Introduction
Osteosarcoma (OS) derives from mesenchymal cells and
is the most common primary malignant bone cancer (1). The incidence rate of OS is ~4.4 per
million worldwide, and incidence is higher in adolescents (15–19
years old) (2). OS accounts for
~5% of all malignant tumors in children and adolescents (3). The principal therapeutic strategies
for patients with OS are surgical resection, radiotherapy and
multidrug chemotherapy (4).
Although substantial improvements have been achieved in the
treatment of OS, the therapeutic outcomes remain poor due to the
high recurrence rate and the metastatic features of OS (5). Various factors, including genetic
mutations and environmental ionizing radiation, are associated with
the development and progression of OS (6). However, the molecular mechanisms
underlying OS pathogenesis remain unclear. Therefore, improving the
understanding of the molecular processes underlying the initiation
and development of OS may contribute to the identification of novel
therapeutic strategies for the treatment of patients with this
malignancy.
microRNAs (miRNAs) are short (18–25 nucleotides in
length) noncoding RNA molecules that are able to directly bind to
the 3′-untranslated regions (3′-UTRs) of their target genes
inducing translational inhibition and/or mRNA degradation (7). In total, >1,000 miRNAs have been
identified in the human genome (8). miRNAs account for ~3% of all human
genes; however, these short noncoding RNAs are able to modulate the
expression levels of ~66% of all coding human genes (8). Accumulating evidence has demonstrated
that the dysregulation of miRNAs is frequent in human malignancies,
and the altered expression level of miRNAs was identified to be
important in maintaining the malignant phenotype of certain tumor
cells (9–11). In particular, various miRNAs were
identified to be downregulated in OS, including miR-103 (12), miR-187 (13) and miR-365 (14). These downregulated miRNAs may serve
as tumor suppressors or oncogenes based on the biological function
of their target genes (15).
Therefore, the identification of novel miRNAs involved in OS
initiation and development may contribute to the development of
effective therapeutic strategies to improve the outcomes of
patients with OS.
Previous studies demonstrated that miRNA-466
(miR-466) is dysregulated in various types of human cancer, and it
was identified to be associated with cancer progression (16–18).
However, whether miR-466 is involved in the initiation and
progression of OS remains unclear. Therefore, the present study
aimed to examine the expression level of miR-466 in OS tissues and
cell lines, investigating the function and the molecular mechanisms
associated with miR-466 in OS in order to identify a novel
potential therapeutic target to treat patients with OS.
Materials and methods
Tissue collection
The present study was approved by The Ethics
Committee of The Ningbo No. 6 Hospital. Written informed consent
was obtained from all patients for the use of their clinical
tissues. In total, 26 pairs of OS and matched adjacent normal
tissues were collected from patients (17 males, 9 females; age
range, 13–45 years) who received surgical resection at The Ningbo
No. 6 Hospital between March 2015 and June 2017. Patients who
received radiotherapy or chemotherapy treatment before surgery were
excluded from the study. The collected tissues were snap-frozen in
liquid nitrogen and stored at −80°C until further analysis.
Cell lines
A normal human osteoblast (hFOB1.19) and four human
OS (SAOS-2, HOS, MG-63 and U2OS) cell lines were purchased from The
American Type Culture Collection (Manassas, VA, USA). Dulbecco's
modified Eagle's medium (DMEM) supplemented with 10% fetal bovine
serum (FBS), 100 U/ml penicillin and 100 µg/ml streptomycin (all
from Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) was
used to culture the cell lines. All cells were maintained at 37°C
in a 5% CO2 humidified atmosphere.
Cell transfection
The miR-466 mimics and miRNA negative control mimics
(miR-NC) were purchased from Shanghai GenePharma Co., Ltd.
(Shanghai, China). To promote miR-466 overexpression, cells were
seeded into 6-well plates with a density of 6×105
cells/well one day prior to transfection. Subsequently, miR-466
mimics and miR-NC were transfected. IRS1 overexpression plasmid
pCMV–IRS1 and the empty control plasmid pCMV were purchased from
Hunan Nanhua Ai Shi Purin Biotechnology Co., Ltd. (Changsha,
China). pCMV–IRS1 was transfected to overexpress IRS1, whereas
empty pCMV plasmid was used as the negative control. Cells were
transfected with miR-466 mimics (100 pmol), miR-NC (100 pmol),
pCMV–IRS1 (4 µg) or pCMV (4 µg) using Lipofectamine®
2000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Following an 8-h incubation at 37°C, the
culture medium was replaced with fresh DMEM containing 10% FBS.
Cell Counting kit-8 (CCK-8) assay, reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
cell invasion assay were conducted after 24, 48 and 48 h,
respectively. A total of 72 h post-transfection, western blot
analysis was performed.
RNA isolation and RT-qPCR
Total RNA was isolated from cells and clinical
tissues using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. For
miR-466 detection, total RNA was reverse-transcribed using a TaqMan
microRNA RT kit (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The temperature protocol for RT was as follows: 16°C for 30
min, 42°C for 30 min and 85°C for 5 min. The expression level of
miR-466 was measured using a TaqMan microRNA PCR kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.), and RNA, U6 small
nuclear 6 was used as the internal control to normalize the
expression level of miR-466. The cycling conditions were as
follows: 50°C for 2 min, 95°C for 10 min; followed by 40 cycles of
denaturation at 95 C for 15 sec and annealing/extension at 60°C for
60 sec; and one cycle of final extension at 60°C for 60 sec and 4°C
for 30 sec. For the quantification of the mRNA expression level of
IRS1, cDNA was synthesized from total RNA using a M-MLV reverse
transcriptase (Takara Biotechnology Co., Ltd., Dalian, China), and
the cDNA was subsequently amplified using a SYBR Premix Ex Taq II
kit (Takara Biotechnology Co., Ltd., Dalian, China). GAPDH was used
as the internal reference gene to normalize the expression level of
IRS1. The RT reaction comprised 100 ng total RNA, 2.5 µl primers
(2.5 µM), 7.5 µl RNase-free H2O, 4 µl 5X RT Buffer, 2 µl
dNTP Mix, 2 µl 100 mM DTT and 0.5 µl MMLV Reverse Transcriptase.
