Introduction
Allergic rhinitis (AR) is a common disease of the
upper airway that typically manifests as sneezing, nasal
congestion, rhinorrhea and pruritus and affects the quality of life
of patients (1). It is estimated
that 10–40% of the population is affected by AR worldwide (2,3).
Moreover, the incidence of AR is increasing globally every year
(4). In Europe, for example, an
analysis of Polish data showed a rise in the prevalence of AR from
4.8% in 2003 to 7.7% in 2012 (5).
In Asia, a survey of self-reported AR in the general Chinese adult
population demonstrated that the prevalence of AR increased between
2005 and 2011 from 11.1 to 17.6% (6). In addition, the Korean National
Health and Nutrition Examination Survey reported an increase of the
prevalence of AR from 1.0% in 1998 to 17.1% in 2017 (7,8).
Despite increasing research, the specific pathogenesis of AR
remains to be fully elucidated.
Increasing evidence indicates that nasal epithelial
cell dysfunction may be the major cause of nasal allergic
inflammation (9,10). The nasal epithelium is the first
barrier against allergen infiltration and epithelial cell-derived
cytokine milieu activates different types of immune cell, thereby
acting as a master immune regulator in allergic inflammation
(11–13). Thymic stromal lymphopoietin (TSLP)
is one of the primary epithelial-derived cytokines in T helper type
2 (Th2) reactions (14). TSLP
programs dendritic cells to activate the Th2 inflammatory response
and is therefore considered a key epithelial-derived molecule in
the sensitization/priming phase of allergic airway disease
(15,16). Moreover, TSLP activates immune
cells, such as type 2 innate lymphoid cells and basophils, to
sustain Th2 polarized immunity (17). Numerous studies have reported that
NF-κB is a key transcription factor in the regulation of TSLP
expression (18–20). However, the reasons for the
aberrant activation of NF-κB and the ensuing increase in TSLP
expression in AR are still poorly understood.
Ubiquitination is a common post-translational
modification involved in numerous aspects of protein function, such
as degradation, localization and protein-protein interactions
(21). Deubiquitination is the
reverse process mediated by deubiquitinating enzymes (DUBs), that
serve a key role in biological activities, such as inflammation,
signal transduction and the immune response (22,23). Ubiquitin-specific peptidase
(USP)25 is a key deubiquitylase belonging to the USP superfamily
that is primarily involved in inflammation and the immune response
(24,25). TNF receptor-associated factor 3
(TRAF3) is one of the key signal transduction molecules that
regulates activation of the NF-κB signaling pathway. Furthermore,
USP25 serves a key role in regulating NF-κB activation associated
with inflammation or the immune response by altering the
ubiquitination of TRAF3. Zhong et al (26) reported that USP25 may inhibit the
degradation of TRAF3 during activation of the toll-like receptor
(TLR)4 signaling pathway, thereby achieving balance of innate
immune response. Moreover, Lin et al (27) demonstrated that USP25 promotes
antiviral responses via stabilization of TRAF3 expression.
Therefore, the present study evaluated the role of USP25 in AR and
whether USP25 regulates TSLP expression in nasal epithelial
cells.
Materials and methods
Subjects
A total of 25 patients with AR and 15 healthy
volunteers were recruited for the present study at the Renmin
Hospital of Wuhan University (Wuhan, China) between June 2020 and
August 2020. Characteristics of the subjects are presented in
Tables SI and SII. Nasal mucosal samples were obtained
from the inferior turbinate tissue of patients with AR and healthy
volunteers. The diagnosis of AR was based on The Chinese Society of
Allergy Guidelines for Diagnosis and Treatment of Allergic Rhinitis
(28). Inclusion criteria
included being diagnosed with AR, being between the ages of 18–60
years, and having the ability to provide informed consent. The
exclusion criteria were: respiratory infection, uncontrolled
asthma, autoimmune diseases, and receipt of immunotherapy or
corticosteroids within the past month. All participants were
requested to rate the severity of their rhinitis symptoms,
including sneezing, nasal congestion, rhinorrhea and nasal itching,
using the visual analog scale (score, 0–10) (29). The present study was approved by
the Ethics Committee of Renmin Hospital of Wuhan University
(approval no. WDRY2018-K014). All participants provided written
informed consent.
Animals
A total of 30 female wild-type (WT) C57BL/6 mice
(age, 6–8 weeks; weight, 20–25 g) were purchased from Shulaibao
Biotechnology Co., Ltd (http://shulb.com/). A total of 15 female USP25
knockout (KO) C57BL/6 mice (age, 6–8 weeks; weight, 20–25 g) were
donated by Dr Bo Zhong at the State Key Laboratory of Virology,
College of Life Sciences, Wuhan University (Wuhan, China). Mice
were housed in a specific-pathogen-free biohazard containment
facility with a 12/12-h dark/light cycle and moderate humidity
(45–55%) at room temperature (22±1°C) in the Animal Experiment
Center of The Renmin Hospital of Wuhan University. Food and water
were freely available to the mice. All experimental procedures
involving animals were approved by the Animal Care and Use
Committee of The Renmin Hospital of Wuhan University (approval no.
WDRM-20211005).
Ovalbumin (OVA)-induced AR mouse
model
USP25 KO and WT mice were randomly assigned into
control and AR groups (n=6 mice/group). AR mouse model induced by
OVA was established as previously described (30,31). Briefly, mice were treated with
intraperitoneal injection of 300 µl phosphate-buffered saline (PBS)
containing 100 µg OVA (Sigma-Aldrich; Merck KGaA) and 1 mg aluminum
hydroxide on days 0, 7 and 14. On days 21–34, mice were treated
intranasally with 100 mg/ml OVA solution (40 µl/mouse) daily. The
control group was injected with the same dose of PBS and
intranasally treated with 40 µl PBS solution on days 21–34. Nasal
symptoms evaluated in mice included the frequency of sneezes and
nasal rubbing. For 10 min following the last nasal treatment with
OVA, nasal rubbing and sneezing frequency were assessed in each
mouse by two independent, blinded investigators. The mean of all
observations per group was used as the final result. At 24 h
following the last nasal treatment, all mice were anesthetized
using an intraperitoneal injection of sodium pentobarbital (50
mg/kg). Blood samples (1 ml/mouse) were collected from mice under
anesthesia, as well as nasal lavage fluid (NLF) samples (1
ml/mouse). Following blood and NLF sample collection, the mice were
sacrificed using cervical dislocation and the noses were collected
for further analysis.
Histological analysis
Nasal mucosal tissue was fixed using 4%
paraformaldehyde at room temperature for 24 h and embedded in
paraffin. Subsequently, paraffin-embedded tissue was sectioned (5
µm). Sections were stained using hematoxylin and eosin (H&E)
and periodic acid-Schiff (PAS) to evaluate eosinophil infiltration
and goblet cell hyperplasia, respectively. The number of
eosinophils and goblet cells in four randomly selected fields of
view under a light microscope at ×400 magnification was quantified
by two independent investigators and the mean value was
determined.
Immunohistochemistry
Nasal mucosal tissue was fixed with 4%
paraformaldehyde for 24 h at room temperature and embedded in
paraffin for sectioning into 5 µm. The paraffin-embedded sections
were deparaffinized with xylene, rehydrated with graded alcohol and
heated with citrate buffer solution (10 mmol/l, pH 6.0) for 10 min
for antigen retrieval. Subsequently, the slides were soaked in 3%
hydrogen peroxide solution for 15 min at room temperature to quench
the endogenous peroxidase activity. The slides were blocked with
10% normal goat serum (Wuhan Servicebio Technology, Co., Ltd.) for
15 min at room temperature. Then, the slides were incubated with
primary antibodies targeting USP25 (1:200; Santa Cruz
Biotechnology, Inc.) or TSLP (1:500; cat. no. ab188766; Abcam) at
4°C overnight. Following primary incubation, sections were
incubated with horseradish peroxidase (HRP)-conjugated secondary
antibody (1:100; cat. no. sc-2357; Santa Cruz Biotechnology, Inc.)
for 1 h at room temperature. Immunoreactivity was visualized using
3,3-diaminobenzidine (DAB) and hematoxylin was used for
counterstaining at 37°C for 25 sec. Subsequently, slides were
dehydrated, cleaned and sealed. A total of three slides from each
mouse were selected for evaluation. The expression and localization
of USP25 and TSLP in the nasal septum were assessed in four
randomly selected fields of view under a light microscope at ×400
magnification by two independent investigators who were blinded to
sample identities.
