Introduction
Laryngeal cancer is the most common type of head and
neck tumor. The most common pathological type of laryngeal cancer
is laryngeal squamous cell carcinoma (LSCC), accounting for ~96%
(1). LSCC is a highly invasive
malignant tumor, which accounts for ~2.4% of the newly diagnosed
cases of malignancy worldwide each year (2,3). The
pathogenesis of the disease is associated with several factors,
including the external environment, genetic factors, poor lifestyle
habits and human papillomavirus infection (4,5). At
present, the treatment of LSCC primarily involves surgical
resection, radiotherapy and chemotherapy, which can be used to cure
LSCC at early stages (6). However,
due to concealed clinical manifestations of LSCC, the majority of
patients are diagnosed at the middle or advanced stages, which are
associated with a poor prognosis, high mortalities and serious
complications (7,8). The occurrence and development of LSCC
are complex processes involving multiple genes and signaling
pathways. For example, high expression of reversion-inducing
cysteine-rich protein with Kazal motifs gene is closely related to
the invasion, metastasis, pathological differentiation and clinical
stages of LSCC cells (9). Cell
cycle arrest induced by p21-activated kinase 4 could activate the
ATM/CHEK1/CHEK2/p53 signaling pathway and inhibit the occurrence
and development of LSCC cells (10). Therefore, an in-depth study into
the regulation of related genes may provide a novel method for
cancer diagnosis and treatment.
Commonly found in eukaryotic cells, long non-coding
RNAs (lncRNAs) are a novel class of non-coding RNAs of >200
nucleotides in length (11,12).
lncRNAs can regulate protein-coding genes through a diverse range
of mechanisms, including regulation of transcription, cell
proliferation and differentiation, and they have been closely
associated with the occurrence and development of numerous types of
cancer, including breast, pancreatic, colorectal and gastric cancer
(13,14). In fact, lncRNAs have been reported
to serve an important role during LSCC occurrence, development and
metastasis (15,16). For example, one study demonstrated
that lncRNA H19 functioned as either a tumor promoter or suppressor
in different tumor cells, including breast epithelial, glioblastoma
and fibroblast cells (17). Luo
et al (18) reported that
H19 regulated the occurrence of LSCC through competitively binding
to insulin-like growth factor (IGF)-2 and serving as a microRNA
(miR) precursor that was positively related to disease progression.
Li et al (19) discovered
that the expression levels of HOX transcript antisense RNA (HOTAIR)
were associated with the clinical stage and tumor differentiation
of LSCC. In addition, upregulated expression levels of HOTAIR were
associated with a lower survival rate of patients with LSCC
(19). Feng et al (20) identified that metastasis associated
lung adenocarcinoma transcript 1 (MALAT1) was upregulated in LSCC
and the expression levels of MALAT1 were closely associated with
the degree of tumor differentiation, lymph node metastasis and
pathological differentiation. Fer-1-like family member 4 (FER1L4)
was also identified to serve as a tumor suppressor gene in several
types of tumor (21). For
instance, the knockdown of FER1L4 in hepatocellular carcinoma (HCC)
promoted cell proliferation and invasion (22); in colon cancer, the overexpression
of FER1L4 inhibited the progression by targeting miR-106a-5p
(23); in esophageal squamous cell
carcinoma (ESCC), the expression levels of FER1L4 were
downregulated in the ESCC tissues compared with the normal tissues;
and the overexpression of FER1L4 significantly suppressed ESCC cell
proliferation and migration, and induced apoptosis (24). In addition, FER1L4 demonstrated a
significant inhibitory effect on various other types of cancer,
including lung (25), prostate
(26) and gastric cancer (27). These results indicated that the
downregulated expression levels of FER1L4 may be related to the
formation of numerous types of cancer, which suggests that FER1L4
has a broad research value. However, to the best of our knowledge,
no study to date has reported on the expression levels and
mechanism of action of FER1L4 in LSCC.
In the present study, Cell Counting Kit-8 (CCK-8),
colony formation, flow cytometry, cell migration/invasion assays
and western blotting were used to evaluate the effect of FER1L4 on
the viability, proliferation, apoptosis, migration, invasion and
the expression levels of AKT/ERK signaling pathway-related
proteins, respectively, of Tu 686 cells. In addition, the mechanism
of FER1L4 in LSCC was preliminarily discussed, which may provide a
novel potential therapeutic target for the development of drugs for
the treatment of LSCC.
Materials and methods
Cell culture
Four LSCC cell lines (AMC-HN-8, Tu 686, M4E and M2E)
and one human bronchial epithelial cell line (HBE135-E6E7) were
used in the present study. AMC-HN-8 (cat. no. BNCC338377) and Tu
686 (cat. no. BNCC100479) cells were obtained from the BeNa Culture
Collection; Beijing Beina Chunglian Biotechnology Research
Institute. M4E (cat. no. JN-2244) and M2E (cat. no. JN-2245) cells
were provided from Shanghai Jining Industrial Co., Ltd. HBE135-E6E7
cells (ATCC CRL-2741) were purchased from the American Type Culture
Collection. LSCC cell lines were cultured in DMEM low glucose
(Hyclone; Cytiva) supplemented with 10% FBS (Hyclone; Cytiva) and
100 U/ml penicillin/streptomycin (Gibco; Thermo Fisher Scientific,
Inc.). The HBE135-E6E7 cell line was cultured in RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS.
All cells were cultured in a 5% CO2 incubator at 37°C.
Cells were selected for following experiments when they were in the
logarithmic phase.
Cell transfection
The FER1L4 sequence was synthesized by Shanghai
GenePharma Co., Ltd., and cloned into the pcDNA3.1 vector
(plasmid-FER1L4; Invitrogen; Thermo Fisher Scientific, Inc.). The
corresponding empty pcDNA3.1 vector [plasmid-negative control (NC)]
was used as the NC. Untransfected cells were used as the control.
Tu 686 cells (5×104 cells/well) in the logarithmic phase
were seeded into a six-well plate and these plasmids were
transfected into the Tu 686 cells at a final concentration of 100
nM using Lipofectamine® 2000 reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). Following transfection for 48 h at room
temperature, the transfection efficiency was analyzed using reverse
transcription-quantitative PCR (RT-qPCR).
CCK-8 assay
Tu 686 cells were seeded into 96-well plates at a
density of 5,000 cells per well. Following incubation for 24, 48
and 72 h at 37°C, 10 µl CCK-8 solution (Beijing Solarbio Science
& Technology Co., Ltd.) was added to each well and incubated in
a humidified atmosphere of 95% O2 and 5% CO2
at 37°C for 2 h, according to the manufacturer's protocols. The
absorbance of each well at 450 nm was subsequently measured using a
microplate reader (BioTek Instruments, Inc.).
Colony formation assay
Tu 686 cells were harvested 48 h post-transfection
and incubated in six-well plates at a density of 5,000 cells per
well; the medium was changed every 4 h. Following 14 days of
incubation in a humidified incubator at 37°C with 5%
CO2, the cells were rinsed with cold PBS, fixed with 4%
methanol for 15 min and then stained with 0.5% crystal violet for
20 min at room temperature. The colonies (>50 cells) were
visualized under a confocal microscopy (Nikon Corporation).