The temperature protocol for RT was as follows: 95°C for 2 min;
followed by 20 cycles at 94°C for 1 min, 55°C for 1 min and 72°C
for 2 min; and 72°C for 5 min. The PCR cycling conditions were as
follows: 5 min at 95°C, followed by 40 cycles at 95°C for 30 sec
and 65°C for 45 sec; and one cycle of final extension at 95°C for
30 sec, 65°C for 45 sec and 4°C for 30 sec. Data analysis was
performed using the 2−ΔΔCq quantification method
(19). The primers were designed
as follows: miR-466, forward 5′-ATGGTTCGTGGGATACACATACACGCA-3′,
reverse 5′-GCAGGGTCCGAGGTATTC-3′; U6, forward
5′-GCTTCGGCAGCACATATACTAAAAT-3′, reverse
5′-CGCTTCACGAATTTGCGTGTCAT-3′; IRS1, forward
5′-CAGGCAGAATGAAAGACCTAAATG-3′, reverse
5′-AGACGTGAGGTCCTGGTTGTG-3′; and GAPDH, forward
5′-CAAGGTCATCCATGACAACTTTG-3′ and reverse
5′-GTCCACCACCCTGTTGCTGTAG-3′.
CCK-8 assay
The proliferative ability of OS cells was determined
using the CCK-8 assay. The transfected cells were collected at 48 h
post-transfection and plated into 96-well plates at a density of
3×103 cells/well. Following incubation for 0, 24, 48 and
72 h, 10 µl CCK-8 reagent (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) was added to each well, and incubated for 2 h at
37°C with 5% CO2. The absorbance was detected at a
wavelength of 450 nm with an ELISA plate reader (Model 550; Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Cell invasion assay
Transfected cells were harvested following a 48-h
incubation at 37°C and resuspended in FBS-free DMEM. In total,
5×104 transfected cells were seeded in the upper
compartment of Transwell® chambers coated with
Matrigel® (all from Becton, Dickinson and Company,
Franklin Lakes, NJ, USA). In total, 600 µl DMEM containing 10% FBS
was plated in the lower compartments. Following a 24-h incubation,
the non-invasive cells were removed from the upper surface of the
Transwell chambers using a cotton swab. The invaded cells were
subsequently fixed with 100% methanol at room temperature for 30
min and stained with 0.5% crystal violet solution at room
temperature for 30 min. The number of invasive cells was counted in
five independent fields per well using an inverted light microscope
(Olympus Corporation, Tokyo, Japan).
Target prediction of miR-466
miRDB (http://mirdb.org/) and TargetScan (Release 7.2;
http://www.targetscan.org/) were used to
predict the potential targets of miR-466.
Luciferase reporter assay
IRS1 was predicted as one of the principal targets
of miR-466. To test this interaction, the 3′-UTR of IRS1 containing
the wild-type or mutant miR-466 binding sites were synthesized by
Shanghai GenePharma Co., Ltd., and inserted into pMIR-GLOTM
Luciferase vectors (Promega Corporation, Madison, WI, USA). Cells
were plated in 24-well plates and incubated until they reached a
confluence of 60–70%. The co-transfection of luciferase reporter
plasmids and miR-466 mimics or miR-NC was performed using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The
transfected cells were incubated at 37°C under 5% CO2
for 48 h. Firefly and Renilla luciferase activities were
measured using a Dual-Luciferase® Reporter Assay system
(cat. no. E1910; Promega Corporation) according to the
manufacturer's protocol. Firefly luciferase activity was normalized
to Renilla luciferase activity.
Western blot analysis
Total protein was isolated from tissue specimens or
cells using radioimmunoprecipitation assay buffer (Beyotime
Institute of Biotechnology, Haimen, China). A bicinchoninic protein
assay kit (Beyotime Institute of Biotechnology) was used to measure
the protein concentration. An equal amount of total protein (30 µg)
was loaded in each lane, and proteins were separated by 10%
SDS-PAGE gel and transferred to polyvinylidene difluoride membranes
(EMD Millipore, Billerica, MA, USA), and subsequently were blocked
with 5% non-fat milk diluted in Tris-buffer containing 0.1%
Tween-20 (TBST) for 2 h at room temperature. Following blocking,
the membranes were incubated overnight at 4°C with anti-IRS1
antibody (cat. no. ab40777; 1:1,000; Abcam, Cambridge, UK) or
anti-GAPDH antibody (cat. no. ab181603; 1:1,000; Abcam). Following
washing with TBST, the membranes were incubated for 2 h at room
temperature with a horseradish peroxidase-conjugated goat
anti-rabbit immunoglobulin G secondary antibody (cat. no. ab6721;
1:5,000; Abcam, Cambridge, UK). Following three washes with TBST,
the protein signal was visualised using enhanced
chemiluminescence-plus reagents (GE Healthcare, Chicago, IL, USA),
according to the manufacturer's protocol. The protein expression
levels were quantified using Quantity One software (version 4.62;
Bio-Rad Laboratories, Inc.).
