Introduction
Preeclampsia is a condition characterized by high
blood pressure and proteinuria that develops during pregnancy
(1–10). Preeclampsia affects 2.5–10% of all
pregnancies (1–8) and causes serious complications for
both the mother and the fetus. According to the World Health
Organization (WHO) report, 14% of maternal deaths occur due to
hypertensive disorders, ranking second overall (11); moreover, preeclampsia can cause
serious complications, such as placental abruption, hemolysis,
elevated liver enzymes, low platelet count (HELLP) syndrome, and
disseminated intravascular coagulopathy (DIC) (2–7,9,10).
In addition, infants of mothers with preeclampsia are at twice the
risk of neonatal death as those without preeclampsia and have a
higher probability of admission to the neonatal intensive care unit
due to preterm birth or low birth weight (12–14). Accordingly, prevention and
treatment strategies for patients with preeclampsia have been one
of the most important topics in obstetrics.
The pathogenesis of preeclampsia has long been
shrouded in mystery but has slowly become clearer. Preeclampsia
occurs only in the presence of the placenta (2,10,15), and delivery is the ultimate
treatment (2–4,15),
which strongly suggests a relationship between the placenta and
preeclampsia. Although inadequate remodeling of the spiral artery
could be one of the mechanisms for the development of preeclampsia,
this condition cannot be explained by this single mechanism, and
other mechanisms could be present in the development of
preeclampsia (2,4,10,15). For example, an increased level of
hypoxia-inducible family (HIF) 1-α in the placenta of preeclamptic
women has been reported, suggesting an association between
preeclampsia and hypoxia in the placenta (16).
Preeclampsia is classified into two types:
early-onset (disease onset before 34 weeks) and late-onset diseases
(disease onset after 34 weeks) (1). Early-onset preeclampsia is reported
to show a poorer prognosis in the neonate period and more frequent
intrauterine fetal growth retardation compared with late-onset
(12). Sezer et al
reported no significant association between angiogenic factors,
such as placental growth factor, vascular endothelial growth
factor, and HIF-1α, in the placenta, and the number of weeks at
preeclampsia onset (17).
However, it has been speculated that early- and late-onset
preeclampsia have different developmental mechanisms (7,12).
Although several research studies have addressed the pathogenesis
of preeclampsia, especially the differences between early- and
late-onset preeclampsia (7,12,17), these differences have not yet been
fully elucidated. CD200 belongs to the immunoglobulin superfamily,
and its signaling leads to immune tolerance (18–20). Immune tolerance plays important
roles in pregnancy, and the association between CD200 and
preeclampsia has been addressed in a study (21). However, difference of CD200
expression between early- and late-onset preeclampsia has not yet
been analyzed.
The purpose of this study was to clarify the
differences between early- and late-onset preeclampsia using
comprehensive gene expression and immunohistochemical analyses.
Materials and methods
Patient selection
This study enrolled patients with preeclampsia, from
January 2014 to December 2020, at Kansai Medical University
Hospital. Preeclampsia was defined as hypertension (systolic blood
pressure of ≥140 mmHg and/or diastolic ≥90 mmHg) and proteinuria
(1+ in randomly collected urine or >0.3 g in a 24-h interval)
(3,5,22)
We excluded patients aged <20 years at delivery, and those with
chorioamnionitis, fetal anomaly, twin pregnancy, and placental
abruption. Patients with preeclampsia were classified into the
following two groups according to the gestational age of onset of
preeclampsia: the early-onset group (gestational age <34 weeks)
and the late-onset groups (gestational age ≥34 weeks) (1).
This study was conducted in accordance with the
Declaration of Helsinki and was approved by the Institutional
Review Board of Kansai Medical University Hospital (approval no.
2018138). Before December 2018, informed consent was obtained from
patients by implementing the opt-out method, owing to the
retrospective design of the study, with assurance of no risk to the
participants. Information regarding this study, such as the
inclusion criteria and the opportunity to opt out, was provided
through the institutional website (https://www.kmu.ac.jp/hirakata/hospital/2671t800000124re-att/a1625627306468.pdf).
Patients who went into delivery after January 2019 provided written
informed consent for sample collection and its subsequent
analysis.
