Introduction
Rheumatoid arthritis (RA) is a common systemic
inflammatory autoimmune disease that mainly affects human joints,
manifesting as joint swelling, pain and deformation (1). RA affects ~1 in 200 adults worldwide
and occurs 2–3 times more frequently in females than in males
(2–4). It is characterized by tumor-like
expansion of the synovium, angiogenesis and destruction of the
adjacent articular cartilage and bone (5). Angiogenesis, a major component of
invasive pannus, is considered an essential step in the abnormal
proliferation of synovial cells and maintains a chronic
inflammatory microenvironment by supplying oxygen and nutrients to
tissues and recruiting immune cells (6,7).
Disease-modifying antirheumatic drugs are the most well-established
treatment option for RA. Immunosuppressants are the main choice of
disease-modifying antirheumatic drug and may control symptoms in
40–50% of patients (8).
Analgesics, such as non-steroidal anti-inflammatory drugs, may
reduce joint pain in patients; however, these exert no effects on
disease improvement (9). Moreover,
clinical drug use may be limited due to associated adverse side
effects (10). Thus, current
research is focused on traditional herbal remedies for the
treatment of RA (11,12), as novel therapeutic strategies for
patients with RA are required.
Anemone flaccida Fr. Schmidt is mainly
distributed in the mountainous areas of southern China. The dried
rhizome, known as DIWU, is widely used as a Traditional Chinese
Medicine (TCM) in the treatment of joint pain and fractures and the
strengthening of bones (13). The
main method of preparation of Anemone flaccida Fr. Schmidt
for use in TCM includes decoction or soaking in wine. A previous
pharmacological study demonstrated that the main components of
triterpenoid saponins exhibit anti-inflammatory, anti-rheumatic and
anti-tumor properties (14).
However, to the best of the authors' knowledge, there are no
studies focusing on the potential of ethanol extract of Anemone
flaccida Fr. Schmidt (EAF) in the treatment of RA. Thus, the
present study aimed to explore the inhibitory effects of EAF on
synovial hyperplasia and pannus formation in RA using a
collagen-induced arthritis (CIA) rat model.
Materials and methods
EAF preparation
Anemone flaccida Fr. Schmidt used in the
present study was collected from Yichang city (Hubei, China) and
authenticated by Professor Keli-Chen (Department of Identification
of Traditional Chinese Medicine, Hubei University of Chinese
Medicine). The extraction method was as follows: Anemone
flaccida Fr. Schmidt was dried and pulverized. A total of 500 g
Anemone flaccida Fr. Schmidt powder was extracted twice
using 500 ml absolute ethyl alcohol, following shaking in an
incubator at 37°C for 24 h. The supernatant was collected following
centrifugation (1,200 × g; 25°C) for 5 min, passed through filter
paper and concentrated and dried via evaporation at room
temperature for 48 h. Subsequently, 9.6 g of dry powder was
extracted and the overall percentage of Anemone flaccida Fr.
Schmidt was 1.92%. Anemone flaccida Fr. Schmidt was stored
at −20°C for subsequent experiments.
Secondary metabolite detection
Sample extracts were analyzed using a UPLC-ESI-MS/MS
system [UPLC: Shimadzu Corporation Nexera X2; MS: Applied
Biosystems 4500 (AB4500) quadrupole-linear ion trap (QTRAP)]. The
analytical conditions were as follows: Column, Agilent SB-C18 (1.8
µm, 2.1×100 mm); mobile phase, solvent A, pure water with 0.1%
formic acid and solvent B, acetonitrile with 0.1% formic acid.
Sample measurements were performed using a gradient program with
95% A and 5% B starting conditions. Within 9 min, a linear gradient
to 5% A and 95% B was programmed and a composition of 5% A and 95%
B was maintained for 1 min. Subsequently, a composition of 95% A
and 5% B was adjusted within 1.1 min and maintained for 2.9 min.
The flow velocity was set as 0.35 ml per minute; column oven was
set to 40°C; and injection volume was set to 4 µl. The effluent was
alternatively connected to an ESI-triple QTRAP-MS.
LIT and triple quadrupole (QQQ) scans were acquired
on a QTRAP mass spectrometer, AB4500 QTRAP UPLC/MS/MS System,
equipped with an ESI Turbo Ion-Spray interface, operating in
positive and negative ion mode and controlled using Analyst 1.6.3
software (Shanghai AB SCIEX Analytical Instrument Trading Co.). ESI
source operation parameters were as follows: Ion source, turbo
spray; source temperature, 550°C; ion spray voltage (IS), 5,500 V
(positive ion mode)/-4,500 V (negative ion mode); ion source gas I
(GSI), 50 psi; gas II (GSII), 60 psi; curtain gas (CUR), 25.0 psi;
and high collision-activated dissociation (CAD). Instrument tuning
and mass calibration were performed using 10 and 100 µmol/l
polypropylene glycol solutions in QQQ and LIT modes, respectively.
QQQ scans were acquired as MRM experiments with collision gas
(nitrogen) set to medium. DP and CE for individual MRM transitions
were performed with further DP and CE optimization. A specific set
of MRM transitions were monitored for each period, according to the
metabolites eluted within this period.
Animals
Animal experiments were carried out at the Animal
Experiment Center of Hubei University of Chinese Medicine
(registration no. syxk 2017-0067) following approval from the Hubei
Experimental Animal Research Center (approval no. SCXK 2022-0012)
and approval from the Laboratory Animal Ethics Committee of Hubei
Academy of Chinese Medicine Sciences (approval no. HUCMS
202208001). Animal experimental procedures were in accordance with
the National Regulations on the Management of Experimental Animals,
Government of China. Male Wistar rats (age, 6–8 weeks; weight,
160±10 g; SPF grade; cat. no. 42000600043481) obtained from the
Hubei Laboratory Animal Research Center were housed at 22±3°C, in
30–70% relative humidity, with a 12/12 h light/dark cycle. Standard
rodent feed and water were provided for all animals ad
libitum. Rats were fed for five days prior to the commencement
of experimental procedures for acclimation.
Induction of arthritis and drug
administration
The RA rat model was induced as previously described
(15). Briefly, Bovine type II
collagen (cat. no. 01025A; Dalian Meilun Biology Technology Co.,
Ltd.) was dissolved in 0.05 mol/l acetic acid (2.0 mg/ml) and
emulsified with complete Freund's adjuvant (CFA; cat. no. F5881-10
ml; Sigma-Aldrich; Merck KGaA) at a ratio of 1:1. The prepared
emulsifier was injected intradermally into the base of the rat tail
at 100 µg per rat. A second immunization with the same dose was
administered 7 days later. Redness and swelling of the ankle of the
rats was observed and arthritis score was evaluated and recorded.
Briefly, 15 days after the first immunization, outliers were
excluded according to arthritis score. A total of 20 rats remained
and these were randomly divided into four groups (5 rats in each
group): i) CIA (200 mg/kg); ii) vehicle (200 mg/kg); iii) EAF-L
(200 mg/kg); and iv) EAF-H (400 mg/kg). A further five rats that
did not receive immunization were used as the blank control group.
Notably, the body weight of all rats was recorded every 7 days.
From the 15th day, groups were treated as follows:
i) Control and CIA groups, daily intraperitoneal injection of
saline; ii) vehicle group, daily intraperitoneal injection of equal
volume of DMSO; and iii) EAF group, daily intraperitoneal injection
of EAF 200 or 400 mg/kg.
Following immunization, all four paws of the rats
were observed and the arthritic condition was evaluated based on
the redness and swelling every three days. Redness and swelling was
scored according to the following criteria: 0, without redness and
swelling; 1, localized redness or swelling (wrist/ankle); 2,
swelling or redness expanded to the palm or sole; 3, redness and
swelling in all joints; and 4, skin bursting, joint dysfunction or
distortion. Clinical assessments were completed by two independent
researchers. The sum of arthritis index scores in the limbs was
used to evaluate the severity of arthritis, with higher scores
indicating more severe arthritis. The humane endpoints in this
experiment were based on the following criteria: No sign of healing
after ulcer treatment, abnormal gait or posture that prevented them
from feeding, spontaneous vocalization or squeaking and quivering
when picked up or handled, significant weight loss (15%) (16).
