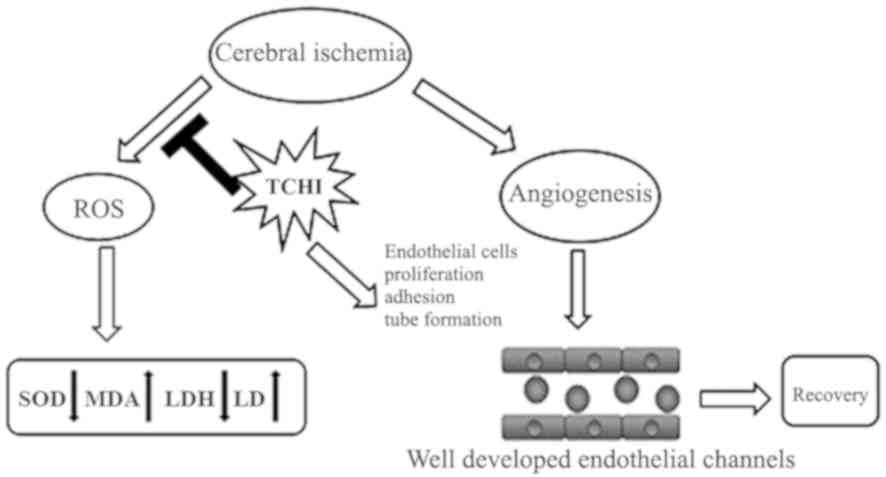
Introduction
Brain ischemia, also known as cerebral ischemia or
ischemic stroke, is a condition involving insufficient blood flow
to the brain to meet metabolic demand and is a leading cause of
mortality worldwide, causing serious long-term disability (1,2). The
treatment strategies of brain ischemia, particularly acute ischemic
stroke, currently include thrombolytic, antiplatelet aggregation
and neuroprotective therapy, among which thrombolytic therapy
remains the sole effective treatment. However, there is a lack of
effective therapies to improve functional recovery in the cerebral
post-ischemic phase, while the treatment for ischemic stroke is
restricted to a narrow therapeutic time window and holds the risk
of hemorrhage (3). Thus, the
mechanism of cerebral ischemia and the treatment of post-stroke
patients remain under investigation (2).
Oxidative stress, characterized by excessive
production of reactive oxygen species (ROS), is a recognized
mechanism of cerebral ischemic injury (4,5). As
a result, antioxidants are considered to be a conventional
therapeutic strategy in the treatment of cerebral ischemic injury.
Superoxide dismutase (SOD) is a first-in-line endogenous defense
against ROS and specific scavengers of the superoxide anion, which
eliminates oxygen-free radicals to prevent excessive superoxide
anion concentration damage and serves an important role in
maintaining the normal physiological activity of the body.
Furthermore, as the product of lipid peroxidation induced by
oxygen-free radicals, the malondialdehyde (MDA) content in tissues
effectively reflects the degree of cell damage and free radical
attacks in the body (6). A number
of studies have confirmed that SOD, lactate dehydrogenase (LDH) and
MDA levels in brain tissue were abnormal following cerebral
ischemia (7,8). In addition, recent studies
demonstrated that the promotion of angiogenesis and the formation
of new blood vessels from pre-existing vessels may be a novel
strategy to reduce the infarct volume and improve neurobehavioral
recovery following ischemic stroke (2,9).
Enhancement of endothelial cell functions, including adhesion,
migration, proliferation and differentiation, is critical for
inducing neovascularization to provide the required nutrients,
oxygen and blood-flow for ischemic tissues during the process of
angiogenesis.
Troxerutin, is a flavonol used as a vascular
protective and antioxidative agent, and cerebroprotein hydrolysate
includes brain protein hydrolysate, sialic acid. The troxerutin and
cerebroprotein hydrolysate injection (TCHI) has been approved by
the State Food and Drug Administration of China for the
amelioration of cerebral ischemic conditions based on
neuroprotective effects exerted in clinical practice (10). Troxerutin has the effect of
lowering blood viscosity, inhibiting platelet aggregation,
promoting the formation of collateral circulation, improving
microcirculation and eliminating free radicals, and can further
effectively inhibit the formation of thrombi and promote the repair
of damaged nerve tissue (11).
Bayer et al (12)
demonstrated that sialic acid increases blood vessel formation.
Other studies have suggested that sialic acid interacts with
extracellular matrix (ECM) components and growth factors,
regulating cell adhesion, migration and proliferation (13). Endothelial cells express several
integrin heterodimers, including αvβ3, α5β1 and αvβ5. Among these,
integrin β3 is a critical cell adhesion molecule in angiogenesis
(14). The expression of integrin
β3 on the endothelial cell surface activates and promotes
endothelial cell proliferation, thereby promoting angiogenesis
(15). A previous clinical study
suggested that TCHI improves neurological recovery in patients with
acute cerebral infarction (16).
It was further demonstrated that TCHI supports a shortening of coma
duration, and improves the quality life and long-term outcomes
(17). Therefore, it is speculated
that TCHI protects against cerebral ischemic injury via attenuation
of oxidative stress or promotion of angiogenesis. However, the
detailed mechanism underlying the effectiveness of TCHI in
cerebrovascular diseases requires further investigation.
In the present study, experimental in vitro
and in vivo models were employed to investigate the
underlying mechanisms of TCHI in the protection of cerebral tissues
from ischemic injury.
Materials and methods
Drug
TCHI (drug batch no., 160602; Shandong Buchang
Pharmaceutical Co., Ltd., Heze, China) is a compound preparation
made with sterilized water, troxerutin
(C33H42O19) and porcine brain
extracts. The components of TCHI include troxerutin (40 mg/ml),
active peptides, a variety of amino acids and a variety of
gangliosides (100 µg/ml), with a total nitrogen content of 0.5
mg/ml. Edaravone (drug batch no., 170704; Nanjing Xiansheng
Dongyuan Pharmaceutical Co., Ltd., Nanjing, China), a
neuroprotective drug that has the properties of a free radical
scavenger and could potentially reduce oxidative stress, was used
to help with recovery following a stroke and to treat amyotrophic
lateral sclerosis.
Animals
Male SD rats (n=66, 250 ± 20 g, 6–8 weeks old) were
purchased from the Experimental Animal Center of Xi'an Jiaotong
University (Xi'an, China) and housed in a room with a 12-h
light-dark cycle maintained at 22±2°C and with a relative humidity
of 60±2%). Food and water were supplied to all rats ad
libitum. All animal studies were conducted in accordance with
the Guide for the Care and Use of Laboratory Animals, and approved
by the Ethics Committee of Xi'an Jiaotong University, School of
Medicine (Xi'an, China).
Middle cerebral artery occlusion
(MCAO)
A modified model of MCAO was used to achieve
permanent focal ischemia, as described previously (18,19).
Briefly, animals were anesthetized by intraperitoneal injection of
7% chloral hydrate (350 mg/kg), and the right side of the common
carotid artery was exposed and isolated. The middle cerebral artery
(MCA) was occluded by inserting a monofilament nylon suture
(diameter 0.265 mm) into the internal carotid artery. When
resistance was encountered, the insertion was stopped, and the
ischemia time was counted. The length of the nylon line inserted
was 18–20 mm. The wound was sutured, and the rat was closely
monitored for post-operative recovery.
Groups and drug administration
Six groups of rats (11 rats/group) were included in
the present study, as follows: Sham-operated group, in which rats
underwent the same surgical procedure as the model group, with the
exception of insertion of the nylon suture; MCAO model group, in
which rats were subjected to ischemia; three TCHI groups, in which
animals received 0.5, 1.0 and 2.0 ml/kg TCHI respectively (this
drug is metabolized in mice and the concentration at each dose is
described, rather than the total concentration of the three doses
as this description is more similar to the human dosage regimen),
following MCAO; and positive control group, in which rats received
edaravone (5 mg/kg) treatment following MCAO. Edaravone is a
synthetic antioxidant agent that neutralizes free radicals, and can
be used to relieve reperfusion injury associated with cerebral
ischemia and oxidation (20). TCHI
and edaravone were administered three times after surgery, at 6 and
24 h; Sham-operated group and MCAO model group were administered of
Saline immediately after surgery, at 6 and 24 h.
