Introduction
Cartilage is a type of tissue characterized by poor
self-repair capacity due to the absence of blood vessels and
nervous tissue. Such tissues do not heal spontaneously after
sustained extensive damage due to trauma or similar events and
eventually lead to the onset of osteoarthritis and impaired
activities of daily living. Treatments to address cartilaginous
tissue damage include bone perforation, osteochondral column
transplantation, and autologous cultured cartilage transplantation.
Although each of these methods has been successful to some extent,
problems related to the number of procedures required and the
quality of regenerated tissue prevail (1). Therefore, treatment methods that can
overcome these issues are crucial for effective therapy.
In recent years, regenerative medical techniques,
including transplantation of cartilaginous tissue cultured from
stem cells, have been regarded as promising new therapeutic
options. Among these, induced pluripotent stem cells (iPSCs) have
garnered attention as a potential cell source, where clinical
applications of iPSCs, such as the production of corneal and
cardiac muscle sheets, continue to progress. iPSCs exhibit high
self-renewal capacity and have excellent potential as a cell source
owing to their ability to undergo cell division while remaining
undifferentiated, even after several divisions. The formation of
cartilaginous tissues from iPSCs has been reported in various
studies (2–8). However, regenerative medicine
utilizing iPSCs faces several obstacles, such as the associated
high costs, long culture periods, risk of oncogenesis, and
compromised tissue purity (9).
The application of biochemical stimulation in
conjunction with recombinant proteins is a common method for
inducing tissue differentiation from stem cells. However, in recent
years, it has been revealed that mechanical signaling via physical
stimulation is also important for morphogenesis/differentiation.
Physiologically, chondrocytes exist in a hypoxic environment,
wherein this environment is essential for their growth,
differentiation, and survival (10). A previous study reported that
chondrocyte differentiation was promoted under hypoxic conditions
during the production of cartilaginous tissues from human embryonic
stem (ES) cells (11). Therefore,
it is possible that a hypoxic environment may also promote
differentiation of cartilaginous tissue from iPSCs.
Based on these reports, we hypothesized that if
cartilaginous tissues could be prepared from iPSCs in a hypoxic
environment, it would be possible to produce such tissues faster
than those cultured in a stable oxygen environment. The objective
of this study was to investigate whether cartilaginous tissues can
be produced from iPSCs under hypoxic conditions and to evaluate the
effects of such an environment on cellular metabolism and purity of
