Introduction
Caffeic acid (3,4-dihydroxycinnamic acid; CaA) is a
natural hydroxycinnamic acid that is found in coffee, argan oil and
barley grain (1). Studies have
shown that CaA has anticancer, anti-oxidant and anti-inflammatory
properties and can be activated both in vitro and in
vivo (2–5). In addition, as a dietary catechol,
CaA can function as an inhibitor of DNA methylation through the
increased formation of S-adenosyl-L-homocysteine via
catechol-O-methyltransferase (COMT)-mediated O-methylation
(6). COMT has multiple functions
in neurological, estrogen-associated and methylation metabolic
pathways, such as catecholamine, estradiol and estrone metabolism
(7,8). Expression of the COMT gene
primarily determines dopaminergic levels in the prefrontal cortex.
Therefore, variations in COMT activity and expression may be
involved in the pathogenesis of various psychiatric and
neurological diseases, including schizophrenia and depression
(9,10). A large amount of research has
focused on a common, single nucleotide polymorphism of the
COMT gene, Val158Met, which results in high activity of the
COMT enzyme, contributing to the pathophysiology of major
depressive disorder (MDD) (11).
Previous studies have suggested that methylation of the COMT
gene impacts its subsequent expression (12,13).
Our previous study showed that phytochemically-stimulated
COMT expression reversed the estrogen-induced inhibition of
COMT via epigenetic mechanisms (14).
A recent study indicated that aberrations in
epigenetic modification, particularly DNA methylation of
5-methylcytosine (5mC) in regions that are differentially
methylated, are associated with depression (15). DNA methylation is initiated and
maintained by DNA methyltransferases (DNMTs), which would be
expected to decrease gene expression. Recent studies have shown
that 5mC can be oxidized by members of the ten-eleven translocation
(TET) protein family to form 5-hydroxymethylcytosine (5hmC), which
could mediate DNA hydromethylation and increase gene expression
(16,17). These epigenetic enzymes, including
methyl CpG binding proteins, DNMTs and TETs, can bind to DNA and
thus regulate gene expression. It has been reported that alteration
in the expression of neurogenesis-associated genes via methylation
regulation is associated with depression in a rat model (18).
Brain-derived neurotrophic factor (BDNF) is a member
of the neurotrophin family of growth factors. Previous research has
shown that expression of Bdnf was decreased in psychiatric
disorders, such as depression (19), ultimately leading to atrophy of the
hippocampus. Bdnf transcription is controlled by eight
promotors, leading to different mRNAs containing one of the eight
untranslated 5′-exons (I to VIII) and the 3′ encoding exon (IX)
(20). The promotor IV region
contains a cAMP responsive element as the key regulatory component
(21), the expression of which is
a good indicator of total Bdnf expression in the brain
(22). The Bdnf promotor IV
is also epigenetically regulated, through both DNA methylation and
hydroxymethylation (23).
In the present study, it was hypothesized that CaA
could influence gene-specific methylation status in the brain of a
rat model with chronic unpredictable mild stress (CUMS),
potentially mediated by the epigenetic effects of CaA on the
transcription of Dnmt and/or Tet genes. Therefore,
the aim of the present study was to investigate antidepressant-like
activity via the epigenetic mechanisms of CaA focusing on the
hippocampus and prefrontal cortex using a well-established model of
CUMS (24,25).
Materials and methods
Animals
A total of 40 male Wistar rats (age, 4 weeks; mean
weight ± SD, 100±10 g), were purchased from Shanghai SLAC
Laboratory Animal Co., Ltd. The size of cages was 460×300×160 mm
and animal designs were set up as previously described (26) (Table
SI; Appendices S1 and S2). Briefly, after 2 weeks of
acclimatization the rats were first trained over a period of 3
weeks to consume a 1% sucrose solution. Then, the rats were
randomly and equally divided into four groups: A control group, a
CUMS model group, a model group treated with the antidepressant
paroxetine (Sigma-Aldrich; Merck KGaA), and a model group treated
with CaA (Sigma-Aldrich; Merck KGaA). Paroxetine and CaA were
administered intraperitoneally at doses of 10and 50 mg/kg for at
9:00 a.m. every day for 4 weeks, respectively. Rats in the control
(no treatment) and treated groups were exposed to a series of CUMS
and behavioral tests, including the sucrose preference test (SPT)
and the forced swimming test (FST) (26). During these tests, food consumption
and body weight were evaluated as an indicator of depression as
previously described (26) (data
not shown). The present study was approved by The Nanjing Medical
University Institutional Animal Care and Use Committee (Nanjing,
China).
Sample collection and preparation
After 4 weeks, 300 mg/kg 10% chloral hydrate
solution per rat was injected intraperitoneally. No rats exhibited
signs of peritonitis following administration of the anesthetic,
and rats were euthanized by dislocation of the cervical vertebrae.
The heart was exposed and heartbeat was assessed to confirm death.
No mortality occurred outside of planned euthanasia. The
hippocampus and the prefrontal cortex were dissected from the brain
of sacrificed animals by a trained expert technician on ice, fixed
with 4% formaldehyde overnight at room temperature, and were
subsequently paraffin-embedded, or frozen with liquid nitrogen and
stored at −80°C for subsequent experiments.
Immunofluorescence (IF) and
immunohistochemistry (IHC) analyses
After paraffin embedding, 3-µm thick sections were
obtained, dewaxed and washed in water. Then, sections were immersed
in xylene and dehydrated in graded concentrations of absolute
ethanol (95, 85, 75 and 50%) at room temperature. For IHC (5mC) and
IF (5hmC), sections were blocked for 30 min at room temperature
with 5% BSA (Beyotime Institute of Biotechnology) and incubated
overnight at 4°C with anti-5mc (1:200; cat. no. 28692; Cell
Signaling Technology, Inc.) and anti-5hmC (1:200; cat. no. 39770;
Active Motif, Inc.) antibodies, respectively. Afterwards, sections
were washed three times with PBS and incubated for a further 1 h at
room temperature with horseradish peroxidase-labeled goat
anti-rabbit antibody (1:1,000; cat. no. GB23303; Wuhan Servicebio
Technology Co., Ltd.) in blocking buffer (Beyotime Institute of
Biotechnology). For IHC, nuclei were stained with Harris
hematoxylin for 2 min at room temperature. Staining intensity for
IHC was digitalized with Panoramic SCAN (3DHISTECH Ltd.) (light
microscopy mode). Each immunohistochemically stained slide was
scanned with a 20× objective and images were captured at ×10
magnification. For further evaluation of IHC imaging, 5hmC level
was quantified by integrated optical density (IOD) of positive
brown staining using Image-Pro Plus version 6.0 software (Media
Cybernetics, Inc.).
For IF, an Alexa-488 labeled secondary antibody
(1:300; cat. no. GB25301; Wuhan Servicebio Technology Co., Ltd.) in
blocking buffer (Beyotime Institute of Biotechnology) was used for
2 h at 4°C, and nuclei were stained with DAPI at room temperature
for 1 h. Images for IF were captured using a Zeiss LSM 700B
confocal microscope and the magnification was ×400. The proportion
(%) of 5hmC-positive foci was then quantified and calculated by the
positive (green) fluorescent intensity normalized by positive
staining area using Image-Pro Plus version 6.0 software (Media
Cybernetics, Inc.). All IF experiments were performed in at least
three independent biological replicates.
Quantification of mRNA levels of
epigenetic modulators and specific genes using reverse
transcription-quantitative (RT-q)PCR
Total RNA was extracted using the FastPure
Cell/Tissue Total RNA Isolation kit (Vazyme Biotech Co., Ltd.) and
reverse transcribed into cDNA using the PrimeScript™ RT reagent kit
(Takara Biotechnology Co., Ltd.) following the manufacturer's
instructions. mRNA levels of Dnmt1, Dnmt3a, Tet1-3, methyl CpG
binding protein 2 (Mecp2), Bdnf and Comt genes were quantified
using SYBR Green-based qPCR using 2X SYBR Green PCR mix (Takara
Biotechnology Co., Ltd.) according to the manufacturer's
instructions. Primer sequences are listed in Table I (15,19,26).
