Introduction
Bronchopulmonary dysplasia (BPD) is the most
critical complication in preterm neonates and is an independent
risk factor affecting long-term cognitive development (1–6).
Inflammation is a common pathway leading to the BPD phenotype
(7,8). Current treatments, such as mechanical
ventilation, dexamethasone therapy or diuretics, have shown limited
improvement in the prevalence of BPD (4–6).
Notably, stem cell-based paracrine cytokine treatment, with its
anti-inflammatory and immunoregulatory ability (9,10)
has been regarded as a promising therapy for BPD in preclinical
models and clinical studies (10–12).
Our previous study demonstrated that autologous cord blood
mononuclear cells (ACBMNCs), which are rich in stem cells, could
substantially prevent moderate or severe BPD in surviving very
preterm neonates, and that the immunomodulatory effect of MNCs
contributed in mitigating the severity of BPD (13). Stem cells exert paracrine effects
by secreting various bioactive substances. Angiopoietin-like
protein 7 (Angptl7) is a glycoprotein that shares sequence homology
with angiopoietins, important modulators of angiogenesis (13,14).
Angptl7 has been shown to be one of the most abundant paracrine
cytokines secreted by stem cells, which is capable of stimulating
human hematopoietic stem and progenitor cell expansion (15,16).
In our previous study, it was revealed that Angptl7 was capable of
stimulating human hematopoietic stem and progenitor cell expansion,
and increasing the repopulation activities of human hematopoietic
progenitors (15,16). In addition, Angptl7-deficient mice
were generated using transcription activator-like effector
nuclease-mediated gene targeting, and it was demonstrated that
hematopoietic stem cell compartments in Angptl7-null mice were
compromised (16). Parri et
al (17) reported that Angptl7
is a proangiogenic factor in human differentiated endothelial cells
and can be specifically upregulated under inflammatory
conditions.
Inflammation-induced impaired lung angiogenesis and
alveolar growth cause BPD (6,8). As
Angptl7 potentially enhances angiopoiesis and has anti-inflammatory
effects (16–18), it was hypothesized that Angptl7 may
ameliorate BPD severity. The present study used serum samples from
the ACBMNCs intervention study cohort (cohort 1) (13) to compare Angptl7 levels between the
ACBMNCs infusion and control groups. In addition, the present study
investigated the association between cord blood Angptl7 levels and
BPD incidence in a cohort of very preterm neonates (cohort 2). The
hypothesis was further verified in a mouse model: Angptl7 was
administered to mice with lipopolysaccharide (LPS)-induced lung
injury, and the protective effects of Angptl7 on lung angiopoiesis
and inflammation was assessed.
Materials and methods
Ethics approval
Guangdong Women and Children Hospital Ethics
committee approved the present study (approval no. 202101030 for
the study involving humans; approval no. 202001031 for the animal
experiments; Guangzhou, China).
Cohort 1: Angptl7 level detection in very preterm
neonates treated with ACBMNCs
In our previous trial (13), an ACBMNCs infusion was administered
to a very preterm neonate cohort (cohort 1). A decrease in moderate
or severe BPD was observed in the ACBMNCs infusion group. Angptl7
levels were measured in preserved samples of cord blood and unused
blood from routine clinical blood tests using an ELISA kit (cat.
no. E8974h; Wuhan EIAab Science Co., Ltd.), according to the
manufacturer's protocol. Patient inclusion and exclusion criteria
for cohort 1 have been described previously (13). Briefly, 29 and 33 patients were
enrolled in the ACBMNCs and control groups, respectively; the
details of patient enrollment are shown in our previous study
(13).
Cohort 2: Association of cord blood Angptl7
levels and BPD
Patient enrollment
Singleton infants (n=112) born between November 2017
and March 2020 at Guangdong Women and Children Hospital were
included in the present study. The inclusion criteria were as
follows: i) Born in the study hospital, ii) singleton birth, iii)
<32 gestational weeks, and iv) consent was obtained from the
parents. Exclusion criteria included: i) Major congenital
abnormalities and ii) severe perinatal asphyxia (defined as an
Apgar score of 0–3 for >5 min, cord blood gas pH <7.00, or
both) (19).
Clinical data collection for cohort 2
Maternal clinical information included age,
gestational diabetes mellitus, pregnancy-induced hypertension,
antenatal steroids administration, preeclampsia, histological
chorioamnionitis and cholestasis (20). Neonatal clinical data included the
following: i) Demographic data, including sex, gestational age
(GA), delivery mode, birth weight, small for GA, length, head
circumference, Apgar score in 1 and 5 min; ii) clinical outcomes
data, including BPD and its severity, necrotizing enterocolitis
(NEC), retinopathy of preterm (ROP), late-onset sepsis, anemia,
invasive mechanical ventilation and total respiratory support
duration, postnatal steroids administration, red blood cells
infusion and length of hospital stay.
Clinical definitions used in the present
study
BPD was defined as treatment with oxygen >21% for
≥28 days using the diagnostic criteria proposed in 2001 by the
Eunice Kennedy Shriver National Institute of Child Health and Human
Development (21). Cranial
ultrasonography was recommended between the 14 and 28th day of
life, and between 34 and 36 weeks of postmenstrual age if the
infant was still hospitalized in the study center at that time. NEC
was diagnosed during surgery, at autopsy, or by the detection of
pneumatosis intestinalis, hepatobiliary gas or free intraperitoneal
air on radiography. All stages of ROP were recorded according to
the international classification (19). Late-onset sepsis was defined as
positive blood or cerebrospinal fluid cultures 72 h after birth.
Anemia was defined as a hemoglobin level of <140 mg/ml. All
clinical diagnoses were made according to standard reference
(19). Respiratory support
included invasive and non-invasive ventilation and oxygen
therapy.
Cord blood collection and quantification of
cytokine levels
Cord blood was collected after cord clamping and
before the delivery of the placenta as described in a previous
study (22). Cord blood was then
centrifuged at 500 × g for 8 min at 4°C (Beckman Coulter, Inc.).
