Introduction
Ischemic stroke (IS) is one of the most common
disorders and accounts for ~ 80% of strokes. Stroke is the second
leading cause of morbidity (158/100,000/year) (1) and mortality (11.6% of total deaths)
(2) worldwide after
cardiovascular diseases (3). In
general, early blood supply or reperfusion is the most effective
method for treating cerebral ischemia; however, reperfusion after
ischemia can result in severe brain failure, known as cerebral
ischemia/reperfusion (I/R) injury (4,5).
Studies suggest that mitochondria are severely damaged in I/R
injury (6,7). In addition, abnormal oxygen supply
can inactivate the initiation of the tricarboxylic acid (TCA) cycle
and subsequently inhibit mitochondrial oxidative phosphorylation;
this can lead to adenosine triphosphate (ATP) deficiency, thus
inducing mitochondrial dysfunction. Including oxygen-free radical
damage, calcium overload and changes in mitochondrial membrane
potential (MMP), the abnormal opening of the mitochondrial
permeability transition pore (mPTP) and mitochondrial structural
damage (8,9) can directly lead to neuronal necrosis
and loss of brain function (10).
Therefore, the relationship between stroke and mitochondria has
been actively explored and neuroprotection is considered a
promising stroke treatment strategy (11). More effective neuroprotective
measures and drugs for the clinical prevention and treatment of
cerebral I/R injury based on the mitochondrial pathway are
required.
Chinese herbal medicine has long been used in the
treatment of stroke. Its rich drug resources and long-term
treatment experience indicate its great application potential for
stroke treatment (12). Notably,
Gastrodia elata Blume has been used as an anticonvulsant in
eastern countries for several centuries. Clinically, it has been
widely used to treat headache, epilepsy, limb numbness, hemiplegia
and other neurological diseases disorders (13); it has also been approved by the
Chinese Pharmacopoeia (14). A
phenolic component of G. elata, 3,4-dihydroxybenzaldehyde
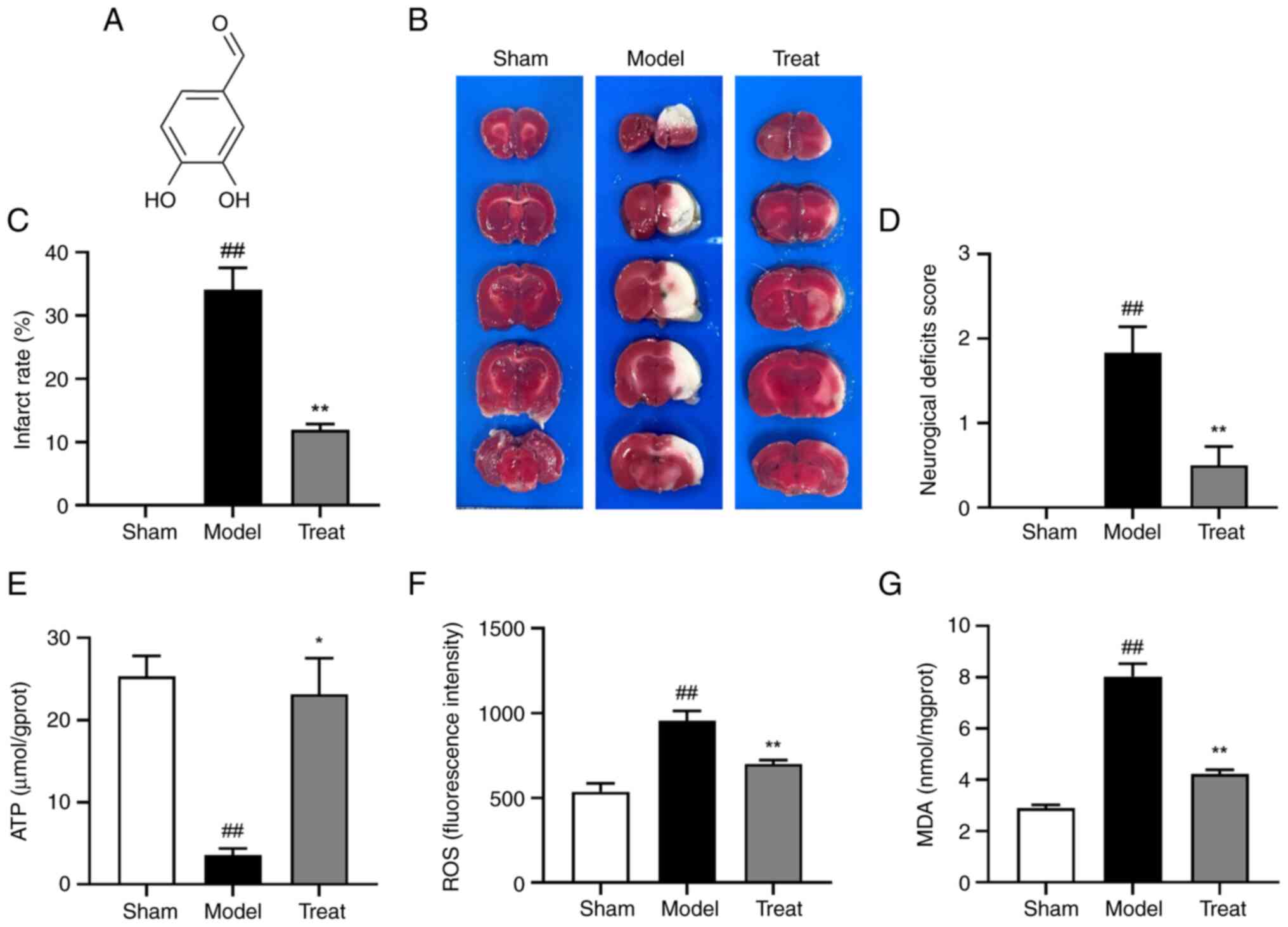
(DBD; Fig. 1A), exhibits
neuroprotective effects for cerebral I/R injury after IS (15,16). Our previous study reported that
DBD can inhibit the activation of MAPK and NF-κB, reduce the
secretion of inflammatory mediators and cytokines and exhibit
antineuritis effects (17). In
addition, the ethyl acetate site of G. elata where DBD is
located can enhance the expression of tight junction proteins and
protect the blood-brain barrier (BBB) (18,19). For middle cerebral artery
occlusion/reperfusion (MCAO/R) rats, neuroprotective effects are
observed by creating anti-oxidative stress and inhibiting apoptotic
pathways (15,20). Recently, Zeng et al
(21) indicated that DBD could
inhibit the hypoxia-inducible factor-1 α/pyruvate dehydrogenase
kinase 1 signaling pathway, alleviate mitochondrial metabolic
disorder in the internal capsule after brain I/R and improve energy
deficiency in vitro and in vivo. These aforementioned
studies indicate that DBD may play an anti-IS role in the brain.
However, previous experiments have not comprehensively explored the
relevant mechanism of DBD in stroke treatment from the metabolite
level; thus, further research is needed.
Metabolomics is a powerful research method in the
field of systems biology. Gene functions and metabolic pathways
associated with phenotypes can be detected by identifying a range
of genes and metabolites (22).
In addition, disease-specific metabolites can be used as biomarkers
for the diagnosis of diseases, thus providing a reference for
clinical precision medicine (23). Mechanistic studies on traditional
Chinese medicine (TCM) pharmacology combined with metabolomics may
help understand the complete metabolic network and be used as an
effective method for identifying the multiple interactions among
TCM components (24).
Accordingly, the present study combined metabolomics and
pharmacology to investigate the neuroprotective effects of DBD
systematically and scientifically on brain I/R injury. Liquid
chromatography-tandem mass spectrometry (LC-MS) has become the
mainstream of metabolomic research because of its analysis speed,
high resolution and high sensitivity (25). Therefore, the present study aimed
to use the LC-MS/MS metabolomics approach to study the brain tissue
metabolome of MCAO/R rats and explore the intervention mechanism of
DBD through energy metabolomic analysis combined with the
biochemical parameters associated with mitochondrial function,
histological observation under an electron microscope, western
blotting, immunofluorescence and other indicators. A comprehensive
study of the neuroprotective effects of DBD revealed potential
biomarkers related to the disorder of the mitochondrial metabolic
pathways. Finally, the present study performed targeted metabolic
profiling and validated potential therapeutic targets.
Materials and methods
Animals
A total of 63 male-specific pathogen-free SD rats
(5–8 weeks-old; 250–280 g weight) were purchased from the Hunan
Shrek Jingda Experimental Animal Co., Ltd. [Laboratory animal
qualification certificate: scxk (Xiang) 2019-0004]. The rats had
free access to food and water; the feeding environment was
maintained in a 12-h light/dark cycle, at a temperature of 20–23°C
and a relative humidity of 40–60%. All animal experiments were
approved by the Animal Ethics Committee of Yunnan University of
Traditional Chinese Medicine (approval no. R-062021088) and the
care and use of experimental animals were in accordance with the
guidelines of the National Institutes of Health. Prior the
experiment, the rats were randomly categorized into sham (Sham),
model (MCAO/R) and treat (MCAO/R + DBD 10 mg/kg) groups, with 21
rats in each group. DBD (≥98% purity) was purchased from Chengdu
Alfa Biotechnology Co., Ltd. Based on the effective doses
determined in our previous study (17), the treat group was continuously
given 10 mg/kg by gavage for 7 days, whereas the model and sham
groups were given the same amount of distilled water by gavage.
MCAO/R model
The MCAO/R model was replicated by the suture method
as described previously (26)
with slight modifications. In brief, rats were anesthetized by
intraperitoneal injection of 2% sodium pentobarbital (40 mg/kg).
The right common carotid artery (CCA) and vagus nerve of the rats
were isolated and a 0.36-mm-diameter polynylon monofilament with a
rounded tip (cat. no. 2636-50A4; Beijing Cinontech Co., Ltd.) was
inserted into the middle cerebral artery from the CCA through the
internal carotid artery; the insertion was stopped if a slight
resistance was encountered (~18–20 mm). After 2 h of ischemia, the
middle cerebral artery was reperfused by gently pulling the suture
back to the CCA. The sham group underwent the same process except
for suture blockage. Neurological scoring was performed at 24 h
after reperfusion. During this period, none of the animals
developed humane endpoint indications, such as non-feeding, dyspnea
and hypothermia, or died prematurely. Neurological deficit was
assessed on a scale of 0–4 points: 0 points: regular activity, no
neurological deficit; 1 point, when the tail is lifted, the left
forelimb is adducted and cannot be fully extended; 2 points, the
body rotates to the left when crawling; 3 points, the body tilts to
the left when crawling; 4 points, inability to walk spontaneously
with a decreased level of consciousness (26). The inclusion criterion for this
model was a neurological score of 1–3; those with scores of 0 or 4
were excluded.
Measurement of cerebral infarct
area
After 24 h of I/R, the rats were injected
intraperitoneally with 2% sodium pentobarbital (150 mg/kg) to
induce deep anesthesia and no response to tail entrapment. Then the
animals were rapidly sacrificed using a decapitation device. The
whole brains were removed and frozen at −20°C for 20 min. Coronal
sections from front to back (2 mm) were made on the brains on ice,
stained with 2% triphenyl tetrazolium chloride (TTC) (cat. no.
