Introduction
Polycystic ovary syndrome (PCOS) is one of the most
prevalent endocrine metabolic disorders affecting 5–10% women of
reproductive age globally (1,2). It
is generally characterized by hyperandrogenism, polycystic ovaries
and anovulation; however, it is also associated with metabolic
dysfunction, cardiovascular disease risk, abnormal granulosa cell
(GC) proliferation and the arrest of follicle growth (3,4). The
clinical symptoms and signs of PCOS include an absent or irregular
menstruation, difficulty in becoming pregnant, acne, and thick,
dark and smooth skin patches (5).
Genetic and environmental factors play a major role in the
pathogenesis of PCOS; however, the underlying molecular mechanisms
remain to be fully elucidated (6).
MicroRNAs (miRNAs or miRs) are small non-coding RNAs
that negatively regulate protein-coding gene expression through RNA
silencing and post-transcriptional regulation (7,8).
Studies have demonstrated that the aberrant expression of miRNAs is
associated with the pathological progression of various diseases,
including cancer, metabolic diseases, inflammation and reproductive
disorders (8–10). However, the function and underlying
molecular mechanisms of miRNAs in follicular development and in the
development of PCOS have not yet been fully elucidated.
miR-132, located in the intron of a non-coding gene
on chromosome 17 in humans, has been reported to play differential
roles in various diseases, such as vascular endothelial
inflammation, gestational diabetes mellitus and periodontitis
(9–11). Recently, it was demonstrated that
miR-132 can mediate tumor initiation and development by regulating
cancer cell proliferation, apoptosis, invasion and migration
(12,13). Furthermore, Wu et al
(14) revealed that miR-132
promoted estradiol synthesis in ovarian GCs via the translational
repression of nuclear receptor subfamily 4 group A member 2.
However, the role and underlying mechanisms of miR-132 in PCOS
remain unclear. Therefore, the aim of the present study was to
determine whether miR-132 is involved in the abnormal viability of
GCs from patients with PCOS and to elucidate the potential
underlying mechanisms.
Materials and methods
Patients and samples
The study population consisted of women referred to
the Reproductive Medicine Center of Shanxi Women and Infants
Hospital (Taiyuan, China) between June 2016 and December 2016. All
subjects were Han ethnic, from the Shanxi Province, in North China.
The study was approved by the Ethics Committee of Shanxi Women and
Infants Hospital Ethics (approval no. 201922021), and all the
participants signed written informed consent for participation in
this study. The blood and follicular fluid samples were obtained
from 26 patients with PCOS and 30 healthy controls. Diagnosis of
PCOS was based on the Rotterdam Criteria (15), including oligo-ovulation and/or
anovulation, excess androgen activity, and ultrasound image of
polycystic ovaries. Patients with endometriosis, congenital adrenal
hyperplasia, hypothyroidism, androgen-secreting tumors, Cushing's
syndrome and other systemic diseases were excluded from the study.
The controls were patients with regular menstrual cycles who were
infertile as a result of tubal factors (primarily tubal obstruction
and abnormal tubal peristalsis) and/or male factors (including
severe oligospermia and severe asthenospermia). All participants
consented to endocrine tests and other routine checks, and the
results are listed in Table I.
 | Table I.Characteristics of patients with PCOS
(n=26) and the controls (n=30). |
Table I.
Characteristics of patients with PCOS
(n=26) and the controls (n=30).
|
Characteristics | PCOS group | Control group | P-value |
|---|
| Age, years | 29.86±2.81 | 30.11±2.95 | 0.75 |
| BMI | 23.74±3.36 | 21.84±3.02 | 0.03 |
| Infertility
duration, years | 4.42±2.76 | 4.78±2.99 | 0.64 |
| FSH, mIU/ml | 6.79±2.18 | 6.96±1.92 | 0.76 |
| LH, mIU/ml | 9.53±6.48 | 4.37±2.11 | <0.001 |
| E2,
pg/ml | 51.12±14.77 | 47.96±19.98 | 0.51 |
| PRL, ng/ml | 14.13±6.48 | 14.41±6.26 | 0.87 |
| TES, ng/dl | 1.14±0.39 | 0.44±0.17 | <0.001 |
Controlled ovarian hyperstimulation
protocol
Both patients with PCOS and control patients
received in vitro fertilization and embryo transfer
treatments, following standard operation procedure. All women
underwent controlled ovarian hyper-stimulation with gonadotropin
releasing hormone agonist long protocol commenced pituitary
suppression with leuprolide acetate (Diphereline 0.1 mg; GenSci
Company) at a dose of 0.05 mg/d, during the mi-luteal phase of the
preceding cycle. Complete pituitary suppression was confirmed by a
serum follicle-stimulating hormone (FSH) level <5 mIU/ml,
luteinizing hormone (LH) level <5 mIU/ml, estradiol
(E2) level <50 pg/ml, bilateral antral follicle
diameter <5 mm, endometrial thickness ≤5 mm. Urofollitropin
(LIVZON) were used at doses ranging between 75 IU/day and 300
IU/day in accordance with patient age, body mass index, size and
number of antral follicles, and serum basic FSH level. The dosage
of urofollitropin was adjusted according to ovarian response, which
was assessed by ultrasound and serum E2 levels.
Recombinant human choriogonadotropin-alfa solution (hCG; Merck
Serono SpA) was administered subcutaneously at the 250 µg dosing
level when at least two follicles with ≥18 mm average diameter were
detected. Oocyte retrieval was performed under the guidance of
transvaginal ultrasounds 34 to 36 h after the hCG injection. Human
GCs were obtained from follicular fluid at the same time.
Cell culture
After oocyte retrieval, all follicular fluids from
each patient were pooled and stored in a tube. The GCs were
prepared and cultured as described previously (16). Briefly, the aspirated follicular
fluid was centrifuged at 1,000 × g for 10 min at 37°C after removal
of oocytes. The cell pellet was resuspended in 1 ml phosphate
buffer saline (PBS). Then, the suspension was overlayed on 1 ml
Ficoll, and centrifuged at 800 × g for 30 min at 37°C. GCs were
aspirated from the interface and washed a few times with PBS. Next,
the isolated and purified GCs (1×105) were cultured in
Dulbecco's modified Eagle medium/nutrient mixture F12 Ham medium
(DMEM/F12; Invitrogen; Thermo Fisher Scientific, Inc.) with 10%
fetal bovine serum (FBS; TBD), 100 µg/ml penicillin and 0.1 mg/ml
streptomycin (MRC) at 37°C with 5% CO2 and 95%
humidity.
Cell transfection
Human granulosa-like tumor cell line, KGN cells,
which have the physiological characteristics of ovarian cells, were
purchased from the American Type Culture Collection. GCs
(1×105) were grown in DMEM/F12 with 10% FBS at 37°C with
5% CO2 and 95% humidity. The miR-132 mimics (cat. no.