RNA extraction
RNA was isolated from the archived samples. Briefly,
for mRNA extraction, 5 µm-thick sections were examined from the
archived formalin-fixed and paraffin-embedded (FFPE) blocks of the
placenta from five patients each in the early- and late-onset
groups, and two blocks from normal controls. For the placental
block, the part with the attached umbilical cord was selected for
each patient. RNA was extracted using a NucleoSpin total RNA FFPE
kit (Macherey-Nagel GmBH & Co. KG), including an on-column
DNase treatment. The quantitative evaluation of RNA was performed
using a Nanodrop 1000 spectrophotometer (Thermo Fisher Scientific,
Inc.). The quality of RNA was evaluated by measuring the ratio of
260/280 nm. We excluded samples in which the total amount of RNA
was <50 ng/µl or the 260/280 ratio was <1.6 (23).
Analysis of comprehensive mRNA
expression profiles
We studied the expression profiles of 770
immune-related genes using the nCounter PanCancer Immune Profiling
Panel (NanoString Technologies, Inc.). The nCounter assay was
performed according to the manufacturer's instructions. The RNA was
hybridized with the probe sets for 16 h at 67°C, and the samples
were processed using an automated nCounter Sample Prep Station
(NanoString Technologies, Inc.). Cartridges containing immobilized
and aligned reporter complexes were subsequently imaged on an
nCounter Digital Analyzer (NanoString Technologies, Inc.) that had
been set at a data resolution of 555 fields of view. Reporter
counts were collected and normalized using the nSolver analysis
software (version 3.0; NanoString Technologies, Inc.). The analysis
between the two groups was performed using a volcano plot after
adjusting for the control group (23). Sequencing data were deposited into
the DNA Data Bank of Japan Sequence Read Archive (accession nos.
SAMD00549508-SAMD00549517).
Immunohistochemistry
The protein expression of CD200 was analyzed by
immunohistochemistry. FFPE blocks of the placenta of the part with
the attached umbilical cord were cut into 4-µm-thick sections.
Subsequently, the samples were deparaffinized and rehydrated.
Immunohistochemical analyses were performed using an autostainer
(Discovery XT System; Roche Diagnostics), according to the
manufacturer's instructions. The primary antibody used was a rabbit
polyclonal antibody against CD200/OX2 (ab203887; Abcam; dilution
1:100). The staining intensity was classified into four categories
as follows: 0=no staining, 1=weak staining, 2=moderate staining,
and 3=strong staining. The scoring was independently done by at
least two researchers blind to the clinical information.
Statistical Analysis
In the analysis of nCounter, statistical software R
version 3.5.2 (R Project, Vienna, Austria) was used to perform
significance analysis of the identification of differentially
expressed genes between normal and preeclampsia placental tissue
samples. The counted raw RNA-seq data were normalized and analyzed
between groups using the R software extension package ‘DESeq’ 2
available in Bioconductor (www.bioconductor.org/packages/release/bioc/html/DESeq2.html).
Genes with an absolute value of log2 fold change (log2FC) >2 and
the adjusted P-value <0.00001 were defined as DEGs. The volcano
plot was visualized using the ggplot2 package in R (24).
Other data were presented as mean ± standard error
and analyzed by unpaired and paired t-tests using Prism 8 computer
software (GraphPad Software). The analysis between the two groups
was performed using the Mann-Whitney test, and the analysis between
the three groups was performed using the Kruskal-Wallis test
(followed by the Dunn or Dunn-Bonferroni posterior test method). A
probability level of <0.05 was considered statistically
significant.
Results
Comprehensive mRNA expression
profiles
Table I summarizes
the clinicopathological features of the 10 patients with
preeclampsia (5 patients each in the early- and late-onset groups)
used for the comprehensive mRNA expression analysis. Gestational
age at onset of preeclampsia was significantly different between
the early-onset (mean 33.2 weeks) and late-onset (mean 35.6 weeks)
groups (P<0.05). Age at delivery, systolic and diastolic blood
pressures, birth weight, and placental weight were not
significantly different between the two groups.
 | Table I.Clinicopathological characteristics
of ten patients with preeclampsia who were studied the mRNA
expression profiling. |
Table I.
Clinicopathological characteristics
of ten patients with preeclampsia who were studied the mRNA
expression profiling.