Wound healing assay
Wound healing assays were used to analyze the cell
migratory abilities. Briefly, Tu 686 cells were plated into
six-well plates at the density of 5×105 cells/ml. Upon
reaching ~100% confluence, a 200 µl-pipette tip was used to gently
scratch the adherent cell layer in the vertical direction of the
well. Then, PBS was used to wash off the non-suspended cells and
100 µg/ml DMEM without serum was added to each well. The cells were
cultured in a CO2 incubator at 37°C for 48 h. The width
of the wound was photographed under a light microscope
(magnification, ×200) and the extent of wound closure was
semi-quantified by ImageJ software 2.0 (National Institutes of
Health) at 0 and 48 h. The cell migration rate was calculated as:
Mobility (%) = (0–48 h scratch distance/initial distance)
×100%.
Invasion assay
For cell invasion experiments, 1×106 Tu
686 cells/well were plated into the upper chamber of a Transwell
plate precoated with Matrigel for 30 min at 37°C (2 mg/ml; 15 µl)
in serum-free DMEM. DMEM (600 µl) containing 10% FBS was plated
into the lower chambers. Following incubation for 24 h at 37°C with
5% CO2, the cells were stained with 0.1% crystal violet
for 20 min at room temperature. Finally, the cells were visualized
under an inverted light microscope (magnification, ×100; Olympus
Corporation). Invasion was semi-quantified by ImageJ software.
Cell apoptotic analysis
Flow cytometry was used to primarily analyze the
proportion of cells in the late apoptosis stage. The digested cells
were centrifuged at 1,000 × g for 10 min at 4°C, and the
supernatant was discarded. Cold PBS was used to collect the Tu 686
cells and wash them twice. Cell apoptosis was analyzed according to
the manufacturer's protocol of the Annexin V-FITC Apoptosis
Detection kit (EBioscience; Thermo Fisher Scientific, Inc.).
Briefly, Annexin V-FITC binding buffer was used to resuspend the Tu
686 cells, which was then diluted to provide a final concentration
of ~1×108 cells/ml. Cells were transferred to the flow
tube and incubated with 5 µl Annexin V-FITC staining solution for
15 min at in the dark at room temperature. Subsequently, the cells
were incubated with 10 µl propidium iodide staining solution for 5
min at 4°C. Apoptotic cells were then analyzed using a flow
cytometer (FC 5000; BD Biosciences) within 1 h, and flow cytometry
data were analyzed using FlowJo software v10.4.2 (FlowJo LLC).
RT-qPCR
Total RNA was extracted from Tu 686 cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. Total RNA was
reverse transcribed into cDNA at 42°C for 1 h using the PrimeScript
RT Reagent kit (Takara Biotechnology Co., Ltd.). The following
thermocycling conditions were used for RT-qPCR: 95°C for 5 sec;
followed by 45 cycles of denaturation at 95°C for 20 sec, annealing
at 58°C for 20 sec, and a final extension at 72°C for 30 sec. qPCR
was subsequently performed using a SYBR Green PCR Master Mix (Roche
Diagnostics) to analyze the expression levels of FER1L4. The
following primers sequences were used: FER1L4 forward,
5′-CCGTGTTGAGGTGCTGTTC-3′ and reverse, 5′-GGCAAGTCCACTGTCAGATG-3′;
GAPDH forward, 5′-CAATGACCCCTTCATTGACC-3′ and reverse,
5′-GACAAGCTTCCCGTTCTCAG-3′. GAPDH was used as the internal loading
control. The 2−ΔΔCq method (28) was used to quantify the mRNA
expression levels.
Western blotting
Total protein was extracted from Tu 686 cells using
RIPA lysis buffer (Beyotime Institute of Biotechnology). Total
protein was quantified using the BCA method and 30 µg protein/lane
was separated via 10% SDS-PAGE. The separated proteins were
subsequently transferred onto a polyvinylidene difluoride membrane
and blocked with 5% skimmed milk overnight at 4°C. The membranes
were then incubated with the following primary antibodies at 4°C
overnight: Anti-matrix metalloproteinase (MMP)-2 (1:1,000; cat. no.
40994; Cell Signaling Technology, Inc.), anti-MMP-9 (1:1,000; cat.
no. 13667; Cell Signaling Technology, Inc.), anti-phosphorylated
(p)-AKT (1:2,000; cat. no. 4060; Cell Signaling Technology, Inc.),
anti-AKT (1:1,000; cat. no. 4691; Cell Signaling Technology, Inc.),
anti-p-ERK (1:2,000; cat. no. 4370; Cell Signaling Technology,
Inc.), anti-ERK (1:1,000; cat. no. 4695; Cell Signaling Technology,
Inc.), anti-p-p38 (1:1,000; cat. no. 4511; Cell Signaling
Technology, Inc.), anti-p38 (1:1,000; cat. no. 8690; Cell Signaling
Technology, Inc.), anti-p-JNK (1:2,000; cat. no. 9255; Cell
Signaling Technology, Inc.), anti-JNK (1:1,000; cat. no. 9252; Cell
Signaling Technology, Inc.), anti-Bcl-2 (1:1,000; cat. no. 15071;
Cell Signaling Technology, Inc.), anti-Bax (1:1,000; cat. no. 2772;
Cell Signaling Technology, Inc.), anti-caspase-3 (1:1,000; cat. no.
9662; Cell Signaling Technology, Inc.), anti-caspase-9 (1:1,000;
cat. no. 9502; Cell Signaling Technology, Inc.) and anti-GAPDH
(1:1,000; cat. no. 5174; Cell Signaling Technology, Inc.).
Following the primary antibody incubation, the membranes were
incubated with HRP-conjugated goat anti-rabbit secondary antibody
(1:5,000; cat. no. A0208; Beyotime Institute of Biotechnology) at
room temperature for 2 h. After washing the membranes with TBS with
0.05% Tween-20, protein bands were visualized using an ECL
detection kit (Cytiva) and analyzed using a Bio-Rad ChemiDoc™ MP
imaging system and analyzed using Image Lab software 6.0.1 (Bio-Rad
Laboratories, Inc.). GAPDH was used as the internal reference
gene.
Statistical analysis
All experiments were repeated at least three times.
