Introduction
Foeniculum vulgare Mill. (F. vulgare),
commonly referred to as fennel, is one of the most widespread
aromatic plants. This species belongs to the Apiaceae family and it
is widely cultivated in different parts of world, including Asia,
North and South America, and the Southern regions of Europe
(1).
Several parts of the plant are edible, including its
leaves, stalks and seeds (fruits), which are a source of a wide
range of phytochemicals such as fatty acids, phenolic compounds and
flavonoids, as well as volatile compounds such as anethole,
estragole and fenchone, which are major phytoconstituents found in
F. vulgare (2,3). Most of these phytochemicals are
found in essential oils (EOs), which can be found in almost any
part of the plant, including the root, stem, seeds and fruits
(4).
F. vulgare fruits are commonly used as a
culinary spice. The EOs from fennel are often used as flavoring
agents, but also as constituents of cosmetic and pharmaceutical
products (5). F. vulgare
is widely used in traditional medicine for its diuretic,
antispasmodic, analgesic, mucolytic and anti-inflammatory functions
(1). The whole plant, as well as
its stems, fruits, leaves and seeds are used to treat a wide range
of ailments of the digestive, reproductive and respiratory systems,
including abdominal pains, constipation, diarrhea, amenorrhea,
fever, flatulence, arthritis, insomnia, irritable colon, liver
pain, mouth ulcer and stomachache (1,6–8).
In ancient China, it was used as remedy for snake bite; in
addition, the infusion of its fruits was used as a carminative,
while its roots have been found to possess efficient purgative
properties. In certain parts of Southern Italy, the decoction of
the fruits of F. vulgare subsp. piperitum was used
for digestive reasons, and chewing its leaves was considered a cure
for mouth ulcers (9).
Furthermore, it has been prescribed as a muscle relaxant, a weak
diuretic, a carminative and a mild stimulant (10).
The genus Foeniculum is found in Italy, with
only one species identified that has been divided into two
subspecies, Foeniculum vulgare subsp. vulgare Miller
and Foeniculum vulgare subsp. piperitum (Ucria)
Coutinho. This distinction is still a subject of debate among
botanists. In fact, based on the difference in distribution, some
botanists believe that they represent two distinct species. The
presence of F. vulgare subsp. piperitum has been
reported in the Central-Southern Mediterranean, but outside this
area, the species is quite rare. Furthermore, this subspecies does
not contain anethole (11) and it
is often confused with a chemotype of F. vulgare var.
vulgare, which presents a bitter, but different taste.
Recently, the chemical compositions of the EOs of
different parts of F. vulgare subsp. piperitum
collected in Sicily were evaluated by gas chromatography (GC) and
GC-mass spectrometry (12). The
results were compared with those of the EOs from the same parts of
F. vulgare subsp. vulgare, collected in the same
region and with those reported in the literature for other
accessions of F. vulgare subsp. piperitum. The EOs of
F. vulgare subsp. vulgare exhibited completely
different compositions, clearly indicating the differences between
the two subspecies.
A number of in vitro and in vivo
studies highlighted how various extracts of F. vulgare
possess antioxidant, anti-inflammatory, anti-mutagenic and
anticancer properties. F. vulgare EOs exhibited
anti-mutagenic effects in mice, as they reduced chromosomal
aberrations induced in mouse bone marrow cells by cyclophosphamide.
This effect was mediated by a reduction of oxidative stress
(13). The anti-tumor activity of
F. vulgare has been reported in different cancer cells, such
as melanoma (14), prostate
(15), lung cancer (16) and hepatocarcinoma cells (17). Ke et al (16) demonstrated that ethanol extract of
F. vulgare seeds induced apoptosis in HCI-H446 and NCI-H661
lung cancer cell lines and inhibited the growth of NCI-446-derived
xenografts by reducing Bcl-2 protein expression. Extracts of F.
vulgare seeds also induced apoptosis and inhibited cell
migration of hepatocarcinoma cells in vitro and
significantly constrained the growth of HCC xenografts in nude mice
by targeting survivin (17). In
addition, extracts of F. vulgare seeds also exerted
anticancer effects on Elrich ascites carcinoma-bearing mice by
modulating lipid peroxidation and potentiating antioxidant defense
(18).
Thus, based on the promising previous results
regarding the anti-cancer potential of F. vulgare, the aim
of the present study was to examine the possible anti-cancer action
of the EOs of F. vulgare subsp. piperitum (FVPEO) in
MDA-MB231 triple-negative breast cancer (TNBC) cells, demonstrating
that FVPEO induces an apoptotic cell death process through the
activation of the NAD(P)H quinone oxidoreductase 1 (NQO1)/p53
axis.
Materials and methods
F. vulgare subsp. piperitum plant
material and fruit EO preparation
Fruits of F. vulgare subsp. piperitum
were collected on the southern slopes of the limestone massif of
Rocca Busambra (Corleone, Palermo, Italy). Typical specimens (PAL
109709), identified by Prof. Vincenzo Ilardi, have been deposited
in Herbarium Mediterraneum Panormitanum of the ‘Orto Botanico’
(Palermo, Italy). A total of 136 g F. vulgare subsp.
piperitum fruits were hydro-distillated for 3 h using
Clevenger's apparatus. The oil (yield 1.36%) was dried with
Na2SO4, filtered and stored in the freezer at
−20°C, until the time of analysis. The chemical composition of
FVPEO was performed as previously reported (12).
Cell cultures, reagents and
chemicals
MDA-MB231 TNBC and estrogen-positive MCF7 cells were
obtained from ‘Istituto Scientifico Tumori’ (Genoa, Italy) and
cultured as monolayers in DMEM medium (cat. no. ECM0749L, Euroclone
SpA) supplemented with 10% (v/v) heat-inactivated FCS (cat. no.
ECS0180L; Euroclone SpA), 1% non-essential amino acids (cat. no.
ECB3054D; Euroclone SpA), 2 mM glutamine (cat. no. ECB3000D,
Euroclone SpA) and 1% penicillin/streptomycin solution (cat. no.
ECB3001D; Euroclone SpA). Cells were plated on 96-well microplates
or on 6-well cell culture plates and allowed to adhere overnight in
culture medium at 37°C in a humidified atmosphere containing 5%
CO2, followed by treatment with FVPEO or vehicle only.
Media and cell culture reagents were purchased from Euroclone SpA.
All other chemicals and reagents were provided by Millipore
Sigma.
Cell viability assessment and
morphological detection of apoptosis
In order to assess the viability of FVPEO-treated
breast cancer cells, an MTT assay was performed, as previously
described (19). Briefly,
8×103 cells/well were plated in 200 µl DMEM in a 96-well
plate and treated. At the end, 4 µl MTT solution (5 mg/ml in PBS)
were added to the cell medium and the incubation was protracted for
2 h at 37°C in the dark. Mitochondria dehydrogenase activity of
viable cells converts MTT to formazan, which is soluble in tissue
culture medium. Cells were then lysed in lysis buffer and
absorbance was read at 570 and 690 nm using an automatic ELISA
plate reader (OPSYS MR; Dynex Technologies).
In order to determine either changes in nuclear
morphology or plasma membrane damage, the cells were stained with
Hoechst 33342 (cat. no. H3570; Invitrogen; Thermo Fisher
Scientific, Inc.), a cell permeant fluorochrome emitting blue
fluorescence when bound to dsDNA and excited by ultraviolet light.
For these assays, cells (8×103) were incubated with
Hoechst 33342 (2.5 µg/ml medium) for 30 min, washed with PBS and
suspended in culture medium prior to FVPEO treatment. Morphological
changes in apoptotic cells, manifesting as chromatin condensation
and fragmentation, were detected by fluorescence microscopy using
an excitation wavelength of 372 nm and emission wavelength of 456
nm. All images were captured by Leica Q Fluoro Software (Leica
Microsystems, Inc.). For this analysis, at least 10 fields were
considered for each sample and apoptotic cells were counted in
random fields at a magnification of ×100.