|
Characteristics | Early-onset
(n=5) | Late-onset
(n=5) | P-value |
|---|
| Age, years | 28 (26–38) | 33 (29–37) | 0.22 |
| Primipara, cases
(%) | 3 (60%) | 5 (100%) | 0.44 |
| Gestational age at
onset, weeks | 33.2
(24.0-33.5) | 35.6
(34.2-38.1) | <0.01 |
| Gestational age at
delivery, weeks | 33.5
(27.5-37.3) | 35.6
(35.3-38.1) | 0.13 |
| Systolic blood
pressure, mmHg | 160 (143–163) | 162 (141–165) | 0.60 |
| Diastolic blood
pressure, mmHg | 96 (89–100) | 109 (70–130) | 0.39 |
| Vaginal delivery,
cases (%) | 3 (60%) | 5 (100%) | 0.44 |
| Birth weight,
g | 1,512
(529–2,394) | 1,806
(1,698–2,715) | 0.15 |
| Placenta weight,
g | 365 (220–480) | 380 (310–510) | 0.69 |
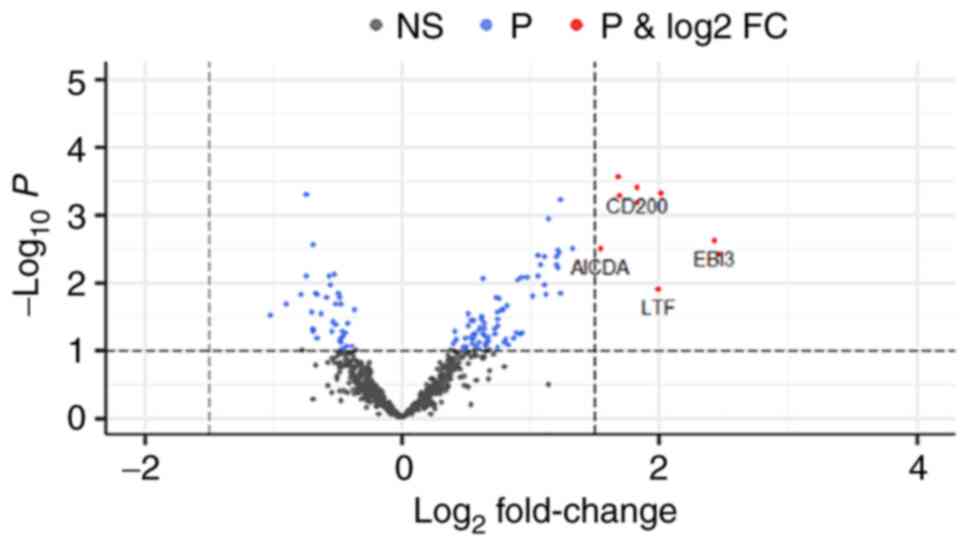
The volcano plot of different gene expression levels
between the early- and late-onset groups is shown in Fig. 1. CD200, AICDA, EBI3, and
lactoferrin were significantly upregulated in the
early-onset group, as compared to the late-onset group (adjusted
P<0.05).
Immunohistochemistry analysis
Table II
summarizes the clinicopathological features of the 53 patients with
preeclampsia (26 and 27 patients in the early- and late-onset
groups, respectively) and 9 normotensive pregnant women (the
control group) included in this analysis. The patients who
performed the comprehensive mRNA expression analysis were also
included in the immunohistochemical analysis. Gestational age at
onset of preeclampsia was significantly earlier in the early-onset
group than in the late-onset group (P<0.05). Gestational age at
delivery was significantly earlier and birth weight was
significantly lower in early-onset group than in the late-onset and
control groups (P<0.05). Systolic and diastolic blood pressures
were significantly higher in the early- and late-onset groups than
in the control group (P<0.05). Other factors were not
significantly different among the three groups.
 | Table II.Clinicopathological characteristics
of 62 patients with preeclampsia, including the ones performed the
immunohistochemistry analysis. |
Table II.
Clinicopathological characteristics
of 62 patients with preeclampsia, including the ones performed the
immunohistochemistry analysis.