the tissue produced.
Materials and methods
Chondrogenic differentiation of human
iPSCs (hiPSCs) in a monolayer culture
The established hiPSC line Toe (cell number
JCRB1338) was maintained in feeder-free medium that included
StemFit AK-02N (Reprocell Inc.) in 6 cm dishes coated with
laminin-511 (Nippi, Inc.). The cultured hiPSCs were then
transferred and maintained in StemFit AK02N in 6-well plates coated
with laminin-511. The iPS cells were confirmed by fluorescent
immunostaining to maintain their undifferentiated potential as
appropriate (Supplemental data; Fig.
S1). The hiPSCs formed high-density cell colonies consisting of
1–2×105 cells 10–15 days after starting maintenance
under feeder-free culture conditions. Subsequently, chondrogenic
differentiation of the iPSCs was initiated. First, the hiPSCs were
differentiated into mesendodermal cells by culturing in Dulbecco's
modified Eagle's medium (DMEM)/F12 (Sigma-Aldrich; Merck KGaA) with
10 ng/ml Wnt3A (R&D Systems), 10 ng/ml activin A (R&D
Systems), 1% insulin-transferrin-selenium (ITS) (Thermo Fisher
Scientific, Inc.), 1% fetal bovine serum (FBS), and 1%
penicillin-streptomycin (P/S) for 3 days. On day 4, the medium was
changed to chondrogenic medium [DMEM with 50 mg/ml ascorbic acid
(Nacalai Tesque), 10 ng/ml BMP2 (PeproTech), 10 ng/ml TGF-β1
(PeproTech), 10 ng/ml GDF5 (PeproTech), 1% ITS, 1% FBS, 2 mM
l-glutamine (Thermo Fisher Scientific, Inc.), 1×10−4 M
non-essential amino acids (Nacalai Tesque), 1 mM sodium pyruvate
(Thermo Fisher Scientific), and P/S]. bFGF (1 ng/ml; Wako Pure
Chemical Industries Ltd.) was added to the medium from days 3 to
14. Two groups of cells were assessed, namely hiPSCs cultured under
normoxic (21% O2, 5% CO2, and 74%
N2) and those under hypoxic conditions (5%
O2, 5% CO2, and 90% N2) in a
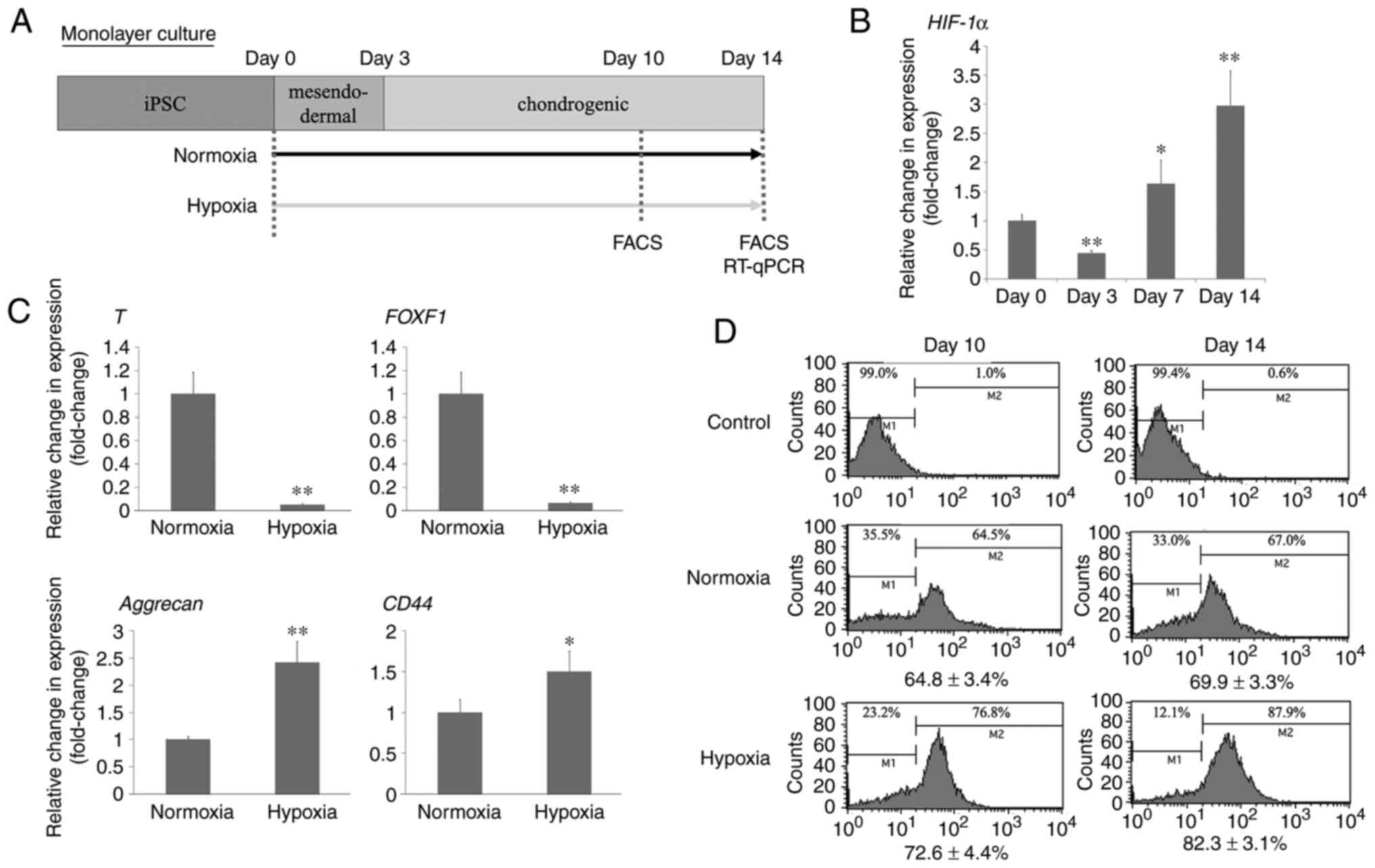
multigas incubator (Model 9200; Wakenyaku Co., Ltd.) (Fig. 1A).
Total RNA extraction and real-time
quantitative reverse transcription-polymerase chain reaction
(RT-qPCR) analysis
Total RNA was extracted from cells using ISOGEN
(Nippon Gene Co., Ltd.). The extracted RNA was reverse-transcribed
using PrimeScript™ RT Master Mix (Takara Bio, Inc.)
according to the manufacturer's instructions. Quantitative
real-time PCR was performed using Step One Plus™
Real-Time PCR System (Applied Biosystems) with a primer probe. Each
20 µl reaction mixture contained 1 µl of cDNA (100 ng), 9 µl
TaqMan™ Fast Advanced Master Mix (Applied Biosystems),
and 0.33 µl of target gene primers (Table I) and probes from the Universal
Probe Library (Roche). The cycle conditions were as follows:
denaturation at 95°C for 15 sec and annealing and extension at 60°C
for 1 min for 40 cycles. Relative changes in gene expression were
calculated using the comparative Ct method and normalized to the
expression of the internal control gene, 18S ribosomal RNA gene.