All amplifications were performed in triplicate using a
LightCycler® 96 (Roche Diagnostics) according to the
manufacturer's instructions with the following cycle parameters:
95°C for 600 sec, followed by 45 repeats of 95°C for 15 sec and
60°C for 60 sec. The 2−ΔΔCq method was used to calculate
the relative expression level of the transcripts normalized to
levels of GAPDH (27).
 | Table I.Primers for quantitative PCR. |
Table I.
Primers for quantitative PCR.
| Gene | Primer sequence,
5′→3′ |
|---|
| Tet1 |
|
|
Forward |
CCGGTCGCCAAGTGGGTGAT |
|
Reverse |
GGTCCACACGCTCACGAACCA |
| Tet2 |
|
|
Forward |
TACCGTACAGCCACCCAAAC |
|
Reverse |
CGTGACTGGAACTGCTCACT |
| Tet3 |
|
|
Forward |
GGACTTCTGTGCCCACGCCC |
|
Reverse |
TCAGGGTGCAGACCACAGTGC |
| Dnmt1 |
|
|
Forward |
GGCCAGCCCCATGAAACGCT |
|
Reverse |
GGGGCGTCCAGGTTGCTTCC |
| Dnmt3a |
|
|
Forward |
TCCAACATGAGCCGCTTGGCG |
|
Reverse |
GGTGGCGGATGACTGGCACG |
| Mecp2 |
|
|
Forward |
CTTGACCTCAATGCTGACGGT |
|
Reverse |
GGGTAGAAAGCCTGGGAGTGT |
| Bdnf P4 |
|
|
Forward |
GCTGCCTTGATGTTTACTTTGA |
|
Reverse |
GCAACCGAAGTATGAAATAACC |
| Total
Bdnf |
|
|
Forward |
GGCCCAACGAAGAAAACCAT |
|
Reverse |
AGCATCACCCGGGAAGTGT |
| Comt |
|
|
Forward |
TCCACAACCTGATCATGGGT |
|
Reverse |
ACATCGTACTTCTTCTTCAGCTGG |
| GAPDH |
|
|
Forward |
TCGGTGTGAACGGATTTGGCCG |
|
Reverse |
CCGTTGAACTTGCCGTGGGT |
Specific promotor methylation and
hydroxymethylation analysis
Chromatin immunoprecipitation was performed using
the chromatin immunoprecipitation (ChIP)-IT Express kit (Active
Motif, Inc.) according to the manufacturer's instructions. A total
of 20–30 mg tissues were fixed with 1% formaldehyde at room
temperature for 15 min and lysed with lysis buffer in ChIP-IT
Express kit (Active Motif, Inc.) to release chromatin. Chromatin
was then enzymatically sheared to obtain chromatin, 100–500 base
pairs (bp) in length, using an Enzymatic Shearing kit (Active
Motif, Inc.). Approximately 50 ng sheared chromatin was then
immunoprecipitated with antibodies against 5mc (1:200; cat. no.
61479) and 5hmc (1:200; cat. no. 39769) (both from Active Motif,
Inc.) overnight at 4°C. Immunoglobulin G was used as a mock
control. DNA released from the reverse crosslink was purified
(Universal DNA Purification kit; cat. no. DP214-02; Tiangen Biotech
Co., Ltd.) according to the manufacturer's instructions. Native
chromatin was used as an initial input sample. The level of DNA
bound to 5mC and 5hmC antibodies was quantified using SYBR-green
based qPCR. Primers were designed to amplify regions of Bdnf
and Comt promotor as follows: ChIP-Bdnf, forward:
5′-TTGTGGCATGGTTCTCAACC-3′ and reverse: 5′-TAGATCTCTGAGAAGAGGTA-3′;
and ChIP-Comt, forward: 5′-TTTGGAGCAGGAGTAGACC-3′ and
reverse: 5′-TTTTAACACGCGCGGGACG-3′. PCR conditions were programmed
as follows: 95°C for 20 sec, followed by 40 cycles 95°C for 3 sec
and 60°C for 30 sec. The levels of bound DNA sequences were then
calculated using the percent input method (2−[Cq (ChIP) - Ct
(Input)] × 100) (14) by
calculating the qPCR signal relative to the input sample.
Statistical analysis
All data are presented as the mean ± standard
deviation. For group comparisons, Kruskal-Wallis tests were used
followed by Dunn's post hoc test. All data were analyzed and
plotted with GraphPad Prism version 6.0 (GraphPad Software).
P<0.05 was considered to indicate a statistically significant
difference.
Results
CaA can reverse changes in the
behavior test induced by the CUMS procedure
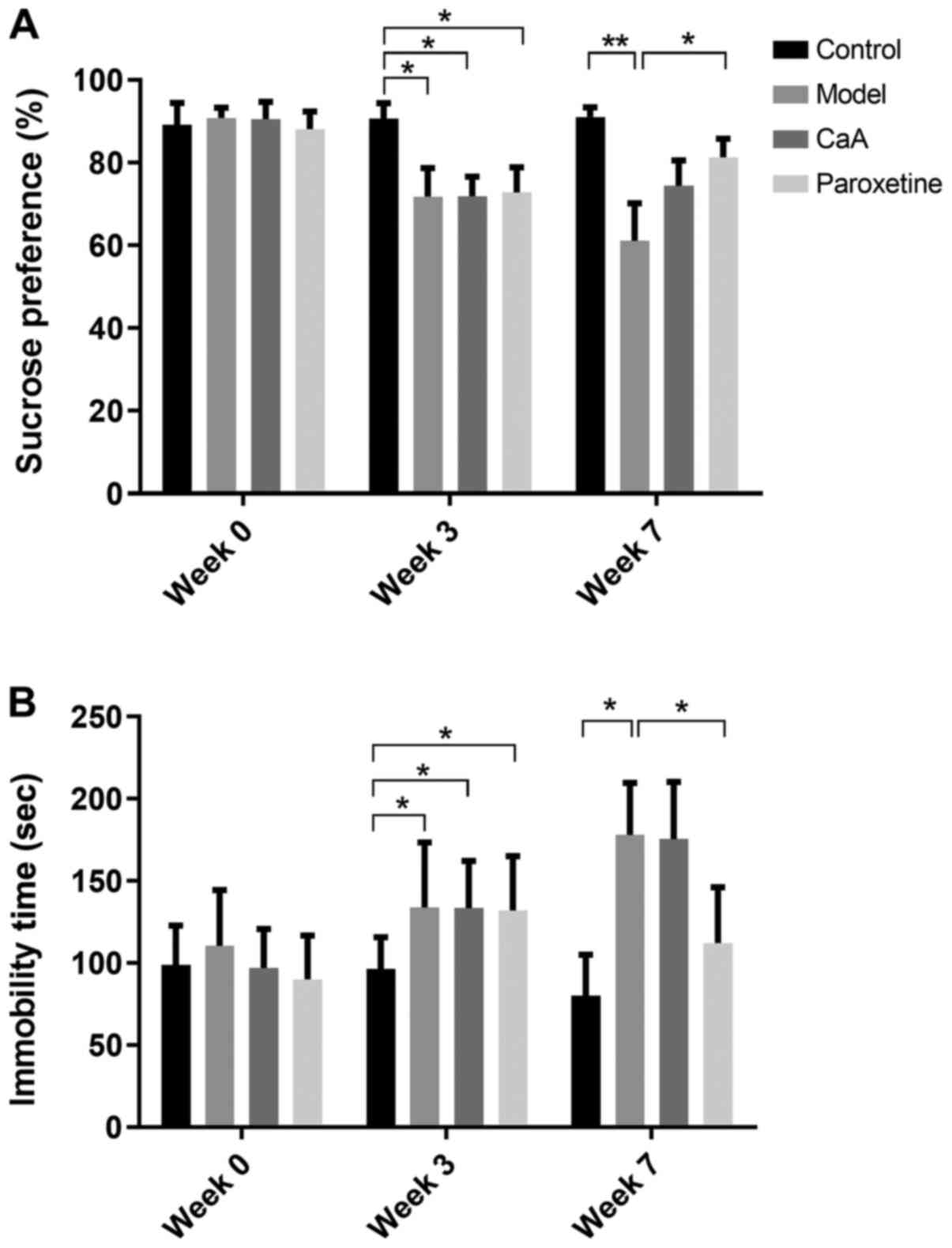
The SP of each rat in the control group decreased in
the first three CUMS procedures. Over the next 4 weeks of the CUMS
procedure, the SP of the model group was continually decreased
compared with that of the control group. Furthermore, SP was
reversed by CaA and paroxetine treatment. During week 3, the model
group, CaA and paroxetine groups were significantly decreased
compared with the control (P<0.05). However, only paroxetine
treatment showed statistical significance when compared with the
model group during week 7 (P<0.05; Fig. 1A). Immobility time in the FST
(26) was clearly increased in the
model group during the CUMS procedure. In the model group, CaA and
paroxetine treatment were significantly increased compared with the
control during week 3. However, paroxetine treatment caused a
significant decrease in immobility time compared with the model
group and CaA treatment had no significant effect on FST during
week 7 (P<0.05; Fig. 1B).