A610558; Sangon Biotech Co., Ltd.) in the dark for 20 min at 37°C
and then fixed in 4% paraformaldehyde for 24 h at room temperature.
The infarcted areas were analyzed with ImageJ 1.52 a (National
Institutes of Health).
Hematoxylin and eosin (HE)
staining
The prepared brain tissue paraffin sections were
heated at 60°C for 3 h. Hematoxylin was administered for 2 min and
then washed off; the sections were stained with eosin dye for 1 min
at room temperature. The samples were dehydrated and dried with
graduated alcohol series, cleared with xylene to make and sealed
with neutral gum. Images were captured using an inverted phase
contrast microscope (IXplore; OM Digital Solutions Corp.) under a
field of view of ×400 magnification. A HE staining kit was used for
histomorphological analysis (cat. no. KGA224; Nanjing KeyGen
Biotech Co., Ltd.).
Immunofluorescence staining
Brain sections were dewaxed, dried at 60°C for 60
min, dewaxed twice with xylene and hydrated with ethanol (100, 95,
80 and 75%) for 5 min each time. Proteinase K working solution (100
µl) was added dropwise to each tissue section and incubated at 37°C
for 30 min. Next, 50 µl of TUNEL fluorescence detection solution
was added to each sample. Then, the sample was incubated at 37°C
for 60 min in the dark. Nuclei were counterstained with DAPI for 5
min at room temperature. Observed the cortex from the cerebral
under a laser confocal microscope (Zeiss LSM; Carl Zeiss AG) and
images captured at 400× magnification (n=3). The areas of interest
were then cropped to approximately 50 µm for presentation. All
analyses were performed in a blinded manner. The TUNEL detection
kit (cat. no. C1090; Beyotime Institute of Biotechnology).
Transmission electron microscopy
After 24 h of I/R, the rats were euthanized and
~1-mm3 of the brain tissue isolated from the ischemic
cortex from the cerebral infarction area was collected, fixed with
4% glutaraldehyde and then post-fixed with 1% osmic acid at 4°C for
2 h. The samples were dehydrated using graduated alcohol series and
acetone, embedded in epoxy resin at 60°C incubator for 48 h, sliced
into 80 nm-thick sections and stained with 4% ethyl citrate uranyl
for 15 min at room temperature. The ultrastructure of mitochondria
was observed and randomly selected fields of view were captured at
400× magnification (n=3) using transmission electron microscopy
(JEM-1400flash; JEOL Ltd.).
Extraction and index determination of
rat brain mitochondria
Based on the regions identified in a previous study
(27), 200 mg of the cortical
tissue of the ischemic hemisphere was collected following MCAO/R
for 24 h. The blood was rinsed with normal saline, dried using a
filter paper, cut into pieces and placed in a 2 ml glass
homogenizer for homogenization. High-purity mitochondria were
extracted using a commercially available mitochondrial extraction
kit (cat. no. SM0020; Beijing Solarbio Science & Technology
Co., Ltd.) according to the manufacturer's instructions and stored
at −80°C. The specific operation steps were as follows:
Centrifugation at 4°C, 1,000 × g for 5 min, transferring the
supernatant to a new centrifuge tube at 4°C, 1,000 × g for 5 min,
continuing to remove the supernatant and transferring to the new
centrifuge tube, 4°C, 12,000 × g for 10 min. The supernatant, after
centrifugation, contained cytoplasmic components, while the
mitochondria precipitated at the bottom of the tube. After removing
the supernatant, 0.5 ml Wash Buffer was added to the mitochondrial
precipitation and centrifuged at 4°C 1,000 × g for 5 min. Then the
supernatant was transferred to a new centrifuge tube for 10 min at
4°C and 12,000 × g. Finally, the supernatant was removed and the
high-purity mitochondria were precipitated at the bottom of the
tube. Subsequently, the mitochondrial purity was evaluated.
Purified mitochondria and non-purified mitochondrial suspension
(mitochondria not treated with wash buffer in mitochondrial
extraction kit and containing other cell tissues) were mixed with
100 µl each of 0.3% Janus green B (mitochondria specific staining;
cat. no. S19083, Shanghai Yuanye Bio-Technology Co., Ltd.) and
0.15% neutral red solution (lysosomal and Golgi staining; cat. no.
DN300; Beijing Dingguo Changsheng Biotechnology Co., Ltd.). The
purified and non-purified mitochondria samples from the cerebral
cortex (n=3) were stained for 10 min at room temperature and then
observed and randomly selected fields of view under an optical
microscope at 400× magnification (DMi1; Leica Microsystems,
Inc.).
A mitochondrial protein Extraction kit (cat. no.
G008-1; Nanjing Jiancheng Bioengineering Institute) was used to
extract total protein from the isolated mitochondria. Then, the
protein content of each sample was determined using a BCA protein
quantification kit (cat. no. PC0020; Beijing Solarbio Science &
Technology Co., Ltd.). Additionally, ATP content in brain tissue
was determined using an ATP assay kit (cat. no. A095-1-1; Nanjing
Jiancheng Bioengineering Institute). The oxidation indexes of brain
tissue and the level of reactive oxygen species (ROS) were detected
by using DCFH-DA as the fluorescent probe (cat. no. E004-1-1;
Nanjing Jiancheng Bioengineering Institute). Malondialdehyde (MDA)
content was detected using the thiobarbituric acid method (cat. no.
BC0025; Beijing Solarbio Science & Technology Co., Ltd.). For
the detection of brain mitochondrial function indicators, the
mitochondrial respiratory chain complex I–IV activity detection kit
(cat. nos. BC0515, BC3235, BC3245 and BC0945; Beijing Solarbio
Science & Technology Co., Ltd.) was used. The electron
transport chain (ETC) contains four multisubunit enzyme complexes,
including mitochondria complex I (nicotinamide adenine dinucleotide
dehydrogenase, NADH dehydrogenase), mitochondria complex II
(succinate dehydrogenase), mitochondria complex III (cytochrome c
reductase) and mitochondria complex IV (cytochrome c oxidase). The
mitochondrial swelling method was used to measure the opening
degree of mPTP. The MMP level was measured by fluorescence
spectrophotometry (cat. nos. GMS10101 and GMS10013.1, Shanghai
Genmed Gene Medicine Technology Co., Ltd.). The content of
cytochrome c (Cyt-c) in brain mitochondria was measured using ELISA
kit (H190-1-1, NanJing JianCheng Bioengineering Inc.).
Western blotting
RIPA lysis buffer (PSMF:RIPA lysis buffer=1:100;
cat. nos. 042121210730; 051021210825; Beyotime Institute of
Biotechnology) was added to the treated brain tissue and lysed on
ice for 20 min and the lysed product was centrifuged at 14,200 × g
for 5 min at 4°C. Protein quantification was performed by the BCA
method. Equal amounts of protein (80 µg) were separated by 8%
SDS-PAGE and transferred to PVDF membranes. Electrophoresis was
performed at 80 V for ~30 min. After the protein marker was
separated and the sample entered the separation gel, the parameter
was changed to a constant voltage of 120 V. After electrophoresis,
the membrane was transferred until the target band reached the
appropriate position. The membrane was blocked with 5% bovine serum
albumin (SW3015, Beijing Solarbio Science & Technology Co.,
Ltd.) at room temperature for 1 h. After blocking, the membrane was
washed twice with TBST buffer (0.1% Tween; cat. no. QN1236; Beijing
Biolab Technology Co., Ltd.). Subsequently, the membrane was
incubated with primary antibodies, including Bax (1:1,000; cat. no.
50599-2-Ig; ProteinTech Group), Bcl-2 (1:500; cat. no. 26593-1-AP;
ProteinTech Group), Caspase-3 (1:1,000; cat. no. 9662; Cell
Signaling Technology, Inc.), O-GlcNAc transferase (OGT; 1:500; cat.
no. 61355, Active Motif, Inc.) and β-Actin (1:25,000; cat. no.
66009-1-Ig; ProteinTech Group, Inc.), overnight at 4°C. Then, it
was incubated with goat anti-rabbit IgG and Rabbit Anti-Mouse IgG
secondary antibodies (both at 1:5,000, cat. nos. ab6721 and
ab97046, Abcam) for 1 h at room temperature. The immunoreactive
bands were developed using enhanced chemiluminescence reagent (cat.
no. A38555; Thermo Fisher Scientific, Inc.) and images were
captured in the Bio-Rad ChemiDoc XRS gel imaging system (Bio-Rad
Laboratories, Inc.). ImageJ Lab V4.0 software (Bio-Rad
Laboratories, Inc.) was used for quantitative analysis.
Brain sample preparation and
extraction
After 2 h of ischemia and 24 h of reperfusion, the
rats were sacrificed and 200 mg of the cortical tissue samples of
the infarcted hemisphere was quickly collected and stored at −80°C
for later use. A total of six brain tissue samples were collected
from each group. After the sample was thawed and homogenized, 0.05
g of the sample was mixed with 500 µl of 70% methanol/water. The
sample was vortexed for 3 min at of 2,500 rpm and centrifuged at
14,200 × g for 10 min at 4°C. Afterwards, 300 µl of supernatant was
collected into a new centrifuge tube and placed in a −20°C
refrigerator for 30 min, Then, the supernatant was centrifuged
again at 14,200 × g for 10 min at 4°C. After centrifugation, 200 µl
of supernatant was transferred through protein precipitation plate
for further LC-MS analysis. Each group of 20 µl samples was mixed
as quality control (QC) samples. Analyzing the coefficient of
variation (CV) of QC samples, the CV value indicates the ratio of
the standard deviation of the original data to the mean of the
actual average data, reflecting the degree of data dispersion.
Furthermore, indicating the stability of experimental data was
tested to ensure QC.
UPLC-MS/MS system conditions
UPLC conditions
Amide method: HPLC was performed under the following
conditions: Column, ACQUITY UPLC BEH Amide (Waters Corporation;
i.d. 2.1×100 mm, 1.7 µm); solvent system, water with 10 mM of
ammonium acetate and 0.3% ammonium hydroxide (A) and 90%
acetonitrile/water (v/v) (B). The gradient was started at 95% B
(0–1.2 min), decreased initially to 70% B (8 min) and then to 50% B
(9–11 min) and finally ramped back to 95% B (11.1–15 min) under the
following conditions: Flow rate, 0.4 ml/min; temperature, 40°C;
injection volume, 2 µl.
ESI-MS/MS conditions
Linear ion trap and triple quadrupole scans were
acquired using a triple quadrupole-linear ion trap mass
spectrometer (QTRAP), QTRAP 6500+ LC-MS/MS System (Sciex),
operating in positive and negative ion mode. The ESI source
operation parameters were as follows: ion source, ESI+/-; source
temperature, 550°C; ion spray voltage 5,500 V (positive) and −4,500
V (negative); curtain gas was set at 35 psi. Tryptophan and its
metabolites were analyzed using scheduled multiple reaction
monitoring. Data acquisitions were performed using Analyst 1.6.3
(Sciex). Multiquant 3.0.3 (Sciex) was used to quantify all
metabolites.
