Introduction
Excessive bone destruction often occurs in
bone-related diseases, such as osteoporosis, rheumatoid arthritis,
and osteomyelitis (1).
Osteoclasts are the only cells that can degrade old bones (2). Thus, osteoclasts are considered to
be clinically important. Many researchers have attempted to
identify new drugs that target osteoclasts for the treatment of
bone loss (3).
Osteoclasts are multinucleated monocyte/macrophage
lineage cells (4). The downstream
signaling pathways of macrophage colony-stimulating factor (M-CSF)
and receptor activator of NF-κB ligand (RANKL) are essential for
osteoclastic differentiation (5).
Upon RANKL stimulation in osteoclast precursors, the recruitment of
cytoplasmic TNF receptor-associated factors (TRAFs) triggers the
activation of downstream signaling pathways, such as nuclear factor
κB (NF-κB) and MAPKs [p38, c-Jun N-terminal kinase, and
extracellular signal-regulated kinase (ERK)] (6,7).
These signaling cascades induce the activation of transcription
factors, such as c-Fos and nuclear factor of activated T cells
(NFATc1) (8–10). NFATc1 is the master transcription
factor for osteoclastogenesis and is involved in the induction of
osteoclast-specific marker genes (11,12).
Coriandrum sativum L. (CSL) is an aromatic
herb that belongs to the Apiaceae family (13). The fresh leaves of CSL are known
as cilantro and have been employed as medicine (14). Although CSL has long been used as
a traditional remedy to treat digestive problems, hyperglycemia,
and other disorders (13,14), its regulatory effect on bone
metabolism has not been investigated. Therefore, we aimed to
determine the effect of the ethanol extract of the aerial part
(stem and leaf) of CSL on osteoclast differentiation in
vitro and in vivo.
Materials and methods
Reagents
The ethanol extract of CSL (reference no. 154) was
provided by the National Institute of Horticultural and Herbal
Science (Jeollabuk-do, Korea). Briefly, the aerial portion of CSL
was extracted with 99.99% ethyl alcohol at 85°C using an
accelerated solvent extractor. The extract was subsequently
filtered and concentrated using a rotary evaporator (JP-SD1000,
Eyela, Tokyo Rikikatai, Japan). The final extract was dissolved in
dimethyl sulfoxide (Sigma-Aldrich) and then diluted in phosphate
buffered saline (PBS). Antibodies against ERK, phospho-ERK, p38,
phosphor-p38, IκB, β-actin, GAPDH and c-Fos were obtained from Cell
Signaling Technology. The antibody against NFATc1 was purchased
from Santa Cruz Biotechnology. All other reagents were purchased
from Sigma-Aldrich.
Co-culture system
All animal experiments were performed in accordance
with the NIH Guide for the Care and Use of Laboratory Animals and
the Association for Assessment and Accreditation of Laboratory
Animal Care of Sookmyung Women's University. For cell harvest, mice
were sacrificed by CO2 (50% vol/min) asphyxiation
followed by cervical dislocation. Primary calvarial osteoblasts
were extracted from the calvariae of neonatal ICR mice (Samtako
Inc.), as previously described (15). Bone marrow cells were extracted
from the long bones of 4- to 6-week-old ICR male mice. To examine
osteoclast differentiation, mouse bone marrow cells
(1×105 cells) were co-cultured with calvarial
osteoblasts (5×103 cells) and 1,25-dihydroxyvitamin
D3 (1,25-(OH)2D3, 10 nM) in the
presence or absence of the ethanol extract of CSL in 96-well
culture plates (Corning). After six days of culture, the cells were
fixed and then stained with tartrate-resistant acid phosphatase
(TRAP) staining solution [0.01% naphthol AS-MX phosphate
(Sigma-Aldrich) and 0.06% Fast Red Violet LB Salt (Sigma-Aldrich)
in 50 mM sodium tartrate dehydrate and 45 mM sodium acetate, at pH
5.0]. TRAP-positive (TRAP+) multinucleated cells (>3
nuclei/cell) were counted as mature osteoclasts.
Bone marrow-derived macrophage (BMM)
culture system
Mice were sacrificed by CO2 (50% vol/min)
asphyxiation followed by cervical dislocation. Bone marrow cells
were extracted from the long bones of 8- to 10-week-old ICR mice
(Samtako Inc., Osan, Korea). Bone marrow cells were cultured for
three days in the presence of M-CSF (30 ng/ml; PeproTech Inc.) to
generate BMMs. To assess osteoclast differentiation, BMMs were
treated with the ethanol extract of CSL, M-CSF (30 ng/ml), and
RANKL (100 ng/ml; PeproTech Inc.). After four days, the cells were
fixed and stained using TRAP.
Cell cytotoxicity assay
Cell viability was determined using the MTT assay.