The results are shown as the average of three samples in which each
sample was assayed in duplicate.
 | Table I.Primers used for reverse
transcription-quantitative PCR. |
Table I.
Primers used for reverse
transcription-quantitative PCR.
| Gene | Direction | Primer sequence
(5′-3′) |
|---|
| 18S rRNA | Forward |
ATGAGTCCACTTTAAATCCTTTAACGA |
|
| Reverse |
CTTTAATATACGCTATTGGAGCTGGAA |
| T
(Brachyury) | Forward |
AGACACGTTCACCTTCAGCA |
|
| Reverse |
GCTCACCAATGAGATGAYCG |
| FOXF1 | Forward |
CAGCCTCTCCACGCACTC |
|
| Reverse |
CCTTTCGGTCACACATGCT |
| Acan | Forward |
GACGGCTTCCACCAGTGT |
|
| Reverse |
TCGAGGGTGTAGCGTGTAGA |
| CD44 | Forward |
GCAGTCAACAGTCGAAGAAGG |
|
| Reverse |
TGTCCTCCACAGCTCCATT |
| HIF-1α | Forward |
TTTTCAAGCAGTAGGAATTGGAA |
|
| Reverse |
TTCCAAGAAAGTGATGTAGTAGCTG |
Flow cytometry
Cells were detached and digested with trypsin to
form a single cell suspension. For labeling of intracellular
antigens, cells were fixed in 4% paraformaldehyde for 30 min at 4°C
and further permeabilized by incubating with 1% (w/v) bovine serum
albumin (BSA) and 0.2% (v/v) Triton X-100 in phosphate buffered
saline (PBS) for 15 min at room temperature. Cells were then
incubated with 10 µl/ml primary Alexa Fluor®
488-conjugated rabbit monoclonal anti-sex-determining region Y box
9 (SOX9) antibody (EPR14335, ab196450; Abcam) diluted in PBS
containing 1% BSA for 30 min at room temperature with light
shielding. Cell labeling was analyzed using a FACSCalibur system
(Becton-Dickinson and Company) with CellQuest software
(Becton-Dickinson and Company).
Chondrogenic differentiation of hiPSCs
in 3D culture
hiPSCs were transferred and maintained in StemFit
AK-02N in 6-well plates coated with Matrigel GFR (Thermo Fisher
Scientific, Inc.). The cells formed high-density colonies
(1-2×105) 10–15 days after the start of maintenance.
Subsequently, chondrogenic differentiation of iPSCs was induced in
the same manner as for monolayer culture. Multilayered nodules were
formed by day 14, and the nodules were physically separated from
the bottom of the dishes to form particles. Then, the particles
were transferred to suspension culture in 3.5 cm non-attachment
culture dishes (Prime surface®; Sumitomo Bakelite Co.,
Ltd.) on day 14 and cultured in chondrogenic medium until day 42.
On day 42, the medium was replaced with conventional medium (DMEM
with 10% FBS and 50 units and 50 mg/ml of penicillin and
streptomycin, respectively). The medium was changed every 2–7 days.
Two groups of cells were assessed, namely hiPSCs cultured under
normoxic and those under hypoxic conditions (5% O2, 5%
CO2, and 90% N2) in a multigas incubator.
Histological and immunohistochemical
analyses
Pellets were fixed in 4% paraformaldehyde (Wako Pure
Chemical Industries Ltd.), embedded in paraffin, and sectioned
(thickness: 5 µm). The sections were stained with hematoxylin and
eosin or safranin O. For immunohistochemical analysis of type 1 and
type 2 collagen, paraffin-embedded sections were deparaffinized in
xylene, rehydrated using a graded alcohol series, and washed with
PBS. Endogenous peroxidase activity was blocked by incubating the
sections in 3% H2O2 in methanol for 5 min.
The sections were incubated at 4°C with mouse polyclonal anti-type
1 collagen antibody (1:150; ab6308; Abcam) or anti-type 2 collagen
antibody (1:50; F-57; Kyowa Pharma Chemical Co., Ltd.) overnight.
After extensive washing with PBS, the sections were incubated in
Histofine Simple Stain Rat MAX-PO (Nichirei Biosciences Inc.) for
30 min at room temperature following the manufacturer protocol.
Immunostaining was detected by 3,3-Diaminobenzidine staining.
Counterstaining was performed with Mayer's hematoxylin. Any three
arbitrary locations stained red with safranin O, which is
considered to indicate advanced chondrogenic differentiation, were
observed at 400×. The number of cell nuclei in the field of section
was counted using ImageJ software (developed by Wayne Rasband), and
the mean value was calculated.
Cartilage-like tissues stained with Safranin O on
day 28 or 56 were qualitatively evaluated using the Bern Score
system (12).