Statistical analysis was performed using GraphPad Prism 8.0
software (GraphPad Software, Inc.) and data are expressed as the
mean ± standard deviation. The two-tailed Student's t-test or a
one-way ANOVA followed by a Tukey's post hoc test were used for the
analyses of the differences between groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
FER1L4 expression levels are
downregulated in LSCC cell lines
To investigate the role of FER1L4 in LSCC, the
expression levels of FER1L4 were first analyzed in different LSCC
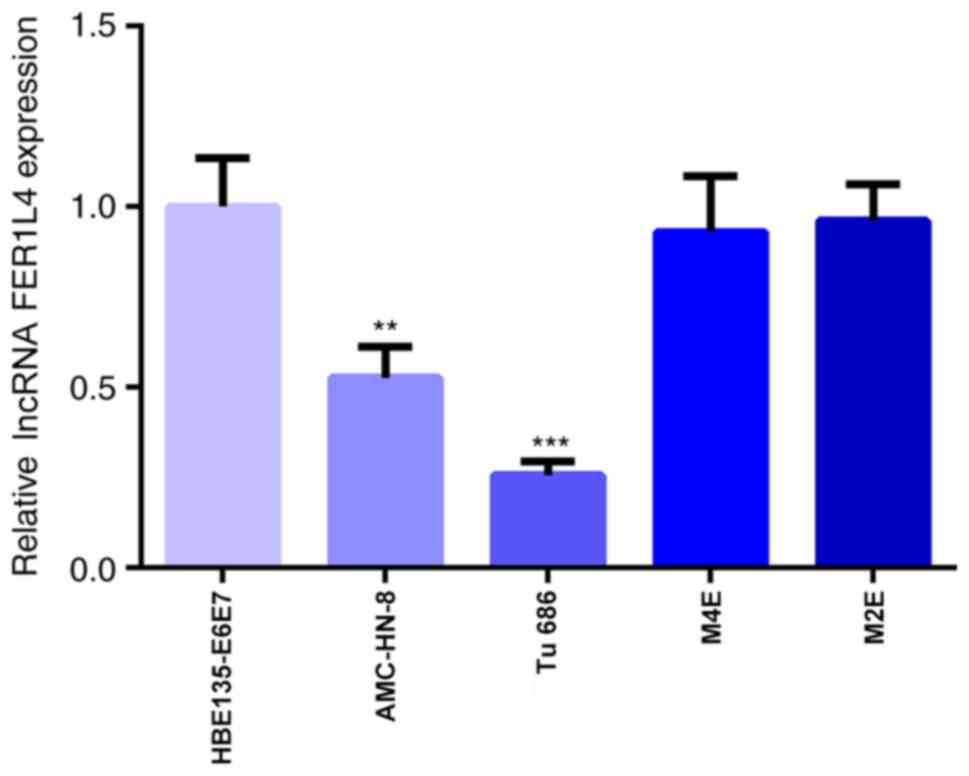
cell lines. The results of the RT-qPCR analysis are presented in
Fig. 1. Compared with the
HBE135-E6E7 cell line, the expression levels of FER1L4 in the LSCC
cell lines, AMC-HN-8 and Tu 686, were significantly downregulated,
while the expression levels of FER1L4 in the other two LSCC cell
lines, M4E and M2E, were not significantly different (Fig. 1). As the expression levels of
FER1L4 were the lowest in Tu 686 cells, these cells were selected
for the subsequent experiments.
Overexpression of FER1L4 inhibits cell
viability and proliferation in the Tu 686 cell line
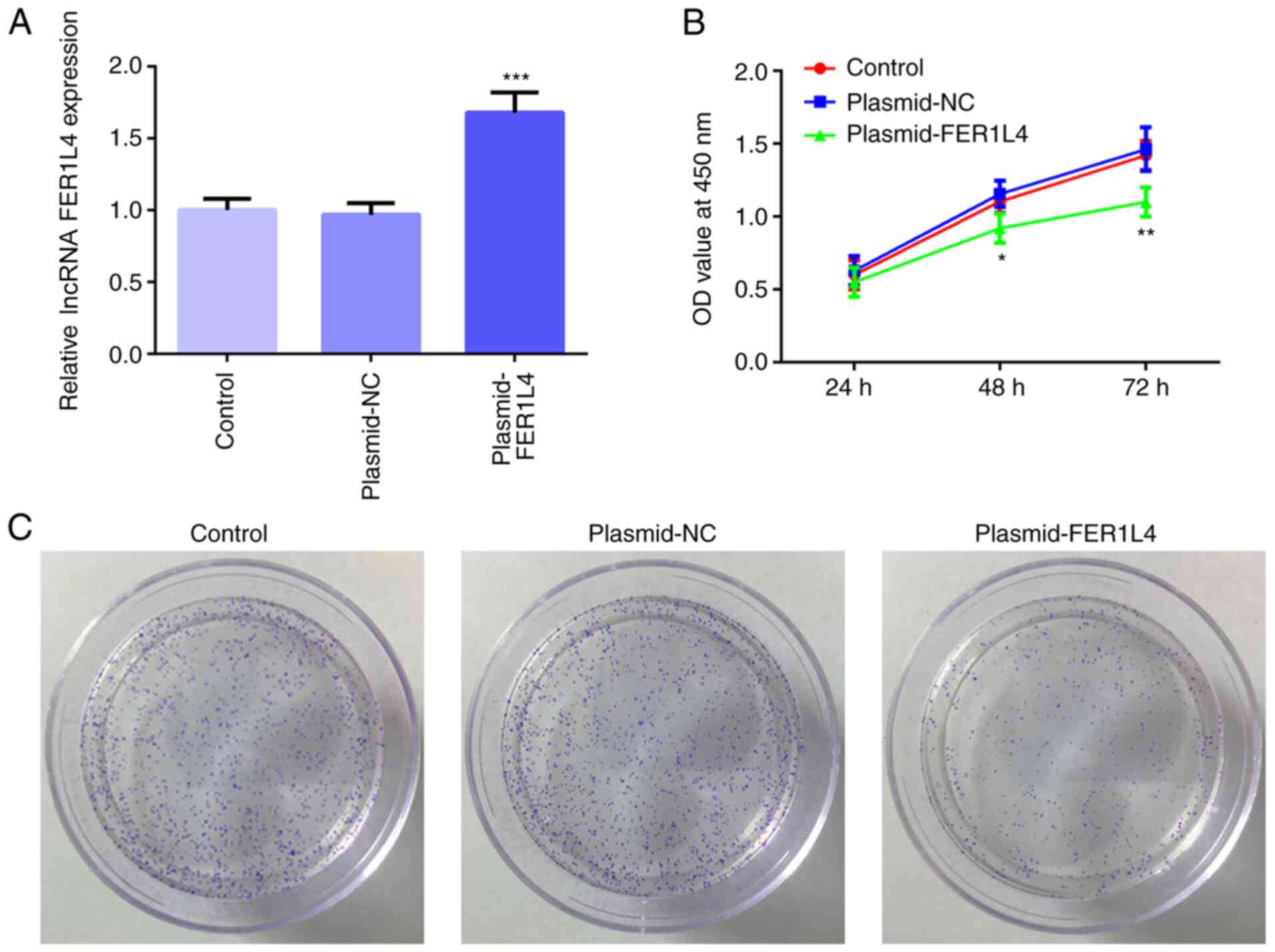
The plasmid-FER1L4 or plasmid-NC were constructed
and transfected into Tu 686 cells; the results demonstrated that
the expression levels of FER1L4 in the plasmid-FER1L4 group were
significantly upregulated compared with the control group, while
there were no significant differences identified in the expression
levels of FER1L4 between the plasmid-NC and control groups
(Fig. 2A). The CCK-8 assay
revealed that the cell viability of the plasmid-FER1L4 group was
significantly decreased after 48 h compared with the control group
(Fig. 2B). In addition, as the
duration of cell culture increased, the cell viability of the
plasmid-FER1L4 group was the most significantly decreased at 72 h
compared with the control group (Fig.
2B). The colony formation assay also demonstrated that the
number of colonies observed in the plasmid-FER1L4 group was
decreased compared with the control and plasmid-NC groups (Fig. 2C). These results suggested that the
overexpression of FER1L4 may inhibit the viability and
proliferation of the Tu 686 cell line.