The apoptotic cell morphology was also studied by
acridine orange and ethidium bromide double staining, as reported
by Liu et al (20).
Analysis of reactive oxygen species
generation
The detection of intracellular reactive oxygen
species production was carried out using
2′,7′-dichlorodihydrofluorescein diacetate (H2-DCFDA)
staining, as previously described (21). H2-DCFDA (cat. no. D399;
Molecular Probe; Thermo Fisher Scientific, Inc.) is a non-polar dye
that can easily cross the cell membrane and can be oxidized to
DCFDA in the presence of reactive oxygen species (ROS) emitting
green fluorescence.
After incubating the cells with FVPEO, the medium
was removed and cells were incubated with 10 µM H2-DCFDA
for 30 min at 37°C. Next, positive cells were analyzed under a
Leica fluorescence microscope (Leica Microsystems, Inc.) with
excitation at 485 nm and emission at 530 nm, as previously
described (22).
Western blot analysis
For western blot analysis, cells were lysed in RIPA
lysis buffer containing 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.1%
sodium dodecyl sulfate, 0.5% sodium deoxycholate, and 1 mM EDTA
supplemented with phosphatase inhibitor mix (Sigma-Aldrich; Merck
KGaA). Extracts were sonicated thrice and protein content was
determined using the Bradford assay (Bio-Rad Laboratories, Inc.)
using a BSA (Sigma-Aldrich; Merck KGaA) standard curve. Next, 30
µg/lane of protein sample were subjected to SDS polyacrylamide gel
electrophoresis and transferred onto a nitrocellulose membrane.
Manganese superoxide dismutase (MnSOD; cat. no. sc-133254; 1:200),
c-Jun (cat. no. sc-1694; 1:200), phospho-c-Jun N-terminal kinases
(pJNK; cat. no. sc-6254; 1:200), γ-H2A histone family member X
(H2AX; cat. no. sc-517348; 1:200), p53 (cat. no. sc-126; 1:200),
pro-caspase-3 (cat. no. sc-65497; 1:200) and PARP-1 (cat. no.
sc-53643; 1:200) were detected using specific antibodies produced
by Santa Cruz Biotechnology, Inc. The antibody for Heme oxygenase
(HO-1; cat. no. orb5455; 1:1,000) was provided by Biorbyt Ltd., and
that for NQO1 (cat. no. 3187S; 1:1,000) by Cell Signaling
Technology, while the nuclear factor E2-related factor-2 (Nrf-2)
antibody (cat. no. NBP1-32822, 1:1,000) was purchased from Novus
Biologicals. Next, the nitrocellulose filters were incubated with
anti-rabbit IgG (H+L) HRP conjugate (cat. no. W4011; 1:5,000;
Promega) or Anti-Mouse IgG (H+L) HRP conjugate (cat. no. W4021;
1:5,000; Promega) secondary antibody for 1 h. Protein bands were
detected using ECL™ Prime Western Blotting System (cat.
no. GERPN2232; Cytiva), and quantified using Quantity One software
4.6.6 (Bio-Rad Laboratories, Inc.). The correct protein loading was
examined using immunoblotting for γ-tubulin (cat. no. T3559;
1:2,000, Sigma-Aldrich; Merck KGaA). All blots shown are
representative of at least three separate experiments.
Statistical analysis
Statistical analysis of data was performed using
GraphPad Prism 5.0. software (GraphPad Software Inc.). A Student's
t-test was applied to evaluate significant differences between
untreated and treated samples. For the analysis of multiple groups
a one-way ANOVA test was used. Data are expressed as the mean ± SD.
The statistical significance threshold was set at P<0.05.
Results
Chemical profiling of EO of F. vulgare
subsp. piperitum
Hydro distillation of the fruits of F.
vulgare subsp. piperitum gave a pale-yellow oil. The EO
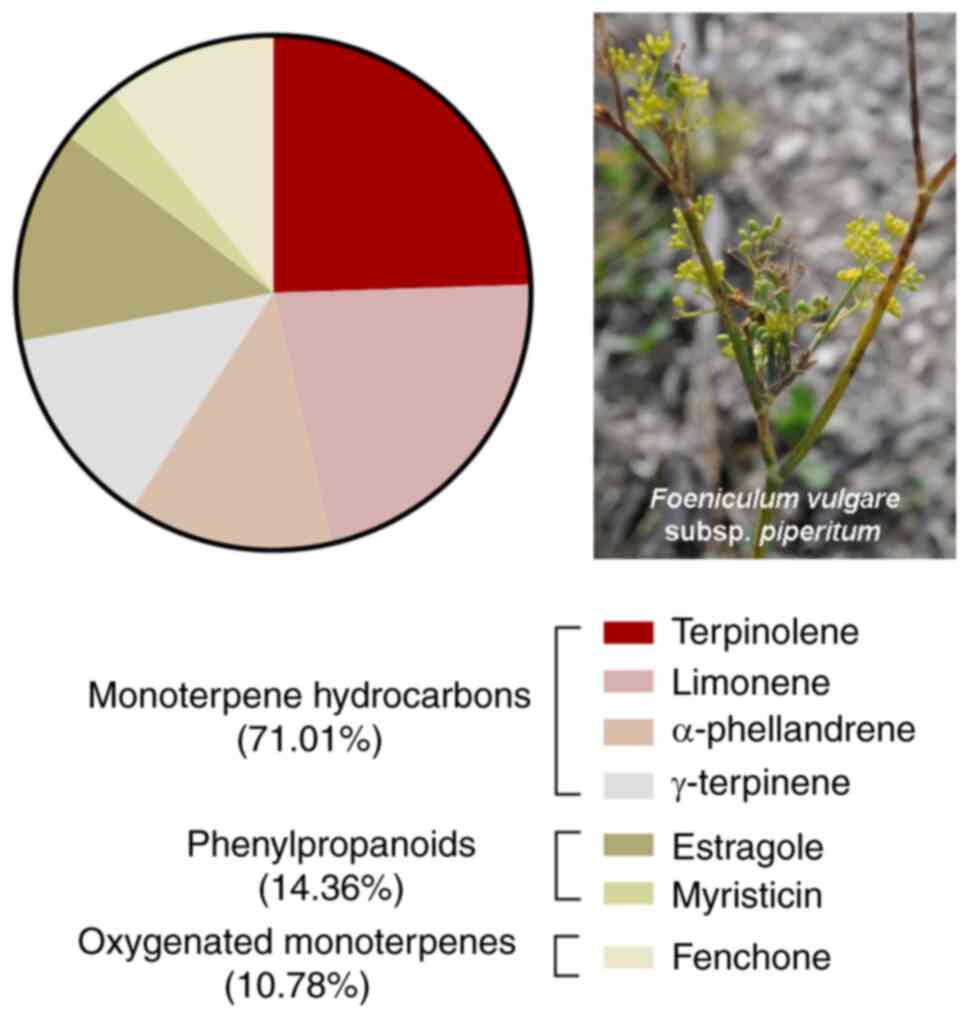
composition was previously reported (12). As shown in the pie chart of
Fig. 1, FVFEO was particularly
enriched in monoterpene hydrocarbons (71.01%), with terpinolene
(20.10%), limonene (17.84%), α-phellandrene (10.53%) and
γ-terpinene (10.43%) as the main components of EO. The
second most abundant class was phenylpropanoids (14.36%), typical
metabolites of F. vulgare subsp. vulgare (12), with estragole (10.96%) and
myristicin (3.09%) as the main products of this class of compounds.