Immunofluorescent staining
For immunohistochemistry of hypoxia-inducible factor
(HIF)-1α, paraffin-embedded sections were deparaffinized in xylene,
rehydrated in a graded alcohol series, and immersed in PBS,
followed by application of a protein block (Dako) for 10 min. The
sections were incubated at 4°C with rabbit polyclonal anti-HIF-1α
antibody (1:250; ab2185; Abcam) for 1 h. After extensive washing
with PBS, the sections were incubated with Alexa Fluor®
568-conjugated goat anti-rabbit IgG (H+L) secondary antibody
(1:500; A-11036; Thermo Fisher Scientific, Inc.) for 30 min at room
temperature. After extensive washing with PBS, the sections were
counterstained using Vectashield mounting medium with
4′,6-diamidino-2-phenylindole (DAPI) (H-1200; Vector
Laboratories).
Statistical analysis
All duplicate and triplicate experiments yielded
almost identical results. All data in this study are expressed as
the mean value ± standard deviation. We used the parametric one-way
analysis of variance to test the differences between groups. The
Tukey-Kramer test was used to determine the differences between
groups when the results were considered significant. For all
analyses, differences at P<0.05 were considered statistically
significant.
Results
Effects of hypoxic stimulation on the
purity of differentiated cartilage
To confirm the effects of hypoxic stimulation on
cell viability, we measured the number of cells in the tissue
specimen on day 14. Although hypoxic stimulation at 5%
O2 did not adversely affect cell viability during the
differentiation of cartilaginous tissue from iPSCs, cell viability
decreased by approximately 80% in the 2% O2 hypoxic
environment (data not shown). Therefore, a hypoxic environment with
5% O2 was used for the subsequent analyses. We also
evaluated the gene expression change in immature mesodermal markers
(T) and chondrogenic markers (sox9). The expression
of mesoderm markers increased during culture in mesoderm medium by
day 3. Thereafter, the expression of mesoderm markers decreased,
and instead, the expression of chondrogenic markers increased after
changing the mesodermal medium to a chondrogenic medium, consistent
with the results of a previous study (8) (Fig.
S2). We observed changes in HIF-1α expression over time under
hypoxic conditions. HIF-1α expression increased as chondrogenic
differentiation progressed from day 3 to day 14 (Fig. 1C). However, on day 3, HIF-1α
expression was decreased rather than increased.
The effect of a hypoxic environment on cartilage
differentiation was investigated using real-time RT-PCR performed
during plate culture. Culturing was carried out in accordance with
the differentiation protocol described in Fig. 1A, and gene expression was assessed
in the group cultured under normoxic conditions and in the group
cultured in a 5% O2 hypoxic environment on day 14 after
the start of culture, the point at which cartilage differentiation
had advanced to some extent, and gene expression was assessed. The
expression of T (Brachyury) and FOXF1, markers
of undifferentiated mesodermal tissue, was markedly reduced in
cultures grown under hypoxic conditions compared to those grown
under normoxic conditions. Additionally, the expression of
Acan, a marker indicating cartilage matrix production, and
CD44, a surface marker of chondrocytes, was significantly
increased in the hypoxic group compared to that in the normoxic
group (Fig. 1B).
To confirm the expression of SOX9, a master
regulator related to chondrocyte differentiation,
fluorescence-activated cell sorting (FACS) targeting SOX9 was
performed on days 10 and 14 in both the groups (cultured under
steady oxygen conditions and in a 5% O2 hypoxic
environment). We observed that the proportion of SOX9-positive
cells not only increased by day 10 under hypoxic culture
conditions, but this number increased even more by day 14 (Fig. 1D).
Effect of hypoxic stimulation on
substrate production during cartilage differentiation
Similarly, we also performed 3D culture using
Matrigel according to the differentiation protocol and established
a suspension culture from day 14. The groups cultured under
normoxic and hypoxic conditions were examined histologically on
days 28 and 56 after the start of differentiation culture.
Histological examination performed on day 28 revealed that only a
portion of the cells grown under normoxic conditions were stained
with safranin O, while the tissue grown under hypoxic conditions
exhibited uniform staining with safranin O and for type 2 collagen
(Fig. 2). Neither tissue
exhibited positive staining for type 1 collagen. In addition, there
was no remarkable difference between the normoxic and hypoxic
groups in terms of the maximum X-axial diameters of the cell
masses, as measured on day 28. By day 56, favorable staining was
obtained with safranin O and for type 2 collagen in both the
normoxia and hypoxia groups, and tissues similar to normal
cartilaginous tissue were produced. The cell phenotype showed
chondrocyte-like cells surrounded by pericellular matrix. On the
high magnification Safranin O staining images, on day 28, the
normoxia group had more cell numbers and less cartilage matrix. On
the other hand, in the hypoxia group, the number of cells at the
margins was high, and matrix production was poor, but
chondrocyte-like cells with cartilaginous lumen were scattered in
the center. On day 56, the number of cells decreased in both
normoxia and hypoxia groups, and the tissue developed a rich
matrix. On day 28, the Bern score, a qualitative score about
cartilage generated in vitro, was higher in the hypoxia
group than in the normoxia group. On the other hand, the normoxia
and hypoxia groups had similarly high scores on day 56 (Fig. 2D-G).