Neurological function assessment
The animal behavior of each rat was carefully
evaluated at 6 and 24 h after surgery. The animals were scored for
neurological damage as follows: 0, normal spontaneous movement; 1,
failure to fully extend contralateral forepaw; 2, circling to
affected side; 3, partial paralysis on affected side; and 4, no
spontaneous motor activity.
Measurement of cerebral infarction
range
At 2 h following the final drug administration, the
brain was carefully removed and cut into six coronal sections with
a thickness of 2 mm. Next, the sections were quickly stained with
triphenyl tetrazolium chloride (TTC; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) solution at temperature of 37°C for 30 min,
followed by incubation with 10% buffered paraformaldehyde for
fixation. Following staining, the non-ischemic tissue was stained
red, while the infarcted area appeared white. The white tissue was
carefully stripped and weighed. The cerebral infarct range was
calculated as the percentage of the infarction tissue weight to the
weight of ischemic hemisphere.
Determination of lactic acid (LD),
LDH, SOD and MDA levels in brain tissue
The rat brain tissue of the surgical side was
weighed, homogenized with normal saline to prepare 10% brain
homogenate and stored in a −20°C refrigerator. The homogenates were
centrifuged at 300 × g for 10 min at 4°C, and the supernatant was
used to measure the LH, MDA, SOD and MDA activities were evaluated
in a 96-well plate using their respective activity assay kits (cat.
nos. A0201, A0031, A001-3 and A003-1; Nanjing Jiancheng
Bioengineering Institute, Nanjing, China) following the
manufacturer's instructions, while the protein content was measured
by biuret method, as described previously (21).
MTT assay
Human umbilical vein endothelial cells (HUVECs) were
provided by the Type Culture Collection of the Chinese Academy of
Sciences (Shanghai, China). RPMI 1640 medium was obtained from
Gibco; Thermo Fisher Scientific, Inc. (Waltham, MA, USA) and fetal
calf serum was purchased from Zhejiang Tianhang Biological
Technology Co., Ltd. (Hangzhou, China). HUVECs were seeded in
96-well plates (3×104 cells/ml) and cultured in a highly
humidified atmosphere of 5% CO2 at 37°C for 24 h.
Subsequently, the medium was replaced with 200 µl medicated medium
containing various concentrations of TCHI (0, 2, 10, 50 and 250
µg/ml). Following incubation for 24 h, the medicated medium was
replaced with 180 µl serum-free medium and 20 µl MTT, and incubated
at 37°C for 4 h. The supernatant was discarded, and the precipitate
was dissolved by adding 150 µl DMSO. The contents of the wells were
dissolved using a shaker for 15 min and the absorbance was measured
using a microplate reader at a wavelength of 490 nm.
Tube formation assay
A 48-well plate was coated with 100 µl Matrigel (BD
Biosciences, Franklin Lakes, NJ, USA) and incubated at 37°C for 30
min to permit solidification. HUVECs (2×104 cells/well
in 500 µl RPMI 1640 medium) and different concentration of TCHI (0,
2, 10, 50 and 250 µg/ml) were added to the plate. Subsequent to
incubation for 24 h, the tube formation was observed by microscopy,
and images of the cells were captured.
Adhesion assay
HUVECs were cultured in RPMI 1640 medium
supplemented with 10% fetal bovine serum, 100 µg/ml Streptomycin
and 100 IU/ml penicillin containing 0, 2, 10, 50 and 250 µg/ml TCHI
in a highly humidified atmosphere of 5% CO2 at 37°C for
24 h. Following incubation for 24 h, HUVECs (2×106
cells/well) were seeded on a Matrigel-coated 24-well plate and
incubated for 1 h. Subsequent to discarding the medium, the plate
was washed with PBS three times and observed under a microscope,
and images were captured to examine cell adhesion.
Cell migration assays
HUVECs (2.5×105/well) were seeded in a
6-well cell culture plate and then incubated at 37°C in a 5%
CO2 atmosphere for 24 h. When confluence reached 90%,
the HUVEC monolayer on the plate was scratched with a 200 µl
pipette tip and washed three times by PBS. Fresh medium containing
various concentrations of TCHI (0, 2, 10, 50 and 250 µg/ml) was
then added. Following incubation at 37°C for 0, 12 and 24 h, images
of the plates were obtained to examine the cell migration.
Chick chorioallantoic membrane (CAM)
assay
Fertilized chicken eggs (Xi'an Xinfengyuan livestock
breeding specialized cooperatives, Xi'an, China) were cleaned with
1% benzalkonium bromide and then incubated at 37°C in an incubator
with 60–70% relative humidity for 7 days. On day 8 of incubation, a
1-cm-diameter window was carefully created on the broader side of
the egg and then sterile gelatin sponges saturated with normal
saline (serving as the control), 10 µg/ml TCHI or recombinant
bovine basic fibroblast growth factor (bFGF; 4,200 IU/ml;
Zhuhaiyisheng Biological Pharmaceutical Co., Ltd., Guangdong,
China) were placed inside the egg, and permeable sticky tape was
immediately placed over the window. bFGF is a cytokine with a
fundamental role in angiogenesis and served as a positive control
drug in the CAM assay. After 3 days of incubation, CAMs were fixed
with 4% paraformaldehyde for 10 min at room temperature. Images of
blood vessels around the gelatin sponges were captured, and the
number of these vessels was counted under a microscope.
Rat aortic ring assay
For the rat aortic ring assay, male SD rats (n=2,
250±20 g, 6–8 weeks old) were purchased from the Experimental
Animal Center of Xi'an Jiaotong University (Xi'an, China) and
housed in a room with a 12-h light-dark cycle maintained at 22±2°C,
with a relative humidity of 60±2%). Food and water were supplied to
all rats ad libitum. Then, 48-well plates were covered with
100 µl Matrigel at 4°C and incubated at 37°C in 5% CO2
for 30 min. The rats were anesthetized with 20% urethane, and then
their aortas were isolated and cleaned of the residual blood in the
lumen and redundant adipose tissue. Aortas were cut into 1-mm long
rings, placed on the Matrigel-covered wells of the plates and
covered with another 100 µl Matrigel. Artery rings were cultured in
the RPMI 1640 complete medium, or with medium containing 2, 10, 50
or 250 µg/ml TCHI. After 6 and 9 days of incubation, the
microvessel growth was measured under an inverted microscope and
images of the artery rings were captured.
RT-PCR
The total RNA was isolated using RNA fast 2000 kit
(Fastagen, Shanghai, China) according to the manufacturer's
protocols. The RNA was subsequently reverse transcribed into cDNA
using Prime Script RT Master Mix Perfect Real-Time kit (DRR036A;
Takara Bio, Inc., Otsu, Japan) according to the manufacturer's
protocols. The primers sequences used (Sangon Biotech Co., Ltd.,
Shanghai, China) were: β3, forward 5′-GCCAGCACCATCTCTTTACC-3′ and
reverse 5′-GCACTCTCTCCCTTTGAGGA-3′, with a length of 112 bp;
β-actin, forward 5′-TGACGTGGACATCCGCAAAG-3′ and reverse
5′-CTGGAAGGTGGACAGCGAGG-3′, with a length of 205 bp. The cycling
protocol for PCR involved incubating the samples at 94°C for 2 min
followed by 35 cycles of denaturation at 94°C for 30 sec, annealing
at 55°C for 30 sec, and extension at 72°C for 30 sec, with a final
cycle of incubation at 72°C for 2 min. The amplification products
were analyzed by electrophoresis (Beijing Junyi, Beijing, China) in
agarose gels and detected under UV illumination (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) after staining with nucleic
acid dye (DuRed; FanBo Biochemicals, Beijing, China). Images were
analyzed using a quantitative analysis system (Quantity One
Analysis Software, version 4.6.2; Bio-Rad Laboratories, Inc.).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistical analysis was performed with SPSS version 17.0 software
(SPSS, Inc., Chicago, IL, USA). One-way analysis of variance was
used to compare differences between groups, and a value of
P<0.05 was considered to denote a statistically significant
difference.