Oxygenated monoterpenes were present at a lower amount (10.78%)
with fenchone (8.83%) as the principal metabolite of this class.
Based on these observations, the anti-tumor potential of FVFEO was
explored.
Effects of FVPEO on the viability of
breast cancer cells
To demonstrate a possible anti-proliferative effect
of the EO of the fruits of F. vulgare subsp.
piperitum (FVPEO), the present study focused on MDA-MB231, a
very aggressive and poorly differentiated breast cancer cell line,
which does not express estrogen, progesterone and human epidermal
growth factor receptor 2 (HER2)/receptors (23). MDA-MB231 cells were treated with
increasing concentrations of FVPEO (125–2,000 µg/ml) for various
periods of time, and the viability was assessed using MTT assay, as
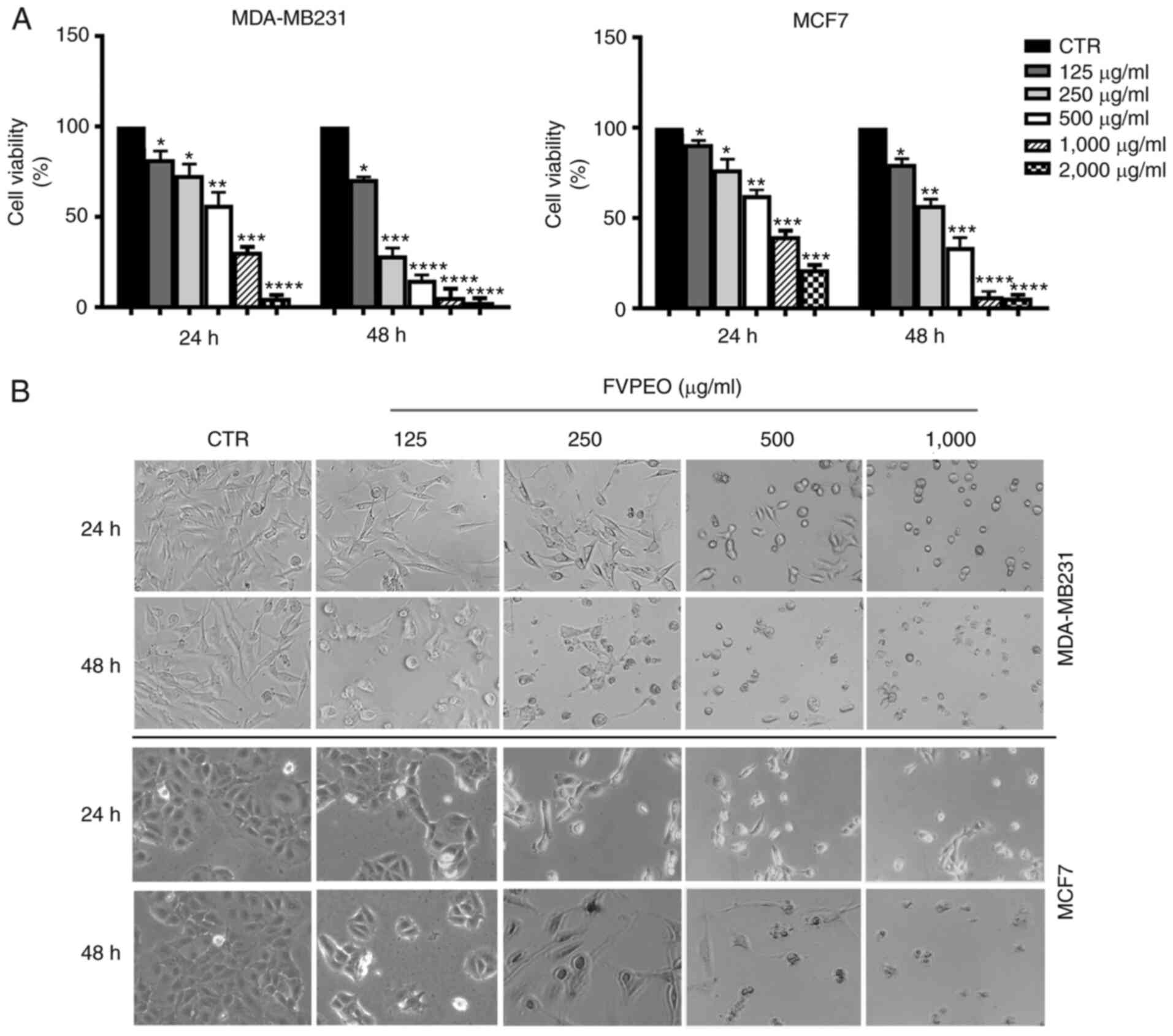
reported in the Materials and methods. As shown in Fig. 2A, the cell survival rate displayed
a marked dose- and time-dependent decrease following FVPEO
treatment, as compared to the untreated control. Following 24 h of
treatment the viability of MDA-MB231 cells was reduced by 20% of
the control with 125 µg/ml FVPEO. Increasing the treatment dose,
the viability diminished progressively and a consistent cytotoxic
effect was reached at the highest concentration examined (only ~5%
of viable cells with 2,000 µg/ml). The cytotoxic effect of FVPEO
was further increased as treatment time increased to up to 48 h,
while the viability was decreased to 15% with 500 µg/ml FVPEO.
Light microscopy findings showed that, following
exposure to FVPEO, MDA-MB231 cells underwent morphological changes.
As shown in Fig. 2B, cells
treated with lower doses (125–250 µg/ml) of FVPEO appeared
elongated, as compared with untreated cells. As the dose of FVPEO
increased, typical morphological changes of apoptotic cells (i.e.
cell shrinkage and roundness) appeared and a marked reduction in
cell number was observed.
A growth inhibition effect was also observed when
FVPEO was tested on MCF7 cells, an estrogen- and
progesterone-positive breast cancer cell line (Fig. 2). Following incubation with FVPEO,
the cell viability declined in a dose- and time-dependent manner,
and cells showed clear signs of death, supporting the anti-cancer
potential of FVPEO.