BMMs (1×104 cells/well) were seeded in a 96-well plate
and incubated with M-CSF (30 ng/ml, R&D) and the ethanol
extract of CSL in α-MEM for 48 h. Thereafter, the MTT solution was
added, and culture was allowed to proceed in the dark. After 5 h,
solubilization buffer (10% SDS in 0.01 M HCl) was added and the
cells were cultured overnight. The viability of the BMMs was
determined by measuring the optical density at 570 nm.
RNA extraction and polymerase chain
reaction (PCR) assay
Total RNA was purified using Easy-Blue (iNtRON
Biotechnology Inc.). cDNA was synthesized from 5 µg of RNA using
the Revert Aid™ first-strand cDNA synthesis kit (iNtRON
Biotechnology Inc., Korea) and amplified reverse
transcription-quantitative PCR (RT-qPCR) or RT-PCR. The primers for
the osteoclastogenic genes were as follows: RANKL:
5′-CCAAGATCTCTAACATGACG-3′ (forward), 5′-CACCATCAGCTGAAGATAGT-3′
(reverse); OPG: 5′-ACGGACAGCTGGCACACCAG-3′ (forward),
5′-CTCACACACTCGGTTGTGGG-3′ (reverse); calcitonin receptor
(CTR): 5′-TTTCAAGAACCTTAGCTGCCAGAG-3′ (forward),
5′-CAAGGCACGGACAATGTTGAGAAG-3′ (reverse); cathepsin K (CTK):
5′-CTTCCAATACGTGCAGCAGA-3′ (forward), 5′-ACGCACCAATATCTTGCACC-3′
(reverse); GAPDH: 5′-AACGGATTTGGTCGTATTGGG-3′ (forward),
5′-CAGGGGTGCTAAGCAGTTGG-3′ (reverse); and β-actin,
5′-TTTGATGTCACGCACGATTTCC-3′ (forward),
5′-TGTGATGGTGGGAATGGGTCAG-3′ (reverse). RT-qPCR analysis was
performed using SYBR® Green PCR Master Mix (Applied
Biosystems) according to the manufacturer's instructions.
Thermocycling was performed using a 7500 Real-time PCR System
(Applied Biosystems) with the following cycling conditions: initial
hold, 95°C for 10 min; followed by 40 cycles of denaturation at
95°C for 15 sec, annealing at 58°C, and extension at 60°C for 1
min. An index mRNA level was assessed using a threshold cycle value
and normalized against glyceraldehyde 3-phosphate dehydrogenase
(GAPDH) expression. The RT-PCR conditions for CTR, cathepsin K, and
β-actin were as follows: an initial denaturation at 94°C for 3 min,
followed by 28 cycles (CTR) or 22 cycles (cathepsin K, β-actin) of
denaturation at 94°C for 30 sec, annealing at 58°C for 45 sec, and
extension at 72°C for 60 sec; and a final extension at 72°C for 10
min.
Western blot analysis
Total cell lysates were separated by SDS-PAGE and
transferred onto Immobilon-P membranes (Millipore). The membranes
were blocked with BSA in PBS-Tween (PBS-T); immunostained with
anti-phospho-ERK (1:1,000), anti-ERK (1:1,000), anti-phospho p38
(1:1,000), anti-p38 (1:1,000), anti-IκB (1:1,000), anti-NFATc1
(1:200), anti-c-Fos (1:1,000), and anti-β-actin (1:4,000); and
incubated with a horseradish peroxidase-conjugated secondary
antibody (1:5,000). The membranes were developed using an advanced
chemiluminescence detection kit (Amersham Biosciences).
Bone resorption assay
BMMs were differentiated on dentine slices in the
presence of M-CSF (30 ng/ml) and RANKL (100 ng/ml) for four days
and then treated with the ethanol extract of CSL for two days. The
dentine slices were stained with toluidine blue (1 µg/ml) (J.T.
Baker). The number of resorption pits on the dentine slices was
counted.
Osteoclast formation in vivo
Mice were housed in specific pathogen-free (SPF)
conditions under a 12 h light/dark cycle. Mice were provided ad
libitum access to food and water. The experiments are
terminated if the mice reach the humane end points; weight loss
≥10% compared with control group and severe necrosis at the
injection site. ICR mice (12-week-old) were injected s.c. with
vehicle (PBS) or lipopolysaccharide (LPS, 0.5 mg) over the
calvarial bone. Mice were also administered daily i.p. injections
of the ethanol extract of CSL (50 mg/kg) (dissolved in DMSO and
corn oil) or vehicle beginning on day 1. On day 6, mice were
euthanized by CO2 (50% vol/min) asphyxiation followed by
cervical dislocation. Whole calvaria was extracted and fixed with
4% paraformaldehyde for 24 h and then stained with TRAP. Image
analysis was performed using ImageJ software (version 1.32;
National Institutes of Health) according to the manufacturer's
protocol.