Statistical analysis
All assays were repeated at least three times, and
data are presented as the mean ± standard deviation. All
statistical analyses were performed using SPSS software (version
16; SPSS, Inc., Chicago, IL, USA). Differences between two groups
were determined using Student's t-test, whereas one-way ANOVA
followed by Student-Newman-Keuls post hoc test was used for the
comparison of multiple groups. Spearman's correlation analysis was
used to examine the correlation between the expression levels of
miR-466 and IRS1 mRNA in OS tissues. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression level of miR-466 is
decreased in OS tissues and cell lines
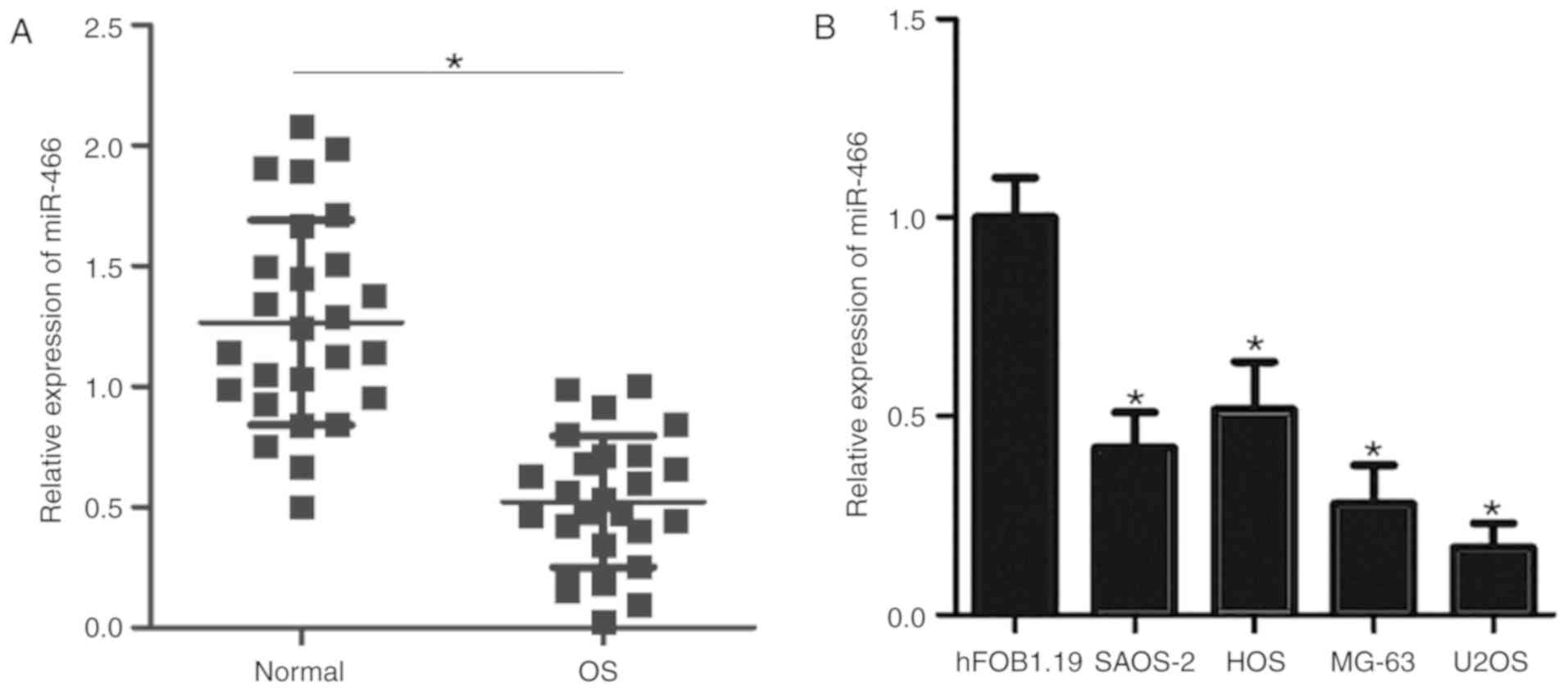
RT-qPCR was performed to quantify the expression
level of miR-466 in the tissue specimens of 26 patients with OS,
and matched adjacent normal tissues were used as a control. The
results suggested that the expression level of miR-466 was
significantly decreased in OS tissues compared with adjacent normal
tissues (Fig. 1A). Subsequently,
the expression level of miR-466 was detected in four OS cell lines
(SAOS-2, HOS, MG-63 and U2OS) and a normal human osteoblast
hFOB1.19 cell line. The expression level of miR-466 was
significantly decreased in all four OS cell lines compared with
hFOB1.19 cells (Fig. 1B). The
present results suggested that miR-466 may serve as an
oncosuppressor gene in the initiation and development of OS.
miR-466 suppresses the proliferation
and invasion of MG-63 and U2OS cells
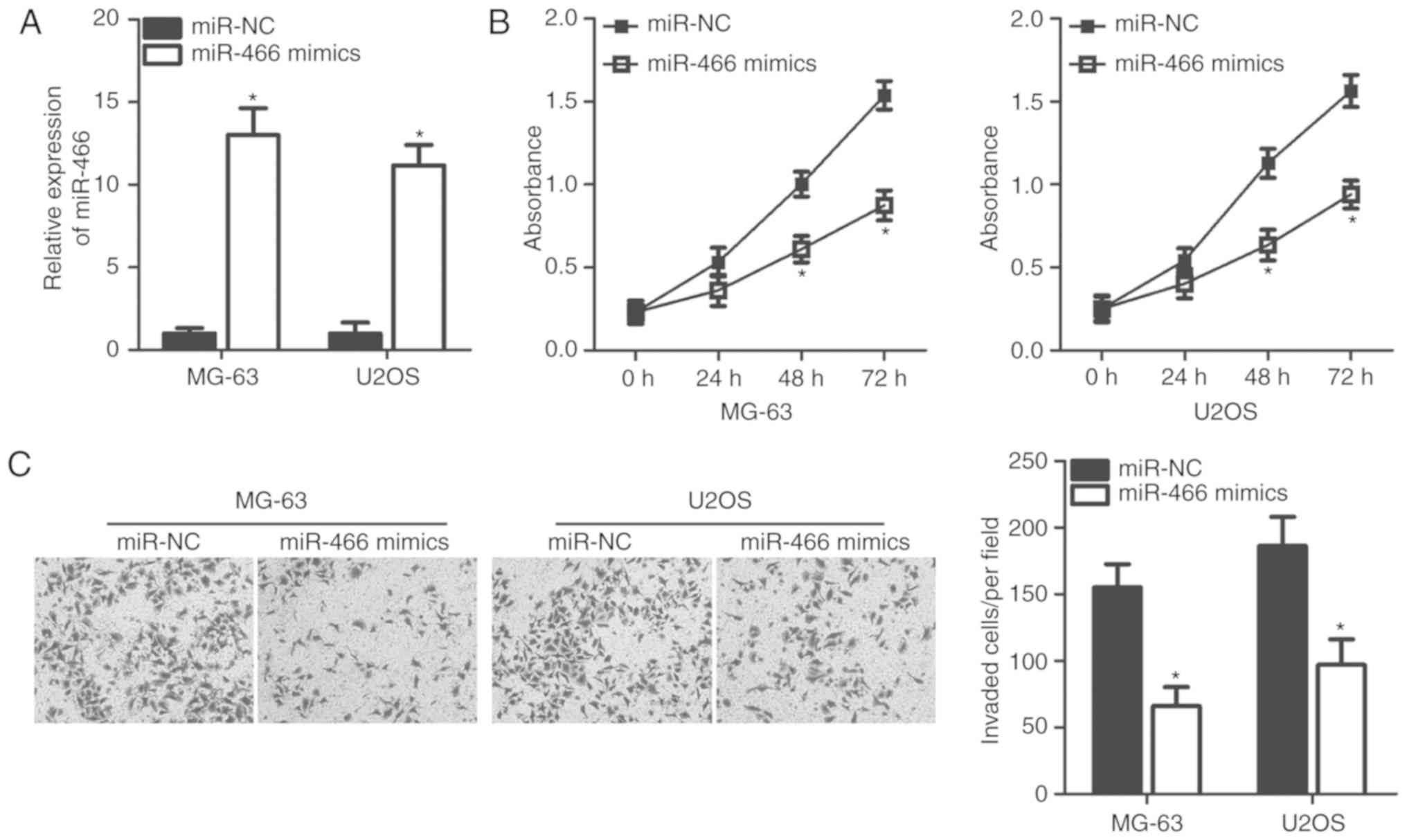
To investigate the role of miR-466 in OS
progression, miR-466 mimics or miR-NC were transfected into OS
cells, and the effects of miR-466 overexpression were determined by
examining the proliferation rate and invasive ability of OS cells.
