Introduction
Coronary heart disease (CHD) is a major
cardiovascular disease and is the leading cause of mortality
worldwide (1). The status of
hyperglycemia causes vascular endothelial dysfunction and injury,
resulting in the development of atherosclerosis. According to the
latest epidemiological data, hyperglycemia is a major risk factor
for patients with CHD and affects the prognosis of ~75% of patients
(2). Previous studies have
suggested that the severity of coronary stenosis in patients with
CHD and hyperglycemia is more severe than that in patients with CHD
and normoglycemia (3,4). With ongoing progress in the
prevention and treatment of CHD, the molecular mechanism underlying
the development of CHD and hyperglycemia has become clear. The
mechanism is mainly related to increased polyol pathway flux,
increased intracellular advanced glycation end product formation,
increased hexosamine biosynthesis pathway, protein kinase C
activation and mitochondrial production of reactive oxygen species
(5); however, there is a lack of
clinically effective biomarkers for predicting coronary artery
lesions.
MicroRNAs (miRNAs/miRs) are a class of non-coding
RNAs 18–25 nucleotides in length, which can accurately regulate
gene expression by directly binding to the 3′-untranslated regions
of their target mRNAs at transcriptional and post-transcriptional
levels. Increasing evidence has suggested that miRNAs are involved
in the regulation of multiple physiological and pathological
processes, including proliferation, metabolism, inflammation,
metastasis and apoptosis (6).
Studies have shown that miRNAs from serum or plasma can be used as
promising biomarkers for diagnosing and monitoring the progression
of cardiovascular diseases, including CHD (7). For example, Bayés-Genis et al
(8) demonstrated that circulating
miR-1254 and miR-1306-5p are associated with risk of death and
hospitalization in patients with heart failure. Based on the
results of a meta-analysis, Zhu et al (9) concluded that serum or plasma miR-133a
may serve as a diagnostic biomarker for patients with acute
myocardial infarction. Wei et al (10) reported that miR-425-5p may serve as
novel biomarker of atrial fibrosis that contributes to atrial
remodeling in patients with atrial fibrillation. Additionally, Pan
et al (11) reported that
peripheral blood miR-15a expression is associated with low-density
lipoprotein cholesterol (LDL-C) and Gensini score, and may serve as
a diagnostic biomarker for patients with coronary artery disease.
Recently, Szydelko and Matyjaszek-Matuszek summarized the role of
miRNAs as novel biomarkers for the development of diabetic coronary
artery disease (12). However, the
low content of most miRNAs in serum limits their clinical
application.
Exosomes are membrane-bound vesicles 30–150 nm in
size, which are present in the majority of bodily fluids and serve
key functions in cell-to-cell communication by acting as a delivery
cargo shuttle for various molecules (13). Exosomes contain various nucleic
acids, including miRNAs, and numerous types of proteins, such as
tumor susceptibility gene 101 (TSG101), membrane CD63,
membrane-anchored heat-shock 70 (HSP70) and ALG2-interacting
protein X. Notably, exosomes have emerged as a promising delivery
system for drugs with lower toxicity and high therapeutic efficacy
(14). Exosomal miRNAs were first
identified in serum, and have also been described in several
biological fluids, such as saliva, urine, cerebrospinal fluid and
synovial fluid (15). In addition,
exosomal miRNAs serve an important role in disease progression by
altering cell signal transduction (16). Notably, exosomes can be stably
stored under different conditions, indicating that exosomal miRNAs
are potential biomarkers for the diagnosis and treatment of
diseases, including CHD (17).
Although the expression of miRNAs in patients with
CHD has been reported (18), to
the best of our knowledge, the relationship between exosomal miRNA
expression and the severity of CHD has not been studied. The
present study aimed to identify the expression profile of
serum-derived exosomal miRNAs in patients with CHD and
hyperglycemia, and to identify effective biomarkers for predicting
coronary artery lesions. The present study may provide evidence for
the use of exosomal miRNAs as novel biomarkers for the severity of
coronary stenosis in patients with CHD and hyperglycemia.
Materials and methods
Study population
In the first stage of the present study, eight
patients with CHD and hyperglycemia (age range, 39–73 years), and
eight age- and sex-matched patients with CHD and normoglycemia (age
range, 42–75 years), who had been admitted to The 960th Hospital of
the Joint Service Support Force of the People's Liberation Army
(Jinan, China) for coronary angiography (CAG) between January 2018
and May 2018 were enrolled to perform a human miRNA microarray
analysis. The clinical characteristics of these patients is shown
in Table SI. In the second stage,
75 patients with CHD and hyperglycemia (age range, 33–76 years;
mean ± SD age, 65.82±8.30 years) and 75 age- and sex-matched
patients with CHD and normoglycemia (age range, 30–78 years; mean ±
SD age, 63.13±9.85 years) were enrolled from The 960th Hospital of
the Joint Service Support Force of the People's Liberation Army
between June 2018 and December 2020 for further validation of the
miRNA microarray results. The study design is shown in Fig. 1. Hyperglycemia was defined as
fasting blood glucose (FBG) ≥6.1 mmol/l. Normoglycemia was defined
as FBG <6.0 mmol/l and oral glucose tolerance test (2-h plasma
glucose) <7.8 mmol/l. The inclusion criteria of patients with
CHD were: At least one major epicardial vessel with >50%
stenosis indicated by CAG and presenting with typical angina. The
exclusion criteria were as follows: i) Age, <18 years; ii)
myocardial infarction within 3 months, cardiac insufficiency, New
York Heart Association grade (19)
≥III, cardiomyopathy, congenital heart disease, heart valve
disease, autoimmune system disease, hematological system disease,
inflammatory disease, lymphatic system disease, active infection,
severe liver and kidney insufficiency, and malignant tumor; iii)
patients with mental illness that were unable to cooperate with
treatments; iv) patients with incomplete medical records. The
present study was approved by The 960th Hospital of the Joint
Service Support Force of the People's Liberation Army (approval no.
202109) and complied strictly with the 2008 Declaration of Helsinki
Principle. Written informed consent was obtained from participants
prior to the collection of samples.