Data processing and statistical
analysis
Unsupervised principal component analysis (PCA) was
performed by statistics function prcomp V1.0.1 (r-project.org)
within R (www.r-project.org). Data were unit
variance scaled before unsupervised PCA. The hierarchical cluster
analysis results of samples and metabolites were presented as
heatmaps with dendrograms using R package pheatmap V1.2.1
(r-project.org). Significantly regulated metabolites among groups
were determined by variable importance in projection (VIP) and
absolute Log2FC (fold change). VIP-values were extracted
from orthogonal partial least squares discriminant analysis
(OPLS-DA) result and generated using R package MetaboAnalystR
V1.0.1 (28). P-value of the
unpaired student's test determined their significance. Metabolites
were annotated using the KEGG database and then mapped to the KEGG
pathway database (http://www.kegg.jp/kegg/pathway.html). Then, the
pathways to which the metabolites with significant modulation were
mapped were fed into metabolite set enrichment analysis and the
P-value of the hypergeometric test determined their
significance.
GraphPad Prism 9.0.0 (GraphPad Software, Inc.) was
used for statistical analyses. The data conform to the normal
distribution. If the variance was homogeneous, then it was analyzed
with Bonferroni's multiple comparisons test using one-way analysis
of variance (ANOVA). If the variance is unequal, then it was
analyzed Dunnett's T3 multiple comparisons test in Welch's ANOVA
test. Non-normally distributed data were analyzed with Dunn's
multiple comparisons test in the Kruskal-Wallis test. All values
were presented as mean ± standard error of the mean. P<0.05 was
considered to indicate a statistically significant difference.
Results
DBD effectively improves MCAO/R in
rats
TTC staining reflects cerebral infarction after
injury and the area of ischemic necrosis is stained white (29). The results showed that obvious
white infarcts in the model group after cerebral I/R and DBD could
significantly reduce the infarct volume (Fig. 1B and C). Compared with the model
group, the neurological function of the rats in the DBD group was
significantly improved (Fig. 1D).
Among them, the ATP content in the brain tissue of MCAO/R rats was
significantly decreased, but this was reversed by DBD (Fig. 1E). In addition, the levels of
oxidative stress-related indicators ROS and MDA increased following
I/R, while DBD could significantly inhibit the generation of ROS
and MDA (Fig. 1F and G). DBD
effectively attenuated brain injury and oxidative stress in MCAO/R
rats while restoring energy supply.
DBD can alleviate mitochondrial damage
induced by MCAO/R
At the heart of mitochondrial dysfunction is ATP
reduction and to determine the effect of DBD on mitochondrial
damage, follow-up experiments were performed. Observation of
mitochondrial ultrastructure in neurons from the cortex tissue was
performed using transmission electron microscopy. It was shown that
I/R resulted in severe swelling of mitochondria and partial
disappearance of cristae, rupture of inner and outer membranes (the
matrix is electron transparent). By contrast, DBD ameliorated this
injury, leaving the inner and outer membranes and ridges largely
intact with only mild swelling (Fig.
2A). Then, high-purity mitochondria were isolated by gradient
centrifugation for detection of related indexes. Microscopic
examination revealed a number of dark reds in non-purified
mitochondria and a single blue-green color in purified mitochondria
(Fig. S1). These results
indicated that the purity of the extracted mitochondria was high.
Following brain I/R, the activity of mitochondrial respiratory
chain enzyme complexes I–IV was inhibited. Mitochondrial
respiratory chain enzyme complexes I and II can oxidize NADH
produced by the TCA cycle and the oxidation of succinate,
respectively and donate electrons to ETC (30). Complex III reduces the mobile
electron carrier Cyt-C, ferries single electrons that are
subsequently transferred from complex III to complex IV, where
molecular oxygen is bound and reduced to water (31). This electron transfer chain
provides an electrochemical gradient across the inner mitochondrial
membrane to drive ATP synthesis. In addition, ROS are mainly formed
by premature leakage of electrons from complexes I, II and III
(31). mPTP was abnormally opened
and MMP level and mitochondrial Cyt-c content were decreased.
However, BDB had a positive effect on mitochondrial health in
MCAO/R rats, manifested by increased activity of mitochondrial
respiratory chain enzyme complexes I–IV (Fig. 2B-E), restored mPTP levels
(Fig. 2F), addition to decreased
MMP loss (Fig. 2G) and increased
mitochondrial Cyt-c content (Fig.
2H). Experiments showed that DBD alleviated cerebral I/R
injury, which may restore cellular energy supply by improving
mitochondrial dysfunction, thereby inhibiting the transfer of
mitochondrial Cyt-c to the cytoplasm, reducing apoptosis and
protecting neurons.
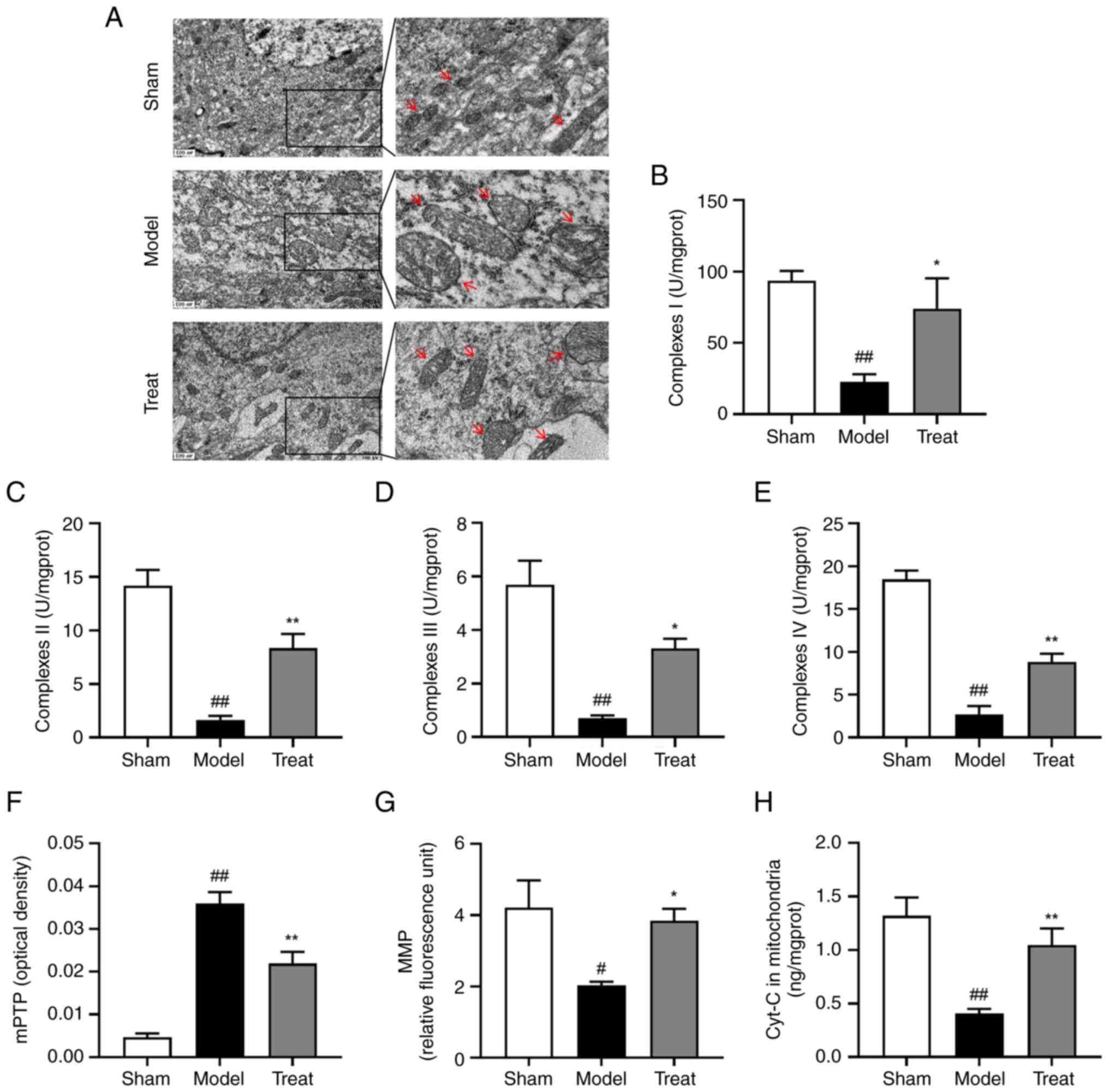 | Figure 2.DBD has a protective effect on
mitochondrial brain damage. (A) Electron microscope observation of
the structure and morphology of mitochondria in different groups of
brain tissues; the right image is the enlarged image in the left
panels. Red arrows indicated typical changes in mitochondrial
morphology. Magnification, ×400; scale bar=500 nm. The activity of
mitochondrial respiratory chain enzyme complexes (B) I, (C) II, (D)
III, and (E) IV in each group. (F) The mitochondrial swelling
method determined the degree of mPTP opening and the higher the
mitochondrial swelling value, the greater the opening degree of
mPTP. (G) MMP levels, a decrease in MMP is an early signal of
apoptosis. (H) mitochondrial Cyt-c content, an essential protein in
apoptosis and an important mediator in the mitochondrial
respiratory chain can reflect apoptosis. All data are presented as
the mean ± standard error of the mean, n=6. Scale bar=500 nm
#P<0.05, ##P<0.01 vs. Sham group;
*P<0.05, **P<0.01 vs. Model group. DBD,
3,4-Dihydroxybenzaldehyde; mPTP, mitochondrial permeability
transition pore; Cyt-c, cytochrome c. |
Metabolomic analysis of MCAO/R rats
following DBD treatment
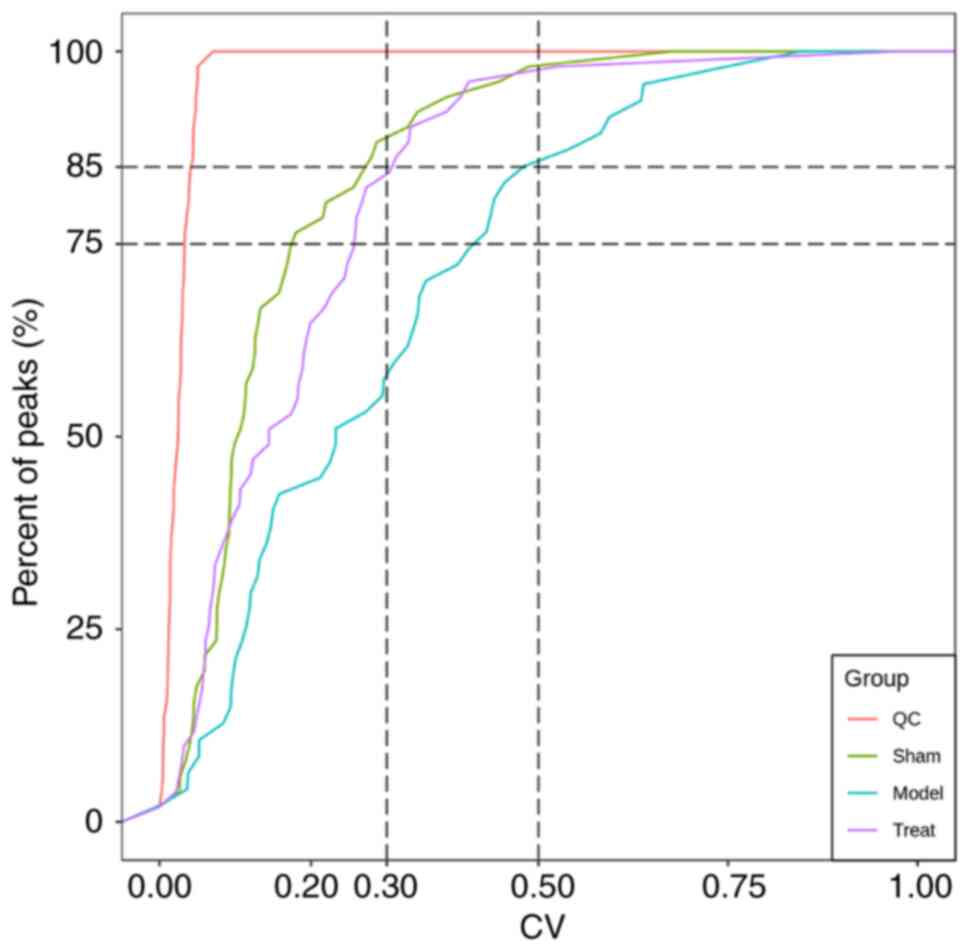
Evaluation of QC samples showed that the proportion
of substances with CV values less than 0.5 in the test and QC
samples were higher than 85%, indicating that the experimental data
was stable (Fig. 3). To
understand the changes on metabolites in I/R of DBD treatment more
clearly, the sham group cortical tissue of rats and model and treat
cerebral cortical tissue of the ischemic hemisphere (n=6) were
collected for metabolic study. Analyst 1.6.3 software (Sciex) was
used to process LC-MS data and to obtained the total ion
chromatogram of all samples (Fig.