MG-63 and U2OS cells exhibited a decreased expression level of
miR-466 compared with SAOS-2 and HOS cells. Therefore, these two
cell lines were used in subsequent experiments. Following
transfection, RT-qPCR analysis identified that the expression level
of miR-466 was significantly upregulated in MG-63 and U2OS cells
following transfection of miR-466 mimics (Fig. 2A). Additionally, CCK-8 assays
suggested that miR-466 overexpression was able to inhibit the
proliferation of MG-63 and U2OS cells at 48 and 72 h (Fig. 2B). Furthermore, cell invasion
assays suggested that overexpression of miR-466 was sufficient to
suppress the invasive abilities of MG-63 and U2OS cells (Fig. 2C). Collectively, the present data
suggested that miR-466 may serve a tumor-suppressive role by
inhibiting cell proliferation and invasion of OS cells.
miR-466 inhibits IRS1 expression by
directly targeting its 3′-UTR
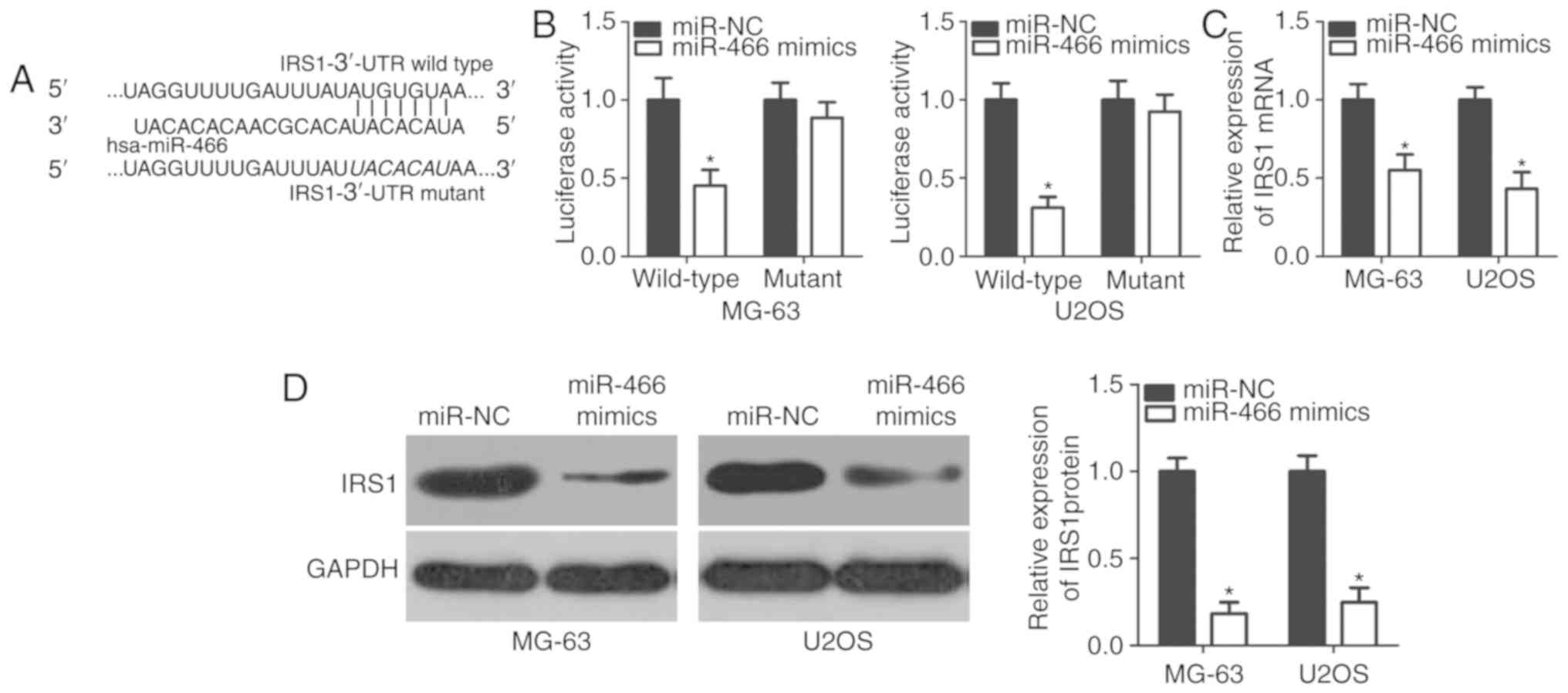
To further investigate the molecular mechanism
underlying the tumor-suppressive function of miR-466 in OS
pathology, bioinformatics analysis was performed to identify the
potential targets of miR-466. Numerous genes were predicted as
potential targets of miR-466, including IRS1, phosphatase and
tensin homolog, insulin-like growth factor (IGF)-like family member
1, SRY-box 6 and metadherin. Among these candidates, IRS1 exhibited
a miR-466 binding sequence at position 482–489 of its 3′-UTR
(Fig. 3A). IRS1 was selected for
further analyses due to the previously observed function of this
gene in OS development and progression (20). In order to test the bioinformatics
analysis in vitro, a luciferase reporter assay was
conducted. Overexpression of miR-466 significantly decreased the
reporter activity of pMIR-GLOTM plasmid containing wild-type IRS1
3′-UTR in MG-63 and U2OS cells (Fig.