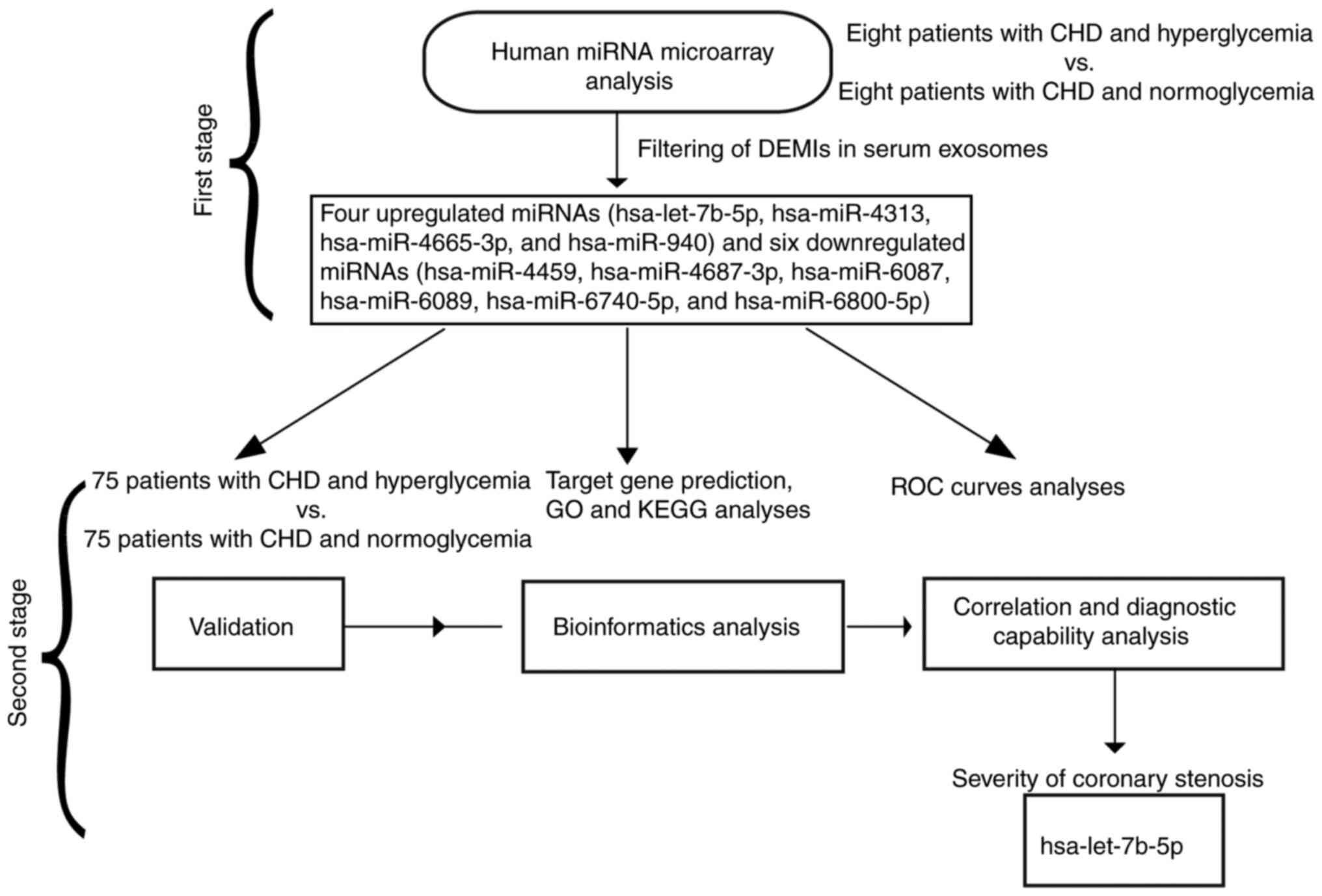 | Figure 1.Flow diagram of the present study. In
the first stage, a human miRNA microarray analysis was performed to
identify the expression profile of exosomal miRNAs in the serum of
patients with CHD and hyperglycemia. In the second stage,
validation, bioinformatics analysis, and correlation and diagnostic
capability analyses were performed to verify hsa-let-7b-5p as an
effective biomarker for differentiating the severity of coronary
stenosis. CHD, coronary heart disease; DEMIs, differentially
expressed miRNAs; GO, Gene Ontology; KEGG, Kyoto Encyclopedia of
Genes and Genomes; miRNA/miR, microRNA; ROC, receiver operating
characteristic. |
Clinical data collection
The clinical baseline data of patients with CHD,
including age, sex, body mass index (BMI), systolic blood pressure
(SBP), diastolic blood pressure (DBP), smoking, drinking, and
history of dyslipidemia and hypertension were collected. The levels
of total cholesterol (TC), triglyceride (TG), LDL-C, high-density
lipoprotein cholesterol (HDL-C), hemoglobin A1C (Hba1c) and FBG
were routinely tested. Based on the Synergy between Percutaneous
Coronary Intervention with Taxus and Cardiac Surgery (SYNTAX) score
(20), the severity of coronary
stenosis in patients with CHD was evaluated, and was divided into
the mild lesion group (SYNTAX score <22) and the moderate to
severe lesion group (SYNTAX score ≥22).
Isolation of exosomes from serum
Peripheral blood (10 ml) was collected from each
participant after fasting for 12 h. The serum was separated by
centrifugation at 2,000 × g for 20 min at 37°C, followed by
centrifugation at 12,000 × g for 5 min at 4°C. Exosomes were
isolated from the serum using an exosome isolation kit (cat. no.
EZB-exo1; EZBioscience), according to the manufacturer's
instructions. Briefly, 1/3 volume of exosome separation reagent was
added to 1 ml serum and was refrigerated at 4°C overnight. The
serum and exosome separation reagent were centrifuged together at
15,000 × g at 4°C for 30 min and supernatants were removed by
aspiration. The pellet containing the exosomes at the bottom of the
tube was resuspended in 200 µl PBS.
Transmission electron microscopy
(TEM)
The exosome pellets were fixed with 4%
paraformaldehyde at 37°C and then placed in a Formvar/carbon
film-coated TEM grid (Thermo Fisher Scientific, Inc.).
Subsequently, samples were fixed by incubation with 1%
glutaraldehyde for 90 min at 37°C, stained with 1% uranyl acetate
for 2 h at 37°C, embedded and polymerized in epoxy resin for 2 h at
37°C, and finally images were captured using a FEI Tecnai T20
transmission electron microscope (Thermo Fisher Scientific,
Inc.).
Western blot analysis
Proteins were extracted from exosomes using an
exosomal protein extraction kit (EZBioscience), according to the
manufacturer's protocol. The concentration of each protein was
detected using a BCA Protein Assay Kit (Shanghai Yeasen
Biotechnology Co., Ltd.). Proteins (35 µg/lane) were loaded and
concentrated on 5% stacking gels, separated by SDS-PAGE on 10%
resolving gels, and then transferred to polyvinylidene difluoride
membranes (MilliporeSigma). After blocking with 5% bovine serum
albumin (Beyotime Institute of Biotechnology) for 2 h at 37°C, the
membranes were incubated with primary human anti-HSP70 (cat. no.
ab181606; 1:1,000), anti-TSG101 (cat. no. ab133586; 1:750) and
anti-CD63 (cat. no. ab134045; 1:2,000) antibodies overnight at 4°C.
Subsequently, the membranes were incubated with goat anti-rabbit
HRP secondary antibody (cat. no. ab6721; 1:2,500) at 37°C for 60
min. All antibodies were obtained from Abcam. Finally, protein
bands were visualized using an ECL kit (Beyotime Institute of
Biotechnology), and were further scanned and analyzed using ImageJ
version 1.8.0 software (National Institutes of Health).
miRNA microarray analysis
The exosomal miRNAs were extracted using the
mirVana™ PARIS™ kit (Ambion; Thermo Fisher Scientific, Inc.),
according to the manufacturer's instructions. For miRNA microarray
analysis, extracted miRNAs were labeled with the cyanine 3-cytidine
bisphosphate labeling kit (Agilent Technologies, Inc.) according to
the manufacturer's protocol, and then hybridized to a human miRNA
microarray (cat. no. G4872A; 8×60 K, version 21.0; Agilent
Technologies, Inc.) at 55°C with constant rotation at 12 × g for 20
h, according to the manufacturer's instructions. Subsequently, the
microarray was rinsed with corresponding wash buffer three times
and scanned using an Agilent G2505C microarray scanner (Agilent
Technologies, Inc.). The raw data were read and converted to
numbers using Feature Extraction Software (version 10.7.1.1;
Agilent Technologies, Inc.), and were further analyzed using
GeneSpring GX software (version 12.5; Agilent Technologies, Inc.).
The signal intensities of the spots on the microarray were
normalized to the total signal intensity and shown as percentages.
An unpaired Student's t-test was used to determine the statistical
significance of differences in microarray signal intensities. The
differentially expressed miRNAs (DEMIs) were screened according to
the following thresholds: Fold change >2 or <0.5; adjusted
P<0.05 (false discovery rate method). Hierarchical clustering
analysis of the dataset of the selected DEMIs was performed using
ClusterProfiler 3.5 version software packages for Windows
(Bioconductor, http://www.bioconductor.org/packages/3.5/bioc/html/clusterProfiler.html)
with Ward's method (21).
Prediction of miRNA target genes and
enrichment analysis
The target genes of DEMIs were predicted using
TargetScan8.0 (https://www.targetscan.org/vert_80/), miRDB
(https://mirdb.org/miRDB/), and Tarbase7.0
(http://diana.imis.athena-innovation.gr/DianaTools/index.php?r=tarbase/index)
databases. For Gene Ontology (GO; http://geneontology.org/) enrichment analysis, the
target genes of the selected DEMIs were mapped to GO terms
(biological process, cellular component and molecular function) in
the dataset, and the gene numbers for every term were calculated.
