Introduction
Atherosclerosis is a chronic inflammatory disease
with high morbidity and mortality worldwide (1,2).
Rupture-prone vulnerable plaques are a key characteristic of
atherosclerosis, caused by disordered lipid metabolism and the
inflammatory response (3,4). However, at present, no effective
therapeutic strategies are available to treat atherosclerosis.
Increasing evidence suggests that aortic adventitia serve a
critical role in the immune response in atherosclerosis (5–9).
Artery tertiary lymphoid organs (ATLOs), which were initially
identified in aged apolipoprotein E (ApoE)−/− mouse
adventitia, may be involved in the regulation of the immune
response to atherosclerosis (6,7,10–13).
Advanced ATLOs consist of distinct immune cell compartments
organized into T-cell areas, B-cell follicles and plasma cell
niches (7,10). It has been identified that ATLOs
recruit naive T cells and generate cluster of differentiation
(CD)4+ and CD8+ T cells, consequently
inducing Treg and memory B cell production. Similar to lymph nodes,
lymph vessels and high endothelial venules in ATLOs specifically
facilitate lymphocyte recruitment and migration. This evidence
suggests that ATLOs may mediate the immune response to
atherosclerosis (11–13). Therefore, it is necessary to focus
on the molecular mechanisms of ATLO development for further
understanding of ATLOs and their potential impact on
atherosclerosis immunity.
Recent advances in microarray methods and data
mining tools offer the possibility to simultaneously analyze
numerous genes associated with the complex mechanisms of ATLOs. The
microarray dataset GSE40156 was used to compare mRNA expression in
the aorta, plaque, adventitia and ATLO, in addition to the blood
and secondary lymphoid organs (sLOs; spleen and renal lymph nodes).
Microarray-based transcriptional profiling was used to evaluate
ATLOs and the associated Kyoto Encyclopedia of Genes and Genomes
(KEGG) pathways. The identified genes provided insights into the
biological processes underlying ATLOs. However, reliably deploying
differentially expressed genes (DEGs) into functionally relevant
mechanisms is challenging. In the present study, the array data of
GSE40156 were downloaded and DEGs associated with KEGG enrichment
were verified by bioinformatics methods. Furthermore, a
protein-protein interaction (PPI) network was constructed and
dissected. Currently, only a limited number of studies concerning
ATLOs have been published, to the best of our knowledge. The
present study highlighted the DEGs and KEGG pathways involved in
the progression of ATLOs, enhancing the understanding of immune
responses in atherosclerosis and identifying the candidate genes
that may be used for the development of novel diagnostic and
therapeutic strategies.
Materials and methods
Affymetrix microarray data
The GSE40156 dataset (12) was downloaded from the Gene
Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/) to determine and
investigate the candidate DEGs that potentially contribute to the
progression of ATLOs. Samples of the ApoE−/− and
wild-type (WT) aorta, ATLO, plaque, adventitia, blood, spleen and
renal lymph node were included in the present study. Additionally,
the raw data and annotation files were collected with reference to
the GPL1261 (Affymetrix Mouse Genome 430 2.0 Array; Affymetrix;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) and GPL8321
platforms for downstream analysis (Affymetrix Mouse Genome 430A 2.0
Array; Affymetrix; Thermo Fisher Scientific, Inc.).
Data processing
The original data were pre-processed with background
correction, normalization and expression calculation using the
‘affy’ package (14) in R (version
3.4.2, http://www.R-project.org/). During probe
selection for further microarray analysis, non-gene-matched probes
were removed, and the probe with the lowest adjusted P-value was
selected where one gene matched multiple probes.
Identification of DEGs
The ‘limma’ package (15) in R was used to identify DEGs
between the different groups. Following probe annotation, gene
expression profiling data were extracted and log2 transformed. The
‘eBayes function’ based on classical Bayesian algorithm (15) was applied to determine DEGs, with
P<0.05 and |log2 fold change (FC)|≥1.5 as cut-off criteria.
Functional and pathway enrichment
analysis
Gene Ontology (GO) and KEGG pathway analyses were
conducted for the DEGs using DAVID (https://david.ncifcrf.gov/). GO classifications
consist of molecular function (MF), biological process (BP) and
cellular component (CC) terms (16,17).
KEGG is a database used to allocate gene sets to their relevant
pathways (18). DEGs were further
classified by GO term and KEGG pathway enrichment, and visualized
using the ‘GOplot’ package in R (16). Adjusted P<0.05 was set as the
cut-off criterion.
PPI network construction and module
selection
Information on predicted or experimental protein
interaction was acquired by means of PPI network construction and
analysis, using the STRING database (19,20).
This database integrates experiments, databases and text mining
data for prediction. The value of interactions between protein
pairs was given as a combined score. DEGs were mapped in the
database with a cut-off value of a combined score of >0.9.
Cytoscape software version 3.6.0 was used to construct the PPI
networks (21). A gene network
module of the interleukin 7 receptor (Il7r) was constructed to
determine its exact biological importance.
Animals
Male ApoE−/− mice (n=10; 3 6 weeks,
21.4±0.5 g; 3 32 weeks, 30.2±0.2 g; and 4 56–60 weeks, 32.6±0.7 g)
were obtained from the laboratory animal center of Huazhong
University of Science and Technology (Hubei, China) and housed in a
controlled environment (20±2°C; 12 h light/dark cycle; 50±10%
humidity) with free access to water and food. The animal protocol
was reviewed and approved by The Institutional Animal Research
Committee of Tongji Medical College (Wuhan, China). All the mice
were sacrificed, and the aorta, renal lymph node (RLN), spleen and
blood were collected and fresh frozen in liquid nitrogen until
required.
Laser capture microdissection (LCM)
procedure
Following the harvesting of the tissues, the aorta
was perfused in situ using EDTA/PBS for 10 min to remove
blood and preserve the adventitia. A dissection microscope was used
to remove adipose tissue and para-aortic ganglia but not lymph
nodes. Later, the aorta was embedded in Tissue-Tek and stored at
−80°C before being cut into sections (10 µm) onto cooled membrane
slides. The slides were dried 10 min at 45°C and stored in a vacuum
desiccator to avoid moisture uptake. LCM were performed with the
Palm MicroBeam laser system (P.A.L.M. Microlaser Technologies AG;
Carl Zeiss AG, Oberkochen, Germany) to microdissect the adventitia,
media and plaque according to their position of appearance under
the LCM microscopy (10). Samples
obtained from LCM were later used for reverse
transcription-quantitative polymerase chain reaction (RT-qPCR).
RT-qPCR validation
Total RNA was isolated by using RNAiso Plus (Takara
Bio, Inc., Otsu, Japan) according to the manufacturer's
instructions. Reverse transcription of RNA was performed using
PrimeScript™ RT reagent Kit (Takara) in 37°C for 15 min, 85°C for 5
sec and kept in 4°C. Quantitative real-time PCR was performed using
PrimeScript™ RT reagent kit (Takara Bio, Inc.) on a StepOnePlus™
Real-time PCR System (Applied Biosystems, Foster City, CA, USA).
Thermal cycling conditions were: 30 sec predenaturation at 95°C,
followed by 40 cycles of 5 sec denaturation at 95°C and 30 sec
annealing at 60°C. Primers were selected using PrimerBank
(https://pga.mgh.harvard.edu/primerbank/) and are
listed in Table I. All PCR was
performed in triplicate, and mRNA fold changes were calculated by
the 2− ΔΔCq method (22) using GAPDH as internal
reference.
 | Table I.Reverse transcription-quantitative
polymerase chain reaction primer information. |
Table I.