3B); however, the reporter activity of the plasmid containing
mutant IRS1 3′-UTR was unaffected following miR-466 overexpression,
suggesting that miR-466 was able to directly target the 3′-UTR of
IRS1. Subsequently, the effect of miR-466 overexpression in OS
cells on the endogenous expression level of IRS1 was investigated.
RT-qPCR and western blot analyses results suggested that miR-466
overexpression decreased the mRNA (Fig. 3C) and the protein (Fig. 3D) expression levels of IRS1 in
MG-63 and U2OS cells. Collectively, the present results suggested
that IRS1 may be a direct target of miR-466 in OS cells.
miR-466 is negatively correlated with
IRS1 expression in OS tissues
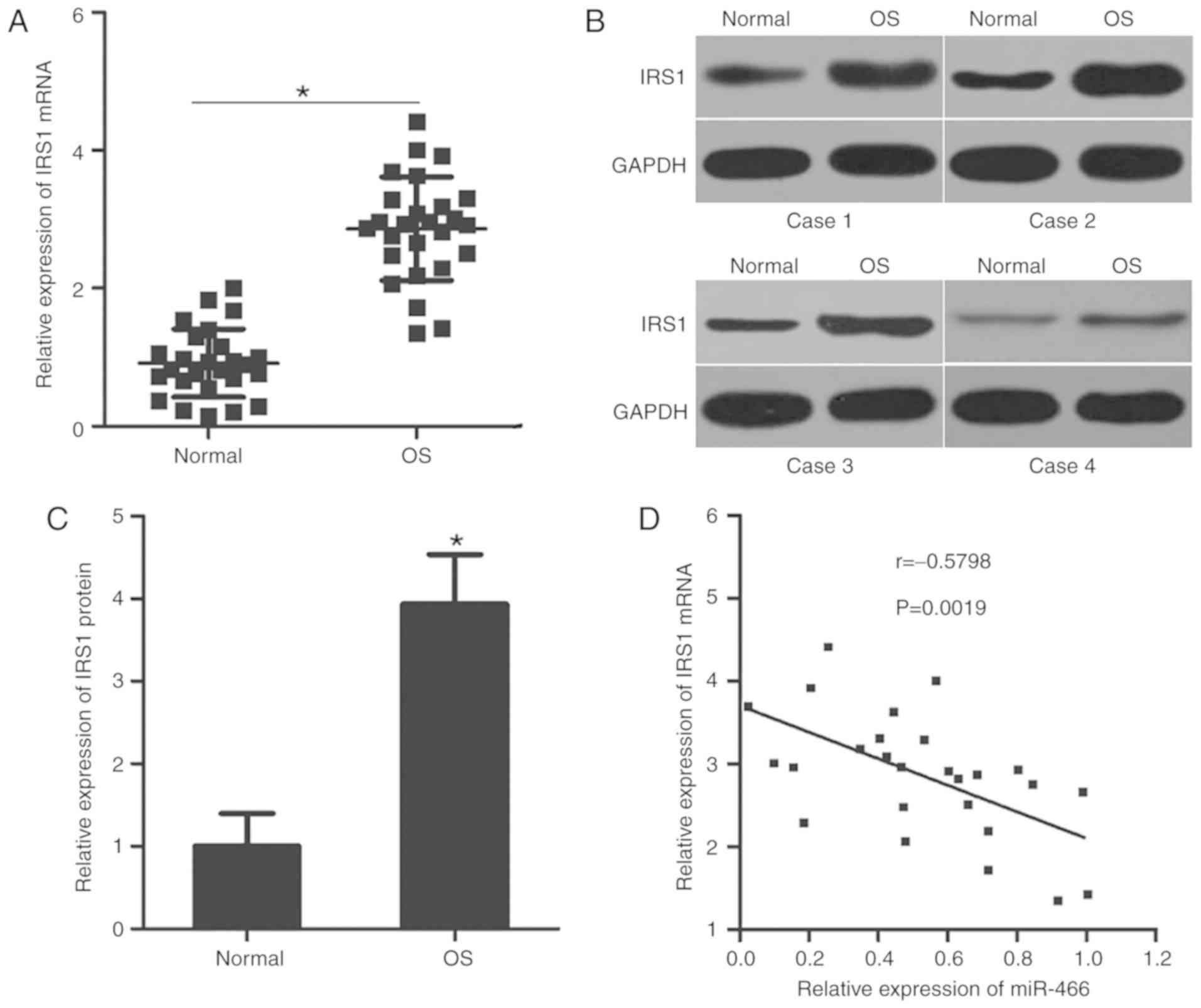
The aforementioned results suggested that IRS1 may
be a direct target gene of miR-466 in OS; therefore, the
association between the expression levels of miR-466 and IRS1 was
investigated in OS clinical tissues. RT-qPCR analysis suggested
that the mRNA expression level of IRS1 was upregulated in OS
tissues compared with adjacent normal tissues (Fig. 4A). Subsequently, the protein
expression level of IRS1 was detected in various OS tissues and
matched adjacent normal tissues using western blot analysis. The
protein expression level of IRS1 was increased in OS tissues
compared with adjacent normal tissues (Fig. 4B and C). Furthermore, Spearman's
correlation analysis identified an inverse correlation between the
mRNA expression levels of miR-466 and IRS1 mRNA in OS tissues
(Fig. 4D). The present results
suggested that the downregulation of miR-466 may, at least in part,
lead to an increase in the expression level of IRS1 in OS
tissues.