Serum was separated from cord blood for the assessment of Angptl7
(ng/ml), vascular endothelial growth factor A (VEGF-A; ng/ml),
interleukin-6 (IL-6; pg/ml), and monocyte chemoattractant protein-1
(MCP-1; pg/ml) concentrations. A minimum of 5 µl serum was used for
analysis using the following ELISA kits: Angptl7, (cat. no.
E8974h), VEGF-A (cat. no. E0143h), IL-6 (cat. no. E0079h) and MCP-1
(cat. no. E0087h) (all from Elabscience Biotechnology, Inc.
Cytokine detection by ELISA was performed as described previously
(23).
Animal model: Effect of Angptl7 on
LPS-induced lung injury
Establishment of LPS-induced lung injury mouse
model
All animal experiments were conducted after approval
by Guangdong Women and Children Hospital Ethics committee, which
conforms to the Guide for the Care and Use of Laboratory Animals
(24). A total of 18 C57BL/6J
wild-type (WT) mice (age, 3 days; weight, 3–4 g; n=6/group) were
obtained from the Guangzhou Institute of Biomedicine and Health
Laboratory. Neonatal mice were used for all experiments. The mice
were fed standard mouse food and water ad libitum, and were
maintained at 21–28°C, 50–60% humidity, and under 12-h light/dark
cycles.
LPS was used to mediate inflammation-induced lung
injury (25,26). Neonatal WT mice were injected
intraperitoneally with 10 mg/kg LPS derived from Escherichia
coli serotype O26:B6 (MilliporeSigma) in the LPS group, or with
an equivalent volume of the vehicle control (normal saline) in the
control group once on postnatal day (PND)3 in the saccular stage of
lung development (24). The mice
in the LPS + Angptl7 group were then injected intraperitoneally
with Angptl7 (500 ng/g; R&D Systems, Inc.). Serum was separated
from cardiac blood samples, which were obtained by cardiac puncture
following pentobarbital sodium injection (50 mg/kg;
intraperitoneal), -via centrifugation at 500 × g for 20 min at 4°C.
Mice in the vehicle, LPS and LPS + Angptl7 groups were euthanized
using CO2, with the volume displacement rate being ~50%
CO2 on PND7 (n=6/group). The lung tissues were then
collected and stored at −80°C.
Fluorescence immunohistochemical
analysis
The lungs from the mice in each group (n=6/group)
were washed with phosphate-buffered saline, fixed for 24 h in 4%
paraformaldehyde in phosphate buffer solution (NaCl, 13.7 mM; KCl,
2.7 mM; Na2HPO4, 0.9 mM;
KH2PO4, 1.8 mM; pH 7.4) at 4°C, and embedded
in paraffin. For immunohistochemistry, 5-µm sections were prepared
from these paraffin-embedded tissues. Paraffin-embedded lung
tissues were deparaffinized in xylene, rehydrated in a descending
series of alcohol and subjected to antigen retrieval at 95°C for
immunohistochemical analysis. After blocking of nonspecific binding
with 5% BSA (cat. no. G5001; Wuhan Servicebio Technology Co., Ltd.)
in room temperature for 30 min, the lung sections were incubated
overnight at 4°C with the following primary antibodies: Anti-von
Willebrand factor (vWF; endothelial-specific marker; cat. no.
GB11020; 1:600) and anti-F4/80 (macrophage-specific marker; cat.
no. GB113373;1:1,000) (all from Wuhan Servicebio Technology Co.,
Ltd.). Subsequently, sections were incubated with secondary
antibodies (for vWF: Cyanine 3-conjugated antibody; cat. no.
GB21303; 1:300; and for F4/80: FITC-conjugated antibody, cat. no.
GB22303; 1:500; both from Wuhan Servicebio Technology Co., Ltd.) at
room temperature 50 min. Antigen-antibody reactions were visualized
using the diaminobenzidine reaction, and image analysis was
performed using Image-Pro Plus software (6.0 version; Media
Cybernetics, Inc.). Three randomly selected high-power fields
(magnification, ×400) of the peripheral pulmonary tissue on each
slide were analyzed. The observers analyzing the slides were
blinded to the experimental conditions. Fluorescence signals were
detected at excitation-emission wavelengths of 590 nm (CY3, red)
and 515–555 nm (FITC, green). The imaging was performed using a
confocal microscope (Nikon Eclipse C1; Nikon Corporation).
Analysis of lung vessel
development
Pulmonary blood vessel development was determined
using immunofluorescence (IF) staining for vWF, as aforementioned.
At least three counts from three random non-overlapping fields
(original magnification, ×400) were performed for each animal
(n=6/group). The number of vessels per field and mean vessel
diameter were calculated and analyzed manually. The observers who
performed the measurements were blinded to the specimen.
Inflammatory cytokine detection and
mRNA expression
A minimum of 5 µl serum was used for analysis using
the following ELISA kits: IL-6 (cat. no. E0079m) and MCP-1 (cat.
no. E0087m) (both from Wuhan EIAab Science Co., Ltd.). The extent
of lung inflammation was assessed by quantifying lung
cytokine/chemokine gene expression. Total RNA was extracted from
frozen lung tissues using the Direct-zol RNA MiniPrep Kit (cat. no.
R2052; Zymo Research Corp.) and was reverse transcribed into cDNA
using Revert Aid First Strand cDNA Synthesis Kit (cat. no. K1622;
Thermo Fisher Scientific, Inc.). Reverse transcription-quantitative
PCR (RT-qPCR) analysis was performed using a 7900HT Real-Time PCR
System with TaqMan Gene Expression Master Mix (cat. no. 4369016)
and TaqMan Gene Expression Assays (all from Applied Biosystems;
Thermo Fisher Scientific, Inc.) for IL-6, MCP-1, IL-10 and GAPDH.
GAPDH was used as the reference gene. The samples were denatured at
95°C for 10 min, followed by 40 cycles at 95°C for 15 sec and 60°C
for 1 min. The 2−ΔΔCq method was used to calculate fold
changes in mRNA expression (27).