Statistical analysis
Data are presented as mean ± standard deviation (SD)
of three independent experiments and were analyzed with Prism 6
software (GraphPad Inc.). Comparisons between two and multiple
groups were performed with the unpaired Student's t-test and
one-way analysis of variance (ANOVA) with a post-hoc Tukey test,
respectively. P<0.05 was considered to indicate a statistically
significant difference.
Results
CSL inhibits osteoclast
differentiation and bone resorption
First, we determined whether the ethanol extract of
CSL modulates osteoclast differentiation using a mouse co-culture
system. In co-cultures of mouse bone marrow cells and osteoblasts,
the presence of 1,25(OH)2D3 induced mature
TRAP-positive (TRAP+) multinucleated osteoclasts (MNCs).
However, the differentiation of these MNCs was substantially
inhibited by treatment with the ethanol extract of CSL (10 µg/ml)
(Fig. 1A). Osteoblasts express
both RANKL and osteoprotegerin (OPG), and the ratio of RANKL to OPG
is known to be critical for osteoclast differentiation (16,17). As the ethanol extract of CSL
exerted an inhibitory effect on osteoclast formation in the
co-culture system, we further investigated whether the addition of
CSL could alter the ratio of RANKL to OPG in osteoblasts. As shown
in Fig. 1B, RT-qPCR revealed that
treatment with 1,25(OH)2D3 increased the mRNA
expression ratio of RANKL to OPG, which was slightly altered by the
addition of CSL. These findings suggest that the ethanol extract of
CSL does not modulate the ratio of RANKL and OPG mRNAs in
osteoblasts.
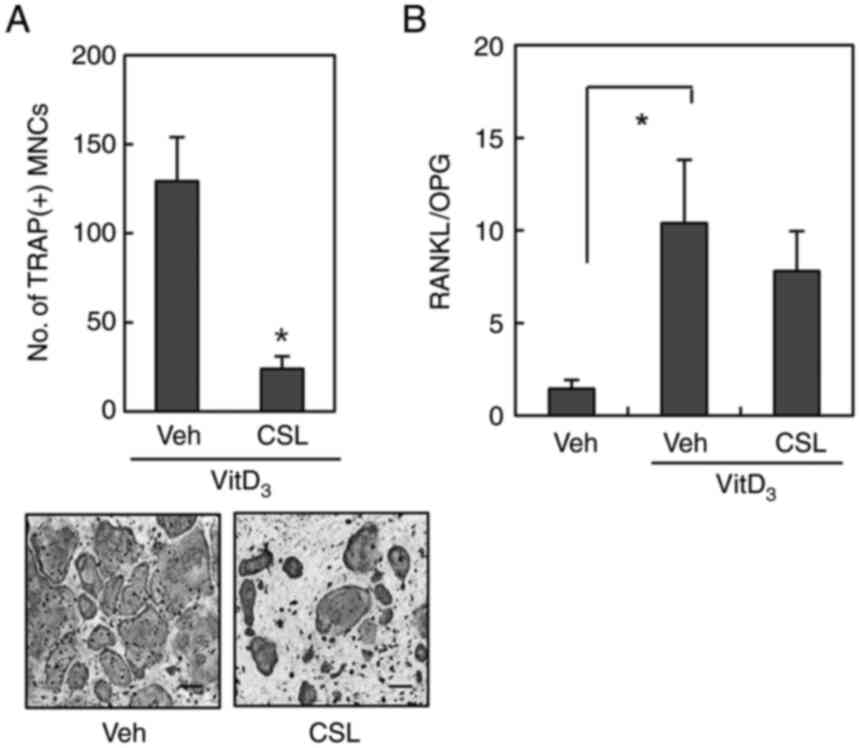 | Figure 1.CSL suppresses osteoclast formation in
a co-culture system. (A) Mouse bone marrow cells and primary
osteoblasts were co-cultured with either Veh or the ethanol extract
of CSL (10 µg/ml) in the presence of
1α,25-(OH)2D3 (10 nM) for six days. After
TRAP staining, TRAP+ MNCs containing three or more
nuclei were counted as osteoclasts. Scale bar, 200 µm. (B) Mouse
primary osteoblasts were pretreated with the ethanol extract of CSL
(10 µg/ml) for 30 min and then cultured with VitD3 for 24 h. The
mRNA expression level was determined using reverse
transcription-quantitative polymerase chain reaction. Data are
expressed as the mean ± SD of at least three independent
experiments. *P<0.05 vs. Veh. CSL, Coriandrum sativum L.; TRAP,
tartrate-resistant acid phosphatase; Veh, vehicle; VitD3,
1α,25-(OH)2D3; OPG, osteoprotegerin; MNCs,
multinucleated osteoclasts. |
As the expression level of RANKL/OPG was not
associated with the inhibitory effect of CSL on osteoclast
formation, we proceeded to determine the effects of CSL on
osteoclastogenesis using primary mouse bone marrow cells. Bone
marrow cells were cultured with M-CSF to generate BMMs, and further
cultured with M-CSF and RANKL for differentiation into osteoclasts.