Reverse transcription-quantitative
polymerase chain reaction primer information.
| Gene | Forward | Reverse |
|---|
| Il7r |
5′-GCGGACGATCACTCCTTCTG-3′ |
5′-AGCCCCACATATTTGAAATTCCA-3′ |
| Cxcl12 |
5′-TGCATCAGTGACGGTAAACCA-3′ |
5′-CACAGTTTGGAGTGTTGAGGAT-3′ |
| Cxcl13 |
5′-GGCCACGGTATTCTGGAAGC-3′ |
5′-ACCGACAACAGTTGAAATCACTC-3′ |
| Cxcl16 |
5′-ACCCTTGTCTCTTGCGTTCTT-3′ |
5′-CAAAGTACCCTGCGGTATCTG-3′ |
| Ccr2 |
5′-ATCCACGGCATACTATCAACATC-3′ |
5′-TCGTAGTCATACGGTGTGGTG-3′ |
| Ccl5 |
5′-TTTGCCTACCTCTCCCTCG-3′ |
5′-CGACTGCAAGATTGGAGCACT-3′ |
| Ccl8 |
5′-TCTACGCAGTGCTTCTTTGCC-3′ |
5′-AAGGGGGATCTTCAGCTTTAGTA-3′ |
| Ccl12 |
5′-ATTTCCACACTTCTATGCCTCCT-3′ |
5′-ATCCAGTATGGTCCTGAAGATCA-3′ |
| GAPDH |
5′-AGGTCGGTGTGAACGGATTTG-3′ |
5′-TGTAGACCATGTAGTTGAGGTCA-3′ |
Statistical analysis
Statistical calculations were performed using
GraphPad Prism 7 (GraphPad Software, Inc., La Jolla, CA, USA).
Experiments were performed in triplicate and data analyzed using an
unpaired Student's t-test or one-way analysis of variance with
Sidak's multiple comparisons test, and are expressed as the mean ±
standard error of the mean. DEGs analyses were performed using
one-way ANOVA with Benjamini-Hochberg correction. P<0.05 was
considered to indicate a statistically significant difference.
Results
Identification of KEGG pathways and
differentially expressed genes in 78-week-old mouse aortas
The GSE40156 dataset was acquired for downstream
analysis. Hierarchical clustering analysis was used to assess the
overall quality of the group data (data not shown). Furthermore,
the DEGs between hyperlipidemic mouse groups were verified (78-week
ApoE−/− aorta samples vs. 78 week normal WT aorta
samples). Following preprocessing, log2-transformed gene expression
data from all samples were extracted for analysis. The DEGs between
the 78-week ApoE−/− aorta and 78-week wild-type aorta
were selected via the classical Bayesian algorithm with
|log2FC|≥1.5 and P<0.05. Gene-unmatched probes were removed, and
if one gene was matched by multiple probes, only the probe with the
lowest adjusted P-value was included. In total, 417 probes (395
upregulated and 22 downregulated, excluding ApoE) were identified
(data not shown). As expected, the 78 week ApoE−/− and
wild-type aorta was able to be distinguished by hierarchical gene
clustering. Subsequently, these 417 probe-matched DEGs were
subjected to KEGG enrichment analysis to determine clusters of
co-expressed genes sharing the same pathway. The results suggested
that the top 10 KEGG pathways significantly enriched by DEGs were
‘chemokine signaling pathway’, ‘phagosome’, ‘staphylococcus aureus
infection’, ‘osteoclast differentiation’, ‘tuberculosis’,
‘rheumatoid arthritis’, ‘cytokine-cytokine receptor interaction’,
‘leishmaniasis’, ‘hematopoietic cell lineage’ and ‘B cell receptor
signaling pathway’. However, ‘phagosome’, ‘staphylococcus aureus
infection’, ‘osteoclast differentiation’, ‘tuberculosis’,
‘rheumatoid arthritis’, ‘leishmaniasis’ and ‘hematopoietic cell
lineage’ had no direct association with immunity in
atherosclerosis. In addition, ‘B cell receptor signaling pathway’
has previously been investigated (11). Therefore, the present study focused
on ‘cytokine-cytokine receptor interaction’ and ‘chemokine
signaling pathway’ for further analysis. In total, 41 DEGs were
included in these two KEGG pathways (Table II).
 | Table II.‘Cytokine-cytokine receptor
interaction’ and ‘chemokine signaling pathway’ DEGs. |
Table II.
‘Cytokine-cytokine receptor
interaction’ and ‘chemokine signaling pathway’ DEGs.
| Group | DEGs |
|---|
| ApoE−/−
vs. WT (78-week aorta) | Vav1, Rac2, Ptk2b,
Prkcb, Prex1, Pik3r5, Pik3cg, Ncf1, Hck, Gngt2, Dock2, Arrb2,
Tnfrsf1b, Tnfrsf13b, Tnfrsf11b, Ltb, Lepr, Il7r, Il6, Il10ra,
Cxcr4, Cxcl9, Cxcl16, Cxcl14, Cxcl13, Cxcl12, Cxcl10, Cxcl1,
Csf2rb, Csf2ra, Ccr5, Ccr2, Ccr1, Ccl9, Ccl8, Ccl7, Ccl6, Ccl5,
Ccl3, Ccl2, Ccl12 |
| ATLO vs.
plaque | Itk, Ccr9, Adcy3,
Tnfsf13b, Tnfrsf17, Tnfrsf13c, Tnfrsf13b, Tnfrsf12a, Tnfrsf11b,
Tgfb2, Pdgfc, Pdgfa, Ngfr, Ltb, Lifr, Lep, Inhbb, Il7r, Il18r1,
Hgf, Ghr, Egfr, Cxcr6, Cxcr5, Cxcl9, Cxcl16, Cxcl13, Cxcl12,
Csf2ra, Ccr6, Ccr2, Ccl8, Ccl5, Ccl4, Ccl3, Ccl2, Ccl12, Ccl11 |
| ATLO vs.