The primers used were as follows: m-IL-6-forward
(F)1, 5′-CAGAAGGAGTGGCTAAGGACC-3′; m-IL-6-reverse (R)1,
5′-GCACTAGGTTTGCCGAGTAG-3′; m-MCP-1-F1, 5′-GAGCTCTCTGGTACTCTTTG-3′;
m-MCP-1-R1, 5′-GTGCATTACAGGGAACAAAC-3′; m-IL-10-F1,
5′-GCTCCAAGAvCCAAGGTGTCT-3′; m-IL-10-R1,
5′-CGGAGAGAGGTACAAACGAGG-3′; m-GAPDH-F1:
5′-GGCCTCCAAGGAGTAAGAAA-3′; m-GAPDH-R1:
5′-GCCCCTCCTGTTATTATGG-3′.
Statistical analysis
Continuous variables are presented as the mean ± SD,
and numbers and percentages are presented for categorical
variables. Differences in continuous variables between two groups
were compared using unpaired Student's t-test. Pearson correlation
coefficients were used to determine the correlation between Angptl7
levels and other variables. Multiple linear regression analysis was
used to estimate the predictive contribution of neonatal/maternal
factors on Angptl7 levels or the contribution of factors on BPD. A
two-way mixed ANOVA and post hoc Tukey's test was used for
comparisons of Angptl7 levels before and after the intervention in
our previous trial. The distribution characteristics of the
variables were estimated using a single-sample Kolmogorov-Smirnov
test. All statistical tests were two-tailed, and P<0.05 was
considered to indicate a statistically significant difference.
For animal studies, all experiments were performed
at least three times in triplicate. Results are expressed as the
mean ± SEM. One-way ANOVA followed by Bonferroni's multiple
comparison test was used to compare groups. P<0.05 was
considered to indicate a statistically significant difference. All
statistical analyses were performed using SPSS 21.0 software (IBM
Corp.).
Results
Angptl7 levels in very preterm
neonates after ACBMNCs intervention in cohort 1
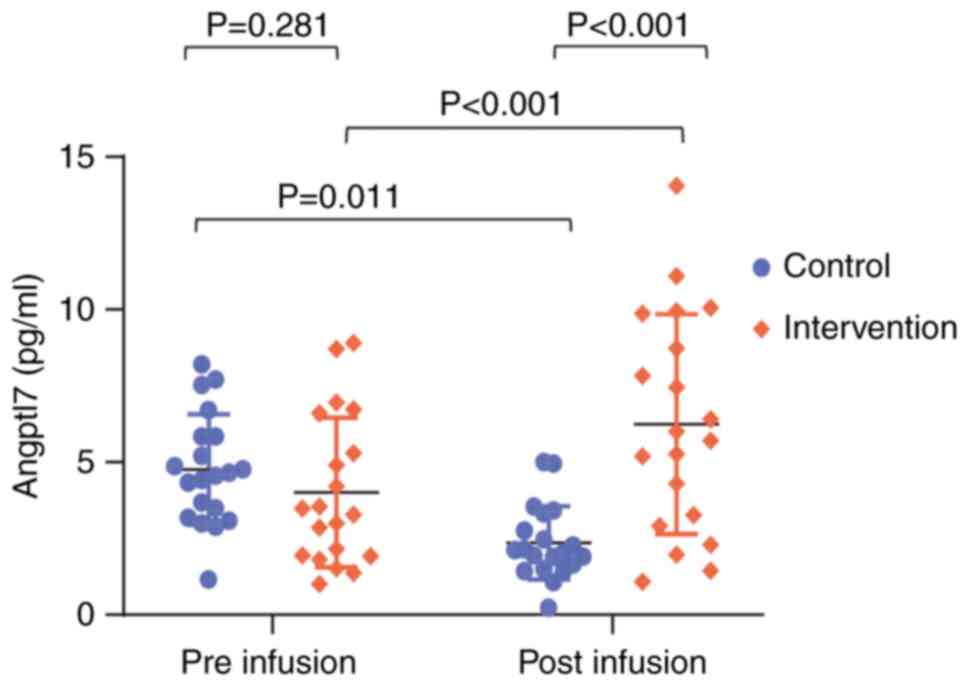
At baseline, no difference in Angptl7 levels was
detected between the groups (control, 4.76±1.82 ng/ml vs. ACBMNC,
4.01±2.45 ng/ml; P=0.281; Fig. 1).
In the control group, Angptl7 levels decreased after intervention
(P=0.011); however, Angptl7 levels were significantly higher after
intervention in the ACBMNCs infusion group compared with those in
the control group (control, 2.35±1.20 ng/ml vs. ACBMNC, 6.24±3.20
ng/ml; P<0.001; n=20/group with available results). The clinical
characteristics of this cohort have been described previously
(13).