Results
TCHI improves neurological outcomes
and attenuates infarct volume following cerebral ischemic injury in
rats
Initially, in order to examine the protective
effects of TCHI in an MCAO animal model, tail-vein injection of
TCHI (0.5, 1 and 2 ml/kg), edaravone (5 mg/kg) or vehicle was
performed, and neurological deficit scores were determined at 0, 6
and 24 h after the MCAO surgery. The results revealed that there
was no neurological deficit in the sham-operated group, while other
groups presented various degrees of neurological deficit symptoms
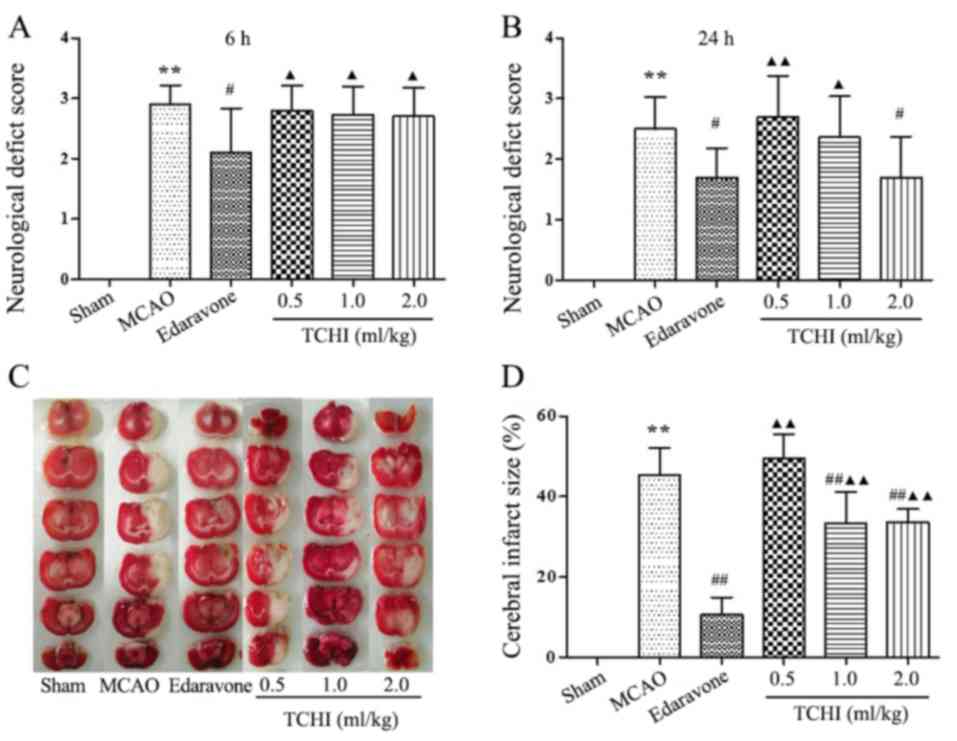
at 6 and 24 h following MCAO surgery (Fig. 1A and B). In addition, there was no
significant difference in neurological deficit scores between the
TCHI dose groups (0.5, 1 and 2 ml/kg) and the MCAO model group at 6
h following surgery (2.80±0.42, 2.80±0.42 and 2.70±0.48 vs.
2.90±0.32, respectively; Fig. 1A);
Compared with the MCAO model and TCHI groups, the edaravone group
significantly reduced neurological deficit scores at 6 h following
surgery (2.90±0.32, 2.80±0.42, 2.80±0.42 and 2.70±0.48 vs.
2.10±0.88, respectively; P<0.05; Fig. 1A). However, compared with the MCAO
model group, the neurological deficit score of the edaravone groups
and 2 ml/kg TCHI group was significantly reduced at 24 h following
surgery (2.50±0.53 vs. 1.70±0.48,1.70±0.67, respectively;
P<0.05; Fig. 1B). Compared with
MCAO model group, 0.5 and 1 ml/kg TCHI groups, edaravone group
significantly reduced neurological deficit scores at 24 h following
surgery (2.50±0.53,2.70±0.67, 2.36±0.67 vs. 1.70±0.48,
respectively; P<0.05; Fig. 1A).
No significant difference was observed in neurological deficit
scores between the 2 ml/kg TCHI group and the edaravone group at 24
h following surgery (1.70±0.67 vs. 1.70±0.48; Fig. 1A). Subsequently, the cerebral
infarct volume was determined using TTC staining. Compared with the
sham-operated group, the infarct volume was significantly increased
in the model group (P<0.01; Fig. 1C
and D), the cerebral infarct volume was 45.35±6.75% in rats of
the MCAO model group, 32.78±7.86 and 33.66±3.19% in rats treated
with 1 and 2 ml/kg TCHI and 10.59±4.40% in rats treated with 5
mg/kg edaravone, respectively. Thus, the results of the present
study revealed that, in MCAO rats treated with TCHI at doses of 1
and 2 ml/kg and 5 mg/kg edaravone, the percentage of the infarct
volume was significantly reduced compared with that in the
untreated model group (P<0.01; Fig.
1C and D). In addition, compared with the edaravone group, the
percentage of the infarct volume was significantly higher compared
with that in the TCHI groups(P<0.01; Fig. 1C and D).
TCHI protects against oxidative
damage
To investigate the potential antioxidative
mechanisms, SOD activity was determined. SOD is one of the most
important endogenous antioxidant enzymes in defending against
oxidative stress. The results revealed that SOD activity was
significantly decreased at 24 h following MCAO surgery, as compared
with the sham group (P<0.01). At concentrations of 1 and 2
ml/kg, TCHI and 5 ml/kg edaravone significantly increased SOD
activity compared with that in the MCAO model group (8.97±0.82,
9.03±0.95 and 9.40±0.89, vs. 6.74±0.61 U/mg, respectively;
P<0.01; Fig. 2A). No
significant difference was observed in SOD activity between the 1
and 2 ml/kg TCHI groups and the edaravone group. The MDA levels in
brain tissues were determined at 24 h following MCAO surgery. The
data demonstrated that the MCAO group exhibited significantly
increased MDA levels compared with the sham group (1.64±0.14 vs.
0.99±0.10 nM/mg; P<0.01). Treatment with TCHI and edaravone
significantly decreased the MDA levels compared with the MCAO model
group (1.33±0.26, 1.26±0.14, 1.03±0.08 and 1.05±0.12, respectively
vs. 1.64±0.14 nM/mg; P<0.01; Fig.
2B). In addition, the MDA level of the edaravone group was
significantly lower than that of the 0.5 and 1 mg/kg TCHI groups.
Although no significant difference in MDA level was observed
between the 2 ml/kg TCHI group and the edaravone group. These
results suggested that TCHI was an effective antioxidant protecting
against cerebral ischemic injury.
