Introduction
Cervical cancer (CC) is a malignancy that occurs in
cervical cells, and causes high morbidity and mortality among
females in China (1,2). It was reported that CC resulted in
>300,000 deaths in women, and >50,000,000 women were
diagnosed with CC in 2018 (3).
Despite advances in screening, diagnosis, vaccination and
treatments, the survival rate for patients with CC remains <50%
(4–6). The low survival rate of CC may result
from the complex mechanisms underlying CC, including multistep
genes, complex regulators and complicated biological processes
(7–9). Therefore, exploring the key molecular
mechanisms underlying CC is important.
Flap structure-specific endonuclease 1 (FEN1) is
located on human chromosome 11q12, and participates in DNA
synthesis and replication (10–14).
In the early 1900s, the function of FEN1 was first identified and
reported (15). During the 1900s,
research focused on the functions of FEN1 at the DNA level
(16). In the late 1990s, it was
reported that FEN1 was downregulated during differentiation of the
HL-60 cell line (a human leukemia cell line) (17). Also in the late 1900s, FEN1 was
identified as a novel cell proliferation marker that could be
stimulated by proliferating cell nuclear antigen (PCNA) (18,19).
FEN1 upregulation has been identified as crucial for the
maintenance of genome stability and for the progression of various
malignancies occurring in prostate, lung, breast, brain, stomach
and pancreatic tissues (20–27).
FEN1 is upregulated in the HeLa cell line, and FEN1 inhibitor
enhances ionizing radiation sensitivity of CC in vivo and
in vitro (28). Previous
studies have investigated the interaction between FEN1 and
microRNAs (miRNAs/miRs) in human breast and hepatocellular cancer
(29–31). However, the interaction between
FEN1 and miRNAs in CC is not completely understood.
miRNAs, small non-coding RNAs that contain ~22
nucleotides, have been reported to serve as crucial players in gene
transcription and gene editing during numerous cancer cell
phenotype alterations (32–35).
In 2006, miR-140-5p was first studied in mouse fibroblasts cells
(3T3), which indicated that miR-140-5p targeted its downstream mRNA
target, histone deacetylase 4, therefore regulating long bone
development (36). In the
following decade, the regulatory function of miR-140-5p was
extensively explored in different types of cancer, such as gastric
cancer, hepatocellular carcinoma and esophageal cancer (37–40).
Moreover, several studies reported the possible cervical
cancer-suppressive role of miR-140-5p via interaction with insulin
like growth factor 2 mRNA binding protein 1, ADAM metallopeptidase
domain 10, origin recognition complex subunit 1 and Smad3 (41–44).
However, how miR-140-5p interacts with FEN1 in CC has not been
previously reported.
The aim of the present study was to explore the
mechanism underlying miRNA and its downstream target genes in
cervical cancer development. Bioinformatics analysis was performed
to identify a core gene that participated in CC cell cycle
progression. The relationship between miR-140-5p and FEN1 was
explored in CC cells. Cytological experiments were conducted to
assess how modulating miR-140-5p and FEN1 expression levels altered
CC cell phenotypes, especially the cell cycle profile.
Materials and methods
Differentially expressed genes (DEGs)
identification and enrichment analysis
The mRNA expression profiles of GSE63514 (24 normal
and 28 cancer; PMID (45),
GSE64217 (2 normal and 2 cancer; www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE64217)
and GSE63678 (5 normal and 5 cancer) (46) were obtained from the Gene
Expression Omnibus (GEO) database (www.ncbi.nlm.nih.gov/gds). DEGs with thresholds of
|log2(FC)|>2 and an adjusted P-value <0.05 were
selected and uploaded to Venny (version 2.1.0;
bioinfogp.cnb.csic.es/tools/venny) for screening common DEGs.
Search Tool for the Retrieval of Interacting Genes/Proteins
(STRING; string-db.org/cgi/input.pl) and Metascape
(metascape.org/gp/index.html#/main/step1), two online tools used
for gene annotation and gene list enrichment analysis, were used to
construct the protein-protein interaction network and analyze the
key biological process. The relative expression of DEGs in cervical
squamous cell carcinoma was assessed using the University of
Alabama Cancer Database (UALCAN; ualcan.path.uab.edu/index.html).
Finally, using data from The Cancer Genome Atlas (www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga),
The Encyclopedia of RNA Interactomes (ENCORI; version 2.0;
starbase.sysu.edu.cn/panCancer.php) (47) was used to identify the key gene
that was closely related to the overall survival outcome of
patients with CC.
Tissue samples and characteristics
record
A total of 40 cervical carcinoma tissues and 40
adjacent healthy tissues (distance from tumor margin, >5 cm)
were obtained from female patients (mean age, 45 years) that
underwent resection at Hanyang Hospital and The Second Affiliated
Hospital of Nanchang University between March 2015 and January
2019. Patients were diagnosed with CC by three independent
pathologists. The clinical characteristics of the patients are
presented in Table I.
 | Table I.Baseline demographics and clinical
characteristics of patients with cervical cancer (n=40). |
Table I.
Baseline demographics and clinical
characteristics of patients with cervical cancer (n=40).
| Characteristic | n (%) |
|---|
| Age (years) |
|
|
<45 | 23 (57.5) |
|
≥45 | 17 (42.5) |
| Histologic
diagnosis |
|
|
Adenocarcinoma | 18 (45) |
|
Squamous carcinoma | 22 (55) |
|
Differentiation |
|
| Low
differentiation | 26 (65) |
| High
differentiation | 14 (35) |
| Pathological
stage |
|
|
I–IIa | 21 (52.5) |
|
IIb-IV | 19 (47.5) |
| Lymph node
metastasis |
|
|
Yes | 13 (32.5) |
| No | 27 (67.5) |
| Vascular
invasion |
|
|
Yes | 16 (40) |
| No | 24 (60) |
Cell lines and cell transfection
Human Uterine Cervical Epithelial Cells (HUCECs;
cat. no. BNCC353405) and five human CC cell lines (HeLa, SiHa,
CaSki, SW756 and C33A) were purchased from BeNa Culture Collection.
Cells were cultured at 37°C with 5% CO2 according to the
manufacturer's protocol: SW756 cells were cultured in Leibovitz's
L-15 medium (American Type Culture Collection); SiHa and C33A cells
were cultured in DMEM (Hyclone; Cytiva); and HUCEC, HeLa and CaSki
cells were cultured in RPMI-1640 medium (Hyclone; Cytiva). In
addition, the 293T cell line was purchased from Sigma-Aldrich
(Merck KGaA) for use in the 3′untranslated region (UTR) assay. 293T
cells were cultured in DMEM supplemented with 2 mM glutamine. All
media was supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.).
A small interfering (si)RNA targeted against FEN1
(si-FEN1), miR-140-5p mimic, miR-140-5p inhibitor, si-negative
control (NC), miR-140-5p mimic NC and miR-140-5p inhibitor NC were
designed and synthesized by Shanghai GenePharma Co., Ltd.. The
sequences are presented in Table
SI. At 50% confluence, HeLa and CaSki cells were transfected
with 50 nM si-FEN1, miR-140-5p mimic, miR-140-5p inhibitor or a
mixture of si-NC, miR-140-5p mimic NC and miR-150-5p inhibitor NC
(the NC group; Figs. S1 and
S2) using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.). At 48 h post-transfection, cells were used for
subsequent experiments.
Reverse transcription-quantitative PCR
(RT-qPCR) and western blotting
Total RNA was extracted from tissues and cells using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.).