ELISA
The protein expression levels of IL-4 (cat. no.
M4000B; R&D Systems, Inc.), IL-5 (cat. no. M5000; R&D
Systems, Inc.), IL-10 (cat. no. M1000B; R&D Systems, Inc.),
IL-13 (cat. no. DY413; R&D Systems, Inc.) and IFN-γ (cat. no.
MIF00; R&D Systems, Inc.) in serum and NLF were assessed using
specific ELISA kits (R&D Systems, Inc.), according to the
manufacturer's protocols. The levels of TSLP in cell culture
supernatant were also assessed using an ELISA kit (cat. no. MTLP00;
R&D Systems, Inc.).
Cell culture and treatment
Human nasal epithelial cells (HNEpCs; RPMI 2650)
were purchased from BeNa Culture Collection (cat. no. BNCC356247;
Beijing Beina Chunglian Institute of Biotechnology). HNEpCs were
cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific,
Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher Scientific,
Inc.) and 1% penicillin-streptomycin (Sigma-Aldrich; Merck KGaA)
and incubated at 37°C in a humidified chamber containing 5%
CO2. Cells were either unstimulated or stimulated with
50 µg/ml house dust mite (HDM) extract (Greer Laboratories, Inc.)
at 37°C for 24 h as previously described (12).
Cell transfection
Small interfering RNA (siRNA) targeting USP25
(si-USP25; antisense, 5′-UAAUUCAGAACUAAUCUUCUA-3′) and TRAF3
(si-TRAF3; antisense, 5′-UAUCCUUAAACACCUUGUCUU-3′) were purchased
from Shanghai GenePharma Co., Ltd. Scrambled sequence siRNA
(Shanghai GenePharma Co., Ltd.) was used as a negative control (NC;
antisense, 5′-GGTACGTCGAGTGAGTTAA-3′). The pcDNA 3.1 plasmids
containing the coding sequences of USP25 (pcDNA-USP25) or TRAF3
(pcDNA-TRAF3) were constructed and purchased from BT Lab. Empty
vectors (Bio-transduction Lab) were used as negative controls.
HNEpCs were seeded into a 6-well plate at a density of
3×105 cells/well. When 70–80% confluence was reached,
siRNAs (10 nM) and plasmids (4 g) were transfected into HNEpCs
alone or in combination using Lipofectamine® 2000
(Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocols. Cells were collected for subsequent experiments 48 h at
37°C following transfection.
Western blotting
Total protein was extracted from cells and nasal
mucosal tissue using RIPA lysis buffer (Wuhan Servicebio
Technology, Co., Ltd.). Protein concentrations were determined
using a bicinchoninic acid kit (Wuhan Servicebio Technology, Co.,
Ltd.). The sample proteins (40 µg/lane) were subjected to
electrophoresis using 10% SDS-PAGE gels. Subsequently, the proteins
were transferred to PVDF membranes (MilliporeSigma) and membranes
were incubated with primary antibodies against USP25 (1:1,000; cat.
no. ab187156; Abcam), TRAF3 (1:1,000; cat. no. ab36988; Abcam),
TSLP (1:1,000; cat. no. ab188766; Abcam) and GAPDH (1:500; cat. no.
sc-47724; Santa Cruz Biotechnology, Inc.) at 4°C overnight.
Following three washes with tris-buffered saline with 0.1%
Tween-20, membranes were incubated with HRP goat anti-rabbit
secondary antibodies (1:10,000; cat. no. GB23303; Wuhan Servicebio
Technology, Co., Ltd.) at room temperature for 1 h. A
Servicebio® super-sensitive enhanced chemiluminescence
(ECL) substrate kit (cat. no. G2020; Wuhan Servicebio Technology,
Co., Ltd.) was used to visualize protein bands and GAPDH was used
as an internal control. Relative protein expression levels were
semi-quantified using ImageJ 1.48v software (National Institutes of
Health).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 8.0 (GraphPad Software, Inc.). All data are presented as the
mean ± standard deviation of a minimum of three independent
experiments. Differences between two groups were assessed using
unpaired Student's t-test. One- or two-way ANOVA followed by
Tukey's post hoc test was used for comparisons between multiple
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
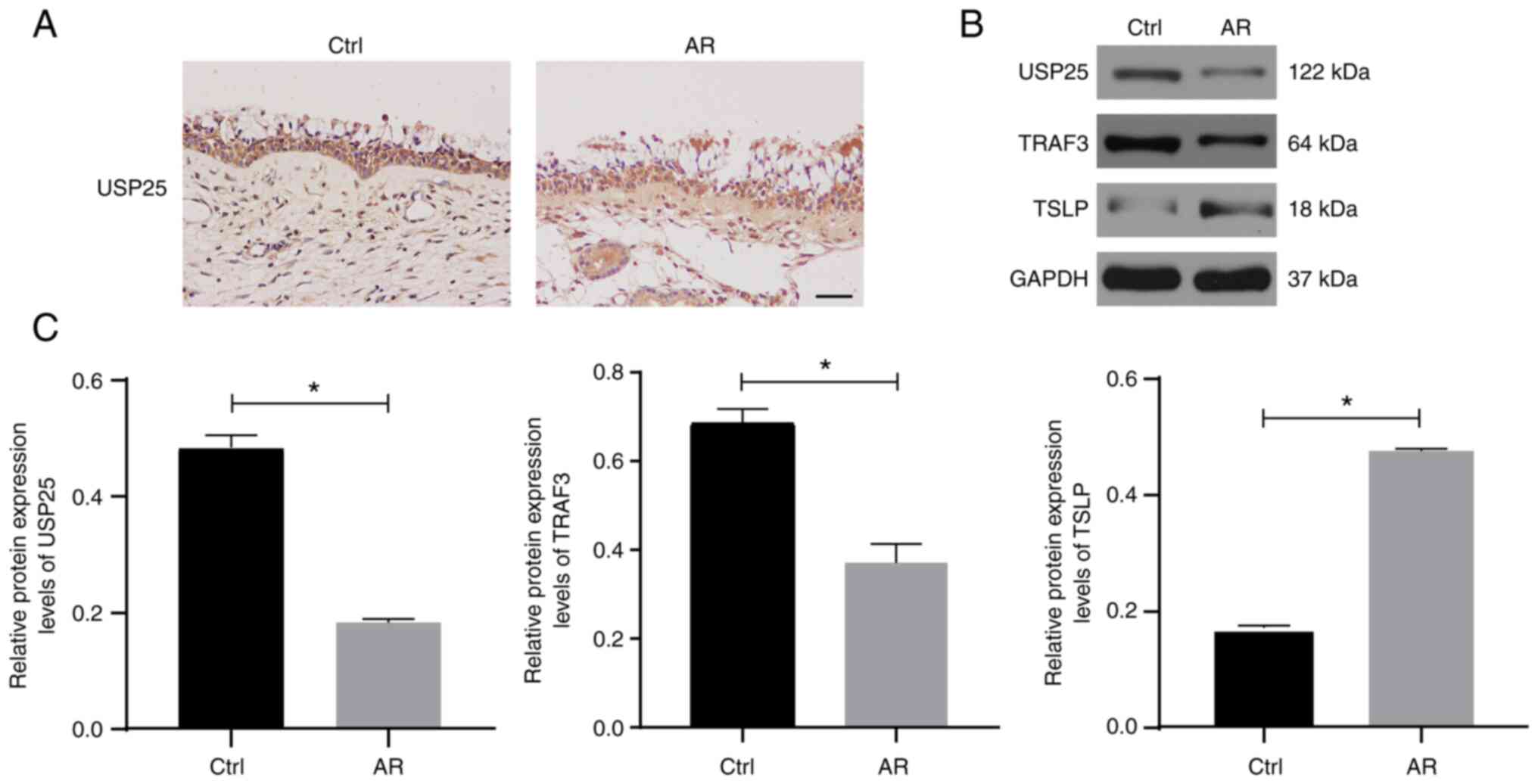
USP25 expression is decreased in the
nasal mucosa of patients with AR and expression of TSLP is
increased
The protein expression of USP25 was analyzed using
paraffinized sections of nasal biopsies obtained from patients with
AR and healthy controls subjected to immunohistochemical staining.