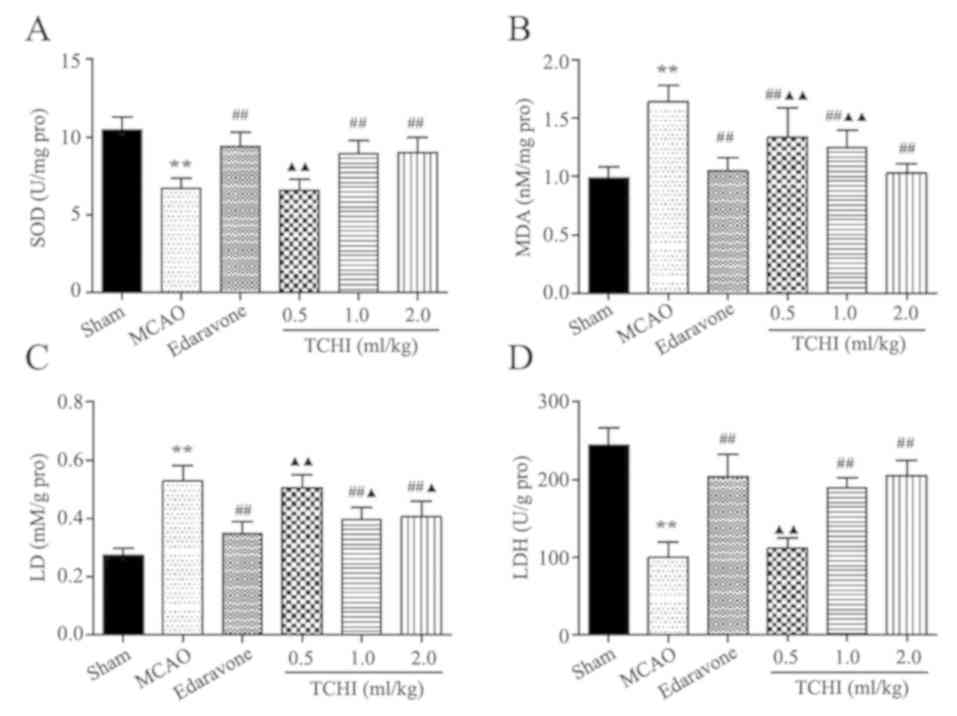 | Figure 2.Effect of TCHI on SOD, MDA, LD and
LDH levels following MCAO. Representative levels of (A) SOD, (B)
MDA, (C) LD and (D) LDH at 24 h following MCAO. Data are expressed
as the mean ± standard error of the mean (n=10-11/group).
**P<0.01 vs. sham group; ##P<0.01 vs. MCAO group;
▲P<0.05 and ▲▲P<0.01 vs. edaravone
group. TCHI, troxerutin and cerebroprotein hydrolysate injection;
SOD, superoxide dismutase; MDA, malondialdehyde; LD, lactic acid;
LDH, lactate dehydrogenase; MCAO, middle cerebral artery
occlusion. |
TCHI decreases LDH and LD levels in
the brain tissue of rats with MCAO
The levels of LDH and LD were also detected in rat
brain tissues. The results revealed that LD levels significantly
decreased in the TCHI treatment groups (1 and 2 ml/kg) and the
edaravone group compared with those in the model group (0.39±0.04,
0.41±0.05 and 0.34±0.04, vs. 0.53±0.05 mM/g, respectively;
P<0.01; Fig. 2C). The LD level
of the TCHI groups was significantly higher compared with the
edaravone group (0.51±0.05,0.39±0.04 and 0.41±0.05 vs. 0.34±0.04
mM/g, respectively; Fig. 2C). In
addition, at the doses of 1 and 2 ml/kg TCHI and 5mg/kg edaravone,
LDH levels were significantly increased compared with those in the
model group (189.59±12.88, 205.71±19.37 and 204.34±28.09, vs.
100.69±19.63 U/g, respectively; P<0.01; Fig. 2D). The LDH level of the 0.5 mg/kg
TCHI group was significantly lower compared with the edaravone
group (102.20±12.50 vs. 204.34±28.09, P<0.01; Fig. 2D) although no significant
difference was observed in LDH level between the 1 and 2 ml/kg TCHI
groups and the edaravone group. The above results suggested that
TCHI had a significant effect on the correction of acidosis in the
brain.
TCHI increases HUVEC proliferation and
tube formation
The effects of TCHI on endothelial cell
proliferation were assessed by an MTT assay. It has previously been
reported that troxerutin enhances thymocyte viability and reduces
apoptosis at a concentrations of 0.625–10 µg/ml, and the peak of
viability was observed when a dose of 10 µg/ml was used (22). Concentrations between 2 and 250
µg/ml TCHI were used for the treatment of HUVECs in the current
study. Treatment with TCHI (2, 10 and 50 µg/ml) significantly
increased HUVEC proliferation compared with the control group
(0.76±0.13, 0.80±0.08 and 0.79±0.14, vs. 0.63±0.09, respectively;
P<0.05, P<0.01 and P<0.05, respectively), while 250 µg/ml
TCHI exerted no marked effects on the cell proliferation (Fig. 3A). In addition, the effects of TCHI
on HUVEC tube formation were investigated with a Matrigel-based
in vitro assay. The results revealed that TCHI (10, 50 and
250 µg/ml) significantly stimulated tubule formation compared with
that observed in the control (19.5±2.65, 17.5±2.08 and 11.5±2.65,
vs. 4.50±1.29, respectively; P<0.01; Fig. 3B and C). These data indicated a
promoting effect of TCHI on HUVECs during angiogenesis.
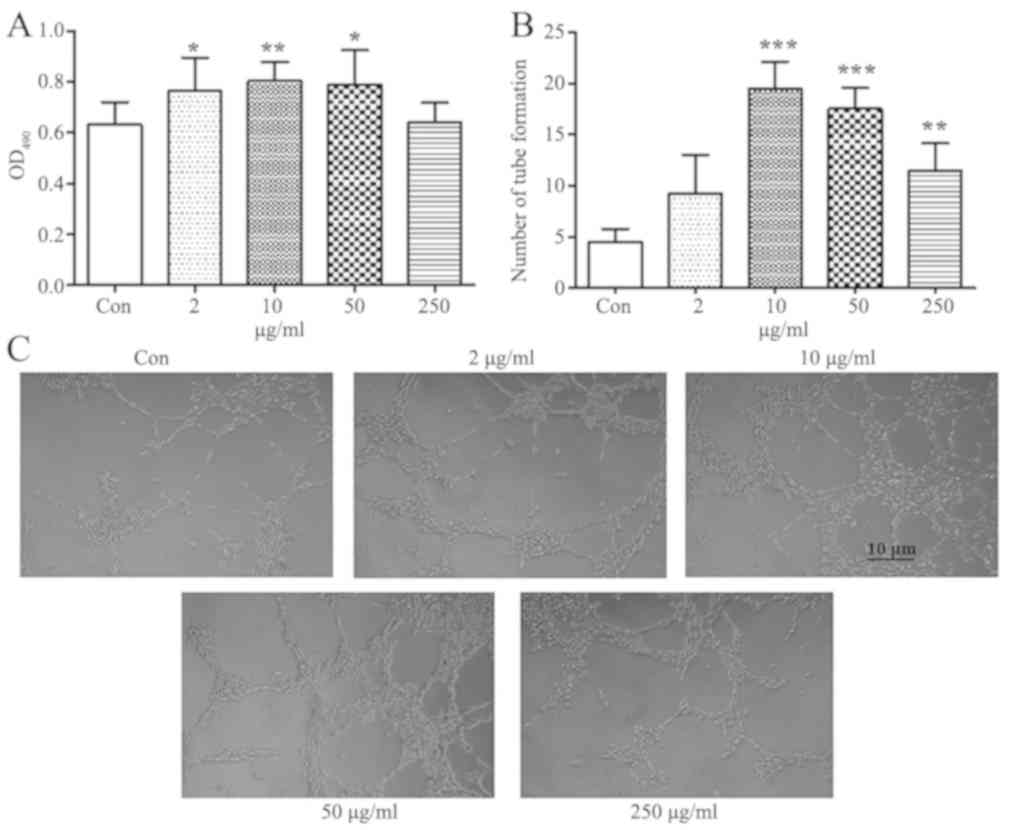 | Figure 3.Effects of TCHI on human umbilical
vein endothelial cell proliferation and tube formation. (A) Cell
proliferation following exposure to TCHI at 2, 10, 50 or 250 µg/ml
for 24 h was assessed by an MTT assay. (B) Quantification and (C)
cell images (magnification, ×100) of capillary-type tube formation
in cells cultured on a layer of Matrigel and incubated with medium
containing 2, 10, 50 or 250 µg/ml TCHI at 37°C for 24 h. Data are
expressed as the mean ± standard error of the mean. *P<0.05,
**P<0.01 and ***P<0.001, vs. control group. TCHI, troxerutin
and cerebroprotein hydrolysate injection; MTT,
methylthiazolyldiphenyl-tetrazolium bromide; OD, optical
density. |
TCHI increases HUVEC adhesion and
migration
The adhesion and migration abilities of endothelial
cells are essential to vascular development and angiogenesis. To
further investigate the proangiogenic activities of TCHI, an
endothelial cell adhesion assay was performed. As presented in
Fig. 4A and B, with the exception
of the concentration of 250 µg/ml TCHI, treatment with 2, 10 and 50
µg/ml TCHI significantly enhanced HUVEC adhesion compared with that
in the control (174.78±2.47, 184.50±1.52 and 177.81±4.85, vs.