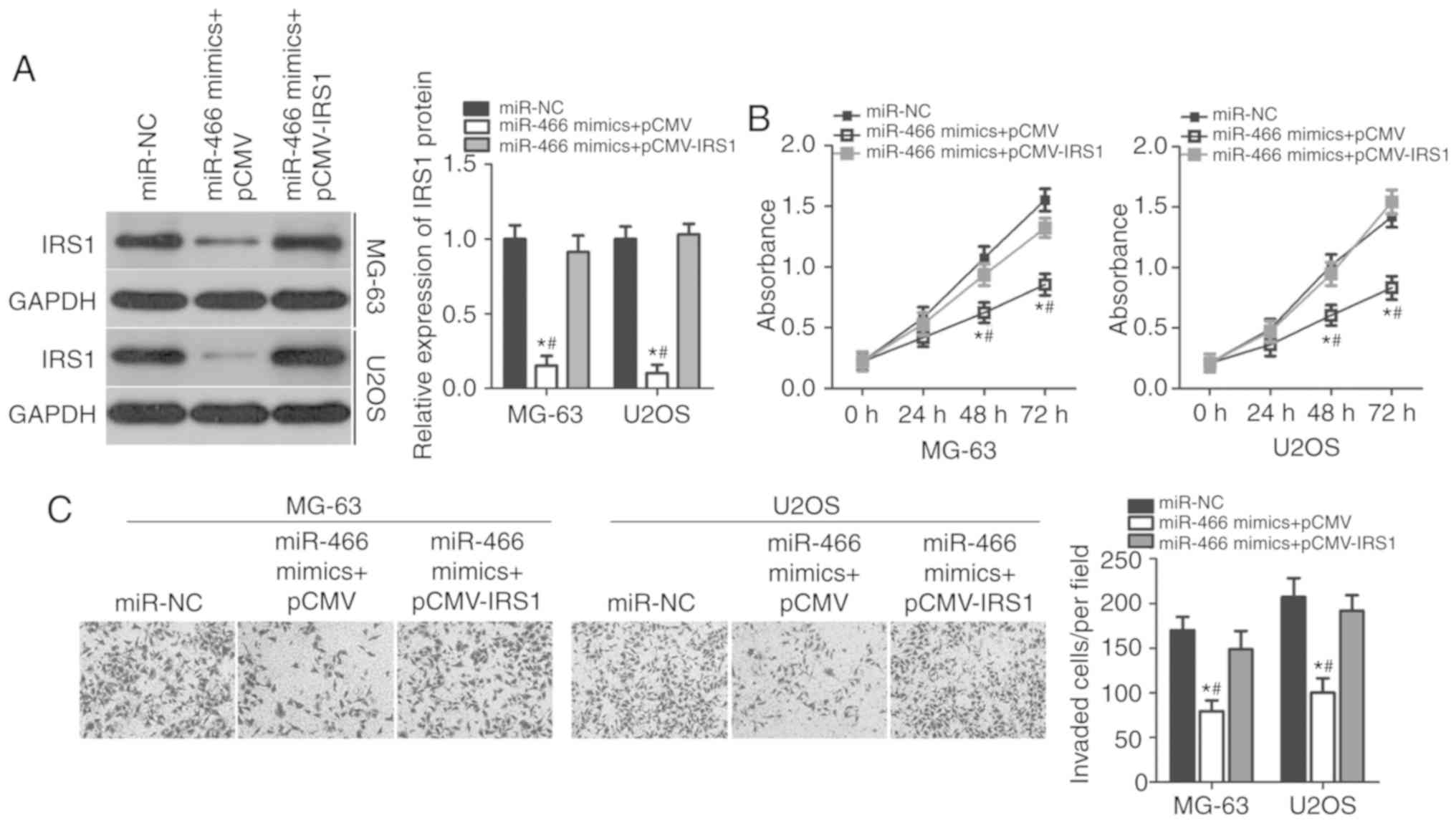
IRS1 mediates the tumor-suppressing
roles of miR-466 in OS cells
Rescue experiments were performed to investigate
whether the suppressive effects of miR-466 overexpression in OS
cell proliferation and invasion were mediated by IRS1 inhibition.
Empty pCMV plasmid or IRS1 overexpression plasmid (pCMV–IRS1)
without its 3′-UTR was co-transfected with miR-466 mimics into
MG-63 and U2OS cells. Western blot analysis suggested that the
downregulation of the protein expression level of IRS1 caused by
miR-466 overexpression was restored in MG-63 and U2OS cells
following co-transfection with pCMV–IRS1 (Fig. 5A). CCK-8 and cell invasion assays
were performed in MG-63 and U2OS cells following co-transfection
with miR-466 mimics and empty pCMV or pCMV–IRS1. Overexpression of
IRS1 suppressed the inhibitory effects of miR-466 overexpression on
the proliferation at 48 and 72 h (Fig.
5B) and invasion (Fig. 5C) of
MG-63 and U2OS cells. The present data suggested that miR-466
overexpression inhibited proliferation and invasion of OS cells by
decreasing the expression level of IRS1, and that the inhibition of
IRS1 was important for the suppressive effects of miR-466 in OS
cells.