The results demonstrated that USP25 protein was primarily expressed
in epithelial cells of the nasal mucosa. Moreover, markedly weaker
immunostaining of USP25 was observed in nasal mucosal sections of
patients with AR compared with control sections (Fig. 1A). The protein expression levels
of USP25, TRAF3 and TSLP were semi-quantified in the nasal mucosal
tissue of patients with AR and healthy subjects using western
blotting. The results demonstrated that the protein expression
levels of USP25 and TRAF3 were significantly lower in the nasal
mucosal tissue of patients of AR than those of the control group,
whereas protein expression levels of TSLP were significantly higher
than those of the control group (Fig.
1B and C).
USP25 knockdown increases TSLP
expression in the nasal mucosa of OVA-induced mice
To assess whether USP25 knockdown was associated
with elevated TSLP protein expression levels, an AR animal model
was constructed using USP25 KO and WT mice. A summary of the animal
experimental procedures is presented in Fig. 2A. Consistent with the results
observed in patients with AR, both USP25 and TSLP were localized in
mouse nasal mucosal epithelial cells. Immunostaining of TSLP in the
nasal mucosal tissue of WT mice with AR was significantly enhanced
compared with the control group. Moreover, immunostaining of TSLP
in nasal mucosa sections was markedly increased in OVA-induced
USP25 KO mice (Fig. 2B).
Furthermore, western blotting demonstrated that TSLP protein
expression levels were significantly higher in nasal mucosa of
OVA-induced WT mice compared with control mice and protein
expression levels of TSLP and TRAF3 in the nasal mucosa were
further increased in OVA-induced USP25 KO mice compared with the
vehicle control (Fig. 2C-F).
Collectively, these results suggested that USP25 may negatively
regulate protein expression levels of TSLP.
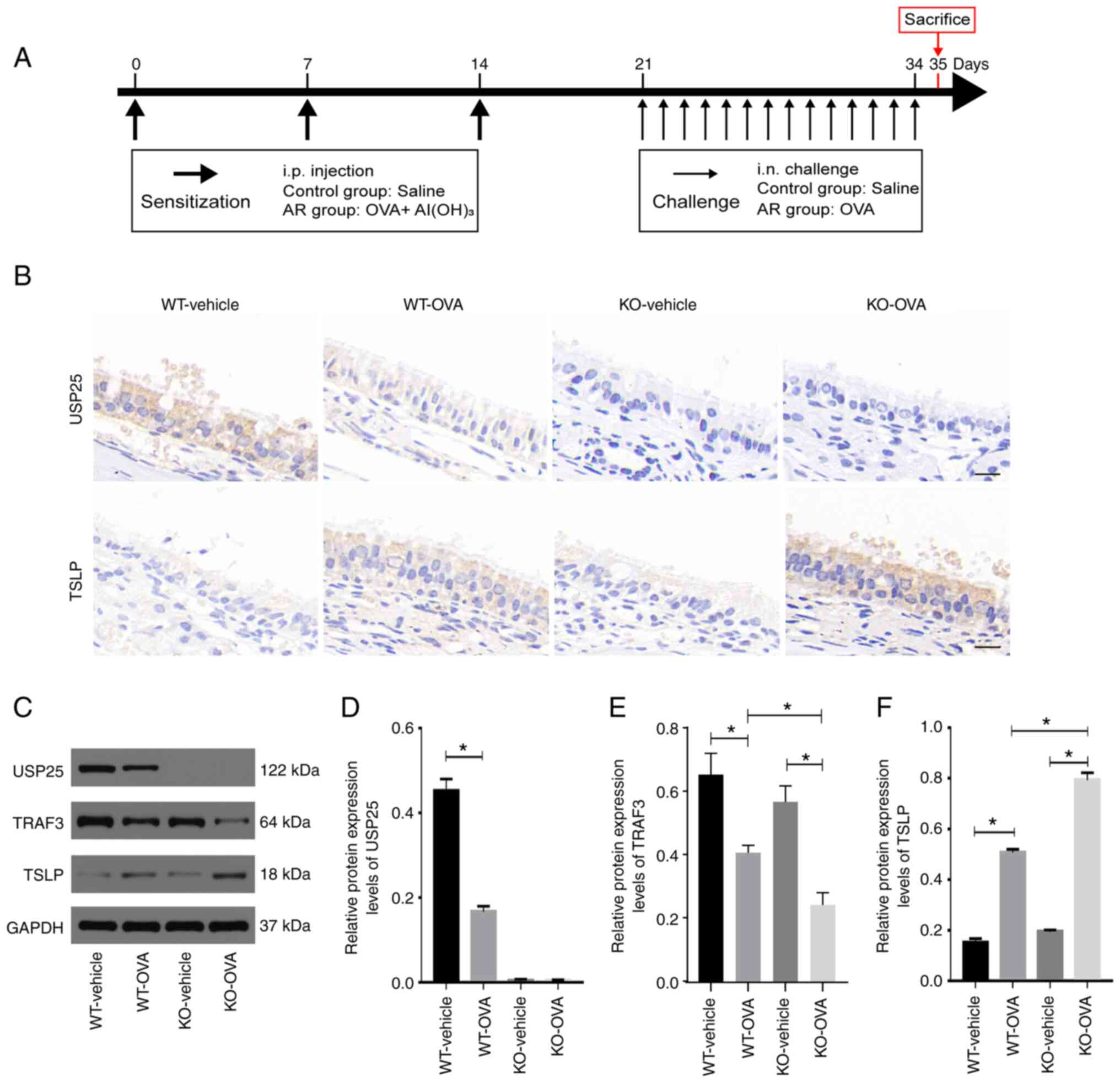 | Figure 2.USP25 knockdown increases protein
expression levels of TSLP in the nasal mucosa of OVA-induced mice.
(A) Schematic experimental protocol for OVA-induced AR in USP25 KO
and WT mice. (B) Representative immunohistochemical images of USP25
and TSLP staining. (C) Protein expression levels of (D) USP25, (E)
TRAF3 and (F) TSLP in nasal mucosal tissue were assessed using
western blotting. Semi-quantitative analysis of band intensity.