167.07±3.73, respectively; P<0.05 or P<0.01). Furthermore,
the mean migration distances during wound closure and cell
migration were observed at 0, 12 and 24 h using scratch assays. The
results suggested that low levels of HUVEC migration were observed
in the vehicle-treated control, while TCHI (2, 10, 50 and 250
µg/ml) strongly enhanced HUVEC migration compared with the control
(51.00±6.08, 59.63±1.10, 48.87±1.90 and 44.10±0.53%, vs.
21.80±0.10%; P<0.01; Fig. 4C and
D).
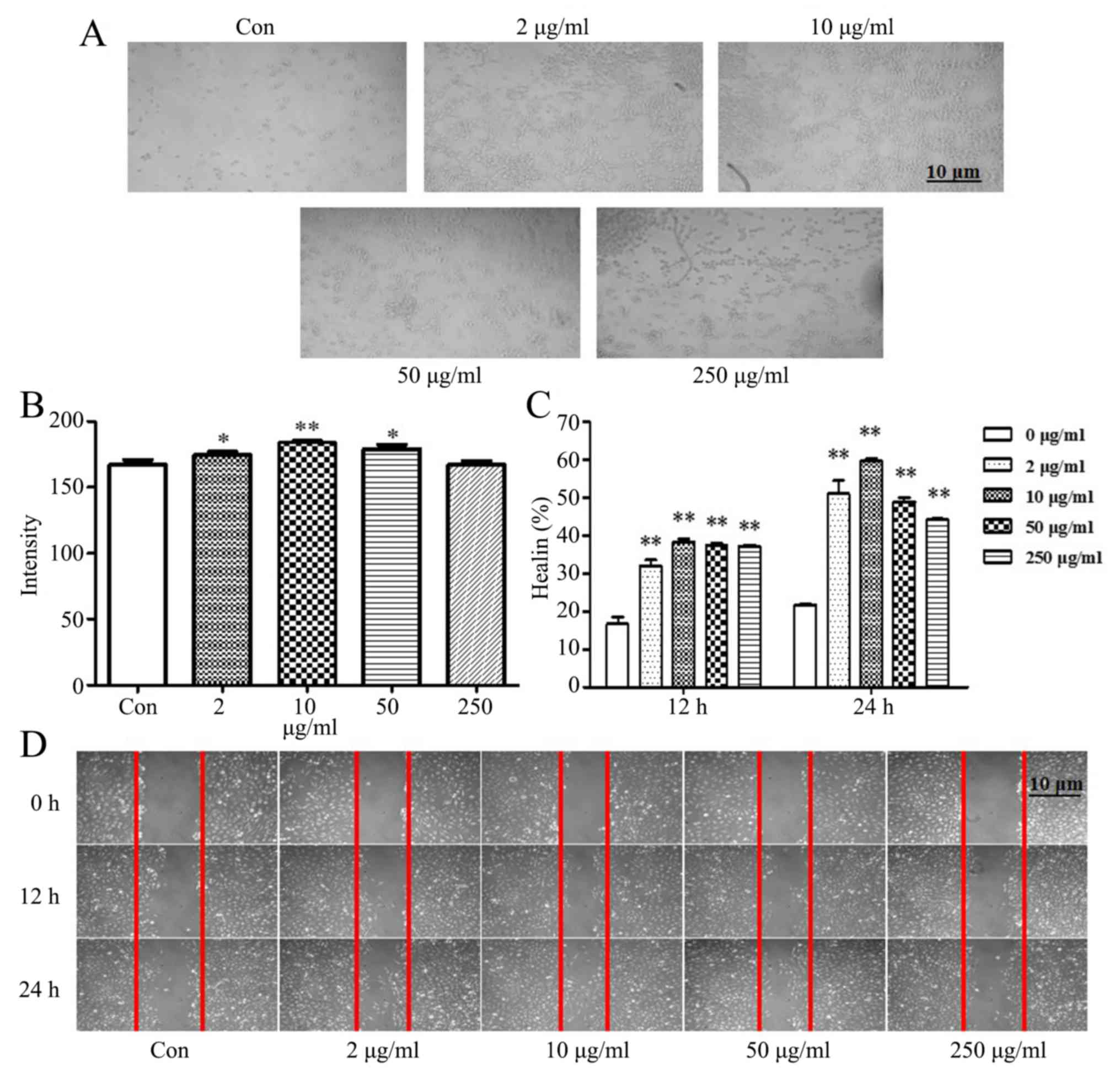 | Figure 4.Effects of TCHI on HUVEC adhesion and
migration. (A) HUVECs were exposed to TCHI at a concentration of 2,
10, 50 or 250 µg/ml for 24 h, seeded on a Matrigel-coated 24-well
plate, incubated for 1 h and observed under a microscope. (B)
Adhesion and (C) migration of HUVECs were significantly enhanced by
TCHI treatment. (D) Cell migration was examined by a wound healing
assay, during which the confluent HUVEC monolayer was wounded with
a 200-µl pipette tip and treated with TCHI (2, 10, 50 or 250
µg/ml). At 0, 12 and 24 h, wound healing was visualized with a
digital camera. *P<0.05 and **P<0.01 vs. Control. TCHI,
troxerutin and cerebroprotein hydrolysate injection; HUVEC, human
umbilical vein endothelial cells. |
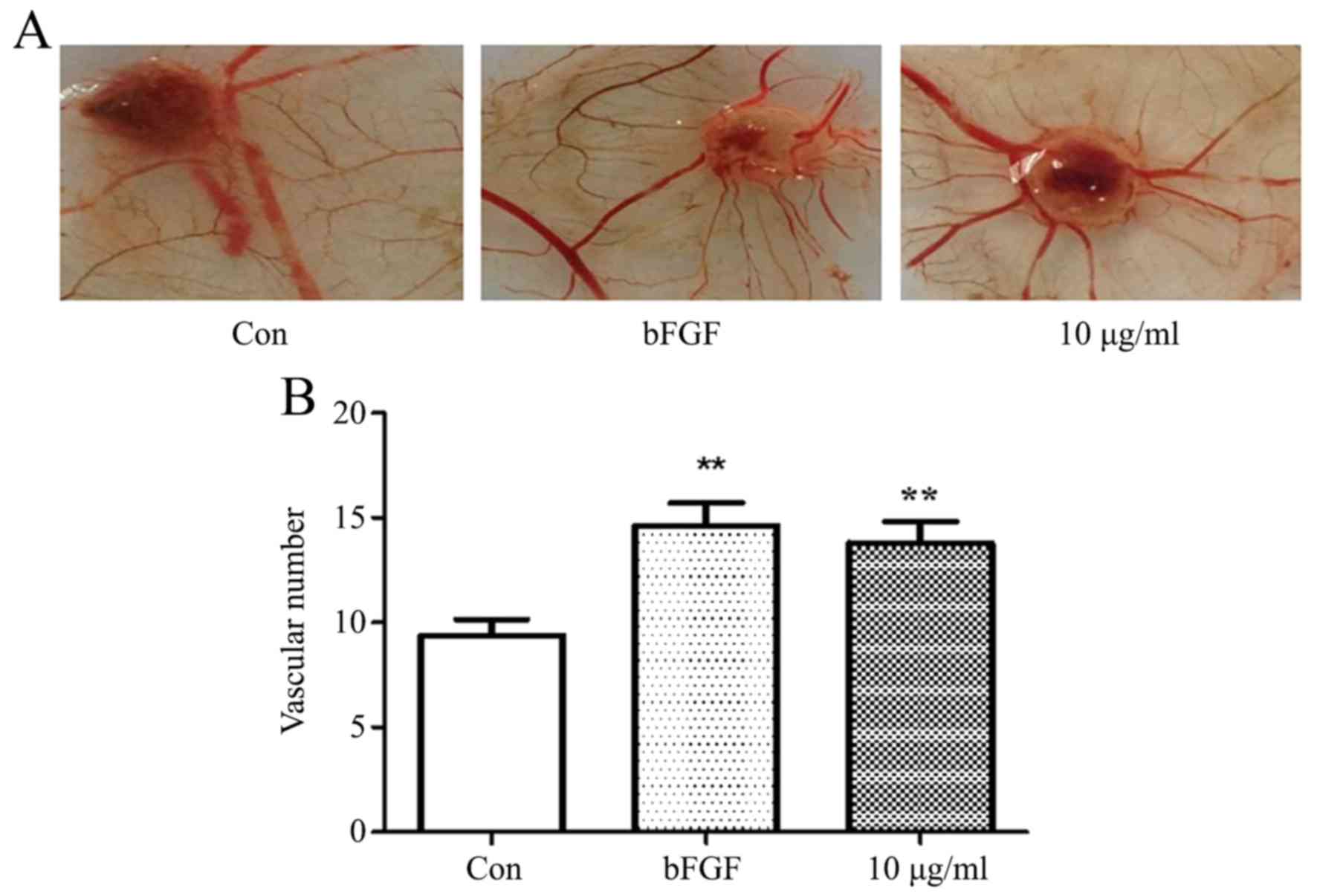
TCHI promotes angiogenesis in CAM
assay
A CAM model was used to confirm the role of TCHI in
angiogenesis. As presented in Fig. 5A
and B, the blood vessel density significantly increased by
55.32 and 46.81% following exposure to 5 ng/ml bFGF or 10 ng/ml
TCHI for 3 days, respectively, as compared with that in the
untreated control group (14.60±3.50 and 13.80±3.19, vs. 9.40±2.37;
P<0.01; Fig. 5A and B)
suggesting a positive impact of TCHI on CAM in angiogenesis.
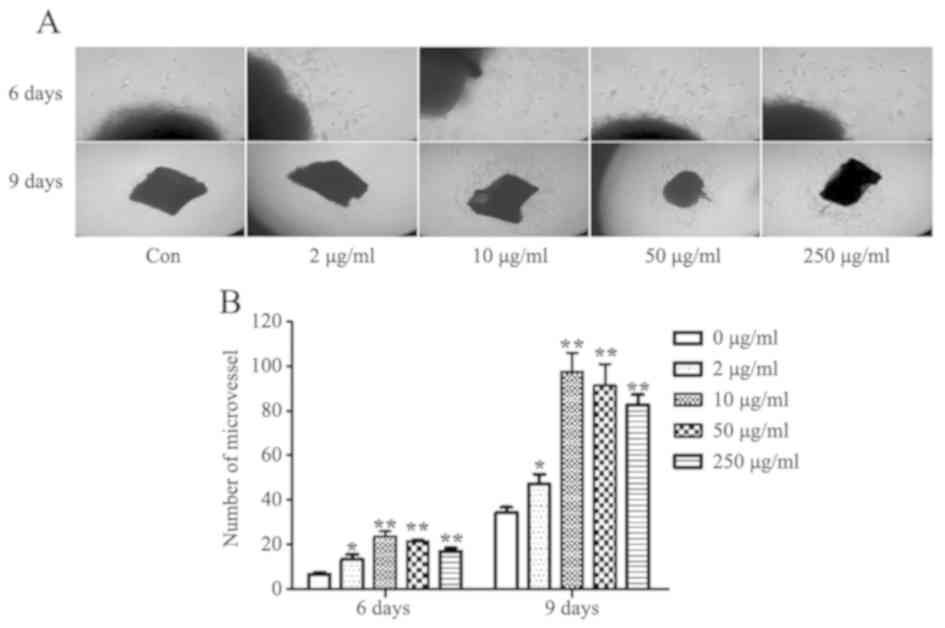