| Characteristic | Control group
(n=9) | Early-onset group
(n=26) | Late-onset group
(n=27) | P-value |
|---|
| Age, years | 35 (24–43) | 34 (24–46) | 33 (23–40) | 0.59 |
| Primipara, cases
(%) | 9 (100%) | 17 (65.3%) | 17 (63.0%) | 0.08 |
| Gestational age at
onset, weeks | ND | 31.3
(24.0–33.6) | 35.6
(34.0–40.5) |
<0.01a |
| Gestational age at
delivery, weeks | 40.2
(37.0–41.2) | 34.0
(27.0–38.0) | 37.1
(34.5–40.5) |
<0.01b |
| Systolic blood
pressure, mmHg | 116 (79–147) | 163 (138–220) | 156 (129–192) |
<0.01c |
| Diastolic blood
pressure, mmHg | 64 (47–84) | 102 (82–125) | 98 (70–124) |
<0.01c |
| Vaginal delivery,
cases (%) | 0 (0%) | 5 (19.2%) | 7 (25.9%) | 0.24 |
| Birth weight,
g | 2,750
(2,450–3,585) | 1,514
(529–2,584) | 2,355
(1,554–3,315) |
<0.01b |
| Placenta weight,
g | 510 (350–655) | 350 (220–630) | 405 (260–690) |
<0.01b |
Table III
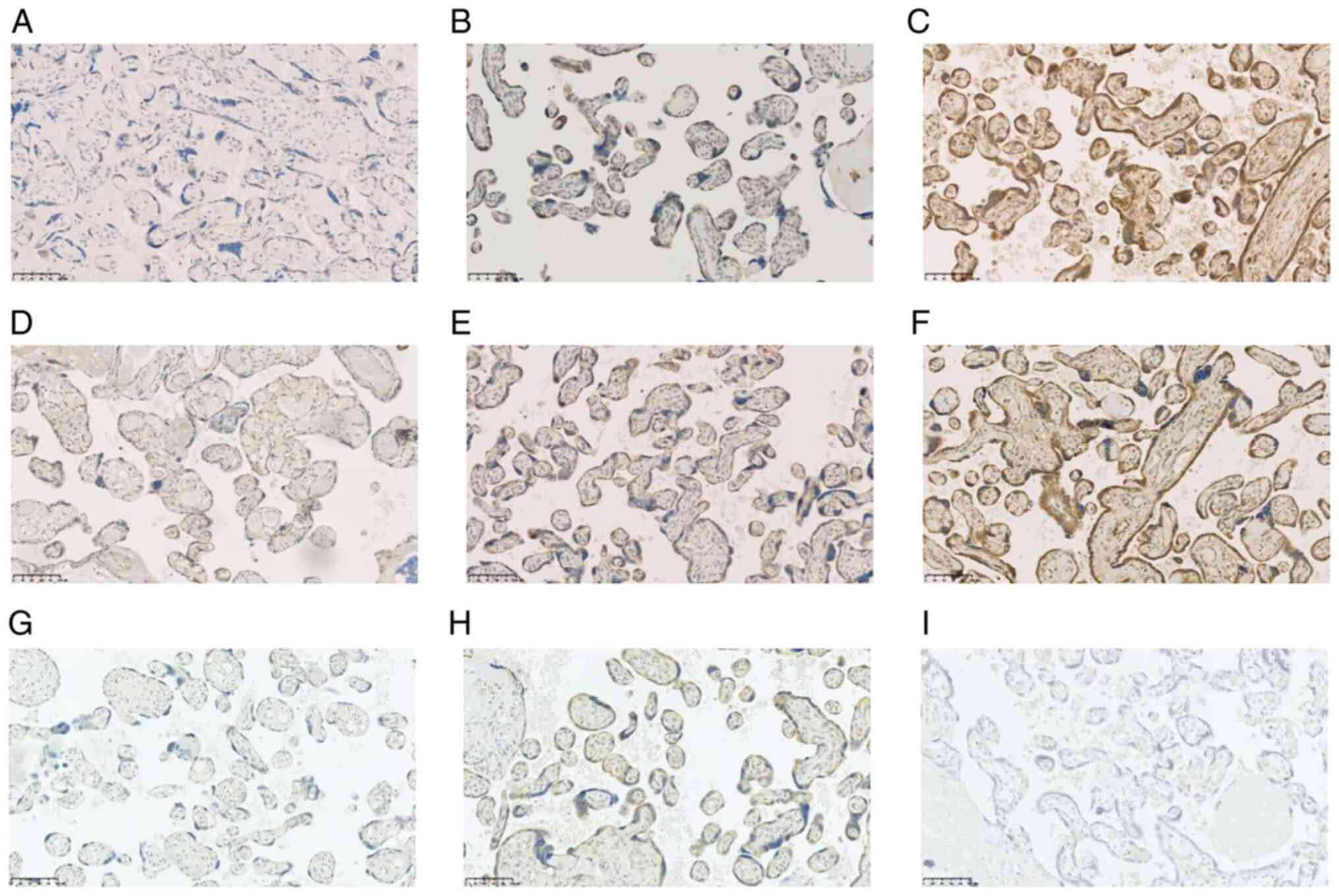
summarizes the results of the immunohistochemical analysis. CD200
was expressed in the syncytial trophoblasts (Fig. 2). The staining intensity of CD200
was significantly stronger in the early-onset group than in the
late-onset and control groups (P<0.05). There was no significant
difference in the staining intensity of CD200 between the
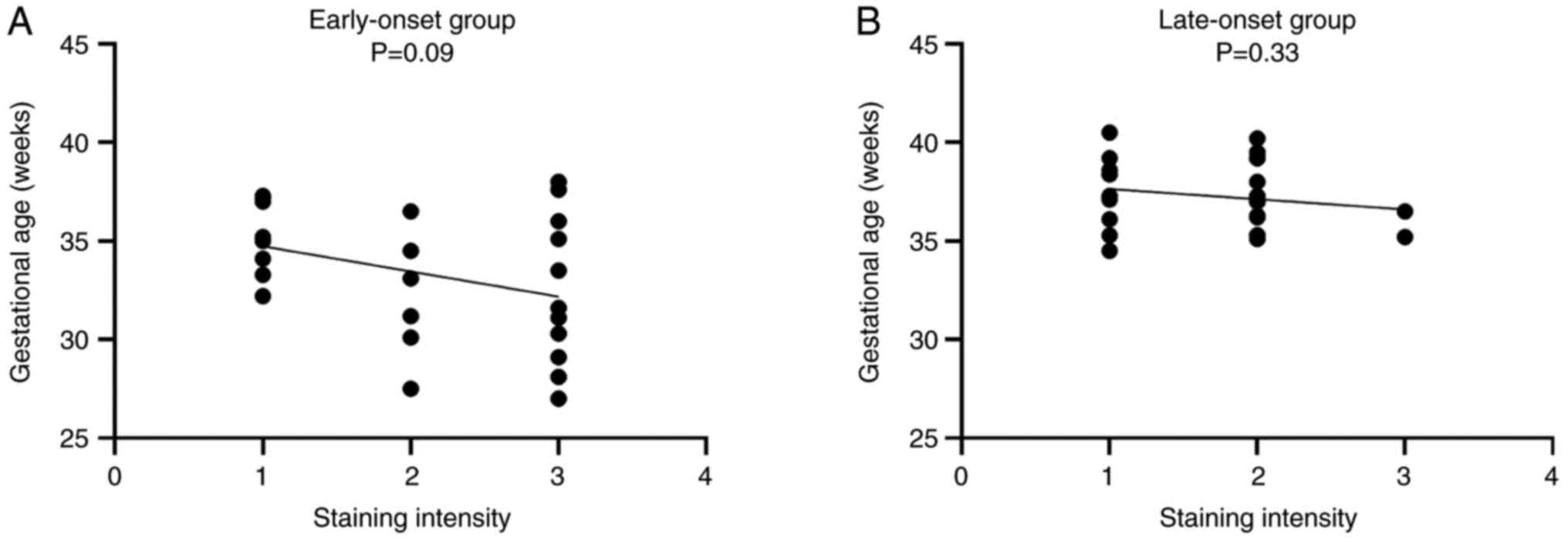
late-onset and control groups. Moreover, in the early-onset group,
the staining intensity of CD200 showed a stronger tendency in
patients with an earlier gestational age compared to those with a