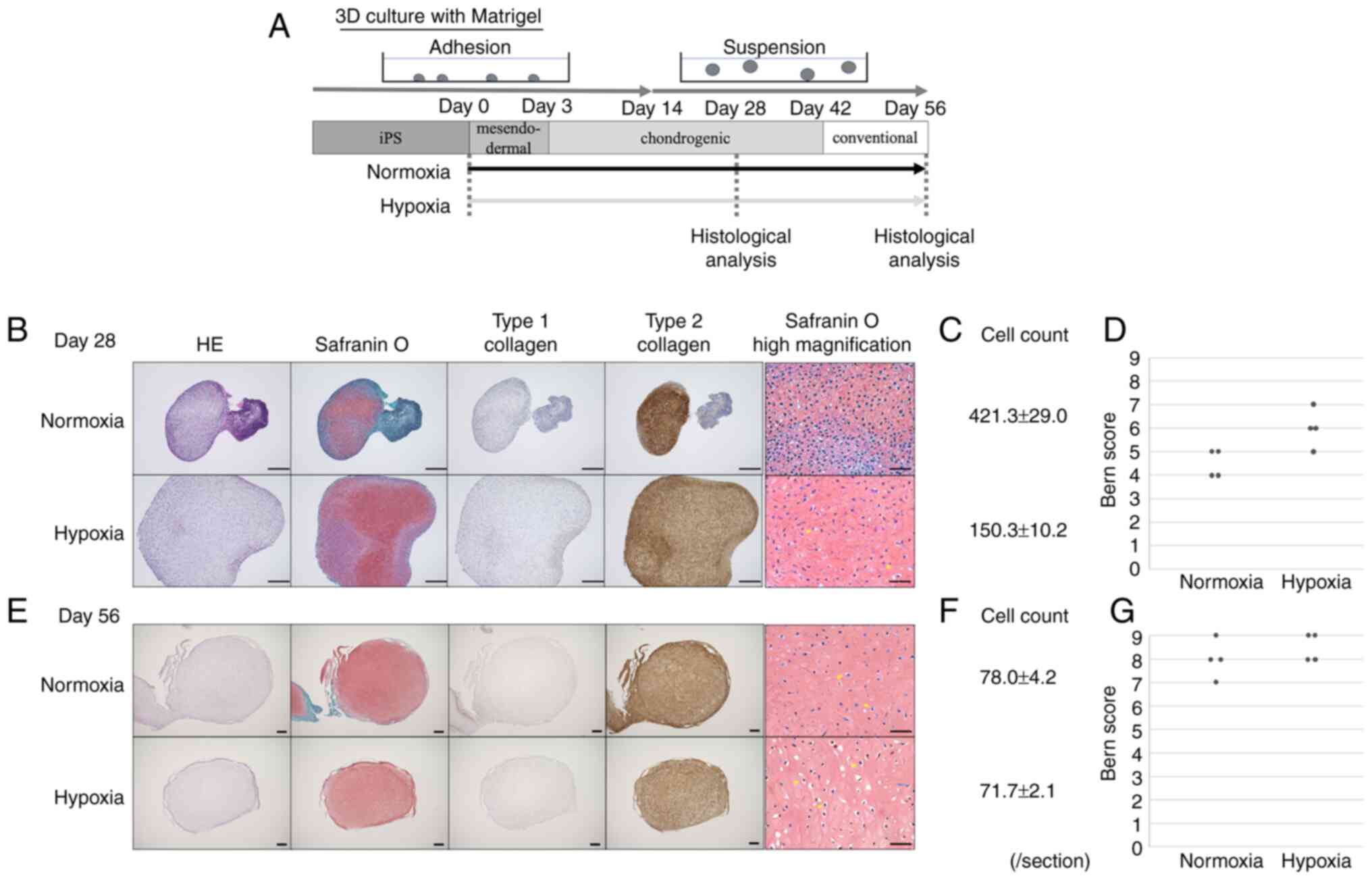 | Figure 2.Hypoxic conditions promotes purity of
cartilage-like tissue. (A) hiPSCs cultured in 3D culture under
normal oxygen or 5% O2 hypoxic conditions during
cartilage differentiation for 28 or 56 days. (B-F) Histological
analysis and quantification of mid-sagittal sections using HE and
safranin O, and immunohistochemical analysis using anti-type 1 or 2
collagen antibodies to visualize chondrogenic differentiation on
(B) day 28 (magnification, ×40) and (E) day 56 (magnification,
×20), [HE, Safranin, Type 1 and 2 collagen scale bar, 500 µm;
safranin O high magnification scale bar, 50 µm (magnification,
×400)]. The yellow arrows indicate chondrocyte-like cells and the
pericellular matrix. Number of cell nuclei in the high magnificent
section (magnification, ×400) on (C) day 28 or (F) 56 was counted.