219600), inhibitor (cat. no. 219300) and negative control (NC; cat.
no. 1022076) were designed and synthesized by Qiagen, Inc. Forkhead
box protein A1 (Foxa1)-small interfering (si)RNA (cat. no. A10001),
control siRNA (si-NC; cat. no. A06001) were designed and
synthesized by Shanghai GenePharma Co., Ltd. Cells were seeded in
6-well plates (2×105 cells/well) 1 day before
transfection to reach a confluency of 90%, and then the medium was
replaced with serum- and antibiotic-free medium. Then, miR-132
mimic, miR-132 inhibitor, and si-Foxa1 were transfected at a final
concentration of 50 nM using Lipofectamine® 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) following the
manufacturer's protocols. At 36 h after transfection, cells were
collected for the following assays.
Cell viability assay
Cell viability was assessed using the Cell Counting
Kit-8 (CCK-8) method. To explore the effect of miR-132 and Foxa1 on
viability, cells transfected with miRNAs or siRNAs were plated in
96-well plates at 5×103 cells/well. Cell viability was
detected at 24, 48 and 72 h after transfection using CCK-8 at 45 nm
according to the manufacturer's instructions (Beyotime Institute of
Biotechnology) at 37°C for 4 h.
TUNEL assay
Apoptosis of GCs was determined by TUNEL staining
using a TUNEL cell apoptosis detection kit (Nanjing KeyGen Biotech
Co., Ltd.) according to the manufacturer's protocol. GCs
(1×104) were cultured directly on coverslips.
Subsequently, GCs were fixed in 4% paraformaldehyde at 4°C for 25
min and then washed thrice in PBS. The cells were incubated in 50
µl permeabilisation solution (0.2% Triton X-100) for 5 min at room
temperature. GCs were then transferred into 50 µl TUNEL reaction
mixture (45 µl equilibrium buffer + 5 µl nucleotide mixture + 1 µl
TdT enzyme) and incubated in a humidified chamber for 60 min in the
dark at 37°C. The reaction was terminated by incubating the GCs in
2X SSC buffer for 15 min at room temperature. The cells were then
washed three times with PBS/PVP. Subsequently, cells were treated
with 0.5 µg/ml of DNase-free RNase. Images were captured using a
BX40 microscope (BX40; Olympus Corporation). The buffy (faint
yellow) nucleus indicated TUNEL-positive cells. A total of five
fields (magnification, ×200) were taken randomly for each sample.
Data are reported as the percentage of TUNEL-positive cells among
the total number of cells. Each experiment was performed in
triplicate. Negative control cells were subjected to the TUNEL
assay without the addition of terminal deoxynucleotidyl transferase
in the reaction mixture. Positive control cells were incubated with
100 µl DNase I solution prior to the TUNEL assay to induce DNA
strand degradation.
RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR) assay
Total RNA of GCs was extracted using the
RNeasy/miRNeasy Mini kit (Qiagen Benelux BV), according to the
manufacturer's instructions. Total RNA (2 ng) was used for reverse
transcription using the OneStep RT-PCT kit (Qiagen Benelux BV),
following the manufacturer's instructions. The primers for miR-132
were the exact sequence of mature miR-132. U6 was used as the
internal control. The primers were purchased from Qiagen Benelux
BV. The thermocycling conditions for miRNA were as follows: 95°C
for 15 min, followed by 95°C for 15 sec, and 60°C for 1 min (40
cycles). For the mRNA expression of Foxa1, GAPDH was used as the
internal control. The primer sequences for Foxa1 and GAPDH were as
follows: Foxa1 sense, 5′-AGGGCTGGATGGTTGTATTG-3′ and antisense,
5′-GCCTGAGTTCATGTTGCTGA-3′; GAPDH sense, 5′-GAAGGTGAAGGTCGGAGTC-3′
and antisense, 5′-GAAGATGGTGATGGGATTTC-3′. The thermocycling
conditions for Foxa1 were as follows: 95°C for 5 min; 35 cycles of
95°C for 1 min, and 60°C for 1 min and 72°C for 1 min; and then
72°C for 7 min. RT-qPCR was performed in triplicate using a
SYBR® Premix Ex Taq Kit (Takara Bio, Inc.), according to
the manufacturer's protocol, on a CFX96 Real-time PCR system
(Bio-Rad Laboratories, Inc.). All reactions were run in triplicate
and gene expression was determined using the 2−∆∆Cq
method (17).
Dual-luciferase reporter assay
Foxa1 was predicted to be a target of miR-132 by the
online database TargetScanHuman 7.1 (www.targetscan.org; Whitehead Institute for Biomedical
Research). Then, the fragment was inserted into the pGL3 luciferase
promoter vector (Promega Corporation) to develop the
Luc-pGL3-Foxa1-3′UTR and Luc-pGL3-Foxa1-mut-3′UTR vectors. Cells
(1×105) in 24-well plates were co-transfected with
Luc-pGL3-Foxa1-3′UTR or Luc-pGL3-Foxa1-mut-3′UTR vector and miR-132
mimics or miR-NC using Lipofectamine 2000 reagent, according to the
instructions of the manufacturer. The Renilla luciferase
reporter vector was transfected as an internal control in each
assay. At 48 h post-transfection, firefly and Renilla
luciferase activities were detected using a dual-luciferase
reporter system (Promega Corporation). The results are expressed as
relative luciferase activity (Firefly/Renilla). All
experiments were performed three times in triplicate.
Western blot analysis
Western blotting was performed to evaluate the
expression of Foxa1. Briefly, the cells were collected and lysed on
ice in RIPA lysis buffer (Beyotime Institute of Biotechnology) with
protease inhibitor, according to the manufacturer's instructions.
The protein concentration of cell lysates was determined using a
BCA kit (Wuhan Boster Biological Technology Co., Ltd.). Equal
amounts of protein lysates (30 µg per lane) were resolved by 10%
SDS-PAGE, and then electrotransferred to PVDF membranes (EMD
Millipore). The membranes were blocked with TBS with 0.1% Tween-20
(TBST)_containing 5% non-fat milk for 2 h at room temperature, and
then incubated with the specific antibodies at 4°C overnight,
including mouse anti-Foxa1 (1:1,000; cat. no. ab40868), mouse
anti-Bax (1:1,000; cat. no. ab3191), mouse anti-Bcl-2 (1:1,000;
cat. no. ab692) and mouse anti-GAPDH (1:200; cat. no. ab8245)
monoclonal antibodies (both from Abcam). After washing with TBST,
the membranes were further incubated with HRP-conjugated goat
anti-mouse IgG (1:2,000; cat. no. BA1051; Wuhan Boster Biological
Technology Co., Ltd.) at 37°C for 1 h, followed by visualization
with an ECL kit (Nanjing KeyGen Biotech Co., Ltd.). Protein
expression levels were semi-quantified using Quantity One software
(version 4.6.7; Bio-Rad Laboratories, Inc.).
