Introduction
Preeclampsia (PE) is a systemic disease that results
from placental defects; this pregnancy-associated disease causes
complications in 5–8% of all pregnancies and increases both
maternal and neonatal morbidity and mortality rates worldwide
(1–3). It is characterized by elevated
maternal blood pressure and proteinuria (4). It is has been established that
trophoblastic dysfunction, such as inadequate migration and
invasion, is one of the primary pathological manifestations of PE
(5); however, the molecular
mechanisms associated with insufficient trophoblastic invasion
remain largely unknown.
MicroRNAs (miRNAs) are a group of small non-coding
RNAs ~22 nucleotides in length, which regulate target gene
expression through either inducing transcript degradation or
inhibiting translation (6).
Several previous studies have demonstrated that various miRNAs are
aberrantly expressed in preeclamptic placentas (7,8), of
which some are involved in trophoblastic invasion, such as miRNA
(miR)-218, miR-520c-3p, miR-520g and miR-203 (9–12).
Notably, miR-491-5p has previously been reported to be upregulated
in hypoxic primary human trophoblasts (13); however, this study did not
investigate the effects of miR-491 on trophoblastic invasion. In
addition, miR-491-5p has been observed to serve as a tumor
suppressor in several types of cancer (14,15);
for example, miR-491-5p expression levels were found to be
decreased in gastric cancer tissues and its upregulation suppressed
gastric cancer metastasis through targeting SNAIL and fibroblast
growth factor receptor 4 (16). Xu
et al (15) demonstrated
that miR-491-5p suppressed cellular invasion through targeting
platelet-derived growth factor receptor α in prostate cancer. Based
on the evidence from these previous studies, it is hypothesized
that miR-491-5p may affect the invasion of trophoblasts. Thus, in
the present study the relative expression levels of miR-491-5p in
placental tissues between patients with PE and normal healthy
pregnant women were investigated, and the role of miR-491-5p in
trophoblast invasion and migration, and its underlying molecular
mechanisms were determined.
Materials and methods
Patient studies
The present study was approved by the Research
Ethics Committee of Hebei Medical University (Tangshan, China) and
informed consent was obtained from all patients. A total of 30
human placental tissues were collected from pregnancies with PE
(mean age, 41.28±4.01 years) who underwent cesarean sections at the
Department of Obstetrics and Gynecology, Tangshan Gongren Hospital,
Hebei Medical University between June 2016 and February 2017. A
total of 30 samples from normal pregnancies (mean age, 39.18±3.02
years) were recruited and used as the control group. All samples
were immediately frozen in liquid nitrogen and stored at −80°C for
subsequent analysis. PE was defined according to the International
Society for the Study of Hypertension in Pregnancy (17). Briefly, patients must have had a
newly onset systolic blood pressure of ≥160 mmHg or a diastolic
blood pressure of ≥110 mmHg on two or more occasions, in addition
to accompanying severe proteinuria (≥3+ or ≥2 g/24 h) at ≥20 week
of gestation. The blood pressure of all patients must have had
returned to normal and symptoms of proteinuria must have
disappeared 6 weeks postpartum. For the control group, women with
renal disease, cardiovascular disease, transient hypertension in
pregnancy, gestational diabetes mellitus, hepatitis, any evidence
of spontaneous abortion, intrauterine fetal death, fetal
chromosomal or other pregnancy complications were excluded from
this study.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from 20 mg tissues using
TRIzol® reagent (Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. Total RNA was reverse
transcribed into cDNA using the PrimeScript™ RT reagent kit (Takara
Biotechnology Co., Ltd.) at 42°C for 30 min and 85°C for 5 sec.
qPCR for miRNA and mRNA was subsequently performed using an ABI
PRISM 7300 sequence detection system and the SYBR®-Green
I Real-Time PCR kit (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The reaction mixtures were denatured at 95°C for 3 min,
followed by 40 cycles of 95°C for 10 sec and 60°C for 30 sec, then
final extension at 72°C for 5 min. The following primer pairs were
used for qPCR: miR-491-5p forward, 5′-ATCCAGTGCGTGTCGTG-3′ and
reverse, 5′-TGCTAGTGGGGAACCCTTC-3′; U6 forward,
5′-TGCGGGTGCTCGCTTCGCAGC-3′ and reverse, 5′-CCAGTGCAGGGTCCGAGGT-3′;
matrix metalloproteinase (MMP)-9 forward,
5′-ACGCAGACATCGTCATCCAGT-3′ and reverse,
5′-GGACCACAACTCGTCATCGTC-3′; GAPDH forward,
5′-AGGTCGGTGTGAACGGATTTG-3′ and reverse,
5′-TGTAGACCATGTAGTTGAGGTCA-3′. The expression levels of miR-491-5p
and MMP9 in the tissues was normalized to those of U6 and GAPDH,
respectively. RT-qPCR assays were performed in triplicate and the
expression levels were calculated using the 2−ΔΔCq
method (18).
Cell culture
The HTR-8/SVneo cell line was purchased from The
Cell Bank of Type Culture Collection of the Chinese Academy of
Sciences. Cells were cultured in RPMI-1640 medium (cat. no.
11875-093; Gibco; Thermo Fisher Scientific, Inc.), supplemented
with 100 U/ml penicillin and streptomycin and 10% FBS, and
maintained at 37°C in a humidified atmosphere with 5%
CO2.