The mean value was calculated (n=4). Cartilage-like tissue stained
with Safranin O on (D) day 28 or (G) 56 was qualitatively evaluated
using the Bern Score system (n=4). HE, hematoxylin and eosin;
hiPSCs, human induced pluripotent stem cells. |
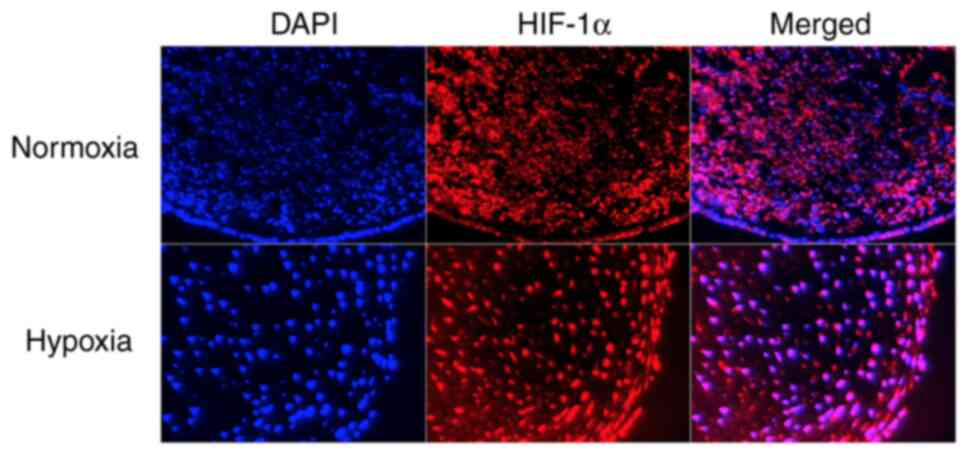
Investigation of the promotive effects
of HIF-1α on cartilage differentiation under hypoxia
Next, we investigated the mechanisms that promote
cartilage differentiation in a hypoxic environment. The expression
of HIF-1α is promoted under hypoxic conditions and accelerates
cartilage differentiation. We also performed 3D culture using
Matrigel according to the differentiation protocol up to day 28,
followed by immunostaining for HIF-1α. The expression of HIF-1α was
observed under both normoxic and hypoxic conditions (Fig. 3). However, nuclei of tissues
cultured under hypoxic conditions were more deeply stained than
those of the tissues cultured under normoxic conditions.
Discussion
In this study, we found that hypoxic culture
conditions not only led to enhanced cartilage matrix production but
also improved cell purity of cartilaginous tissue differentiated
from iPSCs. Using this method, highly pure cartilage-like tissues
may be produced more rapidly and conveniently.
With the industrialization of tissue graft materials
in the field of regenerative medicine, quality assurance and
standardization have been recognized as critical aspects in recent
years. As ES cells and iPSCs maintain their ability to
differentiate, they are excellent cell sources for regenerative
medicine. However, because differentiation is induced in
undifferentiated cells, it is necessary to improve the level of
cell purity. Various studies have been conducted to improve the
degree of purity of tissue differentiation from iPSCs. Hirano et
al were able to increase the purity of islet cell
differentiation from iPSCs by applying a unique culture method
referred to as a closed-channel culture system (13). In addition, Hwang et al
increased the purity of cardiomyocyte differentiation from iPSCs by
adding a small molecule compound to the culturing environment
(14). Various methods for
differentiating iPSCs with a high degree of purity are being
studied in similar ways.
The intra-articular cavity, where chondrocytes are
present, is physiologically hypoxic. Generally, chondrocytes
present in the articular cartilage are found in environments
containing 1–5% O2. Further, they are surrounded by a
thick extracellular matrix, enabling them to remain viable in
increasingly hypoxic environments (15). Reports have also stated that
changes in oxygen concentration are important when preparing
cartilaginous tissue from ES cells (11) and that applying hypoxic
stimulation during cartilaginous tissue differentiation from
mesenchymal stem cells can promote cartilage matrix production in
the resulting chondrocytes (16).
Consistent with these reports, we also observed that the expression
T (Brachyury) and FOXF1, which serve as
markers of undifferentiated mesodermal tissue, declined by day 14
as a result of hypoxic stimulation during cartilaginous tissue
differentiation from iPSCs. Moreover, FACS performed on days 10 and
14 revealed that the proportion of SOX9-positive cells increased. A
previous report found that HIF-1α expression under hypoxic
conditions induced chondrogenic differentiation into articular
cartilage via SOX9, a master regulator related to cartilaginous
tissue (17). Therefore, it has
been considered that, in hypoxic environments, cell differentiation
is promoted via HIF-1α expression, accompanied by decreased levels
of undifferentiated cell markers and an increase in the population
of SOX9-positive chondrocytes.