Subsequently, hypergeometric test was performed to identify
significantly enriched GO terms in the input gene list, and
P<0.05 was set as the cut-off criterion. For Kyoto Encyclopedia
of Genes and Genomes (KEGG; http://www.kegg.jp/) pathway enrichment analysis, the
significantly enriched metabolic or signal transduction pathways
with P<0.05 from target genes of the selected DEMIs were
identified when compared with the whole genome background.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated from exosomes using an
exosome RNA purification kit (EZBioscience), according to the
manufacturer's protocol. RNA was reverse transcribed into cDNA
using a miRNA First Strand cDNA Synthesis (Stem-loop Method) kit
(Sangon Biotech Co., Ltd.), according to the manufacturer's
instructions. For the quantification of miRNA expression levels,
qPCR was performed according to the instructions of the SYBR Green
One™ miRNAs qPCR Detection Kit (BioTeke Corporation), with U6 small
nuclear RNA used as the endogenous reference. The total reaction
system of qPCR was 25 µl, including master mix, primers, cDNA and
RNase-free deionized water, and the thermocycling conditions were
as follows: One cycle at 95°C for 15 min, followed by 40 reaction
cycles at 94°C for 20 sec and 60°C for 34 sec. The primer sequences
are shown in Table I. The relative
expression levels of miRNAs were normalized against the expression
of U6 using the 2−∆∆Cq method (22).
 | Table I.Primers used for reverse
transcription-quantitative PCR. |
Table I.
Primers used for reverse
transcription-quantitative PCR.
| miRNA | Forward primer,
5′-3′ | Reverse primer,
5′-3′ |
|---|
| hsa-let-7b-5p |
TGAGGTAGTAGGTTGTGTGGTT |
CGCAGGGTCCGAGGTATTC |
| hsa-miR-4313 |
AGCCCCCTGGCCCCAAACCC |
CGCAGGGTCCGAGGTATTC |
|
hsa-miR-4665-3p |
CTCGGCCGCGGCGCGTAGCCCCCGCC |
CGCAGGGTCCGAGGTATTC |
| hsa-miR-940 |
AAGGCAGGGCCCCCGCTCCCC |
CGCAGGGTCCGAGGTATTC |
| hsa-miR-4459 |
CCAGGAGGCGGAGGAGGTGGAG |
CGCAGGGTCCGAGGTATTC |
|
hsa-miR-4687-3p |
TGGCTGTTGGAGGGGGCAGGC |
CGCAGGGTCCGAGGTATTC |
| hsa-miR-6087 |
TGAGGCGGGGGGGCGAGC |
CGCAGGGTCCGAGGTATTC |
| hsa-miR-6089 |
GGAGGCCGGGGTGGGGCGGGGCGG |
CGCAGGGTCCGAGGTATTC |
|
hsa-miR-6740-5p |
AGTTTGGGATGGAGAGAGGAGA |
CGCAGGGTCCGAGGTATTC |
|
hsa-miR-6800-5p |
GTAGGTGACAGTCAGGGGCGG |
CGCAGGGTCCGAGGTATTC |
| U6 |
CTCGCTTCGGCAGCACA |
AACGCTTCACGAATTTGCGT |
Statistical analysis
Statistical analysis was performed using SPSS
software (version 27.0; IBM Corp.). The Kolmogorov-Smirnov test was
used to test the normal distribution of all variables. Data
conforming to normal distribution were expressed as the mean ±
standard deviation, and unpaired Student's t-test was used to
assess the significant difference. Data conforming to non-normal
distribution were expressed as the median (interquartile range),
and Mann-Whitney U test was used to assess the significant
difference. Categorical variables were expressed as frequency and
percentage, and were analyzed by χ2 test or Fisher's
exact test. Correlation coefficients between the expression of
selected DEMIs and the levels of biochemical parameters in patients
with CHD and hyperglycemia were determined by Spearman correlation
analysis. The receiver operating characteristic (ROC) curve and
area under the curve (AUC) were analyzed to assess the possibility
of using exosomal miRNAs as diagnostic biomarkers for the severity
of coronary stenosis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Characterization of serum
exosomes
Firstly, the isolated exosomes were assessed by TEM
and western blot analysis. TEM showed that isolated exosomes
exhibited a round-shaped appearance within 30–150 nm, corresponding
to the expected size range of exosomes (Fig. S1A and B). Western blot analysis
showed that the marker proteins, CD63, TSG101 and HSP70, were
enriched in the exosomes, further confirming the successful
isolation of exosomes from serum (Fig. S1C).
Profile of exosomal miRNA expression
in the serum of patients with CHD and hyperglycemia
The miRNA transcript profile was characterized using
a microarray analysis in 16 human serum exosome samples, eight from
patients with CHD and hyperglycemia compared with eight from
patients with CHD and normoglycemia. A total of 10 DEMIs were
identified according to the criteria (Fig. 2A). The 10 DEMIs are shown in a
heatmap (Fig. 2B); among them,
hsa-let-7b-5p, hsa-miR-4313, hsa-miR-4665-3p and hsa-miR-940 were
upregulated, and hsa-miR-4459, hsa-miR-4687-3p, hsa-miR-6087,
hsa-miR-6089, hsa-miR-6740-5p and hsa-miR-6800-5p were
downregulated (Table II; all
P<0.05).
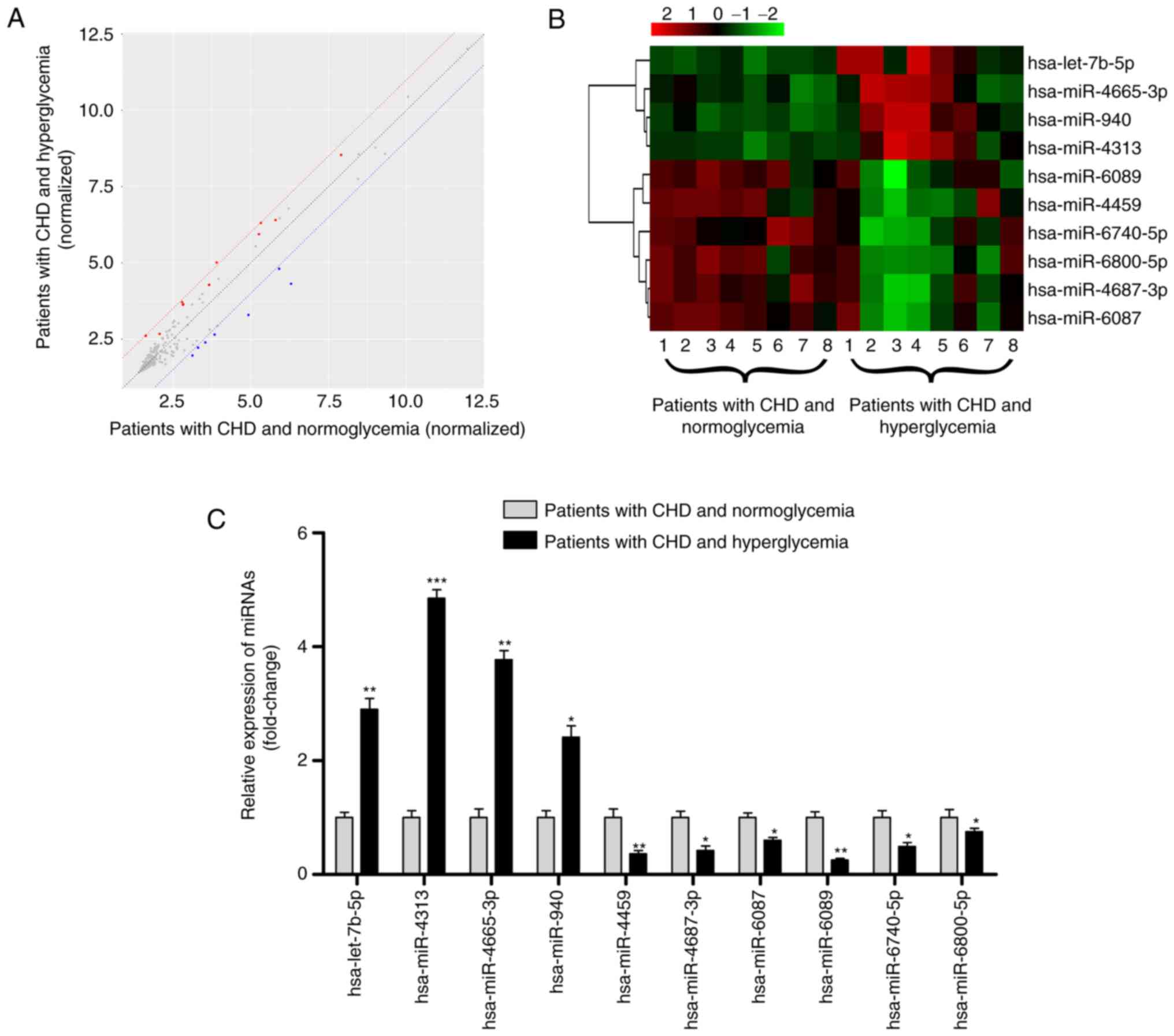 | Figure 2.Profile of exosomal miRNAs in the
serum of patients with CHD and hyperglycemia. (A) Scatter plot
showing the expression profile of exosomal miRNAs between eight
patients with hyperglycemia and eight patients with normoglycemia;
miRNAs above and below the border lines (red and blue) exhibited a
>2 fold-change difference in expression. (B) Clustered heatmap
for the 10 DEMIs, including hsa-let-7b-5p, hsa-miR-4313,
hsa-miR-4665-3p, hsa-miR-940, hsa-miR-4459, hsa-miR-4687-3p,
hsa-miR-6087, hsa-miR-6089, hsa-miR-6740-5p and hsa-miR-6800-5p.