Angptl7 levels in very preterm
neonates in cohort 2
Study population
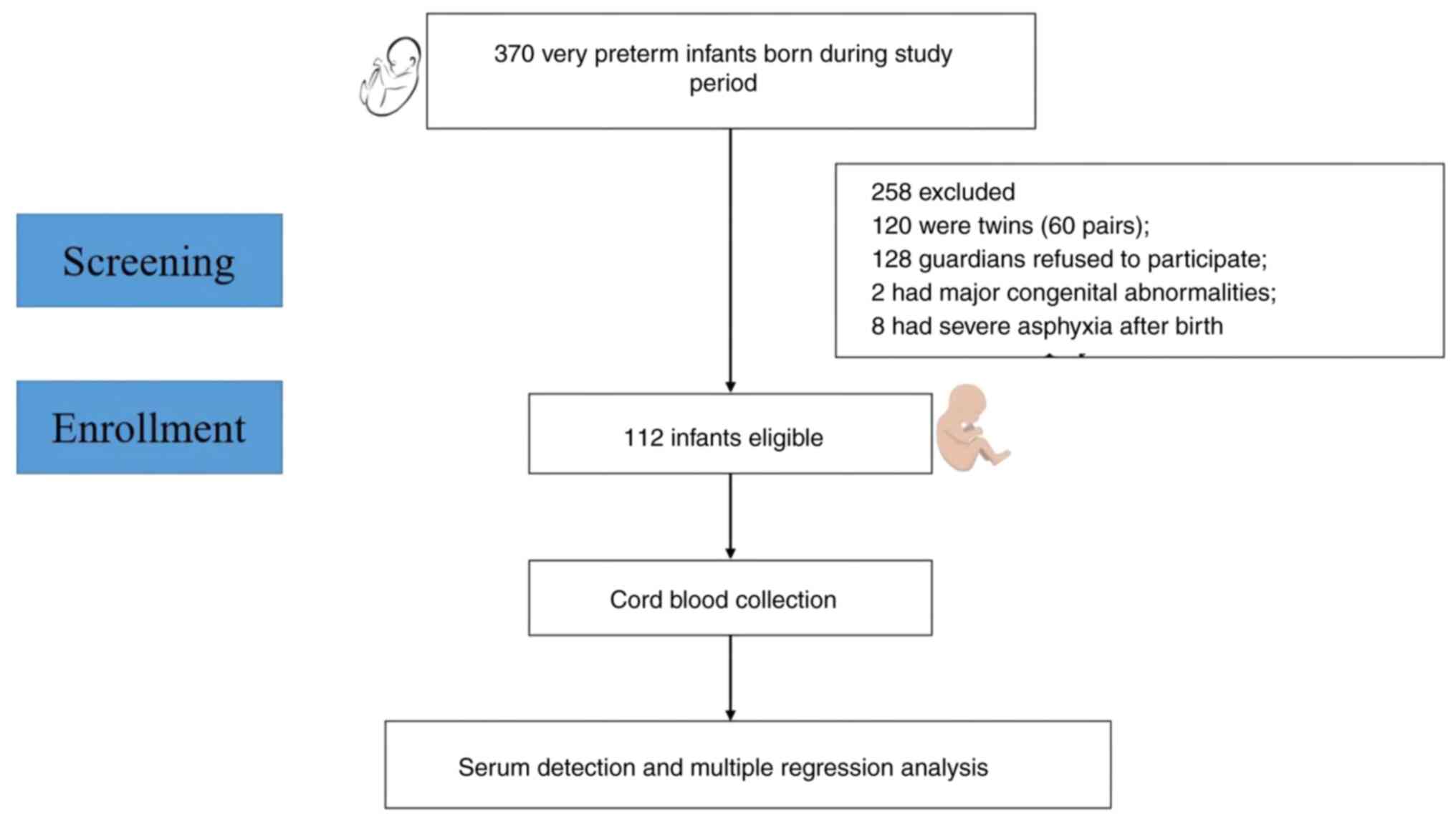
A total of 370 preterm neonates (<32 gestational
weeks) were screened between November 2017 and March 2020. Among
them, 120 were twins, 8 had severe asphyxia, 2 had major congenital
abnormalities, and 128 did not provide consent. Ultimately, 112
patients were enrolled in the present study (Fig. 2). The maternal and neonatal
clinical characteristics are shown in Table I.
 | Table I.Maternal and neonatal clinical
characteristics. |
Table I.
Maternal and neonatal clinical
characteristics.
| A, Maternal
characteristics |
|---|
|
|---|
| Clinical
characteristic | Value |
|---|
| Mean ± SD age,
years | 32.01±5.64 |
| Gestational
diabetes mellitus, n (%) | 29 (25.89) |
| Pregnancy-induced
hypertension, n (%) | 24 (21.43) |
| Antenatal steroids
administration, n (%) | 49 (43.75) |
| Preeclampsia, n
(%) | 22 (19.64) |
| Histological
chorioamnionitis, n (%) | 7 (6.25) |
| Cholestasis, n
(%) | 4 (3.57) |
|
| B, Neonatal
characteristics |
|
| Clinical
characteristic | Value |
|
| Mean ± SD
gestational age, weeks | 29.65±1.55 |
| Gestational age
<28 weeks, n (%) | 18 (16.07) |
| Male sex, n
(%) | 64 (57.14) |
| Cesarean delivery
mode, n (%) | 58 (51.79) |
| Mean ± SD birth
weight, kg | 1.35±0.32 |
| Small for
gestational age, n (%) | 15 (13.39) |
| Mean ± SD birth
length, cm | 38.01±3.64 |
| Meedian (IQR),
Apgar score in 1 min | 9.00 (1.00) |
| Meedian (IQR),
Apgar score in 5 min | 10.00 (1.00) |
| RDS, n (%) | 107 (95.54) |
| Grade
1 | 64 (57.14) |
| Grade
2 | 27 (24.11) |
| Grade
3 | 16 (14.29) |
| BPD, n (%) | 35 (31.25) |
| Grade
1 | 9 (8.04) |
| Grade
2 | 19 (16.96) |
| Grade
3 | 7 (6.25) |
| NEC, n (%) | 19 (16.96) |
| ROP, n (%) | 31 (27.68) |
| Late-onset sepsis,
n (%) | 33 (29.46) |
| IVH, n (%) | 35 (31.25) |
| Mean ± SD
intubation duration, days | 3.64±8.37 |
| Mean ± SD
respiratory support duration, days | 16.96±18.16 |
| Postnatal steroids
administration, n (%) | 18 (16.07) |
| Mean ± SD length of
hospital stay, days | 47.46±22.09 |
Effect of perinatal factors on cord
blood Angptl7 levels
The present study investigated perinatal factors
that may affect cord blood Angptl7 levels in very preterm neonates.
Multiple regression analysis showed that perinatal factors did not
affect the cord blood levels of Angptl7 (Table II). Since GA and birth weight
showed a positive correlation (r=0.238, P=0.015), although this
correlation was weak, only GA was included in the multiple
regression analysis.
 | Table II.Multiple regression analysis model
for perinatal factors on the cord blood levels of Angptl7. |
Table II.