S2, Fig. S3, Fig. S4). In addition, a total of 57
metabolites were detected based on the UPLC-MS/MS detection
platform and database, including 17 nucleotides and their
metabolites, 12 amino acids, 10 Organic acids and their
derivatives, eight phosphate sugars, five phosphoric acids, three
coenzymes and vitamins, one lysophosphatidylethanolamine and amino
acid derivatives.
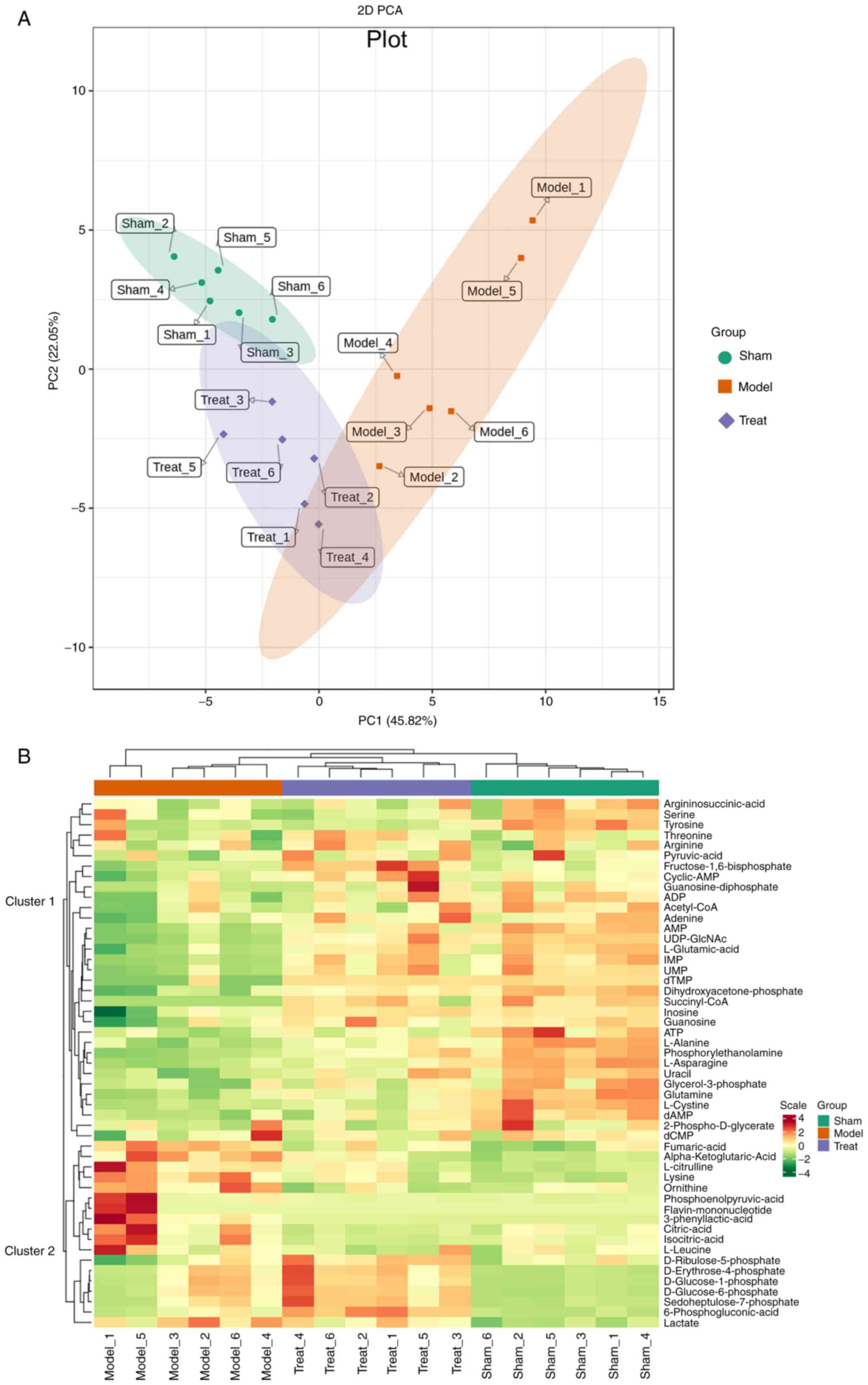
Principal component analysis and heatmap clustering
was used to examine all metabolites in the data. The PCA results
showed clear separation among the different treatments (Fig. 4A), indicating that the metabolites
of samples changed significantly following MCAO/R, consistent with
the I/R phenotype. The change was mainly dominated by the first
principal component (PC1) of the X-axis, which can explain 45.82%
of the characteristics of the original data set. From PC1, it can
be found that the model group and the treatment group are separated
and that there are differences between them.
The differences in the accumulation patterns of
metabolites in the sham-operated group, the model group and the
treatment group can be analyzed by clustering heatmaps (Fig. 4B). The results showed significant
differences in substances in different groups, which were divided
into two clusters. The metabolites in cluster 1 were the highest in
the sham operation group, medium in the treatment group and the
model group. The lowest content, including most nucleotides and
their metabolites, such as adenosine monophosphate (AMP), ATP,
adenosine diphosphate (ADP), guanosine and uracil, decreased
following MCAO/R, which may result from inhibited energy
production. The metabolites in cluster 2 were highest in the model
group, moderate in the treatment group and lowest in the sham
group. Among them, the increased expression of phosphoenolpyruvic
acid in the gluconeogenesis pathway after IS was mainly found in
astrocytes, which can promote acidosis and oxidative damage,
resulting in disturbance of glucose metabolism during I/R (32). The same excess of 3-phenyllactic
acid (PLA) has also been shown to induce ROS production in glial
cell (33). In addition,
ornithine, L-citrulline, L-leucine and other essential amino acids
increased in MCAO/R, which may be the accumulation of protein
synthesis disorders. The different biological replicates also
clustered together, indicating good homogeneity between biological
replicates and high data reliability.
Analysis of differential metabolites
between model group and treatment group
OPLS-DA analysis is a multivariate statistical
analysis method with supervised pattern recognition, which can
effectively eliminate influences irrelevant to the present study to
screen differential metabolites. OPLS-DA was used to perform a
pairwise analysis of the three groups to draw a score map. In this
model, R2X and R2Y represented the
interpretation rate of the X and Y matrices of the built model,
respectively, and Q2 represented the predictive ability
of the model. Q2 were all higher than 0.9, P<0.05,
indicating that the constructed model was suitable (Fig. 5A). The OPLS-DA score plot showed
that there was a clear separation between the model group and the
treatment group (Fig. 5B). Fold
Change (FC) and VIP was used to screen differential metabolites,
metabolites satisfying FC ≥2 and FC ≤0.5 and VIP ≥1 were considered
to have significant differences and the screening results were
displayed in a volcano plot (Fig.
5C). The results showed that in the comparison between the
model group and the treatment group, there were a total of nine
differential metabolites, of which eight metabolites were
upregulated: Succinyl-CoA, UDP-GlcNAc, deoxythymidine monophosphate
(dTMP), AMP, inosine monophosphate (IMP), uridine 5′-monophosphate
(UMP), fructose 1,6-bisphosphate, 6-phosphogluconic acid; 1
metabolite was downregulated: 3-phenyllactic acid (Fig. 5D; Table I).