later gestational age, although the difference was not significant
(P=0.09). However, this trend was not observed in the late-onset
group (P=0.33) (Fig. 3).
 | Table III.Results of the immunohistochemical
analysis for CD200. |
Table III.
Results of the immunohistochemical
analysis for CD200.
| Staining
intensity | Control group
(n=9) | Early-onset group
(n=26) | Late-onset group
(n=27) | P-value |
|---|
| 0 | 0 (0%) | 0 (0%) | 0 (0%) |
<0.01a |
| 1 | 8 (88.9%) | 8 (30.8%) | 11 (40.7%) |
|
| 2 | 1 (11.1%) | 7 (26.9%) | 14 (51.9%) |
|
| 3 | 0 | 11 (42.3%) | 2 (7.4%) |
|
Discussion
Preeclampsia is one of the most important conditions
requiring treatment for pregnant women. Especially, early-onset
preeclampsia is reported to have a poor prognosis in the neonatal
period (12). In particular,
early-onset preeclampsia is more likely to cause intrauterine fetal
growth retardation than late-onset preeclampsia (25). In fact, significant morphological
differences, including villous and vasculature features, have been
reported between the placentas of normotensive pregnant women and
those of pregnant women with early-onset preeclampsia, while the
placentas of pregnant women with late-onset preeclampsia are not
significantly different from those of normotensive pregnant women
with corresponded gestational age (25,26). These facts indicate that early-
and late-onset preeclampsia are not caused by the same mechanism.