Comparison of global 5mC and 5hmC in
the hippocampus and prefrontal cortex
The levels of global 5mC (IHC) and global 5hmC (IF)
in the hippocampus and prefrontal cortex were detected,
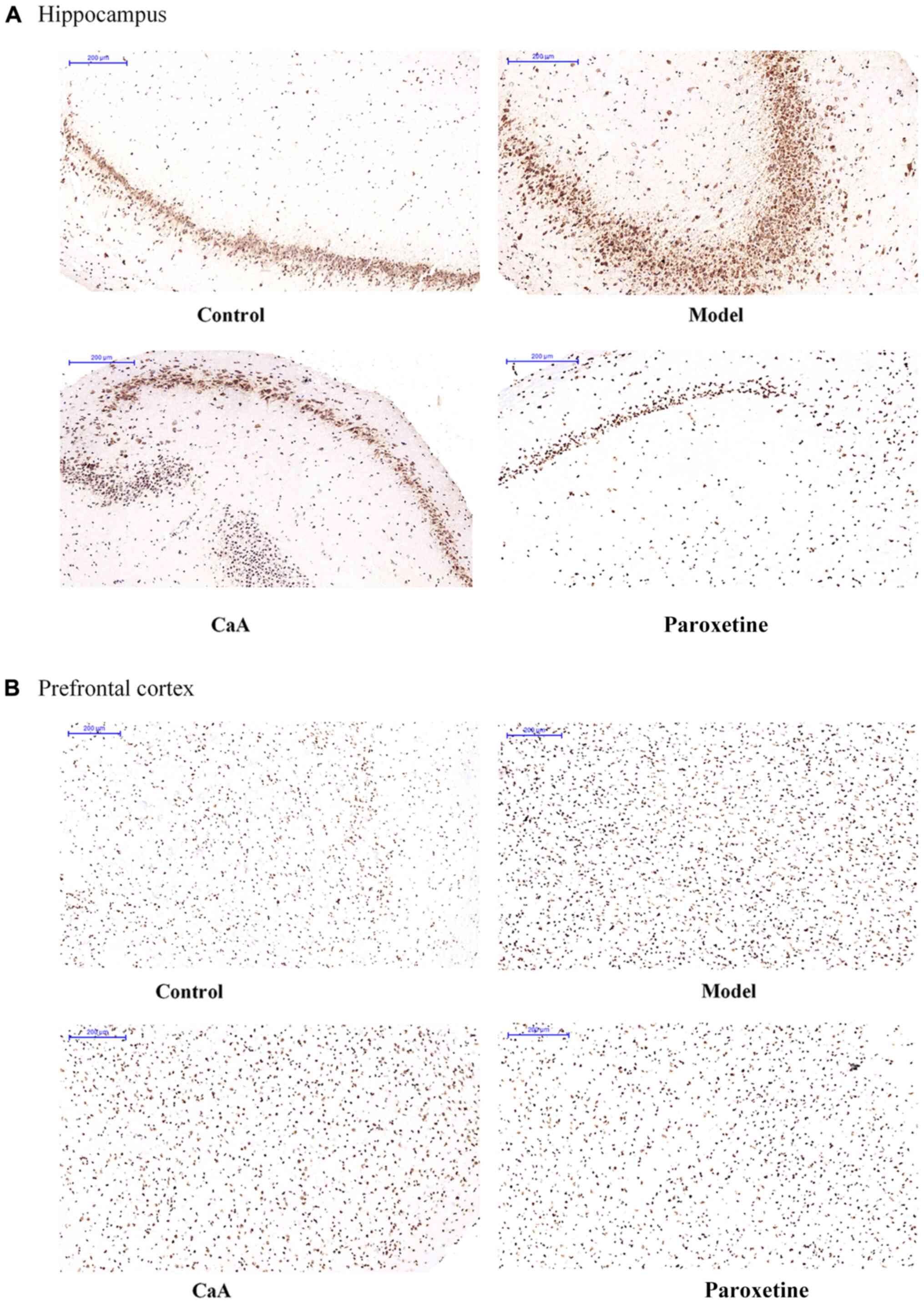
respectively. Immunostaining of 5mC was localized in the nuclei of
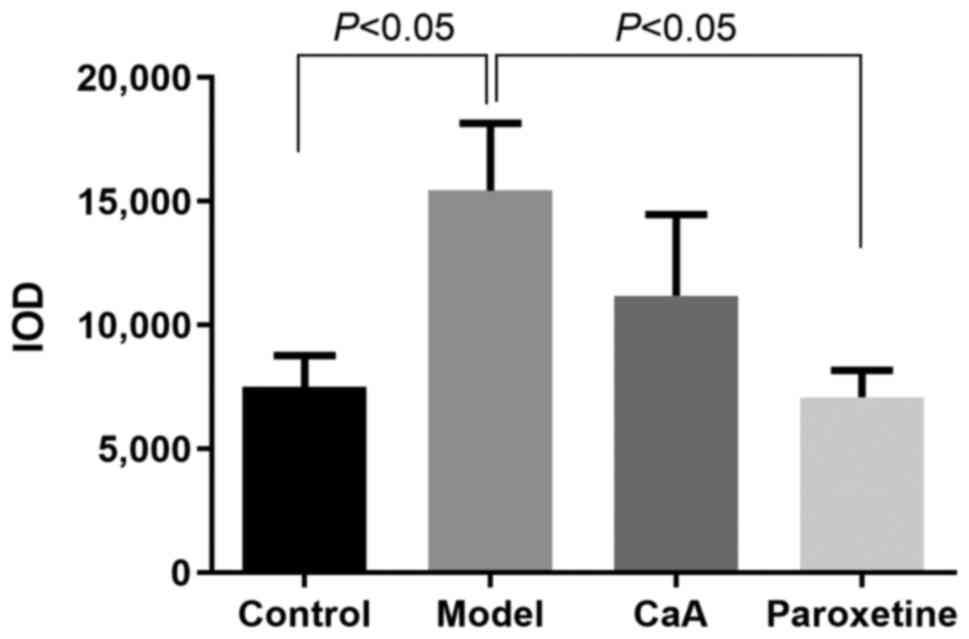
the cells, and was visualized as brown-colored staining (Fig. 2). As presented in Fig. 3, there was a significant difference
in IOD value of 5mC between the model and control groups, and also
between the paroxetine group and the model group (P<0.01 and
P<0.001, respectively). Although the difference was not
statistically significant, 5mC level was slightly lower in the
CaA-treated group compared with the model group. However, in the
prefrontal cortex, there was no significant difference in 5mC level
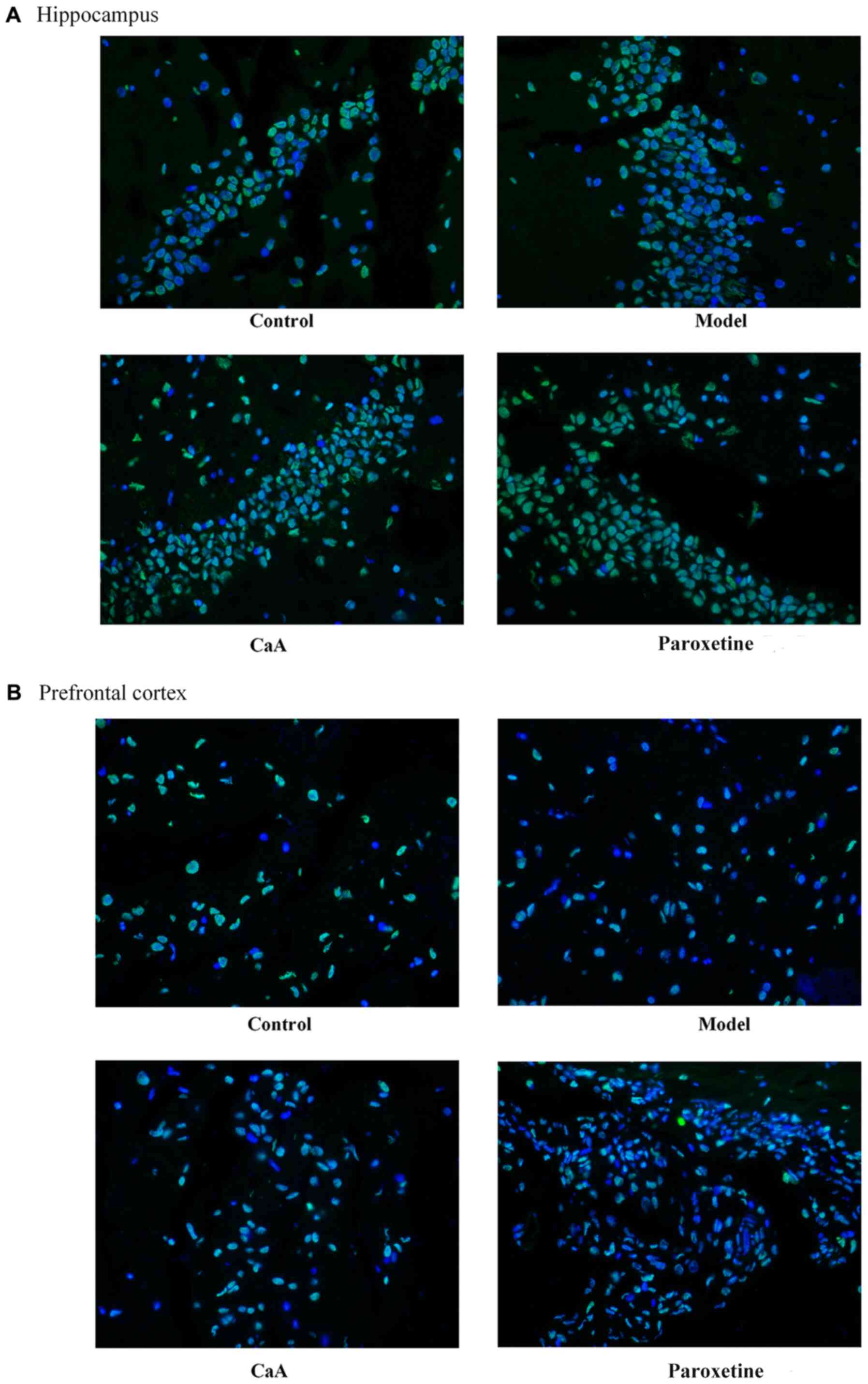
among groups. Levels of 5hmC were confirmed by IF. The levels of
5hmC in the hippocampus were slightly increased in the CaA-treated
group when compared with the model group. Notably, the levels of
5hmC increased in the paroxetine-treated group compared with the
model group in the hippocampus (Fig.4A; Table II). However, there was no
difference in 5hmC levels in the prefrontal cortex when compared
between all groups (Fig. 4B).
 | Table II.Expression levels of 5hmC in the
hippocampus in the control, model, CaA and paroxetine-treated
groups analyzed using immunofluorescent staining. |
Table II.
Expression levels of 5hmC in the