(C) Reverse transcription-quantitative PCR validation of the 10
DEMIs in 75 patients with CHD and hyperglycemia and 75 patients
with CHD and normoglycemia. Data are expressed as the mean ±
standard deviation. *P<0.05, **P<0.01, ***P<0.001 vs.
patients with CHD and normoglycemia. CHD, coronary heart disease;
DEMIs, differentially expressed miRNAs; miRNA/miR, microRNA. |
 | Table II.List of the 10 differentially
expressed miRNAs in the serum of patients with coronary heart
disease and hyperglycemia. |
Table II.
List of the 10 differentially
expressed miRNAs in the serum of patients with coronary heart
disease and hyperglycemia.
| miRNA | Regulation | Fold change | P-value |
|---|
| hsa-let-7b-5p | Up | 2.47 | 0.007a |
| hsa-miR-4313 | Up | 2.60 | 0.015b |
|
hsa-miR-4665-3p | Up | 2.38 | 0.027b |
| hsa-miR-940 | Up | 3.60 | 0.012b |
| hsa-miR-4459 | Down | 0.48 | 0.039b |
|
hsa-miR-4687-3p | Down | 0.52 | 0.006a |
| hsa-miR-6087 | Down | 0.50 | 0.013b |
| hsa-miR-6089 | Down | 0.46 | 0.020b |
|
hsa-miR-6740-5p | Down | 0.50 | 0.013b |
|
hsa-miR-6800-5p | Down | 0.47 | 0.007a |
Validation of exosomal miRNA profile
identified by microarray
The exosomal miRNA profile was further confirmed by
RT-qPCR using samples from 75 patients with CHD and hyperglycemia
and 75 patients with CHD and normoglycemia. The baseline
characteristics of these patients are shown in Table III. The levels of Hba1c and FBG,
the number of stenotic vessels, severity of stenosis and SYNTAX
score were significantly higher in patients with CHD and
hyperglycemia than with those in patients with CHD and
normoglycemia (all P<0.05). There was no significant difference
in other baseline data (all P>0.05), including age, sex, BMI,
SBP, DBP, smoking, drinking, history of dyslipidemia and
hypertension, TC, TG, LDL-C and HDL-C. As shown in Fig. 2C, RT-qPCR results were consistent
with the miRNA microarray analysis results, as increased expression
levels of hsa-let-7b-5p, hsa-miR-4313, hsa-miR-4665-3p and
hsa-miR-940, and decreased expression levels of hsa-miR-4459,
hsa-miR-4687-3p, hsa-miR-6087, hsa-miR-6089, hsa-miR-6740-5p and
hsa-miR-6800-5p were observed in patients with CHD and
hyperglycemia (all P<0.05), thus demonstrating the reliability
of this miRNA profile.
 | Table III.Clinical baseline data of patients
with CHD and hyperglycemia or normoglycemia. |
Table III.
Clinical baseline data of patients
with CHD and hyperglycemia or normoglycemia.
| Characteristic | Patients with CHD
and hyperglycemia (n=75) | Patients with CHD
and normoglycemia (n=75) | P-value |
|---|
| Age, years | 65.82±8.30 | 63.13±9.85 | 0.725 |
| Sex,
female/male | 28/47 | 29/46 | 0.866 |
| BMI,
kg/m2 | 27.16±4.68 | 26.32±4.16 | 0.402 |
| SBP, mmHg | 147.91±14.45 | 148.04±15.41 | 0.331 |
| DBP, mmHg | 83.64±7.76 | 81.57±13.14 | 0.681 |
| Smoking, n (%) | 21 (28.0) | 24 (32.0) | 0.593 |
| Drinking, n
(%) | 13 (17.33) | 17 (22.67) | 0.414 |
| Dyslipidemia, n
(%) | 20 (26.67) | 27 (36.0) | 0.218 |
| Hypertension, n
(%) | 52 (69.33) | 45 (60.0) | 0.232 |
| TC, mmol/l | 4.32±1.09 | 4.16±1.27 | 0.609 |
| TG, mmol/l | 1.47±0.86 | 1.87±1.35 | 0.055 |
| LDL-C, mmol/l | 2.51±0.85 | 2.40±0.93 | 0.252 |
| HDL-C, mmol/l | 1.02±0.21 | 1.14±0.20 | 0.711 |
| Hba1c, % | 8.41±1.06 | 4.19±1.57 |
<0.001a |
| FBG, mmol/l | 8.78±2.10 | 4.49±1.26 |
<0.001a |
| Number of stenotic
vessels | 2.90±0.30 | 2.30±0.80 | 0.046b |
| Severity of
stenosis, % | 78.02±14.15 | 56.42±23.17 | 0.013b |
| SYNTAX score | 29.50 (24.0,
31.50) | 24.0 (21.50,
27.0) |
<0.001a |
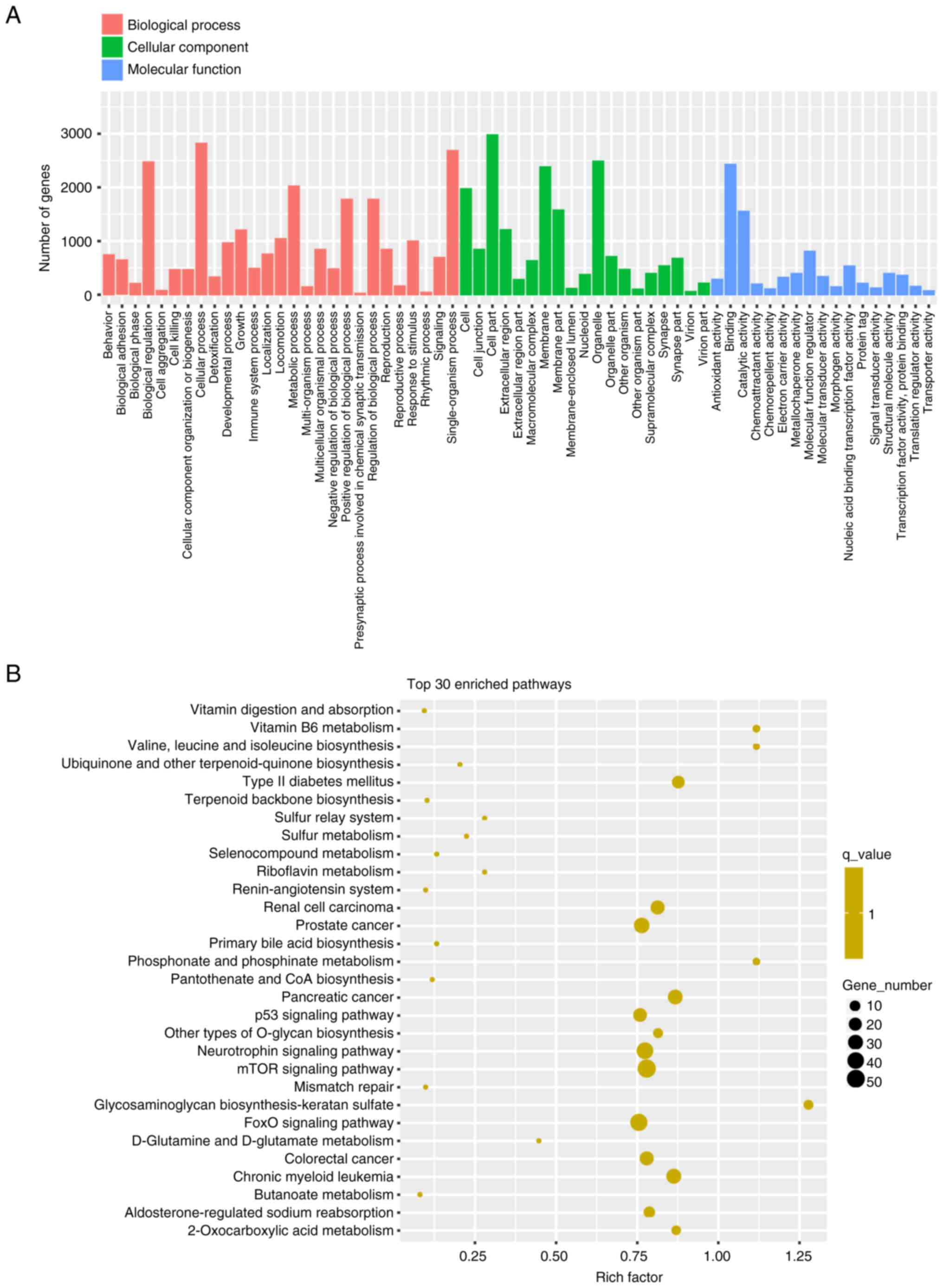
Functional enrichment and pathway
analysis of target genes of the 10 DEMIs
For the GO enrichment analysis, ‘cellular process’,
‘single-organism process’ and ‘biological regulation’ were the top
three enriched terms in biological process; ‘cell part’, organelle’
and ‘membrane’ were the top three enriched terms in cellular
component; and ‘binding’, ‘catalytic activity’ and ‘molecular
function regulator’ were the top three enriched terms in molecular
function (Fig. 3A). The results of
KEGG pathway analysis are shown in Fig. 3B. Several enriched pathways were
identified, including ‘mTOR signaling pathway’, ‘FoxO signaling
pathway’, ‘neurotrophin signaling pathway’, ‘p53 signaling pathway’
and ‘type 2 diabetes mellitus’, which were related to the 10
DEMIs.