Cell transfection
miR-491-5p mimic (5′-AGUGGGGAACCCUUCCAUGAGG-3′),
mimics NC (5′AGAAGCUGUUCCAAGGUGGGCC-3′), miR-491-5p inhibitor
(5′-CCUCAUGGAAGGGUUCCCCACU-3′); and inhibitor NC
(5′-GAACAUCCAGGGUCCCGUUCCU-3′) were purchased from Shanghai
GenePharma Co., Ltd. For plasmid construction (pcDNA-MMP9), MMP9
was cloned into the XhoI and KpnI sites of the
pcDNA3.0 expression vector (Invitrogen; Thermo Fisher Scientific,
Inc.); empty pcDNA3.0 vector (pcDNA-vector) was used as a control.
The coding domain sequences of MMP-9 were inserted into a pcDNA3.0
vector (pcDNA-MMP-9; Invitrogen; Thermo Fisher Scientific, Inc.).
Subsequently, a long-range amplification PCR of MMP9 was performed
with the AccuPrime™ Taq DNA Polymerase High Fidelity mix
(Invitrogen; Thermo Fisher Scientific, Inc.) using forward and
reverse primers. The PCR reaction consisted of 1 cycle at 95°C for
15 sec followed by 30 cycles at 95°C for 5 sec, 60°C for 30 sec and
70°C for 30 sec, followed by final extension at 72°C for 5 min. The
MMP-9 primer sequences were as follows: Sense,
5′-CGCTCGAGGCACCACCACAACATCAC-3′ and antisense,
5′-CGGGTACCACCACAACTCGTCATCGTC-3′. Transfection of
HTR-8/SVneo cells with miRNAs (50 nM) and pcDNA-MMP9 (2 µg) was
performed using Lipofectamine® 2000 reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). Cells were harvested 48 h after
transfection for further experiments.
Cell invasion assays
The invasive abilities of HTR-8/SVneo cells were
analyzed using 8-µm pore Transwell plates, containing membranes
precoated with Matrigel (Becton-Dickinson and Company). Briefly, 48
h after transfection, a total of 1×104 HTR-8/SVneo
cells/well were plated in the upper chambers of Transwell plates in
200 µl serum-free RPMI-1640 medium. The lower chambers were plated
with 500 µl RPMI-1640 medium, supplemented with 10% FBS. Following
incubation at 37°C for 24 h, non-invasive cells in the upper
chamber were removed and the invasive cells on the lower surface of
the chamber were fixed with 4% paraformaldehyde at room temperature
for 30 min and subsequently stained with 0.1% crystal violet
(Sigma-Aldrich; Merck KGaA) for 10 min at room temperature. Stained
cells were counted and photographed using a fluorescent microscope
(Nikon Corporation; ×200 magnification).
Wound healing assay
Upon reaching 70–80% confluence, HTR-8/SVneo cells
were transfected with pcDNA-MMP9, miR-491-5p mimics, miR-491-5p
inhibitor and their respective negative controls for 24 h, and the
wound was subsequently created in the middle of the well using a
200 µl pipette tip. Then, cells were washed twice with PBS and
incubated in serum-free RPMI-1640 medium. The micrographs were
captured using a BX51 Olympus confocal microscope (Olympus
Corporation) at 0 and 24 h (×200 magnification). Image analysis and
quantification was performed using ImageJ version 1.46 software
(National Institutes of Health).
Prediction of the putative targets of
miR-491-3p
The putative targets of miR-491-3p were predicted by
the online software Targetscan 7.0 (targetscan.org) and miRanda
(microRNA.org).
Dual-luciferase reporter assay
The wild-type (wt) and mutant (mut) 3′-untranslated
region (UTR) of human MMP-9 was inserted into the pGL3 control
vector (Promega Corporation). HTR-8/SVneo cells (8×104)
were transfected with the wild-type or mutant vector (200 ng/well),
the pRL-TK Renilla luciferase reporter (10 ng/well),
miR-491-5p mimic or mimic NC (50 nmol/l) using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.). Following
transfection for 48 h, the luciferase activity was measured using
the Dual-Luciferase Reporter Assay system according to the
manufacturer's instructions (Promega Corporation). The pRL-TK
plasmid (Promega Corporation) was used as a normalizing control.
All experiments were performed in triplicate.
Western blotting
Total protein was extracted from HTR-8/SVneo cells
using RIPA lysis buffer (Beyotime Institute of Biotechnology).
Total protein was quantified using a bicinchoninic acid assay kit
(Pierce; Thermo Fisher Scientific, Inc.) and 30 µg protein/lane was
separated via 12% SDS-PAGE. The separated proteins were
subsequently transferred onto a PVDF membrane (GE Healthcare) and
blocked for 1 h at room temperature with 5% non-fat dry milk. The
membranes were incubated with the following primary antibodies
overnight at 4°C: Anti-MMP-9 (1:4,000; cat. no. 13667; Cell
Signaling Technology, Inc.) and anti-β-actin (1:2,000; cat. no.
4970; Cell Signaling Technology, Inc.). Following the primary
antibody incubation, membranes were incubated with HRP-conjugated
Mouse Anti-Rabbit IgG (Light-Chain Specific) (1:10,000; cat. no.