Data are presented as the mean ± standard deviation (n=6). Each
experiment was repeated three times. Scale bar, 50 µm. Statistical
analysis was performed using two-way ANOVA followed by Tukey's post
hoc test. *P<0.05. USP25, ubiquitin-specific peptidase 25; TSLP,
thymic stromal lymphopoietin; OVA, ovalbumin; AR, allergic
rhinitis; TRAF3, TNF receptor-associated factor 3; i.p.,
intraperitoneal; i.n., intranasal; KO, knockout; WT, wild-type. |
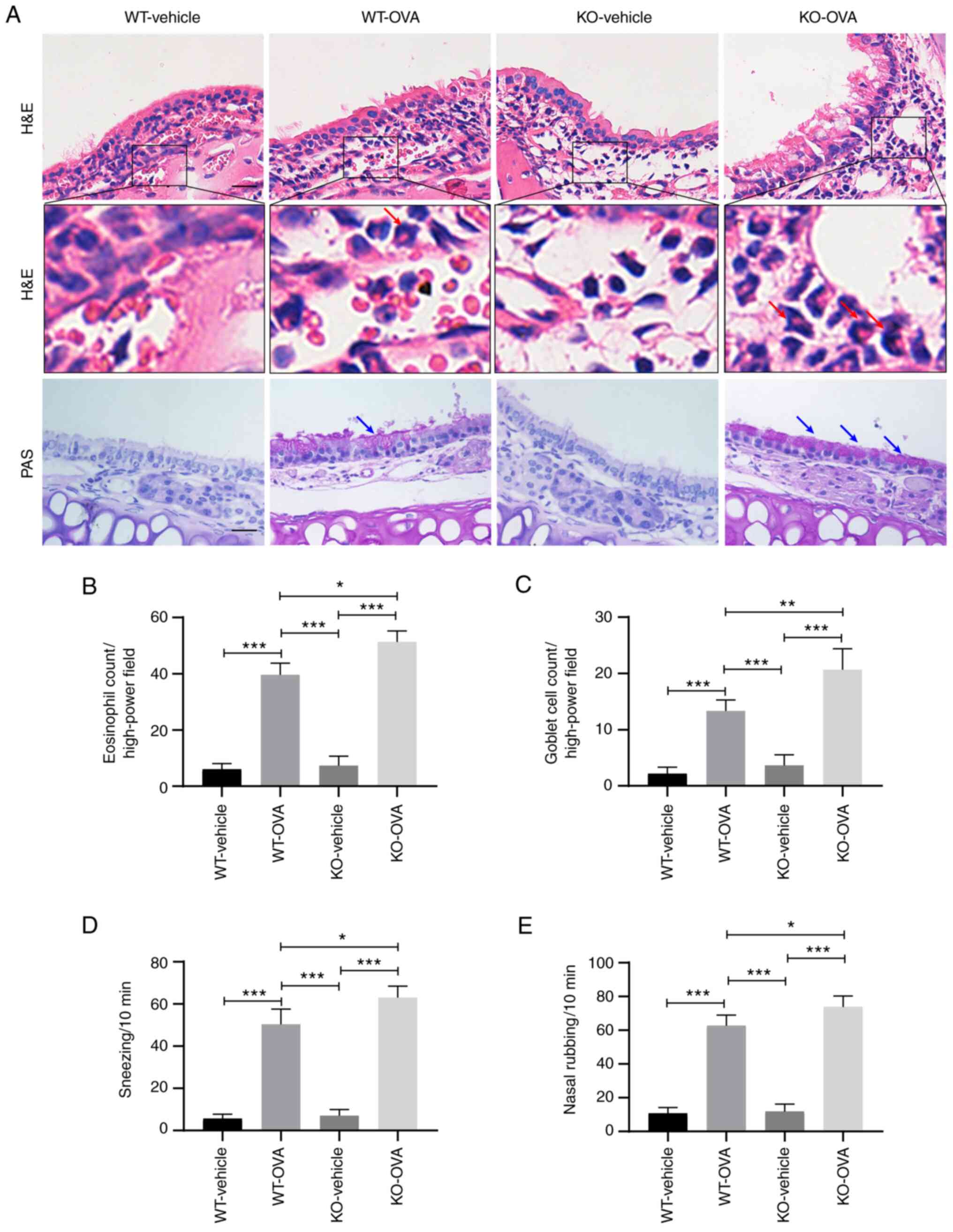
USP25 knockdown exacerbates nasal
mucosal inflammation and nasal symptoms in OVA-induced mice
The primary pathological features of AR include a
large infiltration of eosinophils under the stroma of the nasal
mucosa and increased mucus secretion from goblet cells (2). H&E staining demonstrated that
OVA induction led to significantly increased infiltration of
eosinophils in the nasal mucosa of WT mice compared with WT-vehicle
group and eosinophil infiltration was significantly increased in
OVA-induced USP25 KO mice compared with OVA-induced WT mice
(Fig. 3A and B). Furthermore,
histological analysis of nasal mucosa sections using PAS staining
demonstrated notable goblet cell hyperplasia in OVA-induced WT mice
compared with the WT-vehicle mice and the number of goblet cells
was significantly increased in OVA-induced USP25 KO mice compared
with OVA-induced WT mice (Fig. 3A and
C).
OVA-induced mice exhibited typical nasal allergy
symptoms, including nasal rubbing and sneezing. Compared with
OVA-induced WT mice, OVA-induced USP25 KO mice exhibited
significantly increased levels of both nose rubbing and sneezing
(Fig. 3D and E).
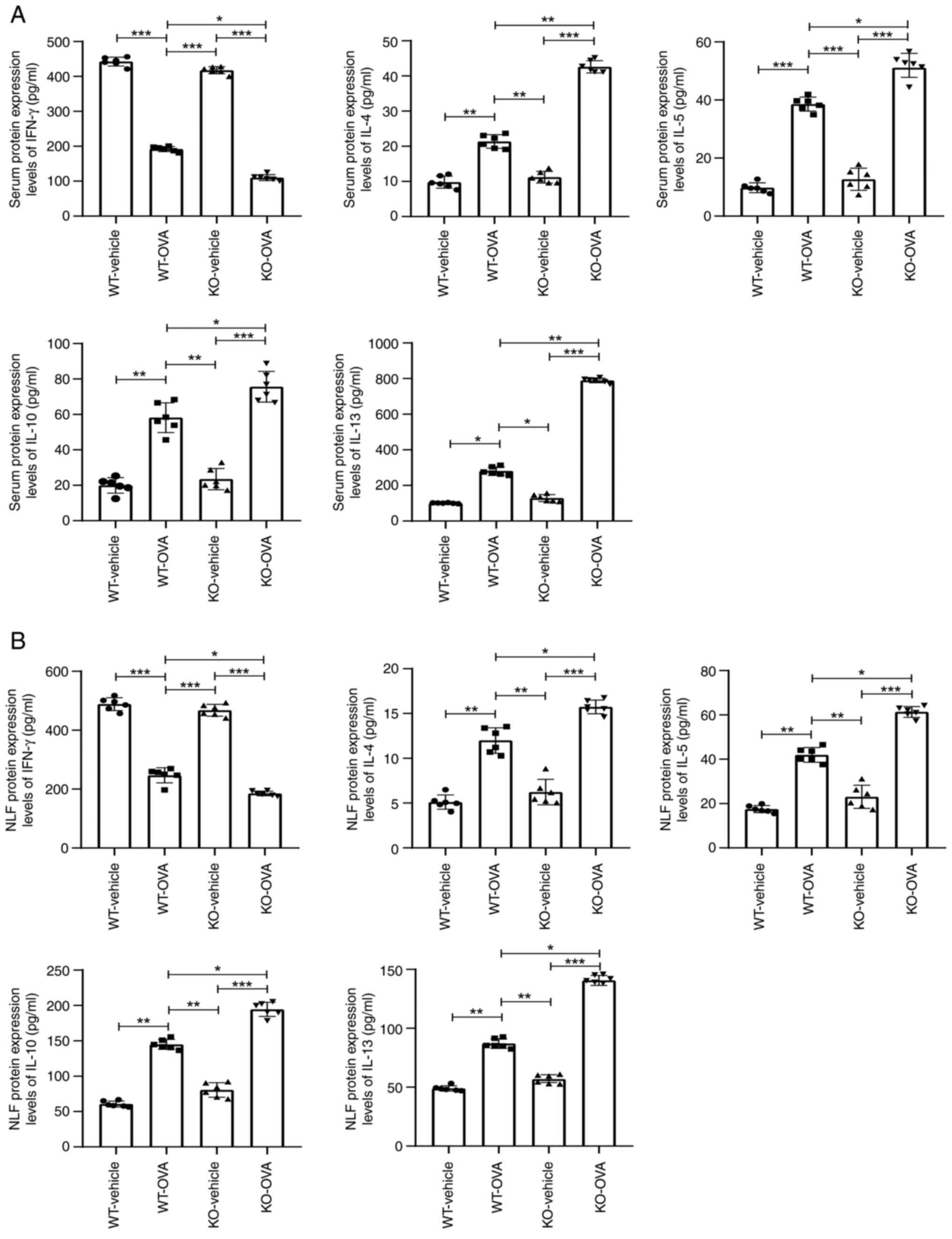
USP25 knockdown exacerbates the
imbalance of Th1/Th2-associated cytokines in mice
TSLP promotes synthesis and release of Th2-type
cytokines, such as IL-4, IL-5, IL-10 and IL-13, and inhibits
production of Th1-type cytokines (17). Therefore, the effect of USP25
knockdown on the production of Th2 cytokines was evaluated. The
protein expression levels of IL-4, IL-5, IL-10 and IL-13 were
significantly increased in both serum and NLF of OVA-induced WT
mice compared with WT-vehicle mice. These protein expression levels
were further elevated in OVA-induced USP25 KO mice compared with
OVA-induced WT mice (Fig. 4A and
B). However, protein expression levels of the Th1 cytokine
IFN-γ were significantly decreased in both the serum and NLF of
OVA-induced WT mice compared with the WT-vehicle mice and further
significantly decreased in USP25 KO mice compared with OVA- induced
WT mice.