FVPEO-induced cytotoxic effect is
counteracted by the antioxidant N-acetylcysteine (NAC) and is
accompanied by ROS generation
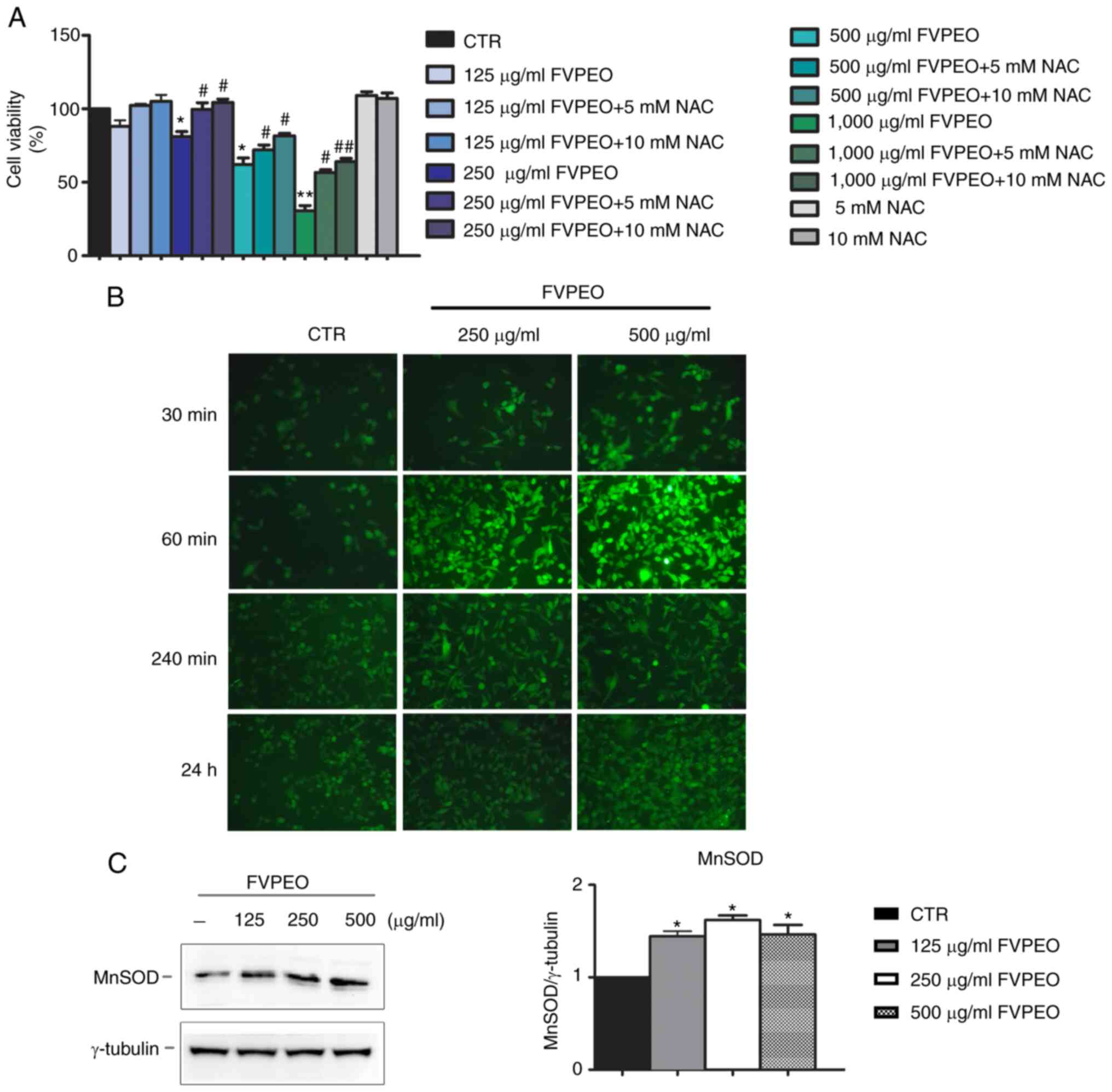
Next, it was explored whether the cytotoxic effect
of FVPEO was dependent on oxidative stress. To this end, MDA-MB231
cells were pre-incubated for 2 h with NAC, a ROS scavenger;
different doses of FVPEO were then added for another 24 h. Our data
demonstrated that the addition of NAC counteracted the cytotoxic
effect of FVPEO. In particular, as shown in Fig. 3A, 10 mM NAC prevented the
cytotoxic effect induced by low concentrations (125–250 µg/ml) of
FVPEO and notably reduced that exerted by high concentrations
(500–1,000 µg/ml). To better explore these effects, the generation
of ROS by H2-DCFDA, a fluorochrome that binds ROS and emits green
fluorescence in its oxidized form, was also evaluated. Using this
experimental approach, a clear rise in green fluorescence,
indicative of ROS production in FVPEO-treated cells, was observed
(Fig. 3B). The increase, which
had already appeared at 30 min of incubation with 250 and 500 µg/ml
FVPEO, peaked at 60 min after application. Western blot analysis
was also performed to evaluate whether FVPEO treatment modified the
level of MnSOD, one of the main cellular antioxidant enzymes
(24). As shown in Fig. 3C, an increased level of MnSOD was
observed following FVPEO incubation.
FVPEO cytotoxic effect is mediated by
oxidative stress and the upregulation of stress-associated
proteins
In light of the observed data demonstrating ROS
production in MDA-MB231-treated cells, it was explored whether the
cytotoxic effect induced by FVPEO can be accompanied by the
activation of stress-related proteins (Fig. 4).
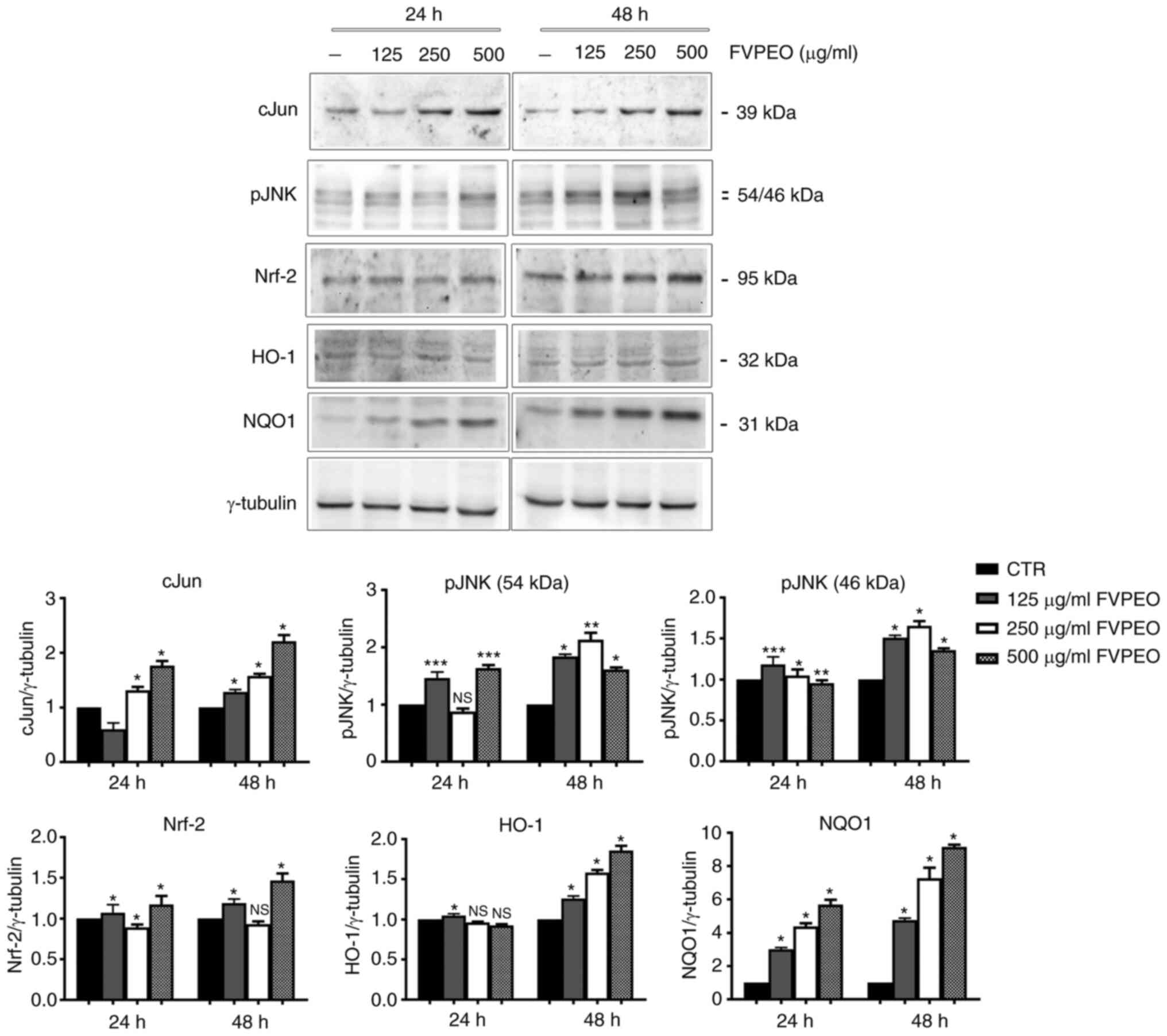 | Figure 4.FVPEO upregulates the level of stress
proteins and antioxidant factors. Cell lysates of MDA-MB231 cells
were prepared following incubation with FVPEO for 24 and 48 h
respectively. The expression of stress proteins (c-Jun and pJNK),
as well as that of antioxidant factors (Nrf2, HO-1 and NQO1), were
analyzed using western blotting using specific antibodies, as
reported in the Materials and methods section. *P<0.05,
**P<0.01, ***P<0.001 vs. control. NS, not significant; FVPEO,
essential oil of Foeniculum vulgare subsp. piperitum
fruits; CTR, control; Nrf-2, nuclear factor erythroid 2-related
factor 2; HO-1, heme oxygenase; NQO1, NAD(P)H quinone
oxidoreductase 1. |
First, the level of c-Jun and pJNK was analysed.