hippocampus in the control, model, CaA and paroxetine-treated
groups analyzed using immunofluorescent staining.
| Group | Mean ± SD | P-value |
|---|
| Control | 0.0804±0.002 | 0.18 |
| Model | 0.0664±0.009 |
0.01a |
| CaA | 0.0823±0.002 | 0.12 |
| Paroxetine | 0.0936±0.011 | 0.01 |
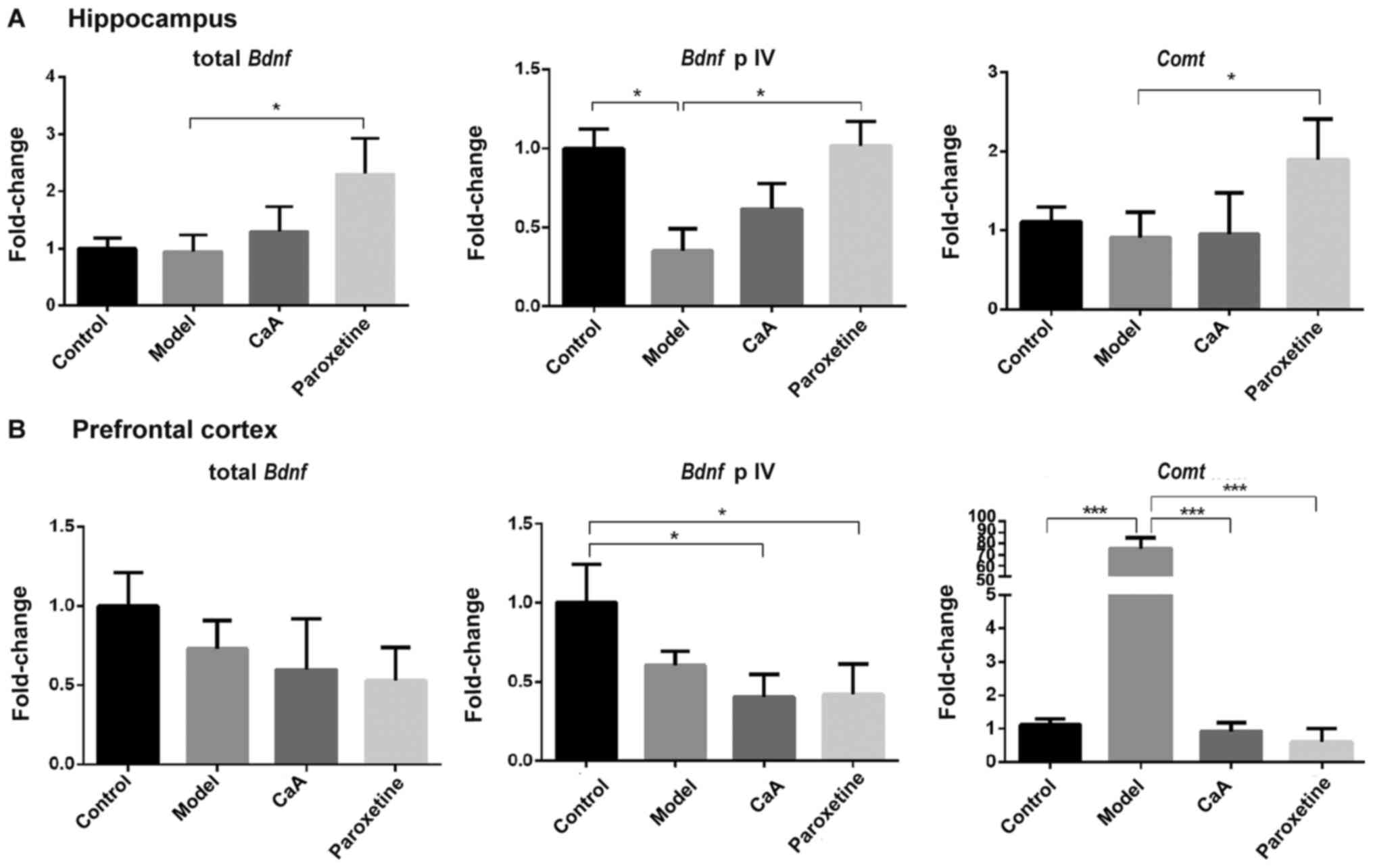
Effects of CaA on the expression of
Bdnf and Comt
The present study assessed whether Bdnf and
Comt expression could be affected by CaA and paroxetine, and
whether these were associated with changes in the regulation of DNA
methylation. In the hippocampus, levels of Bdnf promotor IV
were decreased in the model group compared with the control group
(P<0.05), and CaA caused a non-significant increase in
Bdnf promotor IV levels. However, paroxetine treatment
significantly reversed the inhibitory effects of CUMS. Total
Bdnf and Comt levels were increased in the
paroxetine-treated group compared with the model group (both
P<0.05), although there was no significant difference between
the model group and the CaA-treated group. In the prefrontal
cortex, there was no change in total Bdnf expression levels,
but a significant difference was observed in the expression levels
of the Bdnf promotor IV in CaA and paroxetine treatment
groups vs. control. Notably, levels of Comt mRNA in the
model group were significantly higher when compared with those in
the control group (P<0.001); however, both CaA and paroxetine
treatment significantly decreased Comt mRNA levels compared
with the model group (both P<0.001; Fig.5).