93702; Cell Signaling Technology, Inc.) for 1 h at 37°C. Protein
bands were visualized using an enhanced chemiluminescence kit (GE
Healthcare). Protein expression was quantified using ImageJ
software (National Institutes of Health).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism version 5.01 software (GraphPad Software, Inc.) and data are
presented as the mean ± standard deviation of 3 independent
repeats. Differences between sample groups were analyzed using a
one-way ANOVA and multiple comparisons were performed using a
Tukey's post hoc test. Spearman's rank correlation coefficient
analysis was used for correlation analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
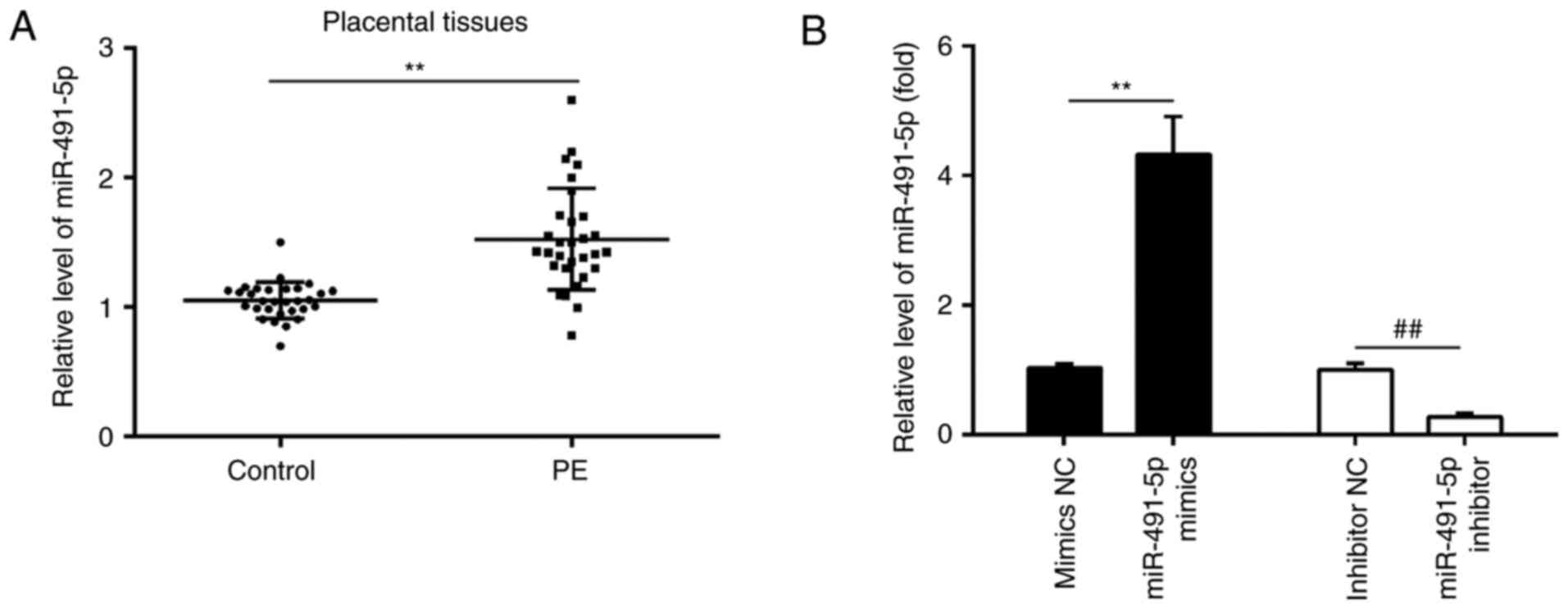
miR-491-5p expression levels are
increased in PE clinical samples
To investigate the role of miR-491-5p in the
development of PE, miR-491-5p expression levels were detected using
RT-qPCR in 30 placental tissues from women with PE and 30 placental
tissues from women who had a healthy pregnancy. The expression
levels of miR-491-5p were significantly increased in placental
tissues from women with PE compared with women in the control group
(Fig. 1A). This indicated that
miR-491-5p may be a novel factor associated with the pathogenesis
of PE. To further establish the role of miR-491-5p in trophoblast
cell invasion, miR-491-5p mimics or miR-491-5p inhibitors were
transfected into HTR-8/SVneo cells. RT-qPCR analysis demonstrated
the significant increase or decrease in cellular miR-491-5p
expression levels in the miR-491-5p mimic- or miR-491-5p
inhibitor-transfected cells, respectively, compared with their
respective NC-transfected cells (Fig.
1B).
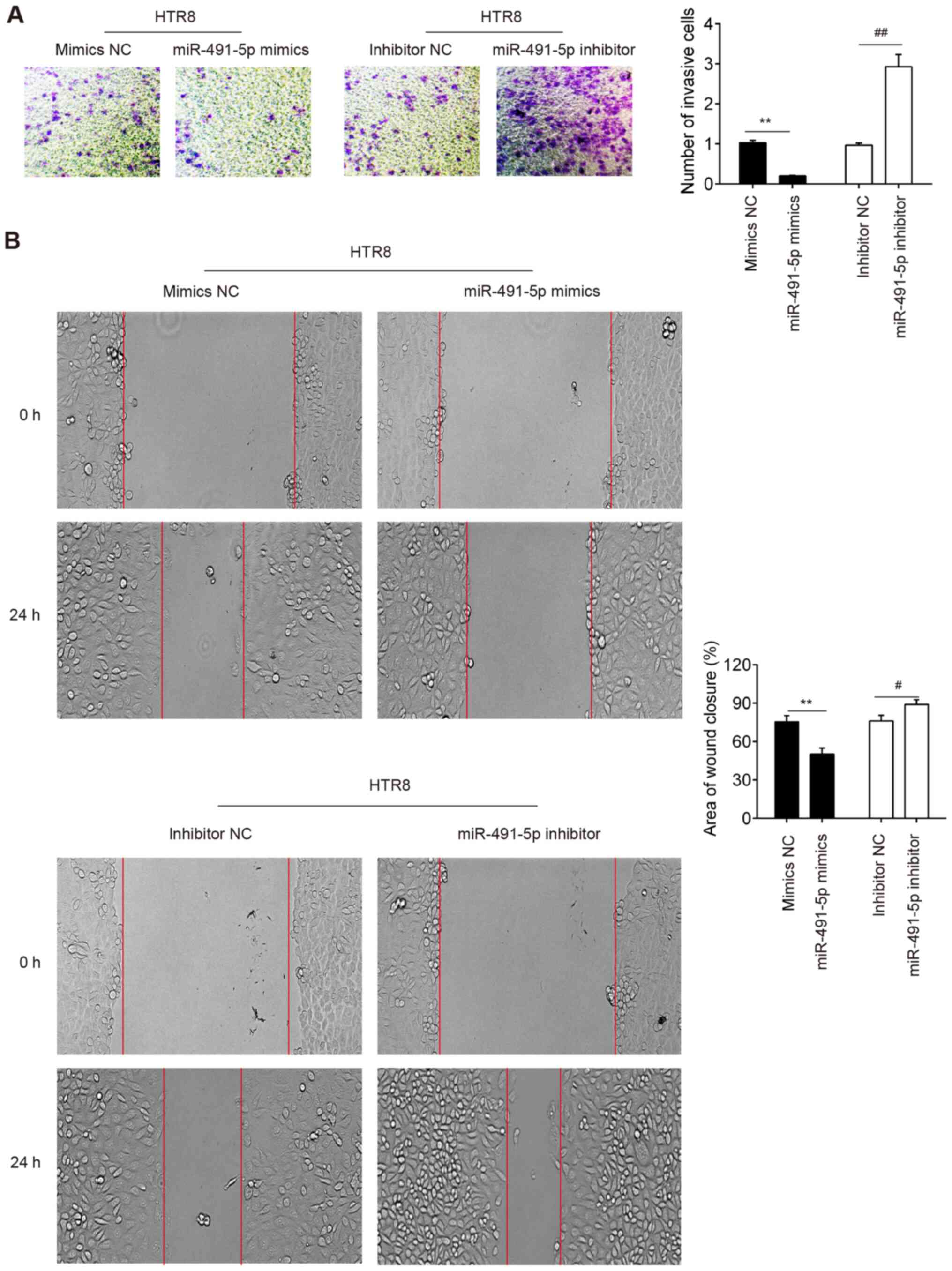
miR-491-5p suppresses the invasive
capacity of trophoblast cells
The invasion of normal trophoblasts is closely
associated with PE (5). Using a
Matrigel invasion assay, it was demonstrated that the
overexpression of miR-491-5p in miR-491-5p mimic-transfected cells
significantly suppressed the invasive ability of HTR-8/SVneo cells
compared with cells transfected with the NC mimic, whereas the
inhibition of miR-491-5p in miR-491-5p inhibitor-transfected cells