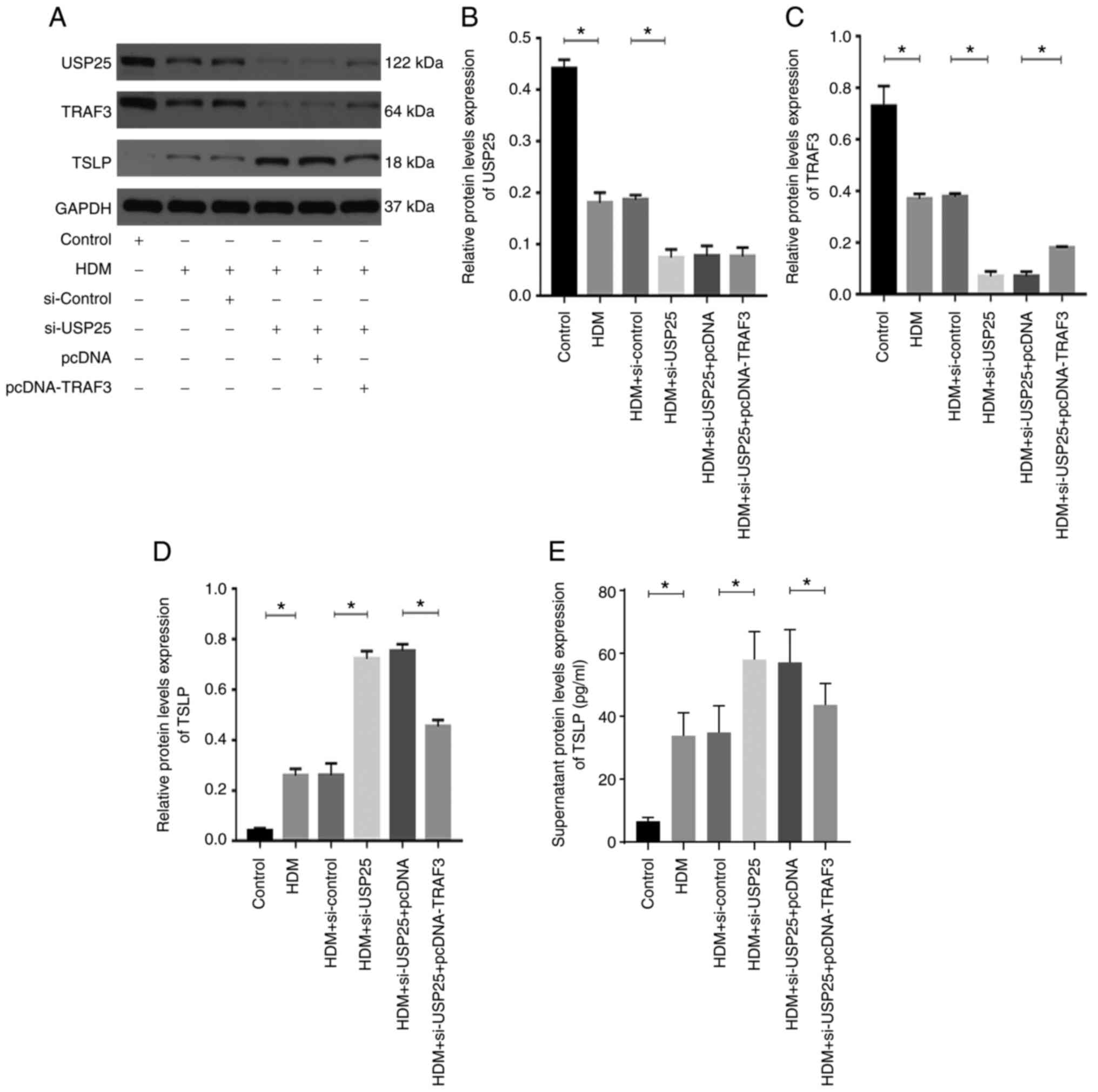
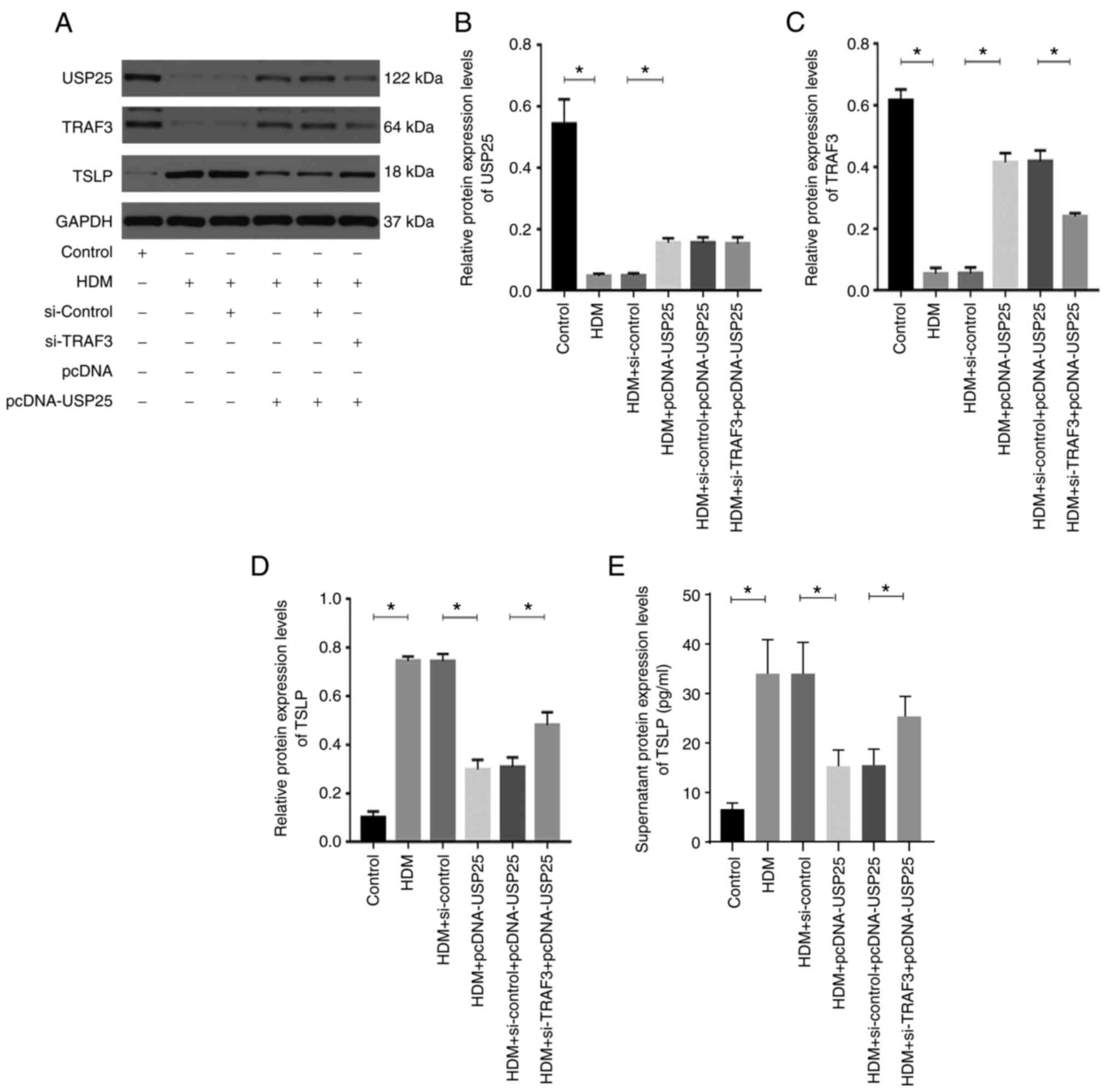
USP25 affects TSLP expression via
regulation of TRAF3 protein in human mucosal epithelial cells
HDMs are one of the most common inhaled allergens
(2). Therefore, HDM extract was
used to stimulate HNEpCs to evaluate the regulatory association
between USP25 and TSLP. Successful transfection of HNEpCs with the
siRNAs or the plasmids was verified by western blotting experiments
(Fig. S1). HDM triggered a
significant increase in TSLP protein expression levels in HNEpCs
and significantly decreased protein expression levels of USP25 and
TRAF3 compared with the control (Fig.
