Introduction
Hypertrophic scars (HSs) are the pathological result
of abnormal tissue repair after deep skin injury, with typical
clinical symptoms manifesting as redness, thickening, stiffening,
itching and tenderness (1). In
patients with severe skin trauma or burn, the incidence of HS is
relatively high (30–72%) (2,3).
When HSs occur in the face, joints, hands and other important
tissues, fibrotic contracture causes functional limitations and
aesthetic disfigurement, which can result in a physiological and
psychological burden on patients (4). At present, there are a number of
treatment strategies available for the prevention and treatment of
HSs, including corticosteroids, cryotherapy, pressure therapy,
laser, radiation and surgery (5),
but the therapeutic effects are not satisfactory. Therefore, the
development of effective therapeutic drugs is important.
HSs are a progressive fibroproliferation disorder
that are characterized by continuous activation of fibroblasts and
excessive extracellular matrix deposition (1). Following skin injury, fibroblasts
surrounding the wound can be transformed into myofibroblasts, which
synthesize and secrete extracellular matrix, serving a substantial
role in wound contraction and physical support (6). Under normal circumstances, when wound
healing is complete, myofibroblasts gradually undergo apoptosis,
resulting in the restoration of the synthesis and degradation of
extracellular matrix to a dynamic equilibrium state. However, under
pathological conditions, due to high levels of inflammation and
oxidative stress, excessive myofibroblasts cannot be removed and
thus remain in a proliferative and activated state, leading to the
synthesis and secretion of large amounts of extracellular matrix,
ultimately generating thick and hardened scars (1,6).
Arctigenin (ATG), a phenylpropanoid
dizbenzylbutyrolactone lignin, is derived from certain plants, such
as Arctium lappa, Torreya nucifera and Bardanae
fructus (7). ATG is primarily
used as a component of compound prescriptions in China and other
Asian countries for the treatment of inflammatory diseases,
including anemopyretic cold, cough, measles and syphilis (8). Numerous studies have demonstrated
that ATG displays a variety of biological activities, including
anti-inflammatory, antioxidant, and antineoplastic properties
(9–12). In addition, ATG has also been
reported to reduce fibrosis in various fibrotic diseases (13–17).
For instance, in oral submucous fibrosis, ATG prevents
arecoline-induced myofibroblast transdifferentiation and
dysregulated activities potentially by downregulating the
LINC00974-mediated TGF-β/phosphorylated (p-) Smad2 signaling
pathway (13). In a peritoneal
fibrosis model using an in vitro cell culture model of
TGF-β1-stimulated human peritoneal mesothelial cells, ATG
suppressed TGF-β1-induced epithelial mesenchymal transition in a
dose-dependent manner, partly by enhancing the activity of the
AMP-activated protein kinase/NF-kB signaling pathway and inhibiting
the expression of plasminogen activator inhibitor-1 (PAI-1)
(14). However, whether ATG can
alleviate hypertrophic scarring is not completely understood.
Materials and methods
Animal and ethics statement
A total of 30 SPF-grade healthy female C57BL/6 mice
(age, 7–8 weeks; weight, 23±3 g) were purchased from Shanghai SLAC
Laboratory Animal Co., Ltd.. Mice were housed at 22–25°C with
50–60% humidity, 12-h light/dark cycles, ad libitum access
to food and water, and adaptive feeding for one week before
conducting experiments. All animal experiments were approved by the
Ethics Committee of Chongqing University Central Hospital (approval
no. 2018–032).
Animal model
A bleomycin (BLM)-induced skin fibrosis murine model
of HS was established as previously described (18). Briefly, a 3 cm2 area of
hair on the back of the mice was removed using depilatory cream.
BLM (Sigma-Aldrich; Merck KGaA) was dissolved in PBS (Wuhan Boster
Biological Technology, Ltd.) and sterilized by filtration. BLM (100
µl; 1 mg/ml) was subcutaneously injected into a 1 cm2
area on the hair removal area using a 27-gauge needle. The
injections were administered daily for 21 consecutive days.
Experimental design
A total of 30 C57BL/6 mice were randomly divided
into three groups (n=10 per group): i) Control (Con); ii) BLM; and
iii) BLM+ATG. HS modeling was established in the BLM and BLM+ATG
groups, whereas the Con group was subcutaneously injected with
saline daily for 21 consecutive days. For the Con group, the same
injection volume was applied to the same hair removal area of
dorsal skin. At 1 day post-bleomycin induction, the BLM+ATG group
was intraperitoneally injected with 3 mg/kg/day ATG (Sigma-Aldrich;
Merck KGaA) for 28 consecutive days (10,15).
ATG was dissolved into a stock solution at a concentration of 12
mg/ml using DMSO and diluted to a total concentration of 0.3 mg/ml
with saline before administration. The Con and BLM group were
intraperitoneally injected with saline (230 µl) daily for 28
consecutive days. At the end of treatment, mice were anaesthetized
with an intraperitoneal injection of pentobarbital sodium solution
(1%; 40 mg/kg) and sacrificed by cervical dislocation prior to
collection of skin tissue samples. Then, skin tissue samples were
collected from the defined area (1 cm2) injected with
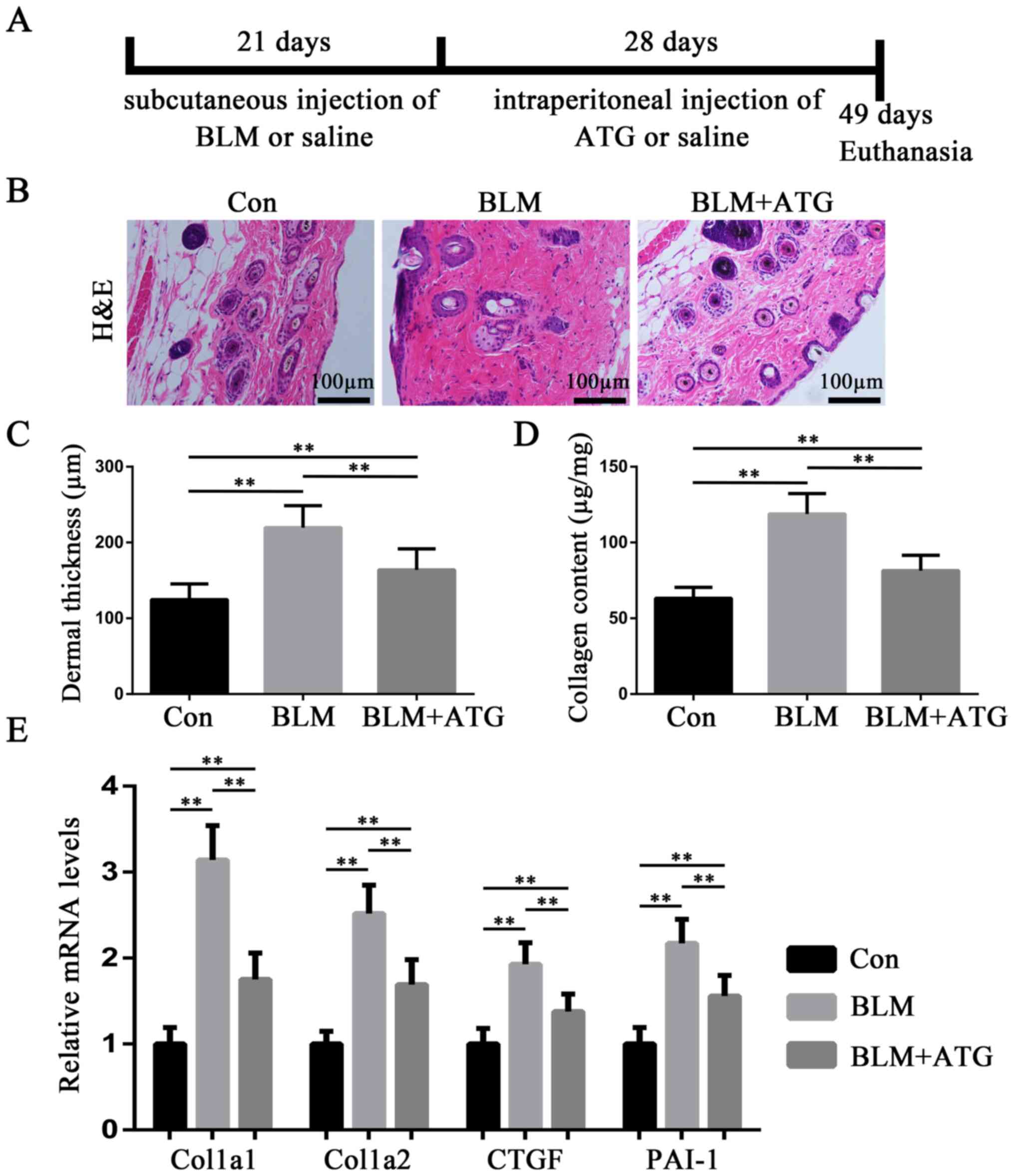
BLM. A schematic diagram of the experiment is presented in Fig. 1A.
Histologic examination
Hematoxylin and eosin (H&E) staining of skin
tissues was performed for pathological examination. Skin tissue
samples were fixed in 4% paraformaldehyde at 4°C for 24 h, embedded
in paraffin and cut into 4-µm thick sections. The sections were
stained with hematoxylin for 5 min and eosin for 3 min at room
temperature. Calculation and analysis of dermal thickness were
performed according to Sekiguchi et al (19). Briefly, dermal thickness was
calculated as the distance from the epidermis-dermis junction to
the dermis-subcutaneous tissue junction. Stained skin tissues were
observed in six randomly selected fields of view using an Axio
Scan.Z1 light microscope (magnification, ×100; Carl Zeiss AG).
ImageJ software (version 1.51, National Institutes of Health) was
used to analyze dermal thickness.
Quantitative detection of collagen
content
Collagen content in skin tissues was measured using
a Sircol Collagen Assay kit (Biocolor, Ltd.) according to previous
reports (20,21) and the manufacturer's instructions.
For analysis, skin was homogenized in 0.5 M acetic acid containing
0.1 mg/ml pepsin (Sigma-Aldrich; Merck KGaA) with TissueLyser II LT
(Qiagen, Inc.) at 4°C, after which the supernatants were collected
through centrifugation (3,000 × g, 10 min, 4°C) and assayed.
Immunofluorescence staining
For immunofluorescence staining, full-thickness skin
samples were dissected, attached to filter paper and fixed in 4%
buffered paraformaldehyde overnight at 4°C. The samples were
embedded in optimal cutting temperature compound and prepared into
10-µm thick sections using a CM3000 cryostat (Leica Microsystems
GmbH). After blocking for 30 min at room temperature with 10% goat
serum (Sigma-Aldrich; Merck KGaA), the sections were incubated with
anti-TGF-β1 (1:100; Santa Cruz Biotechnology, Inc.; cat. no. sc146)
and anti-α-SMA (1:100; Abcam; cat. no. ab32575) primary antibodies
overnight at 4°C. After washing with 0.01 M PBS three times, the
sections were incubated with Alexa Fluor 488 conjugated anti rabbit
secondary antibodies (1:1,000; Abcam; cat. no. ab150077) for 1 h at
room temperature. Subsequently, cell nuclei were stained with DAPI
(Sigma-Aldrich; Merck KGaA) for 10 min at room temperature. Stained
sections were visualized using a TE2000 inverted fluorescence
microscope (magnification, ×200; Nikon Corporation) in at least six
randomly selected fields of view.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from skin tissues using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.).
Total RNA was reverse transcribed into cDNA using the PrimeScript
RT reagent kit (Takara Biotechnology Co., Ltd.). The temperature
protocol for reverse transcription was: 37°C for 15 min, followed
by 85°C for 5 sec. Subsequently, qPCR was performed using SYBR
Premix Ex Taq (Takara Biotechnology Co., Ltd.) and an ABI StepOne
Plus Real-Time PCR machine (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The following thermocycling conditions were used
for qPCR: An initial denaturation at 95°C for 30 sec; followed by
40 cycles at 95°C for 5 sec and 60°C for 30 sec; a final
dissociation at 95°C for 60 sec, 55°C for 30 sec and 95°C for 60
sec. The primer sequences used for qPCR are presented in Table I. The 2−∆∆Cq method was
used to calculate the relative gene expression levels (22).
 | Table I.Sequences of primers used for reverse
transcription-quantitative PCR. |
Table I.
Sequences of primers used for reverse
transcription-quantitative PCR.
| Gene | Sequence
(5′→3′) |
|---|
| Col1a1 | F:
GCTCCTCTTAGGGGCCACT |
|
| R:
ATTGGGGACCCTTAGGCCAT |
| Col1a2 | F:
TCGTGCCTAGCAACATGCC |
|
| R:
TTTGTCAGAATACTGAGCAGCAA |
| CTGF | F:
GGCCTCTTCTGCGATTTCG |
|
| R:
GCAGCTTGACCCTTCTCGG |
| PAI-1 | F:
GGCCTCTTCTGCGATTTCG |
|
| R:
GCAGCTTGACCCTTCTCGG |
| α-SMA | F:
AGAAACGGGACAAACTTCGTC |
|
| R:
GTCTTGCACTGTATAGCCGAG |
| TGF-β1 | F:
CCACCTGCAAGACCATCGAC |
|
| R:
CTGGCGAGCCTTAGTTTGGAC |
| IL-1β | F:
GAAATGCCACCTTTTGACAGTG |
|
| R:
TGGATGCTCTCATCAGGACAG |
| IL-4 | F:
CCCCAGCTAGTTGTCATCCTG |
|
| R:
CAAGTGATTTTTGTCGCATCCG |
| IL-6 | F:
CTGCAAGAGACTTCCATCCAG |
|
| R:
AGTGGTATAGACAGGTCTGTTGG |
| TNF-α | F:
CAGGCGGTGCCTATGTCTC |
|
| R:
CGATCACCCCGAAGTTCAGTAG |
| MCP-1 | F:
TAAAAACCTGGATCGGAACCAAA |
|
| R:
GCATTAGCTTCAGATTTACGGGT |
| GAPDH | F:
AGGTCGGTGTGAACGGATTTG |
|
| R:
GGGGTCGTTGATGGCAACA |
Western blotting
Total proteins were extracted from skin tissues
using RIPA (1% PMSF) tissue lysate (Beyotime Institute of
Biotechnology). Nuclear proteins were extracted using nuclear and
cytoplasmic protein extraction kit (Beyotime Institute of
Biotechnology). A bicinchoninic acid assay kit was used to measure
the protein concentration. Proteins (40 µg per lane) were separated
via 10% SDS-PAGE gels and transferred onto PVDF membranes (EMD
Millipore). Then, the membranes were blocked for 2 h at room
temperature using 5% skimmed milk powder. After blocking, the
membranes were incubated overnight at 4°C with primary antibodies
(all purchased from Abcam) targeted against: α-SMA (1:1,000; cat.
no. ab5694), TGF-β1 (1:1,000; cat. no. ab92486), nuclear factor
erythroid-2-related factor 2 (Nrf2; nuclear; 1:1,000; cat. no.
ab62352), heme oxygenase-1 (HO-1; 1:1,000; cat. no. ab13248) and
GAPDH (1:2,000; cat. no. ab9485). After washing three times with
TBS-T (TBS with 0.1% Tween-20) for 5 min each, the membranes were
incubated with HRP-conjugated secondary antibodies (1:3,000;
ProteinTech Group, Inc.; cat. no. SA00001-2) for 1 h at room
temperature. Protein bands were visualized with ECL WB detection
reagent (Beyotime Institute of Biotechnology) and imaged using the
ChemiDoc™ XRS Imaging System (Bio-Rad Laboratories, Inc.). Protein
expression levels were semi-quantified using ImageJ software
(version 1.51, National Institutes of Health) with GAPDH as the
loading control.
ELISA
The extracted skin tissues were weighed, minced, and
homogenized using a TissueLyser II LT (Qiagen, Inc.) in 10 ml cold
phosphate-buffered saline, which included 1 mmol/l
phenylmethylsulfonyl fluoride (Beyotime Institute of
Biotechnology), 1% protease inhibitors cocktail (Sigma-Aldrich;
Merck KGaA), 0.5% sodium deoxycholate (Sigma-Aldrich; Merck KGaA)
and 1% Triton X-100 (Sigma-Aldrich; Merck KGaA). After incubation
at 4°C for 1 h, the homogenized mixtures were centrifuged at 10,000
× g for 20 min at 4°C. The supernatants were collected and stored
at −80°C prior to use. IL-1β, IL-4, IL-6, TNF-α, and monocyte
chemoattractant protein-1 (MCP-1) contents in skin tissue
homogenate supernatants were measured using ELISA kits (Jingmei
Biotechnology; IL-1β, cat. no. JM-02323M1; IL-4, cat. no.
JM-02448M1; IL-6, cat. no. JM-02446M1; TNF-α, cat. no. JM-02415M1;
and MCP-1, cat. no. JM-02365M1) according to the manufacturer's
protocol.
Determination of oxidative stress
levels
Superoxide dismutase (SOD) activity, glutathione
(GSH) concentration and malondialdehyde (MDA) levels in skin tissue
homogenate supernatants, obtained by the aforementioned method,
were measured spectrophotometrically at 450, 420 and 532 nm
according to previous reports (23,24)
and the manufacturer's protocol (Nanjing Jiancheng Bioengineering
Institute; SOD, cat. no. A001-3-2; GSH, cat. no. A006-1-1; MDA,
cat. no. A003-1-2).
Statistical analysis
Statistical analyses were performed using SPSS
software (version 21.0; IBM Corp.). Data are presented as the mean
± SD. Comparisons among multiple groups were analyzed using one-way
ANOVA followed by the LSD post hoc test. P<0.05 was considered
to indicate a statistically significant difference. All experiments
were repeated at least three times.
Results
ATG alleviates BLM-induced dermal
fibrosis in a murine HS model
To evaluate the antifibrotic effect of ATG on HSs
in vivo, BLM-induced skin fibrosis model mice were treated
with ATG to examine its effects on dermal thickness, collagen
content and extracellular matrix-related gene expression. H&E
staining suggested that BLM significantly induced dermal thickening
compared with the Con group, whereas ATG significantly inhibited
the fibrotic effect of BLM (Fig. 1B
and C). Additionally, Sircol collagen detection also suggested
that BLM-induced increases in collagen content were significantly
suppressed by ATG treatment (Fig.
1D). In addition, Col1a1, Col1a2, CTGF and PAI-1, which are
important fibrotic components in the extracellular matrix, are
overexpressed in HS (25). The
RT-qPCR results indicated that the relative expression levels of
the four aforementioned genes were significantly decreased in the
BLM+ATG group compared with the BLM group (Fig. 1E). Collectively, the results
indicated that ATG inhibited BLM-induced dermal fibrosis in
vivo.
ATG inhibits the transformation of
fibroblasts into myofibroblasts in vivo
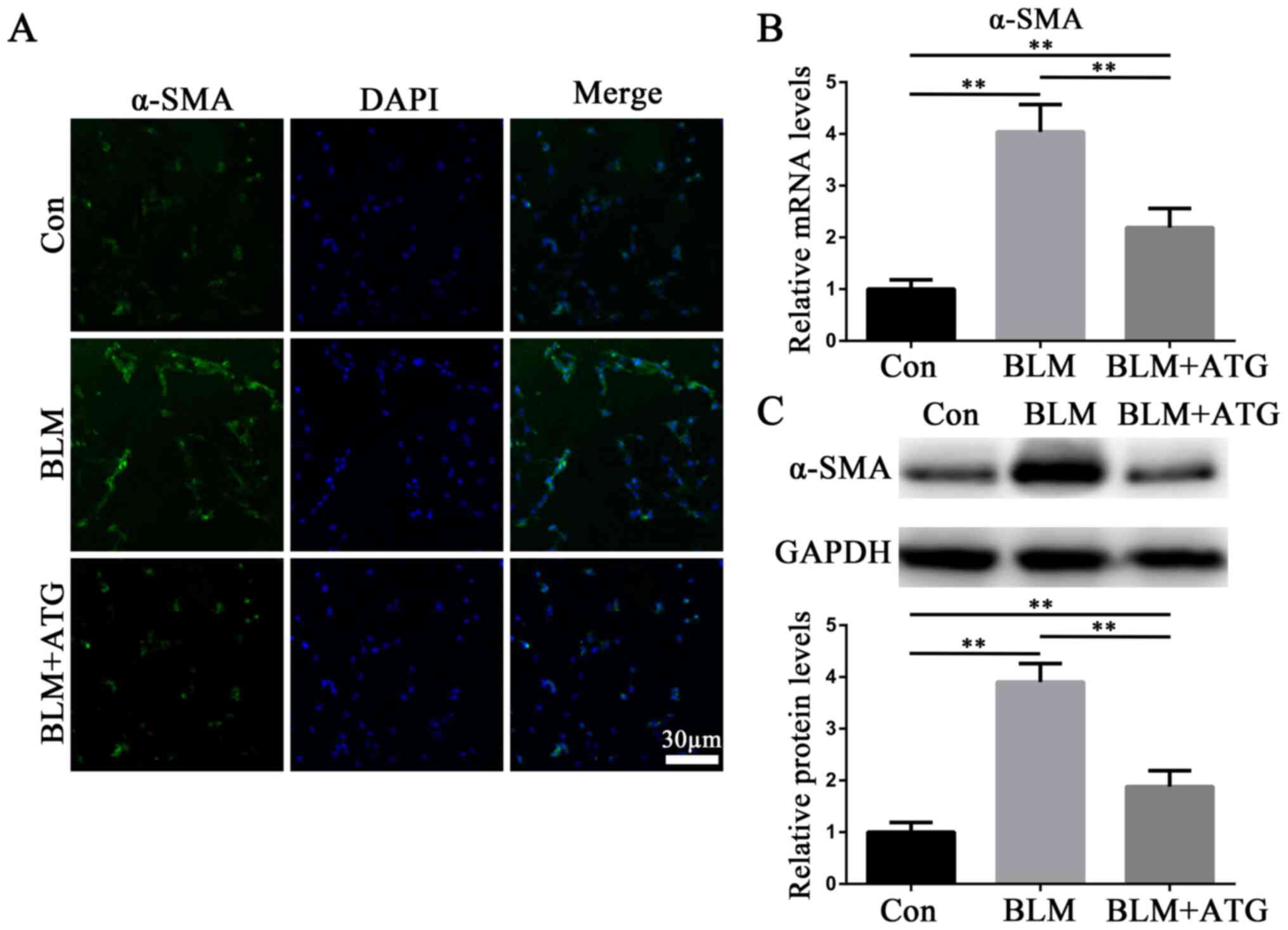
By investigating the mechanism underlying HSs, it
has been reported that myofibroblasts influence skin fibrosis
(6). Myofibroblasts are primarily
derived from activated fibroblasts and also have the
characteristics of smooth muscle cells, with α-SMA serving as
hallmark (26). Therefore, α-SMA
has been demonstrated to serve as the primary biomarker for the
phenotypic transformation of fibroblasts into myofibroblasts
(26). In the present study, the
expression level of α-SMA in skin tissues was detected via
immunofluorescence staining (Fig.
2A), RT-qPCR (Fig. 2B) and
western blotting (Fig. 2C). The
expression level of α-SMA was markedly upregulated in the BLM group
compared with the Con group, whereas α-SMA expression levels were
notably downregulated in the BLM+ATG group compared with the BLM
group (Fig. 2A-C). The results
indicated that ATG inhibited the differentiation of fibroblasts
into myofibroblasts in vivo.
ATG downregulates the expression of
TGF-β1 in BLM-induced skin fibrosis model mice
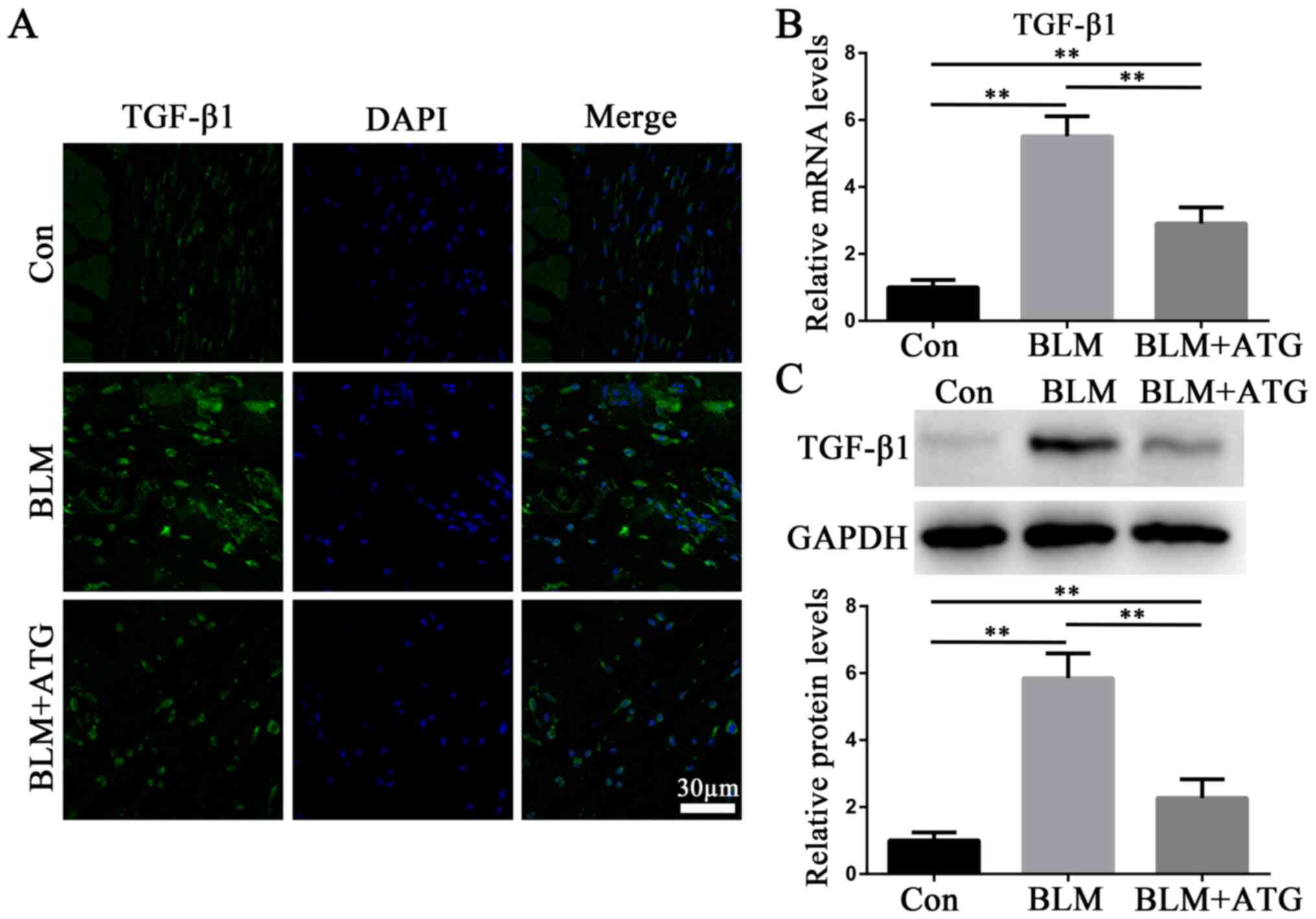
TGF-β1, a profibrotic growth factor, serves as the
primary effector molecule in skin fibrosis (27). Immunofluorescence staining
(Fig. 3A), RT-qPCR (Fig. 3B) and western blotting (Fig. 3C) were performed to analyze the
expression of TGF-β1. Compared with the Con group, BLM markedly
increased TGF-β1 expression levels, which were reversed by ATG
treatment (Fig. 3A-C). The results
suggested that ATG could reverse elevated TGF-β1 expression levels
in BLM-induced skin fibrosis model mice.
ATG reduces the inflammatory response
in BLM-induced skin fibrosis model mice
Increasing evidence has demonstrated that
inflammation is a key mechanism underlying the initiation and
maintenance of pathological fibrosis, including HSs (28,29).
Multiple cytokines, such as IL-1β, IL-4, IL-6, TNF-α and MCP-1,
serve a central role in orchestrating chronic inflammation
(30). To study the
anti-inflammatory effects of ATG, the expression levels of the
aforementioned inflammatory factors were measured. The RT-qPCR
results indicated that the mRNA expression levels of IL-1β, IL-4,
IL-6, TNF-α and MCP-1 were significantly increased in the BLM group
compared with the Con group, but ATG treatment significantly
inhibited BLM-induced mRNA expression levels (Fig. 4A). ELISAs were also conducted to
further investigate the aforementioned results (Fig. 4B). The ELISA results were
consistent with those of RT-qPCR, indicating that reducing the
inflammatory response may serve as a mechanism underlying
ATG-mediated amelioration of skin fibrosis.
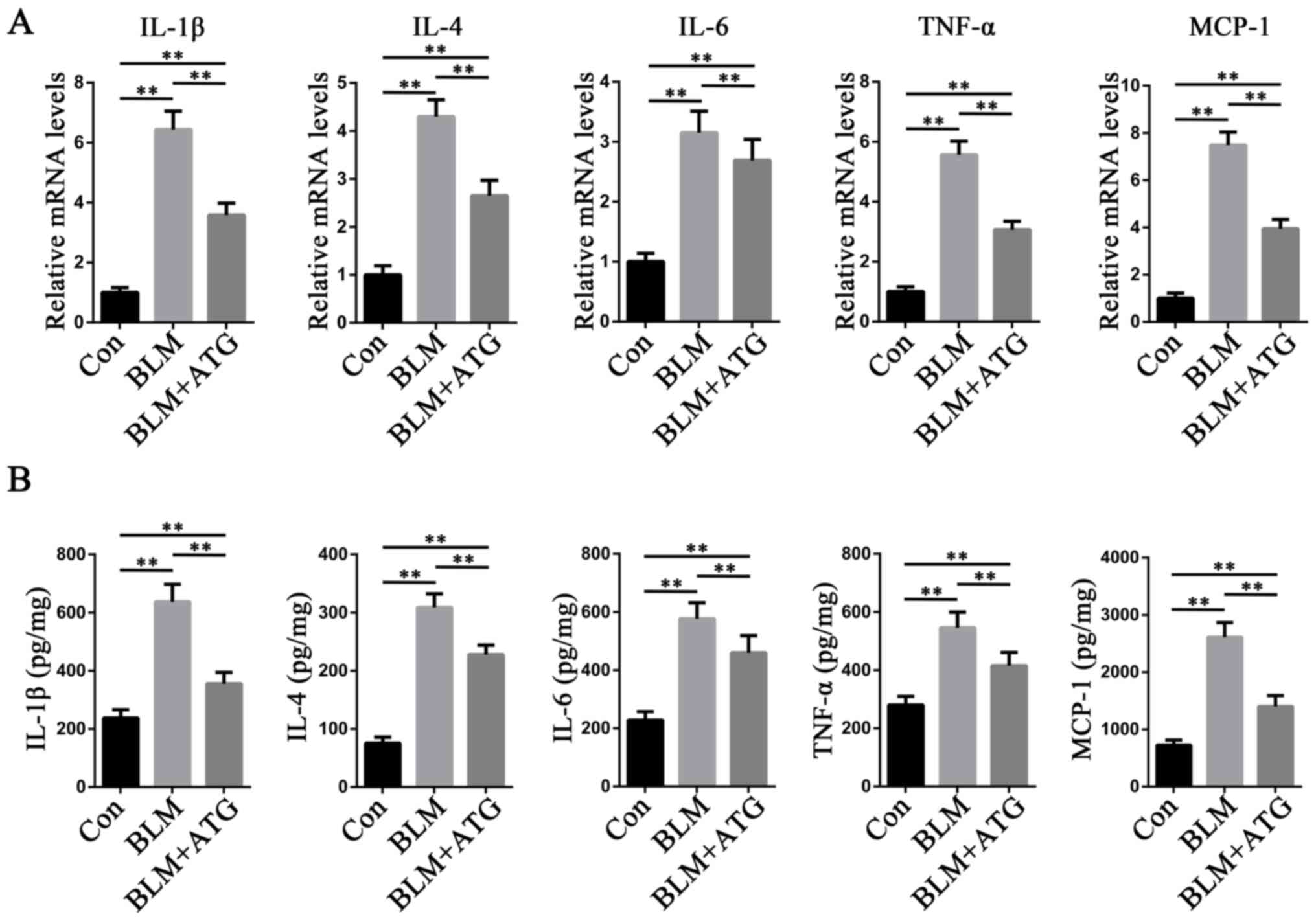 | Figure 4.ATG reduces the inflammation response
in BLM-induced skin fibrosis model mice. (A) mRNA expression levels
of IL-1β, IL-4, IL-6, TNF-α and MCP-1 were measured via reverse
transcription-quantitative PCR. (B) Protein expression levels of
IL-1β, IL-4, IL-6, TNF-α and MCP-1 were measured using ELISAs in
skin tissue homogenate supernatants. **P<0.01. ATG, arctigenin;
BLM, bleomycin; MCP-1, monocyte chemoattractant protein-1; Con,
control. |
ATG reduces oxidative stress in
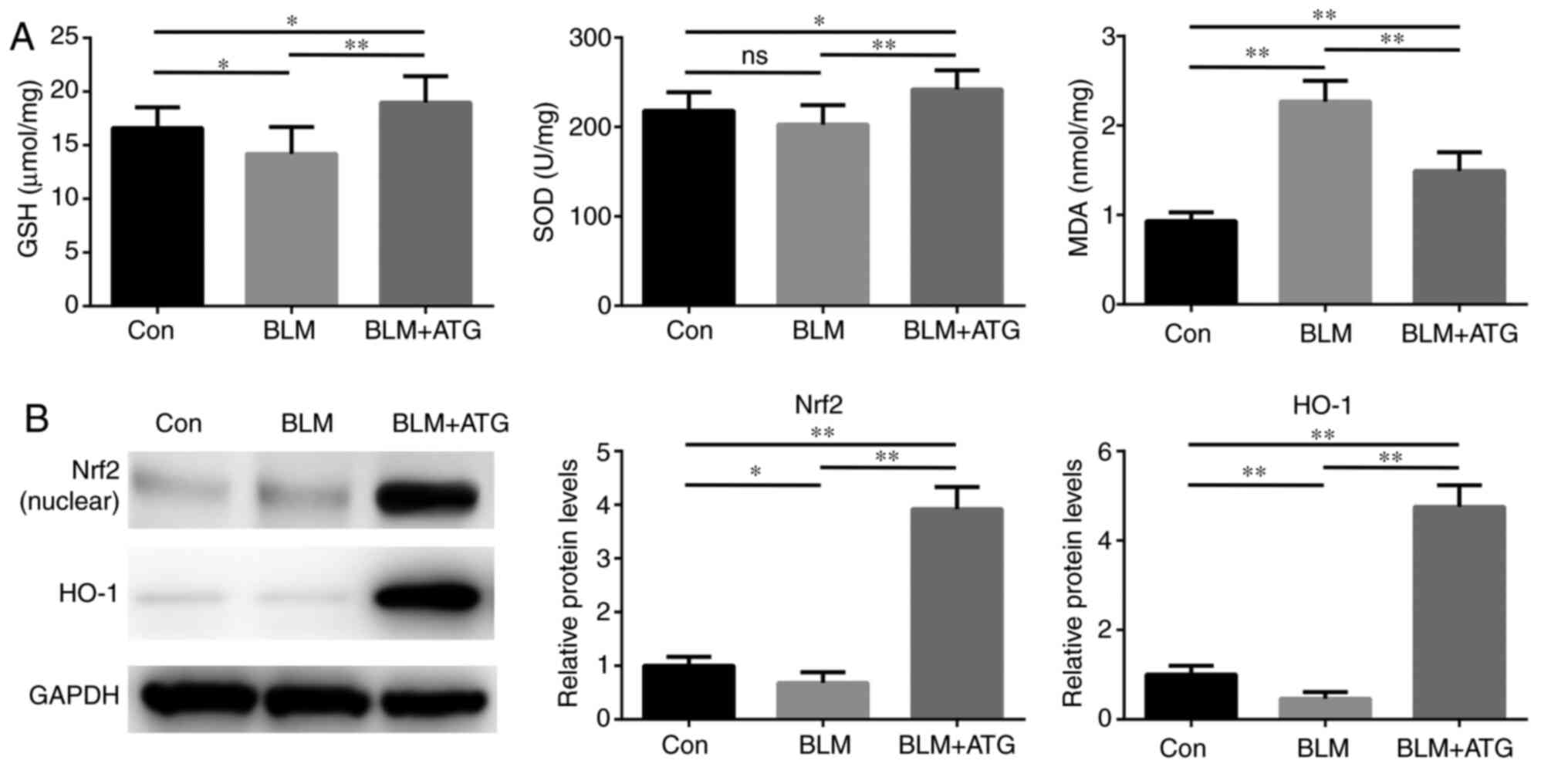
BLM-induced skin fibrosis model mice
Previous studies have demonstrated that elevated
oxidative stress and redox imbalance exacerbate the pathogenesis of
skin fibrosis (31,32). Therefore, identifying the effect of
ATG treatment in modulating oxidative stress requires
investigation. The present study indicated that the BLM+ATG group
displayed significantly increased SOD activity and GSH
concentration, but significantly decreased MDA levels compared with
the BLM group, which may be attributed to the antioxidant effect of
ATG (Fig. 5A).
To further investigate the antioxidant mechanism
underlying ATG, the expression of components of the Nrf2/HO-1
signaling pathway, which serves a critical negative regulatory role
in oxidative stress, was assessed (33,34).
BLM significantly downregulated the protein expression levels of
Nrf2 (nuclear) and HO-1 compared with the Con group (Fig. 5B). Nrf2 and HO-1 expression levels
were significantly increased in the BLM+ATG group compared with the
BLM group. Collectively, the results indicated that ATG effectively
resolved skin fibrosis by reducing oxidative stress via activation
of the Nrf2/HO-1 signaling pathway.
Discussion
HSs are a progressive fibroproliferation disease
characterized by the continuous activation of fibroblasts and
excessive deposition of extracellular matrix (1). At present, no effective treatment
strategy for HS has been established, which is primarily attributed
to the complicated pathological processes mediated by a series of
underlying mechanisms (5). A
variety of biologically active compounds derived from natural
products have been developed into drugs due to their potential
pharmacological activities and reduced side effects (35,36).
Among these, ATG has received increasing attention due to its
reported antifibrotic potential in several diseases, including oral
submucous fibrosis, peritoneal fibrosis, obstructive nephropathy
and liver fibrosis (13–17). However, the antifibrotic effects of
ATG on HSs are not completely understood. To the best of our
knowledge, the present study was the first to provide evidence for
ATG as a potential therapeutic agent against skin fibrosis in HS
via reducing inflammation and oxidative stress.
In the present study, the inhibitory effect of ATG
on skin fibrosis was investigated by performing histological
staining and collagen content determination. Subsequently, the
expression of extracellular matrix-related genes in the skin,
including Col1a1, Col1a2, CTGF and PAI-1, was measured. Col1a1 and
Col1a2 are two genes involved in the biosynthesis of type 1
collagen (37), CTGF is a key
factor in maintaining profibrotic milieu (38) and PAI-1 functions as a suppressor
of fibrinolysis (39). The results
of the present study indicated that Col1a1, Col1a2, CTGF and PAI-1
expression levels were decreased in the BLM+ATG group compared with
the BLM group. The excessive accumulation of extracellular matrix
is attributable to the pathological recruitment of fibroblasts to
injured sites and their transformation to α-SMA-expressing
myofibroblasts (6,26). Although the regulatory mechanisms
underlying fibroblast activation are not completely understood,
numerous studies have demonstrated that TGF-β1 serves as the main
effector (40–42). The present study indicated that ATG
treatment significantly decreased the expression of α-SMA and
TGF-β1 in BLM-induced fibrotic skin, suggesting that the
antifibrotic effect of ATG was associated with suppression of
TGF-β1-mediated ECM gene transcription and fibroblast
activation.
Despite extensive efforts to elucidate the possible
mechanisms underlying fibrosis, the understanding of the initiation
and progression of fibrosis is limited. A number of studies have
demonstrated that inflammation is essential in modulating several
pathological processes of skin fibrosis (28,29).
In the early stage, immune cells are activated by various stimuli,
which leads to the secretion of inflammatory factors that induce
the expression of adhesion molecules on the surface of endothelial
cells. Endothelial cell-specific adhesion molecules interact with
immune cells, recruiting the cells to sites of injury. Therefore,
chronic inflammation may be evoked and sustained by a positive
feedback loop (43). It has been
previously reported that the levels of inflammatory factors are
higher in fibrotic skin compared with healthy skin (29). The results of the present study
supported the aforementioned finding. Moreover, the results
indicated that ATG treatment decreased BLM-induced upregulation of
mRNA expression levels of several inflammatory factors.
Collectively, the results suggested that the antifibrotic effects
of ATG might be related to its anti-inflammatory effects.
Recent studies have demonstrated that oxidative
stress is enhanced in BLM-induced skin fibrosis, which has been
hypothesized to be associated with fibroblast activation and the
generation of inflammatory factors (23,44,45).
MDA is a product of reactive species-induced lipid peroxidation and
is an indicator of oxidative stress (46). In addition, GSH and SOD, which are
intracellular antioxidant enzymes, can be consumed by free radicals
(47). The results of the present
study indicated that ATG reduced oxidative stress in BLM-induced
fibrotic skin, as demonstrated by the upregulation of antioxidants
(GSH and SOD) and downregulation of oxidants (MDA) in the BLM+ATG
group compared with the BLM group. It has been reported that
activation of the Nrf2/HO-1 signaling pathway serves a pivotal role
in antioxidative defense (33,34).
As a redox-sensitive transcription factor, activated Nrf2 can
translocate from the cytoplasm into the nucleus and bind to
antioxidant response elements (AREs) on the HO-1 promoter (48). In the present study, compared with
the BLM group, ATG promoted nuclear translocation of Nrf2 and
increased the expression of HO-1 in BLM-induced fibrotic skin,
suggesting that the antioxidant mechanism underlying ATG might be
associated with activation of the Nrf2-mediated signaling
pathway.
The present study primarily focused on the
antifibrotic effects of ATG on HSs without considering the route of
administration. In the present study, ATG was administered via
intraperitoneal injection, which had the advantage of avoiding the
first-pass metabolism effect and improving bioavailability as a
parenteral administration method. However, intraperitoneal
administration is not a suitable for use in the clinic. Zhong et
al (9) recently reported that
oral gavage is also a suitable application route for ATG, which had
been shown to exert a protective effect in diabetic kidney disease.
In addition, it was hypothesized that if ATG could be formulated
into a plaster and applied to the skin surface, it may display an
improved therapeutic effect; however, low transdermal ability
remains a challenge. In addition, the timing and dose of
administration require further investigation. Moreover, the animal
model of BLM-induced skin fibrosis used in the present study did
not cause skin ulceration, thus, the healing time could not be
assessed. Future studies should establish an animal model of skin
resection to evaluate the effect of ATG on healing time.
In summary, the present study indicated that ATG
ameliorated skin fibrosis in a murine HS model. The antifibrotic
effects of ATG may be mediated in part by reducing inflammation and
oxidative stress. Therefore, the present study suggested that ATG
may serve as a therapeutic agent for HSs.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LJ and YL contributed to the study design. LJ, YD,
WL and YL performed the experiments and analyzed the data. LJ wrote
the manuscript. YD, WL and YL edited the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Chongqing University Central Hospital (approval no.
2018-032).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HS
|
hypertrophic scar
|
|
ATG
|
arctigenin
|
|
BLM
|
bleomycin
|
|
α-SMA
|
α-smooth muscle actin
|
|
Col1a1
|
collagen type I alpha 1 chain
|
|
Col1a2
|
collagen type I alpha 2 chain
|
|
CTGF
|
connective tissue growth factor
|
|
PAI-1
|
plasminogen activator inhibitor-1
|
|
MCP-1
|
monocyte chemoattractant protein-1
|
|
GSH
|
glutathione
|
|
SOD
|
superoxide dismutase
|
|
MDA
|
malondialdehyde
|
|
Nrf2
|
nuclear factor erythroid-2-related
factor 2
|
|
HO-1
|
heme oxygenase-1
|
|
Con
|
control
|
|
H&E
|
hematoxylin and eosin
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
References
|
1
|
Zhang J, Li Y, Bai X, Li Y, Shi J and Hu
D: Recent advances in hypertrophic scar. Histol Histopathol.
33:27–39. 2018.PubMed/NCBI
|
|
2
|
Niessen FB, Spauwen PH, Schalkwijk J and
Kon M: On the nature of hypertrophic scars and keloids: A review.
Plast Reconstr Surg. 104:1435–1458. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tyack Z, Simons M, Spinks A and Wasiak J:
A systematic review of the quality of burn scar rating scales for
clinical and research use. Burns. 38:6–18. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brown BC, McKenna SP, Siddhi K, McGrouther
DA and Bayat A: The hidden cost of skin scars: Quality of life
after skin scarring. J Plast Reconstr Aesthet Surg. 61:1049–1058.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bloemen MC, van der Veer WM, Ulrich MM,
van Zuijlen PP, Niessen FB and Middelkoop E: Prevention and
curative management of hypertrophic scar formation. Burns.
35:463–475. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jinnin M: Mechanisms of skin fibrosis in
systemic sclerosis. J Dermatol. 37:11–25. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cho MK, Jang YP, Kim YC and Kim SG:
Arctigenin, a phenylpropanoid dibenzylbutyrolactone lignan,
inhibits MAP kinases and AP-1 activation via potent MKK inhibition:
The role in TNF-alpha inhibition. Int Immunopharmacol. 4:1419–1429.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gao Q, Yang M and Zuo Z: Overview of the
anti-inflammatory effects, pharmacokinetic properties and clinical
efficacies of arctigenin and arctiin from Arctium lappa L.
Acta Pharmacol Sin. 39:787–801. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhong Y, Lee K, Deng Y, Ma Y, Chen Y, Li
X, Wei C, Yang S, Wang T, Wong NJ, et al: Arctigenin attenuates
diabetic kidney disease through the activation of PP2A in
podocytes. Nat Commun. 10:45232019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu Z, Yan J, Jiang W, Yao XG, Chen J,
Chen L, Li C, Hu L, Jiang H and Shen X: Arctigenin effectively
ameliorates memory impairment in Alzheimer's disease model mice
targeting both β-amyloid production and clearance. J Neurosci.
33:13138–13149. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee JY and Kim CJ: Arctigenin, a
phenylpropanoid dibenzylbutyrolactone lignan, inhibits type I–IV
allergic inflammation and pro-inflammatory enzymes. Arch Pharm Res.
33:947–957. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee JY, Cho BJ, Park TW, Park BE, Kim SJ,
Sim SS and Kim CJ: Dibenzylbutyrolactone lignans from Forsythia
koreana fruits attenuate lipopolysaccharide-induced inducible
nitric oxide synthetase and cyclooxygenase-2 expressions through
activation of nuclear factor-κb and mitogen-activated protein
kinase in RAW264.7 cells. Biol Pharm Bull. 33:1847–1853. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin CY, Hsieh PL, Liao YW, Peng CY, Yu CC
and Lu MY: Arctigenin reduces myofibroblast activities in oral
submucous fibrosis by LINC00974 inhibition. Int J Mol Sci.
20:13282019. View Article : Google Scholar
|
|
14
|
Jin G, Su Y, Dong Q, Zhao X, Zhang L and
Yan X: Arctigenin alleviates TGF-β1-induced epithelial-mesenchymal
transition and PAI-1 expression via AMPK/NF-κB pathway in
peritoneal mesothelial cells. Biochem Biophys Res Commun.
520:413–419. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li A, Zhang X, Shu M, Wu M, Wang J, Zhang
J, Wang R, Li P and Wang Y: Arctigenin suppresses renal
interstitial fibrosis in a rat model of obstructive nephropathy.
Phytomedicine. 30:28–41. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li A, Wang J, Zhu D, Zhang X, Pan R and
Wang R: Arctigenin suppresses transforming growth factor-β1-induced
expression of monocyte chemoattractant protein-1 and the subsequent
epithelial-mesenchymal transition through reactive oxygen
species-dependent ERK/NF-κB signaling pathway in renal tubular
epithelial cells. Free Radic Res. 49:1095–1113. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li A, Wang J, Wu M, Zhang X and Zhang H:
The inhibition of activated hepatic stellate cells proliferation by
arctigenin through G0/G1 phase cell cycle arrest: Persistent
p27(Kip1) induction by interfering with PI3K/Akt/FOXO3a signaling
pathway. Eur J Pharmacol. 747:71–87. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dudgeon C, Shreeram S, Tanoue K, Mazur SJ,
Sayadi A, Robinson RC, Appella E and Bulavin DV: Genetic variants
and mutations of PPM1D control the response to DNA damage. Cell
Cycle. 12:2656–2664. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sekiguchi A, Motegi SI, Fujiwara C,
Yamazaki S, Inoue Y, Uchiyama A, Akai R, Iwawaki T and Ishikawa O:
Inhibitory effect of kaempferol on skin fibrosis in systemic
sclerosis by the suppression of oxidative stress. J Dermatol Sci.
96:8–17. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fujiwara C, Uehara A, Sekiguchi A,
Uchiyama A, Yamazaki S, Ogino S, Yokoyama Y, Torii R, Hosoi M, Suto
C, et al: Suppressive regulation by MFG-E8 of latent transforming
growth factor β-induced fibrosis via binding to αv integrin:
Significance in the pathogenesis of fibrosis in systemic sclerosis.
Arthritis Rheumatol. 71:302–314. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Thoua NM, Derrett-Smith EC, Khan K, Dooley
A, Shi-Wen X and Denton CP: Gut fibrosis with altered colonic
contractility in a mouse model of scleroderma. Rheumatology
(Oxford). 51:1989–1998. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou CF, Yu JF, Zhang JX, Jiang T, Xu SH,
Yu QY and Zhu QX: N-acetylcysteine attenuates subcutaneous
administration of bleomycin-induced skin fibrosis and oxidative
stress in a mouse model of scleroderma. Clin Exp Dermatol.
38:403–409. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Umasuthan N, Bathige SD, Revathy KS, Lee
Y, Whang I, Choi CY, Park HC and Lee J: A manganese superoxide
dismutase (MnSOD) from Ruditapes philippinarum: Comparative
structural- and expressional-analysis with copper/zinc superoxide
dismutase (Cu/ZnSOD) and biochemical analysis of its antioxidant
activities. Fish Shellfish Immunol. 33:753–765. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu Q, Lu J, Lin J, Tang Y, Pu W, Shi X,
Jiang S, Liu J, Ma Y, Li Y, et al: Salvianolic acid B attenuates
experimental skin fibrosis of systemic sclerosis. Biomed
Pharmacother. 110:546–553. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ju W, Zhihong Y, Zhiyou Z, Qin H, Dingding
W, Li S, Baowei Z, Xing W, Ying H and An H: Inhibition of alpha-SMA
by the ectodomain of FGFR2c attenuates lung fibrosis. Mol Med.
18:992–1002. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Verrecchia F, Mauviel A and Farge D:
Transforming growth factor-beta signaling through the Smad
proteins: Role in systemic sclerosis. Autoimmun Rev. 5:563–569.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang J, Qiao Q, Liu M, He T, Shi J, Bai
X, Zhang Y, Li Y, Cai W, Han S, et al: IL-17 promotes scar
formation by inducing macrophage infiltration. Am J Pathol.
188:1693–1702. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shan S, Zhang Y, Wu M, Yi B, Wang J and Li
Q: Naringenin attenuates fibroblast activation and inflammatory
response in a mechanical stretch-induced hypertrophic scar mouse
model. Mol Med Rep. 16:4643–4649. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Christmann RB and Lafyatis R: The cytokine
language of monocytes and macrophages in systemic sclerosis.
Arthritis Res Ther. 12:1462010. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vona R, Giovannetti A, Gambardella L,
Malorni W, Pietraforte D and Straface E: Oxidative stress in the
pathogenesis of systemic scleroderma: An overview. J Cell Mol Med.
22:3308–3314. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shroff A, Mamalis A and Jagdeo J:
Oxidative stress and skin fibrosis. Curr Pathobiol Rep. 2:257–267.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu W, Peng G, Yang F, Zhang Y, Mu Z and
Han X: Sulforaphane has a therapeutic effect in an atopic
dermatitis murine model and activates the Nrf2/HO1 axis. Mol Med
Rep. 20:1761–1771. 2019.PubMed/NCBI
|
|
34
|
Kavian N, Mehlal S, Jeljeli M, Saidu NEB,
Nicco C, Cerles O, Chouzenoux S, Cauvet A, Camus C, Ait-Djoudi M,
et al: The Nrf2-antioxidant response element signaling pathway
controls fibrosis and autoimmunity in scleroderma. Front Immunol.
9:18962018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Katz L and Baltz RH: Natural product
discovery: Past, present, and future. J Ind Microbiol Biotechnol.
43:155–176. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Atanasov AG, Waltenberger B,
Pferschy-Wenzig EM, Linder T, Wawrosch C, Uhrin P, Temml V, Wang L,
Schwaiger S, Heiss EH, et al: Discovery and resupply of
pharmacologically active plant-derived natural products: A review.
Biotechnol Adv. 33:1582–1614. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Won YD, Na MK, Kim CH, Kim JM, Cheong JH,
Ryu JI and Han MH: The frontal skull Hounsfield unit value can
predict ventricular enlargement in patients with subarachnoid
haemorrhage. Sci Rep. 8:101782018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu S, Taghavi R and Leask A: Connective
tissue growth factor is induced in bleomycin-induced skin
scleroderma. J Cell Commun Signal. 4:25–30. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chung EJ, McKay-Corkum G, Chung S, White
A, Scroggins BT, Mitchell JB, Mulligan-Kehoe MJ and Citrin D:
Truncated plasminogen activator inhibitor-1 protein protects from
pulmonary fibrosis mediated by irradiation in a murine model. Int J
Radiat Oncol Biol Phys. 94:1163–1172. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lichtman MK, Otero-Vinas M and Falanga V:
Transforming growth factor beta (TGF-β) isoforms in wound healing
and fibrosis. Wound Repair Regen. 24:215–222. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Selvarajah B, Azuelos I, Platé M,
Guillotin D, Forty EJ, Contento G, Woodcock HV, Redding M, Taylor
A, Brunori G, et al: mTORC1 amplifies the ATF4-dependent de novo
serine-glycine pathway to supply glycine during
TGF-β1-induced collagen biosynthesis. Sci Signal.
12:eaav30482019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lima JDCC, Simoes E, de Castro G, Morais
MRPT, de Matos-Neto EM, Alves MJ, Pinto NI, Figueredo RG, Zorn TMT,
Felipe-Silva AS, et al: Tumour-derived transforming growth factor-β
signalling contributes to fibrosis in patients with cancer
cachexia. J Cachexia Sarcopenia Muscle. 10:1045–1059. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhong L, Simard MJ and Huot J: Endothelial
microRNAs regulating the NF-κB pathway and cell adhesion molecules
during inflammation. FASEB J. 32:4070–4084. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Doridot L, Jeljeli M, Chêne C and Batteux
F: Implication of oxidative stress in the pathogenesis of systemic
sclerosis via inflammation, autoimmunity and fibrosis. Redox Biol.
25:1011222019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhou CF, Zhou DC, Zhang JX, Wang F, Cha
WS, Wu CH and Zhu QX: Bleomycin-induced epithelial-mesenchymal
transition in sclerotic skin of mice: Possible role of oxidative
stress in the pathogenesis. Toxicol Appl Pharmacol. 277:250–258.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Czerska M, Mikolajewska K, Zieliński M,
Gromadzińska J and Wąsowicz W: Today's oxidative stress markers.
Med Pr. 66:393–405. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yang HY and Lee TH: Antioxidant enzymes as
redox-based biomarkers: A brief review. BMB Rep. 48:200–208. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liu Z, Wang J, Huang E, Gao S, Li H, Lu J,
Tian K, Little PJ, Shen X, Xu S and Liu P: Tanshinone IIA
suppresses cholesterol accumulation in human macrophages: Role of
heme oxygenase-1. J Lipid Res. 55:201–213. 2014. View Article : Google Scholar : PubMed/NCBI
|