Multiple regression analysis model
for perinatal factors on the cord blood levels of Angptl7.
| A, Neonatal
variables |
|---|
|
|---|
| Variable | B | 95% CI for B | P-value |
|---|
| Male sex | 0.058 | (−1.196,
2.229) | 0.551 |
| Gestational
age | 0.072 | (−0.386,
0.793) | 0.495 |
| Small for
gestational age | 0.177 | (−0.374,
4.923) | 0.091 |
| Birth length | 0.076 | (−0.176,
0.358) | 0.499 |
| Cesarean delivery
mode | −0.134 | (−2.912,
0.566) | 0.184 |
|
| B, Maternal
variables |
|
|
Variable | B | 95% CI for
B | P-value |
|
| Maternal age | −0.200 | (−0.323,
0.012) | 0.068 |
| Histological
chorioamnionitis | −0.027 | (−3.836,
2.861) | 0.773 |
| Cholestasis | −0.141 | (−7.677,
1.054) | 0.135 |
| Preeclampsia | 0.111 | (−5.131,
7.579) | 0.703 |
| Gestational
diabetes mellitus | 0.125 | (−0.678,
3.163) | 0.202 |
| Pregnancy-induced
hypertension | 0.248 | (−3.643,
8.927) | 0.406 |
| Antenatal steroids
administration | −0.064 | (−2.267,
1.136) | 0.511 |
Association between cord blood Angptl7
levels and outcomes in very preterm neonates
To investigate the association between Angptl7
levels and outcomes in very preterm neonates, the levels of Angptl7
were compared in the very preterm groups between those who were
diagnosed with the following common complications: BPD, NEC,
intraventricular hemorrhage, ROP and late-onset sepsis, and those
who were not. Notably, the cord blood levels of Angptl7 were lower
in neonates who later developed BPD than in those without BPD
(P<0.01; Table III). Whereas,
Angptl7 expression did not differ significantly in other
complications (n=112).
 | Table III.Cord blood levels of Angptl7 and
preterm complications. |
Table III.
Cord blood levels of Angptl7 and
preterm complications.
|
| Mean ± SD cord
blood ANGPTL7, ng/ml (n) |
|
|---|
|
|
|
|
|---|
| Complication | With | Without | P-value |
|---|
| BPD | 7.54±3.88
(n=35) | 9.91±4.43
(n=77) | <0.01 |
| IVH | 9.00±4.66
(n=35) | 9.25±4.29
(n=77) | 0.78 |
| ROP | 10.28±4.90
(n=31) | 8.75±4.13
(n=81) | 0.10 |
| NEC | 8.82±4.73
(n=19) | 9.25±4.34
(n=93) | 0.07 |
| Sepsis | 8.19±4.20
(n=33) | 9.58±4.43
(n=79) | 0.13 |
Cord blood Angptl7 levels and BPD in
very preterm neonates
To further investigate the possible protective
contribution of cord blood Angptl7 against BPD in very preterm
neonates, multiple regression analysis was used, which included
perinatal factors that may affect the incidence of BPD. The results
showed that higher cord blood levels of Angptl7 were an independent
protective factor against BPD (P=0.049, n=112; Table IV).
 | Table IV.Multiple regression analysis model
for perinatal factors on BPD. |
Table IV.
Multiple regression analysis model
for perinatal factors on BPD.
| Variable | B | 95% CI for B | P-value |
|---|
| Male sex | −0.580 | (−0.200,
1.571) | 0.271 |
| Gestational
age | −0.313 | (0.512, 1.044) | 0.085 |
| Small for
gestational age | −0.291 | (0.148, 3.782) | 0.725 |
| Birth length | −0.148 | (0.733, 1.015) | 0.074 |
| Cesarean delivery
mode | −0.210 | (0.289, 2.279) | 0.691 |
| Maternal age | 0.037 | (0.934, 1.153) | 0.489 |
| Histological
chorioamnionitis | −1.035 | (0.061, 2.086) | 0.252 |
| Cholestasis | 0.427 | (0.116,
20.291) | 0.746 |
| Preeclampsia | 0.901 | (0.022,
281.497) | 0.710 |
| Gestational
diabetes mellitus | −0.237 | (0.259, 2.401) | 0.676 |
| Pregnancy-induced
hypertension | −0.622 | (0.005,60.261) | 0.796 |
| Antenatal steroids
administration | −0.745 | (0.174, 1.292) | 0.145 |
| Late-onset
sepsis | −0.445 | (0.222, 1.852) | 0.411 |
| Angptl7 | −0.130 | (0.771, 0.999) | 0.049 |
Correlation of cord blood Angptl7
levels with inflammatory cytokines and VEGF-A
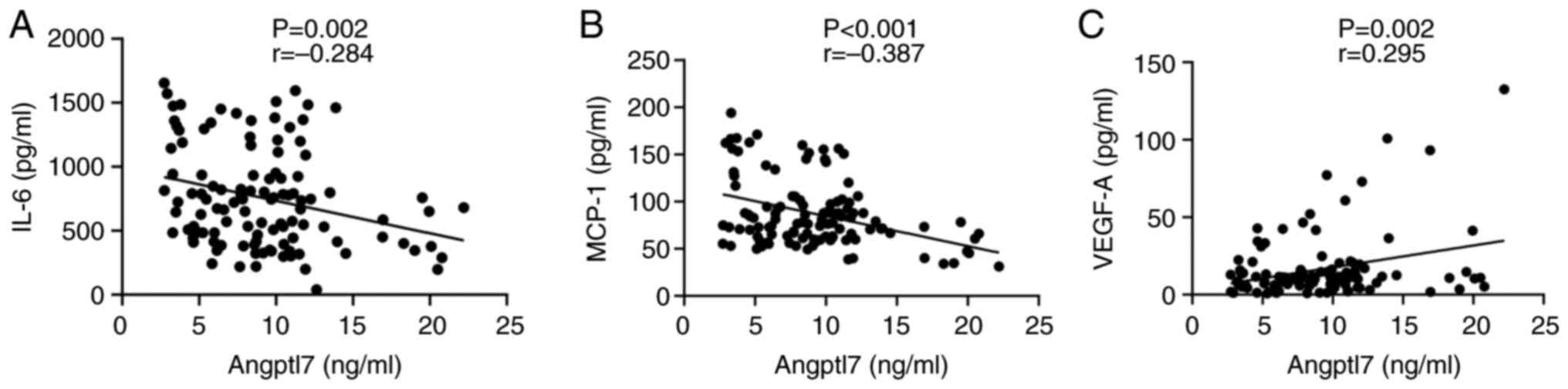
Perinatal inflammation serves a crucial role in the
pathogenesis of BPD and the pro-inflammatory process affects
premature lungs. Angptl7 has important roles in inflammation and
angiogenesis. Therefore, the present study analyzed the correlation
between cord blood Angptl7 levels and common pro-inflammatory
cytokines contributing to the development of BPD, including IL-6,
MCP-1 and VEGF-A. It was observed that the IL-6 levels (r=−0.284,
P=0.002, n=112; Fig. 3a) and MCP-1
levels (r=−0.387, P<0.001, n=112; Fig. 3b) were inversely correlated with
Angptl7 levels. However, the correlation with IL-6 was weak. By
contrast, VEGF-A levels were positively correlated with Angptl7
levels (r=0.295, P=0.002, n=112; Fig.