Statistical analysis
All statistical analyses were performed using
GraphPad Prism 6.0 (GraphPad software, Inc.). Normally distributed
data are presented as the mean ± standard error of the mean. To
check the normality of the distribution, the Shapiro-Wilk test was
performed. Two-tailed Student's t-test was performed for
comparisons of the mean values of two groups; one-way ANOVA
(followed by a Bonferroni post hoc test) was used to determine
differences among the mean values of multiple groups because the
quantitative data followed a normal distribution. P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient characteristics
The characteristics of female patients in the PCOS
group and control group are summarized in Table I. In total, 26 patients with PCOS
were recruited from patients seeking reproductive assistance at the
Reproductive Medicine Center of Shanxi Women and Infants Hospital.
Each patient with PCOS was confirmed clinically. A total of 16
cases of tubal infertility and 14 cases of infertility related to
male factors were included as controls for this study. There was no
significant difference between the PCOS and control groups
regarding age, infertility duration, and levels of FSH,
E2 and PRL. However, compared with the control group,
the BMI, and levels of LH and TES were significantly increased in
the PCOS group (P<0.05)
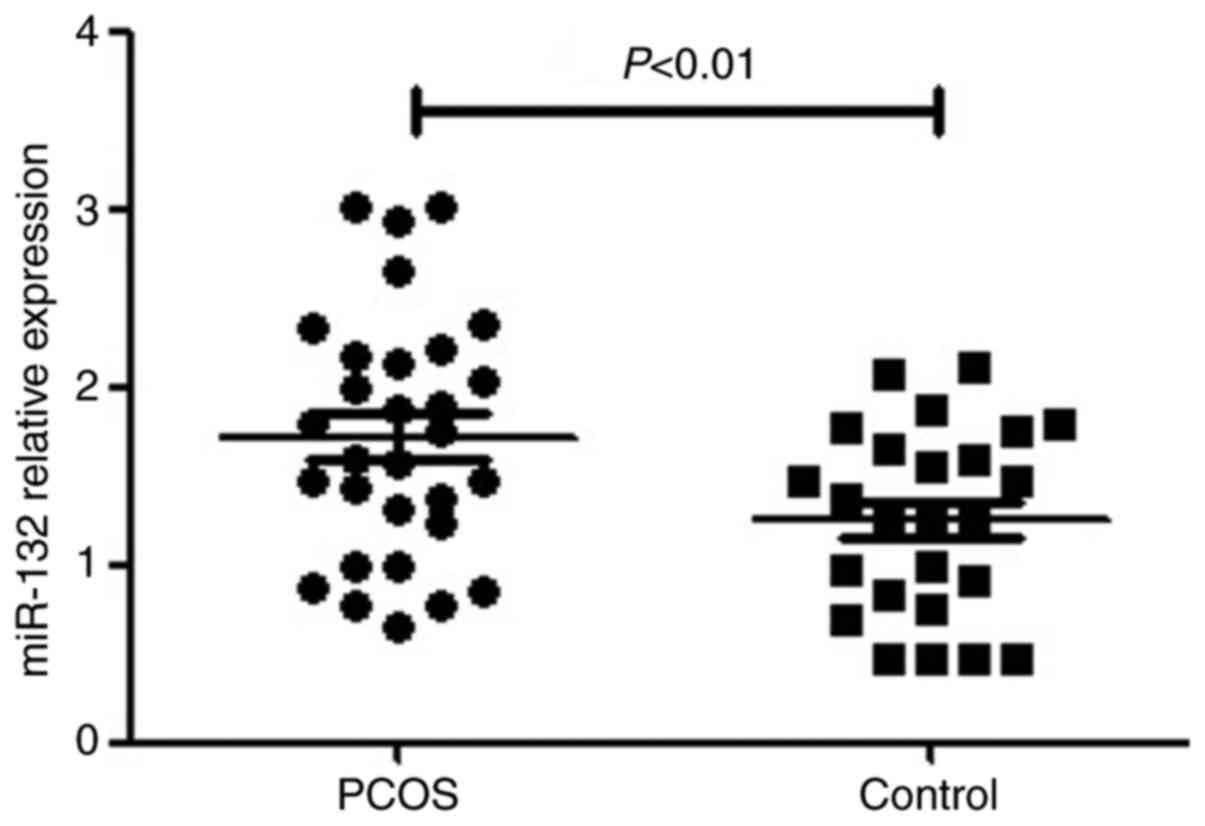
miR-132 expression is upregulated in
human GCs from patients with PCOS
RT-qPCR was performed to detect miR-132 expression
in GCs of 26 patients with PCOS and 30 controls. Compared with the
controls, the expression of miR-132 was significantly upregulated
in the GCs of patients with PCOS (P<0.05; Fig. 1).
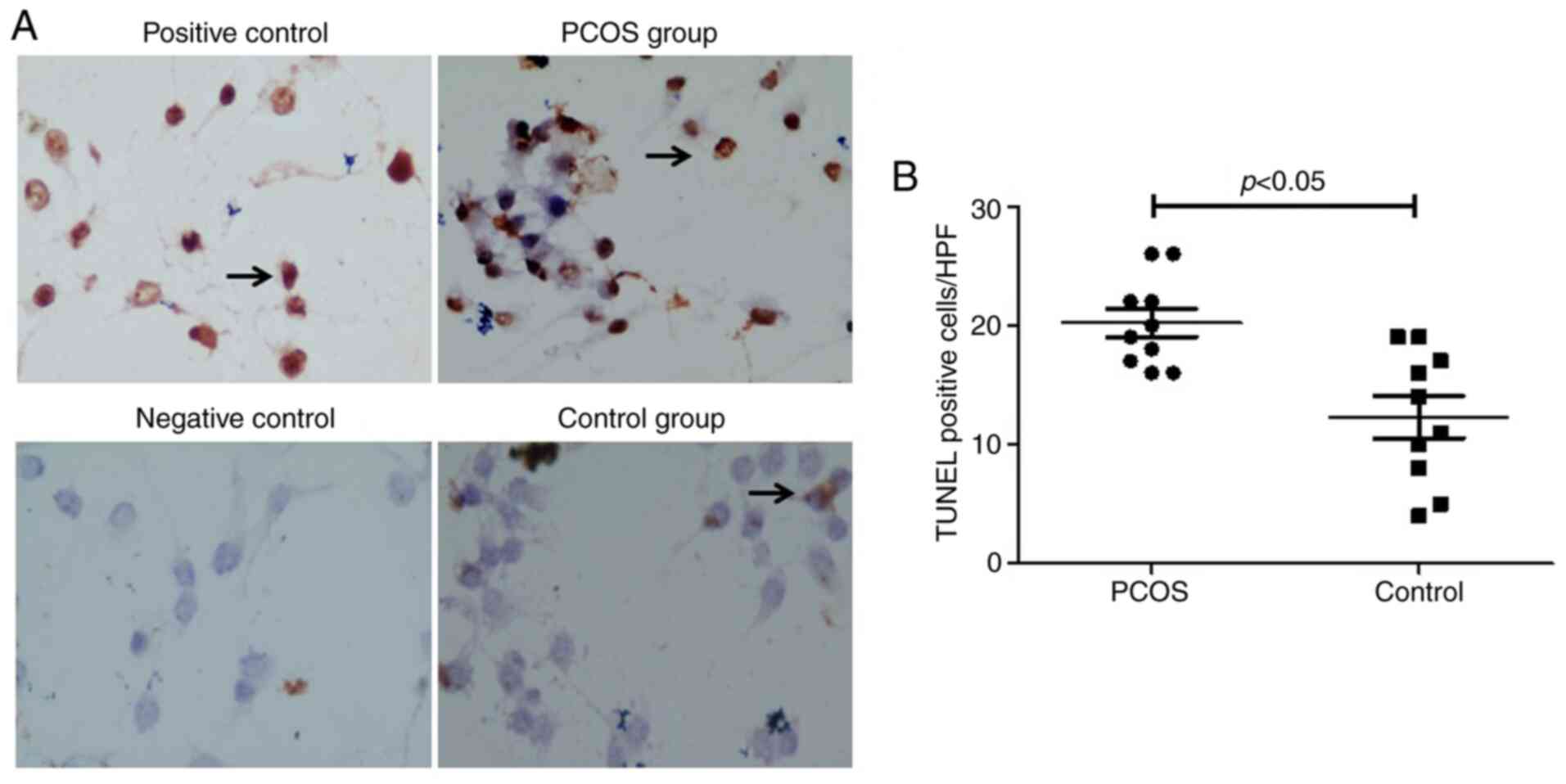
Apoptosis is increased in GCs from
patients with PCOS
In order to determine the GC apoptotic rate in PCOS,
cells were cultured directly on coverslips and stained with a TUNEL
cell apoptosis detection kit. A significantly increased number of
apoptotic nuclei were present in the PCOS group compared with the
control group (P<0.05; Fig. 2A and
B).