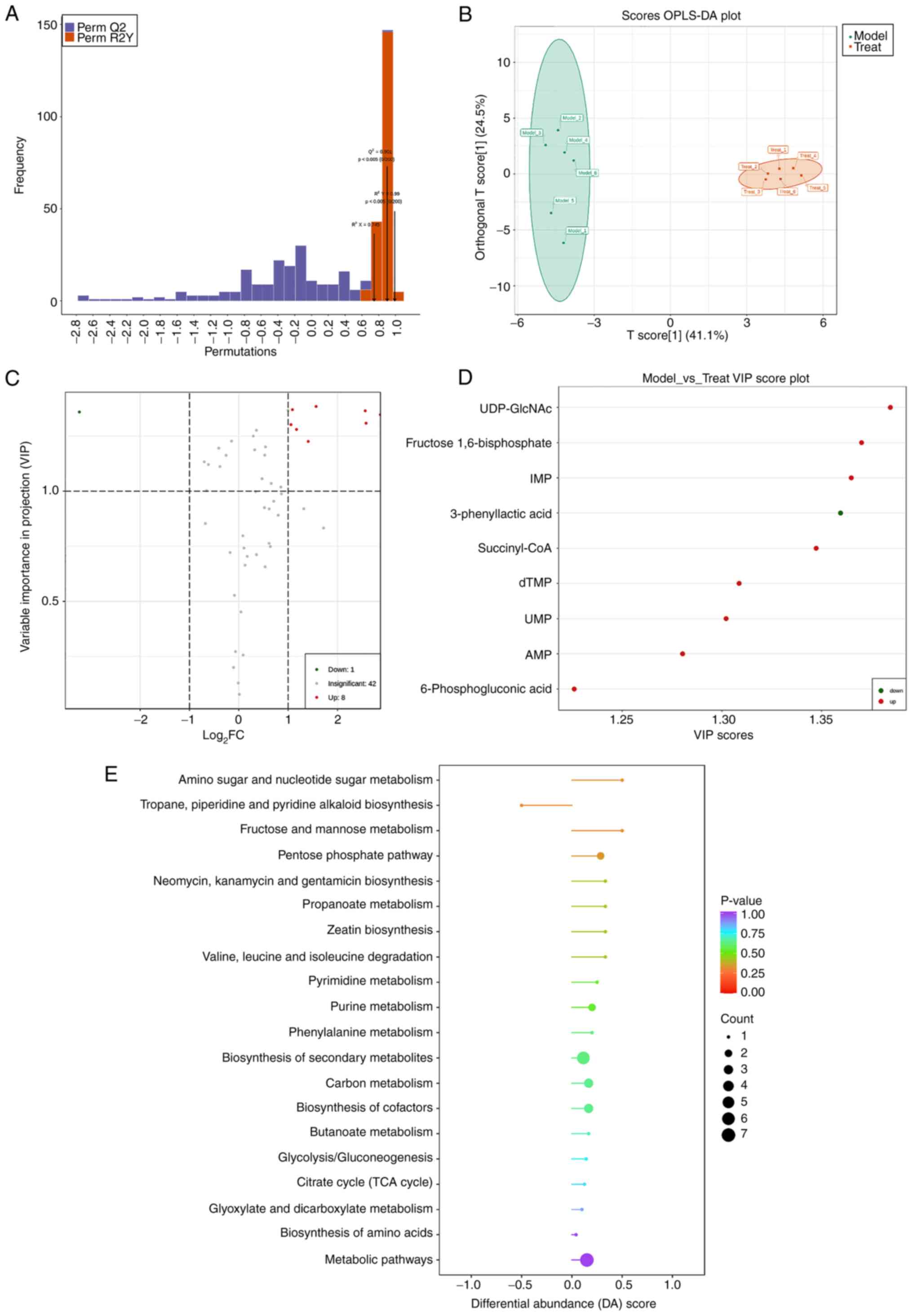 | Figure 5.The enrichment analysis of
differential metabolites and metabolic pathways following MCAO/R
analysis by OPLS-DA. (A) OPLS-DA model validation plot, usually Q2,
is higher than 0.9 and the model is best when P<0.05. (B)
OPLS-DA score plot, representing the gap between Model group and
Treat group. Green: Model group, Red: Treat group. (C) Volcano plot
of differential metabolites, the greater the absolute value of the
abscissa, the greater the fold difference in expression between the
Model group and the Treat group. The greater the ordinate value,
the more significant the differential expression. Green dots:
Downregulated metabolites, red: upregulated metabolites and
visualized in (D) Differential metabolite score plot (VIP≥1). (E)
DA score plot of differential metabolic pathways. The dots are
distributed on the left side of the central axis. The longer the
line segment, the more the pathway's overall expression tends to be
up-regulated; the larger the dots, the more metabolites and vice
versa. The color reflects the size of the P-value; red, the smaller
the P-value. MCAO/R, middle cerebral artery occlusion/reperfusion;
OPLS-DA, orthogonal partial least squares discriminant analysis;
VIP, variable importance in projection; DA, discriminant
analysis. |
 | Table I.Screening results of differential
metabolites in Model group and Treat group. |
Table I.
Screening results of differential
metabolites in Model group and Treat group.
| Metabolites | Formula | RT (min) | VIP | Fold change | P-value | Type |
|---|
| UDP-GlcNAc |
C17H27N3O17P2 | 4.47 | 1.385 | 2.960 |
4.16×10−4 | Up |
| Fructose
1,6-bisphosphate |
C6H14O12P2 | 6.98 | 1.370 | 2.123 |
4.21×10−4 | Up |
| IMP |
C10H13N4O8P | 5.34 | 1.365 | 5.905 |
2.78×10−3 | Up |
| 3-phenyllactic
acid |
C9H10O3 | 0.52 | 1.360 | 0.106 |
4.89×10−2 | Down |
| Succinyl-CoA |
C25H40N7O19P3S | 5.61 | 1.347 | Inf |
5.45×10−3 | Up |
| dTMP |
C10H15N2O8P | 4.62 | 1.309 | 5.973 |
4.16×10−3 | Up |
| UMP |
C9H13N2O9P | 5.21 | 1.302 | 2.080 |
3.35×10−3 | Up |
| AMP |
C10H14N5O7P | 5.25 | 1.280 | 2.248 |
2.06×10−3 | Up |
| 6-Phosphogluconic
acid |
C6H13O10P | 6.13 | 1.226 | 2.650 |
3.65×10−4 | Up |
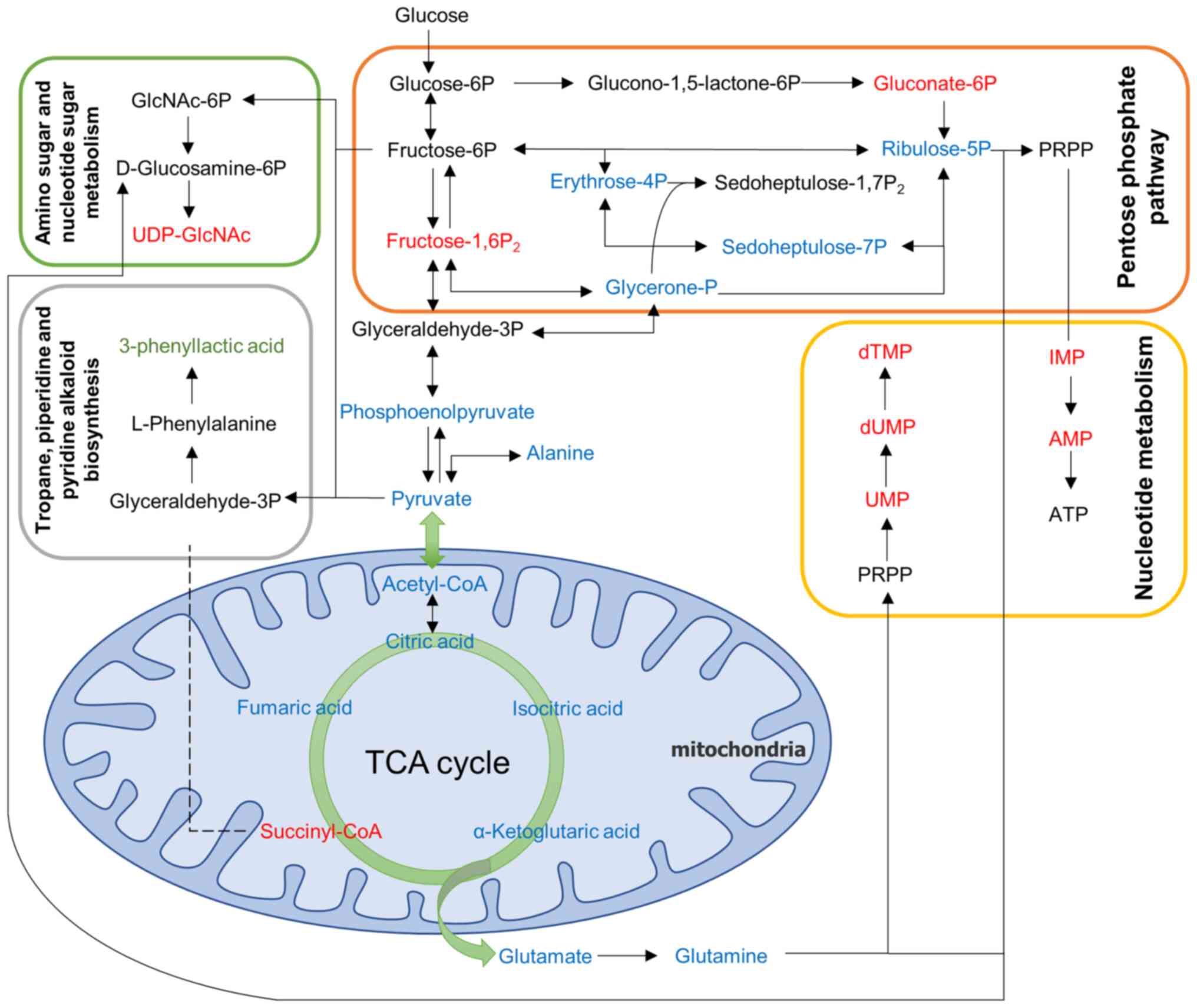
To comprehensively observe changes in metabolic
pathways, the present study employed a pathway-based metabolic
change analysis method, Differential Abundance (DA) Score, which
captures the overall changes of all metabolites in a pathway
(Fig. 5E). The differential
metabolites between the model group and the control group were
mainly annotated and enriched in fructose and mannose metabolism,
tropane, piperidine and pyridine alkaloid biosynthesis, amino sugar
and nucleotide sugar metabolism and pentose phosphate pathway.
These pathways interact and are closely related to I/R. Based on
the results described above, a metabolite overview of key pathways
in brain tissue is shown in (Fig.
6).
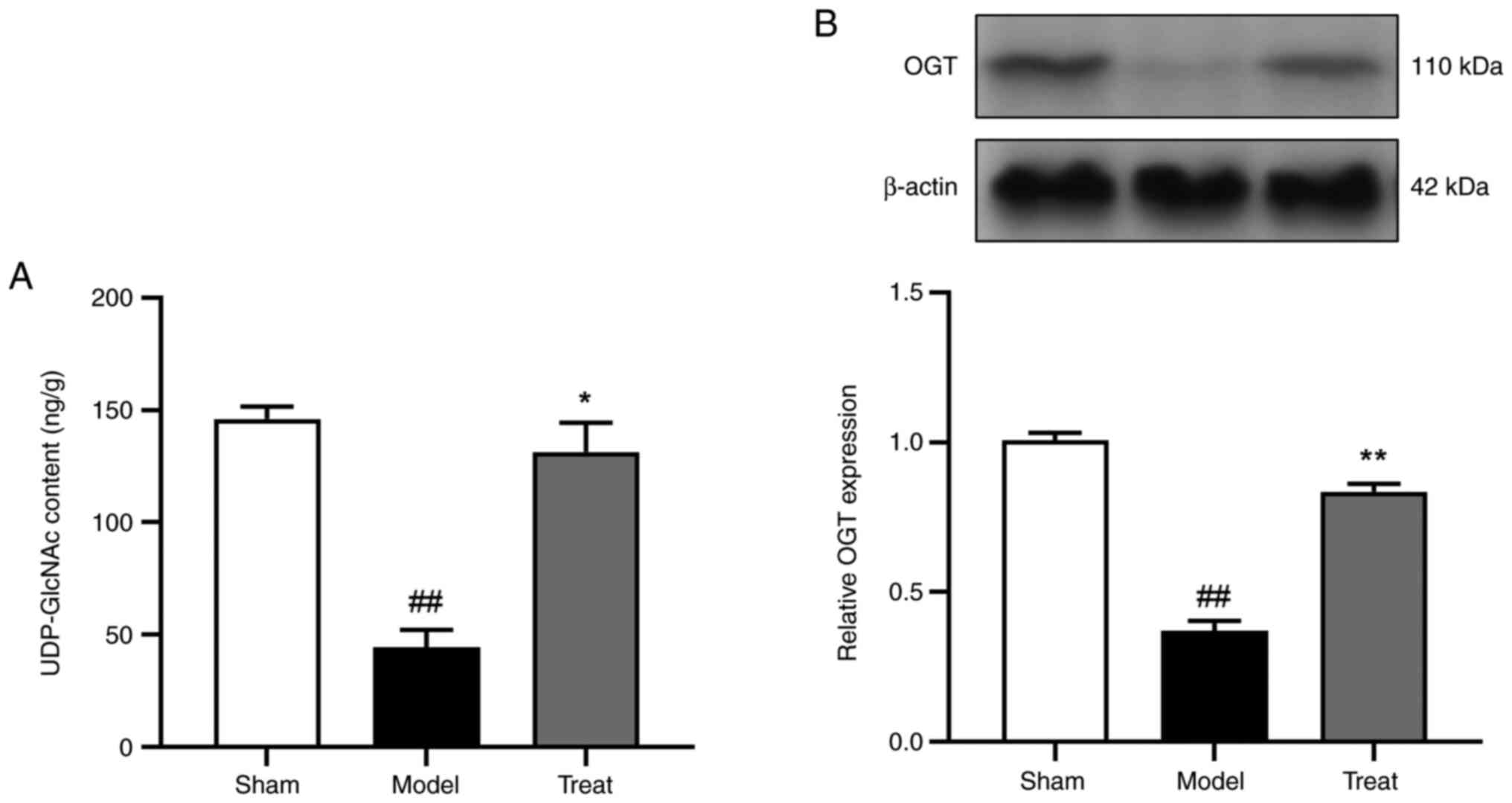
Presentation of quantitative results
of UDP-GlCNAC and verification of OGT expression following DBD
treatment
The most significantly differential metabolite
induced by DBD was UDP-GlcNAc and OGT is the key catalytic enzyme
of UDP-GlcNAc. Subsequently, the content of UDP-GlcNAc in the brain
detected by metabolomics was visualized and the protein expression
of OGTase detected. The experimental results showed that compared
with the sham-operated group, the range of UDP-GlcNAc in the model
group was reduced, which could be improved by DBD (Fig. 7A). Western blotting results showed
that the expression of OGT was significantly increased following
DBD treatment compared with the model group (Fig. 7B). This indicated that DBD
increased the level of UDP-GlcNAc in the brain after IS by
promoting the expression of OGT.