c-Jun is a member of the activating protein transcription factor
that can be activated in response to different extracellular
stimuli, such as pro-inflammatory cytokines, ultraviolet radiation
and several different forms of cellular stress (25). Its activation has been correlated
to the signalling of JNKs, a family of stress-mediated kinases
capable of integrating many different cellular stimuli, including
mitogenic signals, environmental stresses and different apoptotic
insults (26).
The present data provided evidence that FVPEO
treatment caused a modest increase in pJNK, but a consistent
increase in the c-Jun level that was already visible at 24 h at a
dose of 250 µg/ml and further increased with longer periods of
incubation.
The involvement of stress in FVPEO-treated cells was
also confirmed by the upregulation of NF-E2 p45-related factor 2
(Nrf2), a transcription factor that has been considered one of the
main regulatory factors of redox homeostasis that controls a
battery of detoxification and cytoprotective genes (27). In our experimental conditions, an
increase in Nrf-2 content that was associated to an upregulation of
its target genes HO-1 and NQO1 was observed in FVPEO-treated
cells.
FVPEO induces apoptosis in MDA-MB231
cells
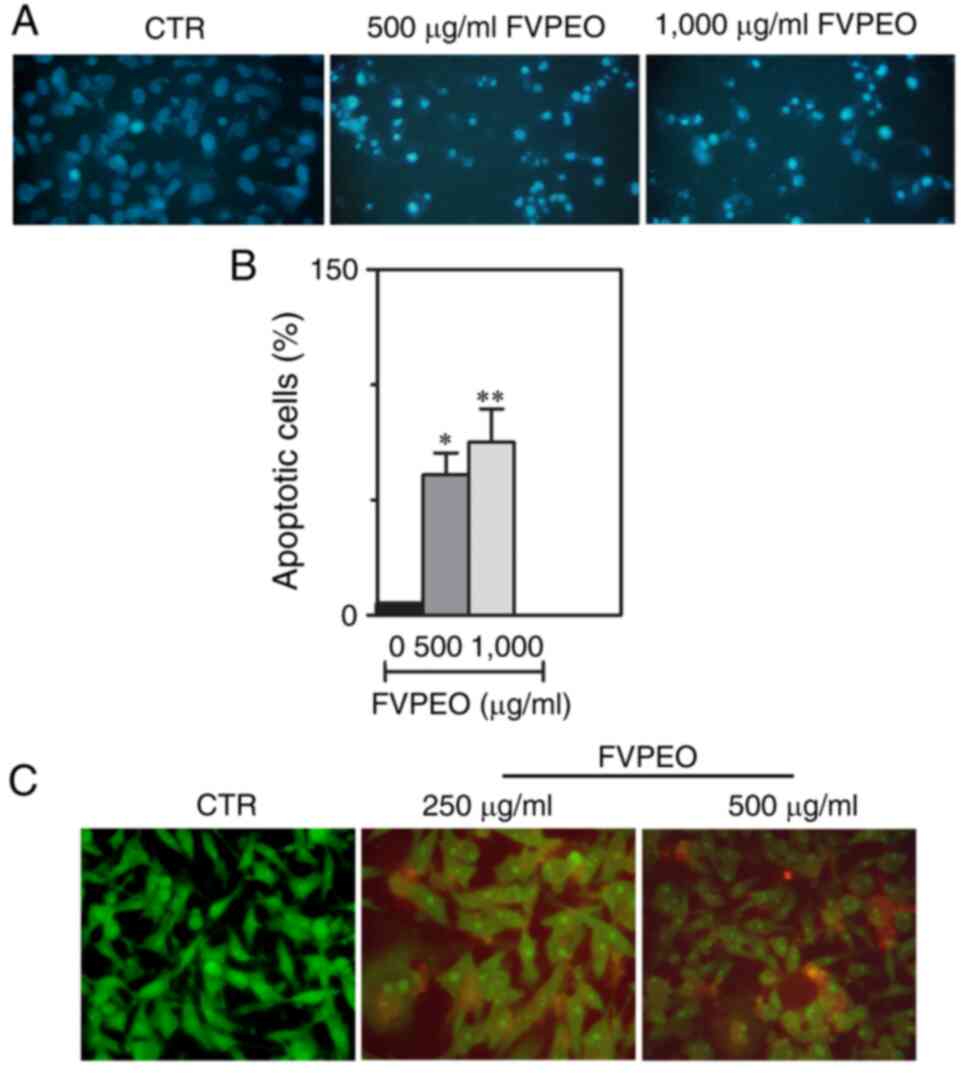
To investigate whether the loss of viability of
MDA-MB231 cells under FVPEO treatment was due to the induction of
apoptosis, cells were stained with Hoechst 33342, a fluorescent dye
that binds to DNA and can identify nuclear apoptotic changes.
According to fluorescence microscopy images, MDA-MB231 cells
treated with FVPEO exhibited a clear nuclear fragmentation and
condensation, as compared with untreated control cells (Fig. 5A). The proportion of cells with
condensed and fragmented nuclei increased as the FVPEO dose
increased (Fig. 5B). Such an
effect was also confirmed by acridine orange/ethidium bromide dual
staining showing the presence of typical morphological features of
apoptosis in MDA-MB231-treated cells (Fig. 5C). Indeed, some FVPEO-treated
cells were positive for a yellow-green acridine orange nuclear
staining (early apoptotic cells), with a considerable proportion of
them (~45%) showing a concentrated orange nuclear ethidium bromide
staining (late apoptotic cells).