Overexpression of FER1L4 inhibits cell
migration and invasion in the Tu 686 cell line
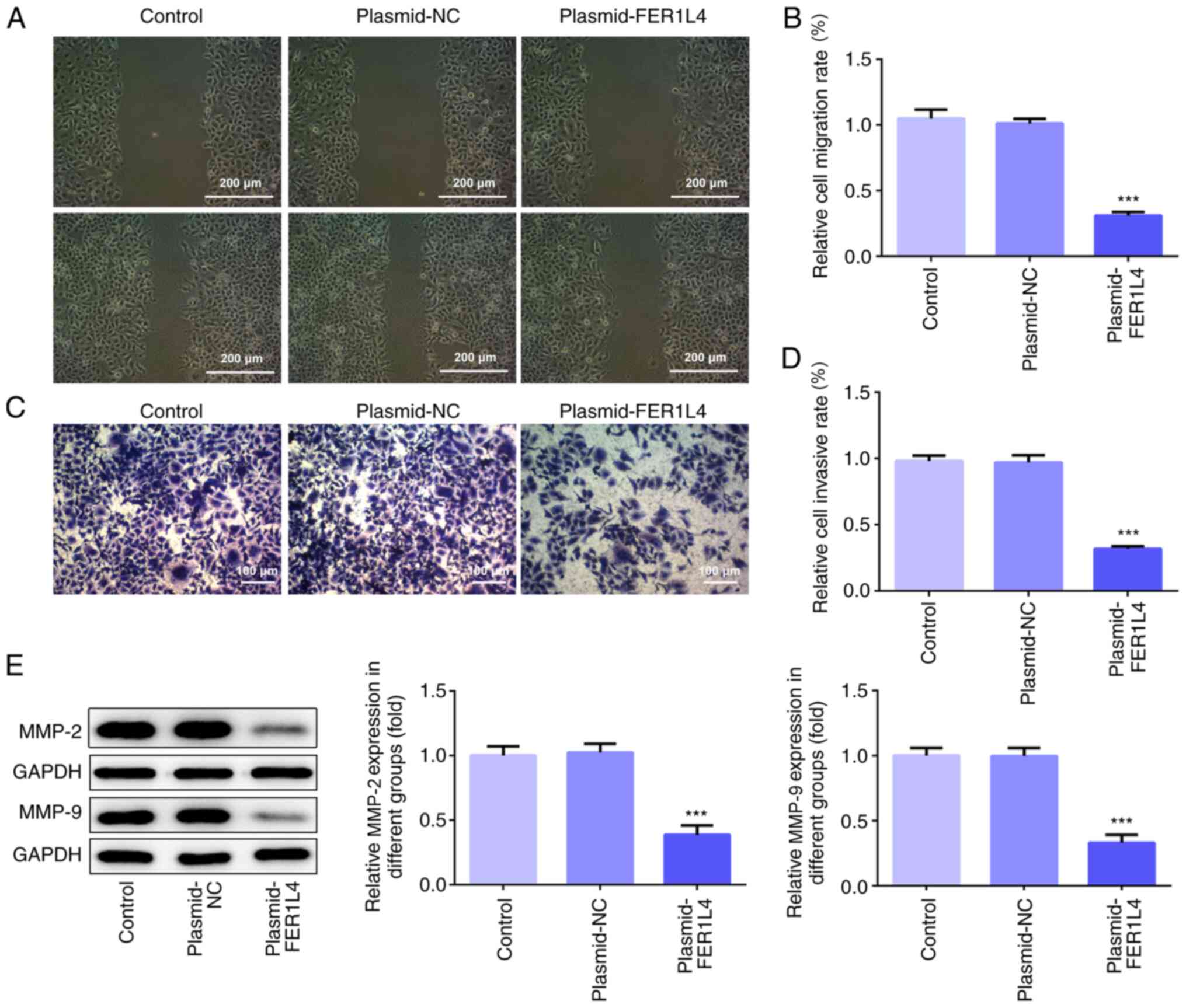
Wound healing and Transwell assays were used to
determine the migratory and invasive ability, respectively, of Tu
686 cells. As shown in Fig. 3A and
B, the results of the wound healing assay demonstrated that the
overexpression of FER1L4 significantly decreased the cell migration
rate and reduced the wound closure speed of Tu 686 cells compared
with the control group. The results of the Transwell assay revealed
that the number of invasive Tu 686 cells in the plasmid-FER1L4
group was significantly decreased compared with the control group
(Fig. 3C and D). In addition, the
expression levels of MMPs were investigated using western blotting.
As shown in Fig. 3E, the
overexpression of FER1L4 significantly downregulated the expression
levels of MMP-2 and MMP-9 compared with the control group. These
results suggested that the overexpression of FER1L4 may inhibit the
migration and invasion of the Tu 686 cell line.
Overexpression of FER1L4 promotes
apoptosis in the Tu 686 cell line
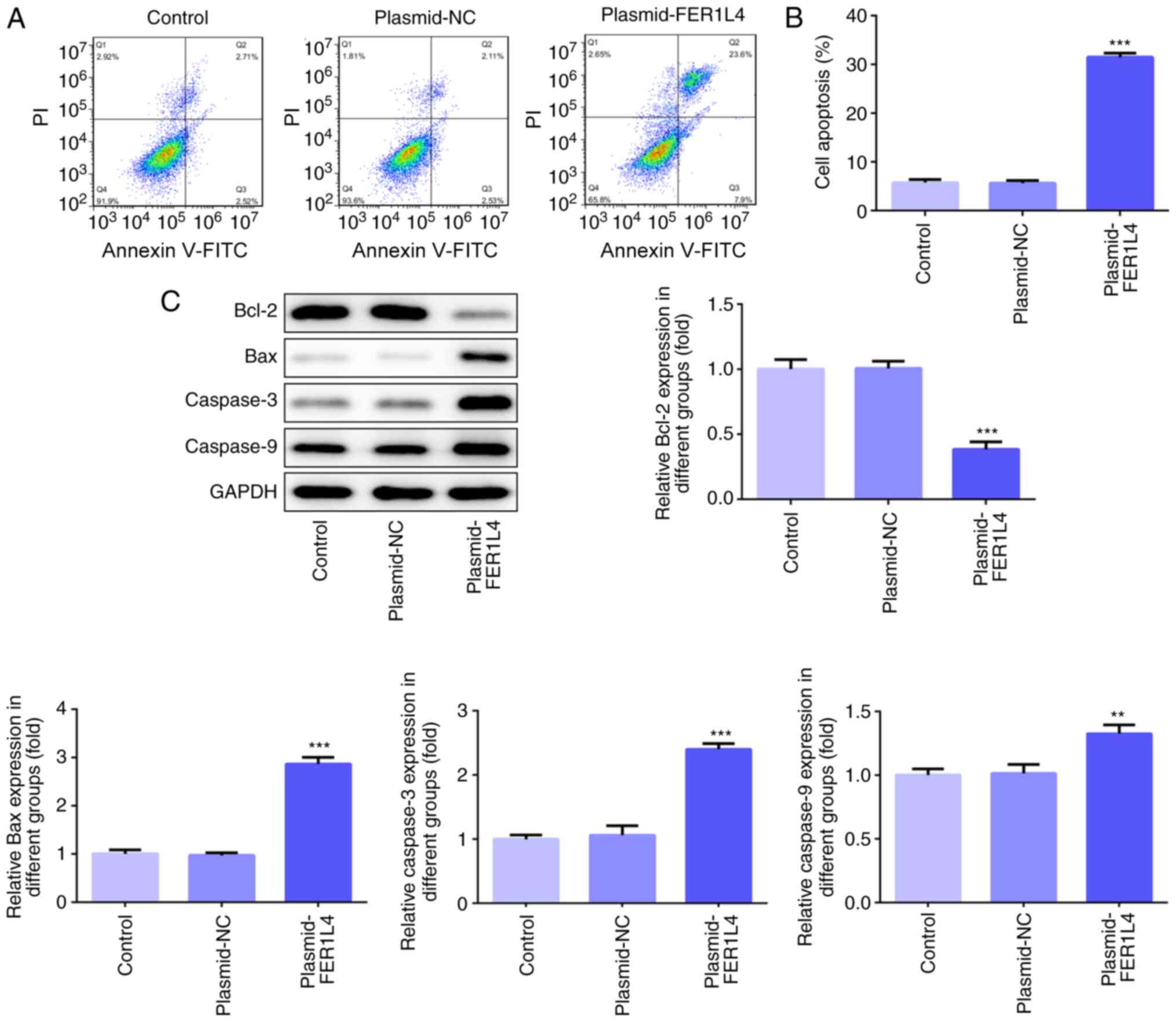
The results of the flow cytometric analysis revealed
that the levels of apoptosis were significantly increased in the
plasmid-FER1L4 group compared with the control group (Fig. 4A and B). The detection of
apoptosis-related proteins using western blotting demonstrated that
FER1L4 overexpression significantly downregulated the expression
levels of Bcl-2, while it significantly upregulated the expression
levels of Bax, caspase-3 and caspase-9 compared with the control
group (Fig. 4C). These results
indicated that the overexpression of FER1L4 may promote the
apoptosis of Tu 686 cells.