significantly increased the invasive ability of cells compared with
NC inhibitor-transfected cells (Fig.
2A). In addition, it was demonstrated that the upregulation of
miR-491-5p expression levels significantly decreased the migratory
ability of HTR8/SVneo cells compared with cells transfected with
the NC mimic (Fig. 2B). In
contrast, the inhibition of miR-491-5p significantly enhanced the
migratory ability of HTR-8/SVneo cells compared with the NC
inhibitor-transfected cells (Fig.
2B). These data suggest that the upregulation of miR-491-5p may
inhibit the invasion and migration of trophoblast cells.
MMP-9 is a direct target of
miR-491-5p
To further elucidate the underlying molecular
mechanisms involved in the miR-491-5p-mediated inhibitory effects
on the invasion of trophoblasts, TargetScan version 7.0
(targetscan.org) (19) and miRanda
algorithm v3.0 (microrna.org/microrna/) (20) was used to predict the target genes
of miR-491-5p. It was discovered that the wt 3′-UTR of the MMP-9
mRNA contained the complementary sequence for miR-491-5p (Fig. 3A). Previous studies have reported
that MMP-9 is critical for regulating trophoblastic invasion
(21,22). To verify whether miR-491-5p
directly bound to MMP-9, a dual-luciferase reporter assay was
performed in HTR8/SVneo cells. The relative luciferase activity of
the wt MMP-9 3′-UTR was significantly reduced in HTR8/SVneo cells
transfected with the miR-491-5p mimic compared with the cells
transfected with the NC mimic, whereas the relative luciferase
activity of the wt MMP-9 3′-UTR was significantly increased in
HTR8/SVneo cells transfected with miR-491-5p inhibitor compared
with cells transfected with the NC mimic (Fig. 3B). In contrast, miR-491-5p did not
affect the luciferase activity when the targeted sequence of MMP-9
was mutated in the miR-491-5p-binding site (Fig. 3B). In addition, western blotting
analysis demonstrated that miR-491-5p overexpression in miR-491-5p
mimic-transfected cells significantly reduced the expression levels
of MMP-9 compared with the NC mimic-transfected cells, whereas
miR-491-5p inhibition significantly increased the expression levels
of MMP-9 in HTR8/SVneo cells compared with NC inhibitor-transfected
cells (Fig. 3C).