Correlation between expression of the
10 DEMIs and biochemical parameters in patients with CHD and
hyperglycemia
The correlation between expression of the 10 DEMIs
and biochemical parameters is shown in Table SII. Results indicated that
hsa-let-7b-5p expression showed a strong positive correlation with
HbA1c levels and SYNTAX score in patients with CHD and
hyperglycemia (all P<0.001). In addition, hsa-miR-940 expression
was positively related to HbA1c level, and hsa-miR-6087 and
hsa-miR-6800-5p expression was negatively related to HbA1c level
(all P<0.01). Furthermore, hsa-miR-4459 expression was
positively related to SYNTAX score (P<0.01). Notably, no
correlation was observed between the expression of the other DEMIs
and HbA1c level or SYNTAX score (all P>0.05). These data
suggested that exosomal hsa-let-7b-5p may be associated with
coronary stenosis in patients with CHD and hyperglycemia.
Diagnostic potential of the 10 DEMIs
for the severity of coronary stenosis
Based on the SYNTAX score, the 75 patients with CHD
and hyperglycemia were divided into two groups, including 22
patients with low SYNTAX score and 53 patients with high SYNTAX
score. RT-qPCR results showed that the expression levels of
hsa-let-7b-5p were significantly increased in the patients with
high SYNTAX score compared with those in the patients with low
SYNTAX score (Fig. 4A;
P<0.001). In addition, the expression levels of hsa-miR-6800-5p
were significantly increased in patients with high SYNTAX score
compared with those in the patients with low SYNTAX score (Fig. 4B; P<0.05). ROC curves of the 10
DEMIs in the diagnosis of the severity of coronary stenosis are
shown in Fig. 5. The results
showed that hsa-let-7b-5p yielded the highest diagnostic accuracy
among the 10 DEMIs in discriminating high SYNTAX score from low
SYNTAX score in patients with CHD and hyperglycemia (Table SIII; P<0.001). These data
demonstrated that exosomal hsa-let-7b-5p has a promising potential
as a biomarker for the diagnosis of the severity of coronary
stenosis.
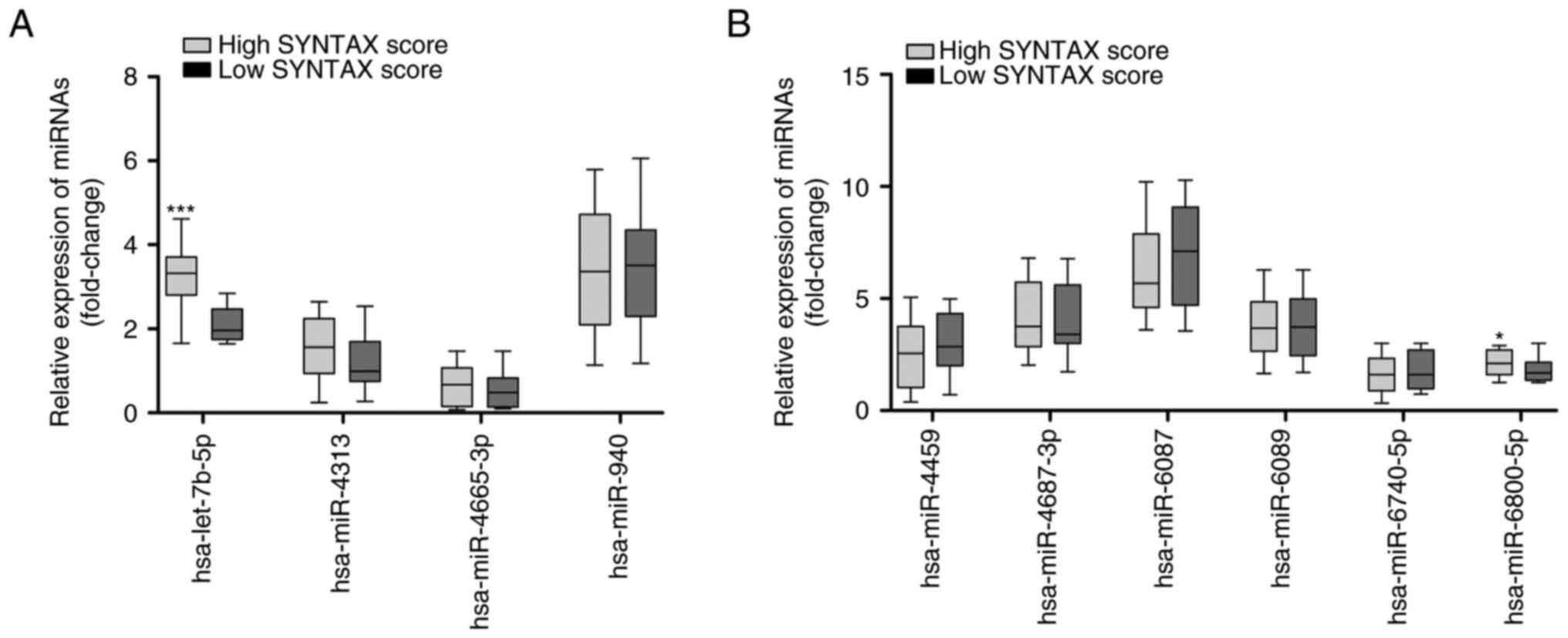 | Figure 4.Expression of the 10 differentially
expressed miRNAs in patients with CHD and hyperglycemia split into
two groups according to SYNTAX score. (A) RT-qPCR analysis of the
expression levels of hsa-let-7b-5p, hsa-miR-4313, hsa-miR-4665-3p
and hsa-miR-940 in 22 patients with low SYNTAX score and 53
patients with high SYNTAX score. (B) RT-qPCR analysis of the
expression levels of hsa-miR-4459, hsa-miR-4687-3p, hsa-miR-6087,
hsa-miR-6089, hsa-miR-6740-5p and hsa-miR-6800-5p in 22 patients
with low SYNTAX score and 53 patients with high SYNTAX score. Data
are presented as box and whisker plots with the minimum, median and
maximum values. *P<0.05, ***P<0.001 vs. low SYNTAX score.