Although it has been recognized that inadequate remodeling of the
spiral artery plays an important role in the development of
preeclampsia, a systemic inflammatory response due to placental
stress, such as hypoxia, is also considered one of the pathogenic
mechanisms (27–29). Microarray studies of placental RNA
have shown that gene expressions of immune and inflammatory systems
in the placenta of preeclampsia patients are different from those
in the normotensive placenta (30). In particular, changes in the
levels of angiogenic factors, such as vascular endothelial growth
factor and placental growth factor, have been strongly associated
with the development of preeclampsia (31). However, the association of these
factors between early- and late-onset preeclampsia has not yet been
analyzed. Thus, we aimed to clarify the differences of the two
subtypes of preeclampsia.
We first used the nCounter immune profiling panel to
analyze differences in RNA expression of 770 genes in the placenta
between early- and late-onset preeclampsia, and the results showed
that four genes, including CD200, were significantly
upregulated in early-onset preeclampsia. Among them, we focused on
CD200, because this protein was found to be expressed in the
placenta in a previous study (21). Following the results of the RNA
expression, we performed the immunohistochemical validation study
in a larger number of patients with preeclampsia.
Immunohistochemical analysis also showed that staining intensity
for CD200 in the trophoblasts was significantly stronger in the
early-onset preeclampsia group than in the late-onset group.
Accordingly, CD200 expression is considered to be significantly
upregulated in patients with early-onset preeclampsia by both RNA
and protein expression levels.
CD200 belongs to the immunoglobulin superfamily and
is expressed on diverse cell types and tissues, from B lymphocytes
in the spleen to neurons in the central nervous system, and
directly and continuously regulates macrophages and granulocytes
through interaction with CD200R (18,19). CD200 signaling has been reported
to inhibit classical macrophage activation (M1 polarization) and
support an immunosuppressive M2 polarized state, resulting in
immune tolerance (19,20). Immune tolerance is an important
intrinsic mechanism in implantation. It has been reported that
CD200 expression was significantly decreased in the villi of
patients with early spontaneous abortion (32), suggesting that CD200 plays
important roles in implantation. In addition, the polarization to
M2 macrophages, which occurs physiologically during pregnancy, is
thought to suppress the development of preeclampsia, and it has
been reported that M1 macrophages were increased in the decidual
tissue in patients with acute atherosis (19). Accordingly, macrophage
polarization plays important roles in pregnancy and its disorders.
Xu et al reported that CD200 expression was significantly
downregulated in preeclampsia compared with normal placenta
(21); however, they did not
stratify the cases into early- and late-onset groups and might have
included more patients with late-onset preeclampsia because the
median gestational week was 36 weeks in their series. In addition,
the period between the onset of preeclampsia and delivery might
influence CD200 expression and macrophage polarization in the
placenta (the data regarding this period was not available in the
study by Xu et al (21).
They also found that Th1 cytokines were upregulated and Th2
cytokines were downregulated in trophoblasts (21). In contrast, the results of the
present study showed that CD200 was significantly upregulated in
the early-onset group. The significance of CD200 expression in the
trophoblasts might differ between early- and late-onset
preeclampsia, although it remains unclear whether CD200 expression
is the mainstream of preeclampsia development. CD200 expression
might be the outcome or main cause of preeclampsia development. It
is possible that excessive macrophage polarization influences the
development of early-onset preeclampsia. In addition, significantly
lower CD200 expression was noted in oocyte donation pregnancies
with preeclampsia compared to those without preeclampsia, and this
was not observed in naturally conceived pregnancies (33). Similarly, in preeclampsia
pregnancies, significantly lower CD200 expression was noted in
oocyte donation pregnancies compared to naturally conceived
pregnancies (33). Thus, CD200
might have a role in the gestation processes of oocyte donation
pregnancies. Moreover, additional mechanisms other than macrophage
polarization might be involved in the development of preeclampsia.
Additional studies are needed to clarify this issue.
There are some limitations in this study. First, we
analyzed the immunohistochemical expression for CD200 in a
relatively large number of patients (53 patients) with
preeclampsia; however, RNA expression profiles analysis was
performed in only 10 patients. Thus, further studies on both RNA
expression and immunohistochemical analysis with a larger sample
size of patients with early- and late-onset preeclampsia are
needed. Second, the present study demonstrated that CD200 was
significantly upregulated in early-onset preeclampsia. Although
CD200 is considered to act in immune tolerance via macrophage
polarization, distribution of M1 and M2 macrophages using CD68 and
CD163 immunostaining in the placenta of patients with early- and
late-onset preeclampsia was not analyzed in the present study.
Thus, the relationship between CD200 expression and distribution of
macrophages in the development of preeclampsia needs to be
clarified.