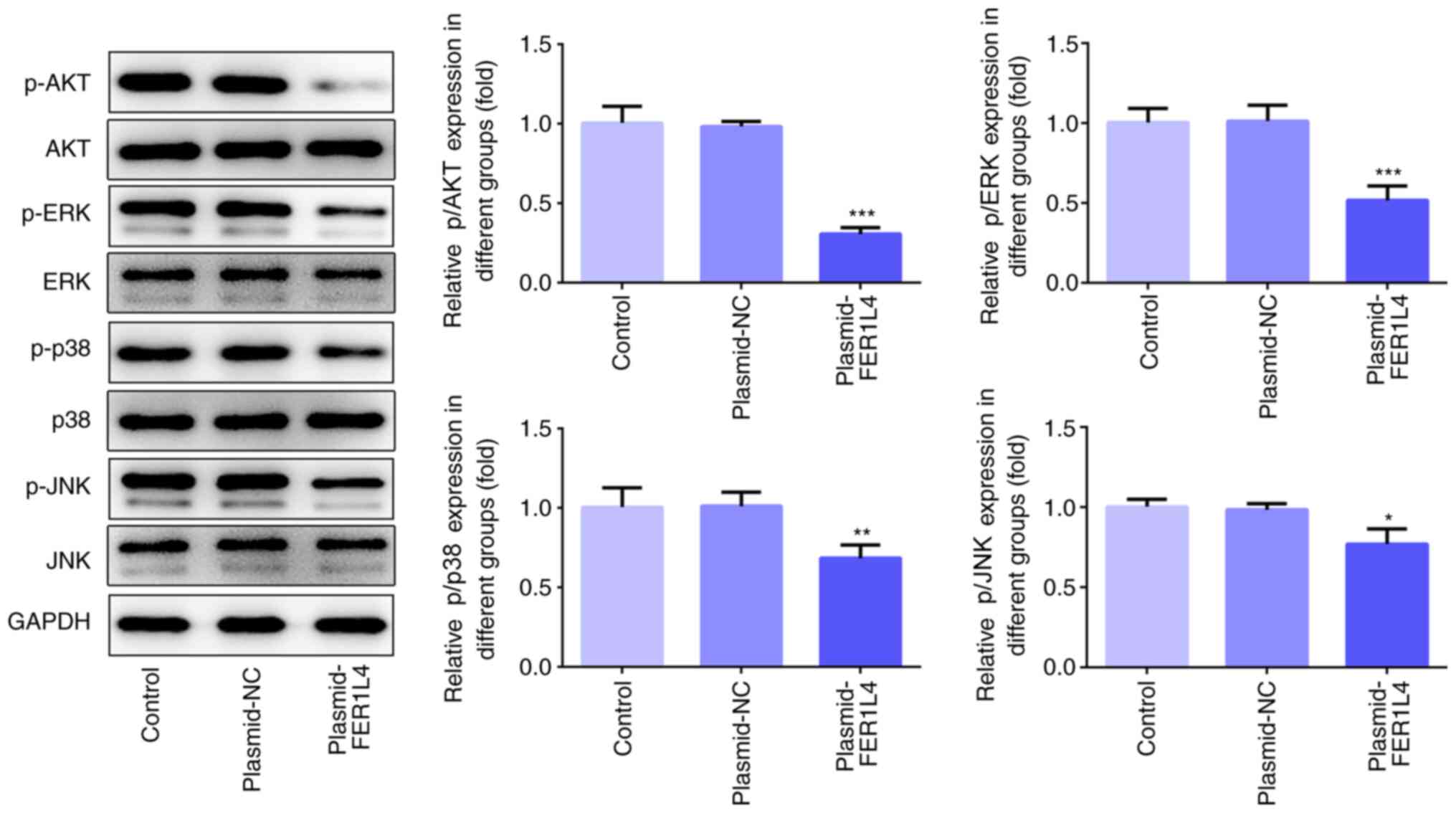
FER1L4 inhibits the AKT/ERK signaling
pathway in the Tu 686 cell line
To further elucidate the regulatory mechanism of
FER1L4 on the proliferation of LSCC cells, the relationship between
FER1L4 and the AKT/ERK signaling pathway was investigated. Compared
with the control group, the overexpression of FER1L4 significantly
downregulated the expression levels of p-AKT/AKT, p-ERK/ERK,
p-p38/p38 and p-JNK/JNK (Fig.
5).