3c), although this correlation was also weak.
Effect of Angptl7 on LPS-induced lung
injury in mice
Angptl7 treatment and pulmonary blood vessel
development
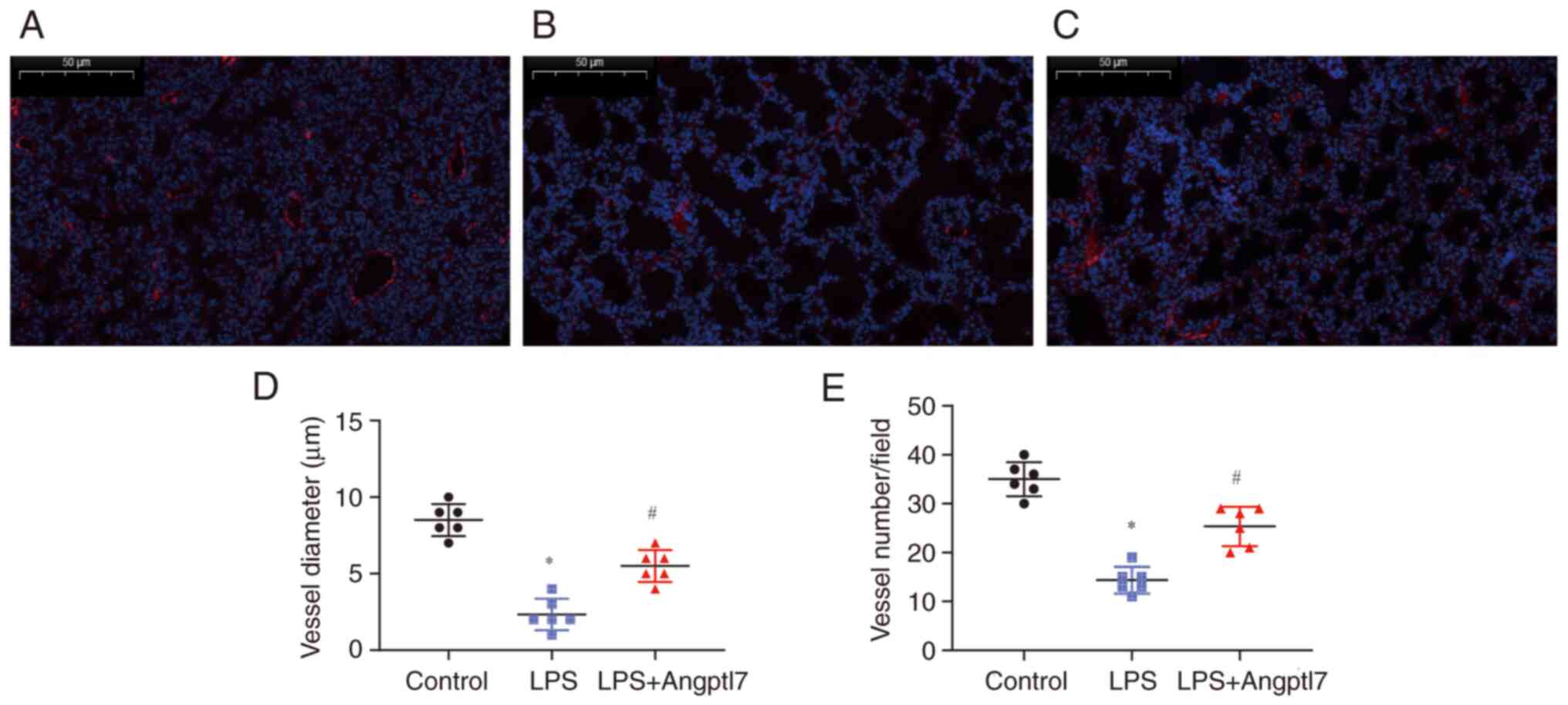
To explore the potential effect of Angptl7 on
LPS-induced peripheral pulmonary vascular impairment, lung sections
prepared from PND7 mice post-LPS-induced lung injury were analyzed
for the expression of vWF. There was an obvious decrease in the
mean vessel diameter in the LPS group (control, 35.00±1.41 µm vs.
LPS, 14.33±1.12 µm; P<0.001; n=6; Fig. 4a, b and d). Angptl7 treatment
ameliorated the LPS-induced vascular diameter impairment (LPS,
14.33±1.12 µm vs. LPS + Angptl7, 25.33±1.65 µm; P<0.001;
Fig. 4b-d). Compared with mice in
the control group, LPS exposure led to significant loss of small
(peripheral) vessels <50 µm in diameter (control, 8.50±0.43 vs.
LPS, 2.33±0.42; P<0.001; n=6), whereas Angptl7 treatment
increased the number of these vessels (LPS, 2.33±0.42 vs. LPS +
Angptl7, 5.50±0.43; P<0.001) (Fig.
4a-c and e).