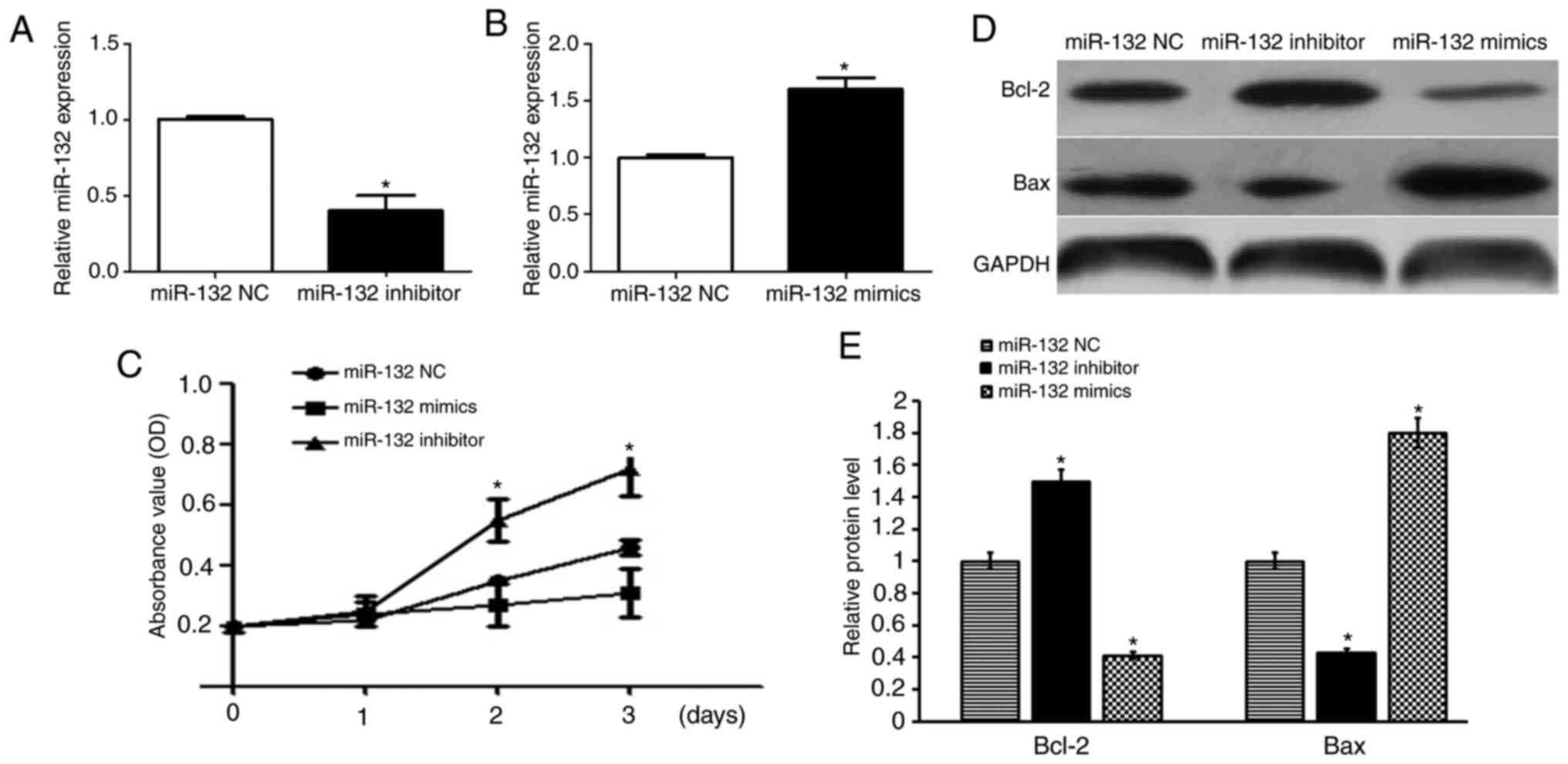
miR-132 negatively regulates cell
growth and viability in GCs
Having noted a significantly higher expression of
miR-132 in GCs of the ovaries from patients with PCOS, it was
proposed that miR-132 may be associated with the growth and
viability of GCs. Therefore, GCs were transfected with miR-132
mimics and miR-132 inhibitor, and the relative miR-132 expression
was verified (Fig. 3A and B). As
shown in Fig. 3C, the CCK-8 assay
revealed that decreased expression of miR-132 in GCs promoted
viability (P<0.05). In contrast to the miR-132 mimics, the
miR-132 inhibitor promoted cell growth (P<0.05; Fig. 3C). To further determine the role of
miR-132 in cell apoptosis of human GCs, western blotting was used
to detect the protein expression levels of Bax and Bcl-2. The
results showed that miR-132 mimics significantly increased Bax
protein expression and decreased protein expression of Bcl-2 when
compared with the control group. Whereas, the miR-132 inhibitor
significantly upregulated the protein expression of Bcl-2 and
decreased the protein expression of Bax when compared with the
control group (P<0.05; Fig. 3D and
E).
