Introduction
Auditory neuropathy spectrum disorder (ANSD) is a
specific form of hearing loss that can affect people of all ages
(1,2). It is normally characterized by
preserved outer hair cell function and abnormal neural conduction
of the auditory pathway (1). As a
result, the inner ear receives sounds successfully, but the cochlea
has a problem with sending the signals from the ear to the auditory
nerve and brain stem (3). Impaired
hearing presents as fluctuations in hearing sensitivity, as well as
speech perception performance, which can eventually lead to social
isolation, depression, and a reduction in professional
capabilities, especially for young children.
It has been reported that disorders of the cochlea
and auditory neuropathies, such as injured inner hair cells (IHCs),
abnormal communication between the IHCs and the auditory nerve, or
the impaired function of the cochlear spiral ganglion neurons
(SNGs) or auditory nerve itself, may account for the
pathophysiological mechanisms of ANSD (4,5).
However, the underlying mechanism involved in this pathology
remains largely unclear.
Several studies have indicated that
apoptosis-inducing factor mitochondrion-associated 1 (AIFM1)
gene expressing AIFM1 protein or AIF plays a vital role in
triggering chromatin condensation and DNA fragmentation to induce
programmed cell death (apoptosis), with a significant role in the
mitochondrial respiratory chain and metabolic redox reactions
(6,7). As a result, the abnormal expression
of AIFM1 gene expression is linked to multiple clinical
phenotypes, such as mitochondrial encephalomyopathy,
spondyloepimetaphyseal dysplasia with mental retardation, X-linked
recessive Charcot-Marie-Tooth disease-4 and ANSD (8,9).
Recently, a high-level association of mutations was reported in the
AIF gene in ANSD. Through the use of whole-exome sequencing
(WES), it has been shown that variants in the AIFM1 gene
were the main cause of familial and sporadic ANSD (10). Immunostaining of the murine inner
ear also indicated a ubiquitous expression of the AIFM1 gene
in IHCs, cochlear outer hair cells (OHCs), and especially SGNs.
Among these cells, SGNs are the first afferent neurons in the
auditory pathway, which makes them the focus of research. However,
the potential regulatory effects of AIF knockdown on the cellular
function of SGNs are rarely reported.
The aim of the present study was to further study
the potential role of AIF dysfunctions in impaired cochlear SNGs
cellular functions. The regulatory effects of AIF on mitochondrial
function and cellular apoptosis were revealed.
Materials and methods
Tissue culture isolation and detection
of AIF expression
The rats were handled in accordance with the NIH
Office of Animal Care and Use Animal Research Advisory Committee,
and were subjected to the lowest possible levels of pain and
discomfort. All animal procedures performed on SPF SD rats were
approved by the local Institutional Animal Care and Use Committee
of the Chinese PLA General Hospital. Spiral ganglia (n=6 for each
time point) were isolated from SD rats on postnatal days 1 (1 d), 4
(4 d), 7 (1 w), 28 (4 w), 56 (8 w) and postnatal year 2. The rats
were housed in SPF animal center (temperature 20–25°C) under
standard acclimatization conditions of 12 h light/dark cycle and
with ad libitum access to water and a commercial standard
chow. Health and behavior monitoring by visual examination was
performed daily.
The isolation procedures were performed as
previously described (11–13). Briefly, for neonatal SD rats (up to
10 days of age), the animals were anesthetized via an intravenous
injection of 30 mg/kg sodium pentobarbital and then decapitated.
For SD rats older than 10 days, the rats were placed in the
euthanasia chamber with readily visible and 100% carbon dioxide was
introduced, during which the flow rate must displace no more than
30% of the chamber volume/minute. The expected time to
unconsciousness is usually within 2 to 3 min. After observation of
each rat for signs of both lack of respiration and faded eye color,
the rat was removed from the cage for further tissue isolation.
During the tissue isolation, the skulls were opened
along the midsagittal (median) plane with a surgical scissor.
Temporal bones from both sides were cut off and placed into in
ice-cold sterile PBS with 2% BSA and 2% glucose under a
stereomicroscope. The cochlear capsule was separated using fine
forceps to expose the membranous labyrinth. To avoid
cross-contamination, the region where the ganglia were in contact
one another was discarded.
To detect the mRNA expression levels of AIF,
RNA isolated from the cochlear capsule tissue was measured by
RT-qPCR. Briefly, total RNA of the micro-dissected samples was
isolated by using an Omega Total RNA kit I (Omega Bio-Tek, Inc.,
Norcross, GA, USA), according to the manufacturer's instructions.
cDNA was synthesized using Takara PrimeScript RT reagent kit with
gDNA Eraser (Takara Biotechnology Co., Ltd., Dalian, China),
according to the manufacturer's instructions. RT-qPCR analysis of
the RNA was performed using a SYBR Premix EX Taq kit (Takara
Biotechnology Co., Ltd.) and RNase-free 96-well PCR plates under
the following cycling conditions: 40 Cycles of 95°C for 15 sec and
58°C for 30 sec.
The forward and reverse primers (Sangon Biotech Co.,
Ltd., Shanghai, China) were used for the specific RNAs in RT-qPCR
analysis: AIF, Forward: 5′-CGGCGGTGTGTGAAAAGAAA-3′ and
5′-GGAGTTCTAGAGGAACACGCC-3′ reverse (199 bp); β-actin, Forward
5′-AACCTTCTTGCAGCTCCTCC-3′ and 5′-CCTTCTGACCCATACCCACC-3′ reverse
(202 bp). β-Actin gene expression was used for normalization and
three independent experiments were performed for each time
points.
Western blotting
The cochlear capsule tissue (n=3) was lysed with
Laemmli Sample Buffer (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). BCA Protein Assay kit (Beyotime Institute of Biotechnology,
Shanghai, China) was used to measure the total protein content of
the lysates. The lysates samples were further heated at 100°C for
10 min and loaded onto 10% sodium dodecyl sulfate polyacrylamide
gels (SDS-PAGE) with equal amounts of total protein. After being
electro-transferred to polyvinylidene fluoride (PVDF) membranes
(Bio-Rad Laboratories, Inc.), the samples were blocked with 1X TBST
with 5% w/v nonfat dry milk at room temperature for 1 h. The
membranes were incubated with primary antibodies overnight at 4°C.
Rabbit monoclonal anti-AIF (1:1,000; Abcam, Cambridge, MA, USA),
and anti-β-actin (1:4,000; Beyotime Institute of Biotechnology)
primary antibodies were used. The blots were then exposed to the
horseradish peroxidase-labelled secondary anti-rabbit antibody
(1:8,000) for 2 h at room temperature. The proteins were visualized
using an enhanced chemiluminescence kit (Thermo Fisher Scientific,
Inc., Waltham, MA, USA). The band signals were detected using a gel
imaging system (Syngene, Frederick, MD, USA). The intensities were
quantified using ImageJ software and the relatively expression
levels of certain proteins were calculated via band intensity
normalization.
SGNs isolation and culture
SGNs were isolated from the micro-dissected
cochlear capsule tissue of newborn rats (1 day) as described above.
The tissues in tubes were dissociated in 0.05% trypsin, 0.37 mM
EDTA, 5 µg/ml each of collagenase type 4 each, papain, and dispase,
and 2,800 U/ml DNase in 1 ml PBS. The tubes were rotated at 37°C
for 45 min, with gentle trituration using a pipette every 15 min.
After 45 min of enzyme digestion, the tissue was finally triturated
5–10 times and centrifuged for 10 min at 4°C at 800 × g. After
discarding the dissociation medium, the cells were re-suspended
gently in Dulbecco's modified Eagle's medium supplemented with B272
ml/ml, BDNF 10 µg/ml and penicillin 100,000 U/l 1% (all from Merck
KGaA, Darmstadt, Germany) and cultured at 37°C in a humidified
incubator with 5% CO2. Cell adhesion and spreading were
observed after 12 and 48 h of culture (14,15).
Immunofluorescence
NF200 immunofluorescence was used to identify the
isolated SGNs (16). Following
culture for 48 h, the SGNs were fixed in 4% paraformaldehyde for 20
min and exposed to 0.2% Triton X-100 in PBS for permeabilization.
The cells were then washed with PBS and incubated with 5% BSA
blocking solution for 20 min. Following incubation with a rabbit
anti-NF200 antibody (1:200; Abcam) overnight at 4°C, the cells were
further treated with Alexa-488-conjugated goat anti-rabbit (1:200;
Sangon Biotech Co., Ltd.) to label the primary antibodies for 30
min at 37°C. The staining results were observed with a fluorescence
microscope (Olympus Corporation, Tokyo, Japan).
siRNA knockdown of AIF in SGNs
To verify the role of AIF in SGNs, we firstly
performed siRNA knockdown of AIF using siRNA oligonucleotides
(oligos) targeting the coding region of AIF. Following culture for
24 h, the SGNs were transfected with siRNA via Amaxa 4D
nucleofector (Lonza Group, Ltd., Basel, Switzerland) using P4
Primary Cell 4D-Nucleofector® X kit L (Lonza Group,
Ltd.), according to the manufacturer's instructions. Half of the
medium was then replaced with fresh culture medium after 4 h and
the cells were incubated in a humidified incubator with 37°C/5%
CO2 until analysis. The siRNA-AIF oligos for AIF
targeting were as follows: Sense
5′-CAGAAGGAGGCUCAGUUCCUCCAAUdTdT-3′, and anti-sense
5′-AUUGGAGGAACUGAGCCUCCUUCUGdTdT-3′; and siRNA-control oligos for
control experiments, Sense 5′-GCAUCGAAUAUUCUACCUUdTdT-3′, and
Anti-Sense 5′-AAGGUAGAAUAUUCGAUGCdTdT-3′ were used. siRNA (15
pmol/sample) was used, and normal siRNA was replaced of with Cy3
labeled siRNA for observation of the transfection efficiency. The
AIF protein contents were analyzed by western blotting. The SGNs
cultured on tissue culture polystyrene (TCPS) without further
treatment were termed the TCPS group, which was used as the blank
comparison group.
Intracellular reactive oxygen species
(ROS) level detection
To evaluate the regulatory effect of the AIF siRNA
knockdown on cellular oxidative stress status, the intracellular
ROS was monitored using 2′,7′-dichlorofluorescein diacetate
(DCFH-DA). Forty-eight hours after siRNA transfection, the SGNs
were treated with DCFH-DA (10 µM) at 37°C for 20 min in the dark.
Next, flow cytometry (BD FACS Calibur; Becton, Dickinson and
Company, Franklin Lakes, NJ, USA) was performed to measure the
fluorescence intensity of the treated cells. The fluorescence of
the oxidation product of DCFH-DA, DCF, was excited at 488 nm and
detected at 535 nm by flow cytometry. Data was acquired and
analyzed using the CELL Quest programme PRO software (Becton,
Dickinson and Company). The percentage of cells with a high DCF
fluorescence of 103−105 units, as measured by
flow cytometry, was used to indicate the intracellular (ROS)
level.
The staining fluorescence of SGNs was also observed
with a fluorescent microscope (Leica Microsystems GmbH, Wetzlar).
Images from 4 random fields in each group were recorded and the
fluorescence density was quantified with ImagePro Plus™
software.
The intracellular malondialdehyde (MDA) levels were
determined by using a Lipid Peroxidation MDA Assay kit (Beyotime
Institute of Biotechnology). To determine the intracellular
malondialdehyde (MDA) levels, the SGNs were lysed with cell lysis
buffer and the total protein content of the lysates was measured
using BCA Protein Assay kit (Beyotime Institute of Biotechnology).
The MDA levels of cell lysis with equal protein contents were
detected according to the manufacturer's instructions and expressed
as µmol/mg protein.
Mitochondrial complexes activity and
adenosine triphosphate (ATP) generation measurement
Twenty-four hours after transfection, cells were
collected and suspended in PBS (pH 7.2). They were then split
through 3 of freeze/thawing cycles. The activity of complex I,
II/III and IV were determined spectrophotometrically using
Mitochondrial complex I, II/III and IV Assay kits, respectively
(Genmed Scientifics Inc., Arlington, MA, USA), according to the
manufacturer's instructions.
In order to determine ATP concentrations, a
colorimetric ATP assay kit (ab83355, Abcam, England) was used. The
cells were lysed according to the manufacturer's instructions and
were placed into a 96-well plate. The absorbance of the reaction
mixture was measured at 550 nm (17).
Mitochondrial membrane potential
The mitochondrial membrane potential (Δψm) was
assessed using MitoProbe™ DiIC1 (5) according to the MitoProbe™ DiIC1
(5) Mitochondrial Membrane
Potential Protocol. The cells were analyzed by flow cytometry with
a 633 nm excitation using emission filters appropriate for Alexa
Fluor 633 dye. The percentages of cells with a high DiIC1
fluorescence (>102 units) measured by flow cytometry
indicated the mitochondrial membrane potential level.
Determining of protein contents
The proteins contents of SGNs 24 h after
transfection were determined by western blotting. Immunoblotting
was performed using the following primary antibodies:
Anti-cytochrome complex (Cyt C) (1:2,000), anti-Bcl-2 (1:1,000),
anti-Bax (1:1,000), anti-poly(ADP-ribose) polymerase (PARP)-1,
anti-superoxide dismutase 1 (SOD)-1 (1:1,000), anti-SOD-2,
anti-Caspase-3 (Casp-3) (1:1,000), anti-C I 39 kDa (1:1,000),
anti-C I 20 kDa (1:1,000), anti-C I 17 kDa (1:1,000), anti-C III 49
kDa (1:1,000), anti-C III 47 kDa (1:1,000), anti-C IV 20 kDa
(1:1,000), anti-PAR (1:500), anti-GAPDH (1:8,000), anti-β-actin
(1:10,000), anti-tubulin (1:10,000), anti-Histone H3 (1:1,000) and
anti-COX IV (1:1,000), which were bought from Cell Signaling
Technology, Inc., Danvers, MA, USA.
For nuclear, mitochondrial and cytosolic protein
enrichment, the cells were fractionated into nuclear, mitochondrial
and cytosolic fractions using the Focus SubCell kit (Geno
Technology, Inc., St. Louis, MO, USA). Briefly, the treated cells
were first harvested and washed in ice-cold SubCell Wash Buffer and
then re-suspended in ice cold FOCUS™ SubCell Buffer-I. Following
mechanic lysis and the addition of SubCell Buffer-II, the lysed
cells were centrifuged for 10 min at 700 × g to pellet the nuclei.
The nuclear pellet was further enriched by gradient centrifugation
and used for further experiments. The cytosolic supernatant with
mitochondria was further centrifuged at 12,000 × g for 15 min at
4°C to pellet the mitochondria for protein content detection.
Finally, the cytosolic supernatant was removed and placed into a
fresh tube. The enriched proteins were analyzed by SDS-PAGE and
western blotting. The relative protein expression levels were
calculated by band intensity normalization to internal control, and
COX IV, Tubulin and histone H3 are used as internal control for the
mitochondrial, cytoplasmic and nuclear fractions respectively
(18–20).
Statistical analysis
Quantitative data are expressed as the means ±
standard deviations (mean ± SD). Statistical analysis was performed
using one-way ANOVA followed by Tukey's post-hoc test for multiple
comparisons (using SPSS software). A P<0.05 was considered to
indicate a statistically significant difference.
Results
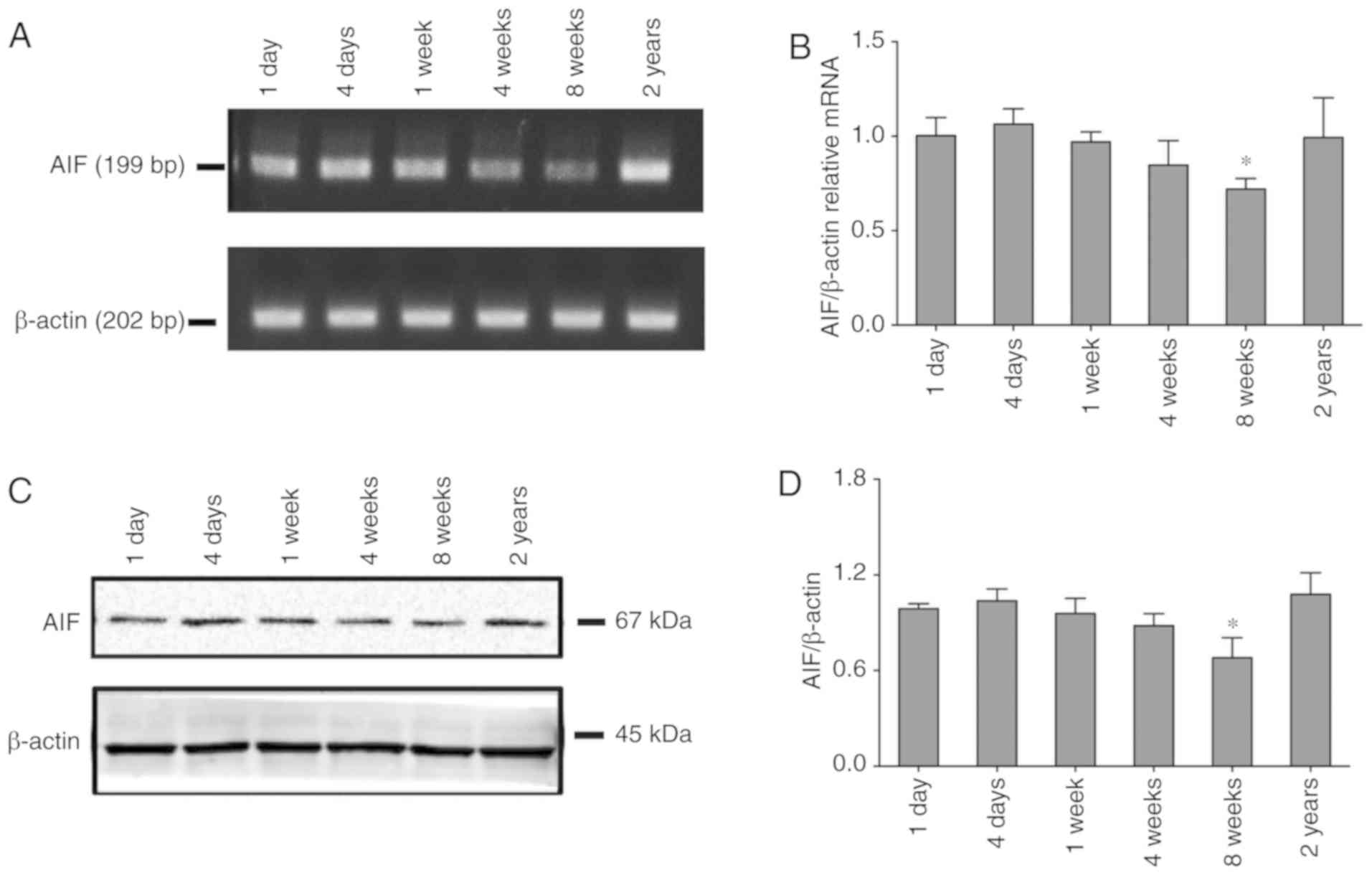
AIF expression decreased during the
stage of growth and development
To characterize the potential role of AIF in the
auditory pathway, we firstly determined the expression levels of
AIF in cochlea spiral tissue from rats at different growth phases.
As shown in Fig. 1A and B, RT-qPCR
results indicated that the mRNA expression levels of AIF decreased
over time during the stage of growth and development. However, the
AIF expression levels in elderly rats were increased to a certain
extent. Western blotting (Fig. 1C and
D) results of AIF protein contents confirmed a similar trend of
the change.
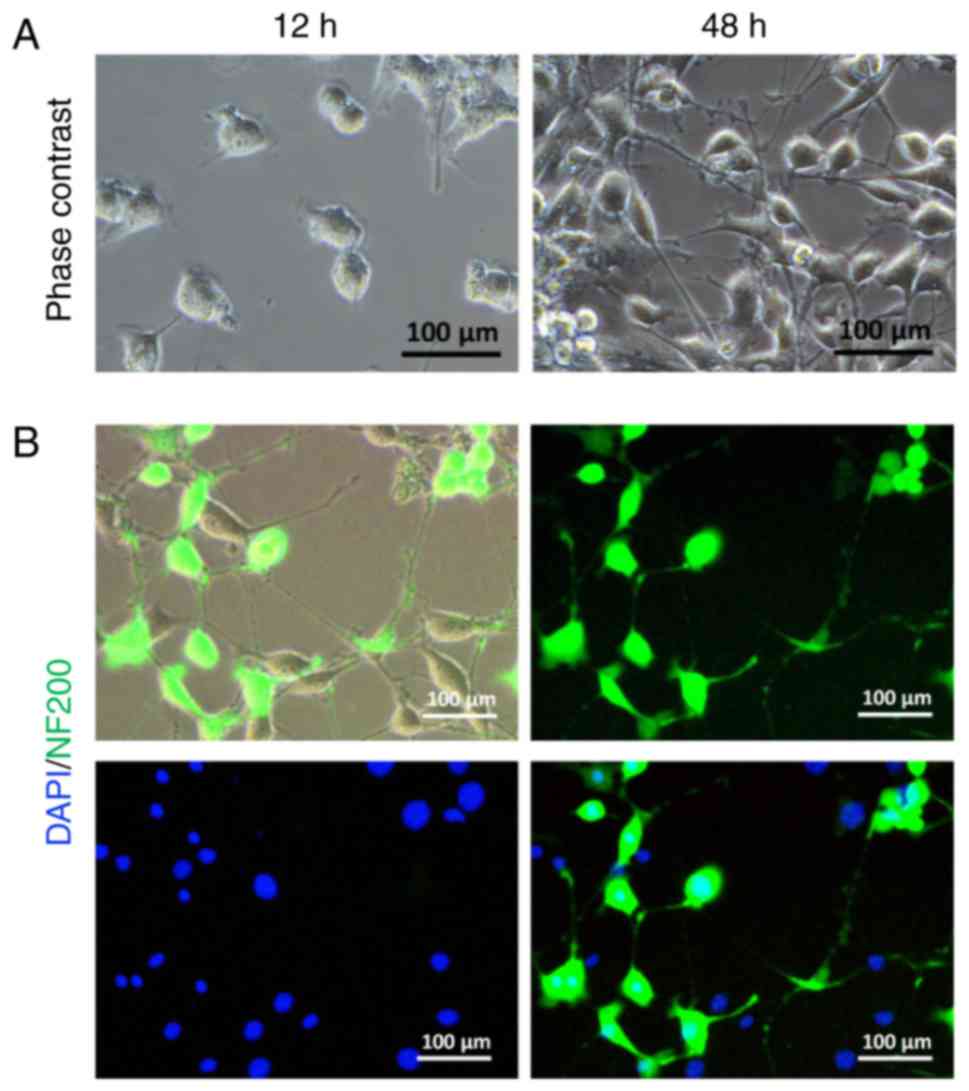
Isolation and identification of
SGNs
Fig. 2A illustrates
an example of cultured SGNs with no obvious neurite after 12 h of
culture in primary growth medium. More neurites emanated from the
cell body after 48 h of culture. Immunofluorescence results
indicated that the surviving SGNs were mostly stained with primary
anti-NF200 antibody (Fig. 2B)
(21).
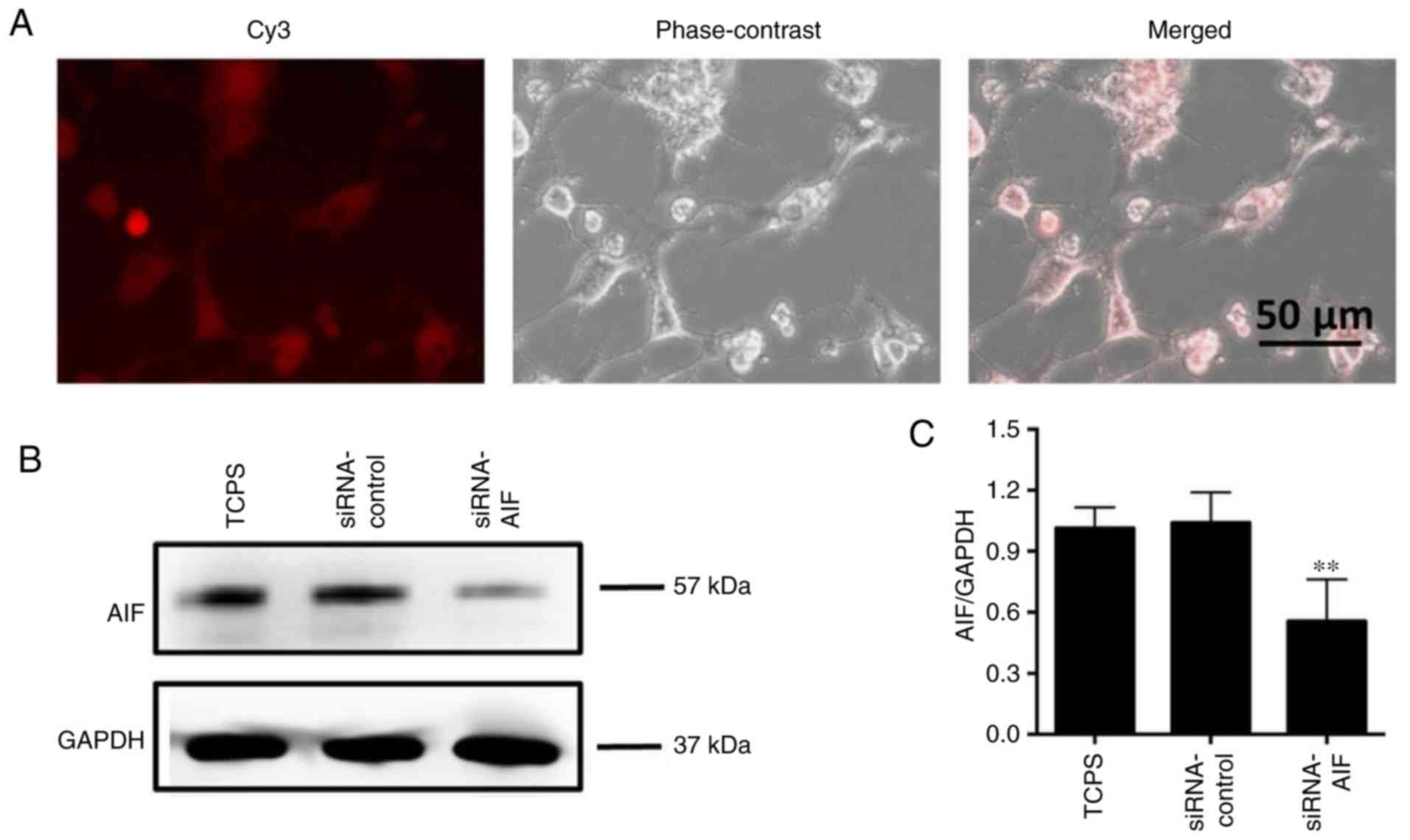
AIF knockdown increases the oxidative
stress status of SGNs
To verify the role of AIF in SGNs apoptosis
execution, AIF siRNA knockdown was performed using siRNA oligos
targeting the coding region of AIF. As shown in Fig. 3A, the siRNAs labeled with Cy3
fluorescent dye were transfected into SGNs at 24 h after treatment.
Compared to normal SGNs or a control siRNA oligo, AIF knockdown
significantly decreased the AIF contents in SGNs (Fig. 3B and C).
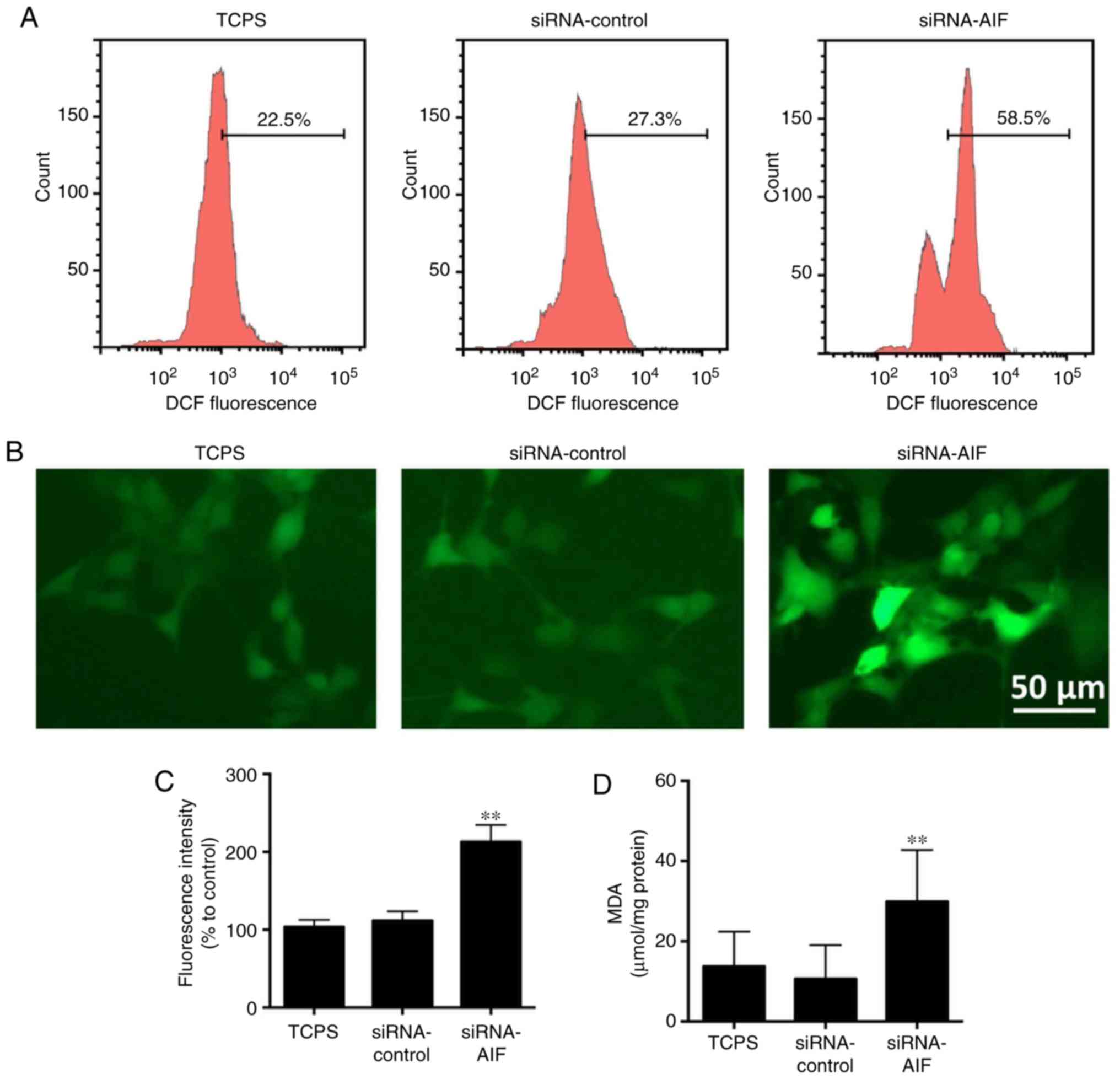
Twenty-four hours after siRNA knockdown, the levels
of reactive oxygen species (ROS) were measured by the cell-permeant
fluorescent indicator 2′,7′-dichlorodihydrofluorescein diacetate
(DCFH-DA). Following treatment with DCFH-DA, intracellular ROS
levels were evaluated by flow cytometry and a fluorescent
microscope (Fig. 4A-C). As
compared to normal SGNs in the TCPS group and control siRNA oligo
in the siRNA-control group, AIF knockdown in SGNs significantly
increased their intracellular ROS levels.
MDA is a mutagenic product of lipid peroxidation,
which is closely associated with intracellular oxidative stress
levels. Generally, cellular MDA levels are low in SGNs, but AIF
knockdown could significantly increase them (Fig. 4D), which also confirmed the high
oxidative stress status in the siRNA-AIF group.
AIF knockdown attenuated mitochondrial
respiration and ATP production
Mitochondrial dysfunction is widely implicated in
cells dysfunction through impaired ATP synthesis. Since
mitochondrial complex I is a major source of mitochondrial ROS
(22), the specific activity of
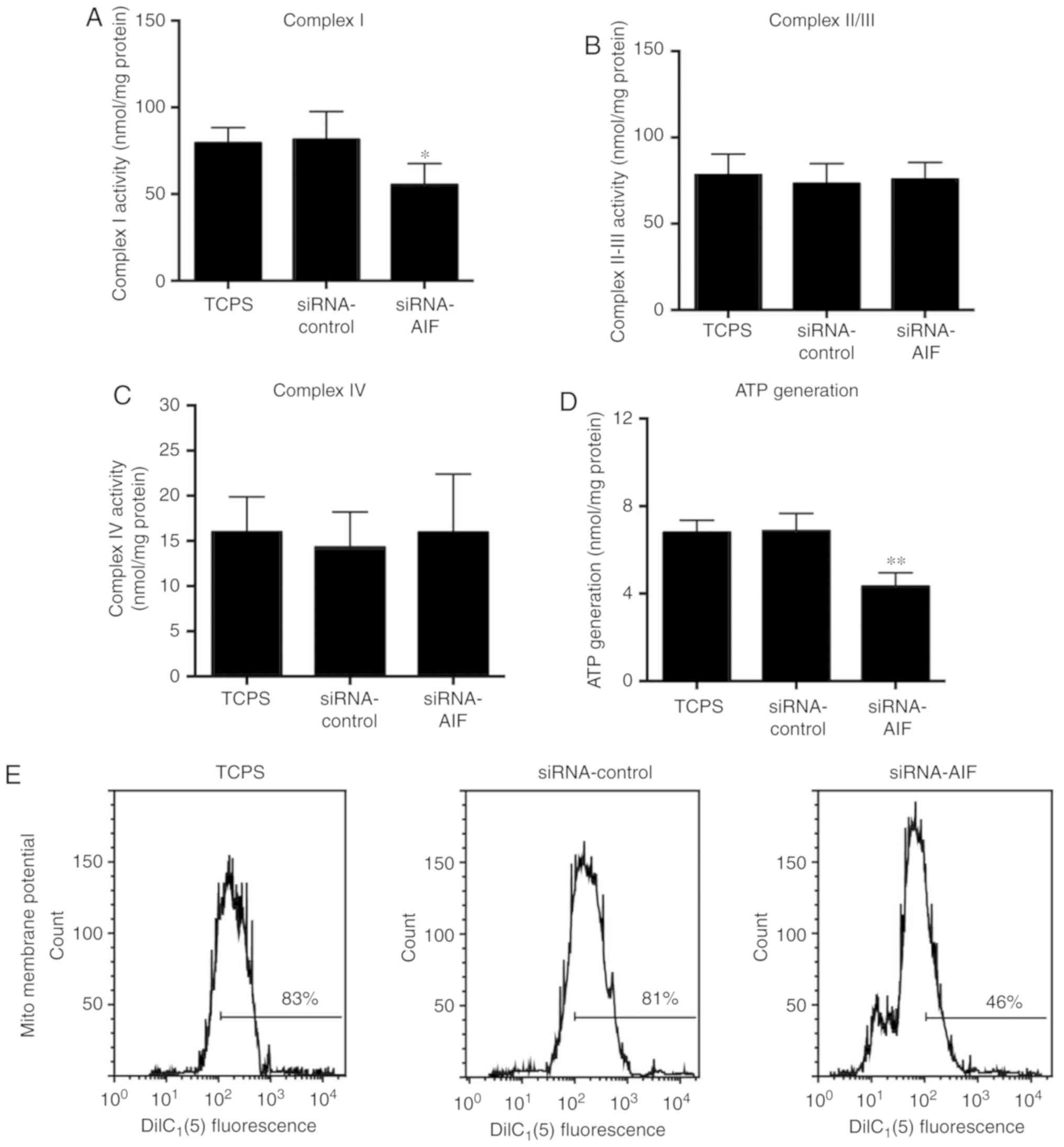
complex I in SGNs was also analyzed. As shown in Fig. 5A and D, complex I activity and ATP
was significantly decreased following AIF knockdown, yet similar
levels of mitochondrial complex II/III (Fig. 5B) and mitochondrial complex IV
(Fig. 5C) were observed between
the three groups. Flow cytometry results (Fig. 5E) of mitochondrial membrane
potential revealed that AIF knockdown markedly decreased the
mitochondrial membrane potential of SGNs.
AIF knockdown impairs SGNs functions
through anti-oxidative and anti-apoptotic bioactivities
To verify whether AIF knockdown had an effect on
anti-oxidative and apoptotic-related factors, western blotting was
performed. As shown in Fig. 6A and
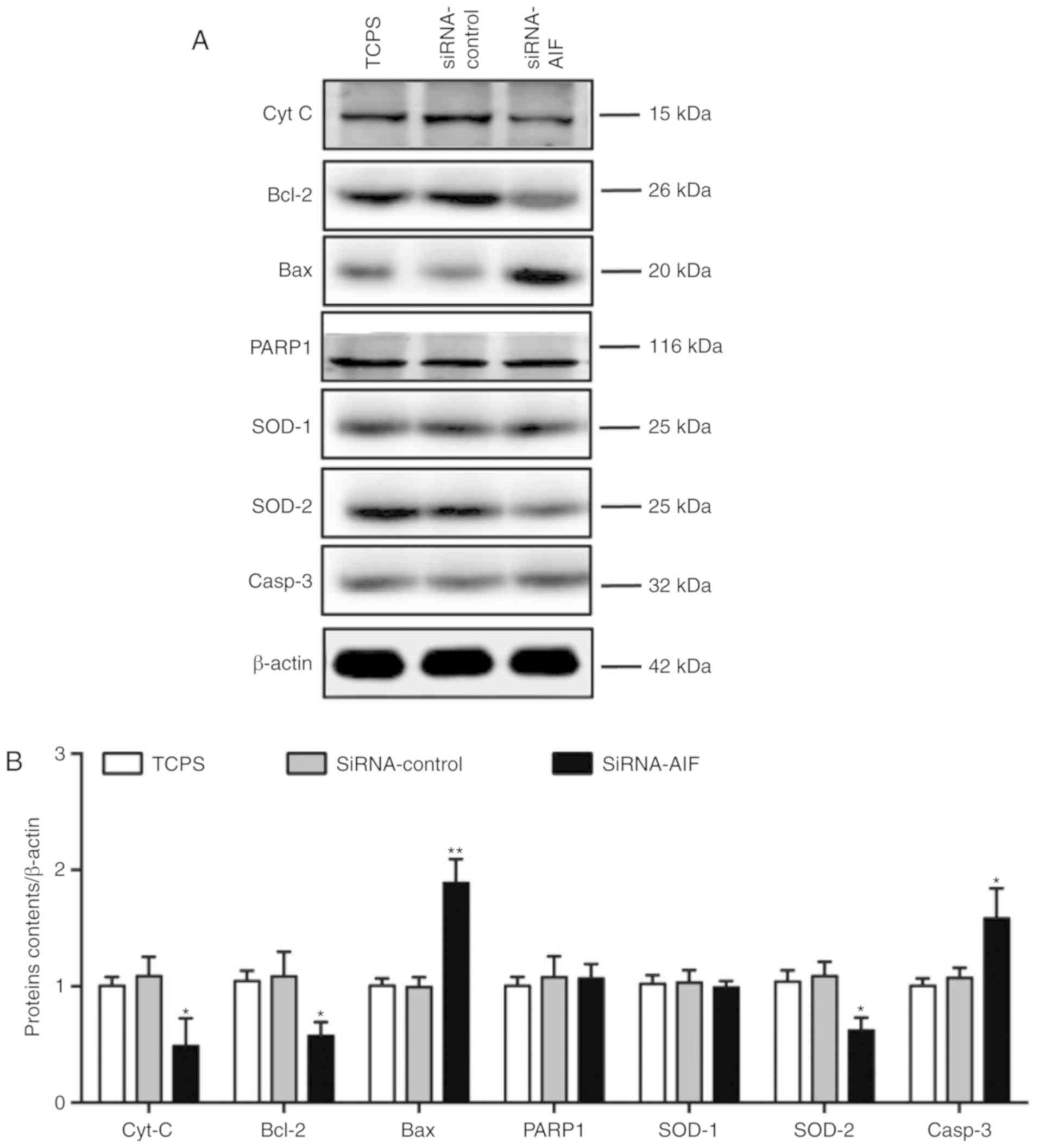
B, AIF knockdown in SGNs resulted in the decrease of
anti-apoptotic factors cytochrome complex (Cyt C) and Bcl-2, and
the increase of pro-apoptotic proteins Bax and Caspase-3 (Casp-3).
Although similar contens of PARP and SOD-1 were observed between
the three groups, anti-oxidative factor SOD-2 in SGNs was decreased
after in SGNs following AIF knockdown. Western blotting analysis
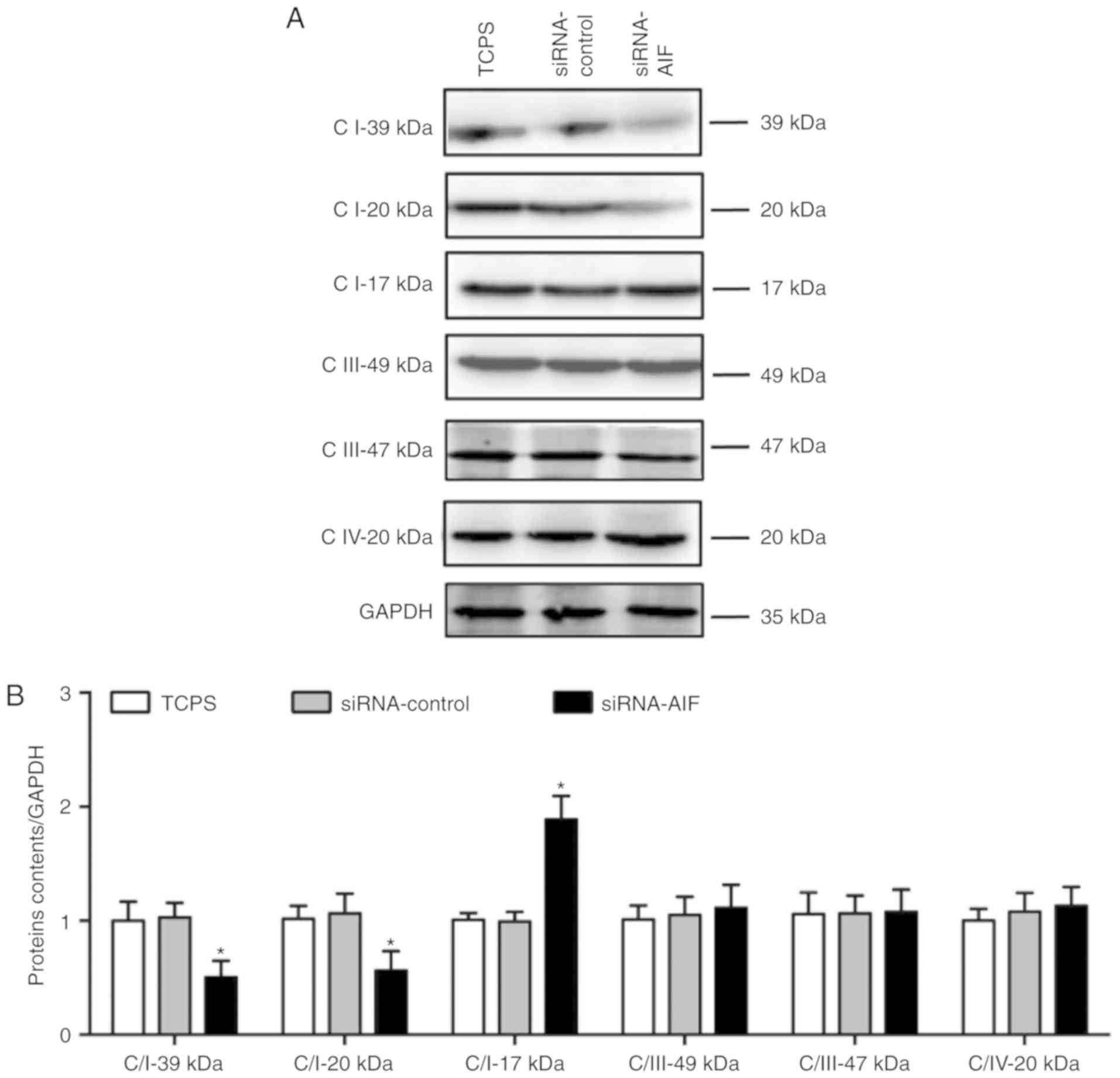
(Fig. 7A and B) of mitochondrial
respiratory complex-related proteins indicated that AIF knockdown
decreased the complex I proteins Complex I 39 kDa and Complex I 20
kDa, but not Complex I 17 kDa, Complex III 49 kDa, Complex III 47
kDa and Complex IV 20 kDa.
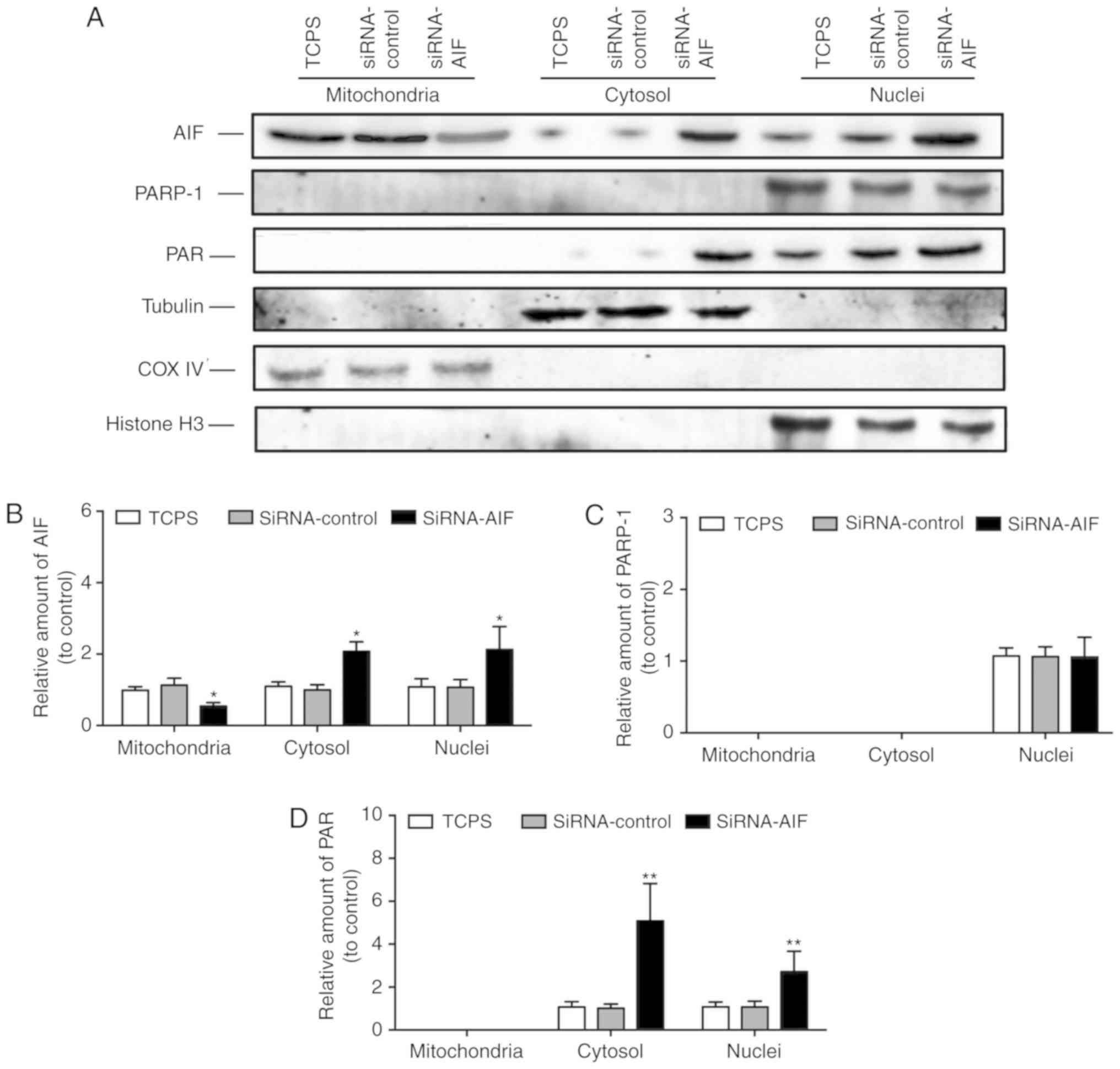
Of note, although AIF knockdown led to a decrease in
the AIF protein content in SGNs, western blotting (Fig. 8A and B) of enriched mitochondrial,
nuclear and cytosolic proteins indicated that mitochondrial
siRNA-AIF was decreased, while nuclear and cytosolic siRNA-AIF were
observed with the increase in AIF protein. Similar levels of PARP-1
expression was observed in the nuclei of the three groups (Fig. 8A and C). However, the cytosolic and
nuclear poly(ADP-ribose) (PAR) polymer levels were increased
following AIF knockdown (Fig. 8A and
D).
Discussion
An improved understanding of the underlying
mechanisms of ANSD is essential for the development of new
prevention and treatment mechanisms. Previously studies have
suggested that the impaired cellular functions of SGNs in the
cochlea play vital roles in the progression of hearing loss, but
the potential mechanism involved remains largely unclear. The
pathological factors inducing programmed cell death (apoptosis) may
be the potential mechanism underlying SGNs dysfunction and cellular
injury. The results the present study revealed new regulatory
effects of the AIF protein on SGNs dysfunctions in vitro.
The knockdown of the AIF expressions with siRNA resulted in high
levels of oxidative stress status, impaired mitochondrial
respiration activity and membrane potential in SGNs. Western
blotting analysis further indicated that AIF knockdown could
decrease the expression levels of anti-apoptotic and anti-oxidative
executors, as well as mitochondrial respiratory chain Complex I
proteins.
AIF is an NAD(P)H oxidase with oxidoreductase
activity. AIF was first identified as a flavoprotein and acts as a
mitochondrial effector of apoptosis. Studies have indicated that
AIF plays various roles in apoptosis, electron transport, and
ferredoxin metabolism, etc. Furthermore, the silencing of the AIF
gene was found to increase ROS generation in the mitochondrion
(15). ROS overproduction induced
high level oxidative stress status, which plays pivotal roles in
various pathologies, especially the apoptosis of impaired cells. In
the present study, we firstly determined the expression levels of
AIF in cochlea spiral tissue from rats at the different growth
phase to primary characterize the potential role of AIF in auditory
pathway. The results indicated the decreased AIF expression levels
of rats as it grows older (during the growth and development
stage). Thus, we further investigated the potential role of AIF
knockdown in cochlear spiral ganglion neurons (SNGs) cellular
functions. As a result, AIF knockdown resulted in a significant
overproduction of ROS, as proven by DCFH-DA staining, and high
levels of lipid peroxidation, as measured by MDA. Western blotting
results showed that anti-apoptotic executors Cyt C and Bcl-2, and
anti-oxidative executor SOD-2, were decreased in SGNs by AIF
knockdown, while pro- apoptotic protein Bax and Casp-3 were
increased. The high oxidative stress status could potentially lead
to injury and apoptosis in SGNs, which may result in dysfunctions
of SGNs.
Normally, the flavoprotein AIF is contained in the
mitochondrial intermembrane space or inner mitochondrial membrane
(23). Upon the induction of
apoptosis, AIF translocates from the mitochondria to the cytosol,
and rapidly relocalizes to the nuclear compartment, where it can
mediate the fragmentation of large-scale DNA (24). Although AIF is not a component of
complex I, AIF-deficiency has been proven to reduce complex I
content, indicating its important roles in the biogenesis and/or
maintenance of mitochondrial respiratory chain complexes function
(25). The present results
indicated that AIF knockdown decreased the complex I activity, ATP
generation and mitochondrial membrane potential in SGNs. These
results suggested that AIF knockdown may induce the mitochondrial
dysfunction.
Of note, although it remains unknown what it is that
can regulate the release of AIF, in the present study, the data
suggested that, even though AIF knockdown resulted in a decrease of
the total AIF protein in SGNs, the release and translocation of AIF
from the mitochondria resulted in high levels of nuclear and
cytosolic AIF. As a result, although the contents of PARP-1 in the
nuclei were similar among the three groups, the PAR polymer levels,
a product of activated PARP-1, were increased in the AIF-knockdown
group (26,27). However, it remains unknown how AIF
knockdown increase the levels of PAR, which conversely leads to AIF
release.
In addition, although the expression levels of AIF
in cochlea spiral tissue were found to be decreased over time
during the stage of growth and development, they were shown to be
increased, to a certain extent, in the elderly. The variation of
AIF expression levels might be closely associated with the
complicated regulatory roles of AIF expression at different
physiological phases, be that its vital mitochondrial role in
healthy cells or its lethal activity in aging-related cell
dysfunctions (28).
As is well known, the X-linked AIFM1 gene encodes
mitochondrial apoptosis inducing factor (AIF) is an FAD-containing
and NADH oxidase and critically important for energy metabolism
(29,30). Many studies indicated that AIF may
regulate mitochondrial function by participating in the assembly
and/or stabilization of the respiratory complexes and contributed
to the normal cellular functions. However, similar as other
mitochondrial factors, the AIF can also be released from
mitochondria during apoptosis, and then migrates to the nucleus,
inducing chromatin condensation and DNA fragmentation by unknown
molecular mechanism. As a result, the AIF, as seen in cytochrome c,
may also has a bifunctional protein with dissociable apoptogenic
and redox properties (31). Thus,
we speculated that the AIF might play certain positive roles during
the early growth and development stage. However, over time, the
potential positive roles of AIF might gradually reduce, and the
expression levels of AIF were decreased by unknown molecular
mechanism. The decrease of AIF expression levels might lead to the
AIF deficiency. The AIF further compromises oxidative
phosphorylation and contributed to the dysfunction and apoptosis of
targeted cells. But, as the rats get older, AIF might play much
more important role in the apoptosis inducing, and the increase of
some apoptotic factors might enhance the expression of AIF, yet
with negative control results of AIF. We thought it will be
important to investigate different potential roles of AIF during
development and aging, in particular, to what extent and through
which exact mechanisms contributes to regulation the expression
levels of AIF as well as its exactly regulation roles. What's more,
the new experiment methods, such as site-specific gene delivery
system, new CRISPR/Cas 9 Technology or site injection of
AIF-activating or AIF-inhibiting molecules could be used for AIF
gene regulation (knockdown or overexpression) study as well as its
in vivo potential regulation effects.
In conclusion, the present data revealed new
regulatory effects of the AIF protein on SGNs dysfunctions in
vitro. It was shown that AIF knockdown with siRNA resulted in
high levels of oxidative stress status, impaired mitochondrial
respiration activity and membrane potential in SGNs. Western
blotting analysis further indicated that AIF knockdown could
decrease the expression levels of anti-apoptotic and anti-oxidative
executors, as well as mitochondrial respiratory chain Complex I
proteins. This study provided a molecular basis for the development
of new prevention and therapeutic strategies for ANSD.
Acknowledgements
Not applicable.
Funding
This study was financially supported by the National
Natural Science Foundation of China (grant no. 81600795) awarded to
LZ. The funders played no role in the study design, data collection
and analysis, decision to publish, or preparation of the
manuscript.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are not publicly available due to the institutional
rules of the PLA General Hospital but are available from the
corresponding author on reasonable request.
Authors' contributions
LZ and JZ performed the experiments and drafted the
manuscript. WW designed the experiments. JW and DH conducted
statistical analysis of the data. ML conceived the study, and
participated in its design and coordination.
Ethics approval and consent to
participate
All animal procedures performed on SPF SD rats were
approved by the local Institutional Animal Care and Use Committee
of the Chinese PLA General Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kaga K: Auditory nerve disease and
auditory neuropathy spectrum disorders. Auris Nasus Larynx.
43:10–20. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Manchaiah VK, Zhao F, Danesh AA and Duprey
R: The genetic basis of auditory neuropathy spectrum disorder
(ANSD). Int J Pediatr Otorhinolaryngol. 75:151–158. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gökdoğan Ç, Altınyay Ş, Gündüz B,
Kemaloğlu YK, Bayazıt Y and Uygur K: Management of children with
auditory neuropathy spectrum disorder (ANSD). Braz J
Otorhinolaryngol. 82:493–499. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moser T and Starr A: Auditory
neuropathy-neural and synaptic mechanisms. Nat Rev Neurol.
12:135–149. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pan H, Song Q, Huang Y and Wang J, Chai R,
Yin S and Wang J: Auditory neuropathy after damage to cochlear
spiral ganglion neurons in mice resulting from conditional
expression of diphtheria toxin receptors. Sci Rep. 7:64092017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yuste VJ, Lorenzo HK and Susin S A: AIFM1
(Apoptosis-Inducing Factor, Mitochondrion-Associated, 1). Atlas of
Genetics and Cytogenetics in Oncology and Haematology. 190–194.
2008.
|
|
7
|
Bano D and Prehn JHM: Apoptosis-inducing
factor (AIF) in physiology and disease: The tale of a repented
natural born killer. EBioMedicine. 30:29–37. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Joza N, Oudit GY, Brown D, Bénit P,
Kassiri Z, Vahsen N, Benoit L, Patel MM, Nowikovsky K, Vassault A,
et al: Muscle-specific loss of apoptosis-inducing factor leads to
mitochondrial dysfunction, skeletal muscle atrophy, and dilated
cardiomyopathy. Mol Cell Biol. 25:10261–10272. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang B, Li X, Wang J, Liu L, Xie Y, Huang
S, Pakhrin PS, Jin Q, Zhu C, Tang B, et al: A Novel AIFM1 Mutation
in a Chinese Family with X-linked Charcot-Marie-Tooth disease type
4. Neuromuscul Disord. 28:652–659. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zong L, Guan J, Ealy M, Zhang Q, Wang D,
Wang H, Zhao Y, Shen Z, Campbell CA, Wang F, et al: Mutations in
apoptosis-inducing factor cause X-linked recessive auditory
neuropathy spectrum disorder. J Med Genet. 52:523–531. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brors D, Bodmer D, Pak K, Aletsee C,
Schäfers M, Dazert S and Ryan AF: EphA4 provides repulsive signals
to developing cochlear ganglion neurites mediated through ephrin-B2
and -B3. J Comp Neurol. 462:90–100. 2010. View Article : Google Scholar
|
|
12
|
Aletsee C, Beros A, Mullen L, Palacios S,
Pak K, Dazert S and Ryan AF: Ras/MEK but not p38 signaling mediates
NT-3-induced neurite extension from spiral ganglion neurons. J
Assoc Res Otolaryngol. 2:377–387. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Szabó Z, Harasztosi C, Szûcs G, Sziklai I
and Rusznák Z: A detailed procedure and dissection guide for the
isolation of spiral ganglion cells of the guinea pig for
electrophysiological experiments. Brain Res Brain Res Protoc.
10:139–147. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu W, Fan Z, Han Y, Zhang D, Li J and
Wang H: Intranuclear localization of apoptosis-inducing factor and
endonuclease G involves in peroxynitrite-induced apoptosis of
spiral ganglion neurons. Neurol Res. 34:915–922. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Apostolova N, Cervera AM, Victor VM,
Cadenas S, Sanjuan-Pla A, Alvarez-Barrientos A, Esplugues JV and
McCreath KJ: Loss of apoptosis-inducing factor leads to an increase
in reactive oxygen species, and an impairment of respiration that
can be reversed by antioxidants. Cell Death Differ. 13:354–357.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jin Y, Kondo K, Ushio M, Kaga K, Ryan AF
and Yamasoba T: Developmental changes in the responsiveness of rat
spiral ganglion neurons to neurotrophic factors in dissociated
culture: Differential responses for survival, neuritogenesis and
neuronal morphology. Cell Tissue Res. 351:15–27. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mendoza EE, Pocceschi MG, Kong X, Leeper
DB, Caro J, Limesand KH and Burd R: Control of glycolytic flux by
AMP-activated protein kinase in tumor cells adapted to low pH 1.
Transl Oncol. 5:208–216. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Teng YD, Choi H, Onario RC, Zhu S,
Desilets FC, Lan S, Woodard EJ, Snyder EY, Eichler ME and
Friedlander RM: Minocycline inhibits contusion-triggered
mitochondrial cytochrome c release and mitigates functional
deficits after spinal cord injury. Proc Natl Acad Sci USA.
101:3071–3076. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Choi GE, Oh JY, Lee HJ, Chae CW, Kim JS,
Jung YH and Han HJ: Glucocorticoid-mediated ER-mitochondria
contacts reduce AMPA receptor and mitochondria trafficking into
cell terminus via microtubule destabilization. Cell Death Dis.
9:11372018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu H, Zhang H, Wu X, Ma D, Wu J, Wang L,
Jiang Y, Fei Y, Zhu C, Tan R, et al: Nuclear cGAS suppresses DNA
repair and promotes tumorigenesis. Nature. 563:131–136. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Whitlon DS, Grover M, Tristano J, Williams
T and Coulson MT: Culture conditions determine the prevalence of
bipolar and monopolar neurons in cultures of dissociated spiral
ganglion. Neuroscience. 146:833–840. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Murphy MP: How mitochondria produce
reactive oxygen species. Biochem J. 417:1–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Otera H, Ohsakaya S, Nagaura Z, Ishihara N
and Mihara K: Export of mitochondrial AIF in response to
proapoptotic stimuli depends on processing at the intermembrane
space. EMBO J. 24:1375–1386. 2014. View Article : Google Scholar
|
|
24
|
Vandenabeele P, Galluzzi L, Vanden Berghe
T and Kroemer G: Molecular mechanisms of necroptosis: An ordered
cellular explosion. Nat Rev Mol Cell Biol. 11:700–714. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vahsen N, Candé C, Brière JJ, Bénit P,
Joza N, Larochette N, Mastroberardino PG, Pequignot MO, Casares N,
Lazar V, et al: AIF deficiency compromises oxidative
phosphorylation. EMBO J. 23:4679–4689. 2014. View Article : Google Scholar
|
|
26
|
Andrabi SA, Kim NS, Yu SW, Wang H, Koh DW,
Sasaki M, Klaus JA, Otsuka T, Zhang Z, Koehler RC, et al:
Poly(ADP-ribose) (PAR) polymer is a death signal. Proc Natl Acad
Sci USA. 103:18308–18313. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu SW, Andrabi SA, Wang H, Kim NS, Poirier
GG, Dawson TM and Dawson VL: Apoptosis-inducing factor mediates
poly(ADP-ribose) (PAR) polymer-induced cell death. Proc Natl Acad
Sci USA. 103:18314–18319. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hangen E, Blomgren K, Bénit P, Kroemer G
and Modjtahedi N: Life with or without AIF. Trends Biochem Sci.
35:278–287. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Susin SA, Lorenzo HK, Zamzami N, Marzo I,
Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler
M, et al: Molecular characterization of mitochondrial
apoptosis-inducing factor. Nature. 397:441–446. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Miramar MD, Costantini P, Ravagnan L,
Saraiva LM, Haouzi D, Brothers G, Penninger JM, Peleato ML, Kroemer
G and Susin SA: NADH oxidase activity of mitochondrial
apoptosis-inducing factor. J Biol Chem. 276:16391–16398. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Maté MJ, Ortiz-Lombardía M, Boitel B,
Haouz A, Tello D, Susin SA, Penninger J, Kroemer G and Alzari PM:
The crystal structure of the mouse apoptosis-inducing factor AIF.
Nat Struct Biol. 9:442–446. 2002. View
Article : Google Scholar : PubMed/NCBI
|