ApoE−/− adventitia | Vav1, Rac2, Prkcb,
Pik3cg, Itk, Hck, Dock2, Arrb2, Tnfsf13b, Tnfrsf18, Tnfrsf13c,
Tnfrsf13b, Ppbp, Ltb, Il7r, Il6, Il21r, Il1r1, Il1b, Il18r1,
Il17ra, Il13ra1, Il10ra, Cxcr6, Cxcr5, Cxcr3, Cxcl16, Cxcl14,
Cxcl13, Cxcl12, Cxcl10, Cxcl1, Csf2rb, Clcf1, Ccr6, Ccr5, Ccr2,
Ccr1, Ccl8, Adcy7, Ccl5, Ccl12 |
| Common genes | Tnfrsf13b, Ltb,
Il7r, Cxcl16, Cxcl13, Cxcl12, Ccr2, Ccl8, Ccl5, Ccl12 |
Common KEGG pathways and DEGs in aorta
and ATLO clusters
ATLOs formed in the plaque-adjacent aorta adventitia
in aged ApoE−/− mice with atherosclerosis. During
atherosclerosis progression and ATLO formation, the adventitia is
reorganized, recruiting lymphocytes and other immune cells,
including B cells, to generate a dense immune cell aggregate
(10). The effects of ATLOs in
atherosclerosis progression have previously been demonstrated in
hyperlipidemic mice (11–13). Furthermore, to assess the
associated KEGG pathways in ATLOs, microarray data of ATLO, plaque
and adventitia, separated from the aorta via laser capture
microdissection technique (10),
were pooled to analyze transcript atlases. The same DEG and KEGG
analysis methods were utilized in ATLO plaque and adventitia
clusters to determine which genes and pathways were co-expressed
(data not shown). In the final analysis, the top 15 KEGG pathways
of aorta clusters (78-week ApoE−/− aorta and WT aorta
clusters) and ATLO clusters (ATLO plaque and adventitia clusters)
were selected, and it was verified that they shared four common
KEGG pathways (Table III). Among
them, ‘cytokine-cytokine receptor interaction’ and ‘chemokine
signaling pathway’ were the top two pathways. Therefore, it was
hypothesized that they were involved in the core mechanism of ATLO
development. Sets of candidate DEGs were obtained from these two
pathways (Table II). Heatmaps of
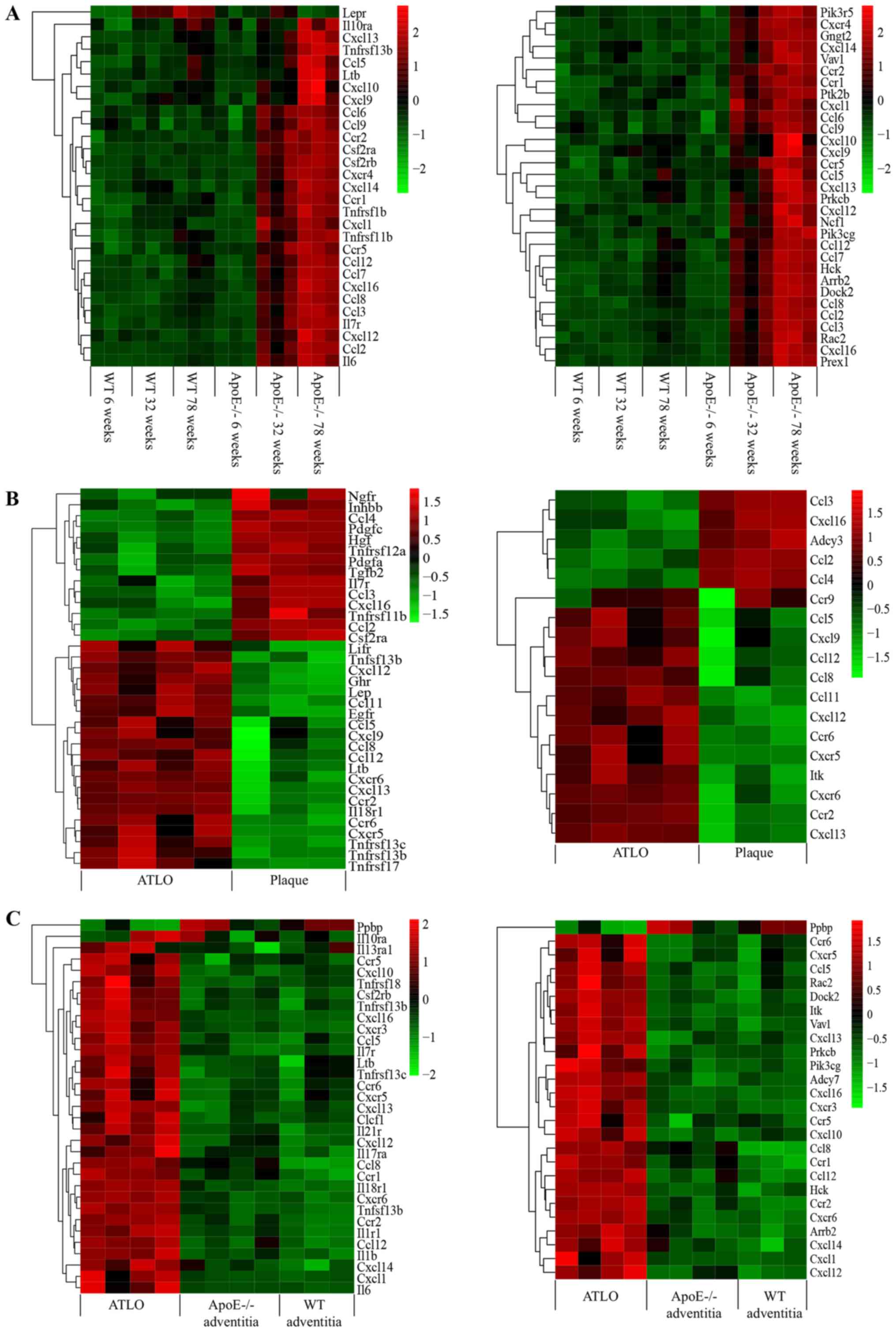
these DEGs in aorta clusters, ATLO plaque clusters and ATLO
adventitia clusters are presented in Fig. 1. In total, 10 DEGs were shared
between these groups: TNF receptor superfamily member 13B,
lymphotoxin-β, Il7r, C-X-C motif chemokine ligand (Cxcl)16, Cxcl13,
Cxcl12, C-C motif chemokine receptor 2 (Ccr2), C-C motif chemokine
ligand (Ccl)8, Ccl5 and Ccl12. Lötzer et al (23) previously reported that tumor
necrosis factor receptor superfamily member 1A/lymphotoxin
β-receptor cross-talk is involved in lymphorganogenic chemokine
protein secretion and tertiary lymphoid organogenesis. Il7r,
Cxcl16, Cxcl13, Cxcl12, Ccr2, Ccl8, Ccl5 and Ccl12 were identified
as candidate genes and may help to elucidate the mechanism of ATLO
formation.
 | Table III.Aorta cluster and ATLO cluster KEGG
enrichment. |
Table III.
Aorta cluster and ATLO cluster KEGG
enrichment.
| Group | KEGG pathways |
|---|
| ApoE−/−
vs. WT (78-week aorta) | ‘Chemokine
signaling pathway’; ‘Phagosome’; ‘Staphylococcus aureus infection’;
‘Osteoclast differentiation’; ‘Tuberculosis’; ‘Rheumatoid
arthritis’; ‘Cytokine-cytokine receptor interaction’;
‘Leishmaniasis’; ‘Hematopoietic cell lineage’; ‘B cell receptor
signaling pathway’; ‘Natural killer cell mediated cytotoxicity’;
‘Leukocyte transendothelial migration’; ‘Lysosome’; ‘Antigen
processing and presentation’; ‘TNF signaling pathway’. |
| ATLO vs.
plaque | ‘Cytokine-cytokine
receptor interaction’; ‘Hematopoietic cell lineage’; ‘Rheumatoid
arthritis’; ‘Complement and coagulation cascades’; ‘Intestinal
immune network for IgA production’; ‘Phagosome’; ‘PI3K-Akt
signaling pathway’; ‘Metabolic pathways’; ‘Lysosome’; ‘ECM-receptor
interaction’; ‘Primary immunodeficiency’; ‘Focal adhesion’;
‘Staphylococcus aureus infection’; ‘Malaria’. |
| ATLO vs.
ApoE−/− adventitia | ‘Metabolic
pathways’; ‘Microbial metabolism in diverse environments’; ‘Carbon
metabolism’; ‘Citrate cycle (TCA cycle)’; ‘Propanoate metabolism’;
‘Cytokine-cytokine receptor interaction’; ‘Valine, leucine and
isoleucine degradation’; ‘Chemokine signaling pathway’;
‘Hematopoietic cell lineage’; ‘Non-alcoholic fatty liver disease
(NAFLD)’; ‘Staphylococcus aureus infection’; ‘Fatty acid
metabolism’; ‘B cell receptor signaling pathway’; ‘Osteoclast
differentiation’; ‘Leishmaniasis’. |
| Common
pathways | ‘Cytokine-cytokine
receptor interaction’; ‘Chemokine signaling pathway’;
‘Hematopoietic cell lineage’; ‘Staphylococcus aureus
infection’. |
Furthermore, Il7r, Cxcl16, Cxcl13, Cxcl12, Ccr2,
Ccl8, Ccl5 and Ccl12 expression in the aorta, ATLO, plaque and
adventitia samples was determined by RT-qPCR (Fig. 2A and B). All of these eight DEGs
displayed stable, gradual upregulation with age in
ApoE−/− mouse aortas. Expression was significantly
different between the 78-week ApoE−/− and 78-week WT
aortas, and between ATLO and plaque or between ATLO and
ApoE−/− mouse adventitia. To the best of our knowledge,
notable atherosclerosis formation occurs at 32 weeks (7). Meanwhile, ATLO neogenesis is affected
by atherosclerosis (10).