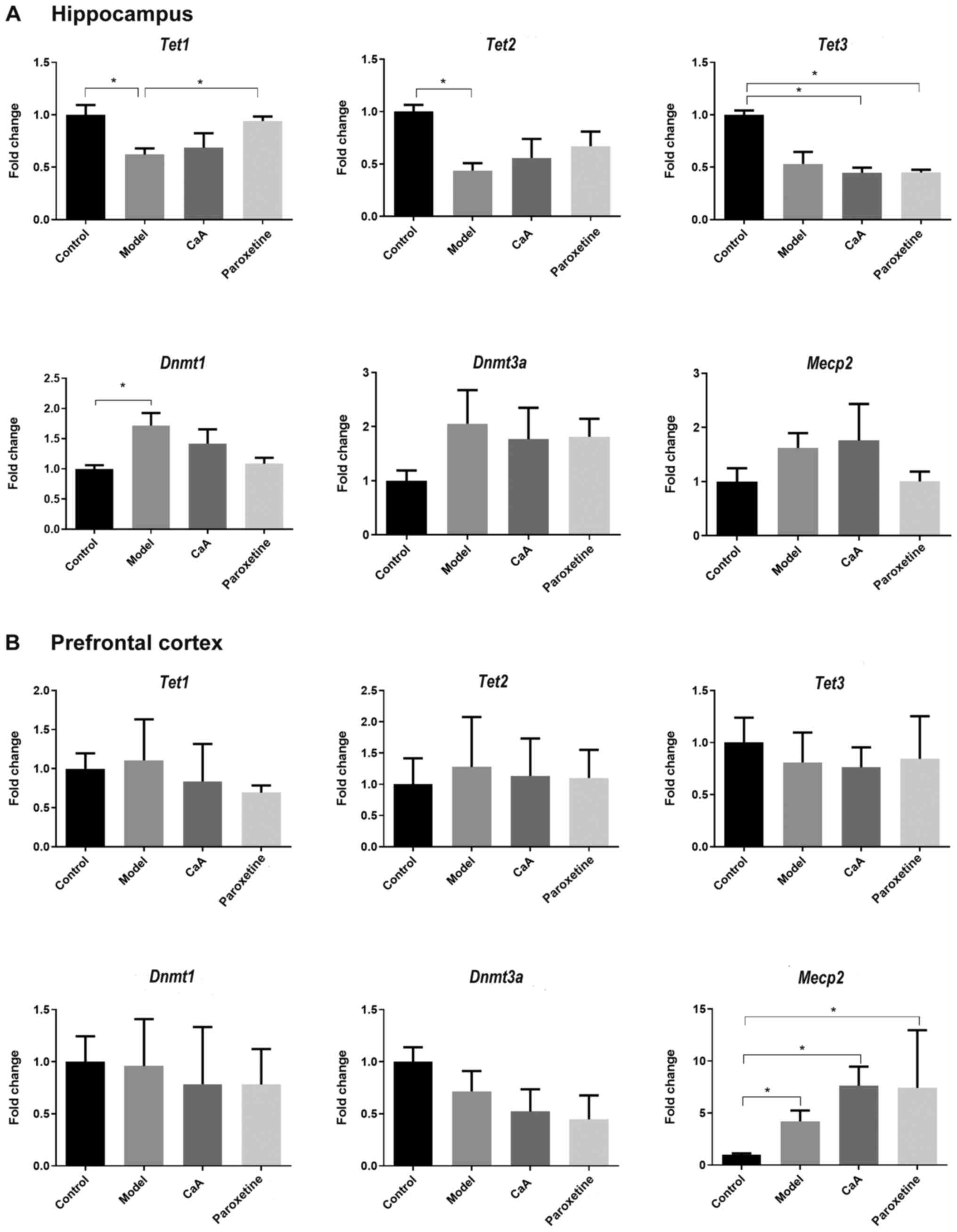
CaA influences the expression of
methylated and hydroxymethylated genes
Dnmt1, Dnmt3a and Mecp2 serve
important roles in the regulation of methylation, while the
Tet1-3 family is involved in the regulation of
hydroxymethylation (28,29). Previous studies have demonstrated
that aberrant epigenetic modification, such as DNA methylation and
hydroxymethylation, is associated with psychiatric disorders,
including depression (30,31). In the present study,
epigenetic-mediated gene expression was detected in the hippocampus
and prefrontal cortex of rats. In the hippocampus, Dnmt1
expression was increased in the model group when compared with the
control group. CaA and paroxetine treatment blocked this increase
but without statistical significance. Tet1 expression was
decreased in the model group (P<0.05) and paroxetine treatment
could reverse this change. MeCP2 belongs to the family of
methyl-CpG-binding domain proteins (MBDs), which bind to methylated
DNA (32). In the present study,
the expression of Mecp2 did not show any difference among
groups. (Fig. 6A). There were no
changes in the expression levels of Dnmt1, Dnmt3a or
Tet1-3 genes in the prefrontal cortex; however, Mecp2
levels were increased in the model, CaA and paroxetine-treated
groups when compared with those in the control group (all
P<0.05; Fig. 6B).