In summary, the present study demonstrated that
CD200 expression was significantly upregulated in patients with
early-onset preeclampsia, although it remains unknown whether
upregulation of CD200 is a cause or effect of development of
early-onset preeclampsia. Thus, further studies are needed to
clarify the mechanism of these conditions for adequate
treatment.
Acknowledgements
The authors would like to thank Mr Ryosuke Yamaka
who is a research assistant of Kansai Medical University (Osaka,
Japan) who provided assistance in this study.
Funding
This research was partially supported by the research grant D2
from Kansai Medical University.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request. Sequencing data were deposited into the DNA Data Bank of
Japan Sequence Read Archive (accession nos. SAMD00549508 and
SAMD00549517; http://ddbj.nig.ac.jp/resource/bioproject/PRJDB14567).
Authors' contributions
HT and HO conceived and designed the study, and
performed gene expression analyses. HT and MI performed
immunohistochemical analyses. HT, MI, AN, TY, SK, YB, AY, YHi, YHa,
TTN, HM, KT and HO acquired and analyzed data. HT, MI, and HO
confirmed the authenticity of all the raw data, and drafted the
manuscript, tables, and figures. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
This retrospective single-institution study was
conducted in accordance with the principles of the Declaration of
Helsinki, and the study protocol was approved by the Institutional
Review Board of the Kansai Medical University Hospital (approval
no. 2018138; Hirakata, Japan). All data are completely anonymized.
The Institutional Review Board waived the requirement for informed
consent due to the retrospective design of the study, using medical
records and archival samples with no risk of identity exposure of
the patients. Moreover, the present study did not include any
minors.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
DIC
|
disseminated intravascular
coagulopathy
|
|
FFPE
|
formalin-fixed and
paraffin-embedded
|
|
HELLP
|
hemolysis, elevated liver enzymes, low
platelet count
|
|
HIF
|
hypoxia-inducible family
|
|
log2FC
|
log2 fold change
|
|
WHO
|
World Health Organization
|
References
|
1
|
von Dadelszen P, Magee LA and Roberts JM:
Subclassification of preeclampsia. Hypertens Pregnancy. 22:143–148.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Walker JJ: Pre-eclampsia. Lancet.
356:1260–1265. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wagner LK: Diagnosis and management of
preeclampsia. Am Fam Physician. 70:2317–2324. 2004.PubMed/NCBI
|
|
4
|
Report of the National high blood pressure
education program working group on high blood pressure in
pregnancy. Am J Obstet Gynecol. 183:S1–S22. 2000. View Article : Google Scholar
|
|
5
|
Wagner SJ, Barac S and Garovic VD:
Hypertensive pregnancy disorders: Current concepts. J Clin
Hypertens (Greenwich). 9:560–566. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kuklina EV, Ayala C and Callaghan WM:
Hypertensive disorders and severe obstetric morbidity in the United
States. Obstet Gynecol. 113:1299–1306. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aneman I, Pienaar D, Suvakov S, Simic TP,
Garovic VD and McClements L: Mechanisms of key innate immune cells
in early- and late-onset preeclampsia. Front Immunol. 11:18642020.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Redman CW and Sargent IL: Latest advances
in understanding preeclampsia. Science. 308:1592–1594. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Norwitz ER, Hsu CD and Repke JT: Acute
complications of preeclampsia. Clin Obstet Gynecol. 45:308–329.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Karumanchi SA, Maynard SE, Stillman IE,
Epstein FH and Sukhatme VP: Preeclampsia: A renal perspective.
Kidney Int. 67:2101–2113. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Say L, Chou D, Gemmill A, Tunçalp Ö,
Moller AB, Daniels J, Gülmezoglu AM, Temmerman M and Alkema L:
Global causes of maternal death: A WHO systematic analysis. Lancet
Glob Health. 2:e323–e333. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lisonkova S and Joseph KS: Incidence of
preeclampsia: Risk factors and outcomes associated with
early-versus late-onset disease. Am J Obstet Gynecol.