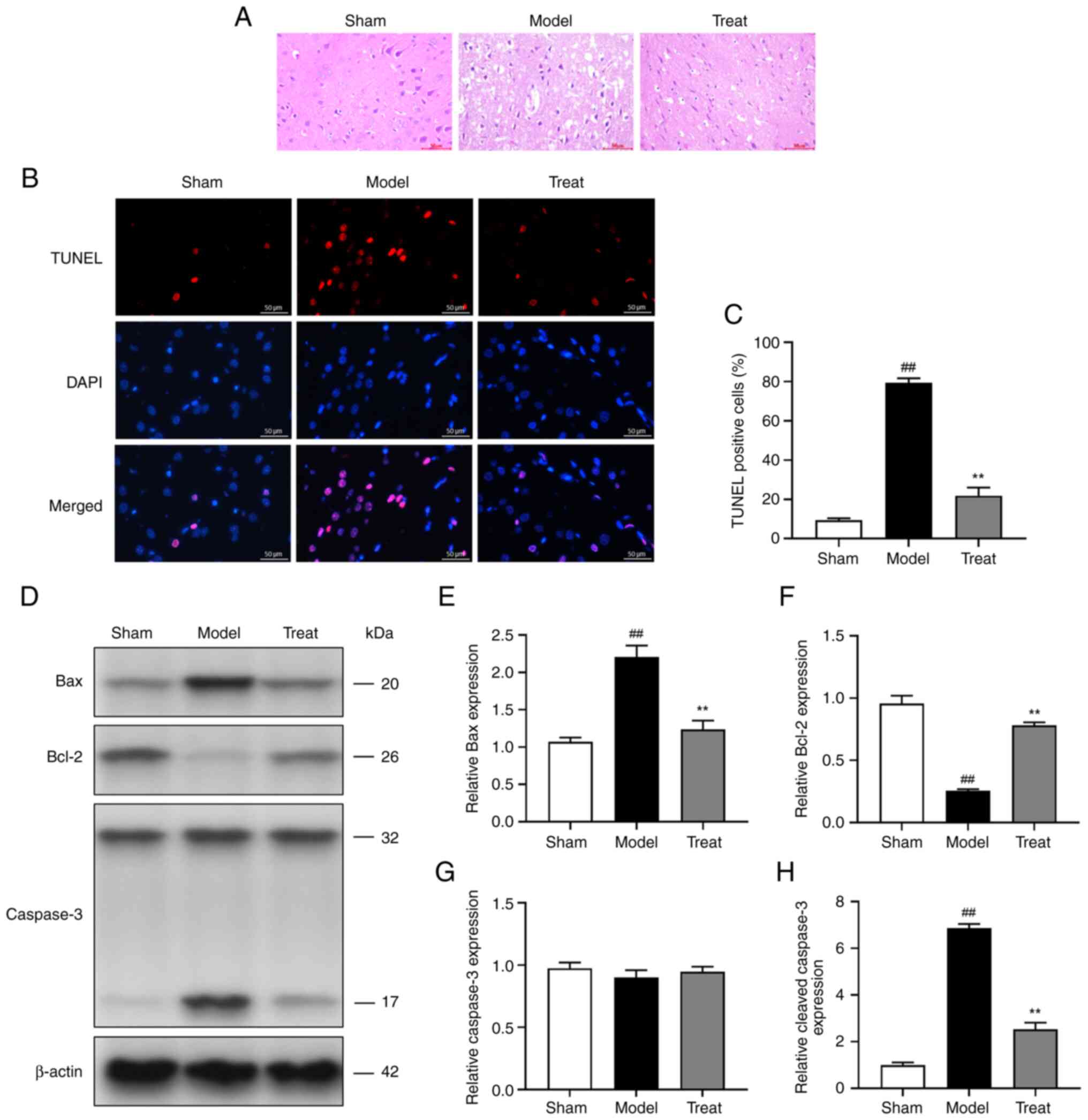
DBD inhibits MCAO/ R-induced apoptosis
of brain cells
Apoptosis is the final and primary determinant of
brain I/R injury. In HE staining, the brain tissue cells of the
rats in the Sham group were arranged neatly and their morphological
structures were normal. In the model group, the gap of cell
structure was widened, nuclear pyknosis appeared, and the fiber
arrangement was disordered, while the treat group exhibited
improved pathology of brain tissue (Fig. 8A). TUNEL staining showed that
MCAO/R decreased the number of neurons and increased the number of
positive apoptotic cells. Following DBD treatment, apoptotic cells
were significantly reduced (Fig. 8B
and C). In addition, the protein expression of genes related to
apoptosis was analyzed by western blotting (Fig. 8D-H). MCAO/R induced the
up-regulation of Bax and cleaved-Caspase-3 and the downregulation
of Bcl-2. However, DBD partially reversed MCAO/R-induced apoptosis
compared with the MCAO/R group. It was hypothesized that DBD
inhibits MCAO/R-induced brain cell apoptosis and reduces neuronal
loss caused by cerebral I/R injury.
Discussion
The present study attempted to identify the
neuroprotective effect of DBD on cerebral I/R injury and the
underlying metabolic pathway by analyzing high-throughput
metabolomic data and using multivariate statistical methods to
elucidate its therapeutic mechanism. Initially, it was demonstrated
that improving mitochondrial dysfunction attenuated brain I/R
injury, which is consistent with previous studies (34,35). DBD could significantly reduce the
neurological deficit and cerebral infarction volume in MCAO/R rats.
In addition, neuronal loss caused by I/R can be reduced by
inhibiting oxidative stress and mitochondrial damage. After
determining the crucial role of mitochondria in cerebral I/R
injury, the present study conducted follow-up studies using
high-purity mitochondria extracted from rat ischemic lateral brain
tissue. The experimental results showed that DBD improved
mitochondrial dysfunction by enhancing the function of the neural
mitochondrial respiratory chain, restoring the level of mPTP,
reducing the loss of MMP and the transfer of mitochondrial Cyt-c to
the cytoplasm, increasing the level of ATP and inhibiting
apoptosis. However, mitochondria are not only the traditionally
considered cellular engine but they also decompose nutrients
through energy metabolism and divide metabolites to maintain redox
homeostasis (36). Thus,
mitochondria deserves our attention in terms of metabolic
functions. The aforementioned studies have shown that DBD can
effectively alleviate reperfusion injury in MCAO/R rats and that
its effect is closely related to that of mitochondria. This result
provides a reliable basis for our later metabolomic research.
The disturbance of energy metabolism is a
significant cause of cerebral ischemia (37). Neuronal activity is closely
associated with mitochondrial function (38). It was hypothesized that energy
metabolism is primarily related to mitochondrial dysfunction and
acts as a key target of cerebral I/R injury. Further research on
the mechanism by which DBD improves mitochondrial energy metabolism
to maintain normal mitochondrial function and morphology is
warranted to break the vicious circle following reperfusion injury.
A number of studies have shown that cerebral I/R injury can peak
within 24 h (27,35). Hence, metabolomics were used to
reveal the neuroprotective effect of DBD and the metabolic pathways
affected by DBD in cerebral I/R injury. The results showed that the
metabolites involved in affected by DBD primarily included amino
acids, nucleotides and their metabolites in brain tissue. DBD
increased succinyl-CoA, UDP-GlcNAc, dTMP, AMP, UMP, IMP, fructose
1,6-bisphosphate and 6-Phosphogluconic acid, and especially
UDP-GlcNAc. By contrast, DBD decreased the expression of PLA.
Pathways mediated by DBD were enriched for
differential metabolites. The results highlighted fructose and
mannose metabolism; tropane, piperidine and pyridine alkaloid
biosynthesis; amino sugar and nucleotide sugar metabolism and
pentose phosphate pathway (PPP). Glycolysis changes from aerobic to
anaerobic pathway in cerebral ischemia because of ischemia and
hypoxia and the PPP is activated as an endogenous antioxidant
mechanism (39).
6-Phosphogluconic acid, the first metabolite of glycolysis and the
initiator of PPP, works with glutathione peroxidase to eliminate
excess ROS following stroke (40). Fructose 1,6-bisphosphate is a
glycolytic intermediate that enhances glycolysis during hypoxia,
preserves cellular ATP stores (41) and reduces oxidative stress
(42). Similarly, activating the
pathways of fructose and mannose metabolism can reduce oxidative
damage in brain tissue, increase brain metabolism and play a
neuroprotective role (43). The
end product of glycolysis is pyruvate, which can enter the
mitochondria to participate in the TAC cycle. Succinyl-CoA is a
product of mitochondrial metabolism of propionyl-CoA, which is a
component of the TAC cycle and it can participate in energy
metabolism (44).
Methylmalonyl-CoA, the major synthase of succinyl-CoA, affects
stroke, dyskinesia and cognitive performance in the basal ganglia
(45). Additionally, the PPP
provides precursors for nucleotide and amino acid biosynthesis.
