Introduction
Cataracts are a major cause of visual impairment
according to the World Health Organization, second only to
uncorrected refractive errors (1). Cataractogenesis is a multifactorial
process caused by aggregation of misfolded crystalline proteins
(2); cataract types are
classified according to the section of the lens exhibiting opacity,
such as cortical, nuclear, and posterior subcapsular cataracts.
Several factors including aging, diabetes, ultraviolet rays, diet,
genetic predisposition, dehydration, oxidative stress, and lipid
peroxidation have been shown to cause cataract formation (3). A study of the global burden of
diseases reported that cataract treatments are lacking in
developing countries and that cataract was the leading cause of
blindness in 2020. There are 15.2 million patients with cataract
aged 50 years and above, accounting for 45.2% of patients who are
blind (4).
Nuclear cataracts typically develop gradually with
age and are the most common morphological form of age-related
cataracts worldwide (5,6). These comprise the most important
category of senile cataract types, as they cause visual
disturbances with increasing higher-order aberration, forward light
scattering, and backward light scattering even in the early stage
(7). Individuals living in
geographic regions with higher temperatures tend to develop
presbyopia earlier (8). Moreover,
our epidemiological studies showed that the prevalence of nuclear
cataracts of grade 1 and above as per the World Health Organization
cataract grading system was significantly higher in tropical or
subtropical regions as compared to that in temperate or subarctic
regions, regardless of race (9,10).
In our previous research, using a large-scale integrated
computational method, we found that the eye lens temperature may
reach 37.0-37.5°C or higher with increasing environmental
temperature. By setting the threshold of the lens temperature at
37.0°C, we found a positive correlation between the cumulative heat
load, which is an indicator of the cumulative temperature
difference above 37°C and nuclear cataract incidence for 10 years;
we also found an association between the time course of the mean
lens temperature rise and nuclear cataract incidence (11). Thus, long-term exposure to a
high-temperature environment may increase the risk of nuclear
cataract development. However, the detailed association between
temperature and nuclear cataracts remains unclear.
A previous study of the human eye showed that the
mean nuclear diameter and thickness were 6.51 ± 0.75 and 2.96 ±
0.33 mm, respectively (12) and
that these values significantly increased with increasing
opacification of the nucleus. Particularly, the thickness and
diameter based on the Lens Opacities Classification System III were
grades 5 and 6 (12). Moreover,
the density of human lens epithelium (HLE) cells of the central
anterior capsule in nuclear cataracts was higher than that in
normal lenses (>NIII), indicating that individual differences in
HLE cell proliferation and density are important underlying causes
of nuclear cataracts (13).
The mitochondria play key roles in regulating cell
proliferation and apoptosis (14). They also provide energy for
anabolic processes, hormone secretion, muscular work, and ionic
pumps (15–17). Most of this energy is derived from
oxidative phosphorylation, during which electrons are transferred
from nicotinamide adenine dinucleotide to oxygen, protons are
extruded, and the energy stored as an inner membrane potential is
dissipated and accumulated as ATP by ATP synthase. Cytochrome c
oxidase (CCO) and sequential oxidoreductive reactions play central
roles in ATP production. Specifically, the inner membrane of
mitochondria contains protein complexes of the respiratory chain
electron transfer system, and electrons pass through complexes I,
III and IV (CCO) to oxygen. In conjugation with this electron
transfer (redox) reaction, protons (hydrogen ions) are pumped from
the inner to the outer membrane, forming a proton electrochemical
gradient across the membrane. This potential energy drives the
rotational motion of complex V (ATP synthase), enabling the
synthesis of ATP. In eukaryotes, the oligomeric enzyme is bound to
the mitochondrial inner membrane with 7–13 subunits. Thus, its
biosynthesis involves coordinate interplay between the nuclear and
mitochondrial genomes. The largest subunits I, II and III, which
represent the catalytic core of the enzyme, are encoded by
mitochondrial DNA and are synthesized within the mitochondria. The
remaining smaller subunits implicated in the regulatory function
are encoded by nuclear DNA and imported to the mitochondria
following their synthesis in the cytosol. Some nuclear coded
subunits are expressed in tissue- and developmental-specific
isologs (18). On the other hand,
complex IV consists of enzymes composed of cytochrome a,
CuA, CuB, and cytochrome a3, and
the three subtypes of mitochondrial cytochrome c oxidase 1, 2 and 3
(CCO1-3) relate this CCO function (19). However, it is not clear enough
which part of complex IV is composed of CCO-1, 2 and 3.
We previously investigated whether mRNA expression
and ATP levels of the three subtypes of mitochondrial cytochrome c
oxidase 1, 2 and 3 vary with cataract type and severity in the
human lens. We found that mitochondrial CCO1-3 mRNA expression in
patients with early-stage cataract was significantly upregulated
compared to that in normal patients, indicating that ATP production
by the lens epithelium is enhanced in early-stage cataracts in
Japanese patients (20).
Increased ATP in mitochondria may contribute to lens opacity,
making it important to investigate changes in cell proliferation
and mitochondrial function in HLE cells under low- and
high-temperature conditions to determine the risk of nuclear
cataract development in high-temperature environments. In this
study, we investigated whether mitochondrial gene expression and
function, ATP production, and cell proliferation capacity were
altered under low- and high-temperature conditions using two
different HLE cell lines, SRA01/04 and iHLEC-NY2.
Materials and methods
Reagents
The mitochondrial isolation kit, CCO assay kit, and
ATP bioluminescent assay kit were obtained from Sigma-Aldrich (St.
Louis, MO, USA). RNase-free DNase and RNeasy Mini kits were
provided by Qiagen (Hilden, Germany). The RNA PCR kit was purchased
from Takara Bio (Shiga, Japan), and the LightCycler FastStart DNA
Master SYBR-Green I was provided by Roche Diagnostics Applied
Science (Mannheim, Germany). The Bio-Rad Protein Assay Kit was
purchased from Bio-Rad Laboratories (Hercules, CA, USA). All other
chemicals used were of the highest commercially available
purity.
Cell lines
The HLE-immortalized cell lines SRA01/04 (RCB1591,
Riken BRC, Tsukuba, Japan) and iHLEC-NY2 were donated by Professor
Ibaraki (21) and Professor
Yamamoto (22). Briefly,
iHLEC-NY2 cells were derived from lens epithelial cells (LECs)
collected during cataract surgery in adult humans. LECs were
cultured in Dulbecco's modified Eagle's medium (DMEM) high-glucose
medium containing 10% fetal bovine serum (FBS). The immortalization
gene was transferred to the pseudo-attachment P site of cultured
LECs, and the cells were cloned to establish the immortalized human
lens epithelial cell line clone NY2 (iHLEC-NY2) (22). The use of iHLEC-NY2 cells was
approved by the Fujita Medical University Recombinant DNA
Experiment Safety Committee (approval no. DP16055) and Kanazawa
Medical University Recombinant DNA Experiment Safety Committee
(approval no. 2020-18). Mycoplasma infections in SRA01/04 and
iHLEC-NY2 cells were detected using a Mycoplasma Detection Kit
(EZ-PCRTM Mycoplasma Test Kit, Biological Industries, Beit Haemek,
Israel) according to the manufacturer's instructions.
Cell culture
SRA01/04 cells were cultured in-low glucose DMEM
(Thermo Fisher Scientific, Waltham, MA, USA) containing 20% (v/v)
heat-inactivated and γ-ray-sterilized FBS (Biological Industries)
and penicillin-streptomycin solution (FUJIFILM Wako Pure Chemical
Corporation, Osaka, Japan). iHLEC-NY2 cells were cultured in
high-glucose DMEM containing 10% FBS, 10 ng/ml basic fibroblast
growth factor, 100× minimum essential medium non-essential amino
acids, 100× GlutaMAX™ (Thermo Fisher Scientific), and
penicillin-streptomycin solution. In computer simulations, when the
ambient temperature was 19–35°C, the estimated lens temperature was
35.0-37.5°C (11). We previously
reported that the prevalence of nuclear cataracts is significantly
higher in tropical regions than in temperate regions (9,10,23). The lens epithelial cell density
and proliferation capacity in vivo may be important
underlying causes of cataracts at the cellular level, particularly
in nuclear cataracts (13).
Therefore, both SRA01/04 and iHLEC-NY2 cells were incubated under
humidified air containing 5% CO2 at 35.0°C or
37.5°C.
Measurement of cell proliferation
The cells were seeded at a density of
2.5×104 cells per well in a cell culture dish (60-mm
diameter, Eppendorf, Hamburg, Germany) and cultured for four days
at 35.0°C or 37.5°C, with the medium replaced every other day. Cell
growth was measured using a Cell Counting Kit-8 (WST-8, FUJIFILM
Wako Pure Chemical Corporation), and the absorbance was measured at
450 nm in a Benchmark Microplate Reader (Bio-Rad Laboratories).
Measurement of cell apoptosis
SRA01/04 and iHLEC-NY2 cells cultured at 35.0 and
37.5°C were removed from the culture dishes and washed with
phosphate-buffered saline. Since iHLEC-NY2 is a GFP-expressing
cell, SRA01/04 and iHLEC-NY2 cells were stained with Annexin V-APC
antibody and Propidium iodide (APC-conjugated Annexin V Apoptosis
Detection Kit, Biolegend, San Diego, CA, USA), and were analyzed by
flow cytometry (CytoFLEXTM Flow Cytometer, Beckman
Coulter, Inc., Brea, CA, USA).
Quantitative real-time RT-PCR
mRNA expression was measured using a LightCycler DX
400, according to our previous report (20). Briefly, total RNA was extracted
from the samples and purified using an RNase-Free DNase Set and
RNeasy Mini Kit. Reverse transcription was performed using an RNA
PCR kit, and PCR was performed using the LightCycler FastStart DNA
Master SYBR-Green I. The RT reaction was carried out at 42°C for 15
min, followed by 95°C for 5 min. The PCR conditions were as
follows: 95°C for 10 min (hot start); 60 cycles at 95°C for 10 sec
(denaturing), 63°C for 10 sec (annealing), and 72°C for 5 sec
(extension). The following specific primers (final concentration 10
pmol) were used: 5′-CCGTCCTAATCACAGCAGTCCTA-3′ and
5′-TGAGGTTGCGGTCTGTTAGTAGT-3′ for CCO-1 (gene ID: 4512, accession
nos.: NC_012920.1, coding sequence: YP_003024028.1, primer
position: 6463-6549); 5′-CCGCCATCATCCTAGTCCTCAT-3′ and
5′-GATCGTTGACCTCGTCTGTTATGT-3′ for CCO-2 (gene ID: 4513, accession
nos.: NC_012920.1, coding sequence: YP_003024029.1, primer
position: 7791-7862); 5′-ACGGCATCTACGGCTCAACA-3′ and
5′-TGGCGGATGAAGCAGATAGTGA-3′ for CCO-3 (gene ID: 4514, accession
nos.: NC_012920.1, coding sequence: YP_003024032.1, primer
position: 9775-9871); and 5′-TGCACCACCAACTGCTTAGC-3′ and
5′-GGCATGGACTGTGGTCATGAG-3′ for glyceraldehyde-3-phosphate
dehydrogenase (gene ID: 2597, accession nos.: NM_002046.7, coding
sequence: NP_002037.2, primer position: 530-616). The differences
in the threshold cycles between the target groups (CCO-1, CCO-2,
and CCO-3) and GAPDH were used to calculate the mRNA expression
levels in the samples.
Measurement of protein
The protein levels in the samples were determined
using the Bradford method with the Bio-Rad Protein Assay Kit with
bovine serum albumin as the standard.
Measurement of CCO activity in
mitochondria
Mitochondria were isolated using a mitochondrial
isolation kit, and CCO activity was measured using a CCO assay kit
according to a previous report (24). Briefly, HLE cells were washed with
ice-cold phosphate-buffered saline, homogenized in pH 7.5 isolation
buffer, and centrifuged at 600 × g for 5 min at 4°C. The
supernatants were centrifuged at 11,000 × g for 10 min at 4°C, and
the pellets were suspended in isolation buffer. The samples were
further centrifuged at 600 × g for 5 min at 4°C, and the
supernatants were centrifuged at 11,000 × g for 10 min at 4°C. The
isolated mitochondria were added to pH 7 buffer consisting of 10 mM
Tris-HCl, 120 mM KCl, and 250 mM sucrose, and the reaction was
initiated by adding ferrocytochrome c (reduced with 0.1 M
dithiothreitol). The decrease in absorbance at 550 nm was measured
for 1 min using a UV2200 spectrophotometer (Shimadzu Corporation,
Kyoto, Japan) according to the manufacturer's instructions. CCO
activity was determined from the decrease in the level of
absorbance at 550 nm and expressed as min/mg protein.
Measurement of ATP
ATP was measured using the luciferin-luciferase
assay method as described previously (18,24,25). Samples were homogenized in 100 µl
saline and centrifuged at 20,400 × g for 15 min at 4°C. The
resulting supernatant was assayed using an ATP bioluminescent assay
kit and luminometer AB-2200 (Atto Corporation, Tokyo, Japan). ATP
levels were expressed as nmol/mg protein.
Measurement of ATPase activity
Both Ca2+-ATPase and
Na+/K+-ATPase activities were measured as
previously described (26).
Briefly, HLE cells were washed with ice-cold pH 7.4
Ca2+, Mg2+-free buffer (290 mOsm) and
harvested using a cell scraper (Iwaki Co., Ltd., Tokyo, Japan). The
composition of the pH 7.4 Ca2+, Mg2+-free
buffer was as follows: 0.5 mM EDTA, 5 mM NaHCO3, 5 mM
KCl, 8 mM Tris, 15 mM HEPES, and 145 mM NaCl in water. The
collected cells were homogenized in 600 ml of hypotonic buffer (pH
7.4) consisting of 10 mM mannitol, 5.75 mM HEPES, and 6.25 mM Tris
base. Unbroken cells were pelleted at a low speed (2,040 × g, 10
min, 4°C), and the supernatant obtained was assayed for ATPase
activity (assessed as Pi liberated from ATP).
Ca2+-ATPase activity was calculated as the difference in
phosphate release measured in the presence or absence of 0.1 mM
Ca2+. Na+/K+-ATPase activity was
calculated as the difference in phosphate release measured in the
presence or absence of 1 mM ouabain. The activity of both plasma
membrane Ca2+-ATPase (PMCA) and sarco (endo) plasmic
reticulum Ca2+-ATPase (SERCA) was measured as
Ca2+-ATPase activity in this study.
Statistical analysis
The data are expressed as the mean ± standard error,
and statistical analysis was performed using unpaired Student's
t-test. The significance level was set at P<0.05.
Results
Effect of high-temperature culture on
growth of SRA01/04 and iHLEC-NY2 cells
The relationship between temperature and cell
proliferation was determined using two HLE cell lines (SRA01/04 and
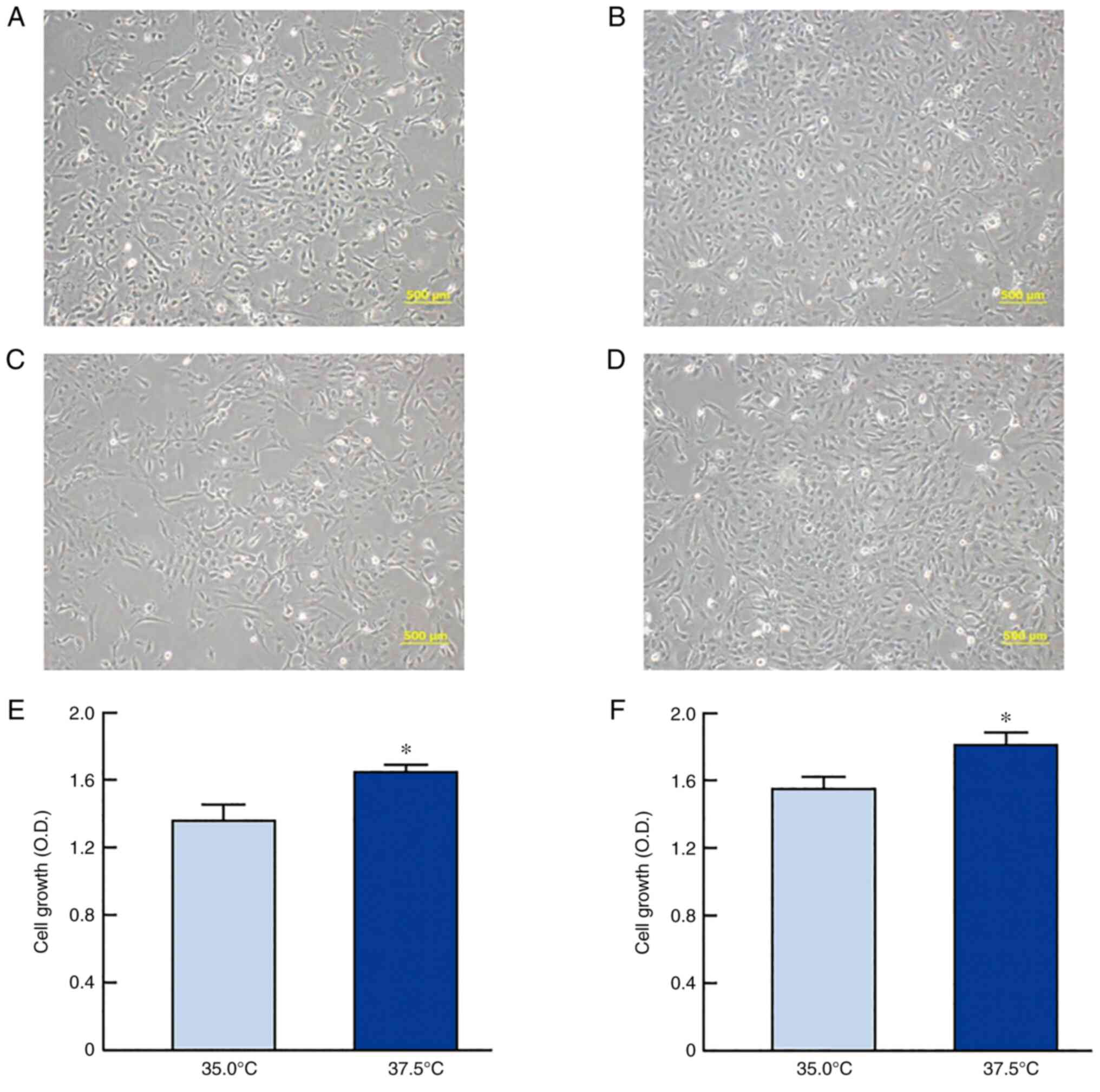
iHLEC-NY2). Fig. 1 shows the
changes in the cell morphology and proliferation of SRA01/04 and
iHLEC-NY2 cells under high- or low-temperature culture. Cell
proliferation was significantly enhanced at high temperature for
both SRA01/04 and iHLEC-NY2 cells, and the growth levels of
SRA01/04 and iHLEC-NY2 cells cultured at 37.5°C were 1.20- and
1.16-fold higher than those at 35.0°C (P<0.05),
respectively.
Activation of mitochondrial function
(CCO activity) in SRA01/04 and iHLEC-NY2 cells under
high-temperature culture
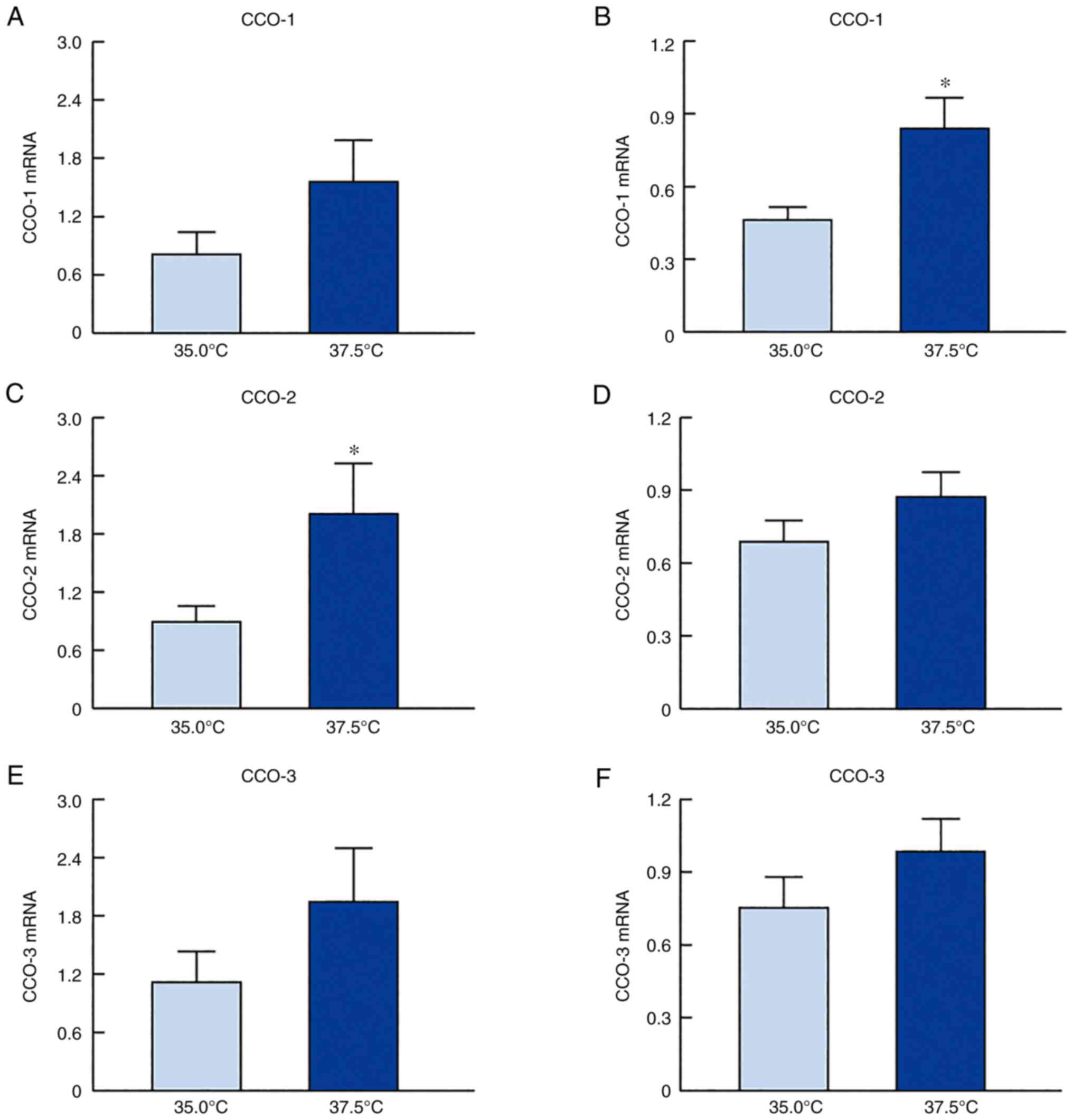
Fig. 2 shows the
changes in the mRNA levels of CCO-1, CCO-2, and CCO-3 in the
SRA01/04 and iHLEC-NY2 cells under low- and high-temperature
cultures. The CCO mRNA expression levels in both cell lines were
higher when the cells were cultured at 37.5°C than when cultured at
35.0°C. Particularly, the level of CCO-2 mRNA expression in
SRA01/04 cells and that of CCO-1 in iHLEC-NY2 cells cultured under
high-temperature conditions (37.5°C) was significantly higher than
those in cells cultured under low-temperature conditions (35.0°C).
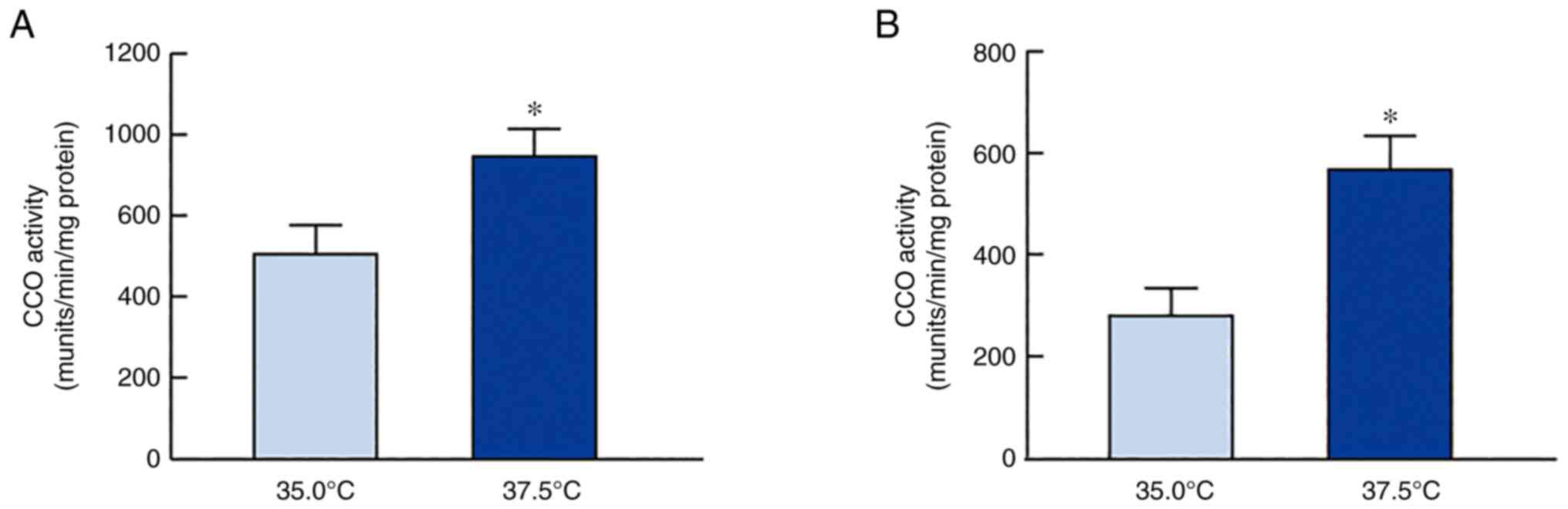
In addition, CCO activity in SRA01/04 and iHLEC-NY2 cells was
measured under low- and high-temperature conditions (Fig. 3). CCO activity was enhanced
following high-temperature culture (37.5°C), with the activity in
SRA01/04 and iHLEC-NY2 showing values 1.48- and 2.02-fold higher
than those at 35.0°C (P<0.05), respectively.
Mitochondria are closely related to apoptosis. We
used immortalized cells, and cell proliferation was slightly slower
at 35.0°C than at 37.5°C; however, the cells still proliferated at
35.0°C. There were a few abnormalities in the cell morphology
(Fig. 1A-D). Because few abnormal
cells were observed, we considered that the difference in
temperature between 37.5°C and 35.0°C had almost no effect on
apoptosis. To corroborate this prediction, we evaluated apoptosis
using an apoptosis detection kit with Annexin V as an indicator.
Almost no apoptotic cells were labeled with Annexin V among the two
cell lines (Fig. S1).
ATP production in SRA01/04 and
iHLEC-NY2 cells under high-temperature conditions
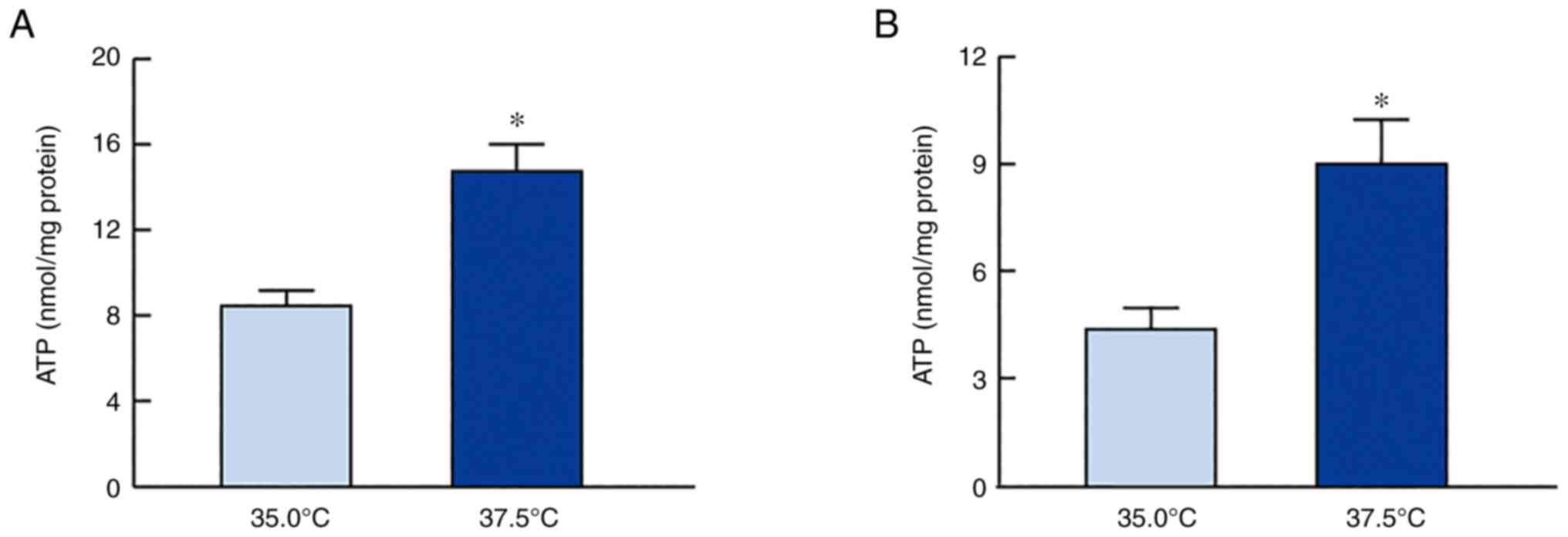
Fig. 4 shows the
effects of low- and high-temperature culture on ATP levels in
SRA01/04 and iHLEC-NY2 cells. The high temperature increased the
ATP levels in SRA01/04 and iHLEC-NY2 cells. The ATP levels in
SRA01/04 cells cultured at 37.5°C were 1.74-fold higher than those
in cells cultured at 35.0°C (P<0.05). In addition, ATP levels in
iHLEC-NY2 cells were significantly higher than those in cells
cultured at 35.0°C. ATP is consumed by ATPase, and the ion balance
is regulated in HLE cells. Therefore, we investigated the changes
in Ca2+-ATPase and Na+/K+-ATPase
activity in SRA01/04 and iHLEC-NY2 cells cultured at low or high
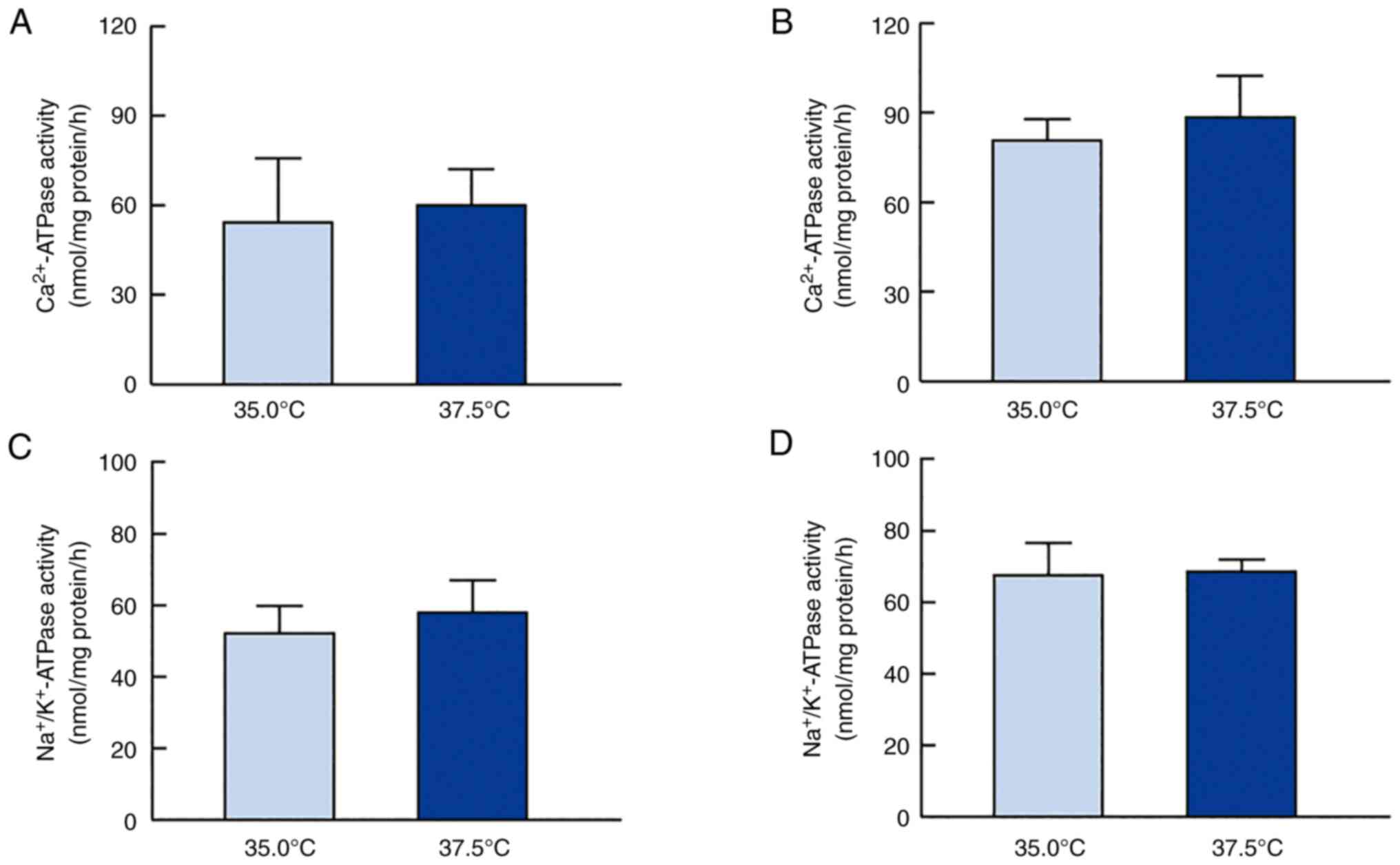
temperature (Fig. 5). Fig. 5A and 5B show the effect of high temperature on
Ca2+-ATPase activity in HLE cells.
Ca2+-ATPase activity did not significantly differ
between HLE cells cultured at 35.0°C or 37.5°C. Fig. 5C and 5D show the changes in
Na+/K+-ATPase activity in SRA01/04 and
iHLEC-NY2 cells cultured at 35.0°C or 37.5°C. As observed for
Ca2+-ATPase activity,
Na+/K+-ATPase activity was similar in HLE
cells cultured at 37.5 and 35.0°C.
Discussion
The effects of global warming have become a major
concern worldwide. The global mean surface air temperatures in
2081-2100 are estimated to be 2.6-4.8°C higher than those in
1986–2005 if greenhouse gas emissions continue to increase on a
high trajectory (RCP 8.5), as predicted by the Representative
Concentration Pathways (RCPs) (27). RCPs were divided into four
scenarios [RCP 2.6 (low), 4.5, 6.0, and 8.5 (high)]. The estimated
temperature of the crystalline lens is affected by the ambient
temperature (surface temperature) around the eye and core
temperature of the body. Therefore, in our previous study (11), simulation analysis using a
supercomputer based on the biothermal transport equation was
performed in a model with an eye tissue resolution of 1 mm based on
the Japanese body model developed by the National Institute of
Information and Communications Technology (28). The results showed that simulated
lens temperatures ranged from 35.0 to 37.5°C at ambient eye
temperatures of 19 to 35°C in a typical environment. In the present
study, cellular experiments were conducted at 35.0 and 37.5°C from
the perspective of lens temperature in vivo. We previously
showed that the estimated temperature of the crystalline lens is
greatly affected by the ambient temperature (surface temperature)
around the eye as well as the core temperature of the body. We
performed computer simulation analysis using a supercomputer based
on the biothermal transport equation, which showed that the
cumulative thermal dose estimation in the crystalline lens was
highly correlated with nuclear cataract prevalence.
An increased cell density via HLE cell proliferation
is the underlying cause of nuclear cataract (13). Moreover, our previous
epidemiological study showed that the risk of nuclear cataract
development is significantly higher in high-temperature
environments (9,10). In this study, we investigated the
relationship between temperature and cell proliferation via
mitochondrial function, which is a nuclear cataract-inducing risk
factor, using HLE cell lines (SRA01/04 and iHLEC-NY2). We found
that expression of the mitochondrial genome (CCO1-3) was enhanced
at high temperature, resulting in sufficient ATP content and cell
proliferation. A previous report comparing the same conditions as
in this study, 35.0 and 37.5°C, reported slower mitotic cell
division in mouse preimplantation embryos at lower temperatures;
this division was accelerated as the temperature was increased from
35.0 to 37.5°C (29). Thus, the
same phenomenon was observed in the lens as in other tissues.
SRA01/04 is a HLE cell line that has been used in
several studies worldwide. The SRA01/04 cell line was derived from
lens epithelial cells isolated and cultured from the lens of 12
infants with retinopathy during prematurity. A large T antigen from
simian vacuolating virus 40 (SV40) was inserted downstream of the
Rous sarcoma virus promoter and transfected into the cultured lens
epithelial cells using the pGEM3Zf plasmid vector to establish the
immortalized HLE cell line SRA01/04 (21).
The iHLEC-NY2 cell line was derived from the lens
epithelial cells of primary culture (30) of a single adult transparent HLE
cell. A plasmid vector designed to specifically introduce the
modified SV40 large T antigen-GFP (mSV40-GFP) into the
pseudo-attachment (att) P site was prepared. Cultured crystalline
lens epithelial cells were transfected by micro-electroporation and
cloned to establish the immortalized human crystalline lens
epithelial cell line iHLEC-NY2 (21).
The difference between SRA01/04 and iHLEC-NY2 cells
is the site at which the immortalizing gene was inserted. Because
the SV40 gene was randomly introduced into SRA01/04 cells, it is
possible that multiple SV40 genes were introduced. As a result,
SV40 may have been introduced into a functional gene region of the
cell. In contrast, in iHLEC-NY2 cells, the SV40 gene was
specifically introduced only into the attP site, which does not
have a function, thus ensuring that the original gene sequence of
the cells was undisturbed. Crystallin proteins expressed in HLE
cells have been detected and differentiated into lens fiber cells
by changing the medium composition (31).
First, we confirmed that the proliferation of
SRA01/04 and iHLEC-NY2 cells was indeed enhanced in
high-temperature culture (Fig.
1). Thereafter, we demonstrated the effects of high-temperature
culture on mitochondrial function by monitoring the expression
level and activity CCO enzyme. This enzyme is the terminal enzyme
of the mitochondrial respiratory chain, which reduces oxygen to
water and pumps protons across the inner mitochondrial membrane
(19), thus playing an important
role in ATP production. CCO contains 13 subunits per monomer with
the mitochondrial genome coding for the largest three catalytic
subunits, whereas 10 subunits, namely 4, 5a, b, 6a, b, c, 7a, b, c
and 8, are coded by nuclear DNA (32,33). The three mitochondrial subunits
are downregulated earlier and to a greater extent than the nuclear
subunits in response to functional inactivation (19). Thus, changes in the expression of
CCO isoforms are related to CCO activity. We measured the CCO
activity in this study and changes in the mRNA expression of the
mitochondrial subunits (CCO1-3) under high-temperature culture and
found that their expression was increased (Fig. 2). The CCO mRNA expression levels
in both cell lines were higher when the cells were cultured at
37.5°C than when cultured at 35.0°C (Fig. 2). Specifically, the level of CCO-2
mRNA expression in SRA01/04 cells and that of CCO-1 in iHLEC-NY2
cells cultured under high-temperature conditions (37.5°C) was
significantly higher than that in cells cultured under
low-temperature conditions (35.0°C) (Fig. 3). In addition, it is necessary to
measure the changes in CCO protein or activity to corroborate the
mRNA data. Therefore, we measured CCO activity, which was enhanced
under high-temperature culture (Fig.
3). These results suggest that the mitochondrial respiratory
pathway in HLE cells was accelerated under high-temperature
conditions. In contrast, the distinct roles of CCO-1, CCO-2, and
CCO-3 in the lens tissue are unclear. In addition, the other 10
subunits (nuclear subunit 4, 5a, b, 6a, b, c, 7a, b, c and 8) may
affect the enhanced CCO activity under high-temperature conditions.
However, it is not yet clear whether other factors (mitochondrial
membrane potential, glycolytic rate, and OXPHOS rate) are involved.
Further studies are needed to clarify this mechanism.
Next, changes in the ATP content in the HLE cells
were measured (Fig. 4).
High-temperature culture enhanced ATP levels in both SRA01/04 and
iHLEC-NY2 cells. These data support that mitochondrial function was
enhanced, as shown in Figs. 2 and
3. Ca2+-ATPase plays a
central role in Ca2+ transport and the maintenance of
low internal Ca2+ concentrations using intracellular
ATP. Further, Na+/K+-ATPase distributes ions
between the intracellular and extracellular spaces and is
responsible for total-body sodium homeostasis. Therefore, when
evaluating ATP production at different temperatures, it is
important to investigate whether these generalized ATPase
activities are altered, as the ATPases consume ATP. However, no
significant difference was observed between different HLE cells and
temperature conditions (Fig. 5).
We found that neither Ca2+-ATPase nor
Na+/K+-ATPase activity was involved in
regulating ATP levels in HLE cells cultured at 37.5 and 35.0°C;
however, further studies are needed to determine whether other
ATPases are involved in the ion balance, such as proton-ATPase
(H+-ATPase).
It has been reported that the enhancement of density
via HLE cell proliferation is the underlying cause of nuclear
cataracts (13) and that
sufficient ATP via mitochondrial function promotes cell
proliferation (14). Taken
together, we hypothesized that the enhanced ATP levels were related
to cell proliferation. However, studies using knockdown or
overexpression of CCO are needed to clarify this hypothesis. The
CCO inhibition observed in this study has been reported to be
reversible, and mitochondrial CCOs are more susceptible to
stimulation (19,31,32). In addition, many studies reported
that mitochondrial mutations in CCO cause disease (34,35). Accordingly, we focused on CCOs to
evaluate the change in mitochondrial function. We showed that the
mitochondrial function and ATP content in HLE cells were enhanced
under high-temperature conditions. Therefore, we hypothesized that
high-temperature conditions increased the ATP content by activating
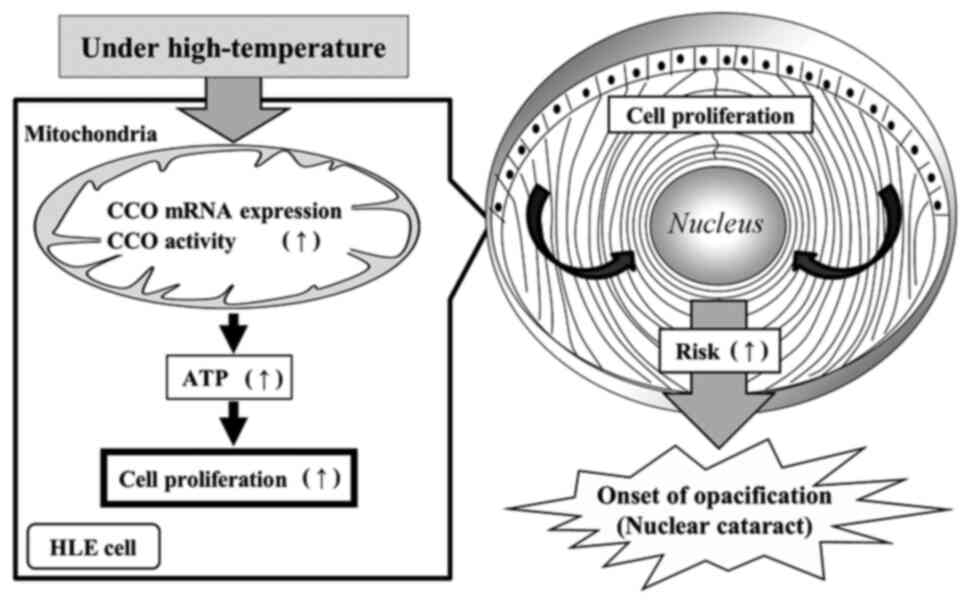
mitochondrial function and accelerating HLE cell proliferation.
Enhanced cell proliferation may lead to a higher cell density in
the central anterior capsule, resulting in an increased risk of
nuclear cataract development (Fig.
6). Unlike other tissues in the body, the crystalline lens does
not contain capillaries; thus, substances involved in nutrient
supply or metabolism such as ions and amino acids move inside the
lens cortex using the intercellular adhesion molecules of the lens
fiber cells or through cell influx such as
Na+/K+ ATPase activity (36). Because the periphery of the lens
is surrounded by the lens capsule, which mainly contains type-4
collagen, cell proliferation and high cell density may affect the
internal hydrostatic pressure of the lens, resulting in a decrease
in nutrient supply and metabolites to the lens nucleus. The
glycolytic and oxidative phosphorylation rates should also be
determined to measure the mitochondrial membrane potential, which
would reveal changes in mitochondrial function. Although there are
reports on the involvement of ATP production and cell division,
there are no data on the oxidative phosphorylation rate (37,38), which will be evaluated in our
future studies.
It is important to perform similar experiments after
HLE cells are exposed to ultraviolet rays, which is another risk
factor for cataract formation (10,39). Additionally, in animal
experiments, as the effect occurs on the lens in the living eye,
the temperature may be affected by the aqueous humor and blood
perfusion. Moreover, further studies are required to determine the
precise mechanisms of cell proliferation and ATP levels in HLE
cells. Therefore, we will investigate the effects of rearing
animals under different environment temperatures on the lens. In
addition, we are currently investigating the changes in ATP
production and cell proliferation in the lens of patients living in
subtropical regions (i.e., Mkuranga, Tanzania; Singapore; Amami,
Kagoshima, Japan; and Sanya, Hainan, China), temperate regions
(Monzen, Ishikawa, Japan; Taiyuan, Shanxi, China; and Shenyang,
Liaoning, China), and subarctic regions (Reykjavik, Iceland).
Another limitation of this study was that the estimated lens
temperature simulated from the ambient temperature around the eye
and body temperature was set as the culture medium temperature
(35.0°C or 37.5°C); however, the influences of other temperatures
on the cells were not verified and should be further investigated.
Second, in an experiment using normal diploid cells (MRC-5), the
authors reported that the cells could be passaged 57.2 times when
at 37.0°C, but only 29.2 times at 40°C, indicating that increasing
the temperature to above 40°C affects cell proliferation (40). Experiments conducted under
high-temperature conditions of 37.5°C or higher have not been
reported, and we identified the upper limit for the
high-temperature condition as 37.5°C. Third, measurement of CCO-1-3
protein by western blotting is important for clarifying the
relationships of the mitochondrial genome. However, we did not
perform this experiment. Instead, we show both the mRNA level and
activity of CCO. Western blotting will be performed in our further
studies.
In conclusion, the expression and activity of CCO in
the mitochondrial genome were enhanced under high-temperature
culture, resulting in sufficient ATP content and cell
proliferation. High cell density via HLE cell proliferation is the
underlying cause of nuclear cataracts (13). Hence, high ATP production via
mitochondrial activation may contribute to HLE proliferation,
resulting in lens opacity. These findings support the results of
our previous epidemiological study on the relationship between the
prevalence of nuclear cataracts and elevated environmental
temperatures (9,10).
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Ms. Mari Seto
(Kanazawa Medical University School of Medicine, Kahoku, Japan) for
English editing and technical support.
Funding
This study was supported by Otsuka Pharmaceutical Co., Ltd.
(research grant program for ‘Relationships among environmental,
body temperatures and cataracts’; grant no. 2020-R-091) and Alcon
Japan Ltd. (grant no. 2021-R-087).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ST participated in the conceptualization,
methodology, data analysis, investigation, and original draft
preparation. NY participated in the conceptualization, methodology,
investigation, resources, data analysis, review-writing and
editing, and visualization. NN participated in the
conceptualization, methodology, data analysis, review-writing and
editing. NoH was involved in provision of resources and
participated in data analysis. SD participated in the methodology
establishment and data analysis. NaH participated in the data
analysis and data interpretation. EK participated in the data
interpretation, writing, reviewing, editing and visualizing the
manuscript. HS participated in the data interpretation, writing the
review and editing, visualization, supervision, project
administration and funding acquisition. NY and NN confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ATP
|
adenosine-5′-triphosphate
|
|
CCO
|
cytochrome c oxidase
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
FBS
|
fetal bovine serum
|
|
HEPES
|
4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid
|
|
HLE
|
human lens epithelial
|
|
iHLEC-NY2
|
immortalized human lens epithelial
cells NY2
|
|
RCP
|
representative concentration
pathway
|
|
Tris-HCl
|
tris(hydroxymethyl)aminomethane
hydrochloride
|
References
|
1
|
World Health Organization (WHO), . Vision
2020: The RIGHT TO SIGHT. Global initiative for the elimination of
avoidable blindness. https://www.who.int/blindness/Vision2020_report.pdfWHO;
Geneva, Switzerland: December 1–2021
|
|
2
|
Moreau KL and King JA: Protein misfolding
and aggregation in cataract disease and prospects for prevention.
Trends Mol Med. 18:273–282. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shenoy AM, Kothadia AD, Shabaraya AR,
Rajan MS, Viradia UM and Patel NH: Evaluation of cataract
preventive action of phycocyanin. Int J Pharm Sci Drug Res.
3:42–44. 2011.
|
|
4
|
GBD 2019 Blindness and Vision Impairment
Collaborators and Vision Loss Expert Group of the Global Burden of
Disease: Study, . Causes of blindness and vision impairment in 2020
and trends over 30 years, and prevalence of avoidable blindness in
relation to VISION 2020: The right to sight: An analysis for the
global burden of disease study. Lancet Glob Health. 9:e144–e160.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vashist P, Talwar B, Gogoi M, Maraini G,
Camparini M, Ravindran RD, Murthy GV, Fitzpatrick KE, John N,
Chakravarthy U, et al: Prevalence of cataract in an older
population in India: the India study of age-related eye disease.
Ophthalmology. 118:272–278. e271–272. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Klein BE, Klein R and Lee KE: Incidence of
age-related cataract over a 10-year interval: The Beaver dam eye
study. Ophthalmology. 109:2052–2057. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee J, Kim MJ and Tchah H: Higher-order
aberrations induced by nuclear cataract. J Cataract Refract Surg.
34:2104–2109. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miranda MN: The geographic factor in the
onset of presbyopia. Trans Am Ophthalmol Soc. 77:603–621.
1979.PubMed/NCBI
|
|
9
|
Sasaki K, Sasaki H, Jonasson F, Kojima M
and Cheng HM: Racial differences of lens transparency properties
with aging and prevalence of age-related cataract applying a WHO
classification system. Ophthalmic Res. 36:332–340. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Miyashita H, Hatsusaka N, Shibuya E, Mita
N, Yamazaki M, Shibata T, Ishida H, Ukai Y, Kubo E and Sasaki H:
Association between ultraviolet radiation exposure dose and
cataract in Han people living in China and Taiwan: A
cross-sectional study. PLoS One. 14:e02153382019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kodera S, Hirata A, Miura F, Rashed EA,
Hatsusaka N, Yamamoto N, Kubo E and Sasaki H: Model-based approach
for analyzing prevalence of nuclear cataracts in elderly residents.
Comput Biol Med. 126:1040092020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ayaki M, Ohde H and Yokoyama N: Size of
the lens nucleus separated by hydrodissection. Ophthalmic Surg.
24:492–493. 1993.PubMed/NCBI
|
|
13
|
Liu X, Liu Y, Zheng J, Huang Q and Zheng
H: Lens epithelial cell proliferation and cell density in human
age-related cataract. Yan Ke Xue Bao. 16:184–188. 2000.PubMed/NCBI
|
|
14
|
Yan XJ, Yu X, Wang XP, Jiang JF, Yuan ZY,
Lu X, Lei F and Xing DM: Mitochondria play an important role in the
cell proliferation suppressing activity of berberine. Sci Rep.
7:417122017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun N, Youle RJ and Finkel T: The
mitochondrial basis of aging. Mol Cell. 61:654–666. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kauppila TES, Kauppila JHK and Larsson NG:
Mammalian mitochondria and aging: An update. Cell Metab. 25:57–71.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sebastian D, Palacin M and Zorzano A:
Mitochondrial dynamics: Coupling mitochondrial fitness with healthy
aging. Trends Mol Med. 23:201–215. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lenka N, Vijayasarathy C, Mullick J and
Avadhani NG: Structural organization and transcription regulation
of nuclear genes encoding the mammalian cytochrome c oxidase
complex. Prog Nucleic Acid Res Mol Biol. 61:309–344. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liang HL, Ongwijitwat S and Wong-Riley MT:
Bigenomic functional regulation of all 13 cytochrome c oxidase
subunit transcripts in rat neurons in vitro and in vivo.
Neuroscience. 140:177–190. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nagai N, Mano Y, Otake H, Shibata T, Kubo
E and Sasaki H: Changes in mitochondrial cytochrome c oxidase mRNA
levels with cataract severity in lens epithelia of Japanese
patients. Mol Med Rep. 19:5464–5472. 2019.PubMed/NCBI
|
|
21
|
Ibaraki N, Chen SC, Lin LR, Okamoto H,
Pipas JM and Reddy VN: Human lens epithelial cell line. Exp Eye
Res. 67:577–585. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yamamoto N, Takeda S, Hatsusaka N,
Hiramatsu N, Nagai N, Deguchi S, Nakazawa Y, Takata T, Kodera S,
Hirata A, et al: Effect of a lens protein in low-temperature
culture of novel immortalized human lens epithelial cells
(iHLEC-NY2). Cells. 9:26702020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sasaki H, Jonasson F, Shui YB, Kojima M,
Ono M, Katoh N, Cheng HM, Takahashi N and Sasaki K: High prevalence
of nuclear cataract in the population of tropical and subtropical
areas. Dev Ophthalmol. 35:60–69. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nagai N and Ito Y: Dysfunction in
cytochrome c oxidase caused by excessive nitric oxide in human lens
epithelial cells stimulated with interferon-gamma and
lipopolysaccharide. Curr Eye Res. 37:889–897. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nagai N and Ito Y: Adverse effects of
excessive nitric oxide on cytochrome c oxidase in lenses of
hereditary cataract UPL rats. Toxicology. 242:7–15. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nagai N, Liu Y, Fukuhata T and Ito Y:
Inhibitors of inducible nitric oxide synthase prevent damage to
human lens epithelial cells induced by interferon-gamma and
lipopolysaccharide. Biol Pharm Bull. 29:2077–2081. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
IPCC (Intergovernmental Panel on Climate
Change): Summary for policymakers, . Climate Change 2013: The
Physical Science Basis, Contribution of Working Group I to the
Fifth Assessment Report of the Intergovernmental Panel on Climate
Change. Stocker TF, Qin D, Plattner GK, Tignor M, Allen SK,
Boschung J, Nauels A, Xia Y, Bex V and Midgley PM: Cambridge
University Press; Cambridge, UK: 2013
|
|
28
|
Nagaoka T, Watanabe S, Sakurai K, Kunieda
E, Watanabe S, Taki M and Yamanaka Y: Development of realistic
high-resolution whole-body voxel models of Japanese adult males and
females of average height and weight, and application of models to
radio-frequency electromagnetic-field dosimetry. Phys Med Biol.
49:1–15. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Walters EA, Brown JL, Krisher R, Voelkel S
and Swain JE: Impact of a controlled culture temperature gradient
on mouse embryo development and morphokinetics. Reprod Biomed
Online. 40:494–499. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yamamoto N, Kato Y, Sato A, Hiramatsu N,
Yamashita H, Ohkuma M, Miyachi EI, Horiguchi M, Hirano K and Kojima
H: Establishment of a new immortalized human corneal epithelial
cell line (iHCE-NY1) for use in evaluating eye irritancy by in
vitro test methods. In Vitro Cell Dev Biol Anim. 52:742–748. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hiramatsu N, Nagai N, Kondo M, Imaizumi K,
Sasaki H and Yamamoto N: Morphological comparison between
three-dimensional structure of immortalized human lens epithelial
cells and Soemmering's ring. Med Mol Morphol. 54:216–226. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kadenbach B, Jarausch J, Hartmann R and
Merle P: Separation of mammalian cytochrome c oxidase into 13
polypeptides by a sodium dodecyl sulfate-gel electrophoretic
procedure. Anal Biochem. 129:517–521. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kuhn-Nentwig L and Kadenbach B: Isolation
and properties of cytochrome c oxidase from rat liver and
quantification of immunological differences between isozymes from
various rat tissues with subunit-specific antisera. Eur J Biochem.
149:147–158. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Afkhami E, Hheidari MM, Khatami M,
Ghadamyari F and Dianatpour S: Detection of novel mitochondrial
mutations in cytochrome c oxidase subunit 1 (COX1) in patients with
familial adenomatous polyposis (FAP). Clin Transl Oncol.
22:908–918. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wangpermtam P, Petmitr S, Punyarit P,
Klongnoi B and Sanguansin S: Down-regulation of mitochondrial NADH
and cytochrome b gene associated with high tumor stages in head and
neck squamous cell carcinoma. Arch Oral Biol. 99:107–112. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nakazawa Y, Donaldson PJ and Petrova RS:
Verification and spatial mapping of TRPV1 and TRPV4 expression in
the embryonic and adult mouse lens. Exp Eye Res. 186:1077072019.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
McMillan SN and Scarff CA: Cryo-electron
microscopy analysis of myosin at work and at rest. Curr Opin Struct
Biol. 75:1023912022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Maeshima K, Matsuda T, Shindo Y, Imamura
H, Tamura S, Imai R, Kawakami S, Nagashima R, Soga T, Noji H, et
al: A transient rise in free Mg2+ ions released from
ATP-Mg hydrolysis contributes to mitotic chromosome condensation.
Curr Biol. 28:444–451. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yoshitomi Y, Osada H, Satake H, Kojima M,
Saito-Takatsuji H, Ikeda T, Yoshitake Y, Ishigaki Y, Kubo E, Sasaki
H and Yonekura H: Ultraviolet B-induced Otx2 expression in lens
epithelial cells promotes epithelial-mesenchymal transition. Biol
Open. 18:bio0356912019. View Article : Google Scholar
|
|
40
|
Thompson KV and Holliday R: Effect of
temperature on the longevity of human fibroblasts in culture. Exp
Cell Res. 80:354–360. 1973. View Article : Google Scholar : PubMed/NCBI
|