Total RNA (1 µg) was reverse transcribed into cDNA using 4 µl 5X
First-Strand Buffer, 2 µl 0.1 M DTT, 1 µl Oligo dT Primer, 1 µl
dNTP Mixture, 1 µl Rnase inhibitor, 1 µl M-MLV reverse
transcriptase (cat. no. 28025013; Thermo Fisher Scientific, Inc.)
and ddH2O to a volume of 20 µl. The following
temperature protocol was used for reverse transcription: At 37°C
for 50 min followed by 70°C for 15 min. Subsequently, qPCR was
performed using an ABI 7300 system (Applied Biosystems; Thermo
Fisher Scientific, Inc.) and the SYBR Green PCR kit (Thermo Fisher
Scientific, Inc.). The following thermocycling conditions were used
for qPCR: 95°C for 10 min; followed by 40 cycles of 95°C for 15 sec
and 60°C for 1 min; and 40°C for 1 min. The sequences of the
primers used for qPCR are presented in Table II. miRNA and mRNA expression
levels quantified using the 2−ΔΔCq method (48) and normalized to the internal
reference genes U6 and GAPDH, respectively.
 | Table II.Sequences of primers used for reverse
transcription- quantitative PCR. |
Table II.
Sequences of primers used for reverse
transcription- quantitative PCR.
| Gene | Sequence
(5′→3′) |
|---|
| FEN1 | F:
AATGGGTGGTTTGAGAGTGGC |
|
| R:
CTGGTCTTCAGGCTCCCTAC |
| GAPDH | F:
GTCAAGGCTGAGAACGGGAA |
|
| R:
AAATGAGCCCCAGCCTTCTC |
| miR-140-5p | F:
TGCGGCAGTGGTTTTACCCTATG |
|
| R:
CCAGTGCAGGGTCCGAGGT |
| U6 | F:
TGCGGGTGCTCGCTTCGGCAGC |
|
| R:
CCAGTGCAGGGTCCGAGGT |
Total protein extraction, protein sample separation
and transfer to the membranes were performed as previously
described (49). Protein
extraction was performed using lysis-M reagent (Roche Diagnostic
GmbH). Protein concentration was measured by performing the BCA
protein assay (Pierce; Thermo Fisher Scientific, Inc.). Proteins
(30 µg) were separated via 10% SDS-PAGE and transferred to
nitrocellulose membranes at 0.25A for 2 h at 4°C in transfer buffer
(25 mM Tris, 200 mM glycine, 20% methanol). After blocking in 5%
non-fat milk for 3 h at room temperature, the membranes were
incubated at 4°C for 8 h with primary antibodies targeted against:
FEN1 (1:2,000; cat. no. ab109132; Abcam) and GAPDH (1:2,500; cat.
no. ab9485; Abcam). Subsequently, the membranes were incubated with
a horseradish peroxidase-labelled secondary antibody (1:2,500; cat.
no. ab205718; Abcam) for 2 h at room temperature. Protein bands
were visualized using ECL (Pierce; Thermo Fisher Scientific, Inc.).
Protein expression levels were semi-quantified using ImageJ
software (version 1.46; National Institutes of Health) with GAPDH
as the loading control.
3′UTR reporter assay
TargetScan Human (version 7.2; www.targetscan.org/vert_72) was used to predict
the complementary sequences between FEN1 3′UTR and miR-140-5p. The
wild-type (WT) 3′UTR of FEN1 mRNA containing the binding site of
miR-140-5p or the mutant (MUT) 3′UTR of FEN1 mRNA containing the
mutated binding site were inserted into the psiCHECK2 vector
(Promega Corporation) to establish the reporter assay constructs.
The WT 3′UTR of FEN1 mRNA was mutated to 5′-CCCGGAUA-3′. 293T cells
(1×105 cells/well) were co-transfected with 0.1 mg
FEN1-WT or FEN1-MUT and 50 nM miR-140-5p mimic or mimic NC using
Lipofectamine 3000. At 48 h post-transfection, a GloMax luminometer
(Promega Corporation) and a dual-luciferase kit (GeneCopoeia, Inc.)
were used to quantify luciferase activities. The activity of
firefly luciferase was normalized to the corresponding
Renilla luciferase activity.
EdU staining assay
Cell proliferation was assessed by performing EdU
staining assay using the EdU Staining Proliferation kit (cat. no.
ab219801; Abcam). HeLa and CaSki cells were seeded
(5×104 cells/well) in 96-well plates. At 48 h
post-transfection, 100 µl EdU (50 µM) was added to each well for 2
h at 37°C. Cells were fixed with 200 µl 1X fixative solution
containing 40% formaldehyde for 15 min at room temperature.
Subsequently, cells were permeated with 200 µl 1X permeabilization
buffer for 20 min at room temperature. After washing with wash
buffer, cells were incubated with 100 µl reaction mix for 20 min at
room temperature. DNA content was stained using 100 µl Hoechst
33342 stock solution (5 µg/ml) for 30 min in the dark at room
temperature. Stained cells were visualized using a fluorescence
microscope (magnification, ×100).
Detection of cell apoptosis and cell
cycle
HeLa and CaSki cells were seeded (1×104
cells/well) into 6-well plates. To assess cell apoptosis, at 48 h
post-transfection, cells were stained using the Annexin V-FITC/PI
cell apoptosis detection kit (Dojindo Molecular Technologies, Inc.)
according to the manufacturer's instructions in the dark. To assess
the cell cycle distribution, at 48 h post-transfection, cells were
fixed and permeabilized with cool 70% ethanol overnight at 4°C.
Cells were centrifuged at 1,000 × g for 5 min at 4°C. Subsequently,
cells were incubated with 50 mg/ml PI (Sigma-Aldrich; Merck KGaA)
and 25 mg/ml RNaseA for 30 min in the dark at 37°C. Following
staining, cells were analyzed using a FACScalibur flow cytometer
(BD Biosciences) and FlowJo software (version 7.6.1; Tree Star,
Inc.) to quantify cell apoptosis and cell cycle distribution.
Assessment of cell migration
The wound healing assay was performed to assess HeLa
and CaSki cell migration. Briefly, transfected cells were plated
into 6-well plates and cultured to 90% confluence. Cells were
cultured in serum-free media for 12 h at 37°C. A sterile pipette
tip was used to create a linear wound in the middle of the cell
monolayer in each well. Following washing with PBS, cells were
cultured in fresh serum-free medium for 48 h at 37°C. The wounds
were observed in six randomly selected fields of view using a light
microscope (magnification, ×100). The migration rates were
calculated by comparing the width of the wound at 0 and 48 h.