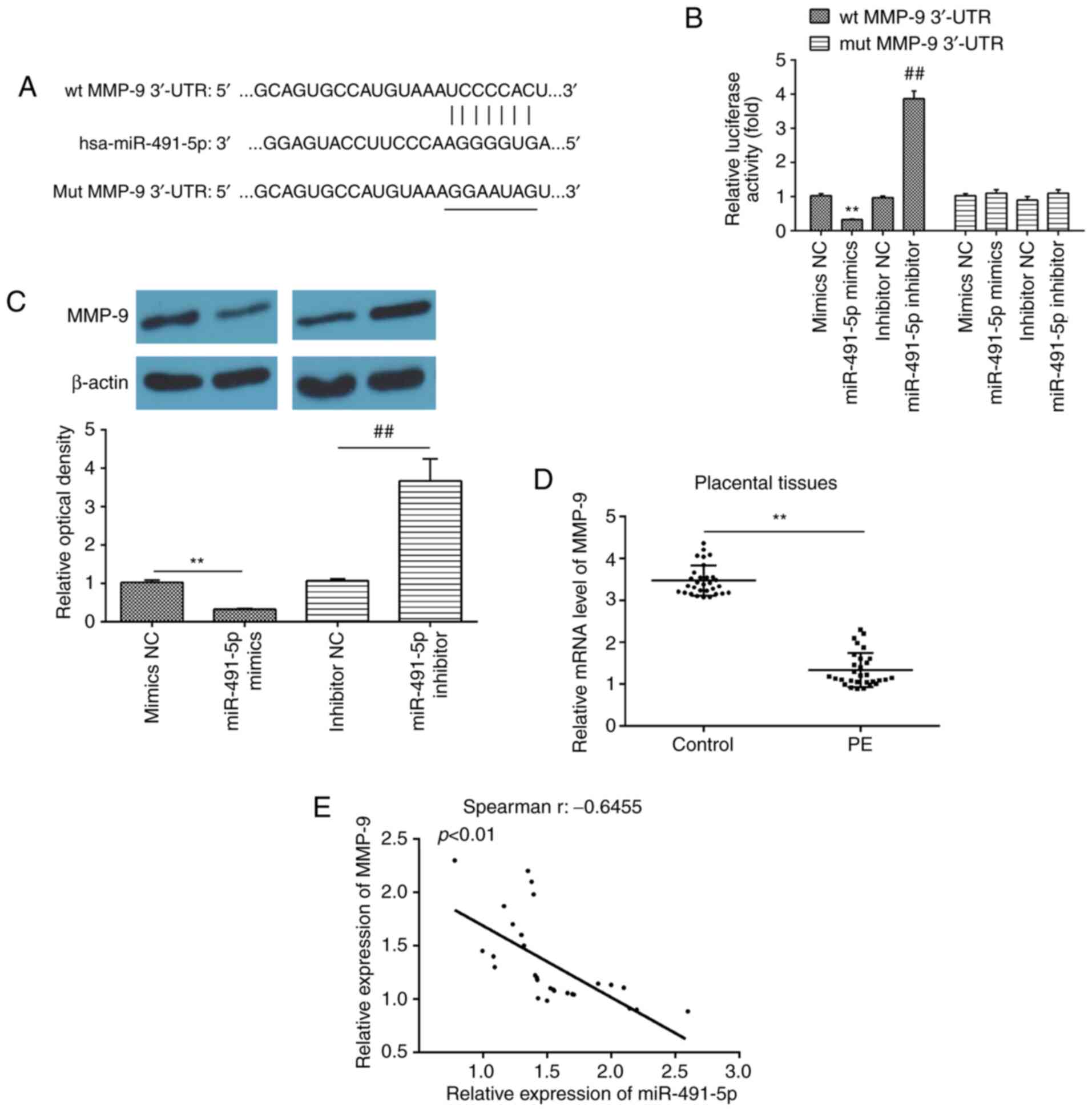 | Figure 3.MMP-9 is a direct target of
miR-491-5p. (A) A putative binding site between miR-491-5p and
MMP-9 was identified. (B) Dual-luciferase reporter assay of
HTR-8/SVneo cells co-transfected with firefly luciferase constructs
containing the wt or mut MMP-9 3′-UTR and miR-491-5p mimic, mimic
NC, miR-491-5p inhibitor or inhibitor NC, as indicated. **P<0.01
vs. mimic NC; ##P<0.01 vs. inhibitor NC. (C)
Expression levels of MMP-9 protein were determined following the
transfection of cells with miR-491-5p mimic, miR-491-5p inhibitor
and their respective NCs using western blotting. **P<0.01 vs.
mimic NC; ##P<0.01 vs. inhibitor NC. (D) Reverse
transcription-quantitative PCR was performed to determine the
expression levels of MMP-9 in PE placentas (n=30) and normal
pregnancies placentas (n=30). **P<0.01 vs. control. (E)
Relationship between miR-491-5p and MMP-9 was analyzed using
Spearman's correlation analysis (r=−0.6455; P<0.01). All data
represent the mean ± standard deviation of three independent
experimental repeats. MMP-9, matrix metalloproteinase 9; miR,
microRNA; wt, wild-type; mut, mutant; UTR, untranslated region; NC,
negative control; PE, preeclampsia. |
To further examine the expression of MMP-9 and its
correlation with miR-491-5p expression in PE, the expression levels
of MMP-9 mRNA were analyzed in all placenta samples used in the
present study. It was observed that the expression levels of MMP-9
were significantly decreased in the placental tissues from women
with PE compared with the women in the control group (Fig. 3D) and miR-491-5p expression was
found to be inversely correlated with the expression levels of
MMP-9 in placental tissues from women with PE (r=−0.6455;
P<0.01; Fig. 3E). These
findings suggested that MMP-9 may be regulated by miR-491-5p in PE
placenta tissues and trophoblast cells.