TRAP staining revealed that the ethanol extract of CSL
significantly decreased the number TRAP+-MNCs in a
dose-dependent manner (Fig. 2A).
Further, the MTT assay showed that the maximum concentration of CSL
(10 µg/ml) exerted minor effects on cell viability, suggesting that
the anti-osteoclastogenic effect of CSL was not attributable to
cellular toxicity (Fig. 2B).
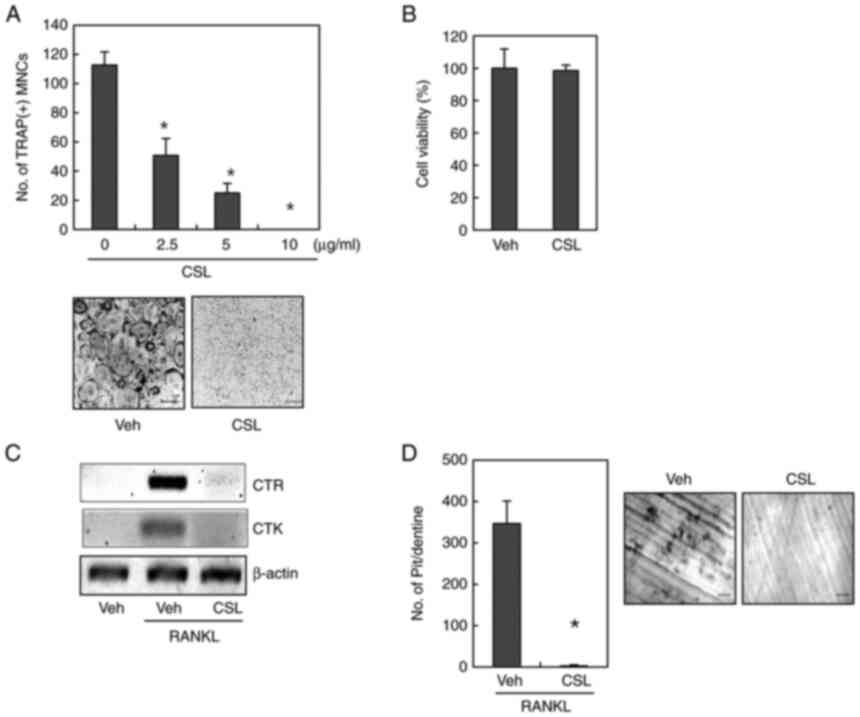 | Figure 2.CSL suppresses RANKL-induced
osteoclast formation and bone resorption. (A) BMMs were incubated
with RANKL (100 ng/ml) and M-CSF (30 ng/ml) in the presence of the
indicated concentration of the ethanol extract of CSL for four
days. After TRAP staining, TRAP+ MNCs were counted. (B)
BMMs were cultured with M-CSF (30 ng/ml) with or without the
ethanol extract of CSL (10 µg/ml) for 48 h; thereafter, an MTT
assay was performed. (C) In BMM cultures, the mRNA expression level
was determined using reverse transcription-polymerase chain
reaction. (D) BMMs were incubated on dentine slices with M-CSF (30
ng/ml) and RANKL (100 ng/ml) for four days followed by the ethanol
extract of CSL (10 µg/ml) for an additional two days. The number of
resorption pits were counted. Scale bar, 200 µm. Data are expressed
as mean ± SD of at least three independent experiments. *P<0.05
vs. 0 µg/ml (A) or Veh (D). CSL, Coriandrum sativum L.; RANKL,
receptor activator of NF-κB ligand; BMMs, bone marrow-derived
macrophages; M-CSF, macrophage colony-stimulating factor; TRAP,
tartrate-resistant acid phosphatase; Veh, vehicle; MNCs,
multinucleated osteoclasts; CTR, calcitonin receptor; CTK,
cathepsin K. |
The expression levels of several marker genes are
upregulated during osteoclast differentiation (11,12). Thus, we further evaluated the
suppressive effect of CSL on osteoclastogenesis by measuring the
mRNA expression levels of osteoclast marker genes, such as
calcitonin receptor and cathepsin K. As shown in
Fig. 2C, the presence of CSL
clearly suppressed the mRNA expression level of calcitonin
receptor and cathepsin K induced by treatment with RANKL
and M-CSF, thereby confirming the anti-osteoclastogenic effect of
CSL.
The main function of active osteoclasts is bone
resorption. As CSL exhibited an anti-osteoclastogenic effect in a
plastic plate, we determined whether the addition of CSL inhibits
the bone-resorbing activity of osteoclasts using dentine slices.
Resorption pits were generated in BMMs in the presence of RANKL and
M-CSF. However, the addition of CSL significantly decreased the
number of pits (Fig. 2D).
Altogether, these results suggests that the ethanol extract of CSL
inhibits the formation of bone-resorbing osteoclasts.
CSL downregulates the RANKL-induced
expression of NFATc1 and c-Fos possibly via the NF-kB and ERK MAPK
pathway
As CSL has been suggested to inhibit RANKL-induced
osteoclast differentiation, we explored the molecular mechanism of
CSL using BMMs. Osteoclast differentiation by RANKL is primarily
regulated by the master transcriptional regulator, NFATc1. c-Fos is
the most well-known positive regulator of NFATc1 (8,10).
Thus, we initially investigated the effects of CSL on the
expression levels of NFATc1 and c-Fos. Treatment with RANKL
stimulated the induction of NFATc1 and c-Fos expression in BMMs,
which was completely abolished by the addition of CSL (Fig. 3A and B). These findings indicate
that the ethanol extract of CSL may inhibit osteoclast
differentiation by downregulating c-Fos and NFATc1 expression.
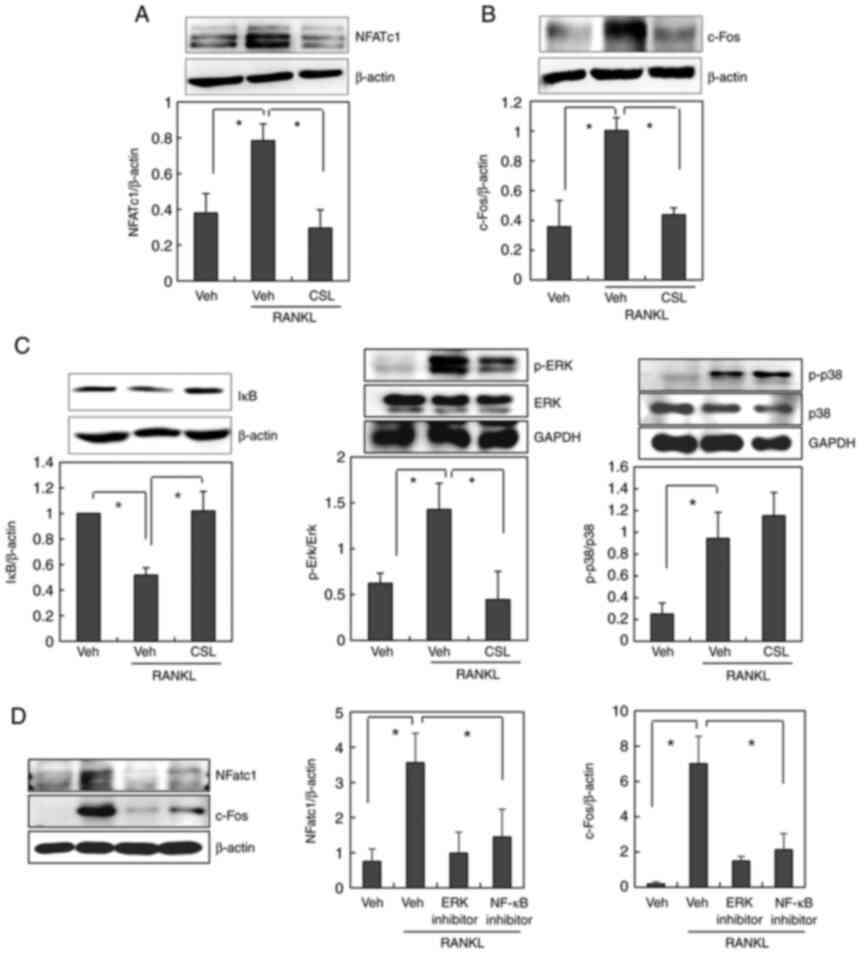 | Figure 3.CSL inhibits the RANKL-induced
expression of c-Fos and NFATc1 via NF-κB and ERK MAPK activation.
(A-C) BMMs were preincubated with or without the ethanol extract of
CSL (10 µg/ml) for 30 min, and then treated with 100 ng/ml of RANKL
for 24 h (for NFATc1 and c-Fos) or 15 min (for IκB, p-ERK and
p-p38). The cell lysates were then subjected to western blot
analysis using (A) NFATc1, (B) c-Fos, (C) IκB, p-ERK, or p-p38
antibodies. (D) BMMs were incubated with or without ERK inhibitor
(U-0126, 10 µM) or NF-κB inhibitor (Bay 11-7082, 10 µM) before
RANKL treatment. The cell lysates were then subjected to western
blot analysis. Data are expressed as mean ± SD of at least three
independent experiments. *P<0.05. CSL, Coriandrum sativum L.;
RANKL, receptor activator of NF-κB ligand; BMMs, bone
marrow-derived macrophages; Veh, vehicle; NFATc1, nuclear factor of
activated T cells; p-, phosphorylated; ERK, extracellular
signal-regulated kinase; NF-κB, nuclear factor-κB. |
Several signaling pathways are involved in
RANKL-induced osteoclast formation. When the involvement of the ERK
and p38 MAPK pathways in the anti-osteoclastogenic effect of CSL
was examined, pretreatment with CSL was found to suppress
RANKL-induced activation of ERK, but not p38 (Fig. 3C). The involvement of the NF-κB
signaling pathways was also assessed by measuring the degradation
level of IκB. As reported previously (18), RANKL stimulates the degradation of
IκB, which implies the activation of NF-κB. Pretreatment with CSL
also suppressed RANKL-induced degradation of IκB (Fig. 3C). We additionally confirmed that
the inhibitor of ERK or NF-kB pathway suppressed RANKL-stimulated
NFATc1 and c-Fos expression (Fig.
3D). Taken together, these results suggest that the ethanol
extract of CSL suppresses RANKL-induced expression of NFATc1 and
c-Fos via the NF-κB and ERK MAPK pathways.
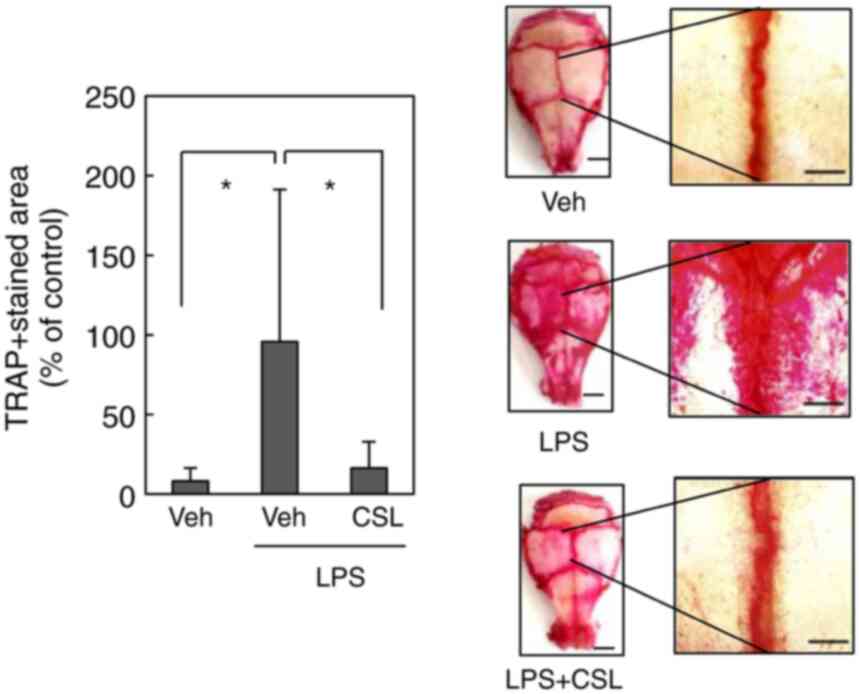
CSL suppresses LPS-induced osteoclast
formation in vivo
As the anti-osteoclastogenic effect of CSL was
observed in vitro, we finally examined the effect of CLS on
osteoclast formation in vivo using a mouse model of bone
destruction (19). Briefly, LPS
was injected into the calvarial bones of mice with or without the
ethanol extract of CSL. TRAP staining of whole calvariae revealed
that the injection of LPS significantly increased the number of
osteoclasts (Fig. 4). Systemic
administration of the ethanol extract of CSL markedly decreased
LPS-induced osteoclast formation in vivo, thereby aligning
with the effects observed in vitro. Altogether, these
results suggest that LPS-induced osteoclast formation was
effectively prevented by the ethanol extract of CSL in
vivo.
Discussion
Metabolic bone disorders are often caused by
excessive bone destruction by hyper-activated osteoclasts. Several
plants have been traditionally used to treat bone loss (20–22); however, little is known regarding
the effects of CSL on bone metabolism. In this study, CSL was
demonstrated to effectively suppress osteoclast differentiation and
bone resorption activity. Among the signaling pathways involved in
osteoclast formation, the addition of CSL abolished the activation
of the NF-κB and ERK MAPK pathways. CSL also inhibited
RANKL-induced NFATc1 expression. As NFATc1 is the master
transcription factor for osteoclast differentiation, CSL is
suggested to modulate osteoclast formation by downregulating the
activation of NF-κB and ERK MAPK and its downstream NFATc1
signaling pathways.
In this study, CSL was found to inhibit osteoclast
formation in co-cultures of mouse primary osteoblasts and bone
marrow cells. CSL was found to have minor effects on the mRNA
expression ratio of RANKL to OPG in osteoblasts treated with
1α,25-(OH)2D3. These findings suggest that
CSL inhibits osteoclast formation by acting directly on osteoclast
precursors. The effect of CSL on bone-forming activity in
osteoblasts needs further verification.
Numerous reports have revealed the therapeutic value
of the aerial parts of C. sativum. These values include
antioxidant and free radical scavenging activities and
metal-chelating and anti-bacterial effects (23,24). Previously, the anti-inflammatory
effect of C. sativum was suggested (25). The aerial part of C.
sativum was found to inhibit LPS-induced iNOS, COX-2, and IL-1β
expression via the MAPK and NF-κB pathways in RAW264.7 macrophage
cells. As LPS stimulates osteoclast formation in RAW264.7
macrophage cells (26), C.
sativum may use this mechanism to suppress osteoclast
formation. The precise molecular mechanism of the inhibitory effect
of CSL on osteoclastogenesis requires further investigation.
According to the previous study, luteolin, vicenin,
ferulic acid, and arbutin were suggested as the main components in
the aerial part of C. sativum (27). Furthermore, rutin was identified
as a major component of C. sativum with anti-inflammatory
properties (25). Luteolin,
ferulic acid, arbutin, and rutin have been reported to prevent bone
loss by inhibiting osteoclast differentiation and function
(28–31). As the whole extract of C.
sativum has been used as a traditional oriental medicine, we
investigated the effect of the crude extract of CSL. However, our
study on the isolation of component of CSL with biological
activities remain limited. A further study is needed to isolate and
validate the components of CSL that prevent osteoclast-related bone
diseases. It would be also of worth to verify the synergistic or
counteractive interaction between the components of CSL with
anti-osteoclastogenic effect.
In conclusion, we reveal that the ethanol extract of
CSL modulates RANKL-induced osteoclast differentiation. This
inhibitory activity of CSL, at least in part, occurs through NF-κB
and ERK MAPK-mediated NFATc1 expression. As many effective drugs
originate from plants, CSL could be a good candidate for the
development of new therapeutics targeting osteoclasts to prevent
various bone diseases.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the National Research
Foundation of Korea (grant no. NRF-2022R1A2C1010419).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
This work was designed by JSS, HYL and MY.
Experiments were performed by JSS and HYL. Data collection,
analysis and interpretation were performed by JS, HL and MY. MY
drafted and revised the manuscript. JSS, HYL and MY confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The animal study protocol was approved by the
Institutional Review Board of Sookmyung Women's University
(approval no. SMWU-IACUC-2109-013).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BMM
|
bone marrow-derived macrophage
|
|
M-CSF
|
macrophage colony-stimulating
factor
|
|
RANKL
|
receptor activator of NF-κB ligand
|
|
OPG
|
osteoprotegerin
|
|
TRAP
|
tartrate-resistant acid
phosphatase
|
|
ERK
|
extracellular signal-regulated
kinase
|
References
|
1
|
Teitelbaum SL: Bone resorption by
osteoclasts. Science. 289:1504–1508. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tanaka Y, Nakayamada S and Okada Y:
Osteoblasts and osteoclasts in bone remodeling and inflammation.
Curr Drug Targets Inflamm Allergy. 4:325–328. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao X, Patil S, Xu F, Lin X and Qian A:
Role of biomolecules in osteoclasts and their therapeutic potential
for osteoporosis. Biomolecules. 11:7472021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boyle WJ, Simonet WS and Lacey DL:
Osteoclast differentiation and activation. Nature. 423:337–342.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Arai F, Miyamoto T, Ohneda O, Inada T,
Sudo T, Brasel K, Miyata T, Anderson DM and Suda T: Commitment and
differentiation of osteoclast precursor cells by the sequential
expression of c-Fms and receptor activator of nuclear factor kappaB
(RANK) receptors. J Exp Med. 190:1741–1754. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Asagiri M and Takayanagi H: The molecular
understanding of osteoclast differentiation. Bone. 40:251–264.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Feng X: RANKing intracellular signaling in
osteoclasts. IUBMB Life. 57:389–395. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Grigoriadis AE, Wang ZQ, Cecchini MG,
Hofstetter W, Felix R, Fleisch HA and Wagner EF: c-Fos: a key
regulator of osteoclast-macrophage lineage determination and bone
remodeling. Science. 266:443–448. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim K, Kim JH, Lee J, Jin HM, Lee SH,
Fisher DE, Kook H, Kim KK, Choi Y and Kim N: Nuclear factor of
activated T cells c1 induces osteoclast-associated receptor gene
expression during tumor necrosis factor-related activation-induced
cytokine-mediated osteoclastogenesis. J Biol Chem. 208:35209–35216.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim JH and Kim N: Regulation of NFATc1 in
osteoclast differentiation. J Bone Metab. 21:233–241. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rho J, Takami M and Choi Y:
Osteoimmunology: Interactions of the immune and skeletal systems.
Mol Cells. 17:1–9. 2004.PubMed/NCBI
|
|
12
|
Walsh MC, Kim N, Kadono Y, Rho J, Lee SY,
Lorenzo J and Choi Y: Osteoimmunology: Interplay between the immune
system and bone metabolism. Annu Rev Immunol. 24:33–63. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Laribi B, Kouki K, M'Hamdi M and Bettaieb
T: Coriander (Coriandrum sativum L.) and its bioactive
constituents. Fitoterapia. 103:9–26. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sahib NG, Anwar F, Gilani AH, Hamid AA,
Saari N and Alkharfy KM: Coriander (Coriandrum sativum L.): A
potential source of high-value components for functional foods and
nutraceuticals-a review. Phytother Res. 27:1439–1456. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Takami M, Cho ES, Lee SY, Kamijo R and Yim
M: Phosphodiesterase inhibitors stimulate osteoclast formation via
TRANCE/RANKL expression in osteoblasts: possible involvement of ERK
and p38 MAPK pathways. FEBS Lett. 31:832–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
O'Brien CA, Gubrij I, Lin SC, Saylors RL
and Manolagas SC: STAT3 activation in stromal/osteoblastic cells is
required for induction of the receptor activator of NF-kappaB
ligand and stimulation of osteoclastogenesis by gp130-utilizing
cytokines or interleukin-1 but not 1,25-dihydroxyvitamin D3 or
parathyroid hormone. J Biol Chem. 274:19301–19308. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Simonet WS, Lacey DL, Dunstan CR, Kelley
M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, et
al: Osteoprotegerin: A novel secreted protein involved in the
regulation of bone density. Cell. 89:309–319. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Darnay BG, Ni J, Moore PA and Aggarwal BB:
Activation of NF-kappaB by RANK requires tumor necrosis factor
receptor-associated factor (TRAF) 6 and NF-kappaB-inducing kinase.
Identification of a novel TRAF6 interaction motif. J Biol Chem.
274:7724–7731. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ha H, Lee JH, Kim HN, Kim HM, Kwak HB, Lee
S, Kim HH and Lee ZH: alpha-Lipoic acid inhibits inflammatory bone
resorption by suppressing prostaglandin E2 synthesis. J Immunol.
176:111–117. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang J, Zheng H and Ma R: Natural
occurring compounds inhibit osteoclastogenesis via targeting
NFATc1-related signaling pathways. Curr Drug Targets. 21:358–364.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu C, He Y, Xu X, He L, He B and Kong L:
The study of natural compounds targeting RANKL signaling pathway
for the treatment of bone diseases. Curr Drug Targets. 21:344–357.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nicolin V, De Tommasi N, Nori SL,
Costantinides F, Berton F and Di Lenarda R: Modulatory effects of
plant polyphenols on bone remodeling: A prospective view from the
bench to bedside. Front Endocrinol (Lausanne). 10:4942019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Harsha SN and Anilakumar KR: In vitro free
radical scavenging and DNA damage protective property of Coriandrum
sativum L. leaves extract. J Food Sci Technol. 51:1533–1539. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mariezcurrena-Berasain MA,
Velázquez-Garduño G, Marín-Mendoza PM, Pliego AB, Vega Castillo LF,
Carranza BV, Khusro A, Ugbogu EA and Salem AZM: Sensitivity of
Coriandrum sativum extract on bacterial pathogens isolated from
digestive system of rabbits, and its role on in vitro cecal gas
production and fermentation. Microb Pathog. 123:18–23. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu TT, Tsai CW, Yao HT, Lii CK, Chen HW,
Wu YL, Chen PY and Liu KL: Suppressive effects of extracts from the
aerial part of Coriandrum sativum L. on LPS-induced inflammatory
responses in murine RAW 264.7 macrophages. J Sci Food Agric.
90:1846–1854. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yim MJ: The role of toll-like receptors in
osteoclastogenesis. J Bone Metab. 27:227–35. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Oganesyan ET, Nersesyan ZM and Parkhomenko
AY: Chemical composition of the above-ground part of Coriandrum
sativum. Pharm Chem J. 41:149–153. 2007. View Article : Google Scholar
|
|
28
|
Kim TH, Jung JW, Ha BG, Hong JM, Park EK,
Kim HJ and Kim SY: The effects of luteolin on osteoclast
differentiation, function in vitro and ovariectomy-induced bone
loss. J Nutr Biochem. 22:8–15. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Doss HM, Samarpita S, Ganesan R and Rasool
M: Ferulic acid, a dietary polyphenol suppresses osteoclast
differentiation and bone erosion via the inhibition of RANKL
dependent NF-kappaB signalling pathway. Life Sci. 207:284–295.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Omori A, Yoshimura Y, Deyama Y and Suzuki
K: Rosmarinic acid and arbutin suppress osteoclast differentiation
by inhibiting superoxide and NFATc1 downregulation in RAW 264.7
cells. Biomed Rep. 3:483–490. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kyung TW, Lee JE, Shin HH and Choi HS:
Rutin inhibits osteoclast formation by decreasing reactive oxygen
species and TNF-alpha by inhibiting activation of NF-kappaB. Exp
Mol Med. 40:52–68. 2008. View Article : Google Scholar : PubMed/NCBI
|