HIF-1α also has an anabolic effect on the metabolism
of cartilaginous tissue. It is known that HIF-1α translocates to
the nucleus in hypoxic environments and regulates the expression of
SOX9 (10,17). Furthermore, a previous study found
that the production of substrates, such as aggrecan and type 2
collagen, can be promoted via HIF-1α expression and by culturing
chondrocytes in a hypoxic environment (18). In this study, HIF-1α expression
increased as chondrogenic differentiation progressed from day 3 to
day 14. The histological examination performed on culture day 28
revealed that safranin O staining and substrate production were
both increased in the cells cultured under hypoxic conditions. By
day 56, it was possible to produce tissue specimens similar to
those cultured in a stable oxygen environment. These findings
indicate that hypoxic cultures may be used to produce high quality
tissue more rapidly. However, HIF-1α expression decreased on day 3
compared to day 0. It has been reported that HIF-1α expression
decreases after prolonged culture under hypoxic conditions in
various cell types (19). HIF-1α
expression might have decreased till day 3, as has been reported in
other cells. Further, HIF-1α expression might have increased due to
the change to a chondrogenic medium and the progress of
chondrogenic differentiation.
Although the study has several merits, there are a
few limitations as well. First, we chose a 5% O2 culture
environment because it is easier to maintain cell viability at this
concentration than in a 2% O2 environment; however,
evaluations of other oxygen concentrations are currently lacking
and are therefore needed. In addition, the period for which hypoxia
should be administered is also a subject that requires further
investigation. Second, we did not evaluate these conditions with
respect to SOX9-negative cells, and therefore the possibility of
contamination by undifferentiated cells cannot be ruled out. Third,
we could not evaluate the role of HIF-1α in chondrogenic
differentiation, including suppression experiments. However, HIF-1α
is a key gene for chondrocytes, and chondrogenic differentiation
did not proceed when HIF-1α was suppressed (10). In addition, we have previously
reported that HIF-1α expression was increased by culturing
chondrocytes under hypoxic conditions, which in turn increased the
production of the cartilaginous matrix (20). These results suggest that HIF-1α
may be involved in the increase in cartilaginous matrix production.
Fourth, as we did not conduct an in vivo transplantation
experiment, we were unable to evaluate the risks of tumorigenesis
or tissue deformation after transplantation.
We believe that the results of this study can be
applied to create high-quality cartilage tissue from iPSCs more
easily and at a lower cost, and more, leading to the advancement of
transplantation medicine using iPSCs.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by JSPS KAKENHI (grant no.
16H05452).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SS, HI, SN, SI and ToK conceptualized the study. SS,
HI, ST, and YA organized the data for paper. SS, YF, TsK and OM
performed formal analysis. SS, YF, SI and ToK examined the study
data. SS, SN, YF, ST, MS and YA designed and performed the
experiments. OM, YA and ToK administrated the project. OM
supervised the study. ToK acquired funding and validated the study
design. SS wrote the original draft of the manuscript. All authors
contributed to manuscript reviewing and editing, and have read and
approved the final manuscript. SS and YA confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
iPSCs
|
induced pluripotent stem cells
|
|
hiPSCs
|
human iPSCs
|
|
FOXF1
|
forkhead box protein F1
|
|
SOX9
|
sex-determining region Y box 9
|
|
HIF-1α
|
hypoxia-inducible factor-1α
|
|
ES cells
|
embryonic stem cells
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
ITS
|
insulin-transferrin-selinium
|
|
FBS
|
fetal bovine serum
|
|
P/S
|
penicillin and streptomycin
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
|
BSA
|
bovine serum albumin
|
|
PBS
|
phosphate-buffered saline
|
|
DAPI
|
4′,6-diamidino-2-phenylindole
|
|
FACS
|
fluorescence-activated cell
sorting
|
References
|
1
|
Roberts S, Menage J, Sandell LJ, Evans EH
and Richardson JB: Immunohistochemical study of collagen types I
and II and procollagen IIA in human cartilage repair tissue
following autologous chondrocyte implantation. Knee. 16:398–404.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Driessen BJH, Logie C and Vonk LA:
Cellular reprogramming for clinical cartilage repair. Cell Biol
Toxicol. 33:329–349. 2017. View Article : Google Scholar
|
|
3
|
Chen YS, Pelekanos RA, Ellis RL, Horne R,
Wolvetang EJ and Fisk NM: Small molecule mesengenic induction of
human induced pluripotent stem cells to generate mesenchymal
stem/stromal cells. Stem Cells Transl Med. 1:83–95. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guzzo RM, Gibson J, Xu RH, Lee FY and
Drissi H: Efficient differentiation of human iPSC-derived
mesenchymal stem cells to chondroprogenitor cells. J Cell Biochem.