Statistical analysis
Statistical analyses were performed using GraphPad
Prism software (version 7.0; GraphPad Software, Inc.). Data are
presented as the mean ± SD of three independent experiments. ENCORI
(47) was used to conduct survival
analysis. Comparisons among multiple groups were analyzed using
one-way ANOVA followed by Dunnett's post hoc test. Comparisons of
gene expression levels between tumor tissues and adjacent healthy
tissues were analyzed using the Wilcoxon matched-paired signed rank
test. Comparisons between two independent groups were analyzed
using the paired Student's t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Identifying genes that are associated
with CC
To select key genes, three gene expression profiles
(GSE63514, GSE64217 and GSE63678) were downloaded from the GEO
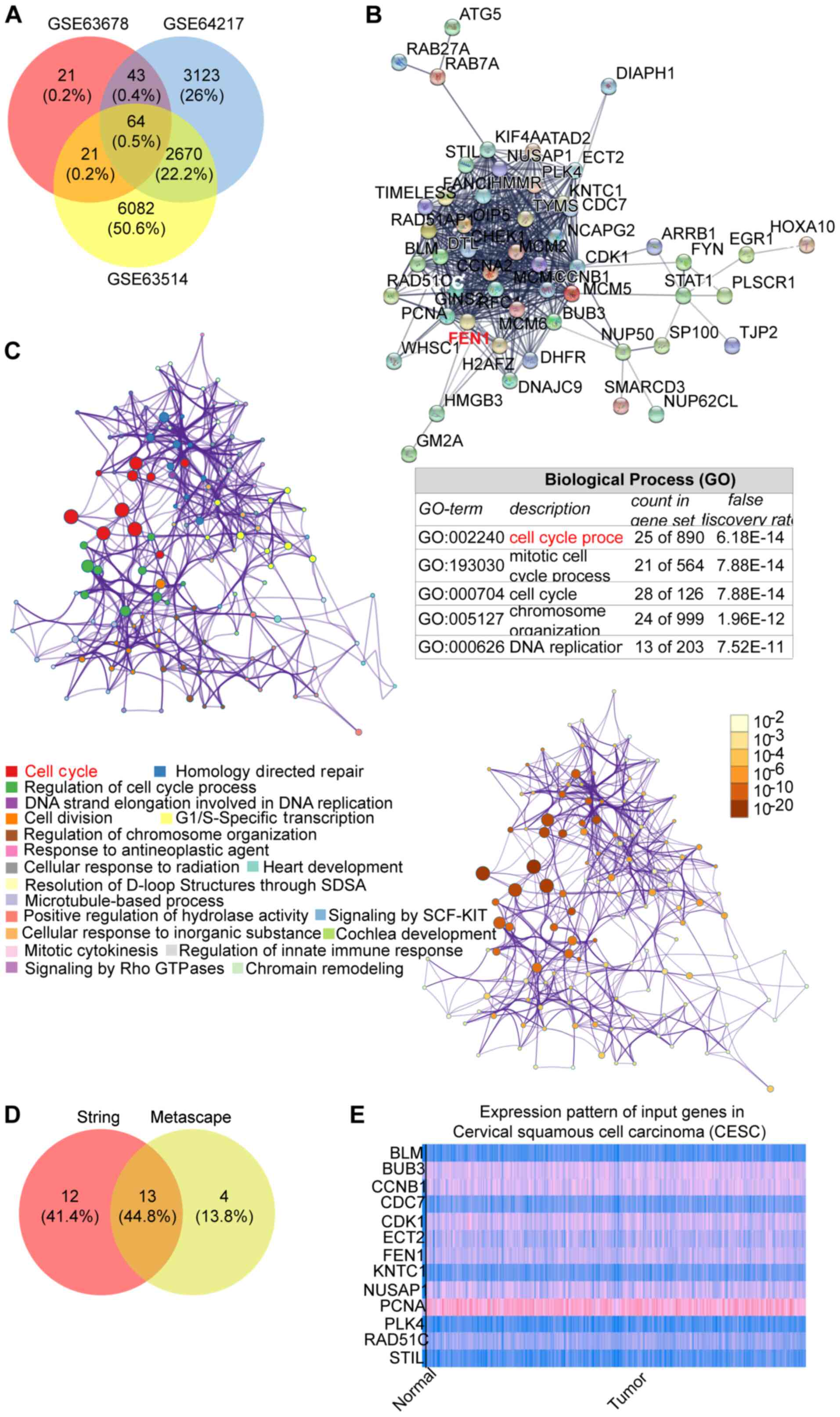
database. A total of 64 common DEGs were identified (Fig. 1A). Subsequently, the gene
interaction network was constructed using STRING and Metascape
algorithms. Both algorithms indicated that the cell cycle was the
key biological process including 25 genes from the STRING algorithm
and 17 genes from the Metascape algorithm (Fig. 1B and C). For further analysis, 13
genes from both algorithms were selected (Fig. 1D). Among the 13 genes, cyclin B1,
cyclin dependent kinase 1, epithelial cell transforming 2, FEN1,
nucleolar and spindle associated protein 1 and PCNA displayed high
expression levels in cervical squamous cell carcinoma compared with
the healthy control, as determined via UALCAN (Fig. 1E). Subsequently, the six
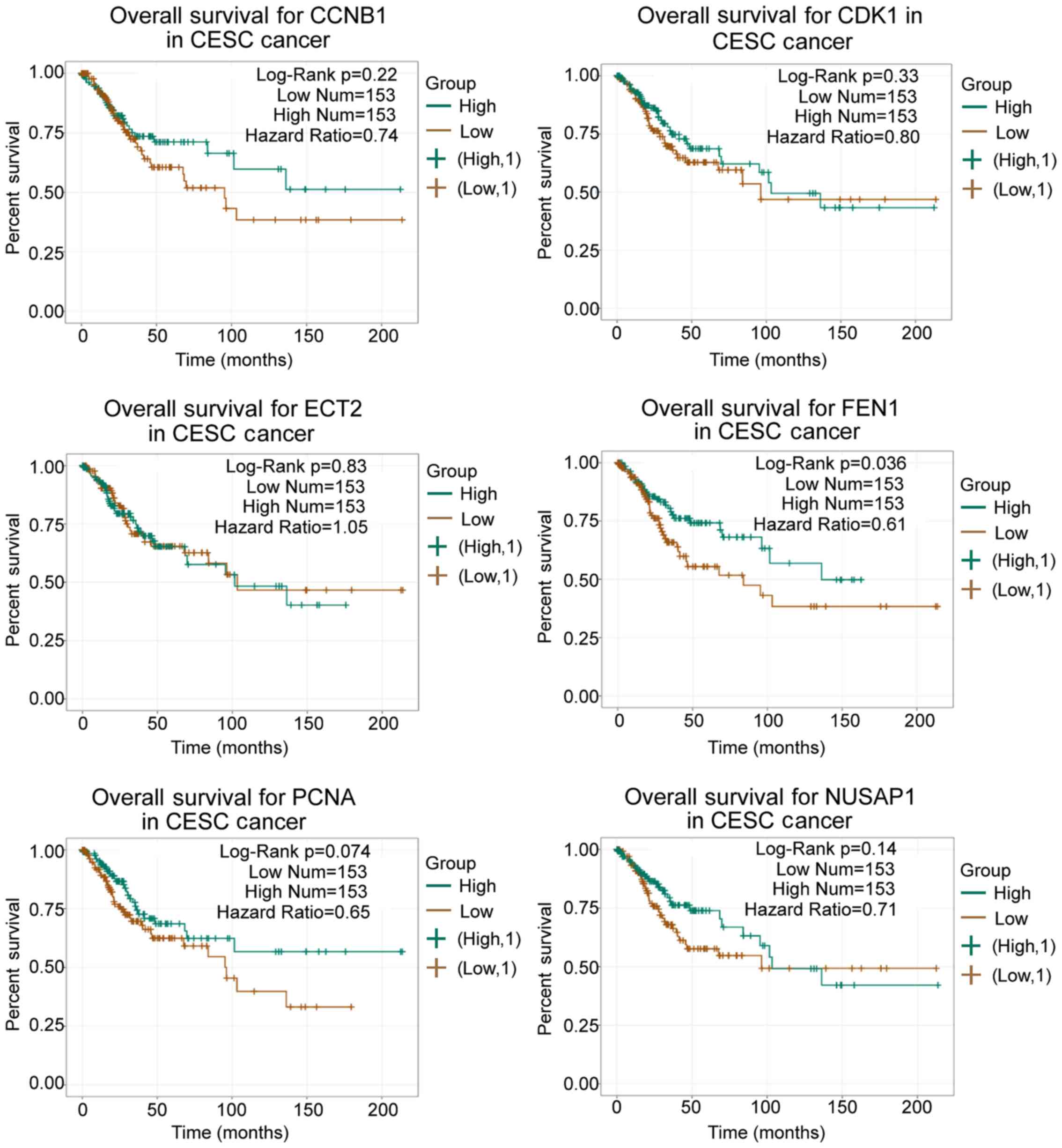
upregulated genes were input into ENCORI to perform prognosis
analysis, which indicated that FEN1 may serve as a significant
prognosis marker in patients with CC (Fig. 2). Previously, FEN1 has been
reported to correlate with poor prognosis in non-small-cell lung
cancer (50,51) and hepatocellular carcinoma
(52). Therefore, FEN1 was
selected as the gene of interest in the present study to
investigate its underlying mechanism in CC.