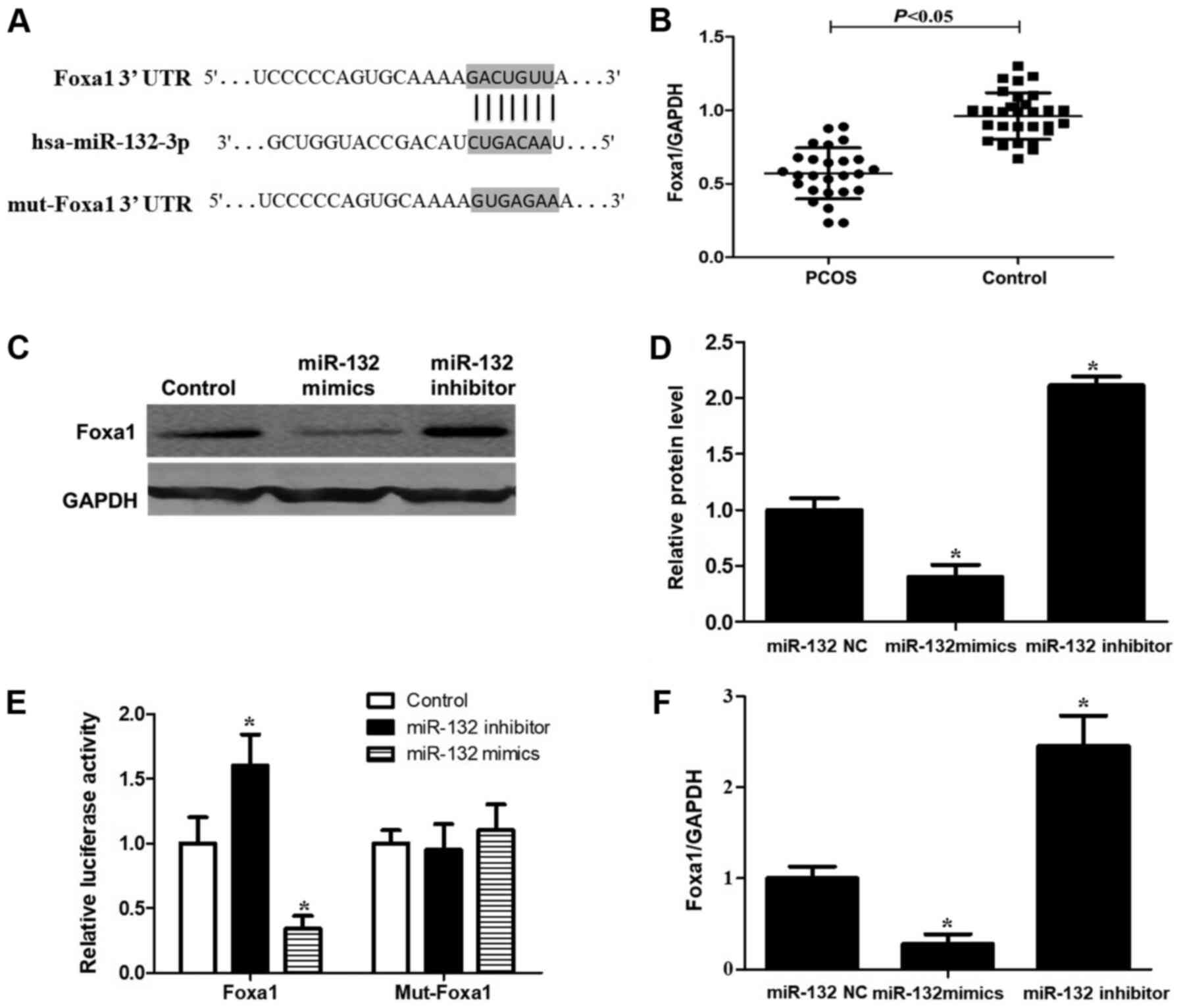
miR-132 directly inhibits Foxa1
expression by binding to its 3′UTR
Foxa1 was predicted to be a target of miR-132 by the
online database TargetScanHuman 7.1 (www.targetscan.org), with the sequence GACUGUUA in its
3′UTR being the predicted binding site (Fig. 4A). A significant downregulation of
Foxa1 expression was detected by RT-qPCR in human GCs from patients
with PCOS (P<0.05; Fig. 4B).
Then, RT-qPCR and western blotting were performed to observe the
expression of Foxa1 at the mRNA and protein levels in GCs
transfected with miR-132 mimics and inhibitor. Compared with the
control group, following the upregulation of miR-132, the mRNA and
protein levels of Foxa1 were significantly decreased. Whereas,
following the downregulation of miR-132, Foxa1 mRNA and protein
levels were significantly increased (P<0.05; Fig. 4C, D and F). To further demonstrate
whether Foxa1 was a direct target of miR-132, Foxa1 3′UTR was
cloned into a luciferase reporter vector and the putative miR-132
binding site in the Foxa1 3′UTR was mutated (Fig. 4E). Compared with the control group,
miR-132 overexpression suppressed the luciferase activity of the
reporter vector with Foxa1-WT; whereas miR-132 knockdown increased
the luciferase activity of the WT vector. Moreover, the luciferase
activity of the MUT vector in GCs was not affected by miR-132
overexpression or knockdown (Fig.
4E). Taken together, these data suggested that the Foxa1 gene
is a direct target of miR-132 overexpression, which inhibits Foxa1
expression in GCs.