114:480–490. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nejadnik H, Diecke S, Lenkov OD, Chapelin
F, Donig J, Tong X, Derugin N, Chan RC, Gaur A, Yang F, et al:
Improved approach for chondrogenic differentiation of human induced
pluripotent stem cells. Stem Cell Rev Rep. 11:242–253. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Teramura T, Onodera Y, Takehara T,
Frampton J, Matsuoka T, Ito S, Nakagawa K, Miki Y, Hosoi Y,
Hamanishi C and Fukuda K: Induction of functional mesenchymal stem
cells from rabbit embryonic stem cells by exposure to severe
hypoxic conditions. Cell Transplant. 22:309–329. 2013. View Article : Google Scholar
|
|
7
|
Umeda K, Zhao J, Simmons P, Stanley E,
Elefanty A and Nakayama N: Human chondrogenic paraxial mesoderm,
directed specification and prospective isolation from pluripotent
stem cells. Sci Rep. 2:4552012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamashita A, Morioka M, Yahara Y, Okada M,
Kobayashi T, Kuriyama S, Matsuda S and Tsumaki N: Generation of
scaffoldless hyaline cartilaginous tissue from human iPSCs. Stem
Cell Reports. 4:404–418. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brown PT, Handorf AM, Jeon WB and Li WJ:
Stem cell-based tissue engineering approaches for musculoskeletal
regeneration. Curr Pharm Des. 19:3429–3445. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schipani E, Ryan HE, Didrickson S,
Kobayashi T, Knight M and Johnson RS: Hypoxia in cartilage:
HIF-1alpha is essential for chondrocyte growth arrest and survival.
Genes Dev. 15:2865–2876. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Koay EJ and Athanasiou KA: Hypoxic
chondrogenic differentiation of human embryonic stem cells enhances
cartilage protein synthesis and biomechanical functionality.
Osteoarthritis Cartilage. 16:1450–1456. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Grogan SP, Barbero A, Winkelmann V, Rieser
F, Fitzsimmons JS, O'Driscoll S, Martin I and Mainil-Varlet P:
Visual histological grading system for the evaluation of in
vitro-generated neocartilage. Tissue Eng. 12:2141–2149. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hirano K, Konagaya S, Turner A, Noda Y,
Kitamura S, Kotera H and Iwata H: Closed-channel culture system for
efficient and reproducible differentiation of human pluripotent
stem cells into islet cells. Biochem Biophys Res Commun.
487:344–350. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hwang GH, Park SM, Han HJ, Kim JS, Yun SP,
Ryu JM, Lee HJ, Chang W, Lee SJ, Choi JH, et al: Purification of
small molecule-induced cardiomyocytes from human induced
pluripotent stem cells using a reporter system. J Cell Physiol.
232:3384–3395. 2017. View Article : Google Scholar
|
|
15
|
Silver IA: Measurement of pH and ionic
composition of pericellular sites. Philos Trans R Soc Lond B Biol
Sci. 271:261–272. 1975. View Article : Google Scholar
|
|
16
|
Leijten J, Georgi N, Moreira Teixeira L,
van Blitterswijk CA, Post JN and Karperien M: Metabolic programming
of mesenchymal stromal cells by oxygen tension directs chondrogenic
cell fate. Proc Natl Acad Sci USA. 111:13954–13959. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Amarilio R, Viukov SV, Sharir A,
Eshkar-Oren I, Johnson RS and Zelzer E: HIF1alpha regulation of
Sox9 is necessary to maintain differentiation of hypoxic
prechondrogenic cells during early skeletogenesis. Development.
134:3917–3928. 2007. View Article : Google Scholar
|
|
18
|
Sanz-Ramos P, Mora G, Vicente-Pascual M,
Ochoa I, Alcaine C, Moreno R, Doblaré M and Izal-Azcárate I:
Response of sheep chondrocytes to changes in substrate stiffness
from 2 to 20 Pa: Effect of cell passaging. Connect Tissue Res.
54:159–166. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ginouvès A, Ilc K, Macías N, Pouysségur J
and Berra E: PHDs overactivation during chronic hypoxia
‘desensitizes’ HIFalpha and protects cells from necrosis. Proc Natl
Acad Sci USA. 105:4745–4750. 2008. View Article : Google Scholar
|
|
20
|
Tsuchida S, Arai Y, Takahashi KA, Kishida
T, Terauchi R, Honjo K, Nakagawa S, Inoue H, Ikoma K, Ueshima K, et
al: HIF-1α-induced HSP70 regulates anabolic responses in articular
chondrocytes under hypoxic conditions. J Orthop Res. 32:975–980.
2014. View Article : Google Scholar : PubMed/NCBI
|