Angptl7 intervention and lung
inflammation
The present study also evaluated the extent of lung
inflammation following treatment with LPS and Angptl7. On PND7, the
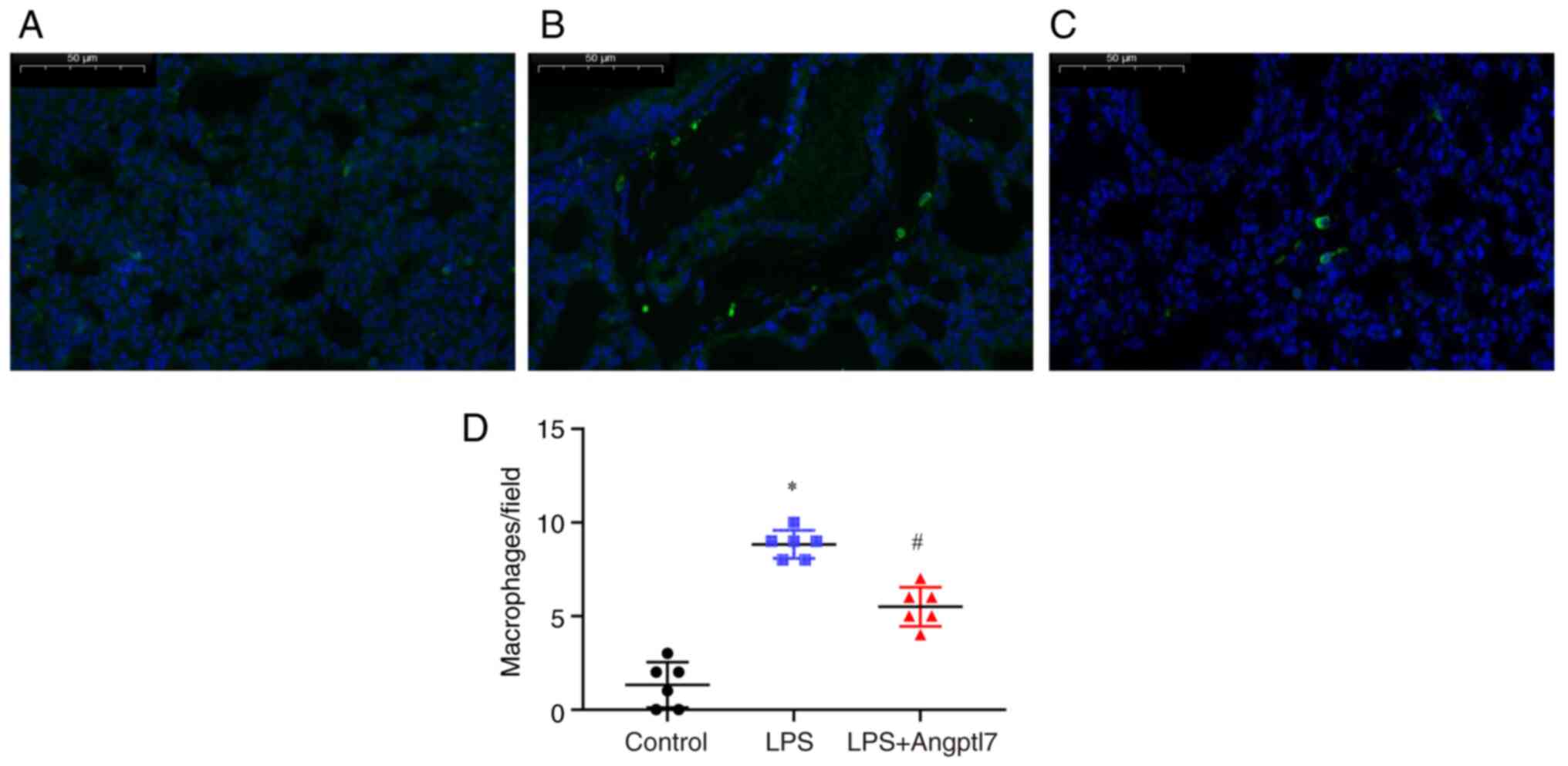
number of inflammatory macrophages infiltrating the lungs were
detected. To compare the number of infiltrating macrophages between
the treatment groups, the lung tissues were stained with
fluorescent antibodies against F4/80 and analyzed by IF microscopy.
WT mice treated with LPS exhibited increased infiltration of
macrophages in their lung tissues compared with vehicle-treated
mice (vehicle, 1.33±0.50 vs. LPS, 8.83±0.31; P<0.001; Fig. 5a, b and d). Angptl7 significantly
suppressed pulmonary macrophage infiltration compared with that in
the LPS group (LPS, 8.83±0.31 vs. LPS + Angptl7, 5.50±0.43;
P<0.001; Fig. 5b-d).
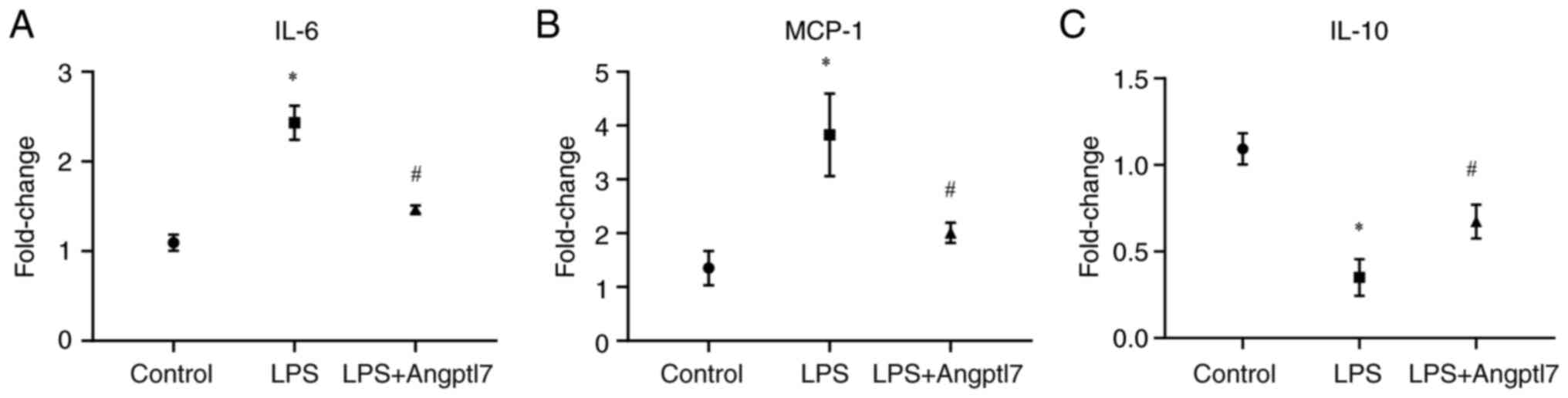
The present study also determined the extent of lung
inflammation by quantifying the production of the proinflammatory
cytokine genes IL-6 and MCP-1, and the anti-inflammatory cytokine
gene IL-10 in lung tissues by RT-qPCR. LPS (10 mg/kg) increased the
mRNA expression levels of IL-6 (control, 1.09±0.05 vs. LPS,
2.43±0.11; P<0.001), and MCP-1 (control, 1.35±0.19 vs. LPS,
3.83±0.44; P=0.0067), whereas Angptl7 lowered the mRNA expression
levels of IL-6 (LPS, 2.43±0.11 vs. LPS + Angptl7, 1.46±0.03;
P<0.001) and MCP-1 (LPS, 3.83±0.44 vs. LPS + Angptl7, 2.01±0.11;
P=0.016) (Fig. 6a and b).
