Introduction
Normal pregnancy involves a special type of
alloimmune tolerance. The fetus, a semi-allograft, escapes from
maternal immune attack, survives and develops until delivery; a
process that is dependent on maternal immune tolerance (1). Abortion results from the immune
rejection of the natural implant by the mother. Normal
physiological pregnancy is similar to allotransplantation; as a
natural allograft, the embryo is not immune to maternal rejection,
but may be the only exception to immune rejection, which reflects
maternal immune tolerance to the embryo. However, pregnancy failure
is primarily caused by maternal immune rejection of the embryo
(1). During successful pregnancy,
maternal decidual cells and fetal trophoblasts can produce various
chemokines, such as CC chemokine ligand 4 and cytokines, including
interleukin (IL)-4/5, which contribute to a unique maternal-fetal
immune environment that prevents fetal alloantigens from inducing
maternal immune attack (2).
Spontaneous abortion occurs before 28 weeks of
gestation, when the embryo or fetus is <1,000 g in weight.
Spontaneous abortions account for 0.4–0.8% of all abortions in
women of childbearing age and account for 10–15% of the total
number of abortions. Early abortions account for the remaining 80%
of total abortions (2). Fetal loss
caused by maternal immune attack has been intensively studied for
years (3). However, the mechanism
of spontaneous abortion is complicated and is not completely
understood.
Programmed cell death 1 (PD-1), also named PDCD1 and
CD279, is a type I transmembrane protein consisting of 288 amino
acid residues that belongs to the B7-CD28 receptor superfamily
(4). PD-1 is expressed on the
surface of bone marrow cells, dendritic cells (DCs), natural killer
cells (NKs), monocytes, CD4-CD8-thymus cells, regulatory T cells, B
cells and antigen-presenting cells (5). The PD-1 gene was identified in
a study conducted by Ishida et al (4) in 1992 aiming to identify the gene
that induces programmed cell death. In 1998, Nishimura et al
(6) reported that mice lacking the
PD-1 gene developed lupoid autoimmune disease, and the
negative immune regulatory function of PD-1 was not present.
Subsequently, the two PD-1 ligands, programmed death-ligand (PD-L)1
and PDL-2, were discovered (7–9).
PD-1 is an inhibitory immunoreceptor that is expressed on the
surface of T cells under certain conditions (10). PD-L1 has a wide tissue expression
profile and is expressed in certain malignant tumor cell lines,
such as ovarian cancer and head and neck squamous cell carcinoma,
which may be related to the tumor immune escape mechanism (11–13).
A number of studies have reported that the PD-1/PD-L signaling
pathways play a role in the negative regulation of the immune
response (14,15). Previous studies have reported that
PD-L1 immunoglobulin (Ig)-modified bone marrow-derived stem cells
(BMSCs) inhibit rat liver transplant rejection and induce liver
transplantation immune tolerance, and they display an improved
effect compared with BMSCs alone (16–18).
However, further investigation into the role of PD-L1 Ig during
spontaneous abortion is required.
The CD40 ligand (CD40L), also known as CD154, is a
member of the tumor necrosis factor superfamily (19). CD40L is mainly expressed on the
surfaces of activated CD4+ T cells, providing
synergistic stimulation signals necessary for the activation of T
and B cells. CD40L is also expressed on the surface of
CD8+ T cells, B cells, macrophages and dendritic cells,
as well as on the surface of non-immune cells, including
endothelial cells and activated platelets (20). Larsen et al (21) reported that blocking the CD40-CD40L
signaling pathway with an anti-CD40L monoclonal antibody (mAb)
could prevent acute rejection and the production of self-reactive
antibodies in a mouse heart transplantation model. Furthermore,
Coenen et al (22)
suggested that anti-CD40L mAbs could induce the proliferation of
CD4+CD25+ T cells in vitro. Blocking
the CD40-CD40L signaling pathway can block the activation of
CD4+ T cells directly, or indirectly by blocking the
activation and maturation of B cells and the production of
alloantigens, reducing the risk of rejection (21,22).
However, a limited number of studies have examined the role of
anti-CD40L mAbs during spontaneous abortion (21–25).
Therefore, the present study aimed to investigate the effects of
PD-L1 Ig and anti-CD40L mAbs on spontaneous abortion in a CBA/J ×
DBA/2 abortion-prone mouse model.
Materials and methods
Animals and groups
A total of 50 female CBA/J, 20 male DBA/2 and 5 male
BALB/c mice (age, 8–10 weeks; weight, 12–15 g) were purchased from
Beijing Vital River Laboratory Animal Technology Co., Ltd. The mice
were maintained under controlled conditions at 19–23°C with 12-h
light/dark cycles and 40–60% humidity, with free access to drinking
water and food. Animal experiments were approved by the
Institutional Animal Care and Use Committee of Kunming Medical
University.
The mice were divided into the following five
groups: the normal group (10 CBA/J mice), the spontaneous abortion
group (10 CBA/J mice), the PD-L1 Ig group (0.1 mg/kg PD-L1 Ig; 10
CBA/J mice), the CD40L mAb group (0.2 mg/kg anti-CD40L mAbs; 10
CBA/J mice) and the PD-L1 Ig + CD40L mAb group (0.1 mg/kg PD-L1 Ig
and 0.2 mg/kg anti-CD40L mAbs; 10 CBA/J mice). The normal group was
mated with male BALB/c mice (n=5). The spontaneous abortion, PD-L1
Ig, CD40L mAb and PD-L1 Ig + CD40 mAb groups were mated with male
DBA/J mice (n=5/group). The day the vaginal plug was observed was
recorded as day 0 of gestation. PD-L1 Ig and/or anti-CD40L mAbs
were injected intraperitoneally into CBA/J female mice at days 4,
6, 8, 10 and 12 of gestation. At day 14 of gestation, the CBA/J
mice were euthanized with an overdose of sodium pentobarbital (150
mg/kg; intraperitoneally). PD-L1 Ig and anti-CD40L mAbs were
purchased from R&D Systems.
Analysis of the embryo absorption
rate
Pregnant CBA/J female mice in each group were
euthanized on day 14 of gestation, and the number of absorbed
embryos and surviving embryos were counted. The embryo resorption
rate was calculated according to the following formula: resorption
rate (%)=the number of absorbed embryos/(the number of absorbed
embryos + the number of viable embryos) ×100.
Isolation of splenocytes
At day 14 of gestation, the spleens of five pregnant
CBA/J mice and five paternal mice from each group were aseptically
removed and mechanically teased out of the stroma in PBS. The cell
suspensions were filtered through a 100-mm pore size nylon mesh and
centrifuged at 1,000 × g for 10 min at 4°C. Subsequently, the
supernatant was removed. Following the addition of Lymphocyte
Isolation Fluid (Beijing Solarbio Science & Technology Co.,
Ltd.), the spleen cells were centrifuged at 800 × g for 30 min at
4°C. After centrifugation, the splenic mononuclear cells were
carefully isolated and washed twice with a 3-fold volume of PBS.
The cells were counted and the cell concentration was adjusted to
1×107 cells/ml with PBS.
Isolation of bone marrow-derived
dendritic cells (BMDCs)
At day 14 of gestation, BMDCs were generated from
five pregnant CBA/J mice from each group. Briefly, femora and
tibiae were removed from CBA/J mice and were mechanically isolated
from the surrounding tissues. The samples were centrifuged at 1,000
× g for 10 min at room temperature and the supernatant was
discarded. Subsequently, 5 m Tris-NH4Cl solution was
added to the cells and the samples were incubated at room
temperature for 2 min to fully lyse the red blood cells. The
samples were centrifuged at 1,000 × g for 5 min at room
temperature, the supernatant was discarded and 5 ml PBS was added
to resuspend the cells. The samples were centrifuged at 1,000 × g
for 5 min at room temperature, the supernatant was discarded and
the samples were washed three times with PBS. RPMI-1640 complete
medium (Logan; GE Healthcare Life Sciences), supplemented with 10%
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.), was
used to adjust the cell concentration to 2×106 cells/ml.
The cells were incubated in a culture dish at 37°C overnight.
Subsequently, the unadhered cells and culture medium were discarded
and the adherent cells were washed twice with PBS. Fresh culture
medium was added to the adherent cells and changed every other day
by removing and replacing half of the volume; the adherent cells
were used for subsequent experimentation.
Flow cytometry
The expression of cell surface molecules was
evaluated using a Sysmex Partec CyFlow® space flow
cytometer (Sysmex Partec GmbH) and FloMax version 2.8 software
(Sysmex Partec GmbH). Cells were fixed with 70% ethanol at 4°C for
2 h and blocked with 2% BSA (Beijing Solarbio Science &
Technology Co., Ltd.) at 4°C for 30 min. The splenic cells were
labeled with both anti-mouse CD25 FITC (cat. no. BG-07312-50-100;
BioGems) and anti-mouse CD4 PE (cat. no. 85-12-0041-81;
eBioscience; Thermo Fisher Scientific, Inc.) in the dark at 4°C for
30 min. The BMDCs were labeled with anti-mouse MHC-II FITC (cat.
no. 85-11-5321-81; eBioscience; Thermo Fisher Scientific, Inc.),
anti-mouse CD80 FITC (cat. no. 85-11-0801-81; eBioscience; Thermo
Fisher Scientific, Inc.) and anti-mouse CD86 FITC (cat. no.
85-11-0862-81; eBioscience; Thermo Fisher Scientific, Inc.) in the
dark at 4°C for 30 min. Subsequently, the cells were centrifuged at
800 × g at 4°C for 5 min and the supernatant was removed. The cells
were washed twice with PBS and resuspended in PBS for flow
cytometry analysis. The control cells were stained with the
corresponding isotype-matched antibody for the same duration and
temperature as the other cells. The isotype-matched antibodies for
CD25, CD4, CD80, CD86 and MHC-II were Rat IgG2a κ Isotype control
(eBR2a)-FITC (cat. no. 11-4321-80; eBioscience; Thermo Fisher
Scientific, Inc.), Rat IgG2b κ Isotype control (eB149/10H5)-PE
(cat. no. 12-4031-82; eBioscience; Thermo Fisher Scientific, Inc.),
Armenian Hamster IgG Isotype control (eBio299Arm)-FITC (cat. no.
11-4888-81; eBioscience; Thermo Fisher Scientific, Inc.), Rat IgG2a
κ Isotype control (eBR2a)-FITC and Rat IgG2b κ Isotype control
(eB149/10H5)-FITC, respectively. Cells were analyzed using a flow
cytometer. The flow cytometry results are presented as the
percentage of cells positive for the surface marker evaluated. The
experiment was repeated three times.
Mixed lymphocyte response
Splenocytes from five pregnant CBA/J mice from each
group on day 14 of gestation were used as responder cells, and
paternal splenocytes were used as stimulator cells. Firstly, 100 µl
responder cells (2×105 cells/well) and 100 µl mitomycin
C (50 µg/ml; Sigma-Aldrich; Merck KGaA)-treated stimulator cells
(2×105 cells/well; stimulator cells were incubated with
mitomycin C at 37°C for 30 min) were aliquoted into 96-well plates.
Responder cells cultured with complete medium alone in 96-well
plates were used as the control. After a 3-day incubation at 37°C,
3H-thymidine (20 µCurie/well) was added to the cells and
incubated for 6 h at 37°C. The cells were harvested onto
glass-fiber paper using a cell harvester, and the count per minute
(cpm) was measured using a liquid scintillation counter. The
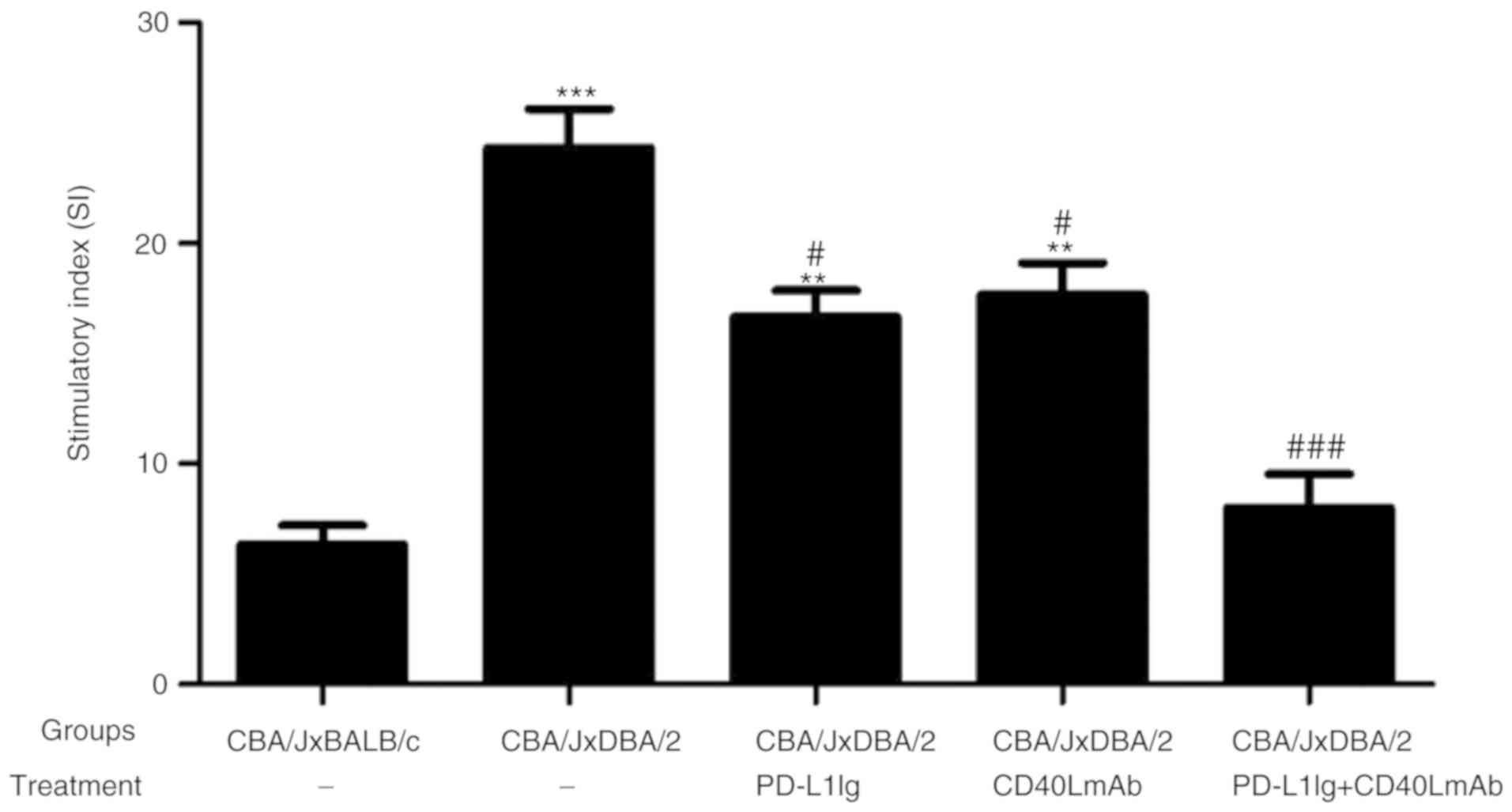
proliferative capacity is presented as the stimulatory index (SI),
calculated according to the following equation: SI=(cpm of
stimulated cultures-cpm of control cultures)/cpm of control
cultures. The experiment was repeated three times.
Western blotting
Total protein was extracted from spleen tissues
isolated from five pregnant CBA/J mice from each group on day 14 of
gestation using RIPA lysis buffer (Beyotime Institute of
Biotechnology). Protein (30 µg/lane) was quantified using a
bicinchoninic acid assay kit (Beyotime Institute of Biotechnology)
and separated by 10% SDS-PAGE and transferred to PVDF membranes.
The membranes were blocked with 10% skim milk at room temperature
for 4 h. Subsequently, the membranes were incubated at 4°C
overnight with the following primary antibodies: Anti-FoxP3 (cat.
no. bs-0269R; 1:1,000; BIOSS), anti-TNF-α (cat. no. A0277; 1:1,000;
ABclonal Biotech Co., Ltd.), anti-IFN-γ (cat. no. bs-0480R;
1:1,000; BIOSS), anti-IL-4 (bs-0581R; 1:1,000; BIOSS) and
anti-β-actin (cat. no. AC026; 1:2,000; ABclonal Biotech Co., Ltd.).
Subsequently, the membranes were incubated with a goat anti-rabbit
IgG horseradish peroxidase-conjugated secondary antibody (1:2,000;
cat. no. bs-0295G-HRP; BIOSS). Protein bands were visualized using
ECL Plus Western Blotting Detection reagents (EMD Millipore). Blots
were performed in triplicate and protein expression was quantified
using ImageJ 2× software (National Institutes of Health) with
β-actin as the loading control.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistical significance was determined by one-way ANOVA followed
by Tukey's post hoc test. All statistical analyses were performed
using GraphPad Prism software (version 5.0a; GraphPad Software,
Inc.). P<0.05 was considered to indicate a statistically
significant difference.
Results
Combined therapy with PD-L1 Ig and
anti-CD40L mAbs reduces the embryo resorption rate of
abortion-prone CBA/J × DBA/2-mated mice
To investigate whether combined treatment with PD-L1
Ig and anti-CD40L mAbs decreases the rate of fetal abortion in
vivo, PD-L1 Ig and/or anti-CD40L mAbs were intraperitoneally
injected into pregnant CBA/J females on days 4, 6, 8, 10, and 12 of
gestation. The embryo resorption rate was determined on day 14 of
gestation. Absorbed embryos (Fig.
1B-D) displayed hemorrhage, ischemia and necrosis, and were
smaller and darker compared with healthy embryos (Fig. 1A and E). Treatment of pregnant
CBA/J females with PD-L1 Ig or anti-CD40L mAbs significantly
reduced the resorption rate compared with the spontaneous abortion
group (Fig. 1F). Combined
treatment with PD-L1 Ig and anti-CD40L mAbs also significantly
reduced the resorption rate compared with the spontaneous abortion
group (Fig. 1F). There was no
significant difference between the normal group and the PD-L1 Ig +
CD40L mAbs group (Fig. 1F). The
results suggested that combined treatment with PD-L1 Ig and
anti-CD40L mAbs was effective in preventing maternal rejection of
the embryo.
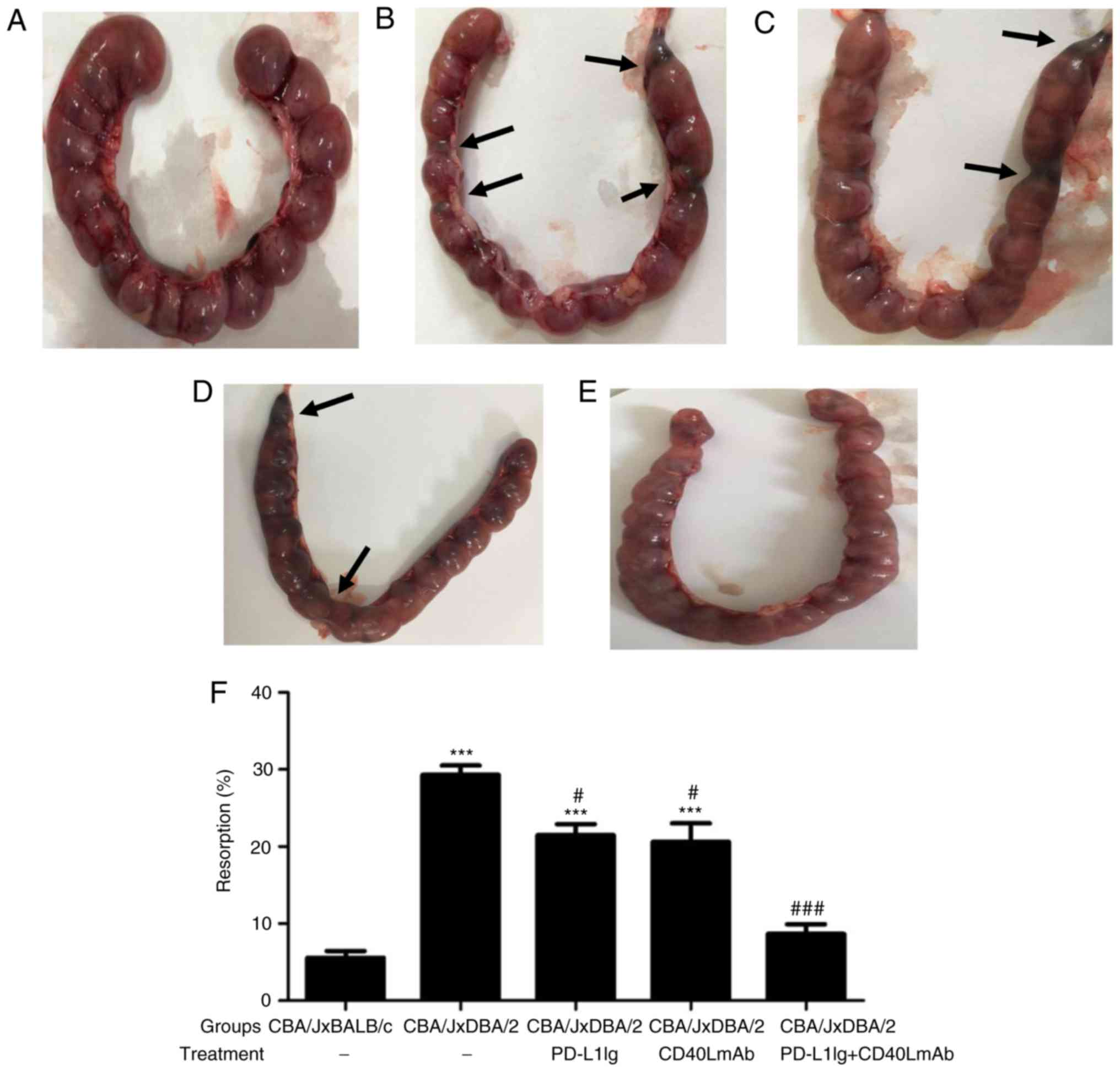 | Figure 1.Combined treatment with PD-L1 Ig and
anti-CD40L mAbs decreases the embryo resorption rate in the CBA/J ×
DBA/2 mating model. Representative images of the number of embryos
per uterus in (A) the normal group, (B) the spontaneous abortion
group, (C) the PD-L1 Ig group, (D) the CD40L group and (E) the
PD-L1 Ig + CD40L group. The black arrows indicate embryos that
displayed hemorrhage, ischemia and necrosis. PD-L1 Ig and/or
anti-CD40L mAbs were injected intraperitoneally into pregnant CBA/J
female mice on days 4, 6, 8 10, and 12 of gestation. (F) Embryo
resorption rates were calculated on day 14 of gestation (n=10).
***P<0.001 vs. the normal group (CBA/J × BALB/c).
#P<0.05 and ###P<0.001 vs. the
spontaneous abortion group (CBA/J × DBA/2). PD-L1, programmed
death-ligand 1; Ig, immunoglobulin; CD40L, CD40 ligand; mAb,
monoclonal antibody. |
Combined treatment with PD-L1 Ig and
anti-CD40L mAbs induces maternal hyporesponsiveness to paternal
antigens in the CBA/J × DBA/2 mating model
To further investigate the inhibitory effects of
PD-L1 Ig and anti-CD40L mAb treatment on the maternal responses to
paternal antigens, a mixed lymphocyte reaction proliferation assay
was performed. Combined treatment with PD-L1 Ig and anti-CD40L mAbs
significantly decreased the proliferation of CBA/J splenocytes in
response to DBA/2 stimulator cells compared with the spontaneous
abortion group. Furthermore, the inhibitory effect of the combined
treatment resulted in lower proliferation of CBA/J splenocytes
compared with the PD-L1 Ig or CD40L mAb group (Fig. 2). The results indicated that
combined treatment with PD-L1 Ig and anti-CD40L mAbs successfully
induced maternal hyporesponsiveness to paternal antigens.
Therefore, the results suggested that combined therapy inhibited
maternal T-cell activation to prevent overactivation of the immune
system in vivo.
Combined treatment with PD-L1 Ig and
anti-CD40L mAbs expands the peripheral
CD4+CD25+ T-cell population in the CBA/J ×
DBA/2 mating model
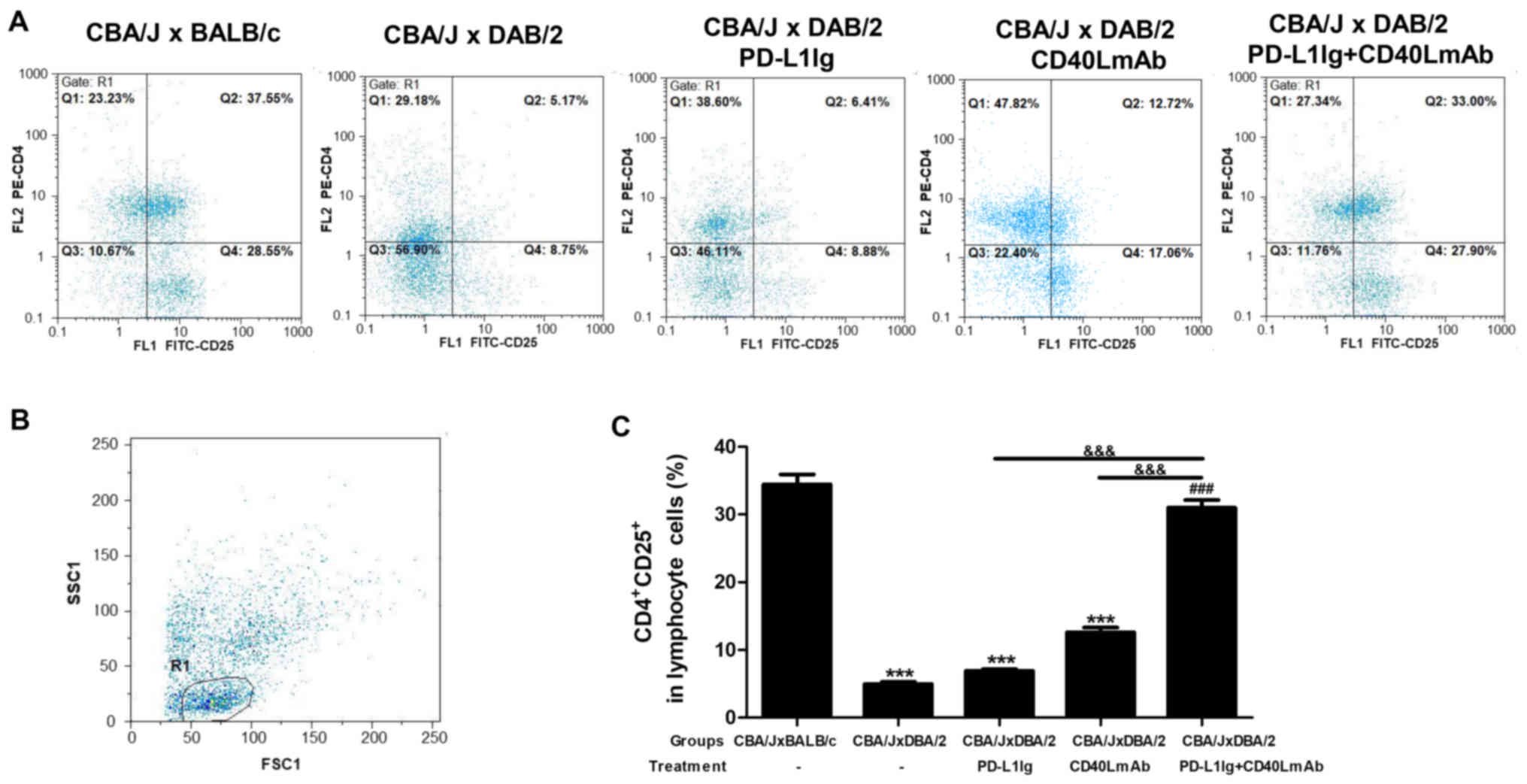
To further investigate the mechanisms involved in
the inhibitory effects of PD-L1 Ig and anti-CD40L mAbs on abortion,
the splenic CD4+CD25+ T-cell population in
pregnant female CBA/J mice was analyzed by flow cytometry (Fig. 3A-C). The spontaneous abortion group
displayed a significant decrease in the percentage of
CD4+CD25+ T cells within the CD4+
T-cell population compared with the normal group (Fig. 3C). The percentage of
CD4+CD25+ T cells within the CD4+
T-cell population in the spleens of the PD-L1 Ig + CD40L mAb group
was significantly increased compared with the spontaneous abortion,
PD-L1 Ig and CD40L mAb groups (Fig.
3C). There was no significant difference between the PD-L1 Ig
or CD40L mAb group, and the normal group (Fig. 3C). The results indicated that
combined treatment with PD-L1 Ig and anti-CD40L mAbs activated the
splenic CD4+CD25+ T cells in the CBA/J ×
DBA/2 mating model.
Combined treatment with PD-L1 Ig and
anti-CD40L mAbs decreases the MHCII+, CD80+
and CD86+ cell populations in BMDCs
To further investigate the mechanisms involved in
the inhibitory effects of PD-L1 Ig and anti-CD40L mAbs on
spontaneous abortion, the MHCII+, CD80+ and
CD86+ cell populations in BMDCs were determined by flow
cytometry. In the spontaneous abortion group, 57.55% of the BMDC
population expressed MHC-II molecules, 62.07% expressed CD80 and
53.73% expressed CD86 (Fig. 4A-C).
The percentage of MHCII+, CD80+ and
CD86+ cells in the spontaneous abortion group was
significantly increased compared with the normal group (Fig. 4C). The PD-L1 Ig, CD40L mAb and
PD-L1 Ig + CD40L mAb groups displayed a significant decrease in the
percentages of MHC-II+, CD80+ and
CD86+ cells compared with the spontaneous abortion group
(Fig. 4C). However, the PD-L1 Ig +
CD40L mAb group displayed the lowest percentages of
MHC-II+, CD80+ and CD86+ cells out
of the three treatment groups. Immature DCs expressed low levels of
MHC-II, CD80 and CD86, and mature DCs expressed high levels of
MHC-II, CD80 and CD86 (26,27).
The results indicated that combined treatment with PD-L1 Ig and
anti-CD40L mAbs decreased DC maturation in the CBA/J × DBA/2 mating
model.
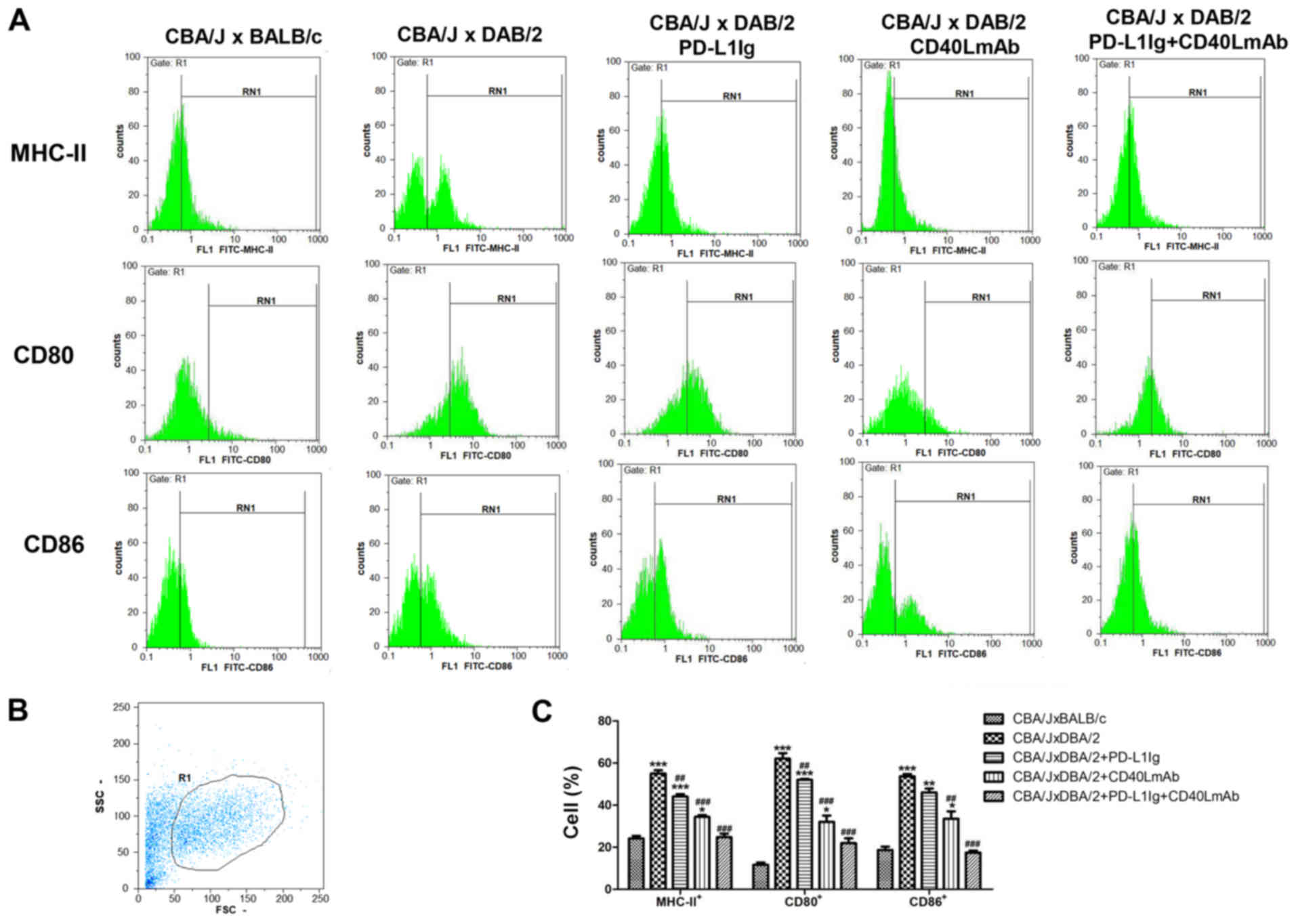 | Figure 4.Decreased MHC-II, CD80 and CD86
expression on BMDCs is induced by combined treatment with PD-L1 Ig
and anti-CD40L mAbs in the CBA/J × DBA/2 model. (A) Number of
MHC-II+, CD80+ and CD86+ cells in
the bone marrow, determined by flow cytometry. (B) Gating strategy
used for flow cytometry, using the FSC/SSC method. R1 represents
the BMDC population. (C) Percentage of MHC-II+,
CD80+ and CD86+ cells. *P<0.05,
**P<0.01 and ***P<0.001 vs. the normal group (CBA/J ×
BALB/c). ##P<0.01 and ###P<0.001 vs.
the spontaneous abortion group (CBA/J × DBA/2). MHC, major
histocompatibility complex; BMDCs, bone marrow-derived dendritic
cells; PD-L1, programmed death-ligand 1; Ig, immunoglobulin; CD40L,
CD40 ligand; mAb, monoclonal antibody; SSC, side scatter; FSC,
forward scatter. |
Combined treatment with PD-L1 Ig and
anti-CD40L mAbs decreases TNF-α and INF-γ expression and increases
IL-4 expression in the CBA/J × DBA/2 mating model
The expression of intracellular cytokines, including
TNF-α, INF-γ and IL-4, in the spleen tissues of pregnant CBA/J
female mice were determined by western blotting (Fig. 5A-D). The levels of TNF-α and INF-γ
in the spontaneous abortion group were significantly increased, and
the level of IL-4 was significantly decreased compared with the
normal group (Fig. 5B-D). The
PD-L1 Ig + CD40L mAb group displayed lower expression levels of
TNF-α and INF-γ, and higher expression levels of IL-4 compared with
the spontaneous abortion, PD-L1 Ig and CD40L mAb groups (Fig. 5B-D). The results suggested that
combined treatment with PD-L1 Ig and anti-CD40L mAbs decreased
TNF-α and INF-γ expression and increased IL-4 expression in the
CBA/J × DBA/2 mating model.
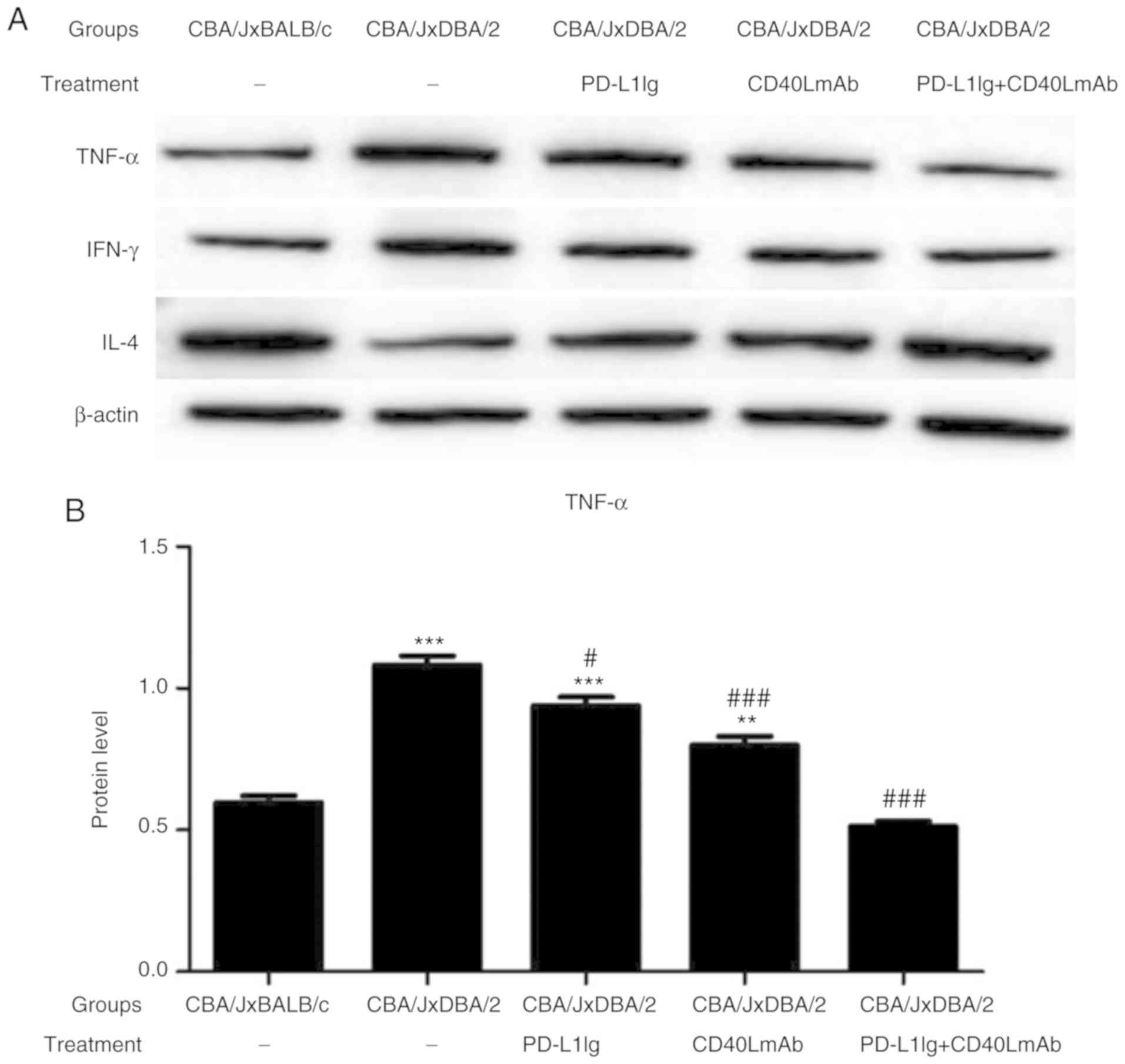 | Figure 5.Combined therapy with PD-L1 Ig and
anti-CD40L mAbs decreases the expression of TNF-α and IFN-γ
expression and increases the expression of IL-4 in the CBA/J ×
DBA/2 model. Protein expression levels were determined by (A)
western blot analysis and quantified for (B) TNF-α, (C) IFN-γ and
(D) IL-4. *P<0.05, **P<0.01 and ***P<0.001 vs. the normal
group (CBA/J × BALB/c). #P<0.05,
##P<0.01 and ###P<0.001 vs. the
spontaneous abortion group (CBA/J × DBA/2). PD-L1, programmed
death-ligand 1; Ig, immunoglobulin; CD40L, CD40 ligand; mAb,
monoclonal antibody; TNF-α, tumor necrosis factor-α; IFN-γ,
interferon-γ; IL-4, interleukin-4. |
Discussion
Normal pregnancy is a complex physiological process
that is similar to successful allotransplantation. The maternal
immune system is stimulated by paternal human leukocyte antigens
(HLA) carried by the fetus, resulting in a corresponding immune
response, however, the mother often develops immune tolerance to
the fetus. If the immune tolerance is disrupted, it can lead to the
occurrence of abortion (1). The
mechanism of maternal and fetal immune tolerance is a focus for
research in the field of reproductive immunology.
PD-L1, in combination with PD-1, can significantly
regulate the expression of cytokines to inhibit the function of T
cells and promote T cell apoptosis (11). PD-L1 combined with PD-1 inhibits
cell proliferation and cytokine production (28,29).
CD40 and CD40L are costimulatory molecules that are involved in the
specific immune response system in vivo, which is required
for the humoral and cellular immune responses of the body. CD40 and
CD40L play a role in B cell activation, proliferation,
differentiation, antibody production and homotypic transformation.
The two molecules also have a regulatory role in T cell activation
and the secretion of effector cytokines (30–32).
Abnormalities in the CD40-CD40L signaling pathway can lead to
pathological reactions, such as inflammation and atherosclerosis,
in the body (33,34). Furthermore, blocking this
costimulatory pathway, by using anti-CD40L mAbs for example, has
been identified as an immunotherapy strategy. Larsen et al
(21) reported that blocking the
CD40-CD40L signaling pathway with anti-CD40L mAbs could prevent
acute rejection and self-reactive antibody generation in a mouse
heart transplantation model.
Therefore, the CD40-CD40L signaling pathway plays a
role in the formation of antibodies in the body and blocking this
pathway can reduce the production of pathogenic autoantibodies or
unrelated antibodies, which might be a novel approach for the
clinical treatment of related autoimmune diseases. Furthermore, it
has been reported that combined treatment of anti-CD40L mAbs and
CTLA-4 Ig in a mouse skin and heart transplantation model, as well
as in a non-human primate kidney transplantation model, could
significantly prolong the survival time of the graft (20,35,36).
However, the effects of combined treatment with PD-L1 Ig and
anti-CD40L mAbs on spontaneous abortion are not completely
understood, and the underlying mechanisms remain unclear.
Spontaneous abortion in abortion-prone CBA/J ×
DBA/2-mated female mice is related to systemic maternal immune
inflammation, increased lymphocyte trafficking, complement
deposition and costimulatory molecules, and the activation of NK
cells, macrophages and T cells in feto-maternal tissues (37–40).
In the present study, an abortion-prone model with CBA/J female
mice and DBA/2 male mice was constructed to investigate the effects
of PD-L1 Ig and anti-CD40L mAb treatment on spontaneous abortion.
CBA/J × BALB/c mating pairs were used to model normal pregnancy. On
days 4, 6, 8, 10 and 12 of gestation, 0.1 mg/kg PD-L1 Ig and/or 0.2
mg/kg anti-CD40L mAbs were injected into pregnant CBA/J female
mice. On day 14 of gestation, the spleens, femora and tibiae were
isolated from pregnant CBA/J female mice for subsequent
experimentation. The resorption rate in the spontaneous abortion
group was higher compared with all other groups. However, combined
treatment with PD-L1 Ig and anti-CD40L mAbs reduced the resorption
rate compared with the PD-L1 Ig or CD40L mAb groups. The
proliferation assay suggested that the peripheral immune cells in
the spleens of pregnant mice in the spontaneous abortion group
displayed a significantly enhanced proliferation response to
paternal antigens compared with the normal group. Furthermore,
combined treatment with PD-L1 Ig and anti-CD40L mAbs during
implantation significantly decreased the proliferation response of
the peripheral immune cells in the spleen to paternal antigens in
the spontaneous abortion group. The combined treatment group
displayed the most significant decrease in proliferation out of the
three treatment groups.
To investigate the potential mechanism involved in
maternal immune tolerance, the splenic
CD4+CD25+ T-cell population was assessed by
flow cytometry. Increasing evidence suggests that regulatory T
cells, in particular CD4+CD25+ regulatory T
cells, play a role in the formation of maternal and fetal tolerance
(41,42). The expansion of
CD4+CD25+ T cells or the augmentation of
their activity can suppress allograft rejection (43,44).
The present study indicated that compared with the normal group,
the proportion of CD4+CD25+ T cells in the
spleens of the spontaneous abortion group was significantly
reduced. This suggested that the number of regulatory T cells in
the spontaneous abortion group was abnormal, which may provide an
explanation for the increased embryo uptake rate in the spontaneous
abortion group compared with the normal group. Combined PD-L1 Ig
and anti-CD40L mAb treatment significantly increased the proportion
of the CD4+CD25+ T cell population compared
with either treatment administered as a monotherapy. Therefore, it
could be hypothesized that combined treatment with PD-L1 Ig and
anti-CD40L mAbs inhibited embryo resorption by increasing the
proportion of CD4+CD25+ T cells in the
spleen.
Dendritic cells (DCs) are the sentinel cells of the
immune system that regulate both innate and acquired immune
responses (45). Mature DCs can
promote the immune response and immature DCs can inhibit the immune
response; therefore, DCs are involved in immune tolerance and
rejection of grafts (46). DCs
were collected from the bone marrow of pregnant mice and the levels
of MHC-II+, CD80+ and CD86+ cells
were determined by flow cytometry. The DCs in the spontaneous
abortion group had higher MHC-II, CD80 and CD86 expression compared
with all other groups, and the DCs in the combined treatment group
had lower MHC-II, CD80 and CD86 expression compared with the
spontaneous abortion, PD-L1 Ig and CD40L mAb groups. The results
suggested that combined treatment with PD-L1 Ig and anti-CD40L mAbs
inhibited the maturation of DCs and increased the number of
immature DCs. Therefore, combined treatment with PD-L1 Ig and
anti-CD40L mAb might have inhibited embryo resorption by inhibiting
DC maturation.
T helper (Th) cells are involved in the immune
tolerance mechanism during pregnancy, and abnormal Th1- and
Th2-type cytokine levels are associated with the occurrence of
abortion. Th2 cytokines are the dominant type during normal
pregnancy, however Th1/Th2-type cytokine balance disorders in
patients experiencing abortions are typically characterized by a
skew toward Th1 bias (47,48). Evidence suggests that fetal
rejection can be prevented by increasing the ratio of Th2 to Th1
cytokines produced by maternal leukocytes (49). In the present study, the expression
of the Th1 cytokines, TNF-α and INF-γ, and the Th2 cytokine, IL-4,
were determined by western blotting. Combined treatment with PD-L1
Ig and anti-CD40L mAbs decreased the production of TNF-α and INF-γ
and increased the expression of IL-4 in the spontaneous abortion
group. The results suggested that combined treatment with PD-L1 Ig
and anti-CD40L mAbs altered the local immune microenvironment to
aid with immune tolerance and further decrease the embryo
resorption rate. Therefore, combined treatment with PD-L1 Ig and
anti-CD40L mAbs decreased the bias towards Th1 cell responses and
increased the bias towards Th2-cell responses to maintain
pregnancy.
In conclusion, in the present study, a normal
pregnancy model was constructed with female CBA/J and male BALB/c
mice and a spontaneous abortion model was constructed with female
CBA/J and male DBA/2 mice. Subsequently, PD-L1 Ig and/or anti-CD40L
mAbs were injected into pregnant CBA/J female mice. The combined
treatment with PD-L1 Ig and anti-CD40L mAbs significantly reduced
the embryo resorption rate by inhibiting MHC-II, CD80 and CD86
expression in DCs, decreasing TNF-α and IFN-γ levels, and
increasing the CD4+CD25+ T cell population
and IL-4 levels; these effects are beneficial to the maintenance of
pregnancy. Thus, these findings indicated that combined treatment
with PD-L1 Ig and anti-CD40L mAbs may result in maternal immune
tolerance and inhibit maternal immune rejection of allogeneic
embryos, improving the outcome of pregnancy in an abortion-prone
mouse model. The combined treatment with PD-L1 Ig and anti-CD40L
mAb also inhibited the maturation of DCs, expanded the peripheral
CD4+CD25+ T cell population and promoted a
shift in cytokine polarization from Th1 to Th2. The results of the
present study may aid in designing therapeutic approaches for
immunological pregnancy complications and also extended the
existing knowledge of how an allograft is tolerated in a foreign
environment. Further investigation into the role of PDL-1 Ig and
anti-CD40L mAbs in uterine immune tolerance is required.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Special
Project of Yunnan Science and Technology Department-Kunming Medical
University Applied Basic Research [grant no. 2017FE467(−062)] and
the Medical Science Leaders Training Project of Yunnan Health
Commission (grant no. D-201633).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GL, LY and WH conceived and designed the study; GL,
LY, DL and JZ performed the experiments; JZ, LD, LX and YL analyzed
the data; LX and YL wrote the manuscript; GL, LY and WH reviewed
and edited the manuscript. All authors read and approved the
manuscript.
Ethics approval and consent to
participate
The animal experiments were approved by the Animals
Ethics Committee of Kunming Medical University and the Guide for
the Care and Use of Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Trowsdale J and Betz AG: Mother's little
helpers: Mechanisms of maternal-fetal tolerance. Nat Immunol.
7:241–246. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Munoz-Suano A, Hamilton AB and Betz AG:
Gimme shelter: The immune system during pregnancy. Immunol Rev.
241:20–38. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bonney EA and Brown SA: To drive or be
driven: The path of a mouse model of recurrent pregnancy loss.
Reproduction. 147:R153–R167. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ishida Y, Agata Y, Shibahara K and Honjo
T: Induced expression of PD-1, a novel member of the immunoglobulin
gene superfamily, upon programmed cell death. EMBO J. 11:3887–3895.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Okazaki T and Honjo T: PD-1 and PD-1
ligands: From discovery to clinical application. Int Immunol.
19:813–824. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nishimura H, Minato N, Nakano T and Honjo
T: Immunological studies on PD-1 deficient mice: Implication of
PD-1 as a negative regulator for B cell responses. Int Immunol.
10:1563–1572. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Carreno BM and Collins M: The B7 family of
ligands and its receptors: New pathways for costimulation and
inhibition of immune responses. Annu Rev Immunol. 20:29–53. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lázár-Molnár E, Yan Q, Cao E, Ramagopal U,
Nathenson SG and Almo SC: Crystal structure of the complex between
programmed death-1 (PD-1) and its ligand PD-L2. Proc Natl Acad Sci
USA. 105:10483–10488. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zak KM, Grudnik P, Magiera K, Dömling A,
Dubin G and Holak TA: Structural biology of the immune checkpoint
receptor PD-1 and its ligands PD-L1/PD-L2. Structure. 25:1163–1174.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nishimura H and Honjo T: PD-1: An
inhibitory immunoreceptor involved in peripheral tolerance. Trends
Immunol. 22:265–268. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dong H, Strome SE, Salomao DR, Tamura H,
Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al:
Tumor-associated B7-H1 promotes T-cell apoptosis: A potential
mechanism of immune evasion. Nat Med. 8:793–800. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iwai Y, Ishida M, Tanaka Y, Okazaki T,
Honjo T and Minato N: Involvement of PD-L1 on tumor cells in the
escape from host immune system and tumor immunotherapy by PD-L1
blockade. Proc Natl Acad Sci USA. 99:12293–12297. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dong H and Chen L: B7-H1 pathway and its
role in the evasion of tumor immunity. J Mol Med (Berl).
81:281–287. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Freeman GJ, Long AJ, Iwai Y, Bourque K,
Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne
MC, et al: Engagement of the PD-1 immunoinhibitory receptor by a
novel B7 family member leads to negative regulation of lymphocyte
activation. J Exp Med. 192:1027–1034. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Carter L, Fouser LA, Jussif J, Fitz L,
Deng B, Wood CR, Collins M, Honjo T, Freeman GJ and Carreno BM:
PD-1:PD-L inhibitory pathway affects both CD4(+) and CD8(+) T cells
and is overcome by IL-2. Eur J Immunol. 32:634–643. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao L, Liu F, Tan L, Liu T, Chen Z and Shi
C: The immunosuppressive properties of non-cultured dermal-derived
mesenchymal stromal cells and the control of graft-versus-host
disease. Biomaterials. 35:3582–3588. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vanikar AV, Trivedi HL, Gopal SC, Kumar A
and Dave SD: Pre-transplant co-infusion of donor-adipose tissue
derived mesenchymal stem cells and hematopoietic stem cells may
help in achieving tolerance in living donor renal transplantation.
Ren Fail. 36:457–460. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hong J, Yeom HJ, Lee E, Han KH, Koo TY,
Cho B, Ro H, Oh KH, Ahn C and Yang J: Islet allograft rejection in
sensitized mice is refractory to control by combination therapy of
immune-modulating agents. Transpl Immunol. 28:86–92. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Foy TM, Aruffo A, Bajorath J, Buhlmann JE
and Noelle RJ: Immune regulation by CD40 and its ligand GP39. Annu
Rev Immunol. 14:591–617. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pinelli DF and Ford ML: Novel insights
into anti-CD40/CD154 immunotherapy in transplant tolerance.
Immunotherapy. 7:399–410. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Larsen CP, Alexander DZ, Hollenbaugh D,
Elwood ET, Ritchie SC, Aruffo A, Hendrix R and Pearson TC:
CD40-gp39 interactions play a critical role during allograft
rejection. Suppression of allograft rejection by blockade of the
CD40-gp39 pathway. Transplantation. 61:4–9. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Coenen JJ, Koenen HJ, van Rijssen E,
Hilbrands LB and Joosten I: Tolerizing effects of co-stimulation
blockade rest on functional dominance of CD4+CD25+ regulatory T
cells. Transplantation. 79:147–156. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vermeiren J, Ceuppens JL,
Haegel-Kronenberger H, De Boer M, Boon L and Van Gool SW: Blocking
B7 and CD40 co-stimulatory molecules decreases antiviral T cell
activity. Clin Exp Immunol. 135:253–258. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Im SH, Barchan D, Maiti PK, Fuchs S and
Souroujon MC: Blockade of CD40 ligand suppresses chronic
experimental myasthenia gravis by down-regulation of Th1
differentiation and up-regulation of CTLA-4. J Immunol.
166:6893–6898. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Law CL and Grewal IS: Therapeutic
interventions targeting CD40L (CD154) and CD40: The opportunities
and challenges. Adv Exp Med Biol. 647:8–36. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lewis KL and Reizis B: Dendritic cells:
Arbiters of immunity and immunological tolerance. Cold Spring Harb
Perspect Biol. 4:a0074012012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pardee AD, Yano H, Weinstein AM, Ponce AA,
Ethridge AD, Normolle DP, Vujanovic L, Mizejewski GJ, Watkins SC
and Butterfield LH: Route of antigen delivery impacts the
immunostimulatory activity of dendritic cell-based vaccines for
hepatocellular carcinoma. J Immunother Cancer. 3:322015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hui E, Cheung J, Zhu J, Su X, Taylor MJ,
Wallweber HA, Sasmal DK, Huang J, Kim JM, Mellman I and Vale RD: T
cell costimulatory receptor CD28 is a primary target for
PD-1-mediated inhibition. Science. 355:1428–1433. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Patsoukis N, Brown J, Petkova V, Liu F, Li
L and Boussiotis VA: Selective effects of PD-1 on Akt and Ras
pathways regulate molecular components of the cell cycle and
inhibit T cell proliferation. Sci Signal. 5:ra462012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Graf D, Müller S, Korthäuer U, van Kooten
C, Weise C and Kroczek RA: A soluble form of TRAP (CD40 ligand) is
rapidly released after T cell activation. Eur J Immunol.
25:1749–1754. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Banchereau J, Bazan F, Blanchard D, Brière
F, Galizzi JP, van Kooten C, Liu YJ, Rousset F and Saeland S: The
CD40 antigen and its ligand. Annu Rev Immunol. 12:881–922. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hanissian SH and Geha RS: Jak3 is
associated with CD40 and is critical for CD40 induction of gene
expression in B cells. Immunity. 6:379–387. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lutgens E, Cleutjens KB, Heeneman S,
Koteliansky VE, Burkly LC and Daemen MJ: Both early and delayed
anti-CD40L antibody treatment induces a stable plaque phenotype.
Proc Natl Acad Sci USA. 97:7464–7469. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schönbeck U, Sukhova GK, Shimizu K, Mach F
and Libby P: Inhibition of CD40 signaling limits evolution of
established atherosclerosis in mice. Proc Natl Acad Sci USA.
97:7458–7463. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yin D, Ma L, Shen J, Byrne GW, Logan JS
and Chong AS: CTLA-41g in combination with anti-CD40L prolongs
xenograft survival and inhibits anti-gal ab production in GT-Ko
mice. Am J Transplant. 2:41–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Saito K, Sakurai J, Ohata J, Kohsaka T,
Hashimoto H, Okumura K, Abe R and Azuma M: Involvement of CD40
ligand-CD40 and CTLA4-B7 pathways in murine acute graft-versus-host
disease induced by allogeneic T cells lacking CD28. J Immunol.
160:4225–4231. 1998.PubMed/NCBI
|
|
37
|
Clark DA, Chaouat G, Arck PC, Mittruecker
HW and Levy GA: Cytokine-dependent abortion in CBA × DBA/2 mice is
mediated by the procoagulant fgl2 prothrombinase [correction of
prothombinase]. J Immunol. 160:545–549. 1998.PubMed/NCBI
|
|
38
|
Girardi G, Yarilin D, Thurman JM, Holers
VM and Salmon JE: Complement activation induces dysregulation of
angiogenic factors and causes fetal rejection and growth
restriction. J Exp Med. 203:2165–2175. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yadav AK, Chaudhari H, Shah PK and Madan
T: Expression and localization of collectins in feto-maternal
tissues of human first trimester spontaneous abortion and abortion
prone mouse model. Immunobiology. 221:260–268. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Clark DA: The importance of being a
regulatory T cell in pregnancy. J Reprod Immunol. 116:60–69. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Aluvihare VR, Kallikourdis M and Betz AG:
Regulatory T cells mediate maternal tolerance to the fetus. Nat
Immunol. 5:266–271. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
42
|
Saito S, Sasaki Y and Sakai M:
CD4(+)CD25high regulatory T cells in human pregnancy. J Reprod
Immunol. 65:111–120. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bacchetta R, Gregori S and Roncarolo MG:
CD4+ regulatory T cells: Mechanisms of induction and effector
function. Autoimmun Rev. 4:491–496. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kingsley CI, Karim M, Bushell AR and Wood
KJ: CD25+CD4+ regulatory T cells prevent graft rejection: CTLA-4-
and IL-10-dependent immunoregulation of alloresponses. J Immunol.
168:1080–1086. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gordon JR, Ma Y, Churchman L, Gordon SA
and Dawicki W: Regulatory dendritic cells for immunotherapy in
immunologic diseases. Front Immunol. 5:72014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Henriques HR, Rampazo EV, Gonçalves AJ,
Vicentin EC, Amorim JH, Panatieri RH, Amorim KN, Yamamoto MM,
Ferreira LC, Alves AM and Boscardin SB: Targeting the
non-structural protein 1 from dengue virus to a dendritic cell
population confers protective immunity to lethal virus challenge.
PLoS Negl Trop Dis. 7:e23302013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Nan CL, Lei ZL, Zhao ZJ, Shi LH, Ouyang
YC, Song XF, Sun QY and Chen DY: Increased Th1/Th2 (IFN-gamma/IL-4)
Cytokine mRNA ratio of rat embryos in the pregnant mouse uterus. J
Reprod Dev. 53:219–228. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Platt JL: New directions for organ
transplantation. Nature. 392 (6679 Suppl):S11–S17. 1998.
|
|
49
|
Lin H, Mosmann TR, Guilbert L,
Tuntipopipat S and Wegmann TG: Synthesis of T helper 2-type
cytokines at the maternal-fetal interface. J Immunol.
151:4562–4573. 1993.PubMed/NCBI
|