5). Moreover, the protein expression levels of USP25 and TRAF3
were further significantly decreased and protein expression levels
of TSLP were further increased in the HDM + si-USP25 group compared
with HDM + si-control group. The TSLP protein expression levels
were significantly reduced in the HDM + si-USP25 + pcDNA-TRAF3
group compared with the HDM + si-USP25 + pcDNA group.
USP25 overexpression significantly increased protein
expression levels of USP25 and TRAF3 and significantly decreased
protein expression levels of TSLP compared with the HDM +
pcDNA-USP25 group, whereas simultaneous TRAF3 knockdown and USP25
overexpression significantly reversed the effect on protein
expression levels of TSLP (Fig.
6). Furthermore, protein expression levels of TSLP in cell
culture supernatant were evaluated. The results demonstrated a
significant increase in TSLP in supernatant following stimulation
with HDM compared with the control. USP25 knockdown significantly
increased protein expression levels of TSLP in supernatant compared
with HDM + si-control group, whereas USP25 overexpression exerted
the opposite effect. Notably, no significant changes in USP25
protein expression levels were observed following TRAF3
overexpression or knockdown, which further indicated that TRAF3
acts downstream of USP25. In conclusion, these results suggested
that USP25 may regulate TSLP expression via TRAF3 protein in nasal
mucosal epithelial cells.
Discussion
Epithelial-derived cytokines, such as TSLP, IL-25
and IL-33, have been previously reported to serve a key role in
allergic disease (32,33). Among these cytokines, TSLP is
regarded as the master switch that initiates and maintains the type
2 immune response in AR. Moreover, TSLP activates multiple types of
immune cell, thereby driving the allergic inflammatory response
(14,15,34). However, it is unclear how TSLP is
regulated and produced in nasal mucosal epithelial cells. To the
best of our knowledge, the present study is the first to
demonstrate that USP25 stabilized TRAF3 protein in the nasal
mucosal epithelium, thereby inhibiting TSLP expression.
Numerous studies have reported the critical role of
NF-κB in TSLP modulation (35–37). Activation of NF-κB in epithelial
cells, including airway epithelial cells and keratinocytes,
stimulates TSLP gene transcription and exacerbates allergic
inflammation (35,36). Lee and Ziegler (37) reported that activated NF-κB binds
to a site −3.8 kb of the TSLP promoter, which promotes TSLP gene
expression in both humans and mice airway epithelial cells.
Furthermore, Cultrone et al (38) reported a novel binding site for
NF-κB located at position −0.37 kb of the TSLP promoter, a key
region for NF-κB-dependent regulation of TSLP in human intestinal
epithelial cells.
It has been reported that NF-κB activation is under
strict regulation of DUBs (39).
Abnormalities in the function or quantity of deubiquitinases has
been reported to lead to development of excessive NF-κB-dependent
inflammatory responses (40). The
present study demonstrated that downregulation of the
deubiquitylase USP25 occurred in nasal mucosal epithelium of both
patients with AR and AR model mice. These results suggested that
USP25 may serve an important regulatory role in AR. To evaluate the
role of USP25 in AR, an AR model was established using USP25
knockout mice. The experimental results demonstrated that USP25
knockdown aggravated nasal symptoms and pathological
manifestations, including markedly increased eosinophil
infiltration and goblet cell hyperplasia in mice. AR is an allergic
disease characterized by Th1/Th2 immune imbalance (2). Therefore, the protein expression
levels of Th1/Th2 cytokines were examined in the serum and NLF in
mice. USP25 knockdown exacerbated the immune imbalance of Th1/Th2
proteins, as demonstrated by the significantly higher levels of Th2
cytokines (IL-4, IL-5, IL-10 and IL-13) and significantly decreased
levels of Th1 cytokines (IFN-γ) in USP25 KO mice. The present study
demonstrated that USP25 knockdown significantly increased TSLP
protein expression levels in nasal mucosal epithelium. It has been
well documented that TSLP directly promotes Th2 cell proliferation
and activation and triggers imbalance of Th1/Th2 cells by affecting
other immune cells such as dendritic cells and type 2 innate
lymphoid cells (17,18). Therefore, flow cytometry
experiments should be performed in future experiments to verify the
Th1 and Th2 cellular distribution. In summary, the results of the
present study suggested that USP25 may serve a role in AR via
regulation of TSLP.
Previous studies reported that USP25 serves as a
signaling switch to regulate the activation of TRAF proteins during
the inflammatory response, which in turn inhibits NF-κB activation
(35,36). TRAF proteins are considered to
serve as signaling adaptor molecules in the NF-κB signaling
pathway, including both canonical and non-canonical NF-κB signaling
pathways (41). TRAF3 is an E3
ubiquitin ligase that transfers activated ubiquitin molecules from
E2 ubiquitin-conjugating enzymes to both itself and downstream
target proteins for ubiquitin-dependent protein degradation
(42). Therefore, TRAF3 serves a
pivotal negative regulatory role in the non-canonical NF-κB
signaling pathway (43).
Accumulation of NF-κB-inducing kinase (NIK) is a key step in
activation of the non-canonical NF-κB signaling pathway (44). Under normal conditions, TRAF3
ubiquitinates NIK, which leads to continuous NIK degradation.
However, under certain physiological and pathophysiological
situations, NIK becomes stable due to TRAF3 degradation, which
results in activation of non-canonical NF-κB signaling.
Furthermore, TRAF3 inhibits activation of IκB kinase by decreasing
NIK protein accumulation, which in turn suppresses canonical NF-κB
signaling activity (45). NIK is
associated with local eosinophil infiltration and enhanced Th2
response in eosinophilic esophagitis (EoE) (46). EoE is a chronic mucosal
inflammatory disease of the esophagus driven by an allergic
inflammatory response to dietary allergens (46). The present study demonstrated
TRAF3 protein expression levels were significantly decreased in the
nasal mucosal epithelium of AR mice and USP25 knockdown further
significantly decreased TRAF3 protein expression levels. Therefore,
it could be hypothesized that NIK may regulate the
epithelium-driven Th2 microenvironment through alterations in TSLP
expression.
