAP with AEG35156. Cancer Lett. 332:215–224.
2013. View Article : Google Scholar : PubMed/NCBI
|