Introduction
Osteoporosis is a common orthopedic disease
featuring a decrease in bone mass and an increase in bone
fragility. It is characterized by low bone mineral density (BMD),
which is usually measured by traditional Dual energy X-ray methods
or optimized methods (1). These
classical methods are able to provide accurate BMD data; however,
they cannot provide information that predicts early bone loss. On
the other hand, if the early decline of bone mass can be
identified, improved measures to prevent the occurrence and
development of osteoporosis may be taken. Certain studies have
focused their attention on exploring new measures to predict BMD by
assessing bone turnover markers (2), C-C motif chemokine ligand
(CCL)11/eotaxin-1 (3), calcium
isotope (4), circular RNAs
(5), metabolites (6), gene expression microarray analyses
(7) and transforming growth
factor-β3 (8). If any of these
factors were revealed to be able to indicate premature bone loss,
they would hold great promise for the prevention of
osteoporosis.
Inflammatory reactions are frequently accompanied by
the occurrence of bone loss. The term osteoimmunology was adopted
by Arron and Choi (9).
Subsequently, it was demonstrated that interleukin (IL)-1β
(10), IL-6 (11–15),
IL-10 (16,17) and IL-13 (18) are closely linked to osteoporosis.
Other biomarkers were also indicated to be associated with BMD. For
instance, early bone loss is frequently accompanied by changes in
the body microenvironments, in particular, changes in serum
cytokine levels, such as CC motif ligand 4 (CCL4)/macrophage
inflammatory protein-1β (MIP-1β) (19), sclerostin (20) and neuropeptide vasoactive
intestinal peptide (21). Based on
this serum screening method, the present study explored which of
the various cytokines may be used as predictors of early bone
loss.
In the present study, a rat model of
ovariectomy-induced bone loss was successfully established to
represent early osteoporosis in humans. The animals demonstrated
progressive bone loss starting from four weeks after surgery. In
the protein array screening, several cytokines in the rat serum
were noted to be associated with the development of osteoporosis.
Specifically, increased serum levels of CCL2 and C-X-C motif
chemokine ligand 1 (CXCL1) were significantly associated with
decreased BMD. Validation in clinical patient samples demonstrated
that the cytokines CCL2 and CXCL1 were also significantly
associated with reduced BMD in humans. Through linear regression
analysis, linear regression equations between these two cytokines
and changes in bone mass were obtained, from which the change in
bone density may be predicted. Overall, the present study suggested
that serum levels of CCL2 and CXCL1 could be used to predict the
decline in BMD at an early stage in individuals in a rapid and
non-invasive manner.
Materials and methods
Establishment of rat model of early
bone loss
Female Sprague Dawley rats (n=24; age, 12 weeks;
body weight, 240±16.9 g) were purchased from the Experimental
Animal Center of the Fourth Military Medical University (Xi'an,
China). The rats were housed with free access to food and water (12
h light/dark cycle, 20°C and 50–55% humidity). The rats were
randomly divided into two groups: An ovariectomy group (OVX, n=12)
and a sham group (Sham, n=12). To establish the early bone loss
model, all 12 rats in the OVX group underwent ovariectomy following
anesthetization by intraperitoneal injection of pentobarbital at a
dose of 40 mg/kg. All 12 rats in the sham group underwent ovary
ablation following the same anesthetization method. At 2, 4, 6 and
8 weeks after the surgical procedures, serum samples were collected
from the animals in each group. All animal experimental procedures
were approved by the Ethics in Experimental Animal Center of the
Fourth Military Medical University (permission code
IACUC-20190112).
Confirmation of early bone loss by
µCT
At 2-week intervals over 2 months following the
ovariectomy or sham operations (time-points of 2, 4, 6 and 8
weeks), each rat was anesthetized with pentobarbital at a dose of
40 mg/kg during µCT scanning. The American Society for Bone and
Mineral Research recommendations for small-animal µCT (22) were followed during the process of
µCT analysis. A pre-clinical Inveon µCT system (Siemens
Healthineers) with a resolution of 8 mm, tube current of 0.1 mA and
tube voltage of 50 kV was used to scan distal femurs. The
three-dimensional quantitative analyses were performed using the
µCT system (Inveon Research Workplace 2.2; Siemens Healthineers).
Scanning regions were confined from the distal metaphysis and
extended 2.0 mm proximally from the proximal tip of the primary
spongiosa. The following three-dimensional indices in the defined
region of interest were determined: BMD, trabecular thickness
(Tb.Th) and relative bone volume over the total volume (BV/TV, %).
The examiner performing the scan analyses was blinded to the
experimental group of the subjects.
Serum cytokine array analyses
Blood samples (1 ml) were obtained from the
retro-orbital veins of each animal at 2, 4, 6 and 8 weeks following
surgery. Rat serum samples collected every two weeks post-surgery
were assessed for the presence of 78 cytokines (Table SI) using the Quantibody Rat
Cytokine Array kit (RayBiotech Inc.) according to the
manufacturer's protocol (Fig.
S1). In brief, antibody array membranes were blocked with
Tris-buffered saline supplemented with 5% skimmed milk and 0.05%
Tween-20 for 1 h, followed by the addition of rat serum to obtain
final 10-fold dilutions. The fluorescence brightness was detected
and quantified on a fluorescent scanner (Axon GenePix; Molecular
Devices, LLC) to determine the cytokine expression levels.
Human BMD detection and blood serum
sample collection
A total of 24 individuals aged 15–75 years from
Xijing Hospital (Xi'an, China) were recruited and provided informed
signed consent, prior to being enrolled in the present study as
human subjects. The experiment was approved by the Ethics Committee
of Xijing Hospital of Fourth Military Medical University (Xi'an,
China; permission code: XJYYLL-2014076). The demographic data of
the patients are presented in Table
SII. The patients had a normal hepatorenal function and were
excluded if they had a history of cardiovascular disease, cancer or
diabetes mellitus. Each human subject signed an informed consent
document prior to study enrollment. None of the subjects had been
diagnosed with any metabolic bone diseases nor had they been
treated with any medications known to affect bone metabolism. In
each subject, the BMD of the lumbar spine (L1-4) was measured using
dual-energy X-ray absorptiometry scanners (QDR4500; Hologic, Inc.).
Blood samples were collected in non-anticoagulated blood collection
tubes at 4°C to obtain serum supernatants, which were then stored
at −80°C for further evaluation using ELISA.
Human serum ELISA and KEGG pathway
maps
Human serum cytokine expression levels (CCL2 and
CXCL1) were measured with ELISA assay kits (cat. nos. P13500 and
P09341; RayBiotech, Inc.) according to the manufacturer's
protocols. The total concentration of each cytokine was estimated
using the Bradford protein assay method. In addition, Kyoto
Encyclopedia of Genes and Genomes (KEGG) pathway analysis was used
to identify possible pathways related to CCL2 and CXCL1 signaling
in osteoporosis (23–25).
Statistical analysis
Statistical analyses were performed with SPSS
software, version 22 (IBM Corp.). Quantitative data are expressed
as the mean ± standard deviation. Statistical tests were performed
by two-way analysis of variance (ANOVA). ANOVA followed by the
Bonferroni post-hoc test was performed for comparisons among
multiple groups. A Pearson correlation was employed to determine
the linear correlation between two variables. P<0.05 was
considered to indicate a statistically significant difference.
Results
Confirmation of early bone loss in an
ovariectomized rat model
In order to track the occurrence of early bone loss,
the BMDs and other bone parameters of rats were measured at two
weeks following surgery. The rat distal femur structure was
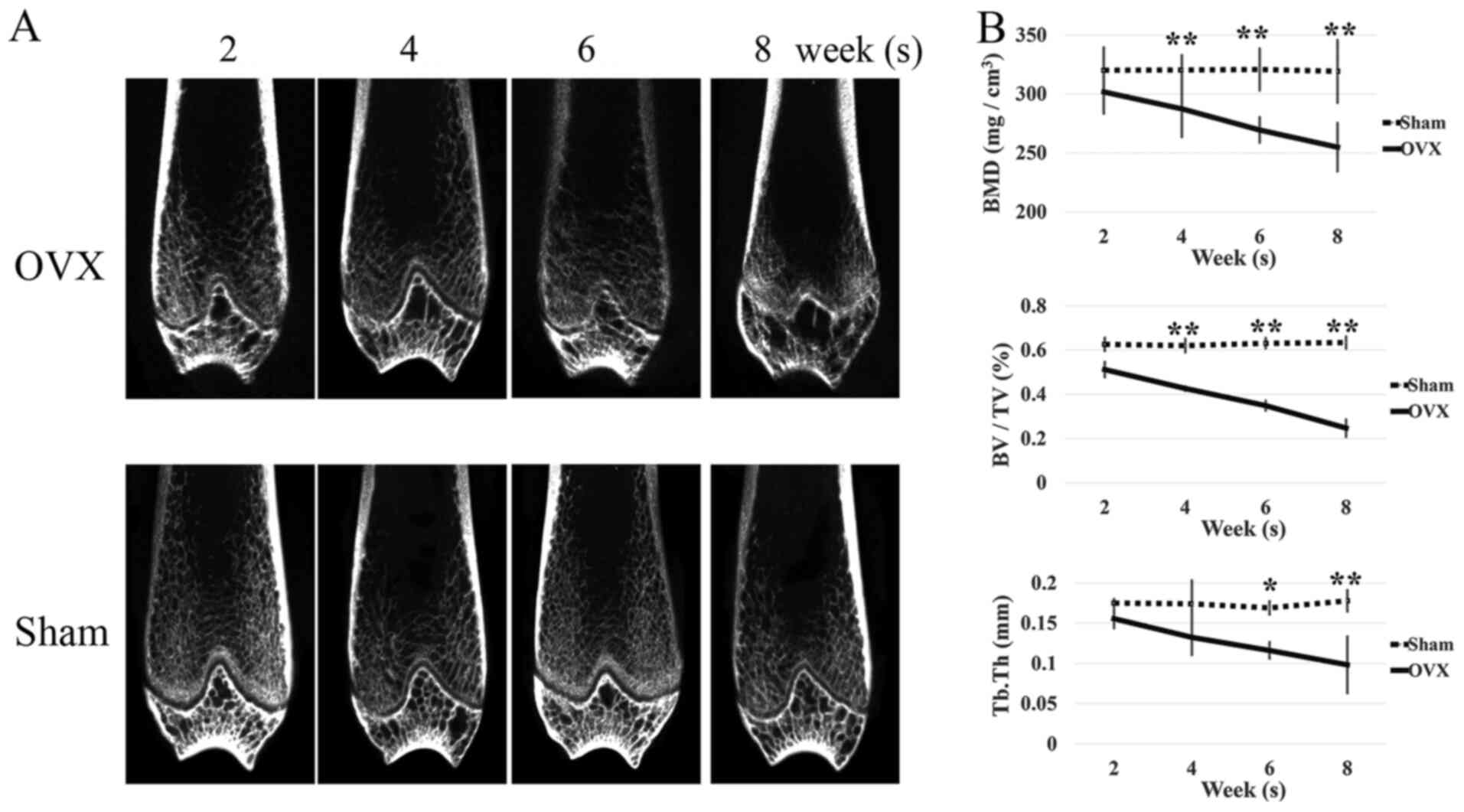
reconstructed based on CT scan images (Fig. 1A). At the fourth week after
surgery, the BMD of the rats in the ovariectomized group began to
decrease significantly. At the eighth week, the BMD decreased by
>15% (Fig. 1B). In the sham
group, the BMD of rats did not change significantly at these
time-points. Along with the decrease of BMD, quantitative analysis
of further bone parameters in the ovariectomized group revealed a
significant decrease in BV/TV at 4–8 weeks (Fig. 1B) and Tb.Th at 6–8 weeks (Fig. 1B) post-surgery (P<0.05).
Screening for cytokines associated
with early bone loss by protein array
A total of 78 serum cytokines were included in the
protein array screening assay. By analysis of fluorescence
intensities, it was revealed that in the OVX group, 20 serum
cytokines increased or decreased steadily from 2 weeks
post-surgery, while they remained unchanged in the Sham group. A
total of 10 cytokines [proopiomelanocortin, β-catenin, CCL2, CXCL1,
tumor necrosis factor super family member 6, follistatin-like 1,
colony-stimulating factor 2, CXCL6, matrix metalloproteinase
(MMP)-8 and hypocretin neuropeptide precursor] were increased
following ovariectomy (Fig. 2A),
whereas another 10 cytokines (brain-derived neurotrophic factor,
C-C motif chemokine receptor 4, C-X-C motif chemokine receptor 4,
GDNF family receptor α4, IL-2, growth hormone 1, MMP-13,
resistin-like β, Toll-like receptor 4 and IL-3) exhibited the
opposite trend (Fig. 2B, Table SIII). The other 58 cytokines
assessed showed insignificant changes between the OVX and the Sham
group over the time-course (Fig.
S2).
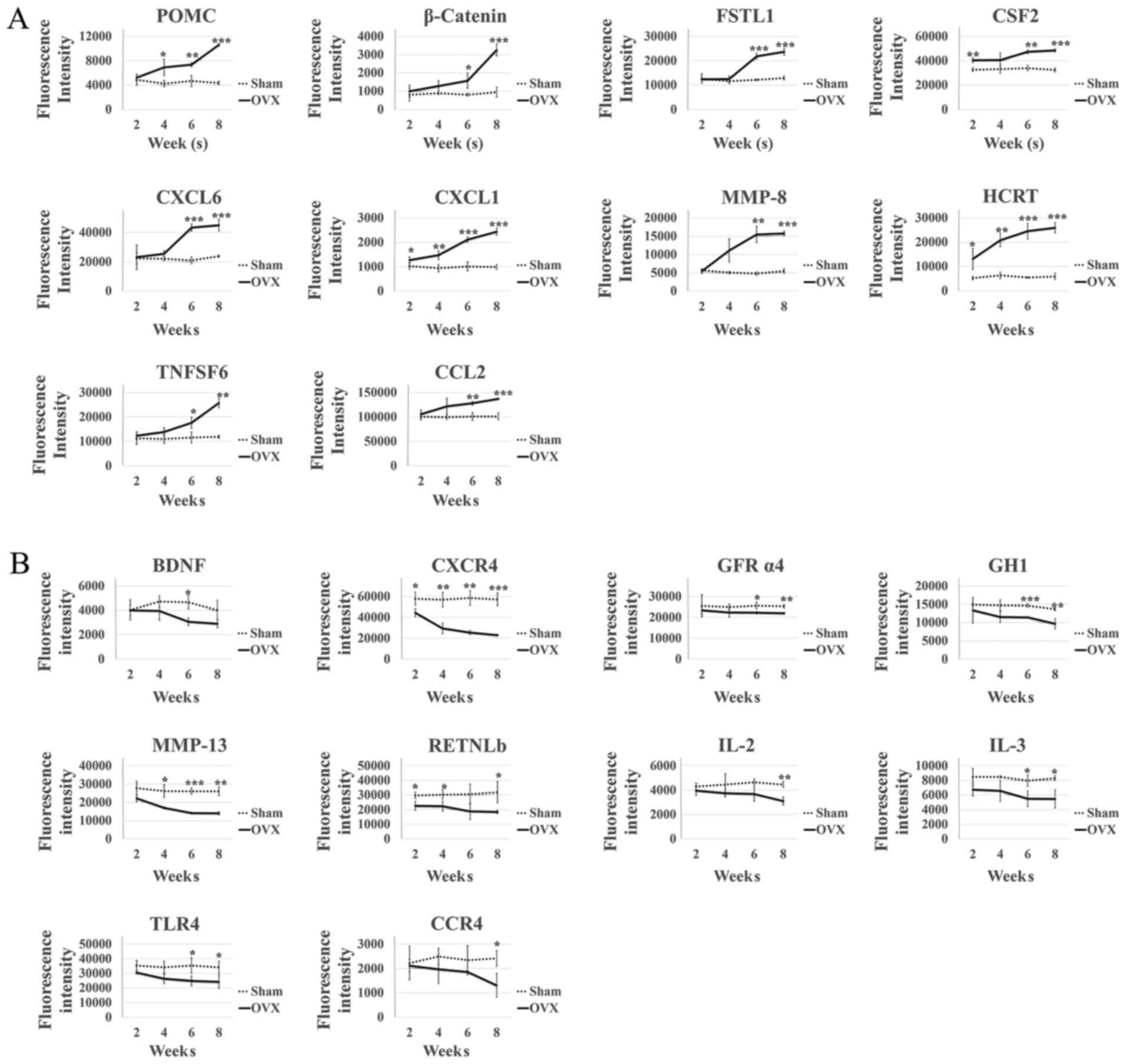 | Figure 2.Screening results of bone
loss-associated biomarkers using protein array. Dynamic alterations
of serum cytokines in the sham or ovariectomized rats at 2, 4, 6
and 8 weeks following surgery. Each sample was repeatedly measured
in three wells in the protein array. The fluorescence intensity was
normalized by means of 8 control wells. (A) The cytokines that
increased following ovariectomy. (B) The 10 cytokines that
decreased following ovariectomy. *P<0.05, **P<0.01,
***P<0.001 vs. sham group (n=3). OVX, ovariectomy group; CCL2,
C-C motif chemokine ligand 2; CXCL1, C-X-C motif chemokine ligand
1; CXCR4, C-X-C motif chemokine receptor 4; MMP, matrix
metalloproteinase; TLR, Toll-like receptor; IL, interleukin; BDNF,
brain-derived neurotrophic factor; GFR, GDNF family receptor; CSF,
colony-stimulatory factor; POMC, proopiomelanocortin; FSTL1,
follistatin-like 1; HCRT, hypocretin neuropeptide precursor; TNSF;
tumor necrosis factor superfamily member; GH, growth hormone. |
Linear regression analyses of
cytokines and bone mass loss in the ovariectomized rat models
After protein array screening, 20 cytokines were
noted to be elevated or decreased in the serum during the early
stage of bone loss in the present model. However, whether they were
also correlated with bone loss progression remained to be
elucidated. To verify the association of these 20 cytokines with
the progression of early bone loss, the correlation between the
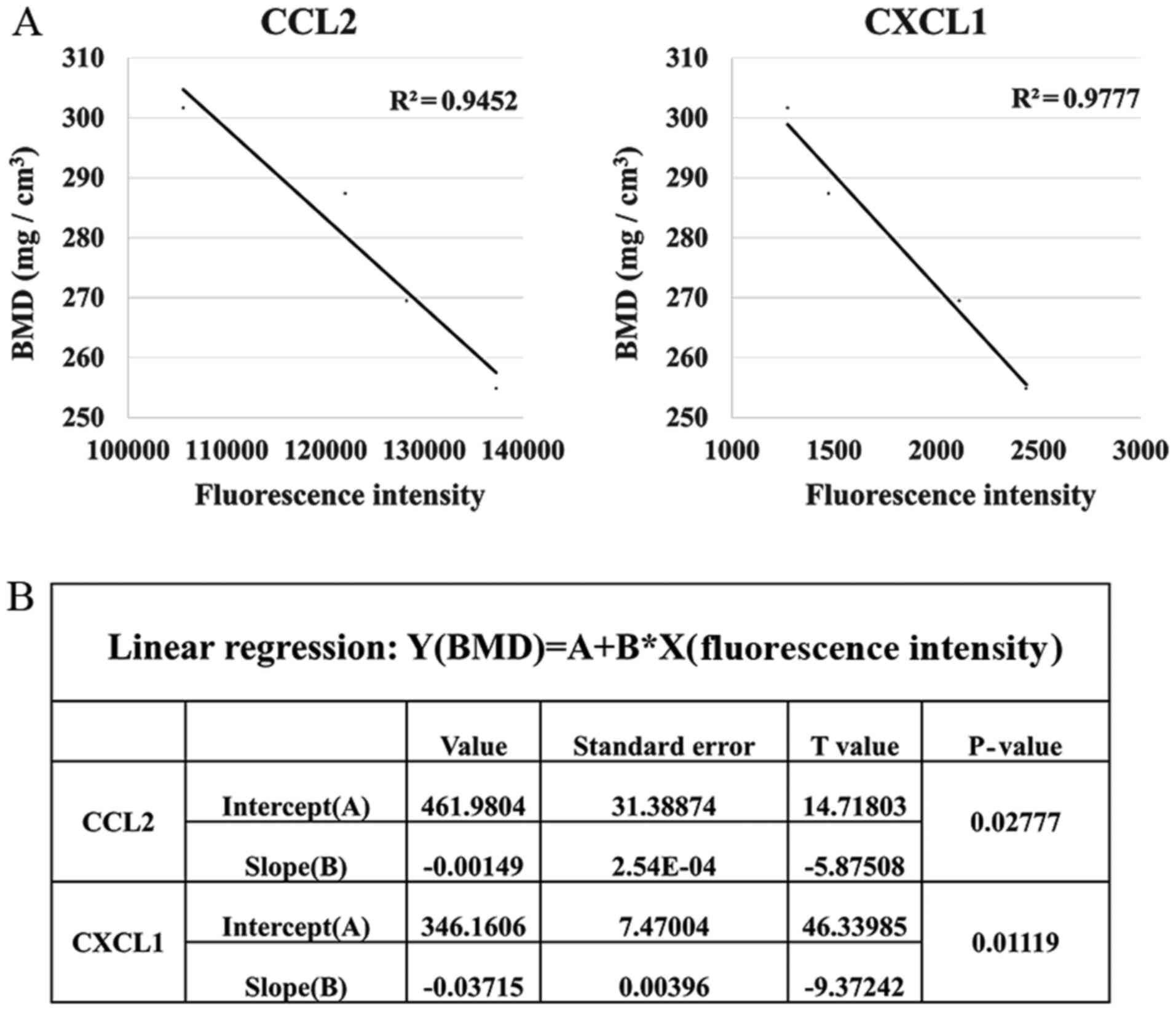
cytokine levels and BMD of ovariectomized rats was analyzed. Among
the 20 cytokines, CCL2 and CXCL1 were inversely correlated with the
BMD of the ovariectomized rats (P<0.05, Fig. 3). By contrast, the other 18
cytokines did not exhibit any significant correlations with BMD
(P>0.05; Fig. S3, Table SIV).
Utility of CCL2 and CXCL1 in
reflecting early bone mass reduction in human serum array
To further verify the clinical significance of the
serum cytokines of CCL2 and CXCL1 as potential predictors of early
bone loss, the present study detected the serum levels of human
CCL2 and CXCL1 in clinical patients (mean age, 52.87 years old;
female/male patients, 18/6; BMD for the subjects is provided in
Table SII, 9 individuals had
osteoporosis, 12 postmenopausal females were included) using
commercially available ELISA kits. Linear regression analysis
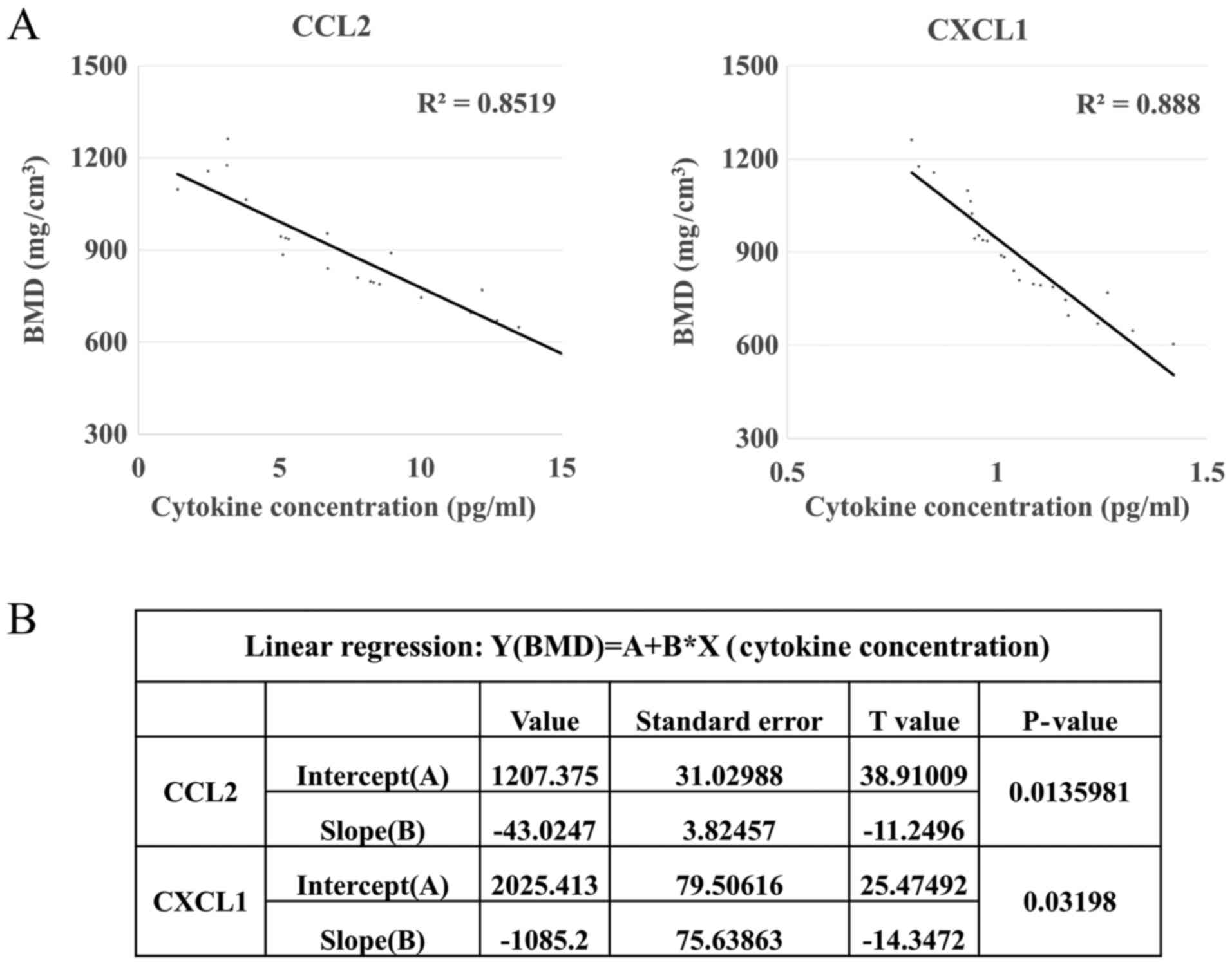
between CCL2 and CXCL1 levels and human BMD was then performed. The
results demonstrated that these two candidate cytokines, CCL2 and
CXCL1, were positively correlated with bone loss in humans
(P<0.05; Fig. 4). The linear
regression equation between CCL2 and BMD was Y (BMD,
mg/cm3)=1,207.375–43.0247*X (CCL2, pg/ml). The linear
regression equation between CXCL1 and BMD was Y (BMD,
mg/cm3)=2,025.413–1,085.2*X (CXCL1, pg/ml). When the
serum levels of CCL2 and CXCL1 increase to 6.2 and 1.0 pg/ml,
respectively, the BMD drops below 940 mg/cm3 (T-value
<-1), which is considered to be indicative of early bone loss in
the clinic. Applying these equations, CCL2 and CXCL1 serum levels
may be used to deduce an individual's BMD value and predict
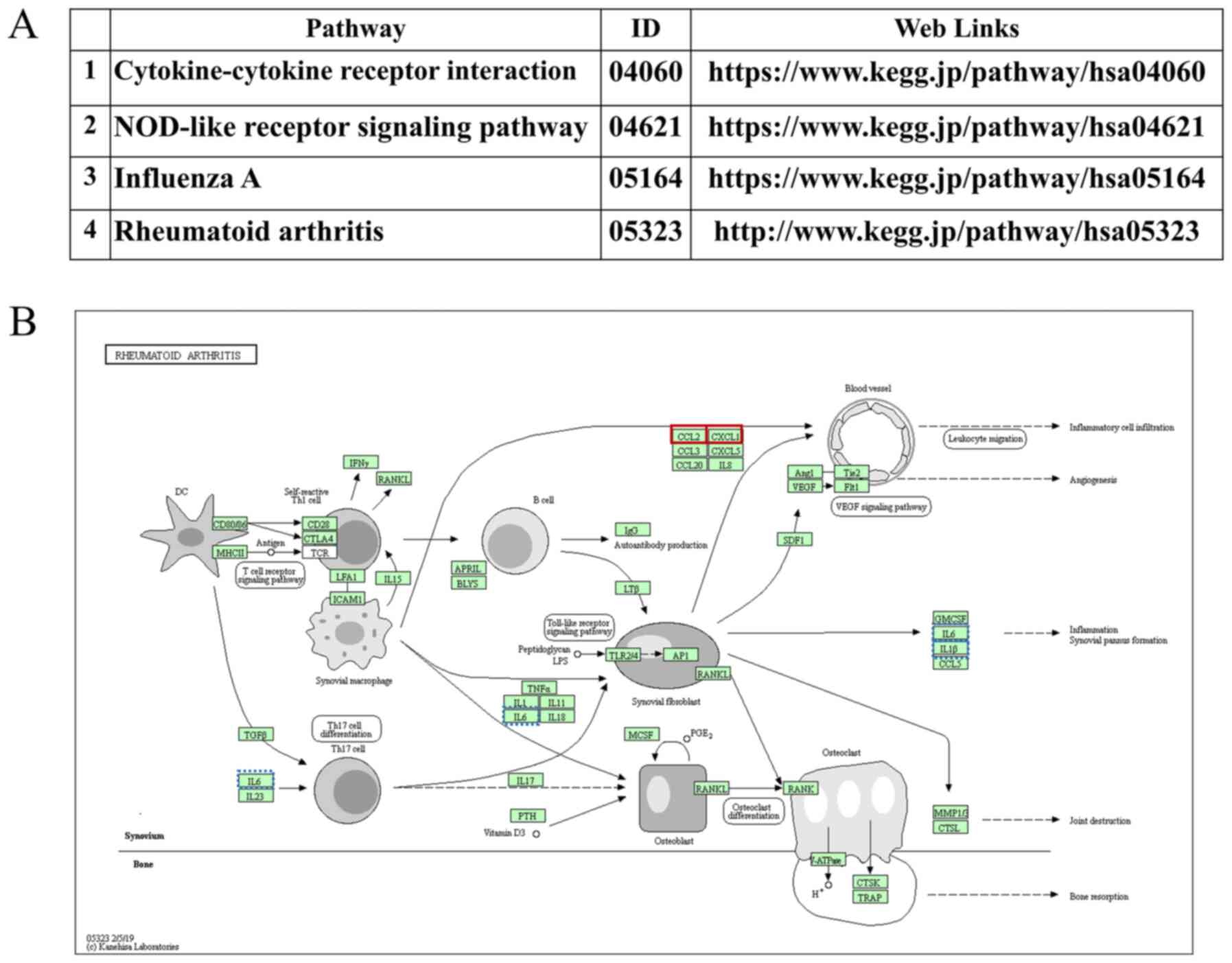
premature bone loss in humans. In addition, KEGG pathway analysis
revealed the involvement of typical osteoporosis-associated ILs in
the same signaling pathways as CCL2 and CXCL1 (Figs. 5 and S4). Furthermore, according to the
present results, these ILs were associated with decreased bone mass
(Fig. S4).
Discussion
Osteoporosis is a common and severe degenerative
disease. If osteoporosis were to be identified at its early stage,
its development could be delayed and it would be possible to avoid
substantial economic losses. In the present study, serum protein
array screening in an ovariectomy-induced rat model of bone loss
revealed that a total of 20 serum cytokines were aberrantly
expressed in parallel with the development of early bone loss. Of
the 20 potential markers, CCL2 and CXCL1 were further validated to
be correlated with bone loss in the ovariectomy-induced
osteoporosis rat model (resembled postmenopausal osteoporosis in
humans). Postmenopausal osteoporosis is one of the most common
skeletal diseases, for which practical methods for early diagnosis
are lacking (3). Of note, in the
present study, CCL2 and CXCL1 increased with the progression of
postmenopausal osteoporosis, which may provide an effective and
promising way to predict postmenopausal early bone loss. Further
statistical analysis of results obtained with human subjects
suggested that this novel prediction method may be used for early
diagnosis of osteoporosis, which may assist in the implementation
of interventions for osteoporosis in a timely manner. The human
subjects of the present study were not only patients with
postmenopausal osteoporosis, but the cohort reflected
age-associated osteoporosis, indicating that these two predictive
markers may be universally applicable for age-associated
osteoporosis.
CCL2 and CXCL1 belong to the superfamily of
chemokines. CCL2 (also known as monocyte chemoattractant protein 1)
is a secreted protein involved in immunoregulatory and inflammatory
processes. CCL2 has roles in systemic inflammation (26), enhancing the efficacy of
immunotherapy (27) and also
promoting the migration of tumor cells and macrophage-like cells
(28). CXCL1 is a member of the
CXC subfamily of chemokines and has a role in inflammation as a
chemoattractant for neutrophils. CXCL1 may promote the
proliferation of neural stem cells (29), restore neutrophil migration
(30) and inhibit
paclitaxel-induced peripheral neuropathy in mice (31). In the inflammatory response, these
two factors may be detected simultaneously, suggesting that they
may also function together. Respective induction of CXCL1 and CCL2
in spinal cord astrocytes and neurons may contribute to neuropathic
pain (32). Neuroinflammation in
chronic pain conditions involves CCL2 and CXCL1 and recent studies
suggested that bone marrow stem cells (BMSCs) produced potent
analgesic effects in animal models of inflammatory pain,
neuropathic pain and cancer pain (33). Microglia-derived IL-1β promoted
CCL2 and CXCL1 expression by Müller cells in focal retinal
degeneration (34).
Current bone-associated studies have indicated a
definite relationship between bone marrow/BMSCs and CCL2. Enhanced
levels of CCL2 were discovered in the bone marrow of septic mice
(35). B-cell acute lymphoblastic
leukemia cell-derived CCL2 was reported to increase periostin
levels in BMSCs (36). The present
study also confirmed the role of CCL2 in bone, as the serum levels
of CCL2 reflected changes in bone loss. Furthermore, a linear
correlation between CXCL1 and the decline in BMD was revealed in
the present study, while there are currently only a few reports on
the correlation between CXCL1 and bone mass (37). The specificity of CCL2 and CXCL2
for osteoporosis may be limited, as they are general chemokines
that may not be specific for osteoporosis. This research should be
further assessed in the future. Through retrieval of pathway
information from KEGG, it was revealed that CCL2, CXCL1 and
numerous ILs are involved in certain pathways together. Previous
studies indicated that IL-1β and IL-6 are significantly associated
with osteoporosis (38). In the
serum cytokine array of the present study, they all displayed a
significant decreasing trend from two to six weeks after surgery,
followed by an increasing trend through to the eighth week. From
these results, it may be expected that during the process of bone
loss, the body's inflammatory response also changes from an initial
decrease to a delayed rise, and finally, there would be significant
inflammation during osteoporosis (39,40).
However, this phenomenon requires to be evidenced in future
studies.
Estrogen deficiency, systemic inflammation and these
two cytokines (CCL2 and CXCL1) are closely linked and these two
cytokines have been reported to respond to systemic inflammation
(26,41). Although estrogen deficiency may
induce higher CCL2 expression levels (42), the present study revealed that the
trends of these two cytokines were still present in age-associated
osteoporosis. Accordingly, it may be more likely that CCL2 and
CXCL1 are indicators of systemic inflammation rather than estrogen
deficiency. Furthermore, studies demonstrated that CCL2 and CXCL1
were involved in physiological bone remodeling, CCL2 was primarily
expressed by bone-forming osteoblasts (43) and these two cytokines mediated
osteoclastogenesis and accelerated osteoclast maturation (37,44–46).
These results may explain why the progress of bone loss was
accompanied by increasing serum levels of CCL2 and CXCL1 in the
present study.
In previous studies exploring BMD prediction
methods, the genetic risk score was assessed (47) and a genome-wide association study
was performed (48), which were
costly and DNA sequencing was tedious. Certain studies have also
made rigorous attempts to identify associations between serum
markers and bone loss. In a 10-year follow-up study based on a
large cohort of human subjects, investigators failed to identify a
significant association between serum testosterone and bone mass
loss (49). The present trial was
based on a small cross-sectional cohort of 24 subjects and there
were limitations of validation and long-term follow-up.
Verification of the present method in a large-sample population and
application of clinical testing in the future may improve the
quality of life of patients while lowering costs and save time.
In previous studies on osteoporosis, real-time bone
density was measured, but there is currently a lack of tools to
monitor its changes, making it difficult to provide accurate
predictions on osteoporosis development. If it were possible to use
serum cytokines for predicting bone mass reduction, this may
provide advantages of convenience, accuracy and efficiency, while
avoiding the risk of radiation damage from other methods. The
present results suggested that serum levels of CCL2 and CXCL1 may
be used as a novel tool for early diagnosis and early intervention
of osteoporosis.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was funded by the National Natural Science
Foundation of China (grant nos. 81772377 and 81730065) and the
Natural Science Foundation of Shaanxi Province (grant no.
2017ZDJC-12).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YH, LY and XS designed the study; YH and LW
performed experiments; ZZ and ZL acquired data; WL, QJ, BG and JF
analyzed and interpreted data; YH and LY wrote the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All applicable international, national, and/or
institutional guidelines for the care and use of animals were
followed. All animal experimental procedures were approved by the
Ethics in Experimental Animal Center of the Fourth Military Medical
University (permission code IACUC-20190112). All procedures
involving human participants were in accordance with the ethical
standards of the institutional and/or national research committee
and with the 1964 Helsinki declaration and its later amendments or
comparable ethical standards. The experiment involving patients was
approved by the Ethics Committee of Xijing Hospital of Fourth
Military Medical University (Xi'an, China; permission code:
XJYYLL-2014076). Informed consent was obtained from all individual
participants included in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
López Picazo M, Humbert L, Di Gregorio S,
González Ballester MA and Del Río Barquero LM: Discrimination of
osteoporosis-related vertebral fractures by DXA-derived 3D
measurements: A retrospective case-control study. Osteoporosis Int.
30:1099–1110. 2019. View Article : Google Scholar
|
|
2
|
Gutierrez-Buey G, Restituto P, Botella S,
Monreal I, Colina I, Rodríguez-Fraile M, Calleja A and Varo N:
Trabecular bone score and bone remodelling markers identify
perimenopausal women at high risk of bone loss. Clin Endocrinol
(Oxf). 91:391–399. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang W, Huang CY, Wang ZP, Xu SS, Qian TY,
Chen YD and Wu WG: Serum C-C motif ligand 11/eotaxin-1 may serve as
a candidate biomarker for postmenopausal osteoporosis. J Med
Biochem. 38:353–360. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eisenhauer A, Müller M, Heuser A, Kolevica
A, Glüer CC, Both M, Laue C, Hehn UV, Kloth S, Shroff R and
Schrezenmeir J: Calcium isotope ratios in blood and urine: A new
biomarker for the diagnosis of osteoporosis. Bone Rep.
10:1002002019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang Y, Xie J and Li E: Comprehensive
circular RNA profiling reveals circ_0002060 as a potential
diagnostic biomarkers for osteoporosis. J Cell Biochem.
120:15688–15694. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang J, Yan D, Zhao A, Hou X, Zheng X,
Chen P, Bao Y and Jia W, Hu C, Zhang ZL and Jia W: Discovery of
potential biomarkers for osteoporosis using LC-MS/MS metabolomic
methods. Osteoporosis Int. 30:1491–1499. 2019. View Article : Google Scholar
|
|
7
|
Yang C, Ren J, Li B, Jin C, Ma C, Cheng C,
Sun Y and Shi X: Identification of gene biomarkers in patients with
postmenopausal osteoporosis. Mol Med Rep. 19:1065–1073.
2019.PubMed/NCBI
|
|
8
|
Haghighizadeh E, Shahrezaee M, Sharifzadeh
S and Momeni M: Transforming growth factor-β3 relation with
osteoporosis and osteoporotic fractures. J Res Med Sci. 24:462019.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Arron JR and Choi Y: Bone versus immune
system. Nature. 408:535–536. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chao TH, Yu HN, Huang CC, Liu WS, Tsai YW
and Wu WT: Association of interleukin-1 beta (−511C/T)
polymorphisms with osteoporosis in postmenopausal women. Ann Saudi
Med. 30:437–441. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wakabayashi H, Kato S, Nagao N, Miyamura
G, Naito Y and Sudo A: Interleukin-6 inhibitor suppresses
hyperalgesia without improvement in osteoporosis in a mouse pain
model of osteoporosis. Calcified Tissue Int. 104:658–666. 2019.
View Article : Google Scholar
|
|
12
|
Harmer D, Falank C and Reagan MR:
Interleukin-6 interweaves the bone marrow microenvironment, bone
loss, and multiple myeloma. Front Endocrinol (Lausanne). 9:7882018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim HJ, Kim HJ, Choi Y, Bae MK, Hwang DS,
Shin SH and Lee JY: Zoledronate enhances osteocyte-mediated
osteoclast differentiation by IL-6/RANKL axis. Int J Mol Sci.
20:14672019. View Article : Google Scholar
|
|
14
|
Edwards CJ and Williams E: The role of
interleukin-6 in rheumatoid arthritis-associated osteoporosis.
Osteoporosis Int. 21:1287–1293. 2010. View Article : Google Scholar
|
|
15
|
Blumenfeld O, Williams FMK, Valdes A, Hart
DJ, Malkin I, Spector TD and Livshits G: Association of
interleukin-6 gene polymorphisms with hand osteoarthritis and hand
osteoporosis. Cytokine. 69:94–101. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tousen Y, Matsumoto Y, Nagahata Y,
Kobayashi I, Inoue M and Ishimi Y: Resistant starch attenuates bone
loss in ovariectomised mice by regulating the intestinal microbiota
and bone-marrow inflammation. Nutrients. 11:2972019. View Article : Google Scholar
|
|
17
|
Kotrych D, Dziedziejko V, Safranow K,
Sroczynski T, Staniszewska M, Juzyszyn Z and Pawlik A: TNF-α and
IL10 gene polymorphisms in women with postmenopausal osteoporosis.
Eur J Obstet Gynecol Reprod Biol. 199:92–95. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ding Q, Zhou H, Yun B, Zhou L, Zhang N,
Yin G and Fan J: Interleukin-13 inhibits expression of cyp27b1 in
peripheral CD14+ cells that is correlated with vertebral bone
mineral density of patients with ulcerative colitis. J Cell
Biochem. 118:376–381. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang XW, Wang F, Qin RZ, Zhou QL and Huang
HX: Elevated serum CCL4/MIP-1β levels in postmenopausal
osteoporosis patients are linked with disease severity. Biomark
Med. 13:17–25. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zheng J, Maerz W, Gergei I, Kleber M,
Drechsler C, Wanner C, Brandenburg V, Reppe S, Gautvik KM,
Medina-Gomez C, et al: Mendelian randomization analysis reveals a
causal influence of circulating sclerostin levels on bone mineral
density and fractures. J Bone Miner Res. 34:1824–1836. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang W, Wang ZP, Huang CY, Chen YD, Yao WF
and Shi BM: The neuropeptide vasoactive intestinal peptide levels
in serum are inversely related to disease severity of
postmenopausal osteoporosis: A cross-sectional study. Genet Test
Mol Bioma. 23:480–486. 2019. View Article : Google Scholar
|
|
22
|
Vollherbst DF, Otto R, Do T, Kauczor HU,
Bendszus M, Sommer CM and Möhlenbruch MA: Imaging artifacts of Onyx
and PHIL on conventional CT, cone-beam CT and MRI in an animal
model. Interv Neuroradiol. 24:693–701. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kanehisa M and Goto S: KEGG: Kyoto
encyclopedia of genes and genomes. Nucleic Acids Res. 28:27–30.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kanehisa M, Sato Y, Furumichi M, Morishima
K and Tanabe M: New approach for understanding genome variations in
KEGG. Nucleic Acids Res. 47(D1): D590–D595. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kanehisa M: Toward understanding the
origin and evolution of cellular organisms. Protein Sci.