To evaluate the direct regulatory association
between USP25 and TRAF3 in AR, in vitro experiments were
performed using HNEpCs. The protein expression levels of USP25 and
TRAF3 were significantly decreased in HDM-stimulated HNEpCs,
whereas TSLP protein expression levels were significantly
increased. Knockdown or overexpression of USP25 significantly
decreased or increased TRAF3 protein expression levels,
respectively, in HNEpCs, whereas TSLP protein expression levels
were negatively associated with expression of USP25 and TRAF3.
These results supported the hypothesis that USP25 relied on TRAF3
to negatively regulate TSLP production in nasal mucosal epithelial
cells. However, the present study did not evaluate the direct
interaction between USP25 and TRAF3. Several studies have reported
the mechanisms underlying USP25-TRAF3 interactions (26,27,47). Notably, USP25 has been reported to
specifically remove the K48-linked ubiquitin chain from TRAF3,
which increases cellular abundance of TRAF3 and balances production
of type I IFNs and inflammatory cytokines following TLR4 activation
(26). Based on domain mapping
analysis, it was reported that USP25 interacted with TRAF3 via its
ubiquitin carboxyl-terminal hydrolase (UCH) domains, thereby
reversing self-ubiquitination of TRAF3 (26). A further study reported that USP25
serves a key role in the antiviral immune response via
stabilization of TRAF3 and TRAF6 proteins during DUB activity
(27). Moreover, Wen et al
(47) reported that USP25
specifically removes K48-linked polyubiquitin chains in TRAF3,
which enhances Kupfer cell-mediated endotoxin tolerance. USP25 also
interacts with other members of the TRAF family. For example, USP25
negatively regulates ubiquitination of TRAF5 and TRAF6 by cleaving
their K63-linked polyubiquitin chains, which inhibits IL-17-induced
activation of NF-κB and inflammatory responses (48). Therefore, it could be hypothesized
that USP25 may negatively regulate production of TSLP protein by
protecting TRAF3 from degradation and inhibiting NF-κB activation.
However, this hypothesis requires further investigation.
There are some limitations in this study. Firstly,
we used USP25 knockout mice in this study. However, USP25 is widely
expressed as a deubiquitinating enzyme in various cell types
throughout the body. Therefore, it is recommended that nasal
mucosa-specific USP25 knockout mice be used for further studies.
Secondly, the present study also did not explore the molecular
mechanisms underlying the USP25 downregulation of nasal epithelium
in AR. These limitations are to be addressed by future
research.
To the best of our knowledge, the present study is
the first to demonstrate that AR is associated with downregulation
of USP25 in nasal mucosal epithelium. USP25 inhibited TSLP
signaling in nasal mucosal epithelial cells by increasing the
cellular abundance of TRAF3, thereby decreasing inflammation in AR.
Therefore, regulation of USP25 expression may be key in controlling
TSLP-mediated type 2 inflammatory responses. Furthermore, USP25 may
serve as a potential therapeutic target for AR.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
The authors would like to thank Dr Bo Zhong (State
Key Laboratory of Virology, College of Life Sciences, Wuhan
University) for generously donating the USP25 KO mice.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 82071017), the National Natural
Science Foundation of Hubei Province (grant no. 2021CFB125) and the
Fundamental Research Funds for the Central Universities (grant no.
2042021kf0093).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WC, HL and YX designed the study. WC and HL wrote
the manuscript. WC, HL, and LT performed all experiments. LT and ZG
contributed to the conception of the paper. PL, DQ, and WZ
performed the data analysis. WZ recruited patients. YX critically
revised the manuscript. All authors confirm the authenticity of all
the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from all
patients and research protocols were approved by the Ethics
Committee of Renmin Hospital of Wuhan University (approval no.
WDRY2018-K014). All experimental procedures involving animals were
approved by The Animal Care and Use Committee of Renmin Hospital of
Wuhan University (approval no. WDRM-20211005).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vandenplas O, Vinnikov D, Blanc PD, Agache
I, Bachert C, Bewick M, Cardell LO, Cullinan P, Demoly P, Descatha
A, et al: Impact of rhinitis on work productivity: A systematic
review. J Allergy Clin Immunol Pract. 6:1274–1286.e9. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brożek JL, Bousquet J, Agache I, Agarwal
A, Bachert C, Bosnic-Anticevich S, Brignardello-Petersen R,
Canonica GW, Casale T, Chavannes NH, et al: Allergic rhinitis and
its impact on asthma (ARIA) guidelines-2016 revision. J Allergy
Clin Immunol. 140:950–958. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang Y and Zhang L: Increasing prevalence
of allergic rhinitis in China, allergy. Asthma Immunol Res.
11:156–169. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Savouré M, Bousquet J, Jaakkola JJK,
Jaakkola MS, Jacquemin B and Nadif R: Worldwide prevalence of
rhinitis in adults: A review of definitions and temporal evolution.
Clin Transl Allergy. 12:e121302022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sozańska B, Błaszczyk M, Pearce N and
Cullinan P: Atopy and allergic respiratory disease in rural Poland
before and after accession to the European union. J Allergy Clin
Immunol. 133:1347–1353. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang XD, Zheng M, Lou HF, Wang CS, Zhang
Y, Bo MY, Ge SQ, Zhang N, Zhang L and Bachert C: An increased
prevalence of self-reported allergic rhinitis in major Chinese
cities from 2005 to 2011. Allergy. 71:1170–1180. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Myong JP, Kim H, Lee K and Chang S: Time
trends of allergic rhinitis and effects of residence on allergic
rhinitis in Korea from 1998 through 2007–2009. Asian Nurs Res
(Korean Soc Nurs Sci). 6:102–106. 2012.PubMed/NCBI
|
|
8
|
Ha J, Lee SW and Yon DK: Ten-year trends
and prevalence of asthma, allergic rhinitis, and atopic dermatitis
among the Korean population, 2008–2017. Clin Exp Pediatr.
63:278–283. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ordovas-Montanes J, Dwyer DF, Nyquist SK,
Buchheit KM, Vukovic M, Deb C, Wadsworth MH II, Hughes TK, Kazer
SW, Yoshimoto E, et al: Allergic inflammatory memory in human
respiratory epithelial progenitor cells. Nature. 560:649–654. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Krohn IK, Seys SF, Lund G, Jonckheere AC,
de Casterlé ID, Ceuppens JL, Steelant B and Hellings PW: Nasal
epithelial barrier dysfunction increases sensitization and mast
cell degranulation in the absence of allergic inflammation.
Allergy. 75:1155–1164. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bergougnan C, Dittlein DC, Hümmer E, Riepl
R, Eisenbart S, Böck D, Griesbaum L, Weigl A, Damialis A, Hartwig
A, et al: Physical and immunological barrier of human primary nasal
epithelial cells from non-allergic and allergic donors. World
Allergy Organ J. 13:1001092020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Park JY, Choi JH, Lee SN, Cho HJ, Ahn JS,
Kim YB, Park DY, Park SC, Kim SI, Kang MJ, et al: Protein arginine
methyltransferase 1 contributes to the development of allergic
rhinitis by promoting the production of epithelial-derived
cytokines. J Allergy Clin Immunol. 147:1720–1731. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang WW, Zhu K, Yu HW and Pan YL:
Interleukin-17A potentiates interleukin-13-induced eotaxin-3
production by human nasal epithelial cells from patients with
allergic rhinitis. Int Forum Allergy Rhinol. 9:1327–1333. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Marković I and Savvides SN: Modulation of
signaling mediated by TSLP and IL-7 in inflammation, autoimmune
diseases, and cancer. Front Immunol. 11:15572020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Soumelis V, Reche PA, Kanzler H, Yuan W,
Edward G, Homey B, Gilliet M, Ho S, Antonenko S, Lauerma A, et al:
Human epithelial cells trigger dendritic cell mediated allergic
inflammation by producing TSLP. Nat Immunol. 3:673–680. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hammad H and Lambrecht BN: Barrier
epithelial cells and the control of type 2 immunity. Immunity.