CHD, coronary heart disease; miRNA/miR, microRNA; RT-qPCR, reverse
transcription-quantitative PCR; SYNTAX, Synergy between
Percutaneous Coronary Intervention with Taxus and Cardiac
Surgery. |
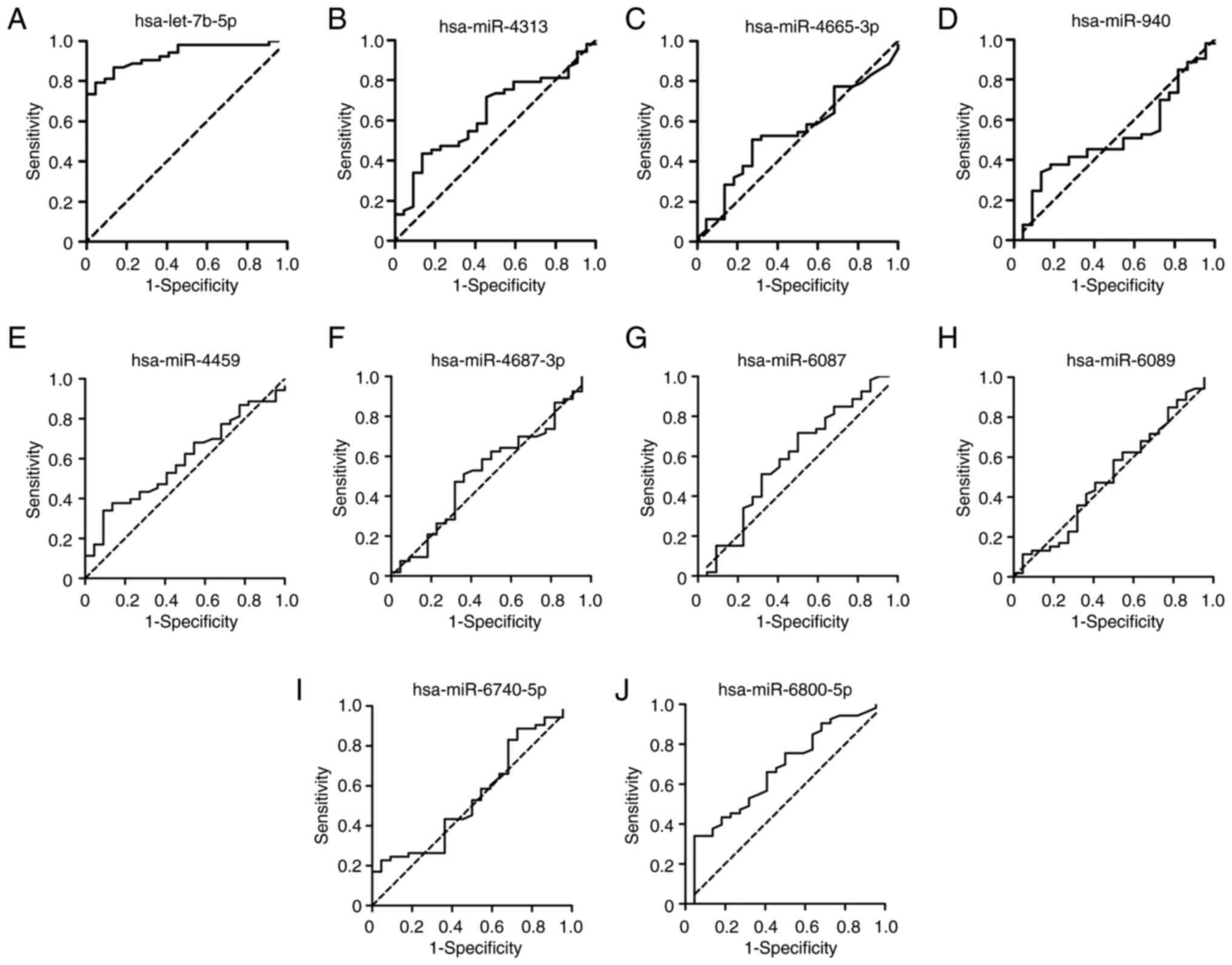 | Figure 5.Diagnostic value of the 10
differentially expressed miRNAs for the severity of coronary
stenosis. Receiver operating characteristic curves were plotted
using the expression levels of (A) hsa-let-7b-5p, (B) hsa-miR-4313,
(C) hsa-miR-4665-3p, (D) hsa-miR-940, (E) hsa-miR-4459, (F)
hsa-miR-4687-3p, (G) hsa-miR-6087, (H) hsa-miR-6089, (I)
hsa-miR-6740-5p and (J) hsa-miR-6800-5p for distinguishing high
SYNTAX score from low SYNTAX score in patients with coronary heart
disease and hyperglycemia. miR/miRNA, microRNA; SYNTAX, Synergy
between Percutaneous Coronary Intervention with Taxus and Cardiac
Surgery. |
Discussion
The incidence of CHD has exhibited an increase in
recent decades, which may be due to the prevalence of obesity and
lifestyle changes (23). CHD is
considered to be caused by atherosclerosis-induced arterial
stenosis and hyperglycemia can exacerbate the progression of
arterial stenosis. An increasing number of studies have
demonstrated that accurate diagnosis of coronary artery lesions is
key to the treatment of CHD (24,25).
CAG is considered the gold standard for detecting coronary stenosis
in routine clinical practice; however, it is expensive and some
patients cannot be tested due to contraindications, such as
hemophilia, severe heart failure, and allergy to iodine and
contrast agents. Therefore, identification of noninvasive
biomarkers for evaluating the severity of coronary stenosis in CHD
is required (26). It has been
suggested that exosomal miRNAs participate in a wide range of
biological processes and their dysregulated levels are associated
with complex phenotypes of human disease (27). In the present study, serum samples
were collected from patients with CHD and hyperglycemia, exosomes
were isolated and DEMIs were detected using human miRNA microarray
analysis. Subsequently, the target mRNAs of the selected DEMIs were
analyzed by bioinformatics analysis. Furthermore, the correlations
and diagnostic values of the selected DEMIs regarding the severity
of coronary stenosis in patients with CHD and hyperglycemia were
analyzed. To the best of our knowledge, the present study was the
first to reveal that upregulated exosomal hsa-let-7b-5p expression
may serve as a noninvasive biomarker the severity of coronary
stenosis, which could provide a novel insight into the diagnostic
capacity of exosomal miRNAs in coronary artery lesions.
miRNAs are transported mainly through exosomes in
the circulatory system. The transfer of miRNAs in these exosomes
prevents them from being degraded, causing miRNAs to circulate
in vivo and act on target cells. Numerous studies have
determined the expression profiles of exosomal miRNAs in human
disease (28). For example, Liu
et al (29) reported 66
DEMIs from exosomes in the serum of patients with esophageal
squamous cell carcinoma and Su et al (30) identified 13 DEMIs from exosomes in
the serum of patients with acute myocardial infarction. In serum
exosomes from patients with endometriosis, 24 DEMIs were identified
by a miRNA microarray (31). By
contrast, investigations into exosomal miRNAs in patients with CHD
and hyperglycemia are rare and limited (32,33).
In the present study, using a human miRNA microarray
analysis, 10 DEMIs, including four upregulated miRNAs
(hsa-let-7b-5p, hsa-miR-4313, hsa-miR-4665-3p and hsa-miR-940) and
six downregulated miRNAs (hsa-miR-4459, hsa-miR-4687-3p,
hsa-miR-6087, hsa-miR-6089, hsa-miR-6740-5p and hsa-miR-6800-5p),
were identified in the serum exosomes of patients with CHD and
hyperglycemia. These results were consistent with those of previous
reports regarding serum exosomes in esophageal squamous cell
carcinoma, acute myocardial infarction and endometriosis (29–31),
suggesting the credibility of the present data. The expression
levels of 10 DEMIs were further validated in 75 patients with CHD
and hyperglycemia. Consistent with a previous study (34), the results revealed that the SYNTAX
score was significantly higher in patients with CHD and
hyperglycemia than in patients with CHD and normoglycemia, thus
confirming the promotion of coronary stenosis by hyperglycemia. A
KEGG pathway enrichment analysis was subsequently conducted, and
the 10 DEMI target genes were significantly enriched in the ‘mTOR
signaling pathway’, ‘FoxO signaling pathway’ and ‘neurotrophin
signaling pathway’. The 10 DEMIs may participate in several
biological processes, such as ‘cellular process’, ‘single-organism
process’ and ‘biological regulation’. These data suggested that the
10 DEMIs may be involved in the pathogenesis of patients with CHD
and hyperglycemia.