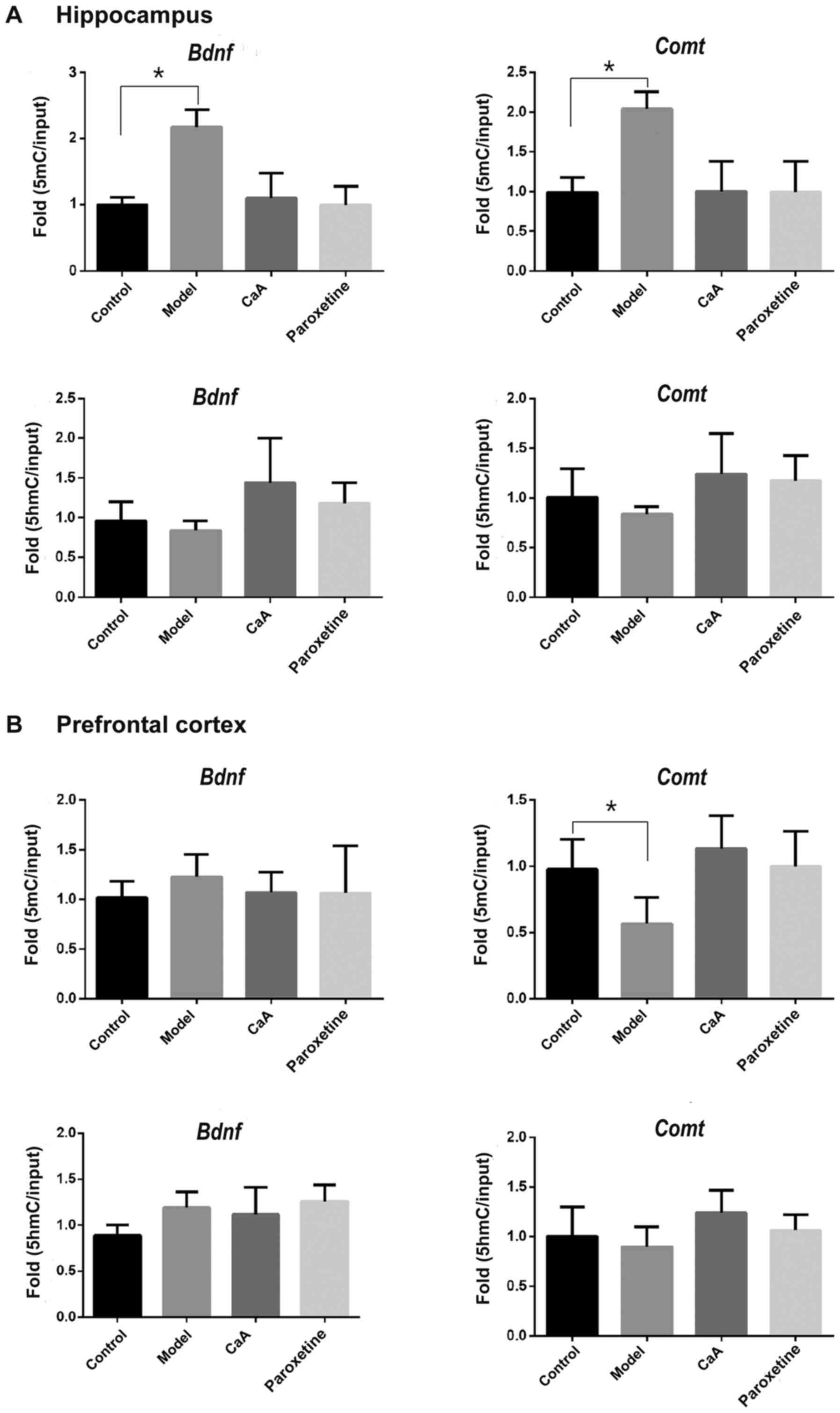
5mC and 5hmC enrichment in the
promotor of Bdnf and Comt
In order to improve the current understanding of how
changes in Bdnf and Comt gene expression may be
associated with methylation levels, the present study predicted
5′-CpG island(s) in the promotor regions of both the Comt
and Bdnf genes using online software (urogene.org/cgi-bin/methprimer/methprimer.cgi)
(33) (Fig. S1). According to the position of
the predicted 5′-CGI(s), the quantities of 5mC and 5hmC in these
regions was determined using ChIP-qPCR. As presented in Fig. 7A, in the hippocampus, levels of 5mC
were increased in the Bdnf and Comt promotors in the
model group compared with the control. However, no changes were
observed in the CaA and paroxetine-treated group. The expression
levels of 5mC were significantly decreased in the Comt
promotor of the prefrontal cortex model compared with those in the
control group (P<0.05; Fig.
7B). Neither of the two promotors showed any changes with
respect to 5hmC levels. There was an increase of 5mC/5hmC in the
Bdnf and Comt gene promotors in the model group
compared with the control group in the hippocampus. CaA treatment
may adjust the balance of 5mC/5hmC in the hippocampus. There was a
decrease of 5mC/5hmC in the Comt gene promotor in the model
group vs. the control group in the prefrontal cortex, which may be
restored by CaA.
Discussion
The results of the present study may provide novel
insight into genetic in depression, and demonstrated that
epigenetic changes for this illness are likely to involve multiple
genes. Extensive studies have indicated that epigenetic
modifications, such as histone acetylation and DNA methylation,
serve a crucial role in the modification of DNA during neuronal
gene expression and memory formation (34,35).
In addition, levels of 5hmC are most abundant in the brain, which
is controlled by the activation of gene transcription (36,37).
In the present study, it was hypothesized that the
antidepressant-like properties of CaA may partially involve changes
at the DNA methylation and hydroxymethylation levels.
In the present study, the CUMS model was selected
for investigation, which is regarded as one of the most valid and
reliable animal models (24,25).
Behavioral data showed that CaA exerted a slight
antidepressant-like effect. In the analysis of global methylation
status, no robust evidence of widespread methylation differences
was observed between the control and CaA groups. However,
non-significant trends towards lower DNA methylation levels in the
CaA group were observed. Furthermore, in the hippocampus,
Dnmt1 and Dnmt3a mRNA levels in the model group were
increased compared with those in the control, and CaA treatment may
lower this trend but this was not significant. Tet1 and
Tet2 mRNA levels were decreased in the model group compared
with those in the control, and this was reversed in the CaA group.
The expression levels of Dnmt1/Dnmt3a and Tet1/Tet2
were associated with the levels of global DNA methylation and
hydroxymethylation, respectively, which were consistent with the
results of the 5mC IHC and 5hmC IF expression analysis in the
hippocampus. A previous epigenetic study has indicated potential
associations between epigenetic patterns and abnormalities in the
hippocampus, which is vulnerable to the pathogenesis of MDD
(38). No obvious changes in
global 5mC and 5hmC levels were observed in the prefrontal cortex
following CaA treatment. The present study revealed that
Bdnf and Comt genes had differential expression
patterns in the hippocampus and prefrontal cortex, respectively.
Specifically, the model group showed decreased Bdnf mRNA
levels, which were restored to within the normal range in the
hippocampus following CaA and paroxetine treatment. These results
are consistent with previous human patient and animal model studies
demonstrating that BDNF deficiency, particularly those lacking
promotor IV-driven Bdnf transcription, underlies depressive
states, and that antidepressant treatments restore this expression
in the hippocampus (39,40). Preclinical studies have established
that hippocampal BDNF expression serves a critical role in the
etiology of depression; however, the exact underlying molecular
mechanisms of this role remain unclear. Notably, the complex
regulation of Bdnf gene transcription may provide the
opportunity to clarify this uncertainty. Keller et al
(40) first reported that lower
Bdnf expression is associated with increased DNA methylation
of Bdnf promotor IV. Therefore, the present study analyzed
the levels of 5mC and 5hmC in the promotor region of the
Bdnf gene following CaA treatment in rats. It was
demonstrated that increased Dnmt1 levels in the model group
were associated with increased levels of 5mC in the Bdnf
gene promotor. There was a decrease in 5hmC levels in the model
group compared with the control group; however, this was not
statistically significant. When these findings were expressed as a
ratio of 5mC/5hmC, the data showed a statistically significant
increase in the Bdnf promotor. These data suggested a shift
in the equilibrium towards methylation in the hippocampus following
CUMS, which may have been driven by the increased quantity of
steady state 5mC in the promotor regions. CaA treatment may restore
the expression of Bdnf by adjusting the balance of 5mC/5hmC
in the hippocampus. Notably, there was a marked increase in
Comt gene expression in the prefrontal cortex in the model
group compared with the control group, and CaA reversed this. There
was also a decrease of 5mC/5hmC, which was shifted towards
hydroxymethylation in the prefrontal cortex. These findings
indicate that the expression of Comt gene can be regulated
by DNA methylation and hydroxymethylation in the brain. COMT
is the primary regulator of dopamine clearance in extra-striatal
regions of the brain, including the prefrontal cortex (13,41).