FEN1 upregulation and miR-140-5p
downregulation in CC
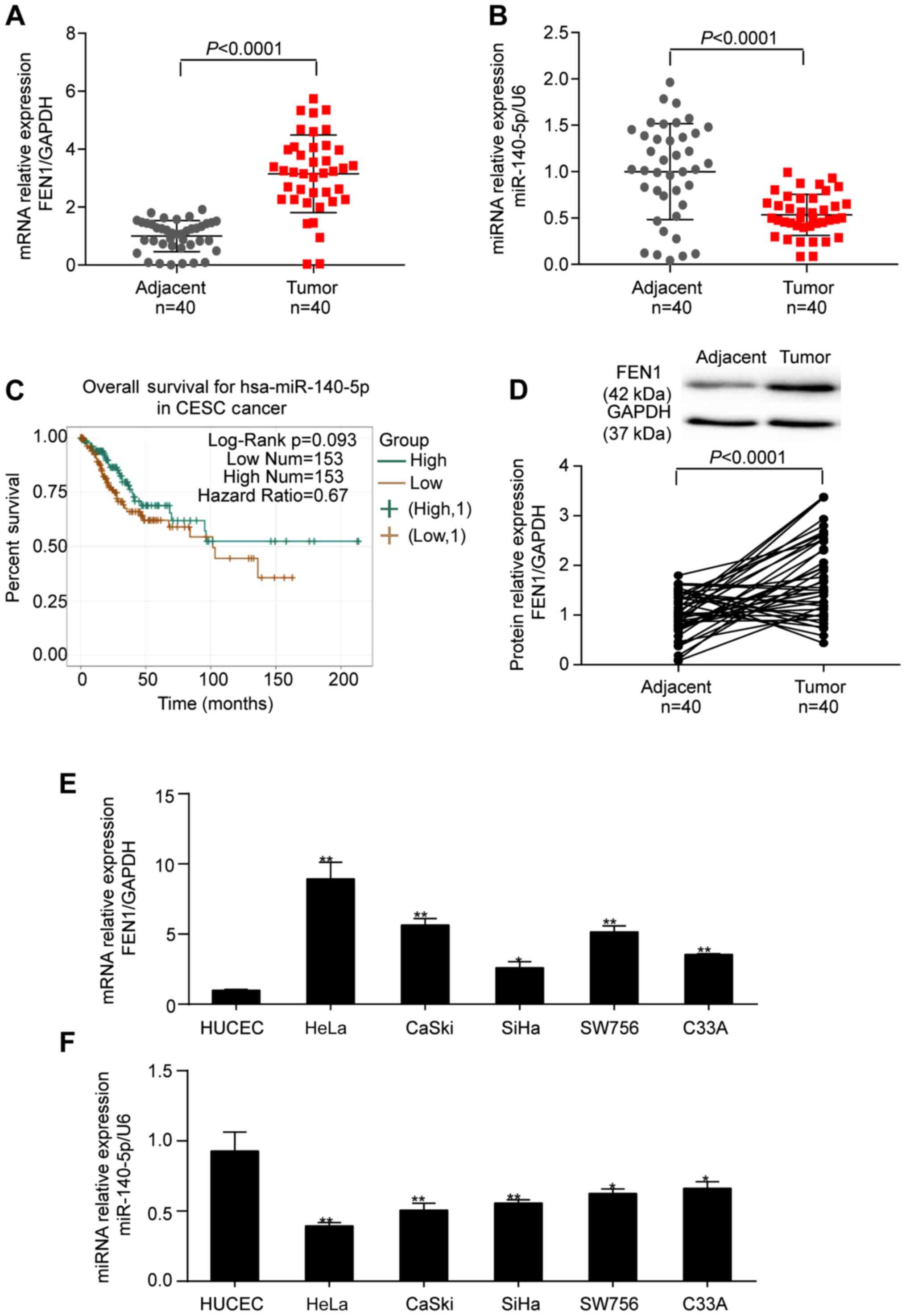
The results indicated that, compared with adjacent
healthy tissues, FEN1 mRNA expression levels were 3.15-fold higher
(Fig. 3A) and miR-140-5p
expression levels were 46.6% lower (Fig. 3B) in CC tissues. Lower miR-140-5p
expression levels were associated with a poorer prognosis outcome
in patients with CC, especially after 100 months, as determined via
ENCORI (Fig. 3C). Consistent with
the RT-qPCR results, the protein expression levels of FEN1 were
1.79-fold higher in CC tissues compared with adjacent healthy
cervical tissues (Fig. 3D). FEN1
and miR-140-5p expression levels in CC cells displayed similar
trends to those in CC tissues (Fig. 3E
and F).
miR-140-5p inhibits FEN1
expression
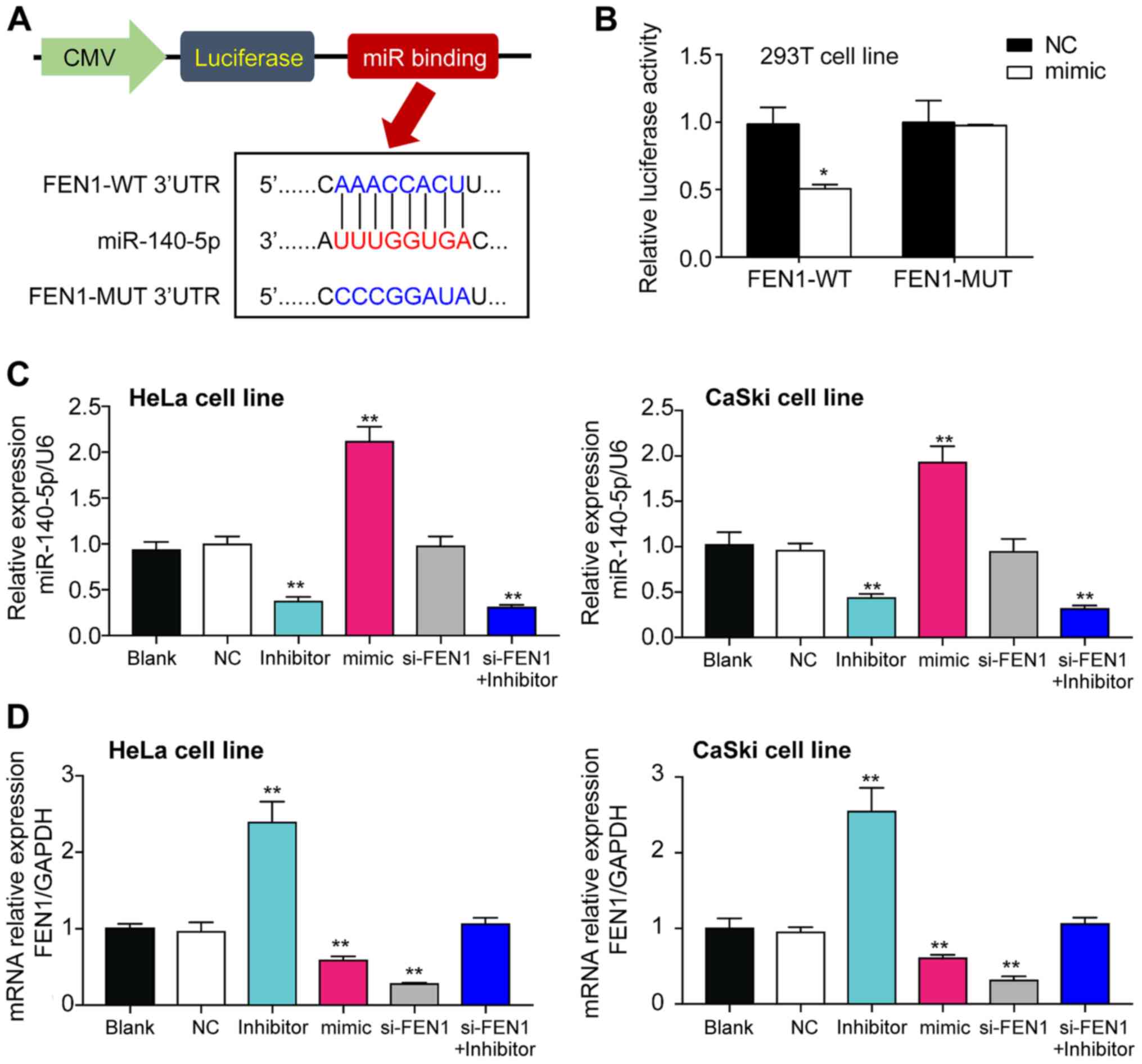
To explore whether FEN1 was the target gene of
miR-140-5p, the TargetScan Human algorithm was used to predict the
complementary sequences between FEN1 3′UTR and miR-140-5p. The
binding site was mutated using the site-directed mutation method
and the mutated sequence is presented in Fig. 4A. The direct regulatory association
between miR-140-5p and FEN1 mRNA 3′UTR was assessed by performing
the 3′UTR reporter assay. The results indicated that luciferase
activities were significantly decreased in 293T cells
co-transfected with miR-140-5p mimic and FEN1-WT compared with 293T
cells co-transfected with mimic NC and FEN1-WT (Fig. 4B). The transfection efficiencies of
miR-140-5p inhibitor and miR-140-5p mimic were confirmed.