Furthermore, the mRNA expression levels of the anti-inflammatory
cytokine gene IL-10 were detected in the lung tissues. It was
observed that exposure to 10 mg/kg LPS decreased the mRNA
expression levels of lung IL-10 (control, 1.09±0.05 vs. LPS,
0.35±0.06; P<0.001), whereas Angptl7 intervention increased the
mRNA expression levels of lung IL-10 compared with those in the LPS
group (LPS, 0.35±0.06 vs. LPS + Angptl7, 0.67±0.06; P=0.018
(Fig. 6c). These results indicated
that Angptl7 may suppress lung inflammation via IL-10
expression.
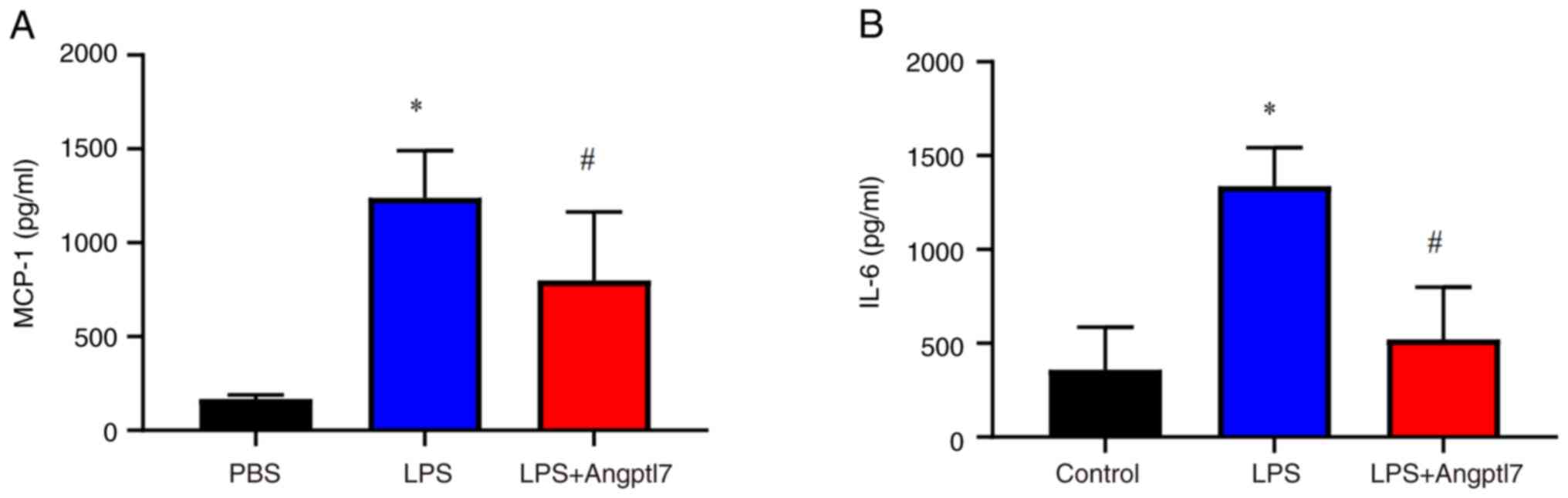
The concentrations of IL-6 and MCP-1 were also
detected in the serum of mice post-LPS-induced lung injury. LPS
stimulation increased the serum levels of IL-6 (control,
359.30±32.32 pg/ml vs. LPS, 1,338.00±83.85 pg/ml; P<0.001) and
MCP-1 (control, 167.00±9.14 pg/ml vs. LPS, 1,239.00±103.00 pg/ml,
P<0.001), whereas Angptl7 decreased the serum levels of IL-6
(LPS, 1,338.00±83.90 pg/ml vs. LPS + Angptl7, 520.90±113.70 pg/ml;
P<0.001) and MCP-1 (LPS, 1,239.00±103.00 pg/ml vs. LPS +
Angptl7, 799.90±148.60 pg/ml; P=0.036) induced by LPS stimulation
(Fig. 7).
Discussion
Inflammatory response-induced impaired angiogenesis
serves an important role in the pathogenesis of BPD (28–31).
Mesenchymal stem cells (MSCs) are potent immunomodulatory cells
capable of alleviating inflammation in experimental BPD (10,32–35).
One important protective mechanism of stem cells is their ability
to reduce lung vascular injury and promote angiogenesis by
secreting and regulating angiogenic proteins (10,36).
Angptl7 is a member of the angiogenin-like protein family that is
abundantly expressed in cord blood stem and angiogenic progenitor
cells (14,37,38).
Important pathological processes involving Angptl7 include
inflammation, apoptosis and angiogenesis (39,40).
Our previous study reported that ACBMNCs could substantially
prevent severe BPD in surviving very preterm neonates and that the
immunomodulatory effect of MNCs contributed to the mitigation of
BