Introduction
Various food-derived peptides have been shown to be
beneficial for human health (1).
Food-derived opioid peptides, which are categorized as exogenous
bioactive peptides, have been expected to have beneficial effects
on psychosomatic disorders, such as depression, anxiety, pain,
eating disorders, and stress-related disorders, and to have no
notable side effects (2). In
general, exogenous food-derived opioid peptides are obtained by the
enzymatic degradation of food in the intestines, and are therefore
resistant to breakdown by intestinal enzymes (2).
Rubiscolin-6 is derived from
D-ribulose-1,5-bisphosphate carboxylase/oxygenase (rubisco), a key
enzyme in the Calvin−Benson carbon fixation cycle in plants.
Rubiscolin-6 is composed of six amino acids:
Tyr-Pro-Leu-Asp-Leu-Phe (3). The
total mass of the rubisco enzymes is ~0.7 Gt in the terrestrial
environment (~3% of total leaf mass) and ~0.03 Gt in the marine
environment (4), and accounts for
approximately 30–50% of the soluble protein present in the green
leaves of plants (5).
Rubiscolin-6 has been shown to have several beneficial effects,
including anti-nociceptive, memory-enhancing, anxiolytic-like, and
anti-depressant effects (3,6–8).
It has also been shown to increase the consumption of a normal
diet, but decrease that of a high-fat diet (9,10).
Rubiscolin-6 is a δ-opioid peptide, and these activities are
thought to be mediated via δ-opioid receptor agonism. Furthermore,
rubiscolin-6 has been shown to cross the blood-brain barrier
(2–4). It well known that δ-opioid receptor
activation contributes to food intake and gastrointestinal function
(11–15). However, the effect of rubiscolin-6
on intestinal motility has not been determined.
Gastric antral motility is closely connected with
food intake, and appetite regulation involves a balance between
hunger and satiety. The stomach responds to the mechanical stimuli
caused by meal volume and composition and transmits information to
the hypothalamus, which is an important area of brain to control
food intake and gastrointestinal function, via the vagal nerve and
gut hormone secretion (16).
Furthermore, gastric motility, such as fundic compliance (gastric
accommodation) and antral contractions (gastric emptying)
contribute to the regulation of the balance between hunger and
satiety (17). The distal
stomach, including the antrum, influences satiety, which is the
feeling to cause the termination of eating (18). Acyl ghrelin, a peptide produced by
the stomach, induces a fasting motor pattern of antral motility in
the fed state (19,20), whereas des-acyl ghrelin, the
precursor of acyl ghrelin, suppresses a fasting motor pattern of
antral motility in the fasted state (21). In addition, acyl ghrelin
stimulates, and des-acyl ghrelin suppresses food intake (21,22).
In the present study, we aimed to investigate the
effects of rubiscolin-6 on the postprandial antral motility of
conscious mice using a manometric method and on the food intake of
ad libitum-fed and overnight-fasted mice. In addition, we
assessed the effects of a δ-opioid receptor antagonist on the
rubiscolin-6-induced changes in antral motility and food
intake.
Materials and methods
Rubiscolin-6 synthesis and
purification
Rubiscolin-6 (YPLDLF) was synthesized by a
solid-phase fluorenylmethyloxycarbonyl (Fmoc)-based strategy and an
automated peptide synthesizer (Model Pioneer; Thermo Fisher
Scientific), as previously described (23). The crude peptide was purified by
reverse-phase high performance liquid chromatography (HPLC, Delta
600 HPLC System) using a Develosil ODSHG-5 column (2×25 cm; Nomura
Chemical Co., Ltd.). High purity of the purified peptide was
confirmed by analytical HPLC and MALDI-TOF MS analysis.
Animals
Seven-week-old male C57BL/6J mice were purchased
from Japan SLC, Inc. The mice were individually maintained in a
pathogen-free facility at 24±2°C and 50±10% humidity, under a
12-h/12-h light/dark cycle (lights on 07:00 a.m. to 07:00 p.m.),
with ad libitum access to sterile standard chow (3.4 kcal/g;
CE-2, CLEA Japan Inc.) and water, in the animal facility of Kobe
Pharmaceutical University. All the animal protocols were approved
by the Kobe Pharmaceutical University Committee for Animal
Experiments (approval no. 2021-063) and performed in accordance
with the relevant guidelines and regulations.
Catheter implantation for manometric
recording
Catheter implantation was performed as previously
reported (24). The mice were
anesthetized by the intraperitoneal (ip) administration of a
mixture of 0.3 mg/kg of medetomidine (Domitor, Meiji Seika Pharma),
4.0 mg/kg of midazolam (Sandoz), and 5.0 mg/kg of butorphanol
(Vetorphale, Meiji Seika Pharma). A mixed anesthetic agent has been
recommended for animal experiments in Japan, replacing ketamine
(25), which has been categorized
as a narcotic drug by the Japanese Narcotics Control Law and is no
longer easy to access for use in animal experiments in Japan. This
mixed anesthetic agent has been used in servals (26), cats (27), and dogs (28) outside of Japan. A silicone tube
(ID 0.3, OD 0.6 mm; Access Technologies) was inserted into the
stomach through a small incision in the gastric body, and the tip
was placed in the gastric antrum. The tube was fixed to the gastric
wall using a purse-string suture, run subcutaneously to emerge in
the dorsal neck, and secured to the skin. After implantation, ip
administration of 0.3 mg/kg of atipamezole (Antisedan; Nippon
Zenyaku Kogyo) was used to reverse the anesthesia and the mice were
allowed to recover in individual cages for 7 days.
Measurement of antral motility and
experimental protocols
The mice were deprived of food for 18 h before the
experiment. On the day of the experiment, they were placed
individually in a black box (150×200×300 mm) with an open top. The
manometric catheter placed in the stomach was connected to an
infusion swivel (375/D/20, Instech Laboratories) on a single-axis
counter-weighted swivel mount (TSB-23, Eicom) to allow free
movement, and then joined to a pressure transducer (DX-100, Nihon
Koden Kogyo). The catheter was then continuously infused with
bubble-free distilled water at 25 µl/h using an infusion pump
(NE-1600, New ERA Pump System). Data were recorded and stored in a
PowerLab (AD Instruments). The basal motor patterns in the antrum
were monitored during the experiments. The mice were given 0.3 g of
laboratory chow, which they all consumed within 30 min. Thirty
minutes after the mice finished eating the pellet, they were
intraperitoneally administered 0.1 ml phosphate-buffered saline
(PBS) pH 7.0 (vehicle), 0.1 or 0.3 mg/kg rubiscolin-6 dissolved in
PBS, 1 mg/kg naltrindole hydrochloride (a δ-opioid receptor
antagonist, 111469-81-9; Tocris Bioscience) in PBS, or a mixture of
0.3 mg/kg rubiscolin-6 and 1 mg/kg naltrindole in PBS. The
percentage motor index (%MI) of the fed motor activity in the
antrum was calculated as (area under the manometric trace for each
20-min period after treatment)/(area under the manometric trace for
the 20-min period immediately before treatment) × 100. Changes in
the mean value of %MI for each 20-min period between 0-20, 20-40,
40-60, 60-80, and 80-100 min after the ip administration of 0.3
mg/kg of rubiscolin-6 or 0.1 ml of vehicle were compared. We
performed catheter implantation in 33 mice to measure the antral
motility, which were maintained without abnormal behavior (e.g.,
immobility, tremors) and euthanized with the inhalation anesthesia
of 6–8% isoflurane (FUJIFUILM Wako Pure Chemical Co.) after the
experiments. The inhalation of anesthesia was maintained until
euthanasia was confirmed by respiratory and cardiac arrest.
Measurement of food intake and
experimental protocols
Measurements of food intake commenced at 10:00 a.m.
in both ad libitum-fed and overnight-fasted mice. Cumulative
food intake was measured 1, 2, 4, and 6 h after the ip
administration of 0.1 ml vehicle, 0.3 mg/kg rubiscolin-6, 1 mg/kg
naltrindole, or a mixture of 0.3 mg/kg rubiscolin-6 and 1 mg/kg
naltrindole. Food intake after the ip administration was also
measured for 30 min in other mice.
Statistical analysis
Data are expressed as mean ± SEM and were analyzed
using Prism software (version 9.3.1.; GraphPad). Multiple groups
were compared using one- or two-way analysis of variance (ANOVA),
followed by Tukey's multiple comparison test. Differences were
considered to be statistically significant when P<0.05.
Results
Effects of rubiscolin-6 on
postprandial motility in antrum
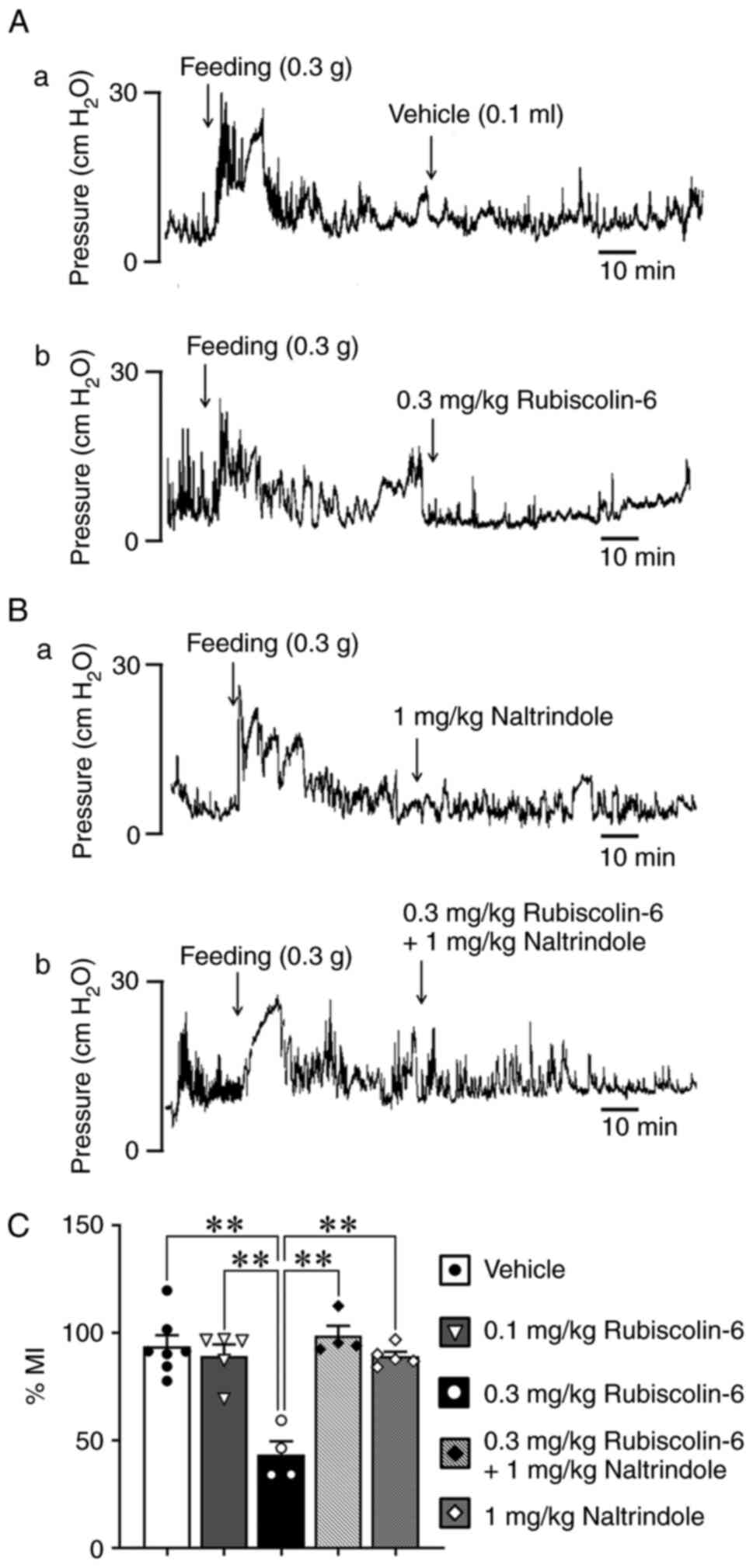
The ip administration of 0.3 mg/kg rubiscolin-6
caused a change in antral motility (Fig. 1Aa, b): the %MI in mice treated
with 0.3 mg/kg rubiscolin-6 was significantly lower than that of
mice treated with vehicle (Fig.
1C). However, no difference in %MI was identified in mice
treated with 0.1 mg/kg of rubiscolin-6. There were no differences
in the traces of mice treated with 1 mg/kg naltrindole, a δ-opioid
antagonist, or the mixture of 0.3 mg/kg rubiscolin-6 and 1 mg/kg
naltrindole (Fig. 1Ba, b): the
%MI of mice treated with the mixture of 0.3 mg/kg rubiscolin and 1
mg/kg naltrindole was similar to that of vehicle-treated mice
(Fig. 1C).
Effect of rubiscolin-6 on food
intake
The cumulative food intake of ad libitum-fed
mice treated with 0.3 mg/kg rubiscolin-6 was significantly higher 4
and 6 h after administration than in the other groups (Fig. 2A). The cumulative food intake of
mice treated with the mixture of 0.3 mg/kg rubiscolin-6 and 1 mg/kg
naltrindole was significantly lower than that of mice treated with
0.3 mg/kg rubiscolin-6 at these time points (Fig. 2A). However, the food intake for 30
min of mice treated with 0.3 mg/kg rubiscolin-6 did not differ from
that of mice treated with vehicle (Fig. 2B). In addition, the food intake
over 30 min of mice treated with the mixture of 0.3 mg/kg
rubiscoln-6 and 1 mg/kg naltrindole was significantly higher than
that of the other groups (Fig.
2B). There were no significant differences among the groups of
fasted mice (Fig. 2C).
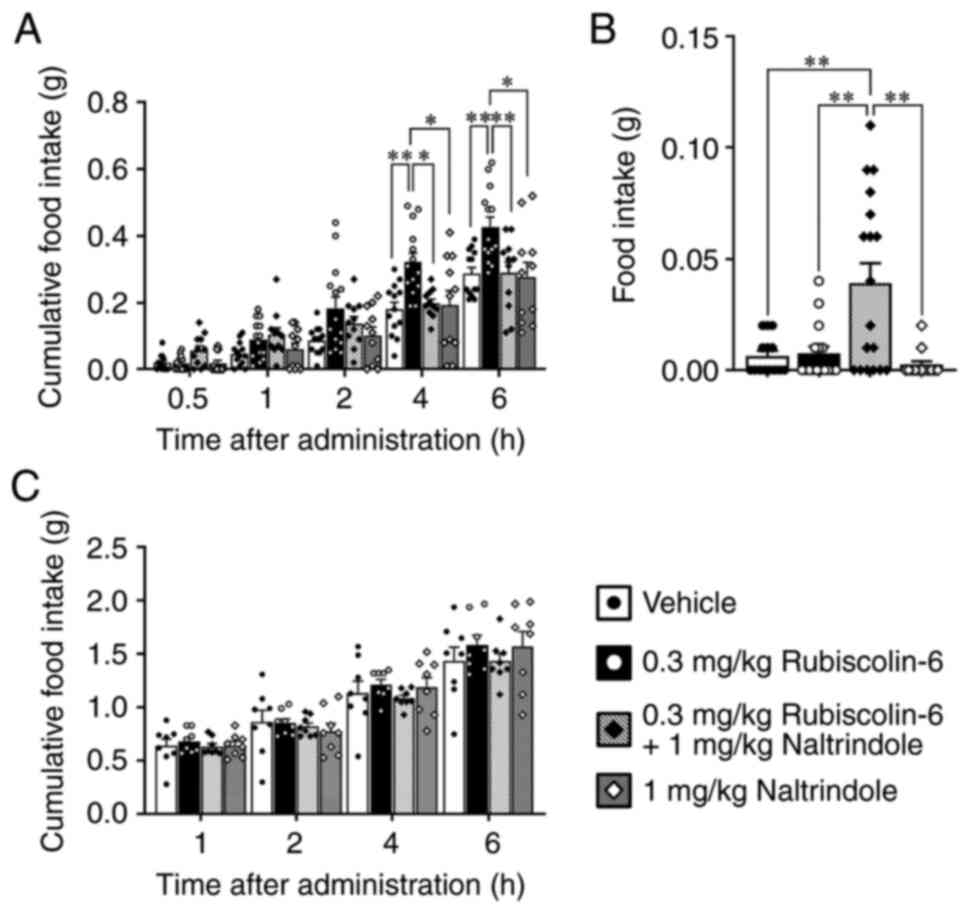 | Figure 2.Effects of rubiscolin-6 and a mixture
of rubiscolin-6 and a δ-opioid receptor antagonist, naltrindole, on
the food intake of mice. (A) Cumulative food intake of ad
libitum-fed mice that underwent intraperitoneal (ip) administration
of vehicle, 0.3 mg/kg rubiscolin-6, 1 mg/kg naltrindole, or a
mixture of 0.3 mg/kg rubscolin-6 and 1 mg/kg naltrindole (n=11-13).
(B) Food intake over 30 min of ad libitum-fed mice that underwent
ip administration of vehicle, 0.3 mg/kg rubiscolin-6, 1 mg/kg
naltrindole, or a mixture of 0.3 mg/kg rubscolin-6 and 1 mg/kg
naltrindole (n=13-18). (C) Cumulative food intake of fasted mice
that underwent ip administration of vehicle, 0.3 mg/kg
rubiscolin-6, 1 mg/kg naltrindole, or a mixture of 0.3 mg/kg
rubscolin-6 and 1 mg/kg naltrindole (n=8). Values are mean ± SEM.
*P<0.05, **P<0.01. |
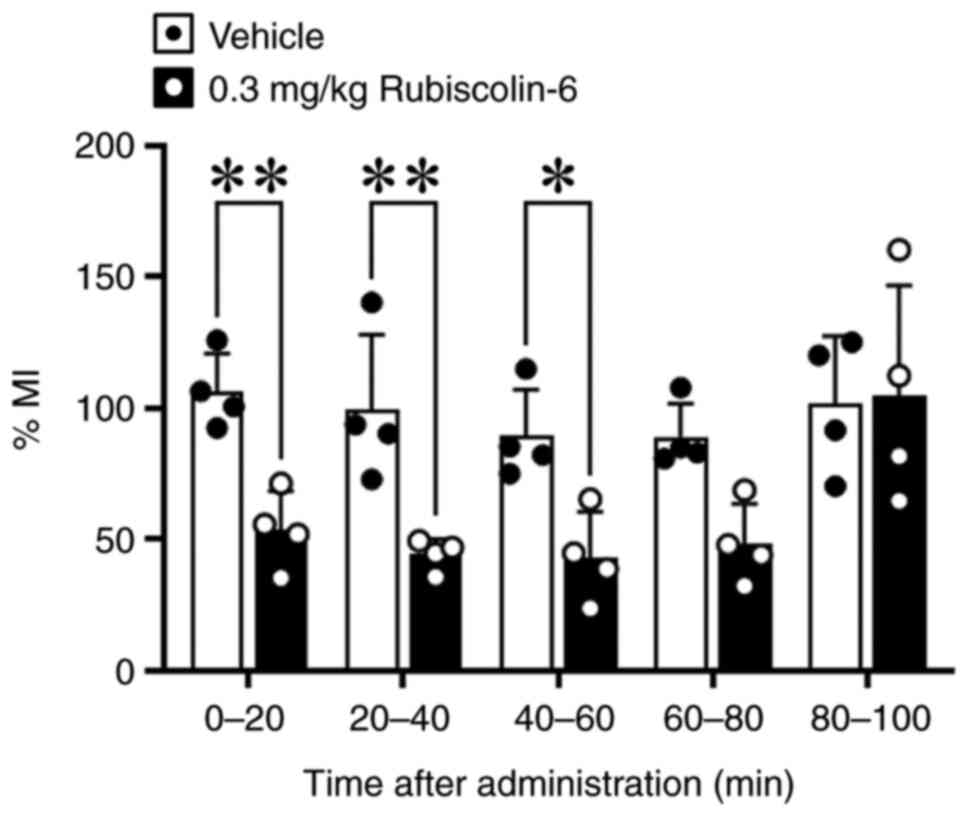
Duration of the inhibitory effect of
rubiscolin-6
Because food intake was high in mice treated with
0.3 mg/kg rubiscolin-6 4 and 6 h after administration, and the food
intake for 30 min after the administration of the mixture of 0.3
mg/kg rubiscolin-6 and 1 mg/kg naltrindole was also high, we
measured %MI over time. We found that %MI was significantly reduced
for each 20-min period between 0-20, 20-40, 40-60 min after the ip
administration of rubiscolin-6 (Fig.
3).
Discussion
Endogenous opioids, such as methionine enkephalin,
leucine enkephalin, β-endorphin, and dynorphin, are expressed in
enteric neurons and mucosal endocrine cells, and these have effects
through µ-, κ-, and δ-opioid receptors (29). Opioid-induced bowel dysfunction is
a well-known adverse effect of opioid use, and opioid-induced
constipation is the most common form of this (15). Many previous studies have
investigated the effect of opioids, especially µ-opioid agonists,
on small intestinal and colonic function, but there have been few
studies of the effects of δ-opioids on stomach motility. Holle and
Steinbach demonstrated that a δ-opioid receptor antagonist, ICI 174
864, increases antral motility in the postprandial state in
conscious dogs (30). In
addition, Ruckebusch et al demonstrated that
D-AIa2-MetS-enkephalinamide (DAMA) and
D-Ala2-D-LeuS-enkephalin (DADLE), which are mixed µ and
δ-opioid agonists, inhibit the motility of the reticulum in sheep
(13). We have shown that
rubiscolin-6 suppresses the postprandial motility of the antrum in
mice, and that this inhibitory effect is reduced by a δ-opioid
receptor antagonist. Thus, rubiscolin-6 inhibits postprandial
antral motility, and this effect may be mediated by the δ-opioid
receptor.
Porreca et al found that the δ-opioid
receptor agonists [D-Pen2, L-Pen5] enkephalin
(DPLPE) and [D-Pen2, D-Pen5] enkephalin
(DPDPE) inhibit gastrointestinal 51Cr transit following
intrathecal administration but not intracerebroventricular (icv)
administration in mice (31). In
addition, Galligan et al demonstrated that icv
administration of DPLPE or DPDPE does not inhibit gastrointestinal
51Cr transit in mice (32). However, Ruckebusch et al
demonstrated that the effects of both µ and δ-opioid opioid
agonists on reticular motility are prevented by the administration
of naloxone, which has high affinity for µ-opioid receptors, but
not by the quaternary parent compound methylnaloxone, which is a
derivative of the opioid receptor antagonist naloxone and cannot
cross the blood−brain barrier (13). Poole et al demonstrated
that δ-opioid receptors localized in the stomach using knock-in
mice carrying a green fluorescent protein linked to Oprd1, which
encodes the δ-opioid receptor (33). In addition, Wittert et al
reported that δ- and κ-opioid receptors, not µ-opioid
receptors, were detected in the stomach, and all three opioid
receptor subtypes were detected in the hypothalamus of rodents
using polymerase chain reaction analysis (34). These results indicate that
δ-opioid receptors in the stomach and µ-receptors in the brain
influence stomach motility. Similarly, the present findings imply
that the inhibitory effect of rubiscolin-6 on antral motility may
be mediated by δ-opioid receptors in the stomach.
Food intake is regulated by the hypothalamus, where
various neurons that secrete orexigenic and anorexigenic peptides
are located. The hypothalamus receives various stimuli from
peripheral tissues via the vagal nerves and hormones secreted into
the blood circulation; e.g., leptin from the adipose tissue,
insulin from the pancreas, and acyl ghrelin from the stomach
(35). Gosnell et al
demonstrated that the icv administration of a selective δ-opioid
receptor agonist, [D-Ser2, Leu5,
Thr6]-enkephalin (DSLET), increases food intake in ad
libitum-fed rats (11).
Israel et al indicated that δ-, κ-, and µ-opioid
receptors contribute to the neuropeptide Y (NPY)-induced food
intake (36). This potent
orexigenic neuropeptide in the hypothalamus regulates feeding
behavior and energy homeostasis (37). Furthermore, Kaneko et al
proposed that the effect of rubiscolin-6 to increase food intake is
mediated by the activation of NPY neurons (10); i.e., the effect of rubiscolin-6 on
food intake might be mediated centrally. However, food intake is
also regulated by gastrointestinal motility (16). Des-acyl ghrelin reduces both
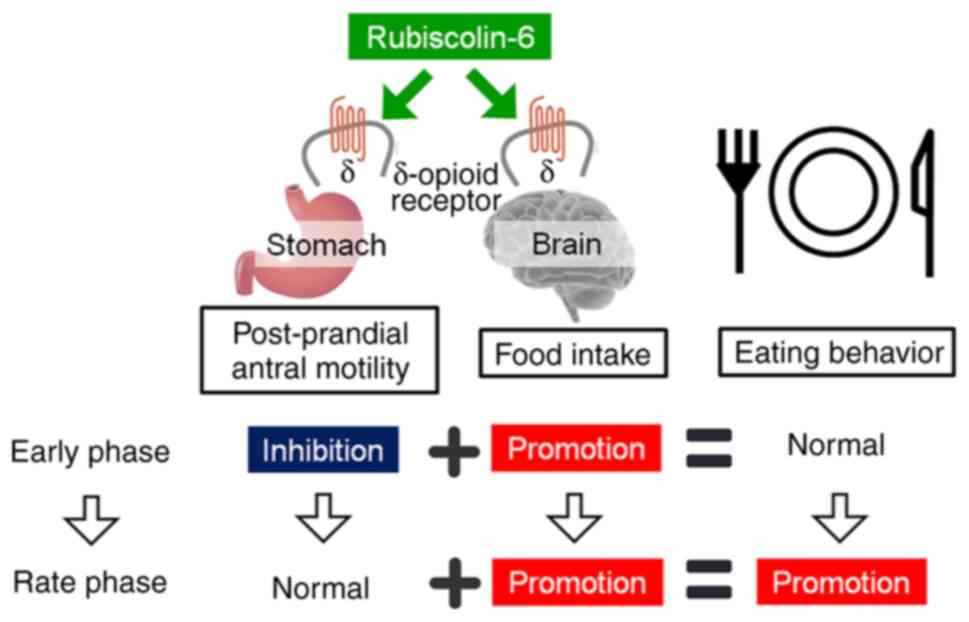
antral motility and food intake (21). In the present study, rubiscolin-6
was shown to significantly reduce postprandial antral motility for
1 h, whereas the orexigenic effect of rubiscolin-6 started to
appear after 2 h, and was significant 4 and 6 h after
administration. In addition, although the administration of
rubiscolin-6 or a δ-opioid receptor antagonist alone had no effect,
a mixture of rubiscolin-6 and δ-opioid receptor antagonist
significantly stimulated food intake for 30 min. These findings
suggest that the inhibitory effect of rubiscolin-6 on antral
motility may predominate over the orexigenic effect and may
contribute to the rapid loss of the effect on food intake. Thus,
the increase in food consumption may appear after the inhibitory
effect on the antral motility disappears (Fig. 4). However, there are a few
limitations of the present study. It is not clear whether the
central effects of rubiscolin-6 are mediated by a direct, neuronal,
or hormonal pathway. The relationship between the inhibitory effect
of rubiscolin-6 on antral motility and its effect on food intake,
and the roles of central and peripheral δ-opioid receptors in these
effects, require further study. The inhibitory effect of
rubiscolin-6 on antral motility may cause stomach discomfort and
upset. The effects of rubiscolin-6 need to be evaluated with
healthy participants and patients with anorexia and cachexia.
We have shown that rubiscolin-6 inhibits
postprandial antral motility for the first time and that it
promotes food intake in the fed state through δ-opioid receptors.
Furthermore, the inhibitory effect of rubiscolin-6 on postprandial
antral motility may delay the appearance of its effect on food
intake. The orexigenic effect of rubiscolin-6 may be applicable to
the treatment of anorexia and cachexia, which are characterized by
severe reductions in food intake and appetite.
Acknowledgements
Not applicable.
Funding
This work was supported in part by grants-in-aid for the Center
for the Advanced Research and Technology of Kobe Pharmaceutical
University from the Association of Private Universities of
Japan.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
IK and AA designed the study. IK synthesized the
peptide. KA carried out all experiments, collected the data,
analyzed the data and wrote the manuscript. IK reviewed the
manuscript. IK, AA and KA confirm the authenticity of all the raw
data. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The animal protocols for this study were approved by
the Kobe Pharmaceutical University Committee for Animal Experiments
(2021–063). Experiments were performed in accordance with the
relevant guidelines and regulations.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chakrabarti S, Guha S and Majumder K:
Food-derived bioactive peptides in human health: Challenges and
opportunities. Nutrients. 10:17382018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tyagi A, Daliri EBM, Ofosu FK, Yeon SJ and
Oh DH: Food-derived opioid peptides in human health: A review. Int
J Mol Sci. 21:88252020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang S, Yunden J, Sonoda S, Doyama N,
Lipkowski AW, Kawamura Y and Yoshikawa M: Rubiscolin, a delta
selective opioid peptide derived from plant Rubisco. FEBS Lett.
509:213–217. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bar-On YM and Milo R: The global mass and
average rate of rubisco. Proc Natl Acad Sci USA. 116:4738–4743.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ellis RJ: The most abundant protein in the
world. Trends Biochem Sci. 4:241–244. 1979. View Article : Google Scholar
|
|
6
|
Yang S, Kawamura Y and Yoshikawa M: Effect
of rubiscolin, a opioid peptide derived from Rubisco, on memory
consolidation. Peptides. 24:325–328. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hirata H, Sonoda S, Agui S, Yoshida M,
Ohinata K and Yoshikawa M: Rubiscolin-6, a delta opioid peptide
derived from spinach Rubisco, has anxiolytic effect via activating
sigma1 and dopamine D1 receptors. Peptides. 28:1998–2003. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mitsumoto Y, Sato R, Tagawa N and Kato I:
Rubiscolin-6, a δ-opioid peptide from spinach RuBisCO, exerts
antidepressant-like effect in restraint-stressed mice. J Nutr Sci
Vitaminol (Tokyo). 65:202–204. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kaneko K, Lazarus M, Miyamoto C, Oishi Y,
Nagata N, Yang S, Yoshikawa M, Aritake K, Furuyashiki T, Narumiya
S, et al: Orally administered rubiscolin-6, a δ opioid peptide
derived from Rubisco, stimulates food intake via leptomeningeal
lipocallin-type prostaglandin D synthase in mice. Mol Nutr Food
Res. 56:1315–1323. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kaneko K, Mizushige T, Miyazaki Y, Lazarus
M, Urade Y, Yoshikawa M, Kanamoto R and Ohinata K: δ-Opioid
receptor activation stimulates normal diet intake but conversely
suppresses high-fat diet intake in mice. Am J Physiol Regul Integr
Comp Physiol. 306:R265–R272. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gosnell BA, Levine AS and Morley JE: The
stimulation of food intake by selective agonists of mu, kappa and
delta opioid receptors. Life Sci. 38:1081–1088. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sudakov SK, Nazarova GA and Alekseeva EV:
Changes in feeding behavior, locomotor activity, and metabolism in
rats upon modulation of opioid receptors in the gastrointestinal
tract. Bull Exp Biol Med. 156:423–425. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ruckebusch Y, Bardon T and Pairet M:
Opioid control of the ruminant stomach motility: Functional
importance of mu, kappa and delta receptors. Life Sci.
35:1731–1738. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Okamoto T, Kurahashi K and Fujiwara M:
Effects of naloxone and opioid agonists on gastric excitatory
responses to stimulation of the vagus nerve in cats. Br J
Pharmacol. 95:329–334. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ketwaroo GA, Cheng V and Lembo A:
Opioid-induced bowel dysfunction. Curr Gastroenterol Rep.
15:3442013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Camilleri M: Peripheral mechanisms in
appetite regulation. Gastroenterology. 148:1219–1233. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Janssen P, Berghe PV, Verschueren S,
Lehmann A, Depoortere I and Tack J: Review article: The role of
gastric motility in the control of food intake. Aliment Pharmacol
Ther. 33:880–894. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sturm K, Parker B, Wishart J,
Feinle-Bisset C, Jones KL, Chapman I and Horowitz M: Energy intake
and appetite are related to antral area in healthy young and older
subjects. Am J Clin Nutr. 80:656–667. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kojima M, Hosoda H, Date Y, Nakazato M,
Matsuo H and Kangawa K: Ghrelin is a growth-hormone-releasing
acylated peptide from stomach. Nature. 402:656–660. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fujino K, Inui A, Asakawa A, Kihara N,
Fujimura M and Fujimiya M: Ghrelin induces fasted motor activity of
the gastrointestinal tract in conscious fed rats. J Physiol.
550:227–240. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen CY, Inui A, Asakawa A, Fujino K, Kato
I, Chen CC, Ueno N and Fujimiya M: Des-acyl ghrelin acts by CRF
type 2 receptors to disrupt fasted stomach motility in conscious
rats. Gastroenterology. 129:8–25. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Asakawa A, Inui A, Kaga T, Yuzuriha H,
Nagata T, Ueno N, Makino S, Fujimiya M, Niijima A, Fujino MA and
Kasuga M: Ghrelin is an appetite-stimulatory signal from stomach
with structural resemblance to motilin. Gastroenterology.
120:337–345. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kairupan TS, Cheng KC, Asakawa A, Amitani
H, Yagi T, Ataka K, Rokot NT, Kapantow NH, Kato I and Inui A:
Rubiscolin-6 activates opioid receptors to enhance glucose uptake
in skeletal muscle. J Food Drug Anal. 27:266–274. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tanaka R, Inui A, Asakawa A, Atsuchi K,
Ataka K and Fujimiya M: New method of manometric measurement of
gastroduodenal motility in conscious mice: Effects of ghrelin and
Y2 depletion. Am J Physiol Gastrointest Liver Physiol.
297:G1028–G1234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kawai S, Takagi Y, Kaneko S and Kurosawa
T: Effect of three types of mixed anesthetic agents alternate to
ketamine in mice. Exp Anim. 60:481–487. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Blignaut CJ, Steenkamp G, Loock D, Emslie
R and Zeiler GE: Immobilization, cardiopulmonary and blood gas
effects of ketamine-butorphanol-medetomidine versus
butorphanol-midazolam-medetomidine in free-ranging serval
(Leptailurus serval). Vet Anaesth Analg. 48:707–715. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Eggers B, Tordiffe ASW, Lamberski N,
Lawrenz A, Sliwa A, Wilson B and Meyer LCR: EVALUATION OF TWO DOSES
OF BUTORPHANOL-MEDETOMIDINE-MIDAZOLAM FOR THE IMMOBILIZATION OF
WILD VERSUS CAPTIVE BLACK-FOOTED CATS (FELIS NIGRIPES). J Zoo Wildl
Med. 51:497–506. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Verstegen J and Petcho A:
Medetomidine-butorphanol-midazolam for anaesthesia in dogs and its
reversal by atipamezole. Vet Rec. 132:353–357. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Holzer P: Opioid receptors in the
gastrointestinal tract. Regul Pept. 155:11–17. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Holle GE and Steinbach E: Different
endogenous opioid effects on delta- and mu-receptor subtypes in
antral and duodenal motility of conscious dogs. Dig Dis Sci.
47:1027–1033. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Porreca F, Mosberg HI, Hurst R, Hruby VJ
and Burks TF: Roles of mu, delta and kappa opioid receptors in
spinal and supraspinal mediation of gastrointestinal transit
effects and hot-plate analgesia in the mouse. J Pharmacol Exp Ther.
230:341–348. 1984.PubMed/NCBI
|
|
32
|
Galligan JJ, Mosberg HI, Hurst R, Hruby VJ
and Burks TF: Cerebral delta opioid receptors mediate analgesia but
not the intestinal motility effects of intracerebroventricularly
administered opioids. J Pharmacol Exp Ther. 229:641–648.
1984.PubMed/NCBI
|
|
33
|
Poole DP, Pelayo JC, Scherrer G, Evans CJ,
Kieffer BL and Bunnett NW: Localization and regulation of
fluorescently labeled delta opioid receptor, expressed in enteric
neurons of mice. Gastroenterology. 141:982–991. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wittert G, Hope P and Pyle D: Tissue
distribution of opioid receptor gene expression in the rat. Biochem
Biophys Res Commun. 218:877–881. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schwartz MW, Woods SC, Porte D Jr, Seeley
RJ and Baskin DG: Central nervous system control of food intake.
Nature. 404:661–671. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Israel Y, Kandov Y, Khaimova E, Kest A,
Lewis SR, Pasternak GW, Pan YX, Rossi GC and Bodnar RJ: NPY-induced
feeding: Pharmacological characterization using selective opioid
antagonists and antisense probes in rats. Peptides. 26:1167–1175.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mercer RE, Chee MJ and Colmers WF: The
role of NPY in hypothalamic mediated food intake. Front
Neuroendocrinol. 32:398–415. 2011. View Article : Google Scholar : PubMed/NCBI
|