TCHI promotes microvessel outgrowth
from the rat aortic ring
A rat aortic ring model was employed to examine the
TCHI-induced angiogenesis in vitro. The results demonstrated
that TCHI (2, 10, 50 and 250 µg/ml) significantly stimulated
microvessel sprouting from the adventitia of cultured aortic rings
by 103.84, 261.54, 230.77 and 161.54% compared with that of the
control after 6 days of treatment (13.25±4.50, 23.50±4.73,
21.50±1.73 and 17.00±2.94, vs. 6.50±1.73, respectively; P<0.05
or P<0.01; Fig. 6A and B).
After 9 days of treatment, the number of microvessel increased by
37.23, 184.67, 166.42 and 140.88% in rats treated with 2, 10, 50
and 250 µg/ml TCHI, respectively, relative to that observed in the
control group (82.50±9.57, 91.25±19.31, 97.50±17.08 and 47.00±9.06,
vs. 34.25±4.92, respectively; P<0.05 or P<0.01; Fig. 6).
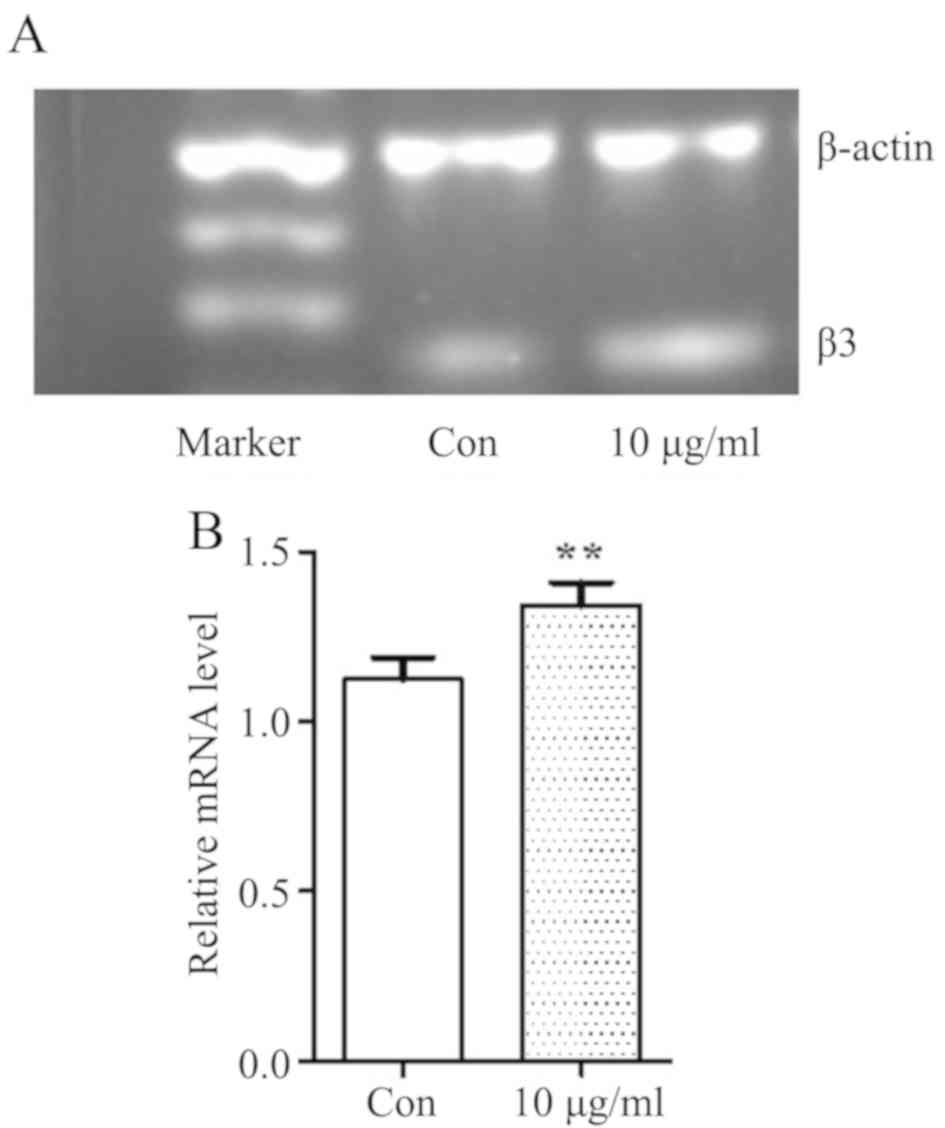
TCHI enhances integrin β3 expression
on HUVECs in vitro
Subsequently, integrin β3 mRNA expression was
detected in order to further investigate the effects of TCHI on
endothelial cells. As discussed earlier, the results demonstrated
that 10 µg/ml TCHI presented a strongest pharmacological action on
endothelial cell functions, including adhesion, migration and
capillary formation. Transcriptional expression of integrin β3 mRNA
on HUVECs following treatment with 10 µg/ml TCHI for 48 h was
investigated by RT-qPCR. It was observed that integrin β3 mRNA
levels were significantly increased in the 10 µg/ml TCHI group as
compared with the control group (1.34±0.08 vs. 1.16±0.09,
respectively; P<0.01; Fig. 7A and
B). This result suggested that TCHI can promote the expression
of integrin β3 in endothelial cells and enhance the adhesion
function of endothelial cells, thus promoting angiogenesis.