As a member of the hsa-let-7 family of miRNAs,
hsa-let-7b-5p acts as a crucial regulator of developmental
processes in human diseases, such as Parkinson's disease, multiple
sclerosis, asthenozoospermia and Crohn disease (35–38).
In the current study, serum-derived exosomal hsa-let-7b-5p was
shown to be upregulated in patients with CHD and hyperglycemia,
which is consistent with a previous study by Zhang et al
(39). Subsequently, the
correlations between expression levels of the 10 DEMIs and
biochemical parameters were analyzed. Only hsa-let-7b-5p expression
was found to have a strong positive correlation with HbA1c levels
and SYNTAX score in patients with CHD and hyperglycemia.
Furthermore, increased hsa-let-7b-5p expression was detected in
patients with high SYNTAX score. Based on these findings, it was
hypothesized that exosomal hsa-let-7b-5p may be associated with
coronary stenosis in patients with CHD and hyperglycemia.
Exosome-derived miRNAs can easily be isolated from
various bodily fluids, indicating potential opportunities for
clinical translation. Exosomal miRNAs have emerged as promising
biomarkers for diagnosis, risk stratification and prognosis
prediction (27). Current research
on the relationships of coronary stenosis and miRNAs is increasing
(40). Ling et al (41) suggested that upregulated miR-122-5p
has the ability to predict the severity of coronary lesions. Li
et al (42) demonstrated
that miR-34a may be a novel biomarker in assistance of the
diagnosis of coronary stenosis. Similarly, miR-221/222 have been
reported to serve as promising biomarkers for the diagnosis of ≥50%
coronary stenosis and the occurrence of acute coronary syndrome
(43). Notably, the present study
found that hsa-let-7b-5p yielded the highest diagnostic accuracy
among the 10 DEMIs in discriminating high SYNTAX score from low
SYNTAX score in patients with CHD and hyperglycemia. Similarly,
previous studies have reported that plasma hsa-let-7b-5p serves as
a biomarker for the diagnosis of heroin use disorders, non-small
cell lung and multiple sclerosis (36,44,45).
These data demonstrated that exosomal hsa-let-7b-5p could be used
as a promising biomarker for differentiating the severity of
coronary stenosis. However, the methods used for exosome isolation
critically impact subsequent analyses due to lack of
internationally standardized methodologies. It is thus strongly
recommended to standardize the isolation procedure before
integrating studies across different laboratories. Similar to all
other biomarkers in CHD, before exosomal hsa-let-7b-5p can be
translated to the clinic, it must be validated in large cohort
studies and accredited by the International Organization for
Standardization.
The present study has some limitations. Firstly, a
relatively small sample size of patients with CHD and hyperglycemia
was assessed and selection bias may exist, thus more investigations
with large clinical samples are required for further validation of
the diagnostic power of exosomal hsa-let-7b-5p for differentiating
the severity of coronary stenosis. Secondly, there was a lack of
continuously monitoring disease progression and follow-up results,
and the relationship between exosomal hsa-let-7b-5p and prognosis
needs further study using regression analysis. Thirdly, the
association of exosomal hsa-let-7b-5p and hyperglycemia requires
further confirmation. Finally, the effects and related signaling
pathways of exosomal hsa-let-7b-5p in coronary stenosis were not
investigated, which could be explored in patients with CHD in
further studies.
In conclusion, the present study screened 10 DEMIs
in the serum of patients with CHD and hyperglycemia using a human
miRNA microarray analysis. Among the DEMIs, exosomal hsa-let-7b-5p
was upregulated in patients with CHD and hyperglycemia, and was
positively correlated with both HbA1c levels and SYNTAX score.
Further analysis showed that exosomal hsa-let-7b-5p could
potentially serve as a noninvasive biomarker for the severity of
coronary stenosis. The present study provides a novel insight into
the diagnostic capacity of exosomal miRNAs in coronary artery
lesions.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by the Medical and Health Science and
Technology Development Planning Project of Shandong Province (grant
no. 202103011061).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are not publicly available due to the institutional
policy that prohibits data sharing, but are available from the
corresponding author on reasonable request.
Authors' contributions
QJ participated in the study conception and design.
SFH participated in the sample collection, experiments, data
collection, statistical analysis and funding support. JF, LLY, BL,
YHH, RMC, CYL, CXZ, JYL, YNW, YQG and HT assisted in the sample
collection, experiments, data collection and statistical analysis.
QJ and SFH prepared the draft of the manuscript. QJ and SFH confirm
the authenticity of all the raw data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was approved by The 960th Hospital
of the Joint Service Support Force of the People's Liberation Army
(Jinan, China; approval no. 202109) and complied strictly with the
2008 Declaration of Helsinki Principle. Written informed consent
was obtained from the participants prior to the collection of
samples.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AUC
|
area under the curve
|
|
CHD
|
coronary heart disease
|
|
DEMIs
|
differentially expressed miRNAs
|
|
GO
|
Gene Ontology
|
|
HSP70
|
heat-shock 70
|
|
KEGG
|
Kyoto Encyclopedia of Genes and
Genomes
|
|
LDL-C
|
low-density lipoprotein
cholesterol
|
|
miRNAs
|
microRNAs
|
|
ROC
|
receiver operating characteristic
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
|
SYNTAX
|
Synergy between Percutaneous Coronary
Intervention with Taxus and Cardiac Surgery
|
|
TEM
|
transmission electron microscopy
|
|
TSG101
|
tumor susceptibility gene 101
|
References
|
1
|
Katta N, Loethen T, Lavie CJ and Alpert
MA: Obesity and coronary heart disease: Epidemiology, pathology,
and coronary artery imaging. Curr Probl Cardiol. 46:1006552021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carneiro AV: Coronary heart disease in
diabetes mellitus: Risk factors and epidemiology. Rev Port Cardiol.
23:1359–1366. 2004.(In English, Portuguese). PubMed/NCBI
|
|
3
|
Goodarzi MO and Rotter JI: Genetics
Insights in the relationship between type 2 diabetes and coronary
heart disease. Circ Res. 126:1526–1548. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schutt K, Muller-Wieland D and Marx N:
Diabetes mellitus and the heart. Exp Clin Endocrinol Diabetes.
127((S 01)): S102–S104. 2019.PubMed/NCBI
|
|
5
|
Battault S, Renguet E, Van Steenbergen A,
Horman S, Beauloye C and Bertrand L: Myocardial glucotoxicity:
Mechanisms and potential therapeutic targets. Arch Cardiovasc Dis.
113:736–748. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Saliminejad K, Khorram Khorshid HR,
Soleymani Fard S and Ghaffari SH: An overview of microRNAs:
Biology, functions, therapeutics, and analysis methods. J Cell
Physiol. 234:5451–5465. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kalayinia S, Arjmand F, Maleki M,
Malakootian M and Singh CP: MicroRNAs: Roles in cardiovascular
development and disease. Cardiovasc Pathol. 50:1072962021.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bayés-Genis A, Lanfear DE, de Ronde MWJ,
Lupon J, Leenders JJ, Liu Z, Zuithoff NPA, Eijkemans MJC, Zamora E,
De Antonio M, et al: Prognostic value of circulating microRNAs on
heart failure-related morbidity and mortality in two large diverse
cohorts of general heart failure patients. Eur J Heart Fail.