Discussion
Accumulating evidence demonstrated that miRNAs are
frequently dysregulated in OS, and the aberrant expression of
miRNAs is associated with OS initiation and progression (21–23).
Therefore, to investigate the expression levels and the functions
of miRNAs associated with OS may provide novel insights into OS
pathogenesis and may facilitate the identification of novel targets
for the diagnosis and treatment of patients with this malignancy.
In the present study, the expression level of miR-466 was
identified to be downregulated in OS tissues and cell lines.
Ectopic overexpression of miR-466 was able to inhibit cell
proliferation and invasion in OS cells. Additionally, IRS1 was
identified as a direct target of miR-466 in OS cells, and its
expression level was significantly upregulated in OS tissues.
Notably, the expression levels of IRS1 and miR-466 were inversely
correlated in clinical samples of OS. Furthermore, IRS1
overexpression significantly suppressed the inhibitory effects of
miR-466 overexpression on the proliferation and invasion of OS
cells. The present results suggested that miR-466 may be a novel
candidate to develop gene therapy strategies to treat patients with
OS.
miR-466 expression has been studied in several types
of human malignancies. The expression of miR-466 is downregulated
in colorectal cancer tissues and cell lines. Decreased miR-466
expression was identified to be associated with tumor size,
Tumor-Node-Metastasis stage, lymph node metastasis and distant
metastasis of colorectal cancer patients (16). Patients with colorectal cancer
exhibiting a decreased expression level of miR-466 presented
decreased overall survival compared with patients with an increased
expression level of miR-466. The decreased expression level of
miR-466 was identified to be a biomarker associated with poor
prognosis in patients with colorectal cancer (16). The expression level of miR-466 was
detected to be decreased in prostate cancer tissues and cell lines
(17). Receiver operating
characteristic curve and Kaplan-Meier analyses demonstrated that
the dysregulated expression of miR-466 may represent a biomarker
for the diagnosis of prostate cancer (17). In addition, alterations in the
expression level of miR-466 may be associated with biochemical
relapse in patients with prostate cancer (17). In contrast, the expression level of
miR-466 was identified to be upregulated in cervical cancer
(18). Increased expression levels
of miR-466 were demonstrated to be associated with lymph node
metastasis (18). These previous
conflicting results suggest that the function of miR-466 may be
tissue-specific. Additionally, the expression level of miR-466 may
be used as an indicator for the early prognosis of patients with
these specific cancer types.
The dysregulation of miR-466 is involved in
important processes underlying tumorigenesis and tumor development
(16,17). A previous paper identified that
miR-466 overexpression inhibited the proliferation and metastasis
of colorectal cancer cells (16).
Additionally, overexpression of miR-466 induced G0/G1 cycle arrest
and promoted apoptosis in colorectal cancer (16). In prostate cancer, miR-466
overexpression inhibited cell proliferation, migration and
invasion, causing cell cycle arrest and promoting cell apoptosis
in vitro. Furthermore, overexpression of miR-466 was able to
decrease tumor growth and bone metastasis of prostate cancer in
vivo (17). These previous
findings suggested that miR-466 may be used as a therapeutic target
to effectively inhibit the development of colorectal and prostate
cancers.
The investigation of the direct targets of miR-466
in OS may be important in the identification of novel genes
involved in OS progression and may further facilitate the
development of targeted therapeutic strategies. IRS1 is a mediator
of oncogenic IGF signaling, and in the present study, IRS1 was
identified to be a direct target of miR-466 in OS. The expression
level of IRS1 is increased in multiple malignant tumor types,
including breast cancer (24),
hepatocellular carcinoma (25),
colorectal cancer (26), gastric
cancer (27) and non-small cell
lung cancer (28). IRS1 serves
pro-carcinogenic roles in tumorigenesis and tumor development, and
regulates a number of biological features, including cell
proliferation, cycle status, apoptosis, metastasis and
epithelial-to-mesenchymal transition (28–30).
In the present study, the expression level of IRS1 was identified
to be upregulated in OS tissues and cell lines. Additionally,
inhibition of IRS1 suppressed the proliferation, colony formation
ability, migration and invasion of OS cells (20). Due to the roles identified for IRS1
in OS pathogenesis, miR-466-mediated IRS1 gene silencing may
represent an effective therapeutic strategy to treat patients with
OS.
Collectively, to the best of the authors' knowledge,
the present study has identified the tumor-suppressive activity of
miR-466 OS. Additionally, the expression level of miR-466 was
significantly downregulated in tumor tissues and cell lines, and
its overexpression was able to inhibit cell proliferation and
invasion. The tumor-suppressive roles of miR-466 in OS cells were
mediated by the silencing of IRS1. Therefore, miR-466 may represent
a novel potential therapeutic target to treat OS. However, one
limitation of the present study was that the sample size was
relatively small. Further experiments analyzing data and samples
from a larger number of subjects are required to confirm the
association between the expression level of miR-466 and the
clinicopathological characteristics of patients with OS.
Acknowledgements
Not applicable.
Funding
Funding information is not applicable.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JC designed the present study, and analyzed and
interpreted the data. YS and JZ performed the CCK-8 and cell
invasion assays. LS and JL conducted RT-qPCR, western blot analysis
and luciferase reporter assay. All the authors read and approved
the final draft.
Ethics approval and consent to
participate
The present study was approved by The Ethics
Committee of The Ningbo No. 6 Hospital, and was performed in
accordance with the Declaration of Helsinki and the guidelines of
The Ethics Committee of The Ningbo No. 6 Hospital. Written informed
consent was obtained from all patients for the use of their
clinical tissues.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Cancer Treat Res. 152:3–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Geller DS and Gorlick R: Osteosarcoma: A
review of diagnosis, management, and treatment strategies. Clin Adv
Hematol Oncol. 8:705–718. 2010.PubMed/NCBI
|
|
3
|
Ta HT, Dass CR, Choong PF and Dunstan DE:
Osteosarcoma treatment: State of the art. Cancer Metastasis Rev.
28:247–263. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferrari S and Palmerini E: Adjuvant and
neoadjuvant combination chemotherapy for osteogenic sarcoma. Curr
Opin Oncol. 19:341–346. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nouri H, Ben Maitigue M, Abid L, Nouri N,
Abdelkader A, Bouaziz M and Mestiri M: Surface osteosarcoma:
Clinical features and therapeutic implications. J Bone Oncol.
4:115–123. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tan ML, Choong PF and Dass CR:
Osteosarcoma: Conventional treatment vs. gene therapy. Cancer Biol
Ther. 8:106–117. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hammond SM: An overview of microRNAs. Adv
Drug Deliv Rev. 87:3–14. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yates LA, Norbury CJ and Gilbert RJ: The
long and short of microRNA. Cell. 153:516–519. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tahara H, Kay MA, Yasui W and Tahara E:
MicroRNAs in cancer: the 22nd hiroshima cancer seminar/the 4th
Japanese Association for RNA interference joint international
symposium, 30 August 2012, Grand Prince Hotel Hiroshima. Jpn J Clin
Oncol. 43:579–582. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Winter J, Jung S, Keller S, Gregory RI and
Diederichs S: Many roads to maturity: microRNA biogenesis pathways
and their regulation. Nat Cell Biol. 11:228–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang X, Lin Y, Peng L, Sun R, Gong X, Du J
and Zhang X: MicroRNA-103 promotes proliferation and inhibits
apoptosis in spinal osteosarcoma cells by targeting p57. Oncol Res.
26:933–940. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xiao Y, Zhao Q, Du B, Chen HY and Zhou DZ:
MicroRNA-187 inhibits growth and metastasis of osteosarcoma by
downregulating S100A4. Cancer Invest. 36:1–9. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu Y, Chu H, Zhou Y, Wang J, Dong C and
Yin R: miR-365 functions as a tumor suppressor by directly
targeting CYR61 in osteosarcoma. Biomed Pharmacother. 98:531–537.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tong F, Ying Y, Pan H, Zhao W, Li H and
Zhan X: MicroRNA-466 (miR-466) functions as a tumor suppressor and
prognostic factor in colorectal cancer (CRC). Bosn J Basic Med Sci.
18:252–259. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Colden M, Dar AA, Saini S, Dahiya PV,
Shahryari V, Yamamura S, Tanaka Y, Stein G, Dahiya R and Majid S:
MicroRNA-466 inhibits tumor growth and bone metastasis in prostate
cancer by direct regulation of osteogenic transcription factor
RUNX2. Cell Death Dis. 8:e25722017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou LL, Shen Y, Gong JM, Sun P and Sheng
JH: MicroRNA-466 with tumor markers for cervical cancer screening.
Oncotarget. 8:70821–70827. 2017.PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhi X, Wu K, Yu D, Wang Y, Yu Y, Yan P and
Lv G: MicroRNA-494 inhibits proliferation and metastasis of
osteosarcoma through repressing insulin receptor substrate-1. Am J
Transl Res. 8:3439–3447. 2016.PubMed/NCBI
|
|
21
|
Ell B and Kang Y: MicroRNAs as regulators
of bone homeostasis and bone metastasis. Bonekey Rep. 3:5492014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kafchinski LA and Jones KB: MicroRNAs in
osteosarcomagenesis. Adv Exp Med Biol. 804:119–127. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim YH, Goh TS, Lee CS, Oh SO, Kim JI,
Jeung SH and Pak K: Prognostic value of microRNAs in osteosarcoma:
A meta-analysis. Oncotarget. 8:8726–8737. 2017.PubMed/NCBI
|
|
24
|
Porter HA, Perry A, Kingsley C, Tran NL
and Keegan AD: IRS1 is highly expressed in localized breast tumors
and regulates the sensitivity of breast cancer cells to
chemotherapy, while IRS2 is highly expressed in invasive breast
tumors. Cancer Lett. 338:239–248. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lai YY, Shen F, Cai WS, Chen JW, Feng JH,
Cao J, Xiao HQ, Zhu GH and Xu B: MiR-384 regulated IRS1 expression
and suppressed cell proliferation of human hepatocellular
carcinoma. Tumour Biol. 37:14165–14171. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Esposito DL, Aru F, Lattanzio R, Morgano
A, Abbondanza M, Malekzadeh R, Bishehsari F, Valanzano R, Russo A,
Piantelli M, et al: The insulin receptor substrate 1 (IRS1) in
intestinal epithelial differentiation and in colorectal cancer.
PLoS One. 7:e361902012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zheng H, Zhang F and Lin X, Huang C, Zhang
Y, Li Y, Lin J, Chen W and Lin X: MicroRNA-1225-5p inhibits
proliferation and metastasis of gastric carcinoma through
repressing insulin receptor substrate-1 and activation of β-catenin
signaling. Oncotarget. 7:4647–4663. 2016.PubMed/NCBI
|
|
28
|
Cao M, Li Y, Lu H, Meng Q, Wang L, Cai L
and Dong X: MiR-23a-mediated migration/invasion is rescued by its
target, IRS-1, in non-small cell lung cancer cells. J Cancer Res
Clin Oncol. 140:1661–1670. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Y, Zhang X, Zou C, Kung HF, Lin MC,
Dress A, Wardle F, Jiang BH and Lai L: miR-195 inhibits tumor
growth and angiogenesis through modulating IRS1 in breast cancer.
Biomed Pharmacother. 80:95–101. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang Y, Hu C, Cheng J, Chen B, Ke Q, Lv Z,
Wu J and Zhou Y: MicroRNA-145 suppresses hepatocellular carcinoma
by targeting IRS1 and its downstream Akt signaling. Biochem Biophys
Res Commun. 446:1255–1260. 2014. View Article : Google Scholar : PubMed/NCBI
|