miR-140-5p inhibitor and miR-140-5p mimic significantly decreased
(by 70%) and increased (by 2-fold) miR-140-5p expression levels,
respectively, compared with the NC group (Fig. 4C). Compared with the NC group,
si-FEN1 significantly decreased FEN1 expression levels by 80%,
miR-140-5p mimic significantly decreased FEN1 expression levels by
~50% and miR-140-5p inhibitor significantly increased FEN1
expression levels by ~2.5-fold in both cell lines (Fig. 4D). Co-transfection of si-FEN1 and
miR-140-5p inhibitor did not significantly alter FEN1 expression
levels.
Suppressive effects of miR-140-5p on
CC cell phenotypes via targeting FEN1
To further explore the mechanism underlying
miR-140-5p in CC, HeLa and CaSki cells were transfected with
miR-140-5p inhibitor, miR-140-5p mimic or si-FEN1. Cell
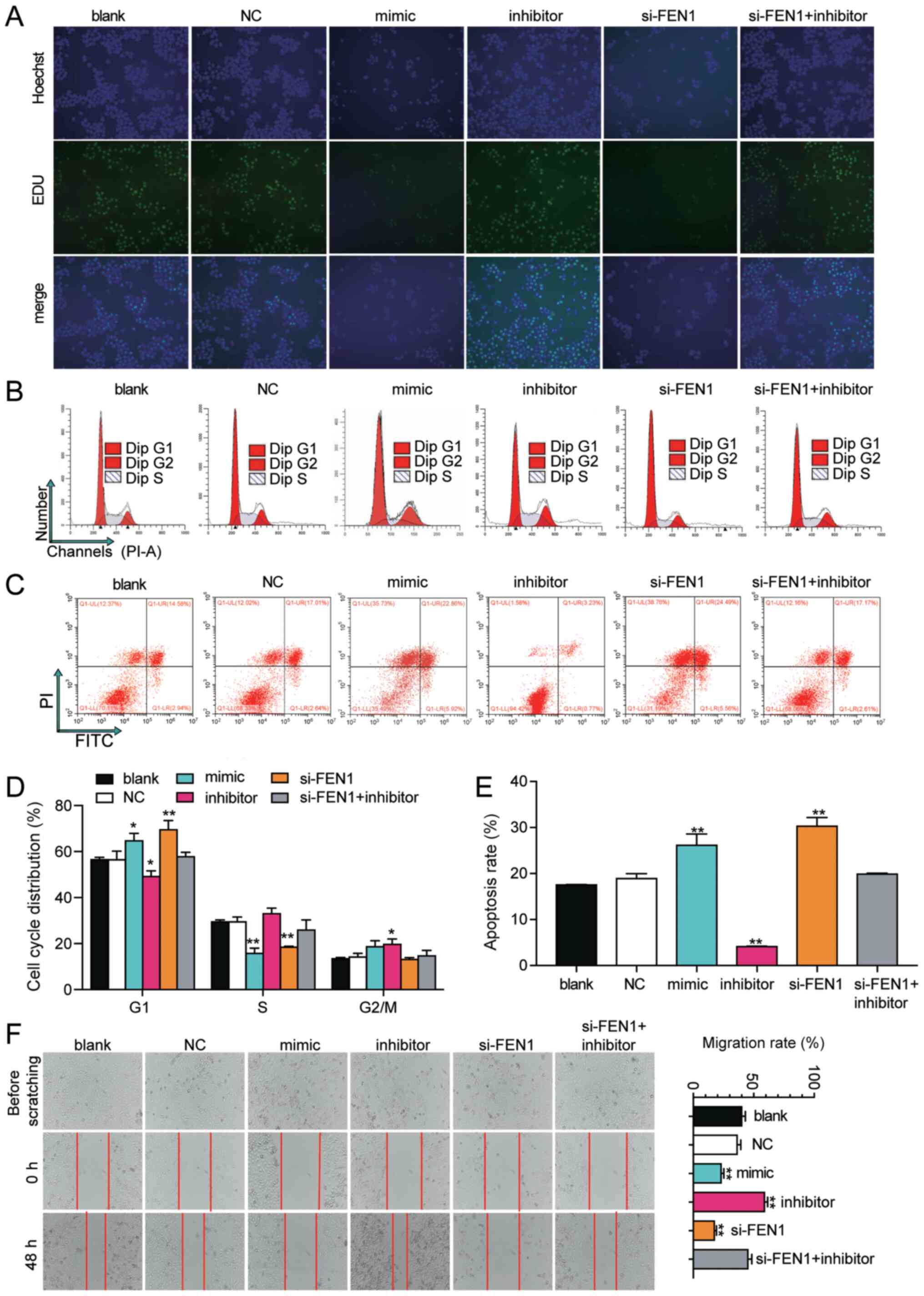
proliferation was impaired by transfection with si-FEN1 or
miR-140-5p mimic, but enhanced by transfection with miR-140-5p
inhibitor compared with blank group. Interestingly, si-FEN1 and
miR-140-5p inhibitor co-transfection did not significantly alter
cell proliferation compared with the blank group (Fig. 5A). Compared with the blank group,
si-FEN1 and miR-140-5p mimic significantly elevated the rate of
cell apoptosis by ~1.5-fold, whereas miR-140-5p inhibitor
significantly decreased the rate of cell apoptosis by ~80% in HeLa
cells. The rate of cell apoptosis in the co-transfection group was
similar to the blank group (Fig. 5C
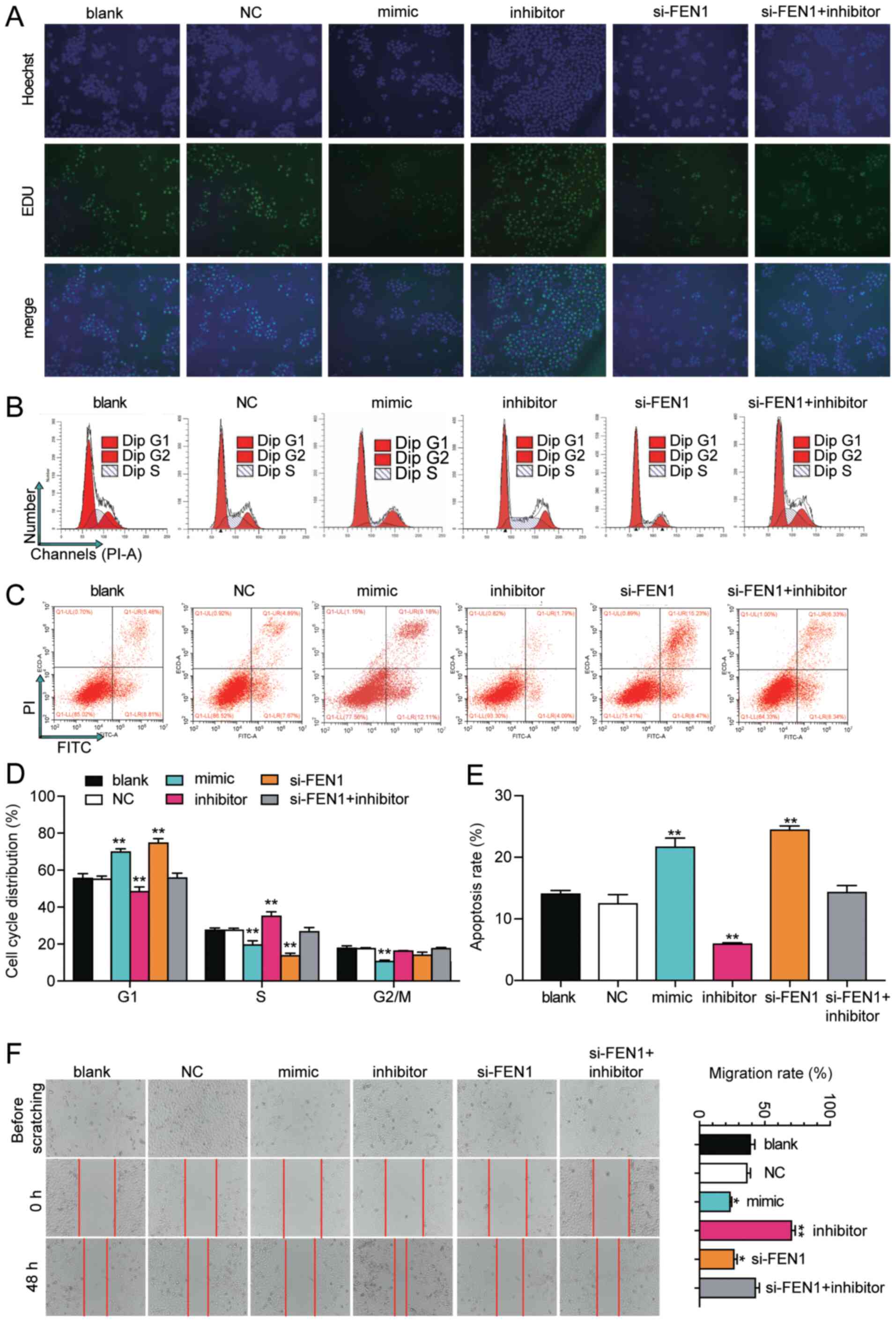
and E). Similarly, in CaSki cells, si-FEN1 and miR-140-5p mimic
significantly increased the rate of cell apoptosis by 74 and 54%,
respectively, whereas miR-140-5p inhibitor significantly decreased
cell apoptosis by 58% compared with the blank group (Fig. 6C and E). The cell cycle profiling
results indicated that si-FEN1 and miR-140-5p mimic significantly
induced cell cycle arrest at the G1 phase, whereas
miR-140-5p inhibitor significantly decreased cell cycle arrest at
the G1 phase in both cell lines compared with the blank
group (Figs. 5B, D, 6B and D). To investigate CC cell
migration, the wound healing assay was performed. The results
suggested that HeLa cell migration in the miR-140-5p inhibitor
group was significantly increased by 1.5-fold at 48 h, whereas cell
migration in the si-FEN1 and miR-140-5p mimic groups was
significantly decreased by 50 and 40% at 48 h, respectively,
compared with the blank group (Fig.
5F). CaSki cell migration in the si-FEN1 and miR-140-5p mimic
groups was significantly decreased by 40 and 50%, respectively, but
significantly increased in miR-140-5p inhibitor group by 70%
compared with the blank group (Fig.
6F). However, cell migration in the co-transfection group was
similar to the blank group.
Discussion
The present study identified a novel crosstalk
between miR-140-5p and FEN1 in human CC. It was hypothesized that
miR-140-5p displayed a CC-suppressing effect, whereas FEN1
displayed the opposite effect. The binding relationship between
miR-140-5p and FEN1 predicted by TargetScan Human was also
verified. The cell experiment results indicated that FEN1 knockdown
inhibited CC cell proliferation, migration and uncontrollable cell
cycle progression, whereas miR-140-5p inhibitor promoted CC cell
proliferation, migration and cell cycle progression compared with
the blank group.
To identify genes that potentially participated in
the development of CC, three GEO datasets were analyzed in the
present study. The bioinformatics analysis indicated that FEN1, in
relation to the cell cycle, was a key gene that served an important
role in CC. FEN1 is upregulated in various types of cancer, and
FEN1 loss-of-function has been reported to inhibit cancer
progression. For instance, He et al (26) reported that FEN1 was upregulated in
lung cancer cells, and FEN1 inhibitor effectively inhibited cell
proliferation and induced DNA damage in vitro. Also, FEN1
promoted breast cancer cell proliferation by mediating DNA
methyltransferase (DNMT)1 and DNMT3a to recover the expression of
miR-200a-5p target genes (31). In
CC, FEN1 was upregulated in HeLa cells, and FEN1 loss-of-function
resulted in significant DNA damage and CC cell proliferation
(28). Besides, the combination of
FEN1 inhibitor and ionizing radiation displayed an enhanced
CC-suppressing effect (28).
Another study demonstrated that the antitumor effect of paclitaxel
was enhanced in CC cells when paclitaxel was used in combination
with FEN1 inhibitor (53).
Therefore, it was hypothesized that FEN1 might contribute to the
development of CC, and FEN1 knockdown might possess a
CC-suppressing effect. The results of the present study indicated
that FEN1 was significantly upregulated in CC tissues and cells
compared with adjacent healthy tissues and HUCECs. FEN1 knockdown
inhibited CC cell phenotypes compared with the blank group,
including inhibiting cell mitosis by halting the cell cycle at the
G1 phase. Similarly, Zhang et al (45) reported that FEN1 knockdown led to
G1/S phase cell cycle arrest in lung cancer cells.
miRNAs have been regarded as negative regulators of
target genes that also affect the progression of different types of
cancer (54–56). miR-140-5p upregulation has been
reported to impair the cancerous phenotypes of CC cells (41–43).
miR-140-5p overexpression suppressed tumor growth and metastasis in
nude mice (44). Interestingly,
the effects of FEN1 and its regulator miR-140-5p in other types of
cancer have been studied. For instance, overexpression of
miR-140-5p and FEN1 in breast cancer resulted in reduced DNA damage
and genome instability compared with miR-140-5p overexpression
alone in breast cancer cell lines (29), suggesting that FEN1 rescued breast
cancer cells from DNA damage and genome instability by compromising
miR-140-5p. Similar results were observed in a hepatocellular
carcinoma study, which reported that miR-140-5p and FEN1
overexpression reduced cell invasion and tumor growth (30). In the present study, the effects of
si-FEN1 were blocked by miR-140-5p inhibitor, indicating a
regulatory association between FEN1 and miR-140-5p. Compared with
the blank group, miR-140-5p knockdown enhanced the malignant
phenotypes of CC cell lines and FEN1 knockdown suppressed CC cell
phenotypes. The results also indicated that miR-140-5p directly
bound to FEN1 mRNA 3′UTR. Therefore, whether miR-140-5p inhibition
could rescue FEN1 knockdown-induced phenotypes was investigated.