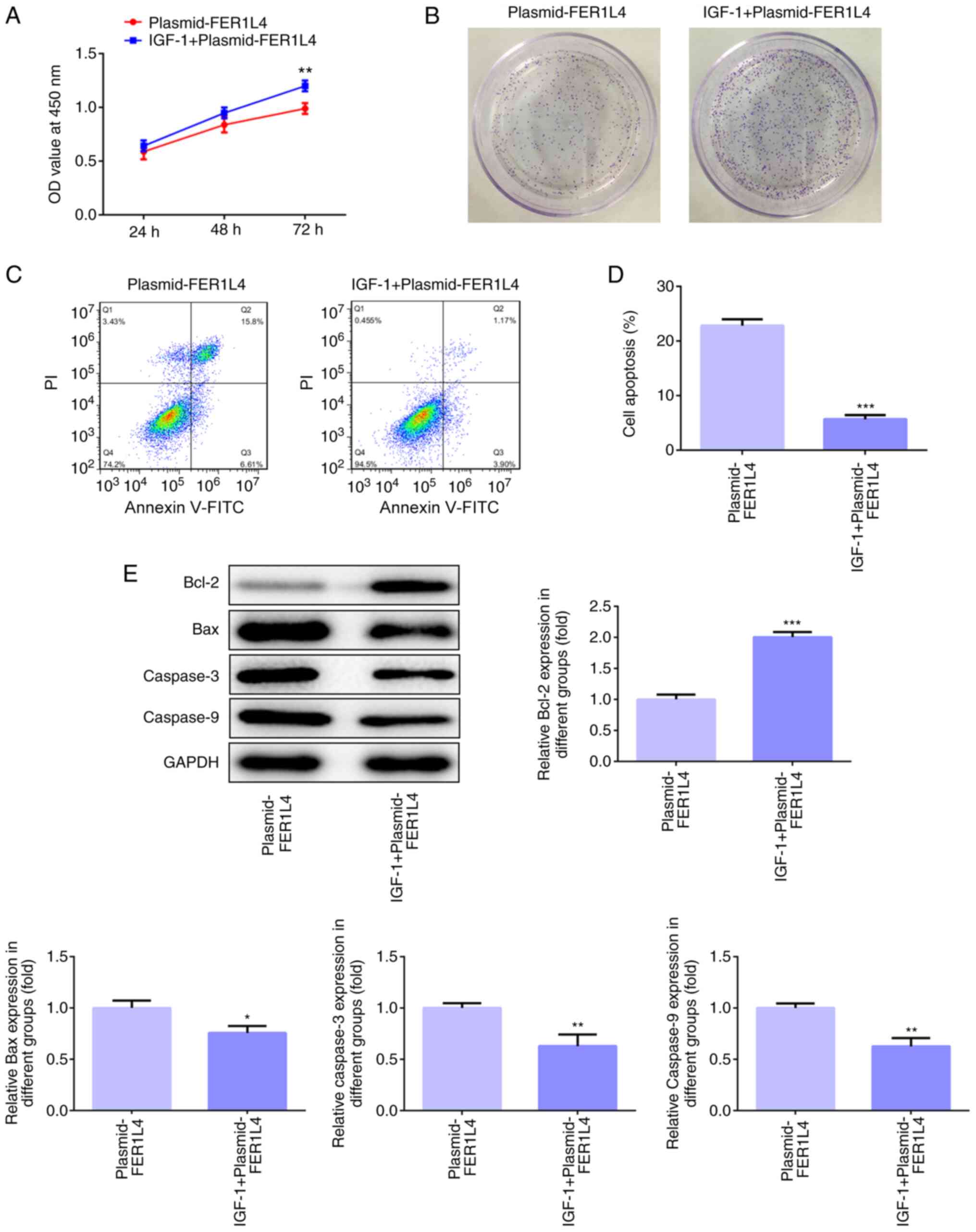
Overexpression of FER1L4 inhibits cell
viability and proliferation and promotes apoptosis by
downregulating the AKT/ERK signaling pathway in the Tu 686 cell
line
To further investigate whether FER1L4 was involved
in regulating the proliferation and apoptosis of LSCC cells via the
AKT/ERK signaling pathway, IGF-1, an agonist of AKT and ERK, was
used to treat the transfected cells for follow-up experiments.
After the plasmid-FER1L4 group was treated with IGF-1, the cell
viability at all times points and the number of colonies formed
increased compared with the plasmid-FER1L4 group (Fig. 6A and B). In the experiments
conducted to examine cell apoptosis, the levels of apoptosis were
significantly reduced in the IGF-1 + plasmid-FER1L4 group compared
with the plasmid-FER1L4 group (Fig. 6C
and D). Western blotting results also demonstrated that the
expression levels of Bcl-2 in the IGF-1 + plasmid-FER1L4 group were
significantly upregulated, while the expression levels of Bax,
caspase-3 and caspase-9 were significantly downregulated, compared
with the plasmid-FER1L4 group (Fig.
6E). Based on these results, it was suggested that the
overexpression of FER1L4 may inhibit the viability and
proliferation, while promoting the apoptosis of Tu 686 cells
through downregulating the AKT/ERK signaling pathway.
Discussion
The present study investigated the expression levels
and biological function of FER1L4 in LSCC cell lines, and the
findings indicated that FER1L4 expression levels in AMC-HN-8 and Tu
686 cells were significantly downregulated. In addition, the
overexpression of FER1L4 significantly suppressed the cell
viability, proliferation, migration and invasion, while inducing
apoptosis. It was further identified that FER1L4 could inhibit the
progression of LSCC by potentially modulating the AKT/ERK signaling
pathway.
LSCC is a malignant type of head and neck cancer
(29). At present, the main
clinical treatment for LSCC is surgical treatment; however,
patients with LSCC often have a poor prognosis and quality of life
due to the lack of effective treatment options (30,31).
Following increased in-depth studies at the gene level, it has been
identified that abnormal gene expression can lead to abnormal
regulatory mechanisms of tumor growth, which subsequently triggers
a large number of malignant cells to proliferate, while further
reducing the occurrence of apoptosis (32,33).
Therefore, understanding the related genes, which are associated
with oncogenesis, and their effects will contribute to the
prevention, treatment and prognosis of LSCC.
It has been confirmed that the abnormal expression
of lncRNA is associated with the pathogenesis of a variety of types
of malignant tumors, including HCC (34), pancreatic cancer (35) and colorectal cancer (36). Thus, the complex regulatory
mechanism of lncRNAs has resulted in widespread interest from
medical researchers (37,38). FER1L4 was discovered to be
expressed in a diverse range of tumors; for example, previous
studies have revealed that FER1L4 inhibited the tumor cell growth,
proliferation, migration and invasion of various types of cancers,
including HCC, ESCC, colon cancer, lung cancer, prostate cancer and
gastric cancer (22–27). In addition, FER1L4 inhibited the
proliferation and migration of HCC cancer cells by regulating the
P13K/AKT signaling pathway (39).
However, to the best of our knowledge, the expression levels and
biological function of FER1L4 in LSCC remain to be investigated.
The present study demonstrated, for the first time, that the
expression levels of FER1L4 were downregulated in the LSCC cell
lines, AMC-HN-8 and Tu 686, in vitro. Further experimental
results illustrated that the overexpression of FER1L4 significantly
induced apoptosis and inhibited cell proliferation, viability,
migration and invasion.
To further elucidate the molecular mechanism of
FER1L4 in regulating the proliferation, apoptosis, invasion and
metastasis of LSCC cells, the present study focused on the
association between FER1L4 and the AKT/ERK signaling pathway in
LSCC. The AKT/ERK signaling pathway is one of the most important
cellular signaling pathways for cell proliferation and it has been
reported to serve an important role in the occurrence and
development of HCC (40),
colorectal cancer (41) and
ovarian cancer (42). The AKT/ERK
signaling pathway has been identified to not only activate
downstream signaling molecules, but also interact with other
signaling molecules and result in a cascade activation (43,44).
However, it is not clear whether FER1L4 also affects the biological
phenotype of cell proliferation, migration, invasion and apoptosis
by mediating the AKT/ERK signaling pathway in LSCC. In the present
study, IGF-1, an agonist of the AKT/ERK signaling pathway (45), was used to regulate the effect of
FER1L4 on cell function. The present study demonstrated that FER1L4
overexpression significantly downregulated the phosphorylation
levels of AKT, ERK, p38 and JNK, which are proteins related to the
AKT/ERK signaling pathway (46),
in Tu 686 cells, whereas IGF-1 reversed the inhibitory effects of
FER1L4 overexpression on the phosphorylation levels of AKT, ERK,
p38 and JNK, and alleviated the effects of FER1L4 overexpression on
cell viability, proliferation and apoptosis. These results
preliminarily suggested that FER1L may inhibit the proliferation,
migration and invasion, while promoting the apoptosis of Tu 686
cells via inhibiting the AKT/ERK signaling pathway. However, there
are a number of limitations of the present study; for example, the
current study did not investigate whether there was a difference in
the role of FER1L4 in vivo and in vitro, whether the
downregulated expression levels of FER1L4 were related to the
prognosis of patients with LSCC or how FER1L4 further affected the
AKT/ERK signaling pathway through the regulation of downstream
genes. Therefore, the expression levels of FER1L4 in LSCC tissues
and a more detailed study of the specific effects and mechanism of
FER1L4 remain to be determined in LSCC.
In conclusion, the present study demonstrated that
FER1L4 overexpression inhibited the proliferation, migration and
invasion, and induced apoptosis of Tu 686 cells, thus inhibiting
the development of LSCC, which was indicated to potentially occur
through inhibiting the AKT/ERK signaling transduction pathway.
These findings suggested that FER1L4 may be a promising biological
target for the gene therapy of LSCC.
Acknowledgements
Not applicable.
Funding
This research study was supported by the Beijing
Natural Science Foundation (7194292), National Natural Science
Foundation of China (Grants NFSC 81770993) and the Fundamental
Research Funds for the Central Universities (2019-JYB-JS-052).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LJ, SL and YX made substantial contributions to the
conception and design of the present study; LJ and SL designed the
experiments; LJ, SL, LL, PH, ZG and ZY performed the experiments;
LJ and SL analyzed the data and wrote the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Almadori G, Bussu F, Cadoni G, Galli J,
Paludetti G and Maurizi M: Molecular markers in laryngeal squamous
cell carcinoma: Towards an integrated clinicobiological approach.
Eur J Cancer. 41:683–693. 2005.PubMed/NCBI
|
|
2
|
Christensen A, Kristensen E, Therkildsen
MH, Specht L, Reibel JP and Homøe P: Ten-year retrospective study
of head and neck carcinoma in situ: Incidence, treatment, and
clinical outcome. Oral Surg Oral Med Oral Pathol Oral Radiol.
116:174–178. 2013.PubMed/NCBI
|
|
3
|
Nunes Da Silva GHB, Miranda MG, Rodrigues
DS, De Souza GR and Ribeiro CV: Epidemiological factors in patients
with larynx cancer treated by surgery, radiotherapy or therapeutic
associations. Arch Otolaryngol Rhinol. 5:43–49. 2019.
|
|
4
|
Edefonti V, Bravi F, Garavello W, La
Vecchia C, Parpinel M, Franceschi S, Dal Maso L, Bosetti C,
Boffetta P, Ferraroni M and Decarli A: Nutrient-based dietary
patterns and laryngeal cancer: Evidence from an exploratory factor
analysis. Cancer Epidemiol Biomarkers Prev. 19:18–27.
2010.PubMed/NCBI
|
|
5
|
Nocini R, Molteni G, Mattiuzzi C and Lippi
G: Updates on larynx cancer epidemiology. Chin J Cancer Res.
32:18–25. 2020.PubMed/NCBI
|
|
6
|
Wu X, Cui CL, Chen WL, Fu ZY, Cui XY and
Gong X: MiR-144 suppresses the growth and metastasis of laryngeal
squamous cell carcinoma by targeting IRS1. Am J Transl Res. 8:1–11.
2016.PubMed/NCBI
|
|
7
|
Forastiere AA, Goepfert H, Maor M, Pajak
TF, Weber R, Morrison W, Glisson B, Trotti A, Ridge JA, Chao C, et
al: Concurrent chemotherapy and radiotherapy for organ preservation
in advanced laryngeal cancer. N Engl J Med. 349:2091–2098.
2003.PubMed/NCBI
|
|
8
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011.PubMed/NCBI
|
|
9
|
Cui J, Hui LI and Linlin LU: Correlation
between RECK gene methylation status and radiosensitivity in
laryngeal squamous cell carcinoma. Chin J Clin Oncol. 5:315–318.
2014.
|
|
10
|
Sun X, Liu B, Wang J, Li J and Ji WY:
Inhibition of p21-activated kinase 4 expression suppresses the
proliferation of Hep-2 laryngeal carcinoma cells via activation of
the ATM/Chk1/2/p53 pathway. Int J Oncol. 42:683–689.
2013.PubMed/NCBI
|
|
11
|
Fang XY, Pan HF, Leng RX and Ye DQ: Long
noncoding RNAs: Novel insights into gastric cancer. Cancer Lett.
356((2 Pt B)): 357–366. 2015.PubMed/NCBI
|
|
12
|
Okazaki Y, Furuno M, Kasukawa T, Adachi J,
Bono H, Kondo S, Nikaido I, Osato N, Saito R, Suzuki H, et al:
Analysis of the mouse transcriptome based on functional annotation
of 60, 770 full-length cDNAs. Nature. 420:563–573. 2002.PubMed/NCBI
|
|
13
|
Geisler S and Coller J: RNA in unexpected
places: Long non-coding RNA functions in diverse cellular contexts.
Nat Rev Mol Cell Biol. 14:699–712. 2013.PubMed/NCBI
|
|
14
|
Jiang C, Li X, Zhao H and Liu H: Long
non-coding RNAs: Potential new biomarkers for predicting tumor
invasion and metastasis. Mol Cancer. 15:622016.PubMed/NCBI
|
|
15
|
Wang KC, Yang YW, Liu B, Sanyal A,
Corces-Zimmerman R, Chen Y, Lajoie BR, Protacio A, Flynn RA, Gupta
RA, et al: A long noncoding RNA maintains active chromatin to
coordinate homeotic gene expression. Nature. 472:120–124.
2011.PubMed/NCBI
|
|
16
|
Yang QQ and Deng YF: Long non-coding RNAs
as novel biomarkers and therapeutic targets in head and neck
cancers. Int J Clin Exp Pathol. 7:1286–1292. 2014.PubMed/NCBI
|
|
17
|
Barsyte-Lovejoy D, Lau SK, Boutros PC,
Khosravi F, Jurisica I, Andrulis IL, Tsao MS and Penn LZ: The c-Myc
oncogene directly induces the H19 noncoding RNA by allele-specific
binding to potentiate tumorigenesis. Cancer Res. 66:5330–5337.
2006.PubMed/NCBI
|
|
18
|
Luo H, Sun Y, Wei G, Luo J, Yang X, Liu W,
Guo M and Chen R: Functional characterization of long noncoding RNA
Lnc_bc060912 in human lung carcinoma cells. Biochemistry.