Therefore, Il7r, Cxcl16, Cxcl13, Cxcl12, Ccr2, Ccl8, Ccl5 and Ccl12
may be key genes involved in atherosclerosis-associated ATLO
development.
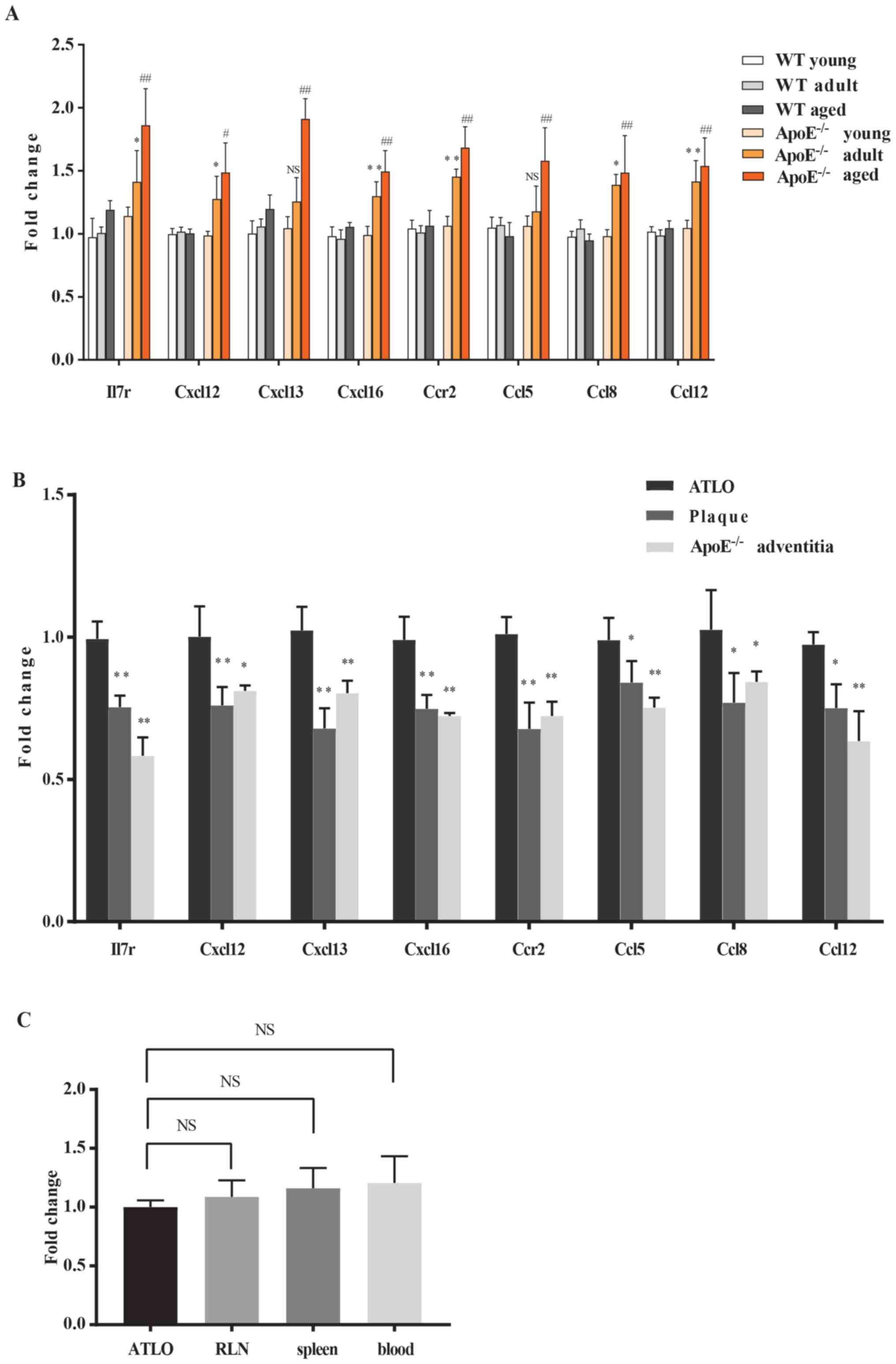 | Figure 2.Reverse transcription-quantitative
polymerase chain reaction analysis of eight identified DEGs in the
aorta and ATLO clusters. (A) DEGs in the aorta from WT and
ApoE−/− mice at 6, 32 and 78 weeks. *P<0.05,
**P<0.01 vs. WT adult; #P<0.05,
##P<0.01 vs. WT aged. NS, not significant. (B) DEGs
in the ATLO with plaque and adventitia. *P<0.05, **P<0.01 vs.
ATLO. (C) Il7r in ATLO with RLN, spleen and blood. NS, not
significant. DEGs analyses were performed using one-way ANOVA with
Benjamini-Hochberg correction. DEGs, differentially expressed
genes; ATLO, artery tertiary lymphoid organ; Il7r, interleukin 7
receptor; WT, wild-type; RLN, renal lymph node; Cxcl, C-X-C motif
chemokine ligand; Ccl12, C-C motif chemokine ligand 12; Ccr, C-C
motif chemokine receptor; ApoE, apolipoprotein E. |
GO enrichment analyses and PPI
network
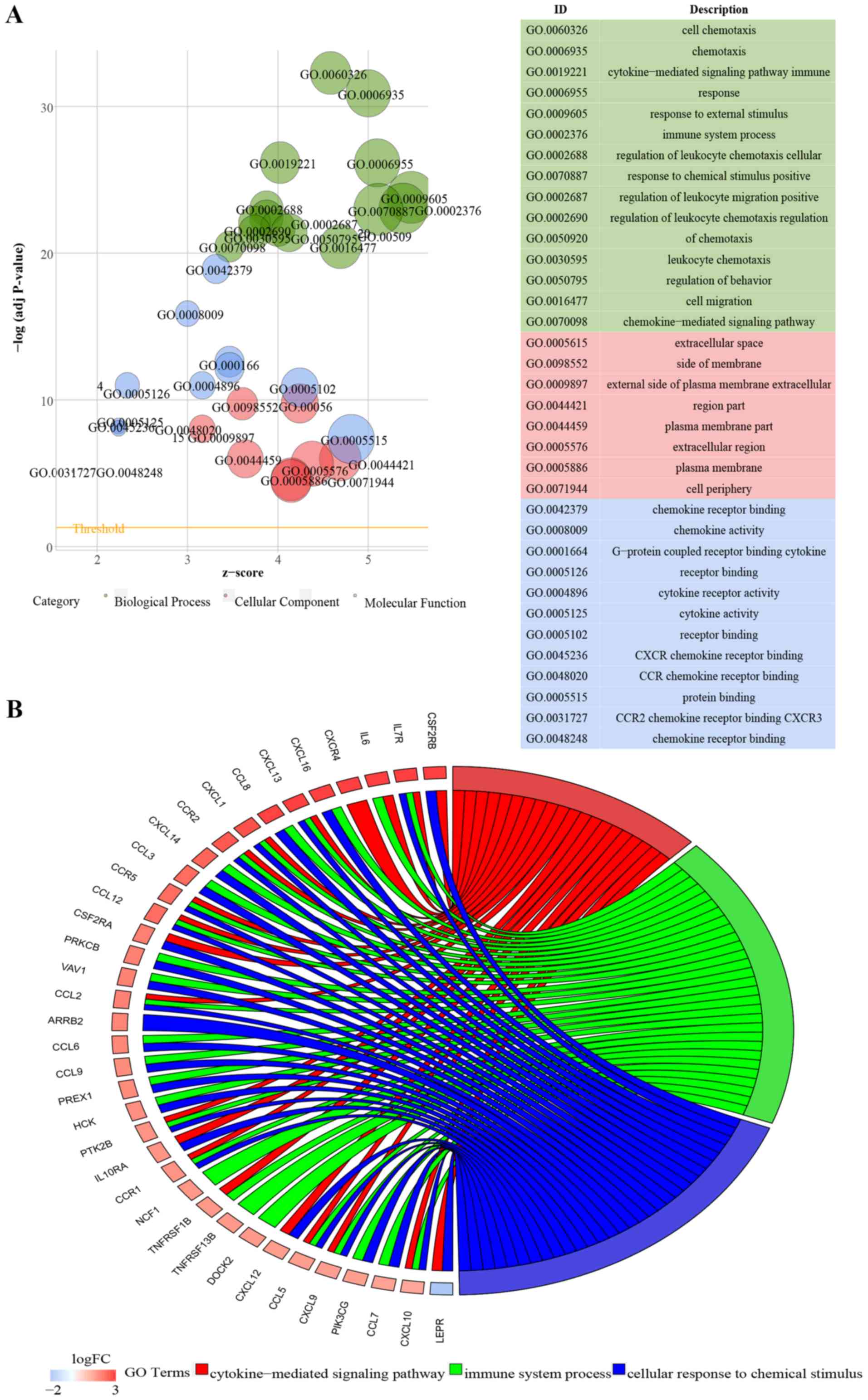
To explore the biological functions involved in
ATLOs, GO analysis was performed (Fig.