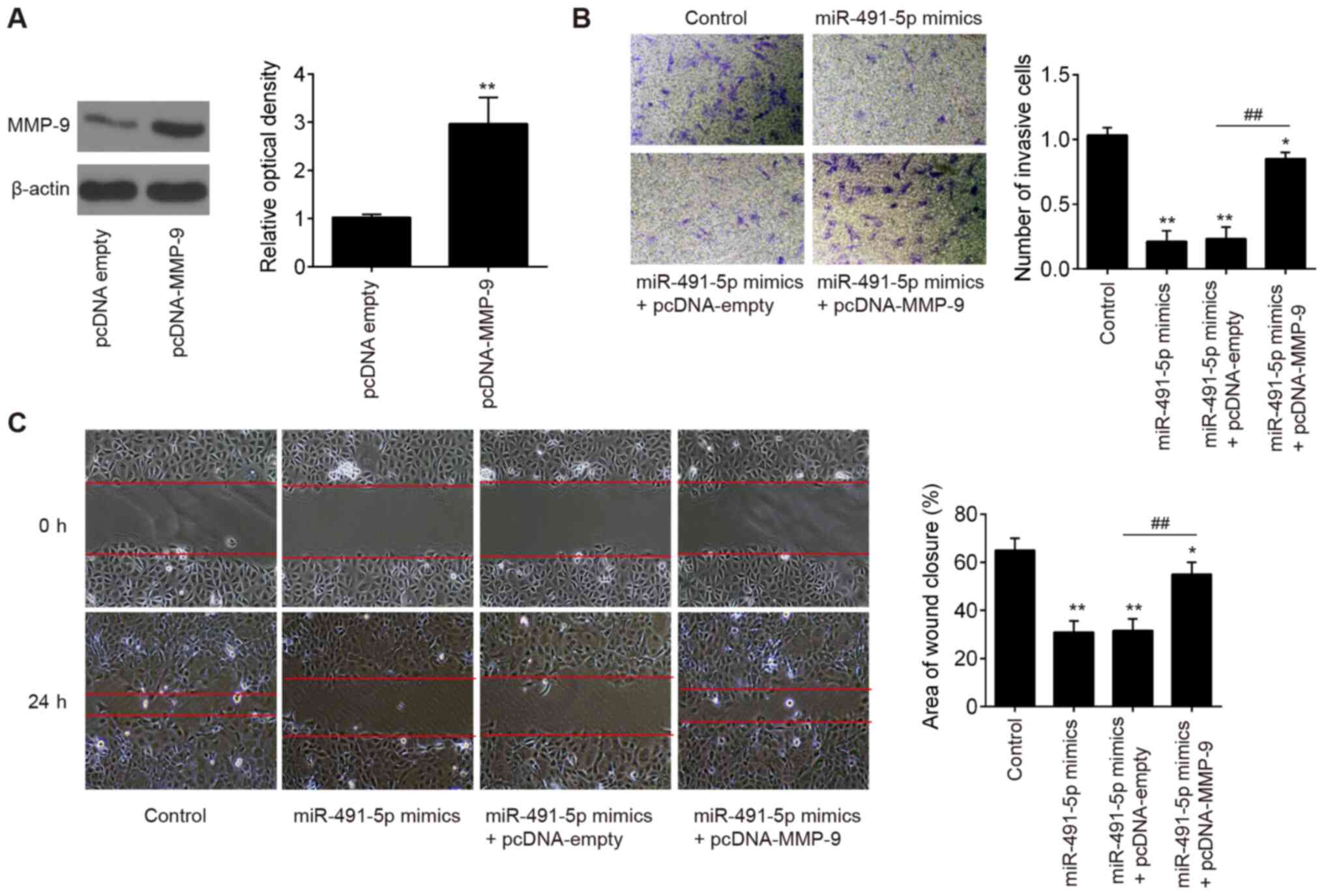
miR-491-5p suppresses the invasive and
migratory ability of trophoblast cells through targeting MMP-9
To determine whether miR-491-5p suppressed the
invasion of trophoblast cells through regulating MMP-9 expression,
MMP-9 was overexpressed in HTR8/SVneo cells using pcDNA-MMP-9
plasmids. Western blotting demonstrated that the expression levels
of MMP-9 were increased in HTR8/SVneo cells following pcDNA-MMP-9
transfection compared with cells transfected with the control
vector (Fig. 4A). Subsequently,
the invasive and migratory ability of HTR8/SVneo cells
co-transfected with miR-491-5p mimics and pcDNA-MMP-9 was examined.
The overexpression of MMP-9 significantly reversed the inhibitory
effects of the miR-491-5p mimic-transfected cells on cell invasion
and migration (Fig. 4B and C).
These data suggested that the upregulation of miR-491-5p may
suppress trophoblast cell invasion and wound healing, at least in
part, through downregulating MMP-9 expression.
Discussion
The present study demonstrated that miR-491-5p
expression levels were increased in the placental tissue from women
with PE, with in vitro analysis further indicating that
miR-491-5p could suppress the invasion and migration of trophoblast
cells through targeting of MMP-9. Collectively, these findings
suggested that the miR-491-5p/MMP9 axis may represent a potential
therapeutic target for the development of novel treatment
strategies for patients with PE.
Previous studies have reported that miRNAs are
aberrantly expressed in PE placenta tissues and are involved in
several pathophysiological processes, including the invasion and
migration of trophoblast cells (8,23–27).
For example, miR-136 was shown to be increased in PE placenta
tissue, where it inhibited the invasion of trophoblast cells
(28); Xue et al (29) found that miR-34a-5p expression
levels were increased in patients with PE, and these upregulated
levels suppressed the invasion and migration of trophoblast cells
through directly targeting SMAD4; Guo et al (30) revealed that miR-423-5p expression
levels were increased in the blood plasma of pregnant women with
PE, which effectively inhibited migration and invasion through
targeting IGF2BP1 in HTR-8/SVneo cells; and Gao et al
(31) found that increased
expression levels of miR-299 suppressed the invasion and migration
of HTR-8/SVneo trophoblast cells through targeting HDAC2 in PE.
These previous findings suggested that miRNAs may be an attractive
therapeutic target against PE. In the present study, it was
discovered that miR-491-5p expression levels were increased in the
placental tissue of patients with PE compared with normal
placentas, suggesting that miR-491-5p may be associated with the
pathogenesis of PE. miR-491-5p has been reported to serve as a
novel regulator of tumor cell function, including cell invasion and
migration (16). Therefore, it was
hypothesized that miR-491-5p may affect the progression of PE
through the regulation of trophoblastic invasion and migration,
which, to the best of our knowledge, had not been previously
investigated. In the present study, it was shown that the invasive
capabilities of HTR8/SVneo cells were suppressed following
miR-491-5p overexpression, whereas they were increased following
miR-491-5p inhibition. Taken together, these results indicated that
the increased expression of miR-491-5p may contribute to PE
development through suppressing invasion and migration in
trophoblasts.
To investigate the potential mechanisms underlying
the miR-491-5p-mediated inhibition of migration and invasion in
trophoblasts, bioinformatics analysis was performed to predict the
putative targets of miR-491-5p; MMP-9 was identified as a potential
target of miR-491-5p. MMP-9, a member of the MMP family, has been
previously demonstrated to mediate trophoblast invasion and
migration (32–34); for example, Rasstrigina et
al (35) reported that the
decreased expression levels of MMP-9 in the placenta contributed to
the reduced invasiveness of trophoblasts. Notably, a previous study
also observed that miR-491-5p suppressed the invasive ability of
gastric, breast and lung cancer cells through targeting MMP-9
(36). Nonetheless, whether MMP-9
was implicated in the inhibitory effects of miR-491-5p on
trophoblastic invasion remained relatively unknown. In the present
study, MMP-9 was validated as a target gene of miR-491-5p in
HTR8/SVneo cells. In addition, MMP-9 expression levels were found
to be decreased in the placental tissue of patients with PE
compared with normal placentas, and an inverse relationship was
observed between MMP-9 and miR-491-5p expression levels.
Furthermore, MMP-9 overexpression reversed the inhibitory effects
of the miR-491-5p mimic on the invasion and wound healing ability
of trophoblast cells. These results suggest that miR-491-5p may
suppress the trophoblastic invasion and migration, at least partly,
through decreasing the expression levels of MMP-9.
In conclusion, the results from the present study
showed that the expression levels of miR-491-5p were increased in
the placental tissues from women with PE and the upregulation of
miR-491-5p could inhibit the invasion and migration of trophoblast
cells, which was partly through targeting MMP-9. These data
suggested that miR-491-5p may be an effective drug target for
therapeutic intervention in PE; however, the present study may be
limited by the fact that the expression levels of miR-491-5p were
only investigated in placental tissues collected from pregnant
women undergoing cesarean sections, which suggested that its
expression levels may not be suitable for the prenatal screening of
patients with PE. In future studies, plasma samples from pregnant
patients should be collected and processed to detect the expression
levels of miR-491-5p in a multi-center study design.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
EL, YZ, JL and DZ all performed the experiments,
analyzed the data and wrote the manuscript. EL conceptualized the
experimental design. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by Research Ethics
Committee of Hebei Medical University. All individuals provided
informed consent for the use of their samples for clinical
research.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Redman CW and Sargent IL: Latest advances
in understanding preeclampsia. Science. 308:1592–1594. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kanasaki K and Kalluri R: The biology of
preeclampsia. Kidney Int. 76:831–837. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Al-Jameil N, Aziz Khan F, Fareed Khan M
and Tabassum H: A brief overview of preeclampsia. J Clin Med Res.
6:1–7. 2014.PubMed/NCBI
|
|
4
|
Vitoratos N, Economou E, Iavazzo C,
Panoulis K and Creatsas G: Maternal serum levels of TNF-alpha and
IL-6 long after delivery in preeclamptic and normotensive pregnant
women. Mediators Inflamm. 2010:9086492010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Meekins JW, Pijnenborg R, Hanssens M,
McFadyen IR and van Asshe A: A study of placental bed spiral
arteries and trophoblast invasion in normal and severe
pre-eclamptic pregnancies. Br J Obstet Gynaecol. 101:669–674. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu P, Zhao Y, Liu M, Wang Y, Wang H, Li
YX, Zhu X, Yao Y, Wang H, Qiao J, et al: Variations of microRNAs in
human placentas and plasma from preeclamptic pregnancy.
Hypertension. 63:1276–1284. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pineles BL, Romero R, Montenegro D, Tarca
AL, Han YM, Kim YM, Draghici S, Espinoza J, Kusanovic JP, Mittal P,
et al: Distinct subsets of microRNAs are expressed differentially
in the human placentas of patients with preeclampsia. Am J Obstet
Gynecol. 196:261 e261–266. 2007. View Article : Google Scholar
|
|
9
|
Takahashi H, Ohkuchi A, Kuwata T, Usui R,
Baba Y, Suzuki H, Chaw Kyi TT, Matsubara S, Saito S and Takizawa T:
Endogenous and exogenous miR-520c-3p modulates CD44-mediated
extravillous trophoblast invasion. Placenta. 50:25–31. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fang M, Du H, Han B, Xia G, Shi X, Zhang
F, Fu Q and Zhang T: Hypoxia-inducible microRNA-218 inhibits
trophoblast invasion by targeting LASP1: Implications for
preeclampsia development. Int J Biochem Cell Biol. 87:95–103. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jiang L, Long A, Tan L, Hong M, Wu J, Cai
L and Li Q: Elevated microRNA-520g in pre-eclampsia inhibits
migration and invasion of trophoblasts. Placenta. 51:70–75. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu F, Wu K, Wu W, Chen Y, Wu H, Wang H
and Zhang W: miR203 contributes to preeclampsia via inhibition of
VEGFA expression. Mol Med Rep. 17:5627–5634. 2018.PubMed/NCBI
|
|
13
|
Mouillet JF, Chu T, Nelson DM, Mishima T
and Sadovsky Y: MiR-205 silences MED1 in hypoxic primary human
trophoblasts. FASEB J. 24:2030–2039. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang J, Ren J, Hao S, Ma F, Xin Y, Jia W,
Sun Y, Liu Z, Yu H, Jia J and Li W: MiRNA-491-5p inhibits cell
proliferation, invasion and migration via targeting JMJD2B and
serves as a potential biomarker in gastric cancer. Am J Transl Res.
10:525–534. 2018.PubMed/NCBI
|
|
15
|
Xu Y, Hou R, Lu Q, Zhang Y, Chen L, Zheng
Y and Hu B: MiR-491-5p negatively regulates cell proliferation and
motility by targeting PDGFRA in prostate cancer. Am J Cancer Res.
7:2545–2553. 2017.PubMed/NCBI
|
|
16
|
Yu T, Wang LN, Li W, Zuo QF, Li MM, Zou QM
and Xiao B: Downregulation of miR-491-5p promotes gastric cancer
metastasis by regulating SNAIL and FGFR4. Cancer Sci.
109:1393–1403. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chaiworapongsa T, Chaemsaithong P,
Korzeniewski SJ, Yeo L and Romero R: Pre-eclampsia part 2:
Prediction, prevention and management. Nat Rev Nephrol. 10:531–540.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Betel D, Wilson M, Gabow A, Marks DS and
Sander C: The microRNA.org resource: Targets and expression.
Nucleic Acids Res. 36((Database Issue)): D149–D153. 2008.PubMed/NCBI
|
|
21
|
Yu Y, Wang L, Liu T and Guan H:
MicroRNA-204 suppresses trophoblast-like cell invasion by targeting
matrix metalloproteinase-9. Biochem Biophys Res Commun.
463:285–291. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liang Y, Lin Q, Luo F, Wu W, Yang T and
Wan S: Requirement of miR-144 in CsA induced proliferation and
invasion of human trophoblast cells by targeting titin. J Cell
Biochem. 115:690–696. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang L, Song G, Liu M, Chen B, Chen Y,
Shen Y, Zhu J and Zhou X: MicroRNA-375 overexpression influences
P19 cell proliferation, apoptosis and differentiation through the
Notch signaling pathway. Int J Mol Med. 37:47–55. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang S, Lai N, Liao K, Sun J and Lin Y:
MicroRNA-210 regulates cell proliferation and apoptosis by
targeting regulator of differentiation 1 in glioblastoma cells.
Folia neuropathol. 53:236–244. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang H, Chao K, Ng SC, Bai AH, Yu Q, Yu J,
Li M, Cui Y, Chen M, Hu JF and Zhang S: Pro-inflammatory miR-223
mediates the cross-talk between the IL23 pathway and the intestinal
barrier in inflammatory bowel disease. Genome Biol. 17:582016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ding GC, Chen M, Wang YX, Rui C, Xu W,
Ding HJ and Shi ZH: MicroRNA-128a-induced apoptosis in HTR-8/SVneo
trophoblast cells contributes to pre-eclampsia. Biomed
Pharmacother. 81:63–70. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jairajpuri DS and Almawi WY: MicroRNA
expression pattern in pre-eclampsia (Review). Mol Med Rep.
13:2351–2358. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ji L, Zhang L, Li Y, Guo L, Cao N, Bai Z,
Song Y, Xu Z, Zhang J, Liu C and Ma X: MiR-136 contributes to
pre-eclampsia through its effects on apoptosis and angiogenesis of
mesenchymal stem cells. Placenta. 50:102–109. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xue F, Yang J, Li Q and Zhou H:
Down-regulation of microRNA-34a-5p promotes trophoblast cell
migration and invasion via targetting Smad4. Bioscience Reports.
39:BSR201816312019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guo L, Liu Y, Guo Y, Yang Y and Chen B:
MicroRNA-423-5p inhibits the progression of trophoblast cells via
targeting IGF2BP1. Placenta. 74:1–8. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gao Y, She R, Wang Q, Li Y and Zhang H:
Up-regulation of miR-299 suppressed the invasion and migration of
HTR-8/SVneo trophoblast cells partly via targeting HDAC2 in
pre-eclampsia. Biomed Pharmacother. 97:1222–1228. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Luo J, Qiao F and Yin X: Hypoxia induces
FGF2 production by vascular endothelial cells and alters MMP9 and
TIMP1 expression in extravillous trophoblasts and their
invasiveness in a cocultured model. J Reprod Dev. 57:84–91. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Singh M, Kindelberger D, Nagymanyoki Z, Ng
SW, Quick CM, Elias KM, Yamamoto H, Fichorova R, Fulop V and
Berkowitz RS: Matrix metalloproteinases and their inhibitors and
inducer in gestational trophoblastic diseases and normal placenta.
Gynecol Oncol. 122:178–182. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Karthikeyan VJ, Lane DA, Beevers DG, Lip
GY and Blann AD: Matrix metalloproteinases and their tissue
inhibitors in hypertension-related pregnancy complications. J Hum
Hypertens. 27:72–78. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rasstrigina IM, Milovanov AP, Fokina TV
and Kadyrov M: The intensity of expression of matrix
metalloproteinases type 2 and type 9 by invasive trophoblast cells
in uncomplicated pregnancy and preeclampsia. Arkh Patol. 76:24–29.
2014.(In Russian). PubMed/NCBI
|
|
36
|
Pirooz HJ, Jafari N, Rastegari M,
Fathi-Roudsari M, Tasharrofi N, Shokri G, Tamadon M, Sazegar H and
Kouhkan F: Functional SNP in microRNA-491-5p binding site of MMP9
3′-UTR affects cancer susceptibility. J Cell Biochem.
119:5126–5134. 2018. View Article : Google Scholar : PubMed/NCBI
|