54:2895–2902. 2015.PubMed/NCBI
|
|
19
|
Li D, Feng J, Wu T, Wang Y, Sun Y, Ren J
and Liu M: Long intergenic noncoding RNA HOTAIR is overexpressed
and regulates PTEN methylation in laryngeal squamous cell
carcinoma. Am J Pathol. 182:64–70. 2013.PubMed/NCBI
|
|
20
|
Feng J, Tian L, Sun Y, Li D, Wu T, Wang Y
and Liu M: Expression of long non-coding ribonucleic acid
metastasis-associated lung adenocarcinoma transcript-1 is
correlated with progress and apoptosis of laryngeal squamous cell
carcinoma. Head Neck Oncol. 4:462012.
|
|
21
|
Xia T, Chen S, Jiang Z, Shao Y, Jiang X,
Li P, Xiao B and Guo J: Long noncoding RNA FER1L4 suppresses cancer
cell growth by acting as a competing endogenous RNA and regulating
PTEN expression. Sci Rep. 5:134452015.PubMed/NCBI
|
|
22
|
Wu J, Huang J, Wang W, Xu J, Yin M, Cheng
N and Yin J: Long non-coding RNA Fer-1-like protein 4 acts as a
tumor suppressor via miR-106a-5p and predicts good prognosis in
hepatocellular carcinoma. Cancer Biomark. 20:55–65. 2017.PubMed/NCBI
|
|
23
|
Yue B, Sun B, Liu C, Zhao S, Zhang D, Yu F
and Yan D: Long non-coding RNA Fer-1-like protein 4 suppresses
oncogenesis and exhibits prognostic value by associating with
miR-106a-5p in colon cancer. Cancer Sci. 106:1323–1332.
2015.PubMed/NCBI
|
|
24
|
Ma W, Zhang CQ, Li HL, Gu J, Miao GY, Cai
HY, Wang JK, Zhang LJ, Song YM, Tian YH and Song YH: LncRNA FER1L4
suppressed cancer cell growth and invasion in esophageal squamous
cell carcinoma. Eur Rev Med Pharmacol Sci. 22:2638–2645.
2018.PubMed/NCBI
|
|
25
|
Gao X, Wang N, Wu S, Cui H, An X and Yang
Y: Long non-coding RNA FER1L4 inhibits cell proliferation and
metastasis through regulation of the PI3K/AKT signaling pathway in
lung cancer cells. Mol Med Rep. 20:182–190. 2019.PubMed/NCBI
|
|
26
|
Huo W, Qi F and Wang K: Long non-coding
RNA FER1L4 inhibits prostate cancer progression via sponging
miR-92a-3p and upregulation of FBXW7. Cancer Cell Int.
20:642020.PubMed/NCBI
|
|
27
|
Sun W, Yang Y, Xu C, Xie Y and Guo J:
Roles of long noncoding RNAs in gastric cancer and their clinical
applications. J Cancer Res Clin Oncol. 142:2231–2237.
2016.PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI
|
|
29
|
Zhang W, Yan Y, Gu M, Wang X, Zhu H, Zhang
S and Wang W: High expression levels of Wnt5a and Ror2 in laryngeal
squamous cell carcinoma are associated with poor prognosis. Oncol
Lett. 14:2232–2238. 2017.PubMed/NCBI
|
|
30
|
Ilm K, Fuchs S, Mudduluru G and Stein U:
MACC1 is post-transcriptionally regulated by miR-218 in colorectal
cancer. Oncotarget. 7:53443–53458. 2016.PubMed/NCBI
|
|
31
|
Wu Y, Jia Z, Cao D, Wang C, Wu X, You L,
Wen S, Pan Y, Cao X and Jiang J: Predictive value of miR-219-1,
miR-938, miR-34b/c, and miR-218 polymorphisms for gastric cancer
susceptibility and prognosis. Dis Markers.
2017:47318912017.PubMed/NCBI
|
|
32
|
Si L, Zheng L, Xu L, Yin L, Han X, Qi Y,
Xu Y, Wang C and Peng J: Dioscin suppresses human laryngeal cancer
cells growth via induction of cell-cycle arrest and MAPK-mediated
mitochondrial-derived apoptosis and inhibition of tumor invasion.
Eur J Pharmacol. 774:105–117. 2016.PubMed/NCBI
|
|
33
|
Morlando M and Fatica A: Alteration of
epigenetic regulation by long noncoding RNAs in cancer. Int J Mol
Sci. 19:5702018.
|
|
34
|
Wang Y, Liu Z, Yao B, Li Q, Wang L, Wang
C, Dou C, Xu M, Liu Q and Tu K: Long non-coding RNA CASC2
suppresses epithelial-mesenchymal transition of hepatocellular
carcinoma cells through CASC2/miR-367/FBXW7 axis. Mol Cancer.
16:1232017.PubMed/NCBI
|
|
35
|
Ding N, Wu H, Tao T and Peng E: NEAT1
regulates cell proliferation and apoptosis of ovarian cancer by
miR-34a-5p/BCL2. Onco Targets Ther. 10:4905–4915. 2017.PubMed/NCBI
|
|
36
|
Peng W, Wang Z and Fan H: LncRNA NEAT1
impacts cell proliferation and apoptosis of colorectal cancer via
regulation of Akt signaling. Pathol Oncol Res. 23:651–656.
2017.PubMed/NCBI
|
|
37
|
Esteller M: Non-coding RNAs in human
disease. Nat Rev Genet. 12:861–874. 2011.PubMed/NCBI
|
|
38
|
Mercer TR, Dinger ME and Mattick JS: Long
non-coding RNAs: Insights into functions. Nat Rev Genet.
10:155–159. 2009.PubMed/NCBI
|
|
39
|
Liu S, Zou B, Tian T, Luo X, Mao B, Zhang
X and Lei H: Overexpression of the lncRNA FER1L4 inhibits
paclitaxel tolerance of ovarian cancer cells via the regulation of
the MAPK signaling pathway. J Cell Biochem. 2018.(Epub ahead of
print).
|
|
40
|
Chen Z, Ma Y, Pan Y, Zuo S, Zhu H, Yu C,
Zhu C and Sun C: Long noncoding RNA RP5-833A20. 1 suppresses
tumorigenesis in hepatocellular carcinoma through Akt/ERK pathway
by targeting miR-18a-5p. Onco Targets Ther. 12:10717–10726.
2019.PubMed/NCBI
|
|
41
|
Ye Q, Cai W, Zheng Y, Evers BM and She QB:
ERK and AKT signaling cooperate to translationally regulate
survivin expression for metastatic progression of colorectal
cancer. Oncogene. 33:1828–1839. 2014.PubMed/NCBI
|
|
42
|
Yao N, Sun JQ, Yu L, Ma L and Guo BQ:
LINC00968 accelerates the progression of epithelial ovarian cancer
via mediating the cell cycle progression. Eur Rev Med Pharmacol
Sci. 23:4642–4649. 2019.PubMed/NCBI
|
|
43
|
Chang C, Xie J, Yang Q, Yang J, Luo Y, Xi
L, Guo J, Yang G, Jin W and Wang G: Serine peptidase inhibitor
Kazal type III (SPINK3) promotes BRL-3A cell proliferation by
targeting the PI3K-AKT signaling pathway. J Cell Physiol.
235:2209–2219. 2020.PubMed/NCBI
|
|
44
|
Meng XP, Ma J, Wang B, Wu X and Liu Z:
Long non-coding RNA OIP5-AS1 promotes pancreatic cancer cell growth
through sponging miR-342-3p via AKT/ERK signaling pathway. J
Physiol Biochem. 76:301–315. 2020.PubMed/NCBI
|
|
45
|
Liu X, Li J and Li X: MiR-142-5p regulates
the progression of diabetic retinopathy by targeting IGF1. Int J
Immunopathol Pharmacol. 34:20587384209090412020.PubMed/NCBI
|
|
46
|
Cordero-Herrera I, Martín MA, Bravo L,
Goya L and Ramos S: Epicatechin gallate induces cell death via p53
activation and stimulation of p38 and JNK in human colon cancer
SW480 cells. Nutr Cancer. 65:718–728. 2013.PubMed/NCBI
|