Histological assessment
Rats were euthanized using an intraperitoneal
injection of sodium pentobarbital (3%; 100 mg/kg). Arthritis
severity was assessed through observing inflammatory infiltration,
pannus and synovial edema in samples obtained from the joints,
following paraffin embedding and H&E staining. The right
hindlimb was collected and fixed in 4% paraformaldehyde for 48 h at
4°C. Following two weeks of slow decalcification, the right
hindlimb was embedded in paraffin and cut into 5-µm thick sections.
Subsequently, H&E staining was carried out according to
standard protocols.
Parts of the sections were used for
immunohistochemical examination and a CD31 antibody (1:200; cat.
no. bs-0195R; BIOSS) was used measure neovascularization. A VEGF
antibody (1:200; cat. no. bs-0279R; BIOSS) was used in sliced
tissue. Primary antibody incubated for 12 h at 4°C, secondary
antibody incubated for 2 h at room temperature. Stained or
protein-labeled specimen slices were observed and a total of five
fields of view were randomly selected and images captured using
light microscopy (CH30RF200; Olympus Corporation) at a high
magnification (×200 and ×400). Positive expression of proteins was
semi-quantified using ImageJ 1.8.0. (National Institutes of
Health).
Rat aortic ring assay
Healthy 8-week-old male Wistar rats were euthanized
using an intraperitoneal injection of sodium pentobarbital (3%; 100
mg/kg) and thoracic aorta segments were collected. Thoracic aorta
segments were cleaned and cut into 1-mm long complete rings and
placed in a 96-well plate pre-coated with Matrigel (Corning, Inc.)
mixed with DMEM (HyClone; Cytiva) medium (1:1; 40 µl/well).
Subsequently, 200 µl medium with 20% FBS (Hangzhou Sijiqing
Biological Engineering Materials Co., Ltd.) was added in each well.
The plate was incubated at 37°C with 5% CO2 for 3 days
until endothelial cells budded in tubular structures around the
vascular ring. Vascular rings were divided into four groups with
five in each group and VEGF and/or EAF was added in each group.
Following incubation for a further seven days, the vascular sprout
was observed and images captured using an inverted microscope
(Eclipse TS 100; Nikon Corporation).
Chick chorioallantois membrane (CAM)
assay
To explore the effects of EAF on angiogenesis in
synovial tissues, a CAM model was used. Briefly, fertilized hen's
eggs were incubated at 37.8°C and 60% relative humidity for five
days. A total of 2 ml albumin was removed under aseptic conditions
and placed in an artificial air chamber. Following two days
incubation, 1-mm3 gelatin sponges soaked in basic
medium, EAF and/or recombinant human VEGF165 protein (VEGF165;
BIOSS) was placed on the CAM through a 1×1 cm2 window,
opened in the large blunt edge of the egg. Subsequently, the window
was sealed using paraffin film for further incubation for three
days. The microvasculature was observed and photographed under a
dissecting microscope (XTL-165-XTWZ; Phenix Optics Co., Ltd.).
Cells and culture methods
Human umbilical vein endothelial cells (HUVECs; cat.
no. C1109) and human fibroblast-like synoviocytes-RA (HFLS-RAs;
cat. no. C1351) were purchased from Shanghai Whelab Biological Co.,
Ltd. Cells were cultured in DMEM (HyClone; Cytiva) containing 5%
FBS (Hangzhou Sijiqing Biological Engineering Materials Co., Ltd.)
and 1% penicillin/streptomycin (Dalian Meilun Biology Technology
Co., Ltd.) and cultured in a humid incubator at 37°C with 5%
CO2.
Cell viability assay
A Cell Counting Kit-8 (CCK-8) assay was used to
detect the effects of EAF on the viability of HFLS-RAs and HUVECs.
HFLS-RAs (3×103 cells/ml) or HUVECs (5×103
cells/well) were seeded in 96-well plates and cultured in normal
growth medium for 24 h. Culture medium was replaced with DMEM,
different concentrations (HUVEC: 5, 7.5, 10, 15, 20, 30 µg/ml;
HFLS-RA: 2.5, 5, 10, 20, 40, 80 µg/ml) of EAF were added and cells
were incubated for 24 or 48 h at 37°C. Following incubation,
culture medium was replaced with 200 µl PBS containing 10% CCK-8
(Dalian Meilun Biology Technology Co., Ltd.). Following a further 2
h incubation at 37°C, the viability of HFLS-RAs and HUVECs was
measured at an absorbance of 490 nm using a microplate reader
(Model 680; Bio-Rad Laboratories, Inc.). Cell viability was
measured using the following formula: Inhibitory rate
(%)=[(1-optical density at 450 nm of the treatment)/optical density
at 450 nm of the control)] ×100%.
Wound healing assay
A wound healing assay was conducted in a six-well
plates to determine the migration of HFLS-RAs. Cells were seeded
and incubated in normal growth medium until 90% confluence was
reached. The cell layer was scratched using a 200-µl pipette tip.
Following washing with PBS twice, 2 ml serum-free medium with
different concentrations of EAF was added to each well and
incubated for a further 24 h. Images were captured at 0 and 24 h
and the start of the wound was marked with a marker under an
inverted microscope (model CK-40; Olympus Corporation). Changes in
wound width were calculated using ImageJ 1.8.0 (National Institutes
of Health).
Migration assay
A Transwell assay was used to detect the vertical
migration of HUVECs and HFLS-Ras (17). Transwell chambers with pore size
8.0 µm were placed in 24-well plates and cells were seeded in the
upper chamber at a density of 2×105 cells/well. Cells
were suspended in serum-free medium following treatment with EAF
and/or VEGF165 in a six-well plate for 24 h at 37°C. The lower
chamber was filled with 700 µl medium and 10% FBS and incubated at
37°C for 12 h at 37°C. Subsequently, cells that failed to migrate
to the lower layer were removed with a cotton swab and cells on the
lower surface were fixed with anhydrous ethanol and stained with
0.5% crystal violet for 5 min at room temperature. The number of
migratory cells in each well was counted in five random fields
using an inverted microscope (model CK-40; magnification, ×100;
Olympus Corporation).
Cell colony formation assay
The effects of EAF on cell proliferation were
detected using a colony formation assay. Briefly, HFLS-RAs were
seeded in a six-well plate at a density of 500 cells/well and
incubated for two days at 37°C. Following treatment with different
concentrations of EAF for 12 h at 37°C, culture medium was replaced
with complete medium. Subsequently, cells were incubated for a
further two weeks at 37°C, colonies were fixed using anhydrous
ethanol for 10 min at room temperature and stained using 0.5%
crystal violet for 5 min at room temperature. The number of
colonies (>50 cells) in each well was quantified using ImageJ
1.8.0. (National Institutes of Health).
Flow cytometry
A FITC-Annexin V/PI apoptosis kit was used to detect
the apoptotic rate of HFLS-RAs following treatment with EAF. Cells
were collected following treatment with different concentrations of
EAF for 24 h and washed twice using cold PBS. FITC-Annexin V (5 µl)
and PI (10 µl) were sequentially added to cells following
resuspension in 400 µl binding buffer. Cells were incubated in the
dark for 15 min at room temperature, following detection using a BD
FACSCalibur flow cytometer (BD Biosciences). The results were
analyzed using FlowJo X 10.0.7 (FlowJo LLC), the apoptotic rate was
calculated by the percentage of early + late apoptotic cells.
Tube formation assay
The formation of tubule-like structures by HUVECs in
Matrigel may reflect their angiogenic capacity; thus, HUVECs were
pre-treated with or without EAF and/or VEGF165 for 12 h at 37°C.
Cells were resuspended in medium with various concentrations of EAF
and/or VEGF165 and seeded in 96-well plate, which had been
pre-coated with a 50 µl layer of Matrigel. Following a further 12-h
incubation at 37°C, images was captured under an inverted
microscope (model CK-40; magnification, ×40; Olympus Corporation).
Tubular structures in five random fields of view were calculated
using ImageJ 1.8.0 (National Institutes of Health).