209:544.e1–544.e12. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Basso O, Rasmussen S, Weinberg CR, Wilcox
AJ, Irgens LM and Skjaerven R: Trends in fetal and infant survival
following preeclampsia. JAMA. 296:1357–1362. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Backes CH, Markham K, Moorehead P, Cordero
L, Nankervis CA and Giannone PJ: Maternal preeclampsia and neonatal
outcomes. J Pregnancy. 2011:2143652011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Burton GJ, Redman CW, Roberts JM and
Moffett A: Pre-eclampsia: Pathophysiology and clinical
implications. BMJ. 366:l23812019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rolfo A, Many A, Racano A, Tal R,
Tagliaferro A, Ietta F, Wang J, Post M and Caniggia I:
Abnormalities in oxygen sensing define early and late onset
preeclampsia as distinct pathologies. PLoS One. 5:e132882010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sezer SD, Küçük M, Döger FK, Yüksel H,
Odabaşı AR, Türkmen MK, Cakmak BÇ, Ömürlü İK and Kınaş MG: VEGF,
PIGF and HIF-1α in placentas of early- and late-onset pre-eclamptic
patients. Gynecol Endocrinol. 29:797–800. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hoek RM, Ruuls SR, Murphy CA, Wright GJ,
Goddard R, Zurawski SM, Blom B, Homola ME, Streit WJ, Brown MH, et
al: Down-regulation of the macrophage lineage through interaction
with OX2 (CD200). Science. 290:1768–1771. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang S, Cherwinski H, Sedgwick JD and
Phillips JH: Molecular mechanisms of CD200 inhibition of mast cell
activation. J Immunol. 173:6786–6793. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Y, Zhang D, Xu L, Dong L, Zheng J, Lin
Y, Huang J, Zhang Y, Tao Y, Zang X, et al: Cell-cell contact with
proinflammatory macrophages enhances the immunotherapeutic effect
of mesenchymal stem cells in two abortion models. Cell Mol Immunol.
16:908–920. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu J, Gu Y, Sun J, Zhu H, Lewis DF and
Wang Y: Reduced CD200 expression is associated with altered Th1/Th2
cytokine production in placental trophoblasts from preeclampsia. Am
J Reprod Immunol. 79:110.1111/aji.12763. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
ACOG Committee on Obstetric Practice, .
ACOG practice bulletin. Diagnosis and management of preeclampsia
and eclampsia. Number 33, January 2002. American college of
obstetricians and gynecologists. Int J Gynaecol Obstet. 77:67–75.
2002.PubMed/NCBI
|
|
23
|
Ryota H, Ishida M, Satoi S, Yanagimoto H,
Yamamoto T, Kosaka H, Hirooka S, Yamaki S, Kotsuka M, Matsui Y, et
al: Clinicopathological and immunological features of follicular
pancreatitis-a distinct disease entity characterised by Th17
activation. Histopathology. 74:709–717. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Anders S and Huber W: Differential
expression analysis for sequence count data. Genome Biol.
11:R1062010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Egbor M, Ansari T, Morris N, Green CJ and
Sibbons PD: Morphometric placental villous and vascular
abnormalities in early- and late-onset pre-eclampsia with and
without fetal growth restriction. BJOG. 113:580–589. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
van der Merwe JL, Hall DR, Wright C,
Schubert P and Grové D: Are early and late preeclampsia distinct
subclasses of the disease-what does the placenta reveal? Hypertens
Pregnancy. 29:457–467. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Staff AC: The two-stage placental model of
preeclampsia: An update. J Reprod Immunol. 134–135. 1–10.
2019.PubMed/NCBI
|
|
28
|
Miller D, Motomura K, Galaz J, Gershater
M, Lee ED, Romero R and Gomez-Lopez N: Cellular immune responses in
the pathophysiology of preeclampsia. J Leukoc Biol. 111:237–260.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dekker GA and Sibai BM: Etiology and
pathogenesis of preeclampsia: Current concepts. Am J Obstet
Gynecol. 179:1359–1375. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Enquobahrie DA, Meller M, Rice K, Psaty
BM, Siscovick DS and Williams MA: Differential placental gene
expression in preeclampsia. Am J Obstet Gynecol. 199:566.e1–11.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shibata E, Rajakumar A, Powers RW, Larkin
RW, Gilmour C, Bodnar LM, Crombleholme WR, Ness RB, Roberts JM and
Hubel CA: Soluble fms-like tyrosine kinase 1 is increased in
preeclampsia but not in normotensive pregnancies with
small-for-gestational-age neonates: Relationship to circulating
placental growth factor. J Clin Endocrinol Metab. 90:4895–4903.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang LQ, Yan CF, Zhao Y, Chu J and Yu XW:
Reduced CD200 and CD200R1 expression in human chorionic villi
contributes to early spontaneous abortion. Acta Obstet Gynecol
Scand. 93:1248–1254. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
van 't Hof LJ, Dijkstra KL, van der Keur
C, Eikmans M, Baelde HJ, Bos M and van der Hoorn MLP: Decreased
expression of ligands of placental immune checkpoint inhibitors in
uncomplicated and preeclamptic oocyte donation pregnancies. J
Reprod Immunol. 142:1031942020. View Article : Google Scholar : PubMed/NCBI
|