28:1947–1951. 2019. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Krause K, Sabat R, Witte Händel E, Schulze
A, Puhl V, Maurer M and Wolk K: Association of CCL2 with systemic
inflammation in Schnitzler syndrome. Br J Dermatol. 180:859–868.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang Y, Zhang X, Yang L, Xue J and Hu G:
Blockade of CCL2 enhances immunotherapeutic effect of anti-PD1 in
lung cancer. J Bone Oncol. 11:27–32. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Higashino N, Koma YI, Hosono M, Takase N,
Okamoto M, Kodaira H, Nishio M, Shigeoka M, Kakeji Y and Yokozaki
H: Fibroblast activation protein-positive fibroblasts promote tumor
progression through secretion of CCL2 and interleukin-6 in
esophageal squamous cell carcinoma. Lab Invest. 99:777–792. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shang Y, Tian L, Chen T, Liu X, Zhang J,
Liu D, Wei J, Fang W, Chen Y and Shang D: CXCL1 promotes the
proliferation of neural stem cells by stimulating the generation of
reactive oxygen species in APP/PS1 mice. Biochem Biophys Res
Commun. 515:201–206. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Salinas-Muñoz L, Campos-Fernández R,
Olivera-Valle I, Mercader E, Fernandez-Pacheco C, Lasarte S,
Pérez-Martín L, Navarro-González MT and Sánchez-Mateos: Estradiol
impairs epithelial CXCL1 gradient in the cervix to delay neutrophil
transepithelial migration during insemination. J Reprod Immunol.
132:9–15. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Manjavachi MN, Passos GF, Trevisan G,
Araújo SB, Pontes JP, Fernandes ES, Costa R and Calixto JB: Spinal
blockage of CXCL1 and its receptor CXCR2 inhibits
paclitaxel-induced peripheral neuropathy in mice.
Neuropharmacology. 151:136–143. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang ZJ, Cao DL, Zhang X, Ji RR and Gao
YJ: Chemokine contribution to neuropathic pain: Respective
induction of CXCL1 and CXCR2 in spinal cord astrocytes and neurons.
Pain. 154:2185–2197. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huh Y, Ji RR and Chen G:
Neuroinflammation, bone marrow stem cells, and chronic pain. Front
Immunol. 8:10142017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Natoli R, Fernando N, Madigan M, Chu-Tan
JA, Valter K, Provis J and Rutar M: Microglia-derived IL-1β
promotes chemokine expression by Müller cells and RPE in focal
retinal degeneration. Mol Neurodegener. 12:312017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Smirnov A, Pohlmann S, Nehring M, Ali S,
Mann-Nüttel R, Scheu S, Antoni AC, Hansen W, Büettner M, Gardiasch
MJ, et al: Sphingosine 1-phosphate- and C-C chemokine receptor
2-dependent activation of CD4+ plasmacytoid dendritic cells in the
bone marrow contributes to signs of sepsis-induced
immunosuppression. Front Immunol. 8:16222017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ma Z, Zhao X, Deng M, Huang Z, Wang J, Wu
Y, Cui D, Liu Y, Liu R and Ouyang G: Bone marrow mesenchymal
stromal cell-derived periostin promotes B-all progression by
modulating CCL2 in leukemia cells. Cell Rep. 26:1533–1543.e4. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hardaway AL, Herroon MK, Rajagurubandara E
and Podgorski I: Marrow adipocyte-derived CXCL1 and CXCL2
contribute to osteolysis in metastatic prostate cancer. Clin Exp
Metastasis. 32:353–368. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Di Munno O and Ferro F: The effect of
biologic agents on bone homeostasis in chronic inflammatory
rheumatic diseases. Clin Exp Rheumatol. 37:502–507. 2019.PubMed/NCBI
|
|
39
|
Lacativa PG and Farias ML: Osteoporosis
and inflammation. Arq Bras Endocrinol Metabol. 54:123–132. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mundy GR: Osteoporosis and inflammation.
Nutr Rev. 65:S147–S151. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Babu H, Ambikan AT, Gabriel EE, Svensson
Akusjärvi S, Palaniappan AN, Sundaraj V, Mupanni NR, Sperk M,
Cheedarla N, Sridhar R, et al: Systemic inflammation and the
increased risk of inflamm-aging and age-associated diseases in
people living with HIV on long term suppressive antiretroviral
therapy. Front Immunol. 10:19652019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ke JY, Kliewer KL, Hamad EM, Cole RM,
Powell KA, Andridge RR, Straka SR, Yee LD and Belury MA: The
flavonoid, naringenin, decreases adipose tissue mass and attenuates
ovariectomy-associated metabolic disturbances in mice. Nutr Metab
(Lond). 12:12015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Siddiqui JA and Partridge NC:
CCL2/Monocyte chemoattractant protein 1 and parathyroid hormone
action on bone. Front Endocrinol (Lausanne). 8:492017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Valerio MS, Herbert BA, Basilakos DS,
Browne C, Yu H and Kirkwood KL: Critical role of MKP-1 in
lipopolysaccharide-induced osteoclast formation through CXCL1 and
CXCL2. Cytokine. 71:71–80. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Khan UA, Hashimi SM, Bakr MM, Forwood MR
and Morrison NA: CCL2 and CCR2 are essential for the formation of
osteoclasts and foreign body giant cells. J Cell Biochem.
117:382–389. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chen W, Foo SS, Taylor A, Lulla A, Merits
A, Hueston L, Forwood MR, Walsh NC, Sims NA, Herrero LJ and
Mahalingam S: Bindarit, an inhibitor of monocyte chemotactic
protein synthesis, protects against bone loss induced by
chikungunya virus infection. J Virol. 89:581–593. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ho-Le TP, Center JR, Eisman JA, Nguyen HT
and Nguyen TV: Prediction of bone mineral density and fragility
fracture by genetic profiling. J Bone Miner Res. 32:285–293. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ho-Le TP, Pham HM, Center JR, Eisman JA,
Nguyen HT and Nguyen TV: Prediction of changes in bone mineral
density in the elderly: Contribution of ‘osteogenomic profile’.
Arch Osteoporos. 13:682018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Orwoll ES, Lapidus J, Wang PY, Vandenput
L, Hoffman A, Fink HA, Laughlin GA, Nethander M, Ljunggren Ö,
Kindmark A, et al: The limited clinical utility of testosterone,
estradiol, and sex hormone binding globulin measurements in the
prediction of fracture risk and bone loss in older men. J Bone
Miner Res. 32:633–640. 2017. View Article : Google Scholar : PubMed/NCBI
|