Nucleotides and their metabolites are involved in basic life
activities such as heredity, development and growth. Different
bases, such as AMP, UMP, thymidine nucleotide (TMP) and IMP, have
become potential therapeutic targets for IS (46,47). In cells, impaired dTMP
biosynthesis can lead to uracil misconjugation and accumulation of
DNA strand breaks, inducing cell cycle arrest and apoptosis
(48). Inosine can directly enter
cells, convert into IMP to synthesize AMP and GMP, participate in
ATP metabolism and then improve the activity of intracellular
enzymes (49). Inosine can
stimulate axonal regeneration in vivo after stroke, reduce
neuronal apoptosis by downregulating the expression of Cyt-c and
induce a protective effect against anti-cerebral I/R injury
(50).
Among the aforementioned metabolites, DBD induces
the most significant differential metabolite UDP-GlcNAc, which is a
nucleotide sugar and the final product of the hexosamine
biosynthesis pathway (HBP) in amino sugar and nucleotide sugar
metabolism (51). UDP-GlcNAc
provides a critical substrate for various biological processes,
indirectly reflecting the nutritional status of cells and promoting
neovascularization (50,52). Based on previous reports,
increasing the amount of UDP-GlcNAc is a direct and effective
method to correct the impaired activation of pro-survival pathways
following ischemia in the aged brain (53). UDP-GlcNAc enables cell survival
under various stresses and induces protective effect against brain
I/R pressure by inhibiting mPTP opening and reducing neuronal
apoptosis (54,55). Therefore, increasing UDP-GlcNAc to
promote cell survival pathways may be a potential neuroprotective
approach for treating stroke. In addition, based on the results of
the present study, the expression of one metabolite (PLA) was
downregulated. Experiments show that PLA interacts with rat brain
mitochondrial hexokinase, which damages glucose metabolism in the
brain and reduces ATP content (56). In addition, PLA has been shown to
increase lipid peroxidation in rodent cerebral cortex and induce
ROS and DNA damage in glial cells (33). In the present study, DBD
ameliorated oxidative stress injury and energy metabolism disorders
in brain mitochondria primarily by interfering with the
aforementioned metabolic pathways and significantly different
metabolites. This increased the production of ATP in the brain,
inhibited neuronal apoptosis and reduced neuronal damage and brain
cell death, which may be the metabolic mechanism through which DBD
alleviates cerebral I/R injury.
It is also noteworthy that UDP-GlcNAc, a metabolite
with a marked increase following DBD treatment, exhibited a
neuroprotective effect. To determine the potential targets of DBD
in the treatment of stroke, the content of UDP-GlcNAc in each
metabolomics group was measured and it was found that DBD could
significantly increase the level of UDP-GlcNAc. Therefore, it was
hypothesized that DBD may inhibit brain cell death by increasing
the level of UDP-GlcNAc. To confirm the mechanism by which
UDP-GlcNAc increases DBD, the activity of OGT, a key metabolic
enzyme of UDP-GlcNAc was confirmed. As UDP-GlcNAc is a direct donor
substrate of OGT, its activity primarily reflects the intracellular
concentration of UDP-GlcNAc. OGT is abundantly expressed in
neurons, primarily in the nucleus and synapse and it plays a role
in regulating neuronal process (57). OGT knockdown significantly reduces
the translocation of dynamin-related protein 1 from the cytoplasm
to mitochondria in the MCAO/R-induced mouse model of brain I/R
injury and enhances mitochondrial fission, thereby leading to
neuronal fission and increased apoptosis (58). Consistent with previous reports,
the present study found that DBD could significantly increase the
expression of OGT, indicating that DBD may increase the level of
UDP-GlcNAc manifested as increased activity of OGT. In addition,
OGT can be a therapeutic target for cerebral I/R.
Apoptosis is the final and significant determinant
of I/R brain injury and inhibition of apoptosis is a crucial step
in treating IS. Mitochondrial dysfunction is closely related to
neuronal apoptosis during brain I/R injury (59). The present study examined the
effect of DBD on mitochondrial apoptosis. TUNEL staining can
accurately reflect the most typical features of apoptosis. The
experimental results showed that DBD can significantly reduce
neuronal apoptosis. The HE staining of brain cells in the model
group showed nuclear pyknosis and disordered arrangement of nerve
fibers, whereas DBD could improve the pathological conditions of
brain tissue. The ratio of anti-apoptotic proteins to pro-apoptotic
proteins regulates cell survival. Bcl-2 is an anti-apoptotic
protein that eliminates excess ROS generated during I/R (60). By contrast, Bax is a pro-apoptotic
protein that causes MMP loss and Cyt-c release(6). Cyt-cis a
crucial mitochondrial molecule (61); once released into the cytoplasm,
it will induce the activation of the apoptotic enzyme activator and
then activate the mitochondrial caspase apoptosis pathway to induce
neuronal apoptosis (62).
Similarly, the results of the present study suggested that DBD can
effectively inhibit brain cell apoptosis and improve mitochondrial
dysfunction by increasing UDP-GlcNAc, thereby alleviating
I/R-induced neural damage. Additionally, apoptosis is inhibited
with increased OGT expression, which may be an essential metabolic
mechanism of DBD in treating IS.
In conclusion, the present study adopted a targeted
energy metabolomic approach to explore the metabolite
characteristics and underlying mechanisms associated with DBD
treatment of cerebral I/R injury. Based on histopathological and
metabolomic results, the present study confirmed the biological
function of UDP-GlcNAc in DBD in the treatment of stroke. Notably,
the regulatory enzyme OTC of UDP-GlcNAc is important for inhibiting
I/R-induced neuronal apoptosis. However, the present study has
certain limitations because of the complexity and variability of
stroke pathogenesis. DBD was also found to be associated with a
variety of differential metabolites and pathways, whereas other
metabolites need further study. Of course, the present study still
updates our understanding of the pathogenesis of brain I/R injury
and the neuroprotective mechanism of DBD and provides a potential
therapeutic target for stroke.
Supplementary Material
Supporting Data
Acknowledgments
Not applicable.
Funding
This study was supported by the National Natural Science
Foundation of China (grant no. 81960733) and the Xingdian Talent
Support Program-Special for Young Talent (2022).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PC conceived the experiment, YL and XD participated
in the experiments, LY analyzed the results, YL wrote the
manuscript and prepared all graphics, and XD revised the
manuscript. YL and XD confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the Animal
Ethics Committee of Yunnan University of Traditional Chinese
Medicine (Kunming, China; approval no. R-062021088).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Feigin VL, Brainin M, Norrving B, Martins
S, Sacco RL, Hacke W, Fisher M, Pandian J and Lindsay P: World
stroke organization (WSO): Global stroke fact sheet 2022. Int J
Stroke. 17:18–29. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
GBD 2019 Stroke Collaborators, . Global,
regional, and national burden of stroke and its risk factors,
1990–2019: A systematic analysis for the global burden of disease
study 2019. Lancet Neurol. 20:795–820. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Feigin VL, Roth GA, Naghavi M, Parmar P,
Krishnamurthi R, Chugh S, Mensah GA, Norrving B, Shiue I, Ng M, et
al: Global burden of stroke and risk factors in 188 countries,
during 1990–2013: A systematic analysis for the global burden of
disease Study 2013. Lancet Neurol. 15:913–924. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lust WD, Taylor C, Pundik S, Selman WR and
Ratcheson RA: Ischemic cell death: Dynamics of delayed secondary
energy failure during reperfusion following focal ischemia. Metab
Brain Dis. 17:113–121. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lim S, Kim TJ, Kim YJ, Kim C, Ko SB and
Kim BS: Senolytic therapy for cerebral ischemia-reperfusion injury.
Int J Mol Sci. 22:119672021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nhu NT, Li Q, Liu Y, Xu J, Xiao SY and Lee
SD: Effects of mdivi-1 on neural mitochondrial dysfunction and
mitochondria-mediated apoptosis in ischemia-reperfusion injury
after stroke: A systematic review of preclinical studies. Front Mol
Neurosci. 14:7785692021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu M, Gu X and Ma Z: Mitochondrial quality
control in cerebral ischemia-reperfusion injury. Mol Neurobiol.
58:5253–5271. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hayashida K, Takegawa R, Shoaib M, Aoki T,
Choudhary RC, Kuschner CE, Nishikimi M, Miyara SJ, Rolston DM,
Guevara S, et al: Mitochondrial transplantation therapy for
ischemia reperfusion injury: A systematic review of animal and
human studies. J Transl Med. 19:2142021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ma Z, Xin Z, Di W, Yan X, Li X, Reiter RJ
and Yang Y: Melatonin and mitochondrial function during
ischemia/reperfusion injury. Cell Mol Life Sci. 74:3989–3998. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vongsfak J, Pratchayasakul W, Apaijai N,
Vaniyapong T, Chattipakorn N and Chattipakorn SC: The alterations
in mitochondrial dynamics following cerebral ischemia/reperfusion
injury. Antioxidants (Basel). 10:13842021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Paul S and Candelario-Jalil E: Emerging
neuroprotective strategies for the treatment of ischemic stroke: An
overview of clinical and preclinical studies. Exp Neurol.
335:1135182021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu T, Wang L, Feng Y, Sun G and Sun X:
Classical Active ingredients and extracts of chinese herbal
medicines: Pharmacokinetics, pharmacodynamics, and molecular
mechanisms for ischemic stroke. Oxid Med Cell Longev.
2021:88689412021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu H, Liu C, Hou J, Long H, Wang B, Guo
D, Lei M and Wu W: Gastrodia elata blume polysaccharides: A
review of their acquisition, analysis, modification, and
pharmacological activities. Molecules. 24:24362019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin LC, Chen YF, Tsai TR and Tsai TH:
Analysis of brain distribution and biliary excretion of a nutrient
supplement, gastrodin, in rat. Anal Chim Acta. 590:173–179. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo C, Wang S, Duan J, Jia N, Zhu Y, Ding
Y, Guan Y, Wei G, Yin Y, Xi M and Wen A: Protocatechualdehyde
protects against cerebral ischemia-reperfusion-induced oxidative
injury via protein kinase cepsilon/Nrf2/HO-1 pathway. Mol
Neurobiol. 54:833–845. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guo Y, Yang JH, He Y, Zhou HF, Wang Y,
Ding ZS, Jin B and Wan HT: Protocatechuic aldehyde prevents
ischemic injury by attenuating brain microvascular endothelial cell
pyroptosis via lncRNA Xist. Phytomedicine. 94:1538492022.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li X, Xiang B, Shen T, Xiao C, Dai R, He F
and Lin Q: Anti-neuroinflammatory effect of
3,4-dihydroxybenzaldehyde in ischemic stroke. Int Immunopharmacol.
82:1063532020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He F, Duan X, Dai R, Wang W, Yang C and
Lin Q: Protective effects of ethyl acetate extraction from
Gastrodia elata blume on blood-brain barrier in focal
cerebral ischemia reperfusion. Afr J Tradit Complement Altern Med.
13:199–209. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Feng J, Xu YL, Meng QT, Yan HW and He FY:
Protective effect of protocatechuic aldehyde on neurovascular unit
nomeostasis damage in rats after cerebral ischemia-reperfusion
Injury. China Pharmacy. 32:1811–1817. 2021.
|
|
20
|
Duan X, Wang W, Liu X, Yan H, Dai R and
Lin Q: Neuroprotective effect of ethyl acetate extract from
Gastrodia elata against transient focal cerebral ischemia in
rats induced by middle cerebral artery occlusion. J Tradit Chin
Med. 35:671–678. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zeng M, Shao C, Zhou H, He Y, Li W, Zeng
J, Zhao X, Yang J and Wan H: Protocatechudehyde improves
mitochondrial energy metabolism through the HIF1alpha/PDK1
signaling pathway to mitigate ischemic stroke-elicited internal
capsule injury. J Ethnopharmacol. 277:1142322021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shah SH, Kraus WE and Newgard CB:
Metabolomic profiling for the identification of novel biomarkers
and mechanisms related to common cardiovascular diseases: Form and
function. Circulation. 126:1110–1120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Medina S, Dominguez-Perles R, Gil JI,
Ferreres F and Gil-Izquierdo A: Metabolomics and the diagnosis of
human diseases-a guide to the markers and pathophysiological
pathways affected. Curr Med Chem. 21:823–848. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lu AP, Bian ZX and Chen KJ: Bridging the
traditional Chinese medicine pattern classification and biomedical
disease diagnosis with systems biology. Chin J Integr Med.
18:883–890. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Beccaria M and Cabooter D: Current
developments in LC-MS for pharmaceutical analysis. Analyst.
145:1129–1157. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cao G, Jiang N, Hu Y, Zhang Y, Wang G, Yin
M, Ma X, Zhou K, Qi J, Yu B and Kou J: Ruscogenin attenuates
cerebral ischemia-induced blood-brain barrier dysfunction by
suppressing TXNIP/NLRP3 inflammasome activation and the MAPK
pathway. Int J Mol Sci. 17:14182016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li J, Yu W, Li XT, Qi SH and Li B: The
effects of propofol on mitochondrial dysfunction following focal
cerebral ischemia-reperfusion in rats. Neuropharmacology.
77:358–368. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chong J and Xia J: MetaboAnalystR: An R
package for flexible and reproducible analysis of metabolomics
data. Bioinformatics. 34:4313–4314. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu Y, Xue X, Zhang H, Che X, Luo J, Wang
P, Xu J, Xing Z, Yuan L, Liu Y, et al: Neuronal-targeted TFEB
rescues dysfunction of the autophagy-lysosomal pathway and
alleviates ischemic injury in permanent cerebral ischemia.
Autophagy. 15:493–509. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rich PR and Marechal A: The mitochondrial
respiratory chain. Essays Biochem. 47:1–23. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nolfi-Donegan D, Braganza A and Shiva S:
Mitochondrial electron transport chain: Oxidative phosphorylation,
oxidant production, and methods of measurement. Redox Biol.
37:1016742020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Geng X, Shen J, Li F, Yip J, Guan L, Rajah
G, Peng C, DeGracia D and Ding Y: Phosphoenolpyruvate carboxykinase
(PCK) in the brain gluconeogenic pathway contributes to oxidative
and lactic injury after stroke. Mol Neurobiol. 58:2309–2321. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Faverzani JL, Steinmetz A, Deon M,
Marchetti DP, Guerreiro G, Sitta A, de Moura Coelho D, Lopes FF,
Nascimento LVM, Steffens L, et al: L-carnitine protects DNA
oxidative damage induced by phenylalanine and its keto acid
derivatives in neural cells: A possible pathomechanism and adjuvant
therapy for brain injury in phenylketonuria. Metab Brain Dis.
36:1957–1968. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cheng H, Lv M, Mi R and Xue G: Amifostine
ameliorates cerebral ischaemia-reperfusion injury via p38-mediated
oxidative stress and mitochondrial dysfunction. Folia Neuropathol.
58:334–346. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li J, Ma X, Yu W, Lou Z, Mu D, Wang Y,
Shen B and Qi S: Reperfusion promotes mitochondrial dysfunction
following focal cerebral ischemia in rats. PLoS One. 7:e464982012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Spinelli JB and Haigis MC: The
multifaceted contributions of mitochondria to cellular metabolism.
Nat Cell Biol. 20:745–754. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang SD, Fu YY, Han XY, Yong ZJ, Li Q, Hu
Z and Li ZG: Hyperbaric oxygen preconditioning protects against
cerebral ischemia/reperfusion injury by inhibiting mitochondrial
apoptosis and energy metabolism disturbance. Neurochem Res.
46:866–877. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen Y, Guo S, Tang Y, Mou C, Hu X, Shao
F, Yan W and Wu Q: Mitochondrial fusion and fission in neuronal
death induced by cerebral ischemia-reperfusion and its clinical
application: A mini-review. Med Sci Monit.
26:e9286512020.PubMed/NCBI
|
|
39
|
Shin TH, Lee DY, Basith S, Manavalan B,
Paik MJ, Rybinnik I, Mouradian MM, Ahn JH and Lee G: Metabolome
changes in cerebral ischemia. Cells. 9:16302020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Imahori T, Hosoda K, Nakai T, Yamamoto Y,
Irino Y, Shinohara M, Sato N, Sasayama T, Tanaka K, Nagashima H, et
al: Combined metabolic and transcriptional profiling identifies
pentose phosphate pathway activation by HSP27 phosphorylation
during cerebral ischemia. Neuroscience. 349:1–16. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Espanol MT, Litt L, Hasegawa K, Chang LH,
Macdonald JM, Gregory G, James TL and Chan PH:
Fructose-1,6-bisphosphate preserves adenosine triphosphate but not
intracellular pH during hypoxia in respiring neonatal rat brain
slices. Anesthesiology. 88:461–472. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Park JY, Kim EJ, Kwon KJ, Jung YS, Moon
CH, Lee SH and Baik EJ: Neuroprotection by
fructose-1,6-bisphosphate involves ROS alterations via p38
MAPK/ERK. Brain Res. 1026:295–301. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Salau VF, Erukainure OL, Koorbanally NA
and Islam MS: Catechol protects against iron-mediated oxidative
brain injury by restoring antioxidative metabolic pathways; and
modulation of purinergic and cholinergic enzymes activities. J
Pharm Pharmacol. 72:1787–1797. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Dobson CM, Gradinger A, Longo N, Wu X,
Leclerc D, Lerner-Ellis J, Lemieux M, Belair C, Watkins D,
Rosenblatt DS and Gravel RA: Homozygous nonsense mutation in the
MCEE gene and siRNA suppression of methylmalonyl-CoA epimerase
expression: A novel cause of mild methylmalonic aciduria. Mol Genet
Metab. 88:327–333. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Andreasson M, Zetterstrom RH, von Dobeln
U, Wedell A and Svenningsson P: MCEE mutations in an adult patient
with Parkinson's disease, dementia, stroke and elevated levels of
methylmalonic acid. Int J Mol Sci. 20:26312019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tanaka T, Ogita A, Usuki Y and Fujita K:
Selective inhibition of embryonic development in starfish by
long-chain alkyl derivatives of UMP, TMP and AMP. Nat Prod Res.
23:1572–1578. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Frenguelli BG and Dale N: Purines: From
diagnostic biomarkers to therapeutic agents in brain injury.
Neurosci Bull. 36:1315–1326. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chon J, Stover PJ and Field MS: Targeting
nuclear thymidylate biosynthesis. Mol Aspects Med. 53:48–56. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Moritz CE, Teixeira BC, Rockenbach L,
Reischak-Oliveira A, Casali EA and Battastini AM: Altered
extracellular ATP, ADP, and AMP hydrolysis in blood serum of
sedentary individuals after an acute, aerobic, moderate exercise
session. Mol Cell Biochem. 426:55–63. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Xu D, Ai Q, Chen X, Wang Z, Wei H, Zhou L,
Mei Z and Ge J: Metabonomics study on naotaifang extract
alleviating neuronal apoptosis after cerebral ischemia-reperfusion
injury. Evid Based Complement Alternat Med.
2022:21124332022.PubMed/NCBI
|
|
51
|
Murakami K, Kurotaki D, Kawase W, Soma S,
Fukuchi Y, Kunimoto H, Yoshimi R, Koide S, Oshima M, Hishiki T, et
al: OGT regulates hematopoietic stem cell maintenance via
PINK1-dependent mitophagy. Cell Rep. 34:1085792021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang ZV, Deng Y, Gao N, Pedrozo Z, Li DL,
Morales CR, Criollo A, Luo X, Tan W, Jiang N, et al: Spliced X-box
binding protein 1 couples the unfolded protein response to
hexosamine biosynthetic pathway. Cell. 156:1179–1192. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang Z, Li X, Spasojevic I, Lu L, Shen Y,
Qu X, Hoffmann U, Warner DS, Paschen W, Sheng H and Yang W:
Increasing O-GlcNAcylation is neuroprotective in young and aged
brains after ischemic stroke. Exp Neurol. 339:1136462021.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Jiang M, Yu S, Yu Z, Sheng H, Li Y, Liu S,
Warner DS, Paschen W and Yang W: XBP1 (X-Box-Binding
Protein-1)-dependent O-GlcNAcylation is neuroprotective in ischemic
stroke in young mice and its impairment in aged mice is rescued by
Thiamet-G. Stroke. 48:1646–1654. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Cheng J, Wu Y, Chen L, Li Y, Liu F, Shao
J, Huang M, Fan M and Wu H: Loss of O-GlcNAc transferase in neural
stem cells impairs corticogenesis. Biochem Biophys Res Commun.
532:541–547. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ziamajidi N, Jamshidi S and Ehsani-Zonouz
A: In-silico and in-vitro investigation on the phenylalanine
metabolites' interactions with hexokinase of Rat's brain
mitochondria. J Bioenerg Biomembr. 49:139–147. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hart GW, Slawson C, Ramirez-Correa G and
Lagerlof O: Cross talk between O-GlcNAcylation and phosphorylation:
Roles in signaling, transcription, and chronic disease. Annu Rev
Biochem. 80:825–858. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhao J, Dong L, Huo T, Cheng J, Li X,
Huangfu X, Sun S, Wang H and Li L: O-GlcNAc transferase (OGT)
protects cerebral neurons from death during ischemia/reperfusion
(I/R) injury by modulating Drp1 in mice. Neuromolecular Med.
24:299–310. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
He Z, Ning N, Zhou Q, Khoshnam SE and
Farzaneh M: Mitochondria as a therapeutic target for ischemic
stroke. Free Radic Biol Med. 146:45–58. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Pan Y, Wang N, Xia P, Wang E, Guo Q and Ye
Z: Inhibition of Rac1 ameliorates neuronal oxidative stress damage
via reducing Bcl-2/Rac1 complex formation in mitochondria through
PI3K/Akt/mTOR pathway. Exp Neurol. 300:149–166. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Bajwa E, Pointer CB and Klegeris A: The
role of mitochondrial damage-associated molecular patterns in
chronic neuroinflammation. Mediators Inflamm. 2019:40507962019.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lai Y, Lin P, Chen M, Zhang Y, Chen J,
Zheng M, Liu J, Du H, Chen R, Pan X, et al: Restoration of L-OPA1
alleviates acute ischemic stroke injury in rats via inhibiting
neuronal apoptosis and preserving mitochondrial function. Redox
Biol. 34:1015032020. View Article : Google Scholar : PubMed/NCBI
|