20:67–75. 2018. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu L, Liu F, Xie H and Feng J: Diagnostic
performance of microRNA-133a in acute myocardial infarction: A
meta-analysis. Cardiol J. 25:260–267. 2018.PubMed/NCBI
|
|
10
|
Wei F, Ren W, Zhang X, Wu P and Fan J:
miR-425-5p is negatively associated with atrial fibrosis and
promotes atrial remodeling by targeting CREB1 in atrial
fibrillation. J Cardiol. 79:202–210. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pan X, He Y, Ling S, Chen Z and Yan G:
MiR-15a functions as a diagnostic biomarker for coronary artery
disease. Clin Lab. 66:2020. View Article : Google Scholar
|
|
12
|
Szydelko J and Matyjaszek-Matuszek B:
MicroRNAs as biomarkers for coronary artery disease related to type
2 diabetes mellitus-from pathogenesis to potential clinical
application. Int J Mol Sci. 24:6162022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang J, Li S, Li L, Li M, Guo C, Yao J
and Mi S: Exosome and exosomal microRNA: Trafficking, sorting, and
function. Genomics Proteomics Bioinformatics. 13:17–24. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li SP, Lin ZX, Jiang XY and Yu XY:
Exosomal cargo-loading and synthetic exosome-mimics as potential
therapeutic tools. Acta Pharmacol Sin. 39:542–551. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li C, Zhou T, Chen J, Li R, Chen H, Luo S,
Chen D, Cai C and Li W: The role of Exosomal miRNAs in cancer. J
Transl Med. 20:62022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Isaac R, Reis FCG, Ying W and Olefsky JM:
Exosomes as mediators of intercellular crosstalk in metabolism.
Cell Metab. 33:1744–1762. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu M, Yuan S, Li S, Li L, Liu M and Wan S:
The exosome-derived biomarker in atherosclerosis and its clinical
application. J Cardiovasc Transl Res. 12:68–74. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang SS, Wu LJ, Li JJ, Xiao HB, He Y and
Yan YX: A meta-analysis of dysregulated miRNAs in coronary heart
disease. Life Sci. 215:170–181. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Caraballo C, Desai NR, Mulder H, Alhanti
B, Wilson FP, Fiuzat M, Felker GM, Piña IL, O'Connor CM, Lindenfeld
J, et al: Clinical Implications of the New York Heart Association
Classification. J Am Heart Assoc. 8:e0142402019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Choi KH, Lee JM, Koo BK, Nam CW, Shin ES,
Doh JH, Rhee TM, Hwang D, Park J, Zhang J, et al: Prognostic
implication of functional incomplete revascularization and residual
functional SYNTAX score in patients with coronary artery disease.
JACC Cardiovasc Interv. 11:237–245. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ward JH: Hierarchical grouping to optimize
an objective function. J Am Stat Assoc. 58:236–244. 1963.
View Article : Google Scholar
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dalen JE, Alpert JS, Goldberg RJ and
Weinstein RS: The epidemic of the 20(th) century: Coronary heart
disease. Am J Med. 127:807–812. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang F, Dong J, Wang W, Wang X, Fu X,
Kumar NC and Zhang T: Evaluation of stenosis severity of coronary
calcified lesions using transluminal attenuation gradient: Clinical
application of 320-row volume CT. Minerva Med. 108:305–316. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sirtori CR, Labombarda F, Castelnuovo S
and Perry R: The use of echocardiography for the non-invasive
evaluation of coronary artery disease. Ann Med. 49:134–141. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cao RY, Yang J, Zheng Y, Li H, Zhao Q,
Ding Y, Li Q, Liu S, Wang L and Zheng H: The potential value of
Copeptin and Pentraxin3 for evaluating the severity of coronary
stenosis in patients with coronary artery disease. Clin Biochem.
87:32–38. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mori MA, Ludwig RG, Garcia-Martin R,
Brandao BB and Kahn CR: Extracellular miRNAs: From biomarkers to
mediators of physiology and disease. Cell Metab. 30:656–673. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
He X, Kuang G, Wu Y and Ou C: Emerging
roles of exosomal miRNAs in diabetes mellitus. Clin Transl Med.
11:e4682021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu S, Lin Z, Zheng Z, Rao W, Lin Y, Chen
H, Xie Q, Chen Y and Hu Z: Serum exosomal microRNA-766-3p
expression is associated with poor prognosis of esophageal squamous
cell carcinoma. Cancer Sci. 111:3881–3892. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Su J, Li J, Yu Q, Wang J, Li X, Yang J, Xu
J, Liu Y, Xu Z, Ji L, et al: Exosomal miRNAs as potential
biomarkers for acute myocardial infarction. IUBMB Life. 72:384–400.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang L, Li H, Yuan M, Li D, Sun C and
Wang G: Serum exosomal MicroRNAs as potential circulating
biomarkers for endometriosis. Dis Markers. 2020:24563402020.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zheng D, Huo M, Li B, Wang W, Piao H, Wang
Y, Zhu Z, Li D, Wang T and Liu K: The role of exosomes and exosomal
MicroRNA in cardiovascular disease. Front Cell Dev Biol.
8:6161612021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Aghabozorgi AS, Ahangari N, Eftekhaari TE,
Torbati PN, Bahiraee A, Ebrahimi R and Pasdar A: Circulating
exosomal miRNAs in cardiovascular disease pathogenesis: New
emerging hopes. J Cell Physiol. 234:21796–21809. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang YX, Zeng RR, Yang Y and Shen Y:
Application of SYNTAX and its derivative scores in the selection of
revascularization strategies for complex coronary heart disease.
Chin Med Sci J. 37:340–348. 2022.PubMed/NCBI
|
|
35
|
Huang Y, Liu Y, Huang J, Gao L, Wu Z, Wang
L and Fan L: Let-7b-5p promotes cell apoptosis in Parkinson's
disease by targeting HMGA2. Mol Med Rep. 24:8202021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mandolesi G, Rizzo FR, Balletta S,
Stampanoni Bassi M, Gilio L, Guadalupi L, Nencini M, Moscatelli A,
Ryan CP, Licursi V, et al: The microRNA let-7b-5p Is negatively
associated with inflammation and disease severity in multiple
sclerosis. Cells. 10:3302021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhou R, Zhang Y, Du G, Han L, Zheng S,
Liang J, Huang X, Qin Y, Wu W, Chen M, et al: Down-regulated
let-7b-5p represses glycolysis metabolism by targeting AURKB in
asthenozoospermia. Gene. 663:83–87. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gong L, Xiao J, Yi J, Lu F and Liu X:
Immunomodulatory effect of serum exosomes from crohn disease on
macrophages via Let-7b-5p/TLR4 Signaling. Inflamm Bowel Dis.
28:96–108. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang Y, Yin B, Shu B, Liu Z, Ding H and
Jia C: Differential expression of microRNA let-7b-5p regulates
burn-induced hyperglycemia. Oncotarget. 8:72886–72892. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Berkan O, Arslan S, Lalem T, Zhang L,
Sahin NO, Aydemir EI, Korkmaz O, Egilmez HR, Cekin N and Devaux Y:
Regulation of microRNAs in coronary atherosclerotic plaque.
Epigenomics. 11:1387–1397. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ling H, Guo Z, Du S, Liao Y, Li Y, Ding C
and Song C: Serum exosomal miR-122-5p is a new biomarker for both
acute coronary syndrome and underlying coronary artery stenosis.
Biomarkers. 25:539–547. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li H, Chen M, Feng Q, Zhu L, Bai Z, Wang
B, Guo Z and Hou A: MicroRNA-34a in coronary heart disease:
Correlation with disease risk, blood lipid, stenosis degree,
inflammatory cytokines, and cell adhesion molecules. J Clin Lab
Anal. 36:e241382022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yu X, Xu JF, Song M, Zhang L, Li YH, Han
L, Tang MX, Zhang W, Zhong M and Wang ZH: Associations of
circulating microRNA-221 and 222 with the severity of coronary
artery lesions in acute coronary Syndrome patients. Angiology.
73:579–587. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu H, Xu W, Feng J, Ma H, Zhang J, Xie X,
Zhuang D, Shen W and Zhou W: Increased expression of plasma
miRNA-320a and let-7b-5p in heroin-dependent patients and its
clinical significance. Front Psychiatry. 12:6792062021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Vadla GP, Daghat B, Patterson N, Ahmad V,
Perez G, Garcia A, Manjunath Y, Kaifi JT, Li G and Chabu CY:
Combining plasma extracellular vesicle Let-7b-5p, miR-184 and
circulating miR-22-3p levels for NSCLC diagnosis and drug
resistance prediction. Sci Rep. 12:66932022. View Article : Google Scholar : PubMed/NCBI
|