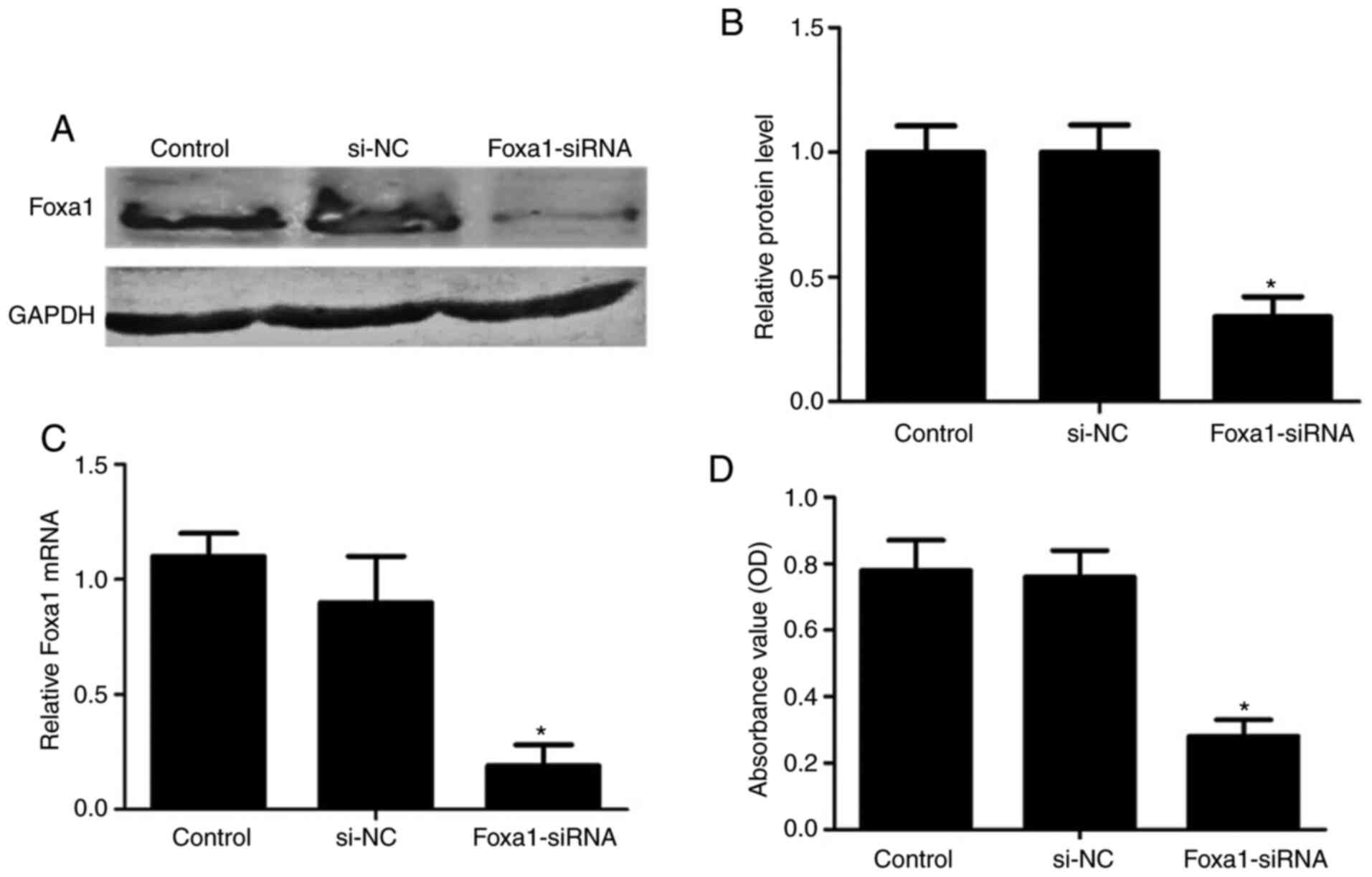
Cytotoxicity assay of silencing
Foxa1in human GCs
To explore the function of Foxa1 in GCs, Foxa1
expression was knocked down by siRNA in human GCs from control
patients. RT-qPCR and western blotting indicted that mRNA and
protein levels of Foxa1 were significantly decreased after 24 h in
GCs transfected with Foxa1-siRNA (P<0.05; Fig. 5A-C). Then, cell viability detection
was performed to evaluate the effect of Foxa1-siRNA on human GCs.
As expected, Foxa1 siRNA-transfected GCs displayed reduced cell
viability compared with si-NC at 48 h post-transfection (P<0.05;
Fig. 5D). These results indicated
that silencing Foxa1 inhibited human GC growth.
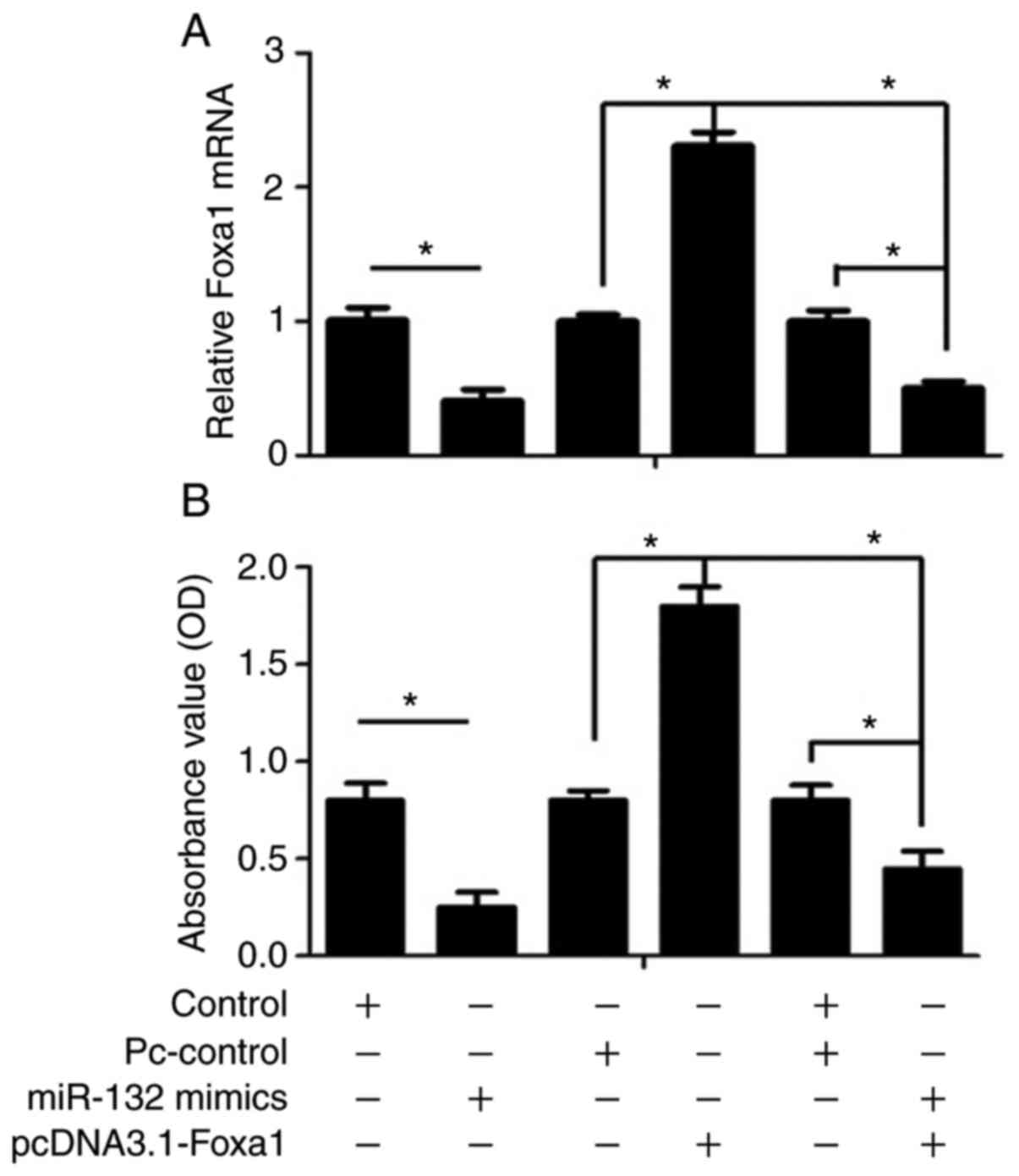
Foxa1 overexpression reverses the
suppressive effect of miR-132 mimics
To further determine the role of miR-132 on cell
viability through the direct targeting of Foxa1 in human GCs, cells
were transfected with pcDNA3.1-Foxa1 or miR-132 mimics. As shown in
Fig. 6A, overexpression of Foxa1
by pcDNA3.1-Foxa1 significantly increased Foxa1 expression in human
GCs compared with the vector control group (P<0.05) Furthermore,
the inhibitory effect of miR-132 mimics on Foxa1 expression was
partially reversed by Foxa1 overexpression. Subsequently, it was
found that the suppressed cell viability following transfection
with miR-132 mimics was attenuated by Foxa1 overexpression in human
GCs (P<0.05; Fig. 6B).