43:29–40. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Toki S, Goleniewska K, Zhang J, Zhou W,
Newcomb DC, Zhou B, Kita H, Boyd KL and Peebles RS Jr: TSLP and
IL-33 reciprocally promote each other's lung protein expression and
ILC2 receptor expression to enhance innate type-2 airway
inflammation. Allergy. 75:1606–1617. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takai T: TSLP expression: Cellular
sources, triggers, and regulatory mechanisms. Allergol Int.
61:3–17. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Meng P, Chen ZG, Zhang TT, Liang ZZ, Zou
XL, Yang HL and Li HT: IL-37 alleviates house dust mite-induced
chronic allergic asthma by targeting TSLP through the NF-κB and
ERK1/2 signaling pathways. Immunol Cell Biol. 97:403–415. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kuroda Y, Yuki T, Takahashi Y, Sakaguchi
H, Matsunaga K and Itagaki H: An acid-hydrolyzed wheat protein
activates the inflammatory and NF-κB pathways leading to long TSLP
transcription in human keratinocytes. J Toxicol Sci. 45:327–337.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Song L and Luo ZQ: Post-translational
regulation of ubiquitin signaling. J Cell Biol. 218:1776–1786.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Budroni V and Versteeg GA: Negative
regulation of the innate immune response through proteasomal
degradation and deubiquitination. Viruses. 13:5842021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Georges A, Gros P and Fodil N: USP15: A
review of its implication in immune and inflammatory processes and
tumor progression. Genes Immun. 22:12–23. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ren Y, Zhao Y, Lin D, Xu X, Zhu Q, Yao J,
Shu HB and Zhong B: The type I interferon-IRF7 axis mediates
transcriptional expression of Usp25 gene. J Biol Chem.
291:13206–13215. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Long C, Lai Y, Li J, Huang J and Zou C:
LPS promotes HBO1 stability via USP25 to modulate inflammatory gene
transcription in THP-1 cells. Biochim Biophys Acta Gene Regul Mech.
1861:773–782. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhong B, Liu X, Wang X, Liu X, Li H,
Darnay BG, Lin X, Sun SC and Dong C: Ubiquitin-specific protease 25
regulates TLR4-dependent innate immune responses through
deubiquitination of the adaptor protein TRAF3. Sci Signal.
6:ra352013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lin D, Zhang M, Zhang MX, Ren Y, Jin J,
Zhao Q, Pan Z, Wu M, Shu HB, Dong C and Zhong B: BInduction of
USP25 by viral infection promotes innate antiviral responses by
mediating the stabilization of TRAF3 and TRAF6. Proc Natl Acad Sci
USA. 112:11324–11329. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cheng L, Chen J, Fu Q, He S, Li H, Liu Z,
Tan G, Tao Z, Wang D, Wen W, et al: Chinese society of allergy
guidelines for diagnosis and treatment of allergic rhinitis.
Allergy Asthma Immunol Res. 10:300–353. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen M, Wu Y, Yuan S, Tang M, Zhang L,
Chen J, Li L, Wu J, Zhang J and Yin Y: Allergic rhinitis
improvement in asthmatic children after using acaricidal bait: A
randomized, double-blind, cross-placebo study. Front Pediatr.
9:7091392021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Deng YQ, Yang YQ, Wang SB, Li F, Liu MZ,
Hua QQ and Tao ZZ: Intranasal administration of lentiviral miR-135a
regulates mast cell and allergen-induced inflammation by targeting
GATA-3. PLoS One. 10:e01393222015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xiang R, Xu Y, Zhang W, Kong YG, Tan L,
Chen SM, Deng YQ and Tao ZZ: Semaphorin 3A inhibits allergic
inflammation by regulating immune responses in a mouse model of
allergic rhinitis. Int Forum Allergy Rhinol. 9:528–537. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Patel NN, Kohanski MA, Maina IW, Workman
AD, Herbert DBR and Cohen NA: Sentinels at the wall:
Epithelial-derived cytokines serve as triggers of upper airway type
2 inflammation. Int Forum Allergy Rhinol. 9:93–99. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Papazian D, Hansen S and Würtzen PA:
Airway responses towards allergens-from the airway epithelium to T
cells. Clin Exp Allergy. 45:1268–1287. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cianferoni A and Spergel J: The importance
of TSLP in allergic disease and its role as a potential therapeutic
target. Expert Rev Clin Immunol. 10:1463–1474. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pan Z, Zhou Y, Luo X, Ruan Y, Zhou L, Wang
Q, Yan YJ, Liu Q and Chen J: Against NF-κB/thymic stromal
lymphopoietin signaling pathway, catechin alleviates the
inflammation in allergic rhinitis. Int Immunopharmacol. 61:241–248.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kumagai A, Kubo T, Kawata K, Kamekura R,
Yamashita K, Jitsukawa S, Nagaya T, Sumikawa Y, Himi T, Yamashita T
and Ichimiya S: Keratinocytes in atopic dermatitis express abundant
ΔNp73 regulating thymic stromal lymphopoietin production via NF-Κb.
J Dermatol Sci. 88:175–183. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee HC and Ziegler SF: Inducible
expression of the proallergic cytokine thymic stromal lymphopoietin
in airway epithelial cells is controlled by NFkappaB. Proc Natl
Acad Sci USA. 104:914–919. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cultrone A, de Wouters T, Lakhdari O,
Kelly D, Mulder I, Logan E, Lapaque N, Doré J and Blottière HM: The
NF-κB binding site located in the proximal region of the TSLP
promoter is critical for TSLP modulation in human intestinal
epithelial cells. Eur J Immunol. 43:1053–1062. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Harhaj EW and Dixit VM: Regulation of
NF-κB by deubiquitinases. Immunol Rev. 246:107–124. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Aksentijevich I and Zhou Q: NF-κB pathway
in autoinflammatory diseases: Dysregulation of protein
modifications by ubiquitin defines a new category of
autoinflammatory diseases. Front Immunol. 8:3992017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shi JH and Sun SC: Tumor necrosis factor
receptor-associated factor regulation of nuclear factor κB and
mitogen-activated protein kinase pathways. Front Immunol.
9:18492018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Häcker H, Tseng PH and Karin M: Expanding
TRAF function: TRAF3 as a tri-faced immune regulator. Nat Rev
Immunol. 11l:457–468. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
He JQ, Saha SK, Kang JR, Zarnegar B and
Cheng G: Specificity of TRAF3 in its negative regulation of the
noncanonical NF-kappa B pathway. J Biol Chem. 282:3688–3694. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cildir G, Low KC and Tergaonkar V:
Noncanonical NF-κB signaling in health and disease. Trends Mol Med.
22:414–429. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zarnegar B, Yamazaki S, He JQ and Cheng G:
Control of canonical NF-kappaB activation through the NIK-IKK
complex pathway. Proc Natl Acad Sci USA. 105:3503–3508. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Eden K, Rothschild DE, McDaniel DK, Heid B
and Allen IC: Noncanonical NF-κB signaling and the essential kinase
NIK modulate crucial features associated with eosinophilic
esophagitis pathogenesis. Dis Model Mech. 10:1517–1527. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wen J, Bai H, Chen N, Zhang W, Zhu X, Li P
and Gong J: USP25 promotes endotoxin tolerance via suppressing
K48-linked ubiquitination and degradation of TRAF3 in Kupffer
cells. Mol Immunol. 106:53–62. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhong B, Liu X, Wang X, Chang SH, Liu X,
Wang A, Reynolds JM and Dong C: Negative regulation of
IL-17-mediated signaling and inflammation by the ubiquitin-specific
protease USP25. Nat Immunol. 13:1110–1117. 2012. View Article : Google Scholar : PubMed/NCBI
|