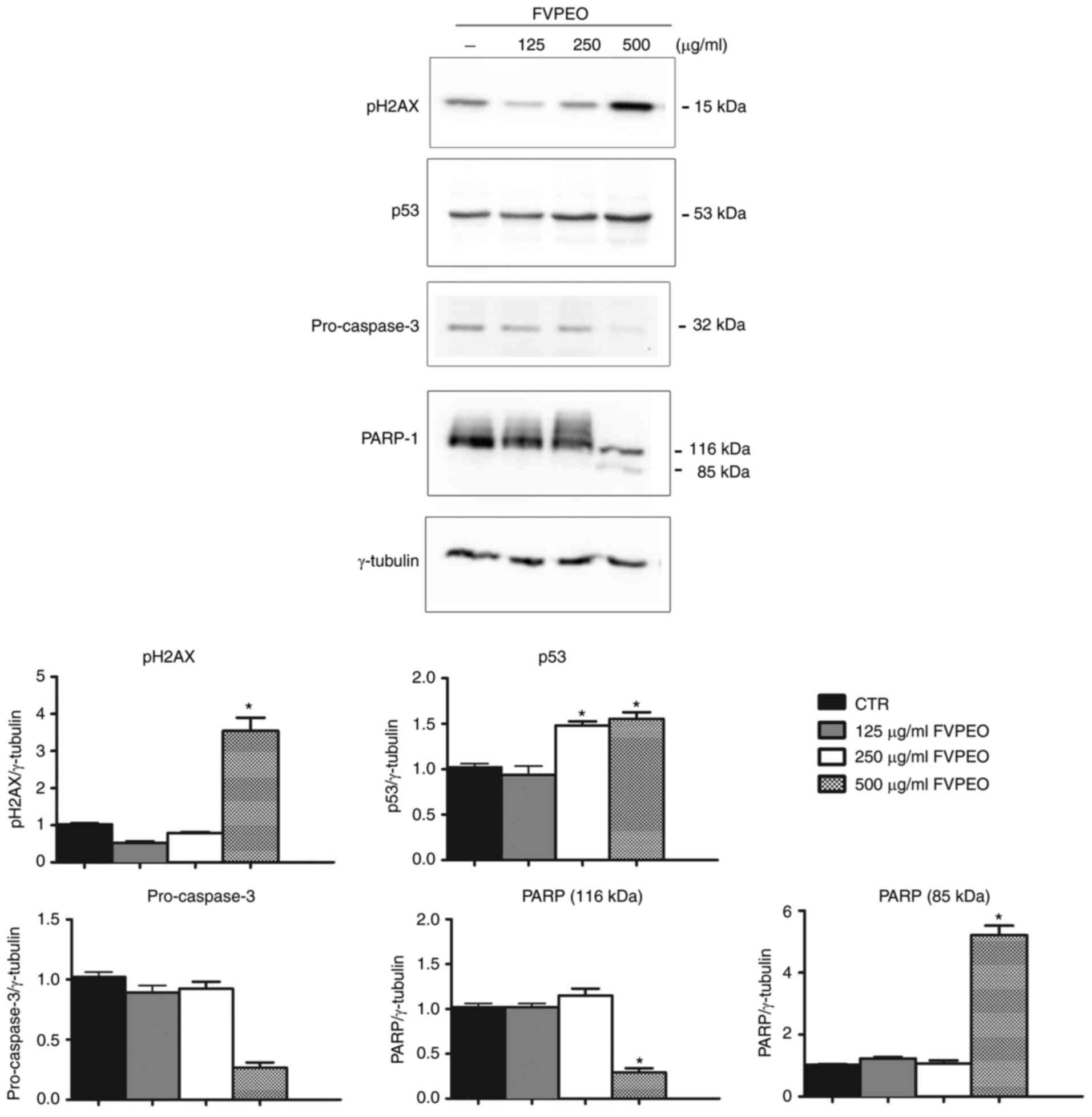
FVPEO induces a p53-dependent
intrinsic apoptotic pathway in MDA-MB231 cells
To further explore the underlying mechanism of
FVPEO-induced apoptosis, it was examined whether the observed
effects and association with DNA injury can be linked to the
recruitment of DNA damage markers. When a double-strand break
occurs in DNA, alteration in chromatin structure promotes the
phosphorylation of the histone variant H2AX at the Ser-139 residue,
a form known as γH2AX (28). This
event is induced by the kinases ataxia-telangiectasia mutated,
ataxia-telangiectasia mutated and Rad3-related protein and
DNA-dependent protein kinase, allowing the formation of H2AX
phosphorylated on serine 139, which is considered a marker of DNA
damage (29). The present data
showed that the treatment of MDA-MB231 cells with FVPEO induced a
strong phosphorylation of H2AX at Ser139 in a dose-dependent manner
(Fig. 6).
Next, possible changes in the level of p53 protein,
a key factor involved in the induction of apoptosis in response to
DNA damage, was examined (30).
As shown in Fig. 6, the level of
p53 markedly increased in MDA-MB231-treated cells compared with the
untreated control.
In the present study, the effect of FVPEO on
caspase-3, a key mediator of apoptosis of mammalian cells, whose
activation by the cleavage of procaspase-3 is responsible for
chromatin condensation, was also analysed (31). These results indicated that FVPEO
treatment induced a dose-dependent decrease in the inactive
procaspase-3, indicating the activation of caspase-3 (Fig. 6). This suggestion was confirmed by
the cleavage of PARP-1, a well-known target of caspase-3 (32), which was observed following FVPEO
treatment. In combination, these data indicated that FVPEO induced
caspase-dependent apoptosis triggered by DNA damage.
Discussion
Nowadays, the global increase in the number of
cancer cases renders the research on specific and targeted medical
therapies urgent. Particular attention has been paid on the plant
kingdom as a possible bio-resource for new phytochemicals that can
be used as preventative or protective compounds in cancer
therapies, either alone or in combination (33–35). Indeed, since their discovery, a
vast array of plant-derived phytochemicals, such as etoposide,
taxol, doxorubicin, topotecan, irinotecan and camptotecin, have
been identified as valuable and highly effective chemotherapeutics
routinely used in clinical practice (36). Over three quarters of anti-cancer
chemotherapeutics currently used in medicine are natural products
or their analogues, chemically modified with active pharmacophores
to enhance their anti-tumor potential (37).
In light of this, the present study was conducted to
evaluate the possible anti-cancer properties of EOs of F.
vulgare subsp. piperitum fruits (FVPEO) grown on the
Sicilian rural areas. Our previous studies highlighted the
composition of the most abundant secondary metabolites present in
FVPEO (12) and since no data are
available on the biological activity of the FVPEO grown in Sicily
thus far, the aim of the present study was to investigate whether
it can exert anti-proliferative effects in TNBC cells.
Breast cancer is one of the most common tumors
affecting women; its incidence tends to rise with age (median age
at diagnosis is 63 for breast cancer patients in United States) and
it is the second leading cause of death (38,39). Based on the presence or absence of
estrogen, progesterone and HER2 receptor, this tumor has been
classified into three distinct subtypes with different incidence
rates among women. In particular, the hormone receptor
positive/Erb-B2 receptor tyrosine kinase 2 (ERBB2) negative form
affects up to 70% of patients, the ERBB2 positive form affects
15–20% of cases, and the TNBC subtype, characterized by the lack of
all receptors, affects ~15% of patients (40). TNBC is a particularly concerning
type of breast cancer, as it is the most aggressive type with a
highly invasive profile that is associated with a poor prognosis
and high resistance to the most common cancer therapies, which
renders its treatment challenging (41).
The present data demonstrated that FVPEO induced a
marked reduction in cell viability in TBNC cells and that effect
was associated with oxidative injury, as evidenced by a consistent
ROS generation. As is well known, ROS are highly reactive molecules
that, when produced at a physiological level in the cell, can
participate in intracellular signaling functioning as redox
messenger (42). However, the
extensive ROS generation is dangerous for the cells, since their
production gets around intracellular scavenger systems, triggering
cell death (43). In accordance
with these observations, the present data provided evidence that
FVPEO treatment induced ROS production, upsurged stress-related
proteins such as c-Jun, pJNK and antioxidant defense systems
represented by Nrf-2 and its targets, MnSOD and NQO1. Of note,
FVPEO induced a dose-dependent effect on pJNK upregulation at 24 h.
Differently, for longer times of exposure (48 h) the maximum effect
of FVPEO on the level of this stress-related factor was observed
with 250 µg/ml, probably because cells underwent apoptotic cell
death at the higher dose.
Nrf-2 is a transcription factor that, under stress
conditions, travels from the cytosol to the nucleus, where it
promotes the basal and stress-inducible expression of a plethora of
cytoprotective enzymes (44)
involved in glutathione metabolism (components of
glutamate-cysteine ligase complex), thioredoxin antioxidant-based
response (thioredoxin, sulfiredoxin), ROS and xenobiotic
detoxification (NQO1, glutathione peroxidase 2 and several
glutathione S-transferases) and iron metabolism (HO-1).
In our experimental conditions, Nrf-2 upregulation
induced by FVPEO treatment was associated with an increased level
of HO-1 and NQO1.
NQO1 has been described as a putative anti-tumor
factor (45) involved in ROS
removal, so that the application of phytochemicals or plant-derived
compounds to promote NQO1 upregulation has been indicated as a
putative chemopreventive strategy for cancer (46). These findings seem to sustain the
mode of action of FVPEO in the breast cancer cells used in the
present study. Indeed, it was demonstrated that FVPEO promoted a
marked DNA condensation and fragmentation through caspase-3
activation and PARP-1 fragmentation. The cell death induced by
FVPEO appears to be correlated with ROS increase, as suggested by
the observation that the effect of FVPEO on the reduction of cell
viability was counteracted by the anti-oxidant NAC. In response to
FVPEO-induced DNA damage, apoptotic cell death was accompanied by
p53 and γH2AX upregulation, two typical markers of DNA damage. In
accordance with Patino-Morales data (47) the increase in the NQO1 and p53
level appeared to be closely linked to one another. These authors
demonstrated the existence of a tight interplay between NQO1 and
p53, aimed at stabilizing p53 half-life and favoring its role in
the induction of apoptotic cell demise.
Mechanistic studies by El-Garawani et al
(48) reported that a combination
of oils of F. vulgare and P. graveolens exerts a
marked cytotoxic effect in breast cancer MCF-7 cells through cell
cycle arrest, while no cytotoxicity was observed in normal human
peripheral blood lymphocytes in vitro. The cytotoxic effect
was attributed by El-Garawani to anethole and estragole, which were
identified as the main constituents of these EOs.
In conclusion, the present data suggested that FVPEO
exerts a marked apoptotic effect on triple-negative breast cancer
cells, which appears to be correlated with ROS increase, whose
level increases the ability of antioxidant systems, such as Nrf2,
HO-1 and NQO1, to counteract them. The increase in the level of the
antioxidant enzyme NQO1 could also favor p53 stabilization induced
by DNA damage, thus contributing to apoptotic cell death.
Acknowledgements
Not applicable.
Funding
This work was supported by a grant from MIUR-ITALY PRIN 2017
(grant no. 2017A95NCJ) and partially sustained by the Fondo
Finalizzato per la Ricerca di Ateneo FFR 2018/2021
(FFR-D15-D'ANNEO, Università degli Studi di Palermo, Palermo,
Italy).
Availability of data and materials
The data used and/or analyzed during the present
study are available from the corresponding authors on reasonable
request.
Authors' contributions
ADA, ML, AM and MB conceived and designed the
experiments. ML, NB and GDDA conducted all the experiments. ML, AM
and ADA acquired and analyzed the data. ML, AM and ADA wrote and
revised the manuscript. ML and ADA confirm the authenticity of all
the raw data. All authors read and approved the final version of
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
FVPEO
|
essential oil of Foeniculum
vulgare subsp. piperitum fruits
|
|
EOs
|
essential oils
|
|
TNBC
|
triple-negative breast cancer
|
References
|
1
|
Badgujar SB, Patel VV and Bandivdekar AH:
Foeniculum vulgare Mill: A review of its botany,
phytochemistry, pharmacology, contemporary application, and
toxicology. Bio Med Res Int. 2014:8426742014.
|
|
2
|
Garg C, Khan SA, Ansari SH, Suman A and
Garg M: Chemical composition, therapeutic potential and
perspectives of Foeniculum vulgare. Pharmacogn Rev.
3:346–352. 2009.
|
|
3
|
He W and Huang B: A review of chemistry
and bioactivities of a medicinal spice: Foeniculum vulgare.
J Med Plants Res. 5:3595–3600. 2011.
|
|
4
|
Díaz-Maroto MC, Pérez-Coello MS, Esteban J
and Sanz J: Comparison of the volatile composition of wild fennel
samples (Foeniculum vulgare Mill.) from central Spain. J
Agric Food Chem. 54:6814–6818. 2006. View Article : Google Scholar
|
|
5
|
Guillén MD and Manzanos MJ: A study of
several parts of the plant Foeniculum vulgare as a source of
compounds with industrial interest. Food Res Int. 29:85–88. 1996.
View Article : Google Scholar
|
|
6
|
Guarrera PM and Savo V: Perceived health
properties of wild and cultivated food plants in local and popular
traditions of Italy: A review. J Ethnopharmacol. 146:659–680. 2013.
View Article : Google Scholar
|
|
7
|
Rahimi R and Ardekani MR: Medicinal
properties of Foeniculum vulgare Mill. in traditional
Iranian medicine and modern phytotherapy. Chin J Integr Med.
19:73–79. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Amini F, Marzban M and Salehi A: The
effect of Foeniculum vulgare on dysmenorrhea; a systematic
review. Planta Med. 82 (Suppl 1):S1–S381. 2016. View Article : Google Scholar
|
|
9
|
Guarrera PM, Salerno G and Caneva G: Folk
phytotherapeutical plants from Maratea area (Basilicata, Italy). J
Ethnopharmacol. 99:367–378. 2005. View Article : Google Scholar
|
|
10
|
Musa Özcan M and Claude Chalchat J: Effect
of collection time on chemical composition of the essential oil of
Foeniculum vulgare subsp. piperitum growing wild in
Turkey. Eur Food Res Technol. 224:279–281. 2006. View Article : Google Scholar
|
|
11
|
Muckensturm B, Foechterlen D, Reduron JP,
Danton P and Hildenbrand M: Phytochemical and chemotaxonomic
studies of Foeniculum vulgare. Biochem Syst Ecol.
25:353–358. 1997. View Article : Google Scholar
|
|
12
|
Ilardi V, Badalamenti N and Bruno M:
Chemical composition of the essential oil from different vegetative
parts of Foeniculum vulgare subsp. piperitum (Ucria)
Coutinho (Umbelliferae) growing wild in sicily. Nat Prod Res. 1–11.
2021.(Epub ahead of print). View Article : Google Scholar
|
|
13
|
Tripathi P, Tripathi R, Patel RK and
Pancholi SS: Investigation of antimutagenic potential of
Foeniculum vulgare essential oil on cyclophosphamide induced
genotoxicity and oxidative stress in mice. Drug Chem Toxicol.
36:35–41. 2013. View Article : Google Scholar
|
|
14
|
Pradhan M, Sribhuwaneswari S, Karthikeyan
D, Minz S, Sure P, Chandu AN, Mishra U, Kamalakannan K,
Saravanankumar A and Sivakumar T: In-vitro cytoprotection activity
of Foeniculum vulgare and helicteres isora in cultured human
blood lymphocytes and antitumour activity against B16F10 melanoma
cell line. Res J Pharm Technol. 1:450–452. 2008.
|
|
15
|
Elkady AI: Anethole inhibits the
proliferation of human prostate cancer cells via induction of cell
cycle arrest and apoptosis. Anticancer Agents Med Chem. 18:216–236.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ke W, Zhao X and Lu Z: Foeniculum
vulgare seed extract induces apoptosis in lung cancer cells
partly through the down-regulation of Bcl-2. Biomed Pharmacother.
135:1112132021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ke W, Wang H, Zhao X and Lu Z:
Foeniculum vulgare seed extract exerts anti-cancer effects
on hepatocellular carcinoma. Food Funct. 12:1482–1497. 2021.
View Article : Google Scholar
|
|
18
|
Mohamad RH, El-Bastawesy AM, Abdel-Monem
MG, Noor AM, Al-Mehdar HA, Sharawy SM and El-Merzabani MM:
Antioxidant and anticarcinogenic effects of methanolic extract and
volatile oil of fennel seeds (Foeniculum vulgare). J Med
Food. 14:986–1001. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lo Galbo V, Lauricella M, Giuliano M,
Emanuele S, Carlisi D, Calvaruso G, De Blasio A, Di Liberto D and
D'Anneo A: Redox imbalance and mitochondrial release of apoptogenic
factors at the forefront of the antitumor action of mango peel
extract. Molecules. 26:43282021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu K, Liu PC, Liu R and Wu X: Dual AO/EB
staining to detect apoptosis in osteosarcoma cells compared with
flow cytometry. Med Sci Monit Basic Res. 21:15–20. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pratelli G, Carlisi D, D'Anneo A, Maggio
A, Emanuele S, Palumbo Piccionello A, Giuliano M, De Blasio A,
Calvaruso G and Lauricella M: Bio-waste products of Mangifera
indica L. reduce adipogenesis and exert antioxidant effects on
3T3-L1 cells. Antioxidants (Basel). 11:3632022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lauricella M, Lo Galbo V, Cernigliaro C,
Maggio A, Palumbo Piccionello A, Calvaruso G, Carlisi D, Emanuele
S, Giuliano M and D'Anneo A: The anti-cancer effect of Mangifera
indica L. peel extract is associated to γH2AX-mediated
apoptosis in colon cancer cells. Antioxidants (Basel). 8:4222019.
View Article : Google Scholar
|
|
23
|
Hero T, Bühler H, Kouam PN,
Priesch-Grzeszowiak B, Lateit T and Adamietz IA: The
triple-negative breast cancer cell line MDA-MB 231 is specifically
inhibited by the ionophore salinomycin. Anticancer Res.
39:2821–2827. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kahl R, Kampkötter A, Wätjen W and
Chovolou Y: Antioxidant enzymes and apoptosis. Drug Metab Rev.
36:747–762. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Meng Q and Xia Y: c-Jun, at the crossroad
of the signaling network. Protein Cell. 2:889–898. 2011. View Article : Google Scholar
|
|
26
|
Chen YR, Meyer CF and Tan TH: Persistent
activation of c-Jun N-terminal kinase 1 (JNK1) in gamma
radiation-induced apoptosis. J Biol Chem. 271:631–634. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bellezza I, Giambanco I, Minelli A and
Donato R: Nrf2-Keap1 signaling in oxidative and reductive stress.
Biochim Biophys Acta Mol Cell Res. 1865:721–733. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao H, Huang X, Halicka HD and
Darzynkiewicz Z: Detection of histone H2AX phosphorylation on
Ser-139 as an indicator of DNA damage. Curr Protoc Cytom.
89:e552019.PubMed/NCBI
|
|
29
|
Burma S, Chen BP, Murphy M, Kurimasa A and
Chen DJ: ATM phosphorylates histone H2AX in response to DNA
double-strand breaks. J Biol Chem. 276:42462–42467. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen J: The cell-cycle arrest and
apoptotic functions of P53 in tumor initiation and progression.
Cold Spring Harb Perspect Med. 6:a0261042016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Porter AG and Jänicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chaitanya GV, Steven AJ and Babu PP:
PARP-1 cleavage fragments: Signatures of cell-death proteases in
neurodegeneration. Cell Commun Signal. 8:312010. View Article : Google Scholar
|
|
33
|
Lauricella M, Emanuele S, Calvaruso G,
Giuliano M and D'Anneo A: Multifaceted health benefits of
Mangifera indica L. (Mango): The inestimable value of
orchards recently planted in sicilian rural areas. Nutrients.
9:5252017. View Article : Google Scholar
|
|
34
|
Emanuele S, Notaro A, Palumbo Piccionello
A, Maggio A, Lauricella M, D'Anneo A, Cernigliaro C, Calvaruso G
and Giuliano M: Sicilian litchi fruit extracts induce autophagy
versus apoptosis switch in human colon cancer cells. Nutrients.
10:14902018. View Article : Google Scholar
|
|
35
|
Allegra M, D'Anneo A, Frazzitta A, Restivo
I, Livrea MA, Attanzio A and Tesoriere L: The phytochemical
indicaxanthin synergistically enhances cisplatin-induced apoptosis
in HeLa cells via oxidative stress-dependent p53/p21waf1
axis. Biomolecules. 10:9942020. View Article : Google Scholar
|
|
36
|
Demain AL and Vaishnav P: Natural products
for cancer chemotherapy. Microb Biotechnol. 4:687–699. 2014.
View Article : Google Scholar
|
|
37
|
Dholwani KK, Saluja AK, Gupta AR and Shah
DR: A review on plant-derived natural products and their analogs
with anti-tumor activity. Indian J Pharmacol. 40:492008. View Article : Google Scholar
|
|
38
|
Ataollahi MR, Sharifi J, Paknahad MR and
Paknahad A: Breast cancer and associated factors: A review. J Med
Life. 8:6–11. 2015.PubMed/NCBI
|
|
39
|
Fahad Ullah M: Breast cancer: Current
perspectives on the disease status. Ahmad A; Breast Cancer
Metastasis and Drug Resistance, : Advances in Experimental Medicine
and Biology. 1152. Springer; Cham: pp. 51–64. 2019, View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Waks AG and Winer EP: Breast cancer
treatment: A review. JAMA. 321:288–300. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Medina MA, Oza G, Sharma A, Arriaga LG,
Hernández Hernández JM, Rotello VM and Ramirez JT: Triple-negative
breast cancer: A review of conventional and advanced therapeutic
strategies. Int J Environ Res Public Health. 17:20782020.
View Article : Google Scholar
|
|
42
|
Emanuele S, D'Anneo A, Calvaruso G,
Cernigliaro C, Giuliano M and Lauricella M: The double-edged sword
profile of redox signaling: Oxidative events as molecular switches
in the balance between cell physiology and cancer. Chem Res
Toxicol. 31:201–210. 2018. View Article : Google Scholar
|
|
43
|
Ghosh N, Das A, Chaffee S, Roy S and Sen
CK: Reactive oxygen species, oxidative damage and cell death.
Chatterjee S, Jungraithmayr W and Bagchi D: Immunity and
Inflammation in Health and Disease; Emerging Roles of
Nutraceuticals and Functional Foods in Immune Support. Elsevier;
pp. 45–55. 2018
|
|
44
|
Tonelli C, Chio IIC and Tuveson DA:
Transcriptional regulation by Nrf2. Antioxid. Redox Signal.
29:1727–1745. 2018. View Article : Google Scholar
|
|
45
|
Mizumoto A, Ohashi S, Kamada M, Saito T,
Nakai Y, Baba K, Hirohashi K, Mitani Y, Kikuchi O, Matsubara J, et
al: Combination treatment with highly bioavailable curcumin and
NQO1 inhibitor exhibits potent antitumor effects on esophageal
squamous cell carcinoma. J Gastroenterol. 54:687–698. 2019.
View Article : Google Scholar
|
|
46
|
Braicu C, Mehterov N, Vladimirov B,
Sarafian V, Nabavi SM, Atanasov AG and Berindan-Neagoe I:
Nutrigenomics in cancer: Revisiting the effects of natural
compounds. Semin Cancer Biol. 46:84–106. 2017. View Article : Google Scholar
|
|
47
|
Patiño-Morales CC, Soto-Reyes E,
Arechaga-Ocampo E, Ortiz-Sánchez E, Antonio-Véjar V,
Pedraza-Chaverri J and García-Carrancá A: Curcumin stabilizes p53
by interaction with NAD(P)H:Quinone oxidoreductase 1 in
tumor-derived cell lines. Redox Biol. 28:1013202020. View Article : Google Scholar
|
|
48
|
El-Garawani I, El Nabi SH, Nafie E and
Almeldin S: Foeniculum vulgare and pelargonium graveolens
essential oil mixture triggers the cell cycle arrest and apoptosis
in MCF-7 cells. Anticancer Agents Med Chem. 19:1103–1113. 2019.
View Article : Google Scholar : PubMed/NCBI
|