The results indicated that the cancer-promoting effect of
miR-140-5p inhibitor on CC was suppressed by si-FEN1. A potential
explanation for the aforementioned effect is that the miR-140-5p
inhibitor may have neutralized part of the endogenous miR-140-5p
that bound to FEN1 mRNA 3′UTR, which freed some FEN1, compensating
for the loss of FEN1 caused by si-FEN1 transfection.
Based on the results of the present study, it was
hypothesized that miR-140-5p was an antitumor regulator in CC via
suppressing FEN1 expression in vitro. However, whether
miR-140-5p and FEN1 serve potential diagnostic and therapeutic
benefits for patients with CC requires further investigation in
vivo. In addition, whether the interaction between miR-140-5p
and FEN1 exists in other CC cell lines should be investigated in
future studies to generalize the axis to a wider range of CC cell
lines. As a potential participant in the cell cycle signaling
pathway, whether FEN1 could regulate the expression of certain cell
cycle-related proteins and how FEN1 regulates cell cycle
progression also require further investigation.
To the best of our knowledge, the present study
suggested for the first time that miR-140-5p binding to FEN1 mRNA
3′UTR suppressed the cancerous phenotypes of HeLa and CaSki cell
lines. Moreover, miR-140-5p knockdown prevented cell cycle arrest
at the G1 phase by targeting FEN1 in HeLa and CaSki cell
lines. Therefore, the present study provided insight into a
potential therapeutic target for CC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SL designed the study and prepared the manuscript.
YG interpreted the data and supervised the experiments. SL and YG
revised the manuscript. Both authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Hanyang Hospital (approval no. AB-20.05/07) and The
Second Affiliated Hospital of Nanchang University (approval no.
NCEFY-04-23). All patients signed written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ding FN, Gao BH, Wu X, Gong CW, Wang WQ
and Zhang SM: miR-122-5p modulates the radiosensitivity of cervical
cancer cells by regulating cell division cycle 25A (CDC25A). FEBS
Open Bio. 9:1869–1879. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bao HL, Liu YN, Wang LJ, Fang LW, Cong S,
Zhou MG and Wang LH: Analysis on mortality of cervical cancer and
its temporal trend in women in China, 2006–2012. Zhonghua Liu Xing
Bing Xue Za Zhi. 38:58–64. 2017.(In Chinese). PubMed/NCBI
|
|
3
|
Cohen PA, Jhingran A, Oaknin A and Denny
L: Cervical cancer. Lancet. 393:169–182. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li H, Wu X and Cheng X: Advances in
diagnosis and treatment of metastatic cervical cancer. J Gynecol
Oncol. 27:e432016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Menderes G, Black J, Schwab CL and Santin
AD: Immunotherapy and targeted therapy for cervical cancer: An
update. Expert Rev Anticancer Ther. 16:83–98. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou Q, Dong J, Luo R, Zhou X, Wang J and
Chen F: MicroRNA-20a regulates cell proliferation, apoptosis and
autophagy by targeting thrombospondin 2 in cervical cancer. Eur J
Pharmacol. 844:102–109. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Peng RQ, Wan HY, Li HF, Liu M, Li X and
Tang H: MicroRNA-214 suppresses growth and invasiveness of cervical
cancer cells by targeting UDP-N-acetyl-α-D-galactosamine:
Polypeptide N-acetylgalactosaminyltransferase 7. J Biol Chem.
287:14301–14309. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ili CG, Brebi P, López J, García P, Leal
P, Suarez E and Roa JC: Genotyping of human papillomavirus in
cervical intraepithelial neoplasia in a high-risk population. J Med
Virol. 83:833–837. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cao XM: Role of miR-337-3p and its target
Rap1A in modulating proliferation, invasion, migration and
apoptosis of cervical cancer cells. Cancer Biomark. 24:257–267.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cooper PR, Nowak NJ, Higgins MJ, Church DM
and Shows TB: Transcript mapping of the human chromosome
11q12-q13.1 gene-rich region identifies several newly described
conserved genes. Genomics. 49:419–429. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lieber MR: The FEN-1 family of
structure-specific nucleases in eukaryotic DNA replication,
recombination and repair. Bioessays. 19:233–240. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Henneke G, Friedrich-Heineken E and
Hubscher U: Flap endonuclease 1: A novel tumour suppresser protein.
Trends Biochem Sci. 28:384–390. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kikuchi K, Taniguchi Y, Hatanaka A, Sonoda
E, Hochegger H, Adachi N, Matsuzaki Y, Koyama H, van Gent DC, Jasin
M and Takeda S: Fen-1 facilitates homologous recombination by
removing divergent sequences at DNA break ends. Mol Cell Biol.
25:6948–6955. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu X, Wilson TE and Lieber MR: A role for
FEN-1 in nonhomologous DNA end joining: The order of strand
annealing and nucleolytic processing events. Proc Natl Acad Sci
USA. 96:1303–1308. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kucherlapati M, Yang K, Kuraguchi M, Zhao
J, Lia M, Heyer J, Kane MF, Fan K, Russell R, Brown AM, et al:
Haploinsufficiency of Flap endonuclease (Fen1) leads to rapid tumor
progression. Proc Natl Acad Sci USA. 99:9924–9929. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ayyagari R, Gomes XV, Gordenin DA and
Burgers PM: Okazaki fragment maturation in yeast. I. Distribution
of functions between FEN1 AND DNA2. J Biol Chem. 278:1618–1625.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim IS: Down-regulation of human FEN-1
gene expression during differentiation of promyelocytic leukemia
cells. Exp Mol Med. 30:252–256. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gary R, Park MS, Nolan JP, Cornelius HL,
Kozyreva OG, Tran HT, Lobachev KS, Resnick MA and Gordenin DA: A
novel role in DNA metabolism for the binding of Fen1/Rad27 to PCNA
and implications for genetic risk. Mol Cell Biol. 19:5373–5382.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Warbrick E, Coates PJ and Hall PA: Fen1
expression: A novel marker for cell proliferation. J Pathol.
186:319–324. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nikolova T, Christmann M and Kaina B: FEN1
is overexpressed in testis, lung and brain tumors. Anticancer Res.
29:2453–2459. 2009.PubMed/NCBI
|
|
21
|
He L, Zhang Y, Sun H, Jiang F, Yang H, Wu
H, Zhou T, Hu S, Kathera CS, Wang X, et al: Targeting DNA flap
endonuclease 1 to impede breast cancer progression. EBioMedicine.
14:32–43. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang K, Xie C and Chen D: Flap
endonuclease 1 is a promising candidate biomarker in gastric cancer
and is involved in cell proliferation and apoptosis. Int J Mol Med.
33:1268–1274. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Iacobuzio-Donahue CA, Maitra A, Olsen M,
Lowe AW, van Heek NT, Rosty C, Walter K, Sato N, Parker A, Ashfaq
R, et al: Exploration of global gene expression patterns in
pancreatic adenocarcinoma using cDNA microarrays. Am J Pathol.
162:1151–1162. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lam JS, Seligson DB, Yu H, Li A, Eeva M,
Pantuck AJ, Zeng G, Horvath S and Belldegrun AS: Flap endonuclease
1 is overexpressed in prostate cancer and is associated with a high
Gleason score. BJU Int. 98:445–451. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Singh P, Yang M, Dai H, Yu D, Huang Q, Tan
W, Kernstine KH, Lin D and Shen B: Overexpression and
hypomethylation of flap endonuclease 1 gene in breast and other
cancers. Mol Cancer Res. 6:1710–1717. 2008.PubMed/NCBI
|
|
26
|
He L, Luo L, Zhu H, Yang H, Zhang Y, Wu H,
Sun H, Jiang F, Kathera CS, Liu L, et al: FEN1 promotes tumor
progression and confers cisplatin resistance in non-small-cell lung
cancer. Mol Oncol. 11:640–654. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zeng X, Che X, Liu YP, Qu XJ, Xu L, Zhao
CY, Zheng CL, Hou KZ and Teng Y: FEN1 knockdown improves
trastuzumab sensitivity in human epidermal growth factor 2-positive
breast cancer cells. Exp Ther Med. 14:3265–3272. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li JL, Wang JP, Chang H, Deng SM, Du JH,
Wang XX, Hu HJ, Li DY, Xu XB, Guo WQ, et al: FEN1 inhibitor
increases sensitivity of radiotherapy in cervical cancer cells.
Cancer Med. 8:7774–7780. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lu X, Liu R, Wang M, Kumar AK, Pan F, He
L, Hu Z and Guo Z: MicroRNA-140 impedes DNA repair by targeting
FEN1 and enhances chemotherapeutic response in breast cancer.
Oncogene. 39:234–247. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li C, Zhou D, Hong H, Yang S, Zhang L, Li
S, Hu P, Ren H, Mei Z and Tang H: TGFβ1-miR-140-5p axis mediated
up-regulation of flap endonuclease 1 promotes
epithelial-mesenchymal transition in hepatocellular carcinoma.
Aging (Albany NY). 11:5593–5612. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zeng X, Qu X, Zhao C, Xu L, Hou K, Liu Y,
Zhang N, Feng J, Shi S, Zhang L, et al: FEN1 mediates miR-200a
methylation and promotes breast cancer cell growth via MET and EGFR
signaling. FASEB J. 33:10717–10730. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hammond SM: An overview of microRNAs. Adv
Drug Deliv Rev. 87:3–14. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Di Leva G, Garofalo M and Croce CM:
MicroRNAs in cancer. Annu Rev Pathol. 9:287–314. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li L, Song Y, Shi X, Liu J, Xiong S, Chen
W, Fu Q, Huang Z, Gu N and Zhang R: The landscape of miRNA editing
in animals and its impact on miRNA biogenesis and targeting. Genome
Res. 28:132–143. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang K, Jin W, Song Y and Fei X: LncRNA
RP11-436H11.5, functioning as a competitive endogenous RNA,
upregulates BCL-W expression by sponging miR-335-5p and promotes
proliferation and invasion in renal cell carcinoma. Mol Cancer.
16:1662017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tuddenham L, Wheeler G, Ntounia-Fousara S,
Waters J, Hajihosseini MK, Clark I and Dalmay T: The cartilage
specific microRNA-140 targets histone deacetylase 4 in mouse cells.
FEBS Lett. 580:4214–4217. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shin VY, Ng EK, Chan VW, Kwong A and Chu
KM: A three-miRNA signature as promising non-invasive diagnostic
marker for gastric cancer. Mol Cancer. 14:2022015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zou J and Xu Y: MicroRNA-140 inhibits cell
proliferation in gastric cancer cell line HGC-27 by suppressing
SOX4. Med Sci Monit. 22:2243–2252. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang H, Fang F, Chang R and Yang L:
MicroRNA-140-5p suppresses tumor growth and metastasis by targeting
transforming growth factor β receptor 1 and fibroblast growth
factor 9 in hepatocellular carcinoma. Hepatology. 58:205–217. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li W, Jiang G, Zhou J, Wang H, Gong Z,
Zhang Z, Min K, Zhu H and Tan Y: Down-regulation of miR-140 induces
EMT and promotes invasion by targeting Slug in esophageal cancer.
Cell Physiol Biochem. 34:1466–1476. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Guo H, Yang S, Li S, Yan M, Li L and Zhang
H: LncRNA SNHG20 promotes cell proliferation and invasion via
miR-140-5p-ADAM10 axis in cervical cancer. Biomed Pharmacother.
102:749–757. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen X, Xiong D, Ye L, Wang K, Huang L,
Mei S, Wu J, Chen S, Lai X, Zheng L and Wang M: Up-regulated lncRNA
XIST contributes to progression of cervical cancer via regulating
miR-140-5p and ORC1. Cancer Cell Int. 19:452019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chang QQ, Chen CY, Chen Z and Chang S:
LncRNA PVT1 promotes proliferation and invasion through enhancing
Smad3 expression by sponging miR-140-5p in cervical cancer. Radiol
Oncol. 53:443–452. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Su Y, Xiong J, Hu J, Wei X, Zhang X and
Rao L: MicroRNA-140-5p targets insulin like growth factor 2 mRNA
binding protein 1 (IGF2BP1) to suppress cervical cancer growth and
metastasis. Oncotarget. 7:68397–68411. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang K, Keymeulen S, Nelson R, et al:
Overexpression of Flap endonuclease 1 correlates with enhanced
proliferation and poor prognosis of non-small-cell lung cancer. Am
J Pathol. 188:242–251. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Pappa KI, Polyzos A, Jacob-Hirsch J,
Amariglio N, Vlachos GD, Loutradis D and Anagnou NP: Profiling of
discrete gynecological cancers reveals novel transcriptional
modules and common features shared by other cancer types and
embryonic stem cells. PLoS One. 10:e01422292015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li JH, Liu S, Zhou H, Qu LH and Yang JH:
starBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA
interaction networks from large-scale CLIP-Seq data. Nucleic Acids
Res. 42((Database Issue)): D92–D97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Salim H, Akbar NS, Zong D, Vaculova AH,
Lewensohn R, Moshfegh A, Viktorsson K and Zhivotovsky B: miRNA-214
modulates radiotherapy response of non-small cell lung cancer cells
through regulation of p38MAPK, apoptosis and senescence. Br J
Cancer. 107:1361–1373. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang K, Keymeulen S, Nelson R, Tong TR,
Yuan YC, Yun X, Liu Z, Lopez J, Raz DJ and Kim JY: Overexpression
of flap endonuclease 1 correlates with enhanced proliferation and
poor prognosis of non-small-cell lung cancer. Am J Pathol.
188:242–251. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
He L, Luo L, Zhu H, Yang H, Zhang Y, Wu H,
Sun H, Jiang F, Kathera CS, Liu L, et al: FEN1 promotes tumor
progression and confers cisplatin resistance in non-small-cell lung
cancer. Mol Oncol. 11:1302–1303. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhang Y, Liu X, Liu L, Chen J, Hu Q, Shen
S, Zhou Y, Chen S, Xue C, Cui G and Yu Z: Upregulation of FEN1 is
associated with the tumor progression and prognosis of
hepatocellular carcinoma. Dis Markers. 2020:25140902020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
He L, Yang H, Zhou S, Zhu H, Mao H, Ma Z,
Wu T, Kumar AK, Kathera C, Janardhan A, et al: Synergistic
antitumor effect of combined paclitaxel with FEN1 inhibitor in
cervical cancer cells. DNA Repair (Amst). 63:1–9. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Minna E, Romeo P, Dugo M, De Cecco L,
Todoerti K, Pilotti S, Perrone F, Seregni E, Agnelli L, Neri A, et
al: miR-451a is underexpressed and targets AKT/mTOR pathway in
papillary thyroid carcinoma. Oncotarget. 7:12731–12747. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Liu H, Ren G, Zhu L, Liu X and He X: The
upregulation of miRNA-146a inhibited biological behaviors of ESCC
through inhibition of IRS2. Tumour Biol. 37:4641–4647. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Li X, Zhang Y, Zhang H, Liu X, Gong T, Li
M, Sun L, Ji G, Shi Y, Han Z, et al: miRNA-223 promotes gastric
cancer invasion and metastasis by targeting tumor suppressor
EPB41L3. Mol Cancer Res. 9:824–833. 2011. View Article : Google Scholar : PubMed/NCBI
|