Discussion
The aim of the present study was to examine whether
miR-132 was involved in the abnormal viability of GCs from patients
with PCOS and to elucidate the underlying mechanisms. It was
observed that the expression of miR-132 was significantly increased
in GCs from patients with PCOS. Furthermore, the results revealed
that the decreased expression of miR-132 was associated with a
increased cell apoptotic index of GCs from patients with PCOS.
Moreover, the dual-luciferase reporter assay revealed that Foxa1
was a direct target for miR-132 and promoted GC viability. Foxa1
overexpression reversed the suppressive effects of miR-132 mimics.
These results thus indicated that miR-132 suppressed cell
viability, and the potential underlying mechanisms are associated
with the targeting and suppression of Foxa1 expression.
Previous studies have demonstrated that miR-132
plays a pro-apoptotic role in a number of cancer types, such as
glioma (18), colorectal cancer
(19,20), osteosarcoma (21), hepatic carcinoma (22), breast cancer (23), pituitary tumor (24) and lung cancer (25). In addition, an increased expression
of miR-132 has been reported in periovulatory mouse GCs following
LH/hCG treatment and enhanced estradiol synthesis in GCs (14,26).
However, a large number of studies on miR-132 have primarily
focused on cancer, and the mechanisms underlying the role of
miR-132 in PCOS have not yet been investigated. To the best of our
knowledge, the present study was the first to report that miR-132
was involved in inhibiting the viability of KGN cells, suggesting
that miR-132 plays crucial roles in the abnormal viability of GCs,
which may lead to the development of PCOS.
Foxa1 is a transcription factor that belongs to the
forkhead family, consisting of the winged-helix DNA-binding domain,
and the N-terminal and C-terminal transcriptional domains, thereby
delineating genomic regions and allowing for the subsequent binding
of other transcription factors, such as the estrogen receptor,
progesterone receptor and androgen receptor (27–29).
Foxa1 is expressed in a variety of organs, including breast, liver,
pancreas and prostate, and can influence the expression of a large
number of genes associated with metabolic processes, the regulation
of signaling and the cell cycle (30,31).
It has been reported that Foxa1 is a direct target of miR-132 in
breast cancer, thyroid cancer and nasopharyngeal carcinoma
(32–34). Consistent with the aforementioned
results, the present study further demonstrated that Foxa1 was a
direct target of miR-132 in KGN cell viability. However, Sang et
al (35), who identified
miRNAs in the human follicular fluid of patients with PCOS,
demonstrated that miR-132 was expressed at significantly lower
levels in the follicular fluid of patients with PCOS compared with
in the healthy controls. The differences were likely due to a
variety of reasons. miR-132 expression in the aforementioned study
was measured in human follicular fluid, whereas in the present
study, miR-132 expression was examined in human GCs. In addition,
the regulatory process of GC proliferation is complex and is
closely related to a variety of factors (36,37).
In conclusion, the findings of the present study
demonstrated that the expression of miR-132 was significantly
increased in patients with PCOS. In addition, the overexpression of
miR-132 inhibited the viability of KGN cells by targeting Foxa1.
These results provided novel evidence for the dysregulated
viability of GCs observed in PCOS. Due to the limitation in the
number of PCOS samples and cell types used, further investigations
are required in order to fully determine the underlying molecular
mechanisms of miR-132 in POCS.
Acknowledgements
Not applicable.
Funding
This study was supported by the Scientific Research
Project of Shanxi Provincial Department of Health (grant no.
201601070), the Initial Scientific Research Fund of PhD at Shanxi
Provincial People's Hospital (grant no. b201635), the Natural
Science Foundation of Shanxi (grant nos. 201901D211519 and
201901D211546), the Natural Science Foundation of Shanxi (grant no.
201901D211546), the Research Project Supported by Shanxi
Scholarship Council of China (grant no. HGKY2019092) and China
Postdoctoral Science Foundation (grant no. 2020M670703).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XC and XJ wrote the manuscript and made substantial
contributions to the design of the present study. XW, JL and XB
contributed to data interpretation and writing of the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Shanxi Women and Infants Hospital Ethics (Taiyuan,
China; approval no. 201922021), and all the participants signed
written informed consent for participation in this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lin J, Huang J, Wang N, Kuang Y and Cai R:
Effects of pre-pregnancy body mass index on pregnancy and perinatal
outcomes in women with PCOS undergoing frozen embryo transfer. BMC
Pregnancy Childbirth. 19:4872019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li S, Qi J, Tao Y, Zhu Q, Huang R, Liao Y,
Yue J, Liu W, Zhao H, Yin H and Sun Y: Elevated levels of
arachidonic acid metabolites in follicular fluid of PCOS patients.
Reproduction. Nov 1–2019.(Epub ahead of print). doi:
10.1530/REP-19-0136.
|
|
3
|
Sagvekar P, Mangoli V, Desai S, Patil A
and Mukherjee S: LINE1 CpG-DNA hypomethylation in granulosa cells
and blood leukocytes is associated with PCOS and related traits. J
Clin Endocrinol Metab. 102:1396–1405. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Makrinou E, Drong AW, Christopoulos G,
Lerner A, Chapa-Chorda I, Karaderi T, Lavery S, Hardy K, Lindgren
CM and Franks S: Genome-wide methylation profiling in granulosa
lutein cells of women with polycystic ovary syndrome (PCOS). Mol
Cell Endocrinol. 500:1106112019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qiu X, Wei Y, Liu C, Ding C and Zhao S:
Hyperandrogen enhances apoptosis of human ovarian granulosa cells
via up-regulation and demethylation of PDCD4. Gynecol Endocrinol.
36:333–337. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li Y, Liu YD, Zhou XY, Chen SL, Chen X,
Zhe J, Zhang J, Zhang QY and Chen YX: MiR-29a regulates the
proliferation, aromatase expression, and estradiol biosynthesis of
human granulosa cells in polycystic ovary syndrome. Mol Cell
Endocrinol. 498:1105402019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Song Y, Yu G, Xiang Y, Li Y, Wan L and Tan
L: Altered miR-186 and miR-135a contribute to granulosa cell
dysfunction by targeting ESR2: A possible role in polycystic ovary
syndrome. Mol Cell Endocrinol. 494:1104782019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen B, Xu P, Wang J and Zhang C: The role
of MiRNA in polycystic ovary syndrome (PCOS). Gene. 706:91–96.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang W, Li X, Ren L, Yuan C, Han Y and
Wang Z: MiR-132 relieves vascular endothelial inflammation and
improve endothelial function in atherosclerosis rats by regulating
SIRT1. Minerva Endocrinol. 45:158–161. 2019.PubMed/NCBI
|
|
10
|
Han Y, Wang F, Shao L, Huang P and Xu Y:
LncRNA TUG1 mediates lipopolysaccharide-induced proliferative
inhibition and apoptosis of human periodontal ligament cells by
sponging miR-132. Acta Biochim Biophys Sin (Shanghai).