Western blot analysis
HUVECs were seeded in a six-well plate and treated
with different concentrations of EAF and/or VEGF165 for 24 h. Total
protein was collected using RIPA lysis buffer (Beyotime Institute
of Biotechnology) containing proteinase inhibitors. Proteins were
quantified using BCA (Beyotime Institute of Biotechnology) and
denatured using 5X loading buffer. A total of 20 µg protein from
each group was separated via SDS-PAGE using a 10% gel and
transferred onto PVDF membranes. Following blocking with 5% skimmed
milk at room temperature for 1 h, membranes were incubated with the
following primary antibodies at 4°C for 12 h: Rabbit anti-VEGF
antibody (1:1,000; cat. no. bs-0279R; BIOSS), anti-VEGF receptor
(VEGFR)1 antibody (1:1,000; cat. no. bs-0170R; BIOSS), anti-PI3K
antibody (1:1,000; cat. no. AF7742; Beyotime Institute of
Biotechnology), anti-phosphorylated (p)-PI3K antibody (1:1,000;
cat. no. AF5905; Beyotime Institute of Biotechnology), anti-AKT
antibody (1:1,000; cat. no. AA326; Beyotime Institute of
Biotechnology), anti-p-AKT1 antibody (1:1,000; cat. no. AF5740;
Beyotime Institute of Biotechnology), anti-mTOR antibody (1:1,000;
cat. no. AF1648; Beyotime Institute of Biotechnology), anti-p-mTOR
antibody (1:1,000; AF5869; Beyotime Institute of Biotechnology).
β-actin was used as the control and detected using the anti-β-actin
antibody (1:1,000; cat. no. K200058M; Beijing Solarbio Science
& Technology Co., Ltd). Following primary incubation, membranes
were incubated with the anti-goat HRP-conjugated immunoglobulin G
secondary antibody (1:1,000; cat. no. SE134; Beijing Solarbio
Science & Technology Co., Ltd.) at room temperature for 2 h.
Proteins were visualized using enhanced chemiluminescent reagent
(Wuhan Servicebio Biotechnology Co., Ltd.) and membranes were
scanned using a gel imaging system (ChemiDoc XRS+; Bio-Rad
Laboratories, Inc.). Protein expression was quantified using Image
Lab software (version 5.0; Bio-Rad Laboratories, Inc.).
Statistical analysis
GraphPad Prism (version 8.00; GraphPad Software,
Inc.) was used for statistical analysis. Data are presented as the
mean ± standard deviation. All experiments were repeated at least
three times. One-way ANOVA followed by Tukey's post-hoc test was
used for comparisons between multiple groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Qualitative chemical analysis using
ultra-high-performance liquid chromatography–high-resolution tandem
mass spectrometry (UHPLC-ESI-HRMS)
UPLC-MS analysis of EAF was performed in the
positive and negative ion mode. The UV detector and total ion
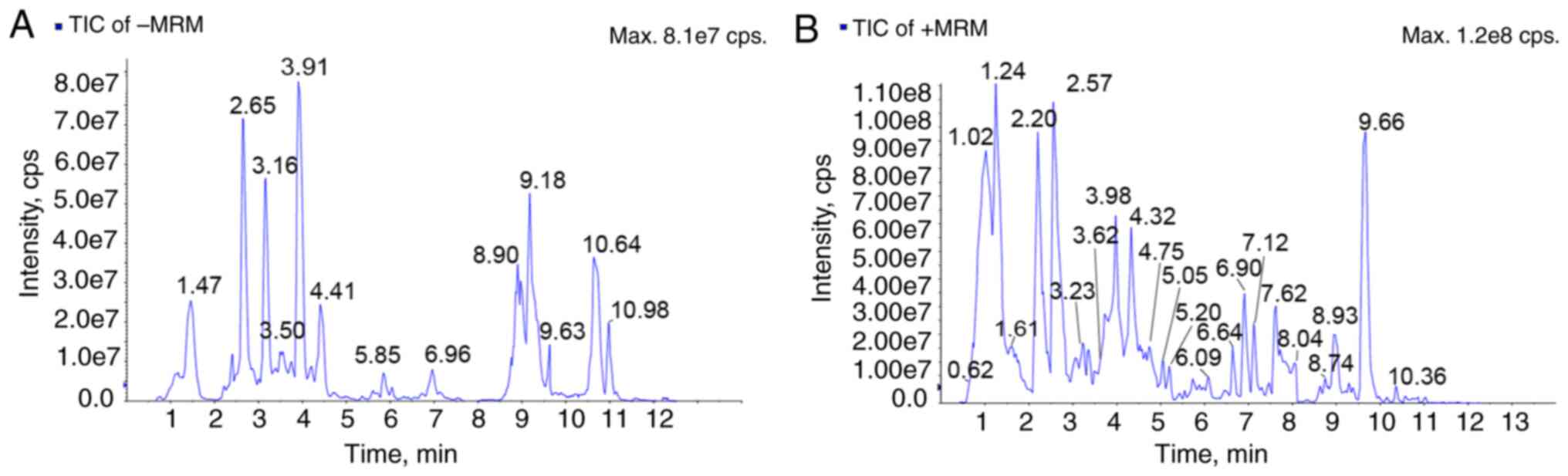
chromatograms of EAF are displayed in Fig. 1. Through the chemical analysis of
EAF, a total of six classes of metabolites were identified,
including: Iridoids glycosides, flavonoids,
phenylpropanoids-derived metabolites, phenylethanoid-derived
metabolites, cinnamic acid-derived metabolites and triterpenes
(Table I). Identification of the
chemical substances in EAF were compared with the main chemical
constituents of Anemone plants described in an internal
database (18). The results of the
phytochemical analysis were consistent with those of previous
studies (13,19).
 | Table I.Qualitative chemical analysis of
extract of Anemone flaccida Fr. Schmidt by
ultra-high-performance liquid chromatography-high-resolution tandem
mass spectrometry. |
Table I.
Qualitative chemical analysis of
extract of Anemone flaccida Fr. Schmidt by
ultra-high-performance liquid chromatography-high-resolution tandem
mass spectrometry.
| Number | Retention time | Formula | Compound type | Ionization
model | Putative
compounds |
|---|
| 1 | 1.45 |
C18H26O11 | Phenolic acids | [M-H]- |
2-Hydroxyphenol-1-O-glucosyl(6→1)
rhamnoside |
| 2 | 2.63 |
C14H20O9 | Phenolic acids | [M-H]- |
glucopyranoside |
| 3 | 2.83 |
C27H36O13 | Phenolic acids | [M-H]- | Citrusin B |
| 4 | 3.61 |
C30H38O15 | Phenolic acids | [M-H]- |
Glucopyranosyl)-feruloyl]glucopyranoside |
| 5 | 3.73 |
C31H41NO11 | Terpenoid
alkaloids | [M+H]+ | Flavaconitine |
| 6 | 3.92 |
C27H30O14 | Flavonoids | [M+H]+ |
Apigenin-7-O-rutinoside
(Isorhoifolin) |
| 7 | 4.04 |
C29H36O15 | Phenolic acids | [M-H]- | Acteoside |
| 8 | 4.04 |
C29H36O15 | Phenolic acids | [M-H]- | Isoforsythoside
A |
| 9 | 4.05 |
C28H36O13 | Lignans | [M-H]- |
Syringaresinol-4′-O-glucoside |
| 10 | 4.48 |
C33H45NO11 | Terpenoid
alkaloids | [M+H]+ | Mesaconitine |
| 11 | 4.52 |
C32H45NO9 | Terpenoid
alkaloids | [M+H]+ | Hemsleyaconitine
B |
| 14 | 4.68 |
C28H32O14 | Flavonoids | [M+H]+ |
Robinson-7-O-Neohesperidin |
| 15 | 6.24 |
C35H54O8 | Terpenoids | [M+H]+ |
3β-[(Arabinosyl)oxy]-19β-hydroxyurs-12,20(30)-dien-28-oic
acid |
| 16 | 6.47 |
C33H40O14 | Flavonols | [M+H]+ |
2′-O-rhamnosyl-icariside II |
| 17 | 6.90 |
C30H48O4 | Triterpene | [M+H]+ | Hederagenin |
| 18 | 9.66 |
C36H56O9 | Terpenoids | [M+H]+ | Oleanolic
acid-GlurA |
| 19 | 9.63 |
C30H46O3 | Terpenoids | [M-H]- | Oleanonic acid |
| 20 | 6.24 |
C41H64O13 | Terpenoids | [M-H]- | Oleanolic
acid-3-O-xylosyl(1→3)glucuronide |
| 21 | 9.29 |
C30H48O4 | Terpenoid | [M-H]- | 2-Hydroxyoleanolic
acid |
EAF reduces joint inflammation in CIA
rats
Animals were treated on the 15th day following the
first immunization and treatment lasted for 21 days. Joint
inflammation clinical scores were recorded every three days and
body weights of rats were recorded every seven days. Results are
displayed in Fig. 2. The degree of
limb swelling and redness in the EAF treatment group was
significantly improved compared with the control groups. Notably,
clinical scores were increased in vehicle and CIA groups and peaked
33 days following the first immunization with no significant
difference between the two groups. Treatment groups were
significantly different from the model group on day 21 and
gradually decreased after peaking on day 24. Clinical scores were
lower in the EAF-high (H) group than in the EAF-low (L) group and
this gap was present on Day 9 and continued until the end of the
experiment. Body weight is displayed in Fig. 2C. All immunized animals exhibited a
significant reduction in body weight compared with the control
group. Results of the present study demonstrated no difference
between the EAF treatment group and the model group. In addition,
results of H&E staining (Fig.
2E) demonstrated that the thickness of joint synovial tissue
was lower in the EAF treatment group compared with the model group
and notable pannus formation was present in the joints of animals
in the model group. By contrast, pannus was significantly reduced
or absent in the EAF-treated group.
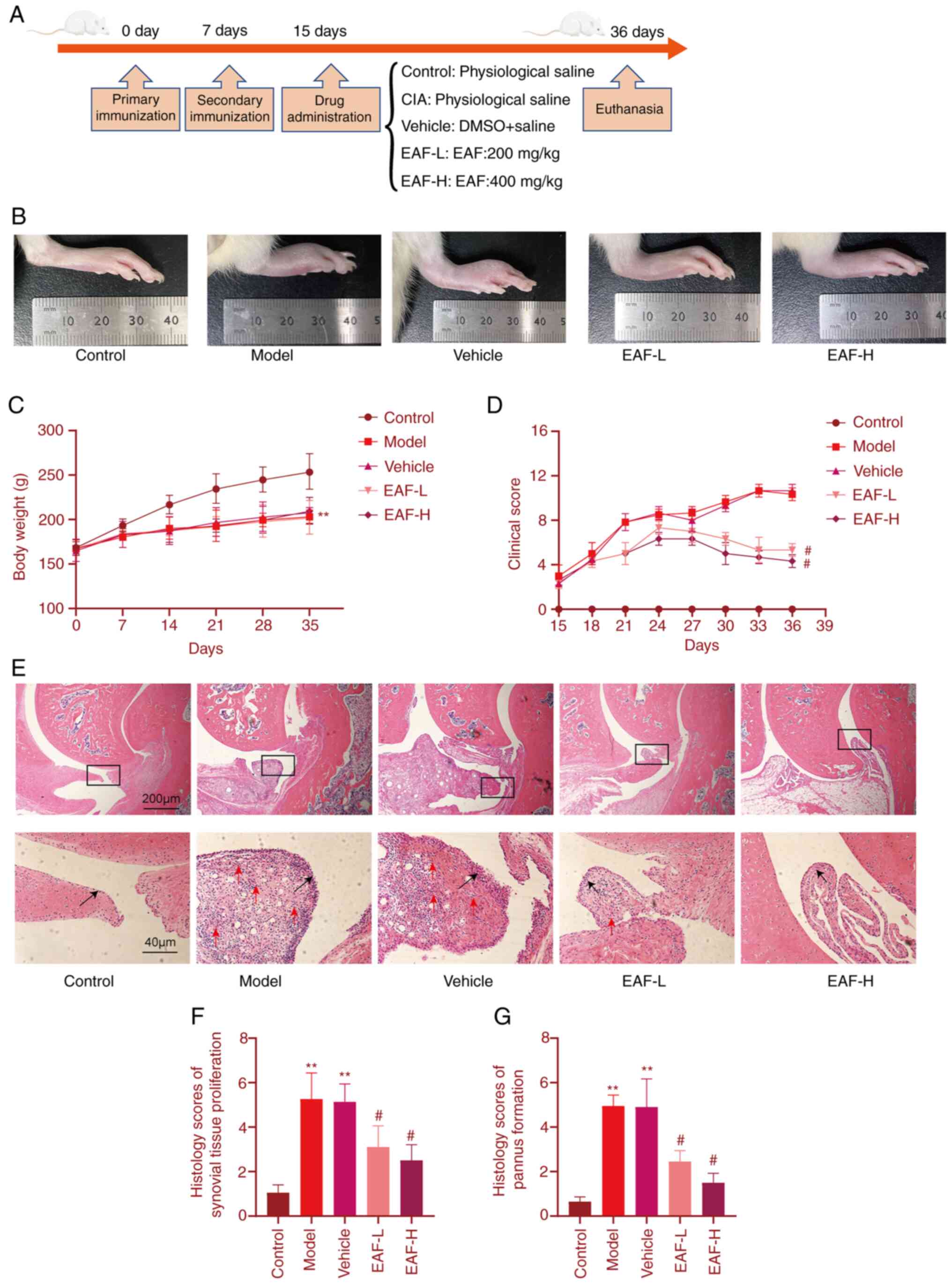 | Figure 2.EAF treatment in CIA rats. (A) Time
course of immunization and drug treatment in the CIA rat model. (B)
Representative image of the right hindlimb of a CIA rat following
21 days of EAF treatment. (C) Body weight of CIA rats was recorded
every seven days. (D) CIA rat limbs were clinically scored every 3
days. 0, without redness and swelling; 1, localized redness or
swelling (wrist/ankle); 2, swelling or redness expanded to palm or
sole; 3, redness and swelling in all joints; and 4, skin
bursting/joint dysfunction or distortion. (E) Representative images
of hematoxylin and eosin staining of CIA rat ankle joint sections
(magnification, ×40). Representative image of synovial tissue
(local magnification, ×200). (F) Histological score of synovial
hyperplasia in each group. (G) Pannus formation score of synovial
tissue in each group. **P<0.01 vs. control group,
#P<0.01 vs. model group. EAF, ethanol extract of
Anemone flaccida Fr. Schmidt; CIA, collagen-induced
arthritis; Control, blank control group; model, CIA group; vehicle,
DMSO with saline group; EAF-L, low dose of ethanol extract of
Anemone flaccida Fr. Schmidt group (200 mg/kg/day); and
EAF-H, high dose ethanol extract of Anemone flaccida Fr.
Schmidt group (400 mg/kg/day). |
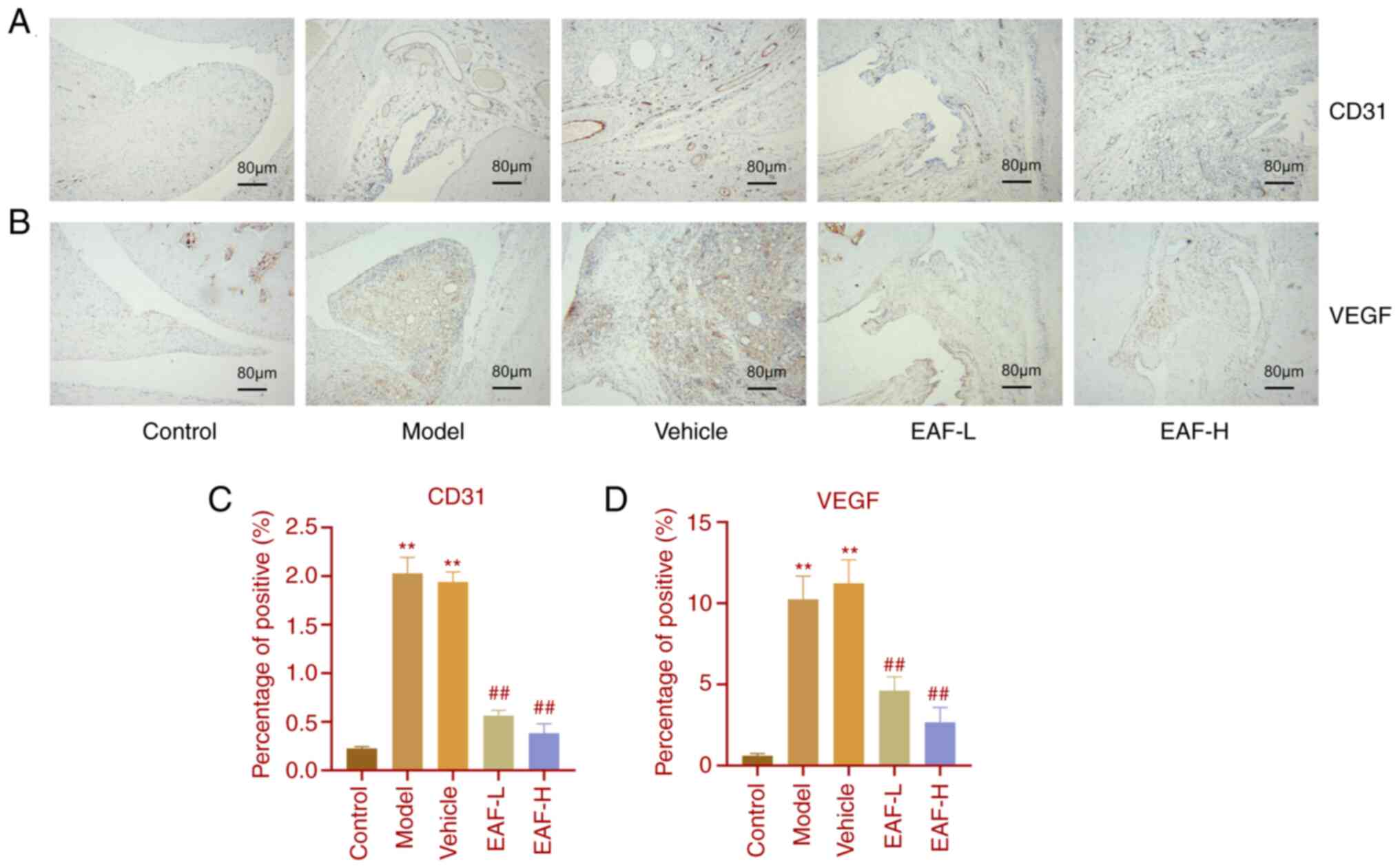
EAF relieves joint synovial
hyperplasia and pannus
H&E staining revealed that EAF treatment
significantly reduced pannus formation in the joint synovium of
arthritic rats. Given the important role of angiogenesis in RA
progression, immunohistochemical staining was performed using joint
tissue sections (Fig. 3A and B).
Compared with the control group, the protein expression levels of
CD31 and VEGF in the model and vehicle groups were significantly
increased. However, CD31 and VEGF expression levels were
significantly reduced in treatment groups compared with the model
group. Moreover, this reduction was more pronounced as the drug
concentration increased.
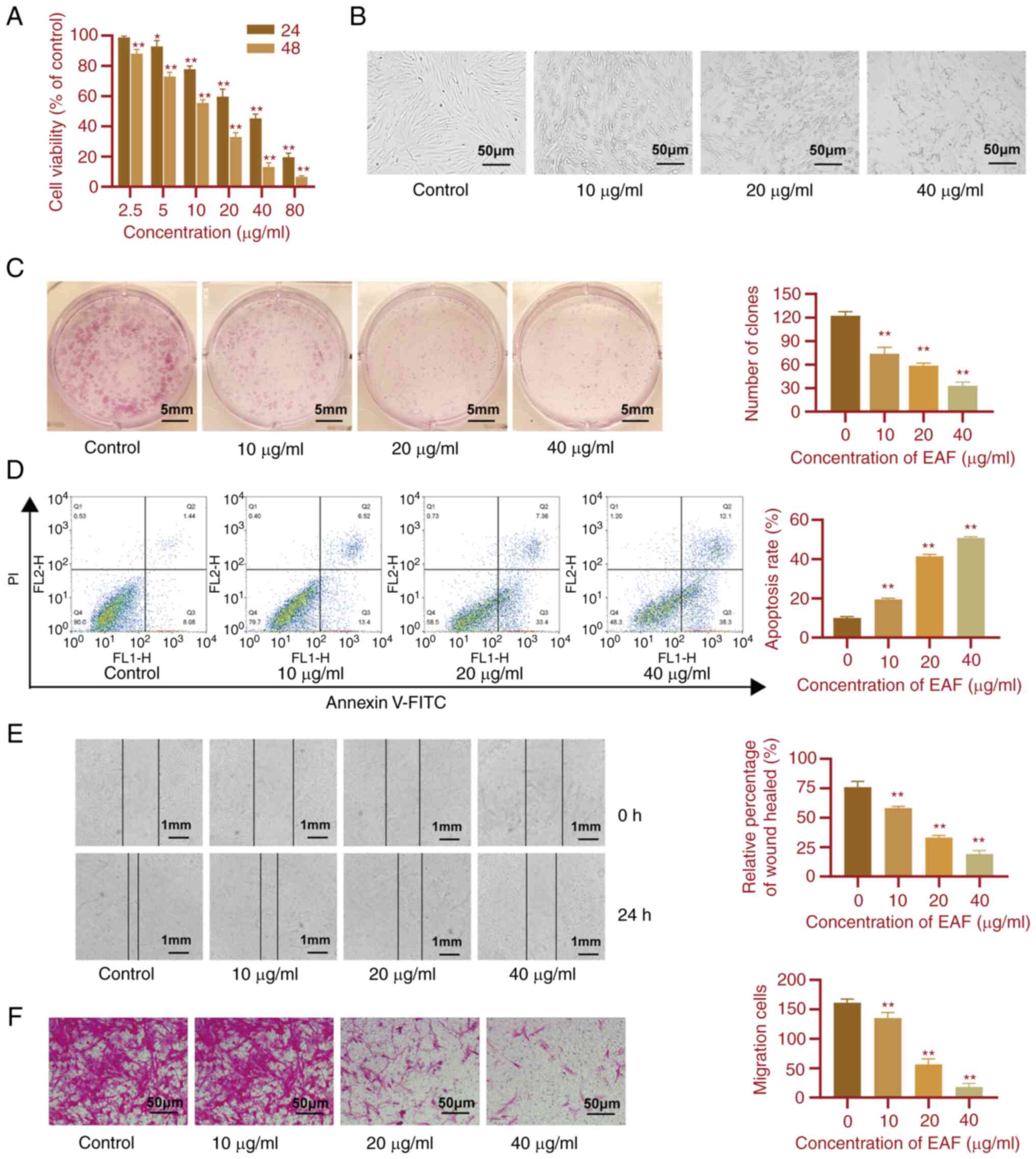
EAF inhibits the proliferation and
migration of synovial cells and promotes apoptosis
To determine the effects of EAF on the synovium in
RA, the effects of EAF on the proliferation, apoptosis and
migration of HFLS-RAs were investigated. Results of the present
study are displayed in Fig. 4.
Following 24 and 48 h EAF treatment, results of the CCK-8 assay
demonstrated that cell viability decreased following increases in
EAF concentration, in a dose-dependent manner (Fig. 4A). Moreover, following 48 h drug
treatment, the cell shape became round and wrinkled as the drug
concentration increased (Fig. 4B).
Results of the colony formation assay highlighted the inhibitory
effects of EAF on cell proliferation. Notably, colony formation was
significantly reduced in the treated groups compared with the
control group and EAF reduced colony formation of HFLS-RAs in a
dose-dependent manner (Fig. 4C).
Results of the flow cytometry analysis demonstrated that EAF
induced the apoptosis of HFLS-RAs (Fig. 4D). Moreover, cell migration was
determined using wound healing and Transwell assays. Results of the
present study demonstrated that migration was significantly
inhibited following treatment with EAF in a dose-dependent manner.
Following 24 h EAF treatment, HFLS-RA migration was significantly
reduced (Fig. 4E and F).
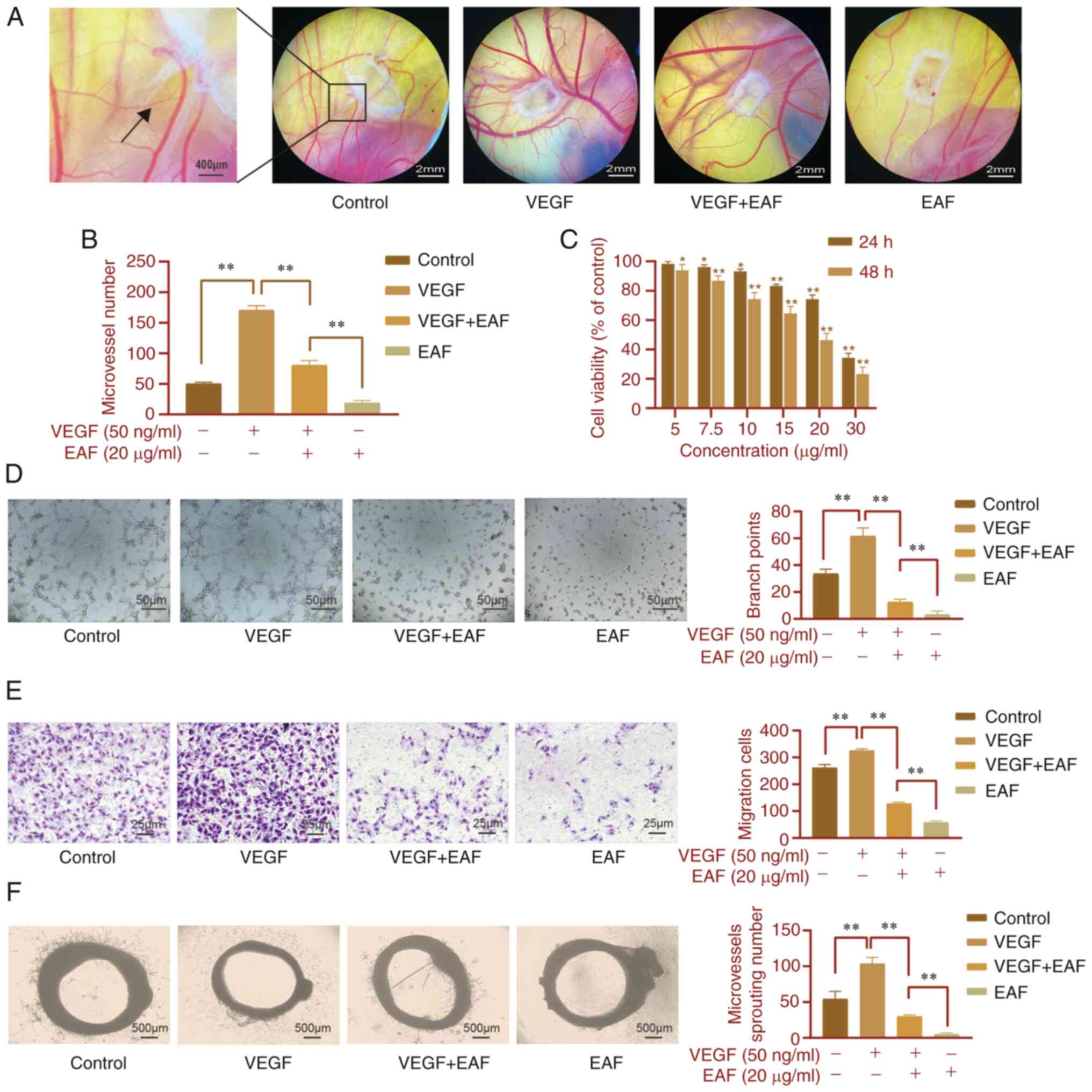
EAF inhibits VEGF-induced angiogenesis
in vitro and in vivo
To further explore the effects of EAF on
angiogenesis, VEGF was used as a positive control in the CAM model
(Fig. 5A). Results of the present
study demonstrated that blood vessels in the CAM in the VEGF group
were significantly increased compared with those in the control
group and blood vessels were distributed in a spoke-like shape with
a sponge-like center. In the VEGF + EAF group, EAF inhibited the
promoting effects of VEGF on blood vessels and the density of blood
vessels was significantly lower than that in the VEGF group.
However, there was no significant difference compared with the
control group. Following EAF treatment alone, angiogenesis was
significantly inhibited and blood vessel density was significantly
reduced compared with the control group (Fig. 5B).
HUVECs were used to explore the effects of EAF on
angiogenesis in vitro and results of the CCK-8 analysis
demonstrated that EAF inhibited the viability of HUVECs in a
dose-dependent manner. This inhibitory effect was more pronounced
from 24 to 48 h of treatment (Fig.
5C). Results of the Transwell assay demonstrated that VEGF
significantly increased the migration of HUVECs; however, treatment
with EAF inhibited this effect. In the EAF + VEGF group, cell
migration was significantly lower than that in the VEGF and control
groups and this inhibitory effect was more pronounced in the EAF
group (Fig. 5E). In addition, VEGF
significantly increased the number of endothelial cells forming
tubules compared with the control group (Fig. 5D and F). However, following
treatment with EAF + VEGF, the number of endothelial cells forming
tubules was significantly reduced compared with the control group.
Following treatment with EAF alone, few microtubules were
formed.
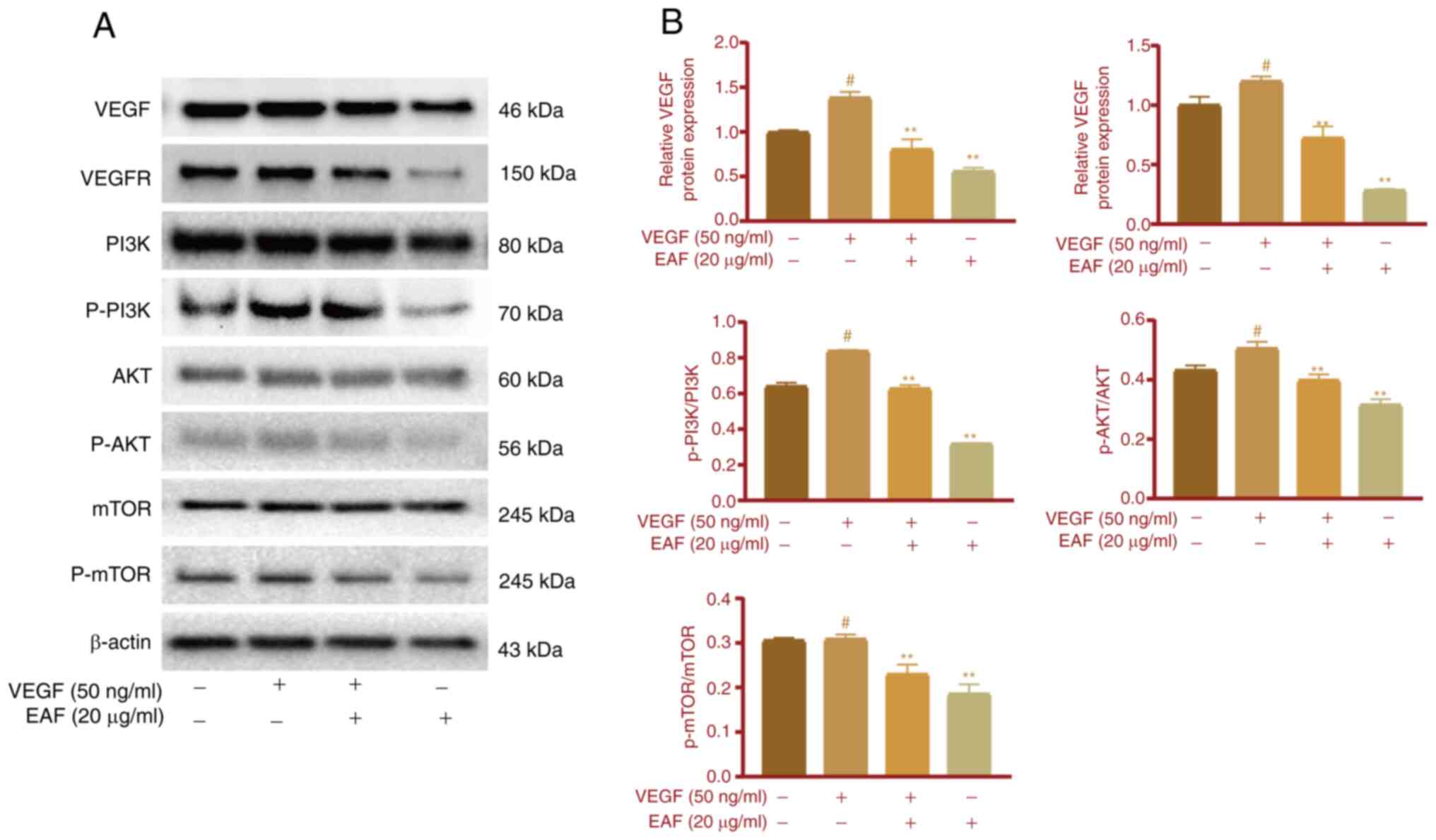
EAF inhibits VEGFR signaling in
HUVECs
The expression of cellular proteins was determined
in HUVECs following EAF treatment. The present study first
demonstrated that EAF exhibited a significant inhibitory effect on
VEGF-induced cell migration and angiogenesis; thus, VEGFR-related
signaling pathways were investigated further. Following the
addition of VEGF in the cell culture, VEGFR expression levels and
the phosphorylation of PI3K and AKT, were increased in HUVECs
(Fig. 6A and B). However, EAF
treatment eliminated this effect and the expression of VEGFR in the
EAF + VEGF group was significantly lower than that in the VEGF and
control groups. Notably, there was no significant difference in the
phosphorylation of PI3K and AKT between EAF + VEGF and control
groups; however, phosphorylation was significantly lower than that
in the VEGF group. In addition, following treatment with EAF alone,
phosphorylation of PI3K and AKT was significantly lower than that
in the control and VEGF groups. Protein expression levels of p-mTOR
were not significantly increased in the VEGF group compared with
the control group; however, these were significantly decreased in
the EAF + VEGF and EAF groups, compared with the control and VEGF
groups.
Discussion
The present study aimed to explore the effects and
mechanisms of EAF in the treatment of RA and revealed the
inhibitory effects on synovial proliferation and angiogenesis.
It provided a novel theoretical basis for the
clinical use of Anemone flaccida Fr. Schmidt.
Liu et al (20) demonstrated that triterpenoid
saponins, the main components of Anemone flaccida,
significantly improved the joint inflammatory response in arthritic
mice and significantly inhibited lipopolysaccharide-induced TNF-α
expression in RAW264.7 cells. Results of a previous study
demonstrated that Anemone flaccida Fr. Schmidt significantly
increased bone mineral density, bone volume fraction and trabecular
thickness in arthritic mice and reduced trabecular separation in
periarticular and extraarticular inflamed joints (21). Anemone flaccida Fr. Schmidt
is effective in the treatment of RA; however, to the best of the
authors' knowledge, no studies have reported the specific effects
on the RA synovium. Consistent with the results of previous
studies, results of the present study demonstrated that the main
components of the ethanolic extract included flavonoids and
phenolic acids. Moreover, results of the present study demonstrated
the inhibitory effects of EAF on intra-articular synovial membrane
proliferation and angiogenesis in RA, both in vitro and
in vivo.
The therapeutic effects of EAF on RA were
investigated using a CIA model in the present study. Compared with
the model group, EAF markedly improved joint redness and swelling
in CIA model rats. Notably, the body weight of CIA model rats was
significantly lower than that of the control group, which may have
been due to a reduction in food intake as a result of arthralgia.
There was no significant difference in body weight between the
model group and the EAF-treated group, which may demonstrate that
EAF did not induce weight loss. Subsequent immunohistochemical and
H&E staining analyses demonstrated significant improvements in
synovial tissue proliferation and inhibition of neovascularization
in RA, following treatment with EAF. Thus, it was hypothesized that
EAF may aid in the treatment of RA via inhibition of rheumatoid
synovial cells and angiogenesis.
Fibroblast-like synoviocytes are derived from
mesenchymal stem cells, rich in rough endoplasmic reticulum, and
are the main component of synovial tissue (22,23).
Synovial fibroblasts exert a variety of pathological changes in RA
through the secretion of inflammatory factors, including
inflammatory infiltration, synovial proliferation and vascular
opacification formation, mediating pathways that play an important
role in cartilage destruction and joint deformation (24,25).
Results of previous studies demonstrate that activated synovial
cells exhibit properties comparable with tumor-like cells, such as
excessive proliferation, migration and insufficient apoptosis
(26,27). RA-FLS destroys cartilage and bone
through migration and invasion, promoting the progression of
arthritis (28). Thus, targeting
RA-FLSs to modulate the aggressiveness or apoptosis of FLS cells
may be a novel therapeutic approach for RA (29,30).
The results of the present study demonstrated that EAF
significantly inhibited the viability of HFLS-RAs, suppressed the
proliferation, migration and invasion and promoted apoptosis in a
dose-dependent manner.
Neovascularization plays a key role in synovial
development in the inflammatory microenvironment of RA (31). Vascular endothelial cells respond
to various inflammatory and growth factors in RA and maintain
proliferation in the synovium through proliferating and migrating
to form new blood vessels (32).
The results of a previous study demonstrated that levels of
pro-angiogenic factors in the RA synovium, including VEGF and
platelet derived growth factor (PDGF), respond to the severity of
RA and therapy (33). In the
present study, both neovascularization and VEGF expression were
significantly reduced in the synovial membrane of CIA rats treated
with EAF. Thus, it was hypothesized that EAF inhibited synovial
angiogenesis and improved arthritic symptoms through suppression of
VEGF expression. A CAM model was established and VEGF-165 was added
to simulate the VEGF-rich environment in arthritis. The results of
the present study demonstrated that VEGF-165 significantly
stimulated angiogenesis in the CAM model; however, EAF also
inhibited angiogenesis in the presence of VEGF-165. The inhibitory
effects of EAF on VEGF-activated endothelial cell migration and
tube formation were examined and results of the present study
demonstrated that EAF markedly inhibited the migration and tube
formation of HUVECs. These results were also observed in the
presence of VEGF-165.
Angiogenesis occurs through endothelial cell
proliferation, differentiation, migration and lumen formation
(34). Notably, VEGF is a key
regulatory factor of angiogenesis (35,36).
VEGF also plays a role in the vascular proliferation and blood
vessel invasion of the synovial lining membrane in RA (37). The binding of VEGF to its receptor
VEGFR activates the PI3K/AKT signaling pathway, which, in turn,
regulates endothelial cell formation of blood vessels (38). Results of previous studies
demonstrated that triterpene saponins markedly inhibit the PI3K/AKT
signaling pathway (39,40). Notably, results of the present
study demonstrated that treatment with VEGF-165 activated the
PI3K/AKT signaling pathway and HUVEC migration and tube formation
were significantly increased. However, these effects were reversed
following EAF treatment.
In conclusion, results of the present study
demonstrated that EAF exerted notable effects on RA. EAF markedly
inhibited synovial proliferation and angiogenesis in arthritic
joints and this was associated with regulation of the
VEGFR/PI3K/AKT signaling pathway (Fig.
7). These findings further revealed the anti-RA mechanism of
EAF and provided a novel theoretical basis for its clinical
application. In addition, the anti-angiogenesis effects of EAF
should be further explored in tumors.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Chinese Academy of
Medical Sciences (CAMS) Innovation Fund for Medical Sciences (grant
no. 2018-I2M-AI-015) and the National Science Foundation of China
(grant no. 81702920).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GZ, DYC and QR designed the study, conceived the
experiments, outlined the experimental scheme, confirmed the
authenticity of all the raw data and revised the manuscript. QR, HY
and XZ performed the experiments and drafted the manuscript. FHW,
XHG and YDX analyzed the experimental data. GZ, QR, FHW, XHG and
YDX reviewed and edited the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The animal experiments were carried following
approval by the Laboratory Animal Ethics Committee of Hubei Academy
of Chinese Medicine Sciences (approval no. 202208001).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sparks JA: Rheumatoid arthritis. Ann
Intern Med. 170:ITC1–ITC16. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aletaha D and Smolen JS: Diagnosis and
management of rheumatoid arthritis: A review. JAMA. 320:1360–1372.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Smith MH and Berman JR: What is rheumatoid
arthritis? Jama. 327:11942022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
van der Woude D and van der Helm-van Mil
AHM: Update on the epidemiology, risk factors, and disease outcomes
of rheumatoid arthritis. Best Pract Res Clin Rheumatol. 32:174–187.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
You S, Koh JH, Leng L, Kim WU and Bucala
R: The tumor-like phenotype of rheumatoid synovium: Molecular
profiling and prospects for precision medicine. Arthritis
Rheumatol. 70:637–652. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cush JJ: Rheumatoid arthritis: Early
diagnosis and treatment. Med Clin North Am. 105:355–365. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim JW, Kong JS, Lee S, Yoo SA, Koh JH,
Jin J and Kim WU: Angiogenic cytokines can reflect the synovitis
severity and treatment response to biologics in rheumatoid
arthritis. Exp Mol Med. 52:843–853. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Radu AF and Bungau SG: Management of
rheumatoid arthritis: An overview. Cells. 10:28572021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abbasi M, Mousavi MJ, Jamalzehi S,
Alimohammadi R, Bezvan MH, Mohammadi H and Aslani S: Strategies
toward rheumatoid arthritis therapy; the old and the new. J Cell
Physiol. 234:10018–10031. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Burmester GR and Pope JE: Novel treatment
strategies in rheumatoid arthritis. Lancet. 389:2338–2348. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu H, Qiu Y, Tasneem S, Daniyal M, Li B,
Cai X, Rahman AU and Wang W: Advancement of natural compounds as
anti-rheumatoid arthritis agents: A focus on their mechanism of
actions. Mini Rev Med Chem. 21:2957–2975. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Y, Chen S, Du K, Liang C, Wang S,
Boadi EO, Li J, Pang X, He J and Chang YX: Traditional herbal
medicine: Therapeutic potential in rheumatoid arthritis. J
Ethnopharmacol. 279:1143682021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu Q, Zhu XZ, Feng RB, Liu Z, Wang GY,
Guan XF, Ou GM, Li YL, Wang Y, Li MM and Ye WC: Crude triterpenoid
saponins from Anemone flaccida (Di Wu) exert anti-arthritic effects
on type II collagen-induced arthritis in rats. Chin Med. 10:202015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu C, Yang Y, Sun D, Wang C, Wang H, Jia
S, Liu L and Lin N: Total saponin from Anemone flaccida fr. Schmidt
prevents bone destruction in experimental rheumatoid arthritis via
inhibiting osteoclastogenesis. Rejuvenation Res. 18:528–542. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Linghu KG, Xiong SH, Zhao GD, Zhang T,
Xiong W, Zhao M, Shen XC, Xu W, Bian Z, Wang Y and Yu H:
Sigesbeckia orientalis L. Extract alleviated the collagen type
II-induced arthritis through inhibiting multi-target-mediated
synovial hyperplasia and inflammation. Front Pharmacol.
11:5479132020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hawkins P, Armstrong R, Boden T, Garside
P, Knight K, Lilley E, Seed M, Wilkinson M and Williams RO:
Applying refinement to the use of mice and rats in rheumatoid
arthritis research. Inflammopharmacology. 23:131–150. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rao Q, Li R, Yu H, Xiang L, He B, Wu F and
Zhao G: Effects of dihydroartemisinin combined with cisplatin on
proliferation, apoptosis and migration of HepG2 cells. Oncol Lett.
24:2752022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hao DC, Gu X and Xiao P: Anemone medicinal
plants: Ethnopharmacology, phytochemistry and biology. Acta Pharm
Sin B. 7:146–158. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao L, Chen WM and Fang QC: Triterpenoid
Saponins from Anemone flaccida. Planta Med. 56:92–93. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu Q, Xiao XH, Hu LB, Jie HY, Wang Y, Ye
WC, Li MM and Liu Z: Anhuienoside C ameliorates collagen-induced
arthritis through inhibition of MAPK and NF-κB signaling pathways.
Front Pharmacol. 8:2992017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kong X, Wu W, Yang Y, Wan H, Li X, Zhong
M, Zhao H, Su X, Jia S, Ju D and Lin N: Total saponin from Anemone
flaccida Fr. Schmidt abrogates osteoclast differentiation and bone
resorption via the inhibition of RANKL-induced NF-κB, JNK and p38
MAPKs activation. J Transl Med. 13:912015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Németh T, Nagy G and Pap T: Synovial
fibroblasts as potential drug targets in rheumatoid arthritis,
where do we stand and where shall we go? Ann Rheum Dis.
81:1055–1064. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhai KF, Duan H, Cui CY, Cao YY, Si JL,
Yang HJ, Wang YC, Cao WG, Gao GZ and Wei ZJ: Liquiritin from
glycyrrhiza uralensis attenuating rheumatoid arthritis via reducing
inflammation, suppressing angiogenesis, and inhibiting MAPK
signaling pathway. J Agric Food Chem. 67:2856–2864. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nygaard G and Firestein GS: Restoring
synovial homeostasis in rheumatoid arthritis by targeting
fibroblast-like synoviocytes. Nat Rev Rheumatol. 16:316–333. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Falconer J, Murphy AN, Young SP, Clark AR,
Tiziani S, Guma M and Buckley CD: Review: Synovial cell metabolism
and chronic inflammation in rheumatoid arthritis. Arthritis
Rheumatol. 70:984–999. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Q, Liu J, Zhang M, Wei S, Li R, Gao
Y, Peng W and Wu C: Apoptosis induction of fibroblast-like
synoviocytes is an important molecular-mechanism for herbal
medicine along with its active components in treating rheumatoid
arthritis. Biomolecules. 9:7952019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Du H, Zhang X, Zeng Y, Huang X, Chen H,
Wang S, Wu J, Li Q, Zhu W, Li H, et al: A novel phytochemical, DIM,
inhibits proliferation, migration, invasion and TNF-α induced
inflammatory cytokine production of synovial fibroblasts from
rheumatoid arthritis patients by targeting MAPK and AKT/mTOR signal
pathway. Front Immunol. 10:16202019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
You S, Yoo SA, Choi S, Kim JY, Park SJ, Ji
JD, Kim TH, Kim KJ, Cho CS, Hwang D and Kim WU: Identification of
key regulators for the migration and invasion of rheumatoid
synoviocytes through a systems approach. Proc Natl Acad Sci USA.
111:550–555. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hu P, Dong ZS, Zheng S, Guan X, Zhang L,
Li L and Liu Z: The effects of miR-26b-5p on fibroblast-like
synovial cells in rheumatoid arthritis (RA-FLS) via targeting EZH2.
Tissue Cell. 72:1015912021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Svensson MND, Zoccheddu M, Yang S, Nygaard
G, Secchi C, Doody KM, Slowikowski K, Mizoguchi F, Humby F, Hands
R, et al: Synoviocyte-targeted therapy synergizes with TNF
inhibition in arthritis reversal. Sci Adv. 6:eaba43532020.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Le THV and Kwon SM: Vascular endothelial
growth factor biology and its potential as a therapeutic target in
rheumatic diseases. Int J Mol Sci. 22:53872021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Baggio C, Boscaro C, Oliviero F, Trevisi
L, Ramaschi G, Ramonda R, Bolego C and Cignarella A: Gender
differences and pharmacological regulation of angiogenesis induced
by synovial fluids in inflammatory arthritis. Biomed Pharmacother.
152:1131812022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Balogh E, Pusztai A, Hamar A, Végh E,
Szamosi S, Kerekes G, McCormick J, Biniecka M, Szántó S, Szűcs G,
et al: Autoimmune and angiogenic biomarkers in autoimmune
atherosclerosis. Clin Immunol. 199:47–51. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sajib S, Zahra FT, Lionakis MS, German NA
and Mikelis CM: Mechanisms of angiogenesis in microbe-regulated
inflammatory and neoplastic conditions. Angiogenesis. 21:1–14.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li Y, Liu Y, Wang C, Xia WR, Zheng JY,
Yang J, Liu B, Liu JQ and Liu LF: Succinate induces synovial
angiogenesis in rheumatoid arthritis through metabolic remodeling
and HIF-1α/VEGF axis. Free Radic Biol Med. 126:1–14. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
MacDonald IJ, Liu SC, Su CM, Wang YH, Tsai
CH and Tang CH: Implications of angiogenesis involvement in
arthritis. Int J Mol Sci. 19:20122018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Malemud CJ: Growth hormone, VEGF and FGF:
Involvement in rheumatoid arthritis. Clin Chim Acta. 375:10–19.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang X, Bove AM, Simone G and Ma B:
Molecular bases of VEGFR-2-mediated physiological function and
pathological role. Front Cell Dev Biol. 8:5992812020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu X, Mi X, Wang Z, Zhang M, Hou J, Jiang
S, Wang Y, Chen C and Li W: Ginsenoside Rg3 promotes regression
from hepatic fibrosis through reducing inflammation-mediated
autophagy signaling pathway. Cell Death Dis. 11:4542020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jang E and Lee JH: Promising anticancer
activities of alismatis rhizome and its triterpenes via p38 and
PI3K/Akt/mTOR signaling pathways. Nutrients. 13:24552021.
View Article : Google Scholar : PubMed/NCBI
|