Elevated levels of COMT may accelerate the metabolism of dopamine
and result in decreased dopamine levels, which may in turn
influence brain function, potentially leading to depression. Of
note, CaA treatment resulted in differential DNA methylation
regulation of Bdnf and Comt genes in the hippocampus
and the prefrontal cortex, respectively. During antidepressant
treatment, epigenetic mechanisms may exert a dynamic and
tissue-specific regulation of gene transcription (42). Therefore, studies investigating
epigenetic mechanisms have improved the current understanding of
the molecular basis for antidepressant treatment in MDD.
In the present study, the effects of CaA on MDD had,
in part, the same trend as the antidepressant, paroxetine. It was
revealed that there were epigenetic changes in the genes
(Bdnf and Comt) associated with depression, which
were reversible with CaA treatment. It has previously been
demonstrated that CaA could enhance the expression of microRNA
(miR)-148a by decreasing Dnmt1 mRNA levels (43). There is also a support that CaA may
act as a histone deacetylase inhibitor for breast cancer treatment
(44). Thus, CaA, as a
phytochemical and a dietary supplement, may be a comprehensive
epigenetic modulator, and may have potential as an antidepressant.
The results of the present study are promising and these trends
require further validation in studies with larger sample size.
However, it should be noted that the present study did not detect
the effects of every epigenetic-associated enzyme on gene
transcription, such as DNMTs/MBDs mediating DNA methylation and
histone deacetylases/histone acetyltransferases. It is hypothesized
that CaA may recruit the binding of the aforementioned enzymes to
specific CpG sites of the gene promotor. These putative bulky
enzymatic complexes may inhibit the binding of transcription
co-activators at the promotor leading to decreased gene expression,
and vice versa. Future mechanistic studies should aim to
elucidate the mechanisms of CaA as a direct or indirect epigenetic
modulator. Further experiments should also investigate the effects
of different CaA dosages and administration methods.
In conclusion, the present results demonstrated that
there were epigenetic changes in the hippocampus and prefrontal
cortex in rats with CUMS. CaA may function as a modulator of DNA
methylation to regulate Bdnf and Comt gene
transcription. Although a limitation of the present study was a
lack of statistical power, the trends observed provide a
mechanistic basis for the use of this phytochemical agent in the
treatment of depression.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was funded by The National Natural
Science Foundation of China (grant nos. 81903365, 81673228 and
81473020), The Natural Science Foundation of Jiangsu Province
(grant no. BK20161571) and The Natural Science Foundation of the
Higher Education Institution of Jiangsu Province (grant no.
16KJA330002).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QW and LL conceived and designed the experiments.
JH, SC, DW and LW performed the experiments. QW, ZZ and SC analyzed
data. QW wrote the manuscript. All the authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was approved by The Nanjing
Medical University Institutional Animal Care and Use Committee
(Nanjing, China) (approval no. NJMU2015/81473020).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Touaibia M, Jean-François J and Doiron J:
Caffeic Acid, a versatile pharmacophore: An overview. Mini Rev Med
Chem. 11:695–713. 2011.PubMed/NCBI
|
|
2
|
Onori P, DeMorrow S, Gaudio E, Franchitto
A, Mancinelli R, Venter J, Kopriva S, Ueno Y, Alvaro D, Savage J,
et al: Caffeic acid phenethyl ester decreases cholangiocarcinoma
growth by inhibition of NF-kappaB and induction of apoptosis. Int J
Cancer. 125:565–576. 2009.PubMed/NCBI
|
|
3
|
Son S and Lewis BA: Free radical
scavenging and antioxidative activity of caffeic acid amide and
ester analogues: Structure-activity relationship. J Agric Food
Chem. 50:468–472. 2002.PubMed/NCBI
|
|
4
|
Koltuksuz U, Mutuş HM, Kutlu R, Ozyurt H,
Cetin S, Karaman A, Gürbüz N, Akyol O and Aydin NE: Effects of
caffeic acid phenethyl ester and epidermal growth factor on the
development of caustic esophageal stricture in rats. J Pediatr
Surg. 36:1504–1509. 2001.PubMed/NCBI
|
|
5
|
Borrelli F, Izzo AA, Di Carlo G, Maffia P,
Russo A, Maiello FM, Capasso F and Mascolo N: Effect of a propolis
extract and caffeic acid phenethyl ester on formation of aberrant
crypt foci and tumors in the rat colon. Fitoterapia. 73 (Suppl
1):S38–S43. 2002.PubMed/NCBI
|
|
6
|
Lee WJ and Zhu BT: Inhibition of DNA
methylation by caffeic acid and chlorogenic acid, two common
catechol-containing coffee polyphenols. Carcinogenesis. 27:269–277.
2006.PubMed/NCBI
|
|
7
|
Ira E, Zanoni M, Ruggeri M, Dazzan P and
Tosato S: COMT, neuropsychological function and brain structure in
schizophrenia: A systematic review and neurobiological
interpretation. J Psychiatry Neurosci. 38:366–380. 2013.PubMed/NCBI
|
|
8
|
Samavat H and Kurzer MS: Estrogen
metabolism and breast cancer. Cancer Lett. 356A:A231–A243.
2015.
|
|
9
|
Matsumoto M, Weickert CS, Beltaifa S,
Kolachana B, Chen J, Hyde TM, Herman MM, Weinberger DR and Kleinman
JE: Catechol O-methyltransferase (COMT) mRNA expression in the
dorsolateral prefrontal cortex of patients with schizophrenia.
Neuropsychopharmacology. 28:1521–1530. 2003.PubMed/NCBI
|
|
10
|
Lin CH, Chaudhuri KR, Fan JY, Ko CI, Rizos
A, Chang CW, Lin HI and Wu YR: Depression and
Catechol-O-methyltransferase (COMT) genetic variants are associated
with pain in Parkinson's disease. Sci Rep. 7:63062017.PubMed/NCBI
|
|
11
|
Egan MF, Goldberg TE, Kolachana BS,
Callicott JH, Mazzanti CM, Straub RE, Goldman D and Weinberger DR:
Effect of COMT Val108/158 Met genotype on frontal lobe
function and risk for schizophrenia. Proc Natl Acad Sci USA.
98:6917–6922. 2001.PubMed/NCBI
|
|
12
|
Swift-Scanlan T, Smith CT, Bardowell SA
and Boettiger CA: Comprehensive interrogation of CpG island
methylation in the gene encoding COMT, a key estrogen and
catecholamine regulator. BMC Med Genomics. 7:52014.PubMed/NCBI
|
|
13
|
Na KS, Won E, Kang J, Kim A, Choi S, Tae
WS, Kim YK, Lee MS, Joe SH and Ham BJ: Differential effect of COMT
gene methylation on the prefrontal connectivity in subjects with
depression versus healthy subjects. Neuropharmacology. 137:59–70.
2018.PubMed/NCBI
|
|
14
|
Wu Q, Odwin-Dacosta S, Cao S, Yager JD and
Tang WY: Estrogen down regulates COMT transcription via promoter
DNA methylation in human breast cancer cells. Toxicol Appl
Pharmacol. 367:12–22. 2019.PubMed/NCBI
|
|
15
|
Bustamante AC, Armstrong DL and Uddin M:
Epigenetic profiles associated with major depression in the human
brain. Psychiatry Res. 260:439–442. 2018.PubMed/NCBI
|
|
16
|
Tahiliani M, Koh KP, Shen Y, Pastor WA,
Bandukwala H, Brudno Y, Agarwal S, Iyer LM, Liu DR, Aravind L, et
al: Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in
mammalian DNA by MLL partner TET1. Science. 324:930–935.
2009.PubMed/NCBI
|
|
17
|
Ito S, D'Alessio AC, Taranova OV, Hong K,
Sowers LC and Zhang Y: Role of Tet proteins in 5mC to 5hmC
conversion, ES-cell self-renewal and inner cell mass specification.
Nature. 466:1129–1133. 2010.PubMed/NCBI
|
|
18
|
Wei Y, Melas PA, Wegener G, Mathé AA and
Lavebratt C: Antidepressant-like effect of sodium butyrate is
associated with an increase in TET1 and in 5-hydroxymethylation
levels in the Bdnf gene. Int J Neuropsychopharmacol.
18:pyu0322014.PubMed/NCBI
|
|
19
|
Angelucci F, Brenè S and Mathé AA: BDNF in
schizophrenia, depression and corresponding animal models. Mol
Psychiatry. 10:345–352. 2005.PubMed/NCBI
|
|
20
|
Aid T, Kazantseva A, Piirsoo M, Palm K and
Timmusk T: Mouse and rat BDNF gene structure and expression
revisited. J Neurosci Res. 85:525–535. 2007.PubMed/NCBI
|
|
21
|
Lubin FD, Roth TL and Sweatt JD:
Epigenetic regulation of BDNF gene transcription in the
consolidation of fear memory. J Neurosci. 28:10576–10586.
2008.PubMed/NCBI
|
|
22
|
Boulle F, van den Hove DL, Jakob SB,
Rutten BP, Hamon M, van Os J, Lesch KP, Lanfumey L, Steinbusch HW
and Kenis G: Epigenetic regulation of the BDNF gene: Implications
for psychiatric disorders. Mol Psychiatry. 17:584–596.
2012.PubMed/NCBI
|
|
23
|
Martinowich K, Hattori D, Wu H, Fouse S,
He F, Hu Y, Fan G and Sun YE: DNA methylation-related chromatin
remodeling in activity-dependent BDNF gene regulation. Science.
302:890–893. 2003.PubMed/NCBI
|
|
24
|
Hill MN, Hellemans KG, Verma P, Gorzalka
BB and Weinberg J: Neurobiology of chronic mild stress: Parallels
to major depression. Neurosci Biobehav Rev. 36:2085–2117.
2012.PubMed/NCBI
|
|
25
|
Willner P: Validity, reliability and
utility of the chronic mild stress model of depression: A 10-year
review and evaluation. Psychopharmacology (Berl). 134:319–329.
1997.PubMed/NCBI
|
|
26
|
Yu X, Qiao S, Wang D, Dai J, Wang J, Zhang
R, Wang L and Li L: A metabolomics-based approach for ranking the
depressive level in a chronic unpredictable mild stress rat model.
Rsc Adv:6:25751–25765. 2016.
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI
|
|
28
|
Jeschke J, Collignon E and Fuks F:
Portraits of TET-mediated DNA hydroxymethylation in cancer. Curr
Opin Genet Dev. 36:16–26. 2016.PubMed/NCBI
|
|
29
|
Rivas MP, Aguiar TFM, Fernandes GR,
Caires-Júnior LC, Goulart E, Telles-Silva KA, Cypriano M, de Toledo
SRC, Rosenberg C, Carraro DM, et al: TET Upregulation Leads to
5-Hydroxymethylation Enrichment in Hepatoblastoma. Front Genet.
10:5532019.PubMed/NCBI
|
|
30
|
Irwin RE, Pentieva K, Cassidy T,
Lees-Murdock DJ, McLaughlin M, Prasad G, McNulty H and Walsh CP:
The interplay between DNA methylation, folate and neurocognitive
development. Epigenomics. 8:863–879. 2016.PubMed/NCBI
|
|
31
|
Gross JA, Pacis A, Chen GG, Drupals M,
Lutz PE, Barreiro LB and Turecki G: Gene-body 5-hydroxymethylation
is associated with gene expression changes in the prefrontal cortex
of depressed individuals. Transl Psychiatry. 7:e11192017.PubMed/NCBI
|
|
32
|
Nan X, Campoy FJ and Bird A: MeCP2 is a
transcriptional repressor with abundant binding sites in genomic
chromatin. Cell. 88:471–481. 1997.PubMed/NCBI
|
|
33
|
Li LC and Dahiya R: MethPrimer: Designing
primers for methylation PCRs. Bioinformatics. 18:1427–1431.
2002.PubMed/NCBI
|
|
34
|
Seo MK, Ly NN, Lee CH, Cho HY, Choi CM,
Nhu LH, Lee JG, Lee BJ, Kim GM, Yoon BJ, et al: Early life stress
increases stress vulnerability through BDNF gene epigenetic changes
in the rat hippocampus. Neuropharmacology. 105:388–397.
2016.PubMed/NCBI
|
|
35
|
Brenet F, Moh M, Funk P, Feierstein E,
Viale AJ, Socci ND and Scandura JM: DNA methylation of the first
exon is tightly linked to transcriptional silencing. PLoS One.
6:e145242011.PubMed/NCBI
|
|
36
|
Klengel T, Pape J, Binder EB and Mehta D:
The role of DNA methylation in stress-related psychiatric
disorders. Neuropharmacology. 80:115–132. 2014.PubMed/NCBI
|
|
37
|
Li W and Liu M: Distribution of
5-hydroxymethylcytosine in different human tissues. J Nucleic
Acids. 2011:8707262011.PubMed/NCBI
|
|
38
|
Na KS, Chang HS, Won E, Han KM, Choi S,
Tae WS, Yoon HK, Kim YK, Joe SH, Jung IK, et al: Association
between glucocorticoid receptor methylation and hippocampal
subfields in major depressive disorder. PLoS One.
9:e854252014.PubMed/NCBI
|
|
39
|
Sakata K, Jin L and Jha S: Lack of
promoter IV-driven BDNF transcription results in depression-like
behavior. Genes Brain Behav. 9:712–721. 2010.PubMed/NCBI
|
|
40
|
Keller S, Sarchiapone M, Zarrilli F,
Videtic A, Ferraro A, Carli V, Sacchetti S, Lembo F, Angiolillo A,
Jovanovic N, et al: Increased BDNF promoter methylation in the
Wernicke area of suicide subjects. Arch Gen Psychiatry. 67:258–267.
2010.PubMed/NCBI
|
|
41
|
Laatikainen LM, Sharp T, Harrison PJ and
Tunbridge EM: Sexually dimorphic effects of
catechol-O-methyltransferase (COMT) inhibition on dopamine
metabolism in multiple brain regions. PLoS One.
8:e618392013.PubMed/NCBI
|
|
42
|
Kim JK, Samaranayake M and Pradhan S:
Epigenetic mechanisms in mammals. Cell Mol Life Sci. 66:596–612.
2009.PubMed/NCBI
|
|
43
|
Li Y, Jiang F, Chen L, Yang Y, Cao S, Ye
Y, Wang X, Mu J, Li Z and Li L: Blockage of TGFβ-SMAD2 by
demethylation-activated miR-148a is involved in caffeic
acid-induced inhibition of cancer stem cell-like properties in
vitro and in vivo. FEBS Open Bio. 5:466–475. 2015.PubMed/NCBI
|
|
44
|
Omene C, Kalac M, Wu J, Marchi E, Frenkel
K and O'Connor OA: Propolis and its Active Component, Caffeic Acid
Phenethyl Ester (CAPE), Modulate Breast Cancer Therapeutic Targets
via an Epigenetically Mediated Mechanism of Action. J Cancer Sci
Ther. 5:334–342. 2013.PubMed/NCBI
|