51:1208–1215. 2019.PubMed/NCBI
|
|
11
|
Zhou X, Xiang C and Zheng X: miR-132
serves as a diagnostic biomarker in gestational diabetes mellitus
and its regulatory effect on trophoblast cell viability. Diagn
Pathol. 14:1192019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wei XC and Lv ZH: MicroRNA-132 inhibits
migration, invasion and epithelial-mesenchymal transition via
TGFβ1/Smad2 signaling pathway in human bladder cancer. Onco Targets
Ther. 12:5937–5945. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang XL, Sun BL, Tian SX, Li L, Zhao YC
and Shi PP: MicroRNA-132 reverses cisplatin resistance and
metastasis in ovarian cancer by the targeted regulation on Bmi-1.
Eur Rev Med Pharmacol Sci. 23:3635–3644. 2019.PubMed/NCBI
|
|
14
|
Wu S, Sun H, Zhang Q, Jiang Y, Fang T, Cui
I, Yan G and Hu Y: MicroRNA-132 promotes estradiol synthesis in
ovarian granulosa cells via translational repression of Nurr1.
Reprod Biol Endocrinol. 13:942015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Azziz R: Controversy in clinical
endocrinology: Diagnosis of polycystic ovarian syndrome: The
Rotterdam criteria are premature. J Clin Endocrinol Metab.
91:781–785. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kaur S, Archer KJ, Devi MG, Kriplani A,
Strauss JF III and Singh R: Differential gene expression in
granulosa cells from polycystic ovary syndrome patients with and
without insulin resistance: Identification of susceptibility gene
sets through network analysis. J Clin Endocrinol Metab.
97:E2016–E2021. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li Y, Zhang J, He J, Zhou W, Xiang G and
Xu R: MicroRNA-132 cause apoptosis of glioma cells through blockade
of the SREBP-1c metabolic pathway related to SIRT1. Biomed
Pharmacother. 78:177–184. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mokutani Y, Uemura M, Munakata K, Okuzaki
D, Haraguchi N, Takahashi H, Nishimura J, Hata T, Murata K,
Takemasa I, et al: down-regulation of microrna-132 is associated
with poor prognosis of colorectal cancer. Ann Surg Oncol. 23 (Suppl
5):599–608. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qin J, Ke J, Xu J, Wang F, Zhou Y, Jiang Y
and Wang Z: Downregulation of microRNA-132 by DNA hypermethylation
is associated with cell invasion in colorectal cancer. Onco Targets
Ther. 8:3639–3648. 2015.PubMed/NCBI
|
|
21
|
Liu Y, Li Y, Liu J, Wu Y and Zhu Q:
MicroRNA-132 inhibits cell growth and metastasis in osteosarcoma
cell lines possibly by targeting Sox4. Int J Oncol. 47:1672–1684.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lei CJ, Li L, Gao X, Zhang J, Pan QY, Long
HC, Chen CZ, Ren DF and Zheng G: Hsa-miR-132 inhibits proliferation
of hepatic carcinoma cells by targeting YAP. Cell Biochem Funct.
33:326–333. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang ZG, Chen WX, Wu YH, Liang HF and
Zhang BX: MiR-132 prohibits proliferation, invasion, migration, and
metastasis in breast cancer by targeting HN1. Biochem Biophys Res
Commun. 454:109–114. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Renjie W and Haiqian L: MiR-132, miR-15a
and miR-16 synergistically inhibit pituitary tumor cell
proliferation, invasion and migration by targeting Sox5. Cancer
Lett. 356:568–578. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li Y, Zu L, Wang Y, Wang M, Chen P and
Zhou Q: miR-132 inhibits lung cancer cell migration and invasion by
targeting SOX4. J Thorac Dis. 7:1563–1569. 2015.PubMed/NCBI
|
|
26
|
Fiedler SD, Carletti MZ, Hong X and
Christenson LK: Hormonal regulation of MicroRNA expression in
periovulatory mouse mural granulosa cells. Biol Reprod.
79:1030–1037. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fu X, Pereira R, De Angelis C,
Veeraraghavan J, Nanda S, Qin L, Cataldo ML, Sethunath V,
Mehravaran S, Gutierrez C, et al: FOXA1 upregulation promotes
enhancer and transcriptional reprogramming in endocrine-resistant
breast cancer. Proc Natl Acad Sci USA. 116:26823–26834. 2019.
View Article : Google Scholar
|
|
28
|
Jing X, Liang H, Hao C, Hongxia L and Cui
X: Analyses of an epigenetic switch involved in the activation of
pioneer factor FOXA1 leading to the prognostic value of estrogen
receptor and FOXA1 co-expression in breast cancer. Aging.
11:7442–7456. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gou L, Zou H and Li B: Long noncoding RNA
MALAT1 knockdown inhibits progression of anaplastic thyroid
carcinoma by regulating miR-200a-3p/FOXA1. Cancer Biol Ther.
20:1355–1365. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stone L: Different FOXA1 classes drive
prostate cancer. Nat Rev Urol. 16:5082019. View Article : Google Scholar
|
|
31
|
BenAyed-Guerfali D, Dabbeche-Bouricha E,
Ayadi W, Trifa F, Charfi S, Khabir A, Sellami-Boudawara T and
Mokdad-Gargouri R: Association of FOXA1 and EMT markers (Twist1 and
E-cadherin) in breast cancer. Mol Biol Rep. 46:3247–3255. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang D, Ren J, Ren H, Fu JL and Yu D:
MicroRNA-132 suppresses cell proliferation in human breast cancer
by directly targeting FOXA1. Acta Pharmacol Sin. 39:124–131. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen X, Li M, Zhou H and Zhang L: miR-132
targets FOXA1 and exerts tumor-suppressing functions in thyroid
cancer. Oncol Res. 27:431–437. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li YL, Zhao YG, Chen B and Li XF:
MicroRNA-132 sensitizes nasopharyngeal carcinoma cells to cisplatin
through regulation of forkhead box A1 protein. Pharmazie.
71:715–718. 2016.PubMed/NCBI
|
|
35
|
Sang Q, Yao Z, Wang H, Feng R, Wang H,
Zhao X, Xing Q, Jin L, He L, Wu L and Wang L: Identification of
microRNAs in human follicular fluid: characterization of microRNAs
that govern steroidogenesis in vitro and are associated with
polycystic ovary syndrome in vivo. J Clin Endocrinol Metab.
98:3068–3079. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kranc W, Budna J, Kahan R, Chachuła A,
Bryja A, Ciesiółka S, Borys S, Antosik MP, Bukowska D, Brussow KP,
et al: Molecular basis of growth, proliferation, and
differentiation of mammalian follicular granulosa cells. J Biol
Regul Homeost Agents. 31:1–8. 2017.PubMed/NCBI
|
|
37
|
Thomas FH and Vanderhyden BC:
Oocyte-granulosa cell interactions during mouse follicular
development: Regulation of kit ligand expression and its role in
oocyte growth. Reprod Biol Endocrinol. 4:192006. View Article : Google Scholar : PubMed/NCBI
|