3). GO terms with an adjusted P<0.05 were considered
significantly enriched. DEGs from aorta clusters, ATLO plaque
clusters and ATLO adventitia clusters were significantly enriched
in the GO terms ‘cytokine-cytokine receptor interaction’ and
‘chemokine signaling pathway’. The top 15 biological process (BP),
eight molecular function (MF) and 12 cellular component (CC) GO
terms were visualized in Fig. 3A, C
and E.
To the best of our knowledge, the association
between Il7r and tertiary lymphoid organogenesis has not been
previously described. The Il7r gene encodes the interleukin 7
receptor, which is a protein found on the surface of various cells
and has been suggested to serve a critical role in development of
Peyer's patches and certain peripheral lymph nodes (24,25).
Thus, the present study focused on Il7r-associated GO terms. The GO
categories of BP associated with Il7r in aorta clusters,
ATLO-plaque clusters and ATLO-adventitia clusters are presented in
Fig. 3B, D, F and Table IV. The common BP GO terms were:
‘cytokine-mediated signaling pathway (GO:0019221)’ and ‘cellular
response to chemical stimulus (GO:0070887)’; thus, it was
hypothesized that these were the principal Il7r-associated
biological functions in ATLO development.
 | Table IV.Interleukin 7 receptor-associated BP
GO terms. |
Table IV.
Interleukin 7 receptor-associated BP
GO terms.
| Group | GO term |
|---|
| Aorta clusters | ‘Cytokine-mediated
signaling pathway’; ‘Immune system process’; ‘Cellular response to
chemical stimulus’. |
| ATLO-plaque
clusters | ‘Cytokine-mediated
signaling pathway’; ‘Cell surface receptor signaling pathway’;
‘Response to cytokine’; ‘Cellular response to chemical stimulus’;
‘Cellular response to cytokine stimulus’; ‘Cellular response to
organic substance’; ‘Signal transduction’. |
| ATLO-adventitia
clusters | ‘Cytokine-mediated
signaling pathway’; ‘Cellular response to cytokine stimulus’;
‘Response to cytokine’; ‘Cellular response to chemical stimulus’;
‘Cell surface receptor signaling pathway’; ‘Positive regulation of
immune system process’; ‘Cellular response to organic substance’;
‘Immune system process’. |
| Common BP GO
terms | ‘Cytokine-mediated
signaling pathway’; ‘Cellular response to chemical stimulus’. |
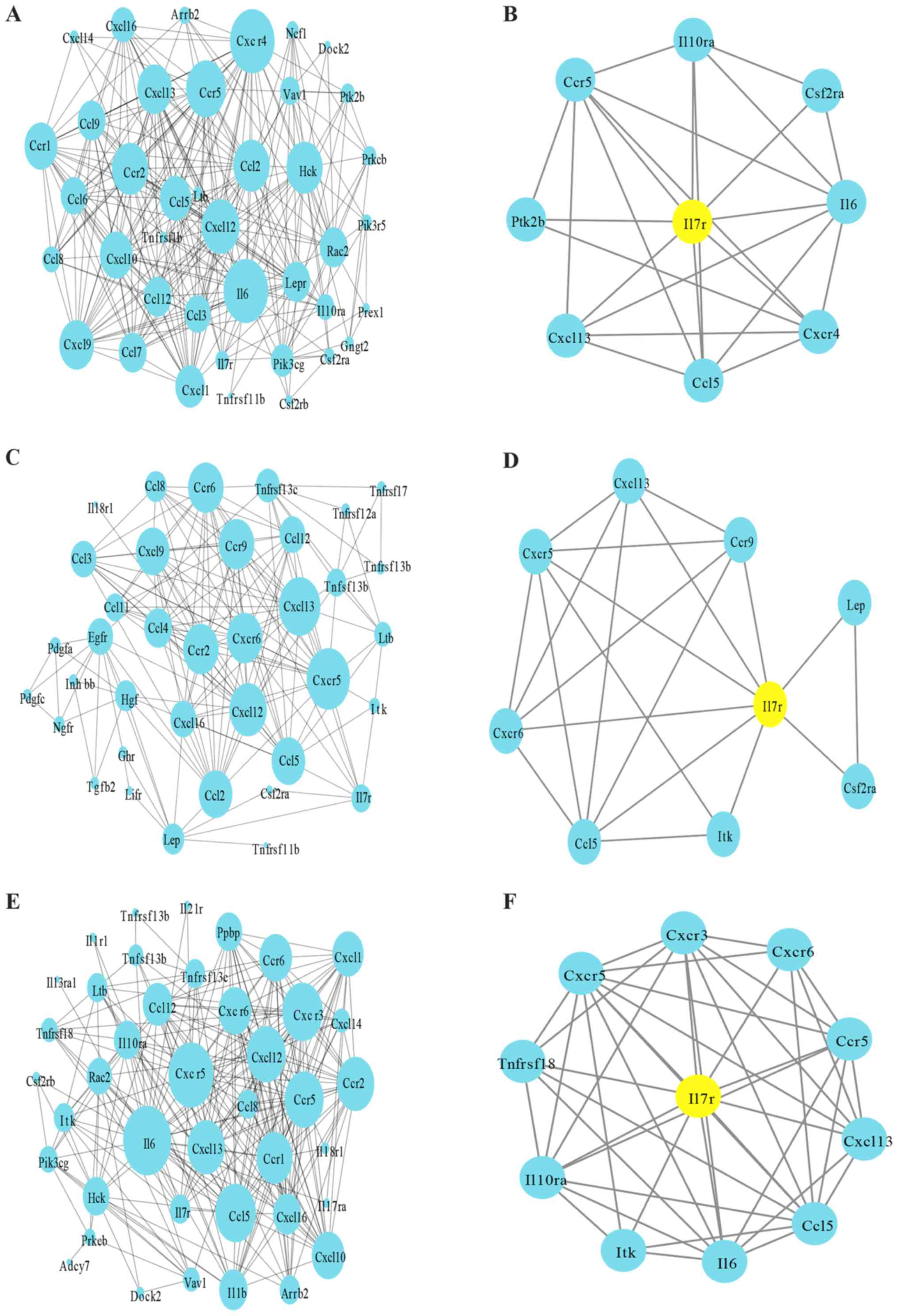
PPI network analysis (Fig. 4) was also used for the purpose of
characterizing core processes at the protein level. As illustrated
in Fig. 4A, C and E, the majority
of the nodes were included in ‘cytokine-cytokine receptor
interaction’ and ‘chemokine signaling pathway’ in three clusters.
For further analysis, Il7r modules were selected from the PPI
network. The results demonstrated that the protein-coding genes in
the network interacted with Il7r (Fig.
4B, D and F). In the aorta clusters, Il7r interacted with
interleukin (Il)6, interleukin 10 receptor subunit-α (Il10ra),
protein tyrosine kinase 2β, Ccr5, colony stimulating factor 2
receptor α-subunit (Csf2ra), Cxcr4, Ccl5 and Cxcl13; in ATLO plaque
clusters, Il7r interacted with leptin, Cxcr5, IL2 inducible T cell
kinase (Itk), Csf2ra, Ccr9, Ccl5, Cxcl13 and Cxcr6; and in
ATLO-adventitia clusters, Il7r interacted with Il6, Tnfrsf18,
Cxcr5, Il10ra, Cxcr3, Ccr5, Itk, Ccl5, Cxcl13 and Cxcr6. Ccl5 and
Cxcl13 were demonstrated to be involved in all of these three
clusters with Il7r. Notably, it was also observed that Ccl5 and
Cxcl13 were associated with the GO terms ‘cytokine-mediated
signaling pathway’ and ‘cellular response to chemical stimulus’,
important Il7r-associated BP GO terms. According to previous
studies, Ccl5 and Cxcl13 are lymphorganogenic chemokines that
trigger the development of elaborate bona fide ATLOs (10,23).
It was subsequently deduced that Il7r may be involved, with Ccl5
and Cxcl13, in cytokine-mediated signaling pathways and the
cellular response to chemical stimuli in the progression of ATLO
development.
Il7r is not involved in ATLO
spleen/blood/RLN clusters
In contrast, spleen-, blood- and RLN-transcript
atlases were noticeably larger, with a more pronounced level of
DEGs in ATLOs (data not shown). Analysis of spleen-, blood- and
RLN-regulated genes revealed that 599 common DEGs remained
(Fig. 5A). The enrichment KEGG
pathways of the 599 DEGs were ‘hematopoietic cell lineage’,
‘rheumatoid arthritis’, ‘complement and ‘coagulation cascades’,
‘intestinal immune network for IgA production’, ‘osteoclast
differentiation’, ‘leishmaniasis’, ‘proteoglycans in cancer’ and
‘viral myocarditis’. ‘Cytokine-cytokine receptor interaction’ and
‘chemokine signaling pathway’ were not identified. The transcript
atlas demonstrated no significant alterations in Il7r expression in
ATLOs vs. spleen, blood, or RLN. To confirm these results, GO terms
and KEGG enrichment were also separately applied for ATLO-RLN
clusters (Fig. 5B-F). It is
noteworthy that the ATLO-RLN clusters still performed a comparable
enrichment in ‘cytokine-cytokine receptor interaction’ (adjusted
P=6.34×10−10) and ‘chemokine signaling pathway;
(adjusted P=4.54×10−6). However, they were less
important in the ATLO-RLN clusters and Il7r was not identified in
the KEGG pathways, indicating that the expression level of Il17r in
ATLOs was no different compared with that in RLN, which was also
validated by the RT-qPCR (Fig.
2C). As Il7r serves a vital role in lymph node development, it
was hypothesized that Il7r may be involved in lymphoid organ
neogenesis in ATLOs and RLN, meaning that there were no identified
differences between ATLOs and RLN. Similar results were found in
ATLO-spleen/blood clusters (data not shown).
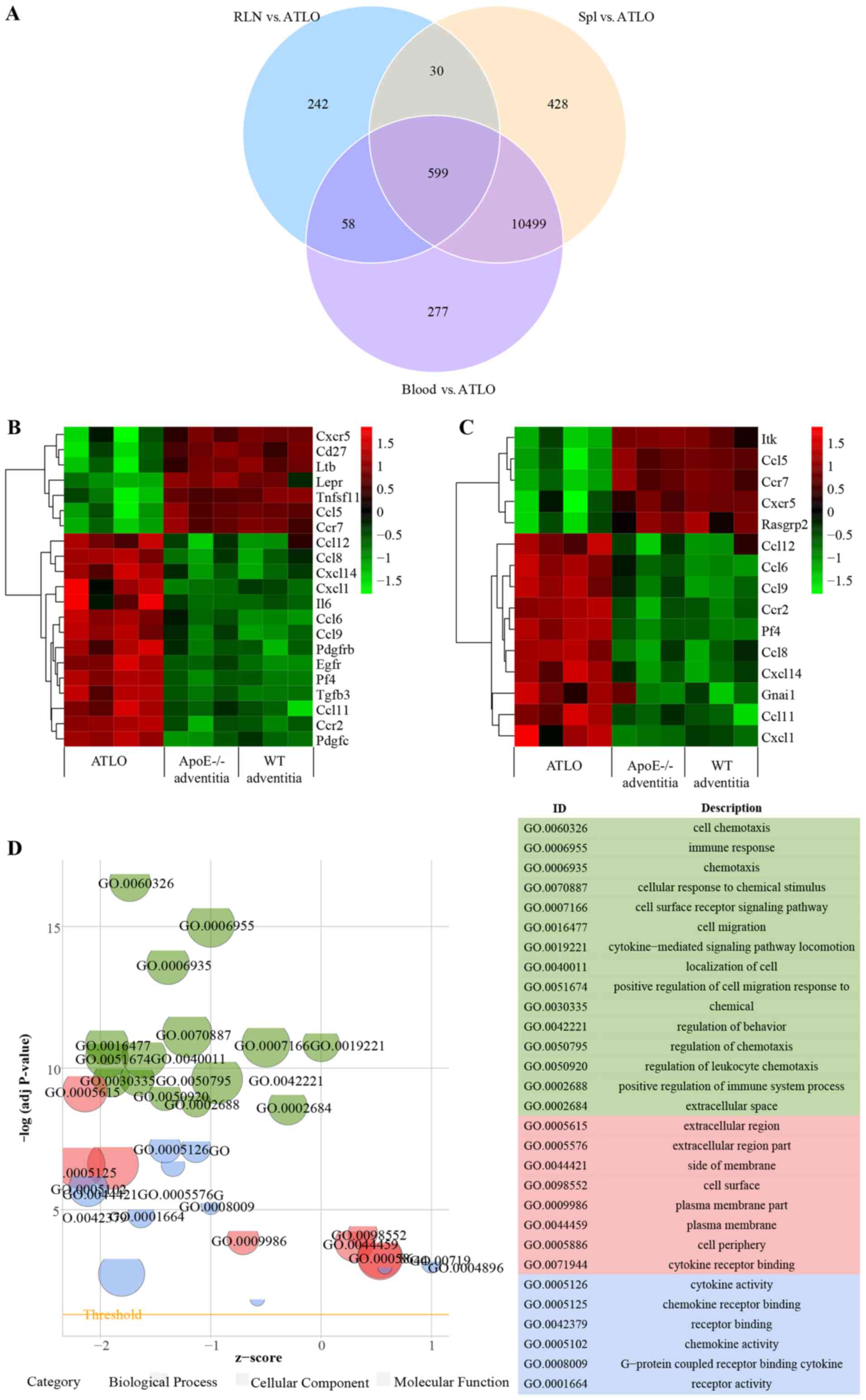 | Figure 5.Functional analysis showing DEGs in
ATLO-spleen/blood/RLN clusters. (A) Venn diagram representation of
the comparative analysis of DEGs in ATLO spleen/blood/RLN clusters.
(B) Heatmaps of DEGs of ‘cytokine-cytokine receptor interaction’ in
ATLO RLN clusters. (C) Heatmaps of DEGs of ‘chemokine signaling
pathway’ in ATLO RLN clusters. The color scale of the raw Z-scores
represents high (red) and low (green) expression. (D) The bubble
plot displays GO enrichment based on DEGs of ‘cytokine-cytokine
receptor interaction’ and ‘chemokine signaling pathway’ between
ATLO and RLN. The y-axis indicates the significance of the term
(-log10 adjusted P-value) and the x-axis indicates the Z-score.
Bubbles indicate the GO terms, with green indicating the BP terms,
red the cellular component terms and blue the molecular function
terms. Bubble size indicates the gene numbers in the GO terms. (E)
Circular plot showing Il7r-associated BP GO terms in ATLO RLN
clusters. The genes are bridged by ribbons to their assigned BP
terms. The dark-to-light blue color indicates the logFC. (F) PPI
network based on these DEGs between ATLO and RLN. The size of a
node is proportional to the connection degree. The nodes in network
represent proteins (indicated by gene names). Lines demonstrate the
associations between proteins. PPI, protein-protein interaction;
DEGs, differentially expressed genes; ATLO, artery tertiary
lymphoid organ; Il7r, interleukin 7 receptor; BP, biological
process; FC, fold change; RLN, renal lymph node; spl, spleen. |
Discussion
It is well known that the immune system is crucially
involved in atherogenesis. However, there remain questions as to
how, when and where cell subset interactions are organized in
disease development. The outermost connective tissue that covers
arteries is highly active and complex. It is termed adventitia and
functions in artery maintenance and homeostasis (5,7).
During aging, the quantity of inflammatory leukocytes, including
monocytes, macrophages and T-cells, are progressively increased in
the adventitia (26), which drives
the development of ATLOs from the appearance of atherosclerotic
plaques (10). ATLO neogenesis
occurs from 32 weeks and is noted in 75% of 78-week-old mice with
atherosclerosis (10). They are
complex plaque-associated lymphoid organs associated with the
immune response, and are crucial in atherosclerosis.
In the present study, ‘cytokine-cytokine receptor
interaction’ and ‘chemokine signaling pathway’ were identified from
aorta and ATLO clusters. These two pathways are of major importance
in guiding immune cells to and within lymphoid and non-lymphoid
tissues (27–29). While the development and
architectural organization of ATLOs requires homeostatic chemokines
or lymphoid cytokines, Il7r appears to be a key gene linking these
events with chemokines or chemokine receptors. Previous studies
have demonstrated that Il7r is involved in lymph node development
and inflammatory disease (24,30).
Consistent with this, in the present study, Il7r exhibited
significant differential expression in the aged ApoE−/−
aorta and ATLOs, compared with WT mice. A limited number of studies
have demonstrated the direct association between Il7r and
atherosclerosis. However, it has been reported that Il7r is
upregulated in atherosclerotic tissues, and Il7r may lead to
atherosclerosis through the inflammatory pathway (10,31),
and is also associated with a number of other inflammatory diseases
such as ulcerative colitis (30),
rheumatoid arthritis (32) and
multiple sclerosis (33). This
evidence suggests that Il7r may also be required for the
progression of atherosclerosis. It is known that the formation of
ATLOs is influenced by plaques and that their sizes increase with
plaque size (10). Thus, it was
hypothesized that Il7r may be involved in
atherosclerosis-associated ATLO development.
In addition to the aforementioned Il7r gene, the
chemokines Cxcl16, Cxcl13, Cxcl12, Ccr2, Ccl8, Ccl5 and Ccl12 were
observed to be candidate genes in ATLO formation, and a number of
these have already been identified in previous studies (10,23,34).
Furthermore, it was identified that they were enriched with Il7r in
‘cytokine-cytokine receptor interaction’ and ‘chemokine signaling
pathway’ in the present study. In the PPI network analysis, it was
demonstrated that Il7r was also co-expressed with Ccl5 and Cxcl13
at the core of cellular processing at the protein level. Ccl5 is
chemotactic for T cells, eosinophils and basophils, triggering the
recruitment of leukocytes into inflammatory sites (35). Chemokine Cxcl13 is essential for
lymph node initiation (36). In
the GO enrichment analysis, Ccl5 and Cxcl13 were also involved in
the two major Il7r-associated BP GO terms, namely
‘cytokine-mediated signaling pathway’ and ‘cellular response to
chemical stimulus’. These two GO terms may be associated with ATLO
development. Combining the results from our analysis, it was
concluded that Il7r may contribute to chemokine recruitment in
atherosclerosis-associated ATLO development.
The present research was a re-analysis based on
available microarray data, rather than the reproduction of
conclusions presented by the original publications. The original
study also used immunostaining and paid more attention to gene and
biological process-associated T/B cell immune processes (10). In the present study, the DEGs and
KEGG pathways in the gene expression profile throughout the
development of ATLOs were focused upon. Il7r acted as a core
component in ATLO lymphogenesis in a hyperlipidemic state. This may
contribute to the understanding of atherosclerotic immunity in the
adventitia.
Nevertheless, certain limitations, such as a lack of
verification at the experimental level, exist in the present study.
Complementary experiments in ATLOs will be performed in the future
for further confirmation.
Acknowledgements
Professor Yuxiang Yang from Wuhan Vocational College
of Software and Engineering provided valuable bioinformatics
technology support and advice.
Funding
This study was supported by the National Natural
Science Foundation of China (grant no. 81270297), and the
Scientific Research Training Program for Young Talents sponsored by
Union Hospital, Tongji Medical College, Huazhong University of
Science and Technology.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XZ and CC conceived and designed the study. FL and
PB analyzed the microarray datasets and interpreted the results. FL
and ND downloaded the gene expression profile from the Gene
Expression Omnibus. ND was also responsible for designing and
supervising the whole project. XZ wrote and edited the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The animal protocol was reviewed and approved by The
Institutional Animal Research Committee of Tongji Medical College
(Wuhan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Brown AJ, Teng Z, Evans PC, Gillard JH,
Samady H and Bennett MR: Role of biomechanical forces in the
natural history of coronary atherosclerosis. Nat Rev Cardiol.
13:210–220. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tabas I and Lichtman AH:
Monocyte-macrophages and T cells in atherosclerosis. Immunity.
47:621–634. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weber C and Noels H: Atherosclerosis:
Current pathogenesis and therapeutic options. Nat Med.
17:1410–1422. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang X, Zou Y, Li L, Chen S, Hou J and Yu
B: Relation of ABO blood groups to the plaque characteristic of
coronary atherosclerosis. Biomed Res Int. 2017:26747262017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Campbell KA, Lipinski MJ, Doran AC,
Skaflen MD, Fuster V and McNamara CA: Lymphocytes and the
adventitial immune response in atherosclerosis. Circ Res.
110:889–900. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mohanta SK, Yin C, Peng L, Srikakulapu P,
Bontha V, Hu D, Weih F, Weber C, Gerdes N and Habenicht AJ: Artery
tertiary lymphoid organs contribute to innate and adaptive immune
responses in advanced mouse atherosclerosis. Circ Res.
114:1772–1187. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yin C, Mohanta SK, Srikakulapu P, Weber C
and Habenicht AJ: Artery tertiary lymphoid organs: Powerhouses of
atherosclerosis immunity. Front Immunol. 7:3872016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Psaltis PJ, Puranik AS, Spoon DB, Chue CD,
Hoffman SJ, Witt TA, Delacroix S, Kleppe LS, Mueske CS, Pan S, et
al: Characterization of a resident population of adventitial
macrophage progenitor cells in postnatal vasculature. Circ Res.
115:364–375. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
He C, Medley SC, Hu T, Hinsdale ME, Lupu
F, Virmani R and Olson LE: PDGFRbeta signalling regulates local
inflammation and synergizes with hypercholesterolaemia to promote
atherosclerosis. Nat Commun. 6:77702015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Grabner R, Lotzer K, Dopping S, Hildner M,
Radke D, Beer M, Spanbroek R, Lippert B, Reardon CA, Getz GS, et
al: Lymphotoxin beta receptor signaling promotes tertiary lymphoid
organogenesis in the aorta adventitia of aged ApoE−/−
mice. J Exp Med. 206:233–248. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Srikakulapu P, Hu D, Yin C, Mohanta SK,
Bontha SV, Peng L, Beer M, Weber C, McNamara CA, Grassia G, et al:
Artery tertiary lymphoid organs control multilayered
territorialized atherosclerosis B-Cell responses in aged
ApoE−/− mice. Arterioscler Thromb Vasc Biol.
36:1174–1185. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu D, Mohanta SK, Yin C, Peng L, Ma Z,
Srikakulapu P, Grassia G, MacRitchie N, Dever G, Gordon P, et al:
Artery tertiary lymphoid organs control aorta immunity and protect
against atherosclerosis via vascular smooth muscle cell lymphotoxin
β receptors. Immunity. 42:1100–1115. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Clement M, Guedj K, Andreata F, Morvan M,
Bey L, Khallou-Laschet J, Gaston AT, Delbosc S, Alsac JM, Bruneval
P, et al: Control of the T follicular helper-germinal center B-cell
axis by CD8(+) regulatory T cells limits atherosclerosis and
tertiary lymphoid organ development. Circulation. 131:560–570.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gautier L, Cope L, Bolstad BM and Irizarry
RA: Affy-analysis of affymetrix genechip data at the probe level.
Bioinformatics. 20:307–315. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW,
Shi W and Smyth GK: limma powers differential expression analyses
for RNA-sequencing and microarray studies. Nucleic Acids Res.
43:e472015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Walter W, Sanchez-Cabo F and Ricote M:
GOplot: An R package for visually combining expression data with
functional analysis. Bioinformatics. 31:2912–2914. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gu W, Sun Y, Zheng X, Ma J, Hu XY, Gao T
and Hu MJ: Identification of gastric cancer-related circular RNA
through microarray analysis and bioinformatics analysis. Biomed Res
Int. 2018:23816802018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Altermann E and Klaenhammer TR: Pathway
voyager: Pathway mapping using the Kyoto encyclopedia of genes and
genomes (KEGG) database. BMC Genomics. 6:602005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Szklarczyk D, Franceschini A, Wyder S,
Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos
A, Tsafou KP, et al: STRING v10: protein-protein interaction
networks, integrated over the tree of life. Nucleic Acids Res.
43:D447–D452. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pang X, Zhao Y, Wang J, Zhou Q, Xu L,
Kang, Liu AL and Du GH: The bioinformatic analysis of the
dysregulated genes and MicroRNAs in entorhinal cortex, hippocampus,
and blood for Alzheimer's Disease. Biomed Res Int.
2017:90845072017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lotzer K, Dopping S, Connert S, Grabner R,
Spanbroek R, Lemser B, Beer M, Hildner M, Hehlgans T, van der Wall
M, et al: Mouse aorta smooth muscle cells differentiate into
lymphoid tissue organizer-like cells on combined tumor necrosis
factor receptor-1/lymphotoxin beta-receptor NF-kappaB signaling.
Arterioscler Thromb Vasc Biol. 30:395–402. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Luther SA, Ansel KM and Cyster JG:
Overlapping roles of CXCL13, interleukin 7 receptor alpha, and CCR7
ligands in lymph node development. J Exp Med. 197:1191–1198. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ribeiro de Almeida C, Hendriks RW and
Stadhouders R: Dynamic control of long-range genomic interactions
at the immunoglobulin κ light-chain locus. Adv Immunol.
128:183–271. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao L, Moos MP, Grabner R, Pedrono F, Fan
J, Kaiser B, John N, Schmidt S, Spanbroek R, Lötzer K, et al: The
5-lipoxygenase pathway promotes pathogenesis of
hyperlipidemia-dependent aortic aneurysm. Nat Med. 10:966–973.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schulz O, Hammerschmidt SI, Moschovakis GL
and Forster R: Chemokines and chemokine receptors in lymphoid
tissue dynamics. Annu Rev Immunol. 34:203–242. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Anders HJ, Romagnani P and Mantovani A:
Pathomechanisms: homeostatic chemokines in health, tissue
regeneration, and progressive diseases. Trends Mol Med. 20:154–165.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jones GW, Hill DG and Jones SA:
Understanding immune cells in tertiary lymphoid organ development:
It is all starting to come together. Front Immunol. 7:4012016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Willis CR, Seamons A, Maxwell J, Treuting
PM, Nelson L, Chen G, Phelps S, Smith CL, Brabb T, Iritani BM and
Maggio-Price L: Interleukin-7 receptor blockade suppresses adaptive
and innate inflammatory responses in experimental colitis. J
Inflamm (Lond). 9:392012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Moreno-Viedma V, Amor M, Sarabi A, Bilban
M, Staffler G, Zeyda M and Stulnig TM: Common dysregulated pathways
in obese adipose tissue and atherosclerosis. Cardiovasc Diabetol.
15:1202016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang R, Yang X, Wang J, Han L, Yang A,
Zhang J, Zhang D, Li B, Li Z and Xiong Y: Identification of
potential biomarkers for differential diagnosis between rheumatoid
arthritis and osteoarthritis via integrative genomewide gene
expression profiling analysis. Mol Med Rep. 19:30–40.
2019.PubMed/NCBI
|
|
33
|
Tavakolpour S: Interleukin 7 receptor
polymorphisms and the risk of multiple sclerosis: A meta-analysis.
Mult Scler Relat Disord. 8:66–73. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ciccia F, Rizzo A, Maugeri R, Alessandro
R, Croci S, Guggino G, Cavazza A, Raimondo S, Cannizzaro A,
Iacopino DG, et al: Ectopic expression of CXCL13, BAFF, APRIL and
LT-beta is associated with artery tertiary lymphoid organs in giant
cell arteritis. Ann Rheum Dis. 76:235–243. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hong M, Puaux AL, Huang C, Loumagne L, Tow
C, Mackay C, Kato M, Prévost-Blondel A, Avril MF, Nardin A and
Abastado JP: Chemotherapy induces intratumoral expression of
chemokines in cutaneous melanoma, favoring T-cell infiltration and
tumor control. Cancer Res. 71:6997–7009. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
van de Pavert SA, Olivier BJ, Goverse G,
Vondenhoff MF, Greuter M, Beke P, Kusser K, Höpken UE, Lipp M,
Niederreither K, et al: Chemokine CXCL13 is essential for lymph
node initiation and is induced by retinoic acid and neuronal
stimulation. Nat Immunol. 10:1193–1199. 2009. View Article : Google Scholar : PubMed/NCBI
|