Discussion
Troxerutin and cerebroprotein hydrolysate have been
observed to have protective effects on acute cerebral infarction in
clinical practice (16). However,
the mechanisms of action remain unclear. The current study
demonstrated that TCHI effectively alleviated neurological symptoms
and cerebral ischemic injury in a rat MCAO model. In addition, the
results demonstrated that the beneficial effects of TCHI treatment
on cerebral ischemia were accompanied by increased LDH and SOD
activities, and decreased LD and MDA levels. In addition, TCHI was
observed to promote angiogenesis in vivo and enhance
endothelial cell function in vitro.
Following cerebral ischemia and tissue hypoxia, the
anaerobic metabolism leads to the production of lactic acid and
protons (23). Accumulation of
lactic acid results in brain edema, blood-brain barrier dysfunction
and free radical production, promoting tissue necrosis by
inhibiting uptake of excitatory amino acids (24). LDH is a key enzyme involved in
energy metabolism, which converts lactic acid into pyruvate by
dehydrogenation (25). The results
in the present study demonstrated that severe brain damage resulted
in a higher neurological deficit score and lower LDH levels. TCHI
treatment increased the LDH levels in brain homogenates and
conversely decreased LD levels, indicating that TCHI reduces the
degree of ischemia necrosis and increases the normal brain cell
numbers.
Free radicals and ROS are increasingly produced
following cerebral ischemia, and oxidative stress is considered as
the basic mechanism of brain injury following cerebral ischemia
(26,27). ROS levels are modulated by
endogenous antioxidant enzymes, including SOD and glutathione
peroxidase (GPx). The antioxidant activity of SOD indirectly
reflects the ability of scavenging ROS. MDA is an end product of
lipid peroxidation and levels reflect the degree of free
radical-induced damage (28).
Studies have revealed that troxerutin significantly decreases MDA
levels and increases GPx activity (29). In the present study, TCHI
significantly increased SOD activity and decreased MDA levels
following ischemic cerebral injury, suggesting that TCHI attenuates
cerebral ischemic injury through the amelioration of oxidative
stress. However, the detailed mechanism requires further
investigation.
It has previously been confirmed that microvessel
density is increased following cerebral ischemia. Post-ischemic
angiogenesis improves cerebral blood perfusion, neurological
recovery and survival of stroke patients (30). Thus, enhancement of brain
revascularization is an important option for the treatment of
cerebral ischemia. Angiogenesis comprises a series of events,
including endothelial cell proliferation, migration, adhesion and
tube formation (31). The effects
of TCHI on endothelial cell functions were determined in the
present study, and the results demonstrated that TCHI significantly
promoted vascular maturation processes in cultured HUVECs,
including cell proliferation, adhesion, migration and tube
formation. TCHI was further revealed to significantly stimulate
microvessel formation in the rat aortic ring in vitro.
Cell-matrix interactions are crucial for cell migration, and
particular ECM proteins have been revealed to regulate endothelial
cell migration (32). The scratch
assay results obtained in the current study indicated that TCHI
treatment promoted endothelial cell migration in a dose-dependent
manner. These results provided strong evidence that TCHI stimulated
angiogenesis at endothelial cell proliferation, migration, adhesion
and tube formation.
The present results further demonstrated that TCHI
stimulated the transcription of integrin β3 mRNA on endothelial
cells. An earlier study confirmed that synergistic interactions
between endothelial cell-specific integrin β3 and vascular
endothelial growth factor serve a key role in angiogenesis
(33). Therefore, the detailed
mechanism of TCHI in promoting angiogenesis was further
investigated. The tube formation and aortic ring assay results
obtained in the current study indicated that TCHI promoted
angiogenesis through tube formation. These results were verified by
a CAM model, in which TCHI increased blood vessel density and
vascular branches. Taken together, these findings implied that TCHI
promoted angiogenesis via enhancing endothelial cell migration and
inducing the formation of vascular networks.
However, a limitation of the present study was that
the association between cerebral ischemia and angiogenesis was not
examined in vivo. This was due to the complexity of cerebral
ischemia and angiogenesis animal models, requiring a much longer
time to demonstrate notable changes in comparison with acute animal
models (34). Furthermore, we have
tried to build animal models, but it is difficult to obtain stable
results from the model group; thus, these in vivo
experiments were delayed for technical reasons and not examined in
the present study. Setting up a reliable animal model is currently
under investigation.
In conclusion, the findings of the present study
suggested that TCHI increased LDH levels and decreased LD levels in
rats with cerebral ischemic injury. TCHI also significantly
increased SOD activity and decreased MDA levels following ischemia
in rat cerebral tissues. In addition, TCHI promoted angiogenesis
through increasing the proliferation and enhancing the functions of
endothelial cells, including adhesion, migration and capillary
formation. Therefore, TCHI reduced experimental ischemic damage
through the amelioration of oxidative stress and angiogenesis
(Fig. 8). These data are a
preliminarily elucidation of the role of TCHI in improving cerebral
ischemic reperfusion injury, and provided a theoretical basis for
the rational use of TCHI in clinical practice.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of China (grants nos. 81071765 and 81372379) the
Fundamental Research Funds of the First Affiliated Hospital of
Xi'an Jiao Tong University (grant no. 2017MS-04) and the Clinical
Research Award of the First Affiliated Hospital of Xi'an Jiao Tong
University (grant no. XJTU1AF-CRF-2016-002).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contribution
WF, SW and WM designed the experiments. WM, XLui,
FT, PZ, KC and XLi performed the experiments. QZ, YZ and XZ
analyzed the data. WF and WM wrote the manuscript. WF and XLi
revised the manuscript. All authors reviewed the final
manuscript.
Ethics approval and consent to
participate
All animal studies were conducted in accordance with
the Guide for the Care and Use of Laboratory Animals and were
approved by the Ethics Committee of Xi'an Jiaotong University,
School of Medicine (Xi'an, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Writing Group Members, Lloyd-Jones D,
Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB,
Ford E, Furie K, et al: Heart disease and stroke statistics-2010
update: A report from the American Heart Association. Circulation.
121:e46–e215. 2011.
|
|
2
|
Soleimannejad K, Rahmani A, Hatefi M,
Khataminia M, Hafezi Ahmadi MR and Asadollahi K: Effects of nigella
sativa extract on markers of cerebral angiogenesis after global
ischemia of brain in rats. J Stroke Cerebrovasc Dis. 26:1514–1520.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gursoy-Ozdemir Y, Yemisci M and Dalkara T:
Microvascular protection is essential for successful
neuroprotection in stroke. J Neurochem. 123 (Suppl 2):S2–S11. 2012.
View Article : Google Scholar
|
|
4
|
Li J, Dong Y, Chen H, Han H, Yu Y, Wang G,
Zeng Y and Xie K: Protective effects of hydrogen-rich saline in a
rat model of permanent focal cerebral ischemia via reducing
oxidative stress and inflammatory cytokines. Brain Res.
1486:103–111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Margaill I, Plotkine M and Lerouet D:
Antioxidant strategies in the treatment of stroke. Free Radic Biol
Med. 39:429–443. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gustaw-Rothenberg K, Kowalczuk K and
Stryjecka-Zimmer M: Lipids' peroxidation markers in Alzheimer's
disease and vascular dementia. Geriatr Gerontol Int. 10:161–166.
2010.PubMed/NCBI
|
|
7
|
Vani JR, Mohammadi MT, Foroshani MS and
Jafari M: Polyhydroxylated fullerene nanoparticles attenuate brain
infarction and oxidative stress in rat model of ischemic stroke.
EXCLI J. 15:378–390. 2016.PubMed/NCBI
|
|
8
|
Apak I, Iltumur K, Tamam Y and Kaya N:
Serum cardiac troponin T levels as an indicator of myocardial
injury in ischemic and hemorrhagic stroke patients. Tohoku J Exp
Med. 205:93–101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nour M, Scalzo F and Liebeskind DS:
Ischemia-reperfusion injury in stroke. Interv Neurol. 1:185–199.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhào H, Liu Y, Zeng J, Li D, Zhang W and
Huang Y: Troxerutin and cerebroprotein hydrolysate injection
protects neurovascular units from oxygen-glucose deprivation and
reoxygenation-induced injury in vitro. Evid Based Complement
Alternat Med. 2018:98596722018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Panat NA, Maurya DK, Ghaskadbi SS and
Sandur SK: Troxerutin, a plant flavonoid, protects cells against
oxidative stress-induced cell death through radical scavenging
mechanism. Food Chem. 194:32–45. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bayer NB, Schubert U, Sentürk Z, Rudloff
S, Frank S, Hausmann H, Geyer H, Geyer R, Preissner KT and Galuska
SP: Artificial and natural sialic acid precursors influence the
angiogenic capacity of human umbilical vein endothelial cells.
Molecules. 18:2571–2586. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chiodelli P, Urbinati C, Mitola S,
Tanghetti E and Rusnati M: Sialic acid associated with αvβ3
integrin mediates HIV-1 Tat protein interaction and endothelial
cell proangiogenic activation. J Biol Chem. 287:20456–20466. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Scatena M and Giachelli C: The
alpha(v)beta3 integrin, NF-kappaB, osteoprotegerin endothelial cell
survival pathway. Potential role in angiogenesis. Trends Cardiovasc
Med. 12:83–88. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mahabeleshwar GH, Feng W, Phillips DR and
Byzova TV: Integrin signaling is critical for pathological
angiogenesis. J Exp Med. 203:2495–2507. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liang KS, Yin CB, Peng LJ, Zhang JL, Guo
X, Liang SY, Zhou XY, Yuan DC, Li GL, Hu FY, et al: Effect of
troxerutin and cerebroprotein hydrolysate injection for the
treatment of acute cerebral infarction: A multi-center randomized,
single-blind and placebo-controlled study. Int J Clin Exp Med.
10:10959–10964. 2017.
|
|
17
|
Wang Z: A controlled, multi-center,
randomized study of efficacy and safety of troxerutin and
cerebroprotein hydrolysate injection for traumatic brain injury.
Chin J Neurosurg. 33:669–672. 2017.(In Chinese).
|
|
18
|
Yang C, Zhang X, Fan H and Liu Y: Curcumin
upregulates transcription factor Nrf2, HO-1 expression and protects
rat brains against focal ischemia. Brain Res. 1282:133–141. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Geng X, Sy CA, Kwiecien TD, Ji X, Peng C,
Rastogi R, Cai L, Du H, Brogan D, Singh S, et al: Reduced cerebral
monocarboxylate transporters and lactate levels by ethanol and
normobaric oxygen therapy in severe transient and permanent
ischemic stroke. Brain Res. 1603:65–75. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hua K, Sheng X, Li TT, Wang LN, Zhang YH,
Huang ZJ and Ji H: The edaravone and 3-n-butylphthalide
ring-opening derivative 10b effectively attenuates cerebral
ischemia injury in rats. Acta Pharmacol Sin. 36:917–927. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Deng WS, Xu Q, Liu YE, Jiang CH, Zhou H
and Gu L: Effects of melatonin on liver function and lipid
peroxidation in a rat model of hepatic ischemia/reperfusion injury.
Exp Ther Med. 11:1955–1960. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xua P, Zhang WB, Cai XH, Qiu PY, Hao MH
and Lu DD: Activating AKT to inhibit JNK by troxerutin antagonizes
radiation-induced PTEN activation. Eur J Pharmacol. 795:66–74.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li P, Shen M, Gao F, Wu J, Zhang J, Teng F
and Zhang C: An antagomir to MicroRNA-106b-5p ameliorates cerebral
ischemia and reperfusion injury in rats via inhibiting apoptosis
and oxidative stress. Mol Neurobiol. 54:2901–2921. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Leng T, Shi Y, Xiong ZG and Sun D:
Proton-sensitive cation channels and ion exchangers in ischemic
brain injury: New therapeutic targets for stroke? Prog Neurobiol.
115:189–209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang J, Deng Z, Liao J, Song C, Liang C,
Xue H, Wang L, Zhang K and Yan G: Leptin attenuates cerebral
ischemia injury through the promotion of energy metabolism via the
PI3K/Akt pathway. J Cereb Blood Flow Metab. 33:567–574. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Alexandrova ML and Bochev PG: Oxidative
stress during the chronic phase after stroke. Free Radic Biol Med.
39:297–316. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Firuzi O, Miri R, Tavakkoli M and Saso L:
Antioxidant therapy: Current status and future prospects. Curr Med
Chem. 18:3871–3888. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang R, Liu YY, Liu XY, Jia SW, Zhao J,
Cui D and Wang L: Resveratrol protects neurons and the myocardium
by reducing oxidative stress and ameliorating mitochondria damage
in a cerebral ischemia rat model. Cell Physiol Biochem. 34:854–864.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Badalzadeh R, Layeghzadeh N, Alihemmati A
and Mohammadi M: Beneficial effect of troxerutin on
diabetes-induced vascular damages in rat aorta: Histopathological
alterations and antioxidation mechanism. Int J Endocrinol Metab.
13:e259692015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang ZG and Chopp M: Neurorestorative
therapies for stroke: Underlying mechanisms and translation to the
clinic. Lancet Neurol. 8:491–500. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kitlinska J, Abe K, Kuo L, Pons J, Yu M,
Li L, Tilan J, Everhart L, Lee EW, Zukowska Z and Toretsky JA:
Differential effects of neuropeptide Y on the growth and
vascularization of neural crest-derived tumors. Cancer Res.
65:1719–1728. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hielscher A, Ellis K, Qiu C, Porterfield J
and Gerecht S: Fibronectin deposition participates in extracellular
matrix assembly and vascular morphogenesis. PLoS One.
11:e01476002016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Somanath PR, Malinin NL and Byzova TV:
Cooperation between integrin alphavbeta3 and VEGFR2 in
angiogenesis. Angiogenesis. 12:177–185. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Salehi A, Zhang JH and Obenaus A: Response
of the cerebral vasculature following traumatic brain injury. J
Cereb Blood Flow Metab. 37:2320–2339. 2017. View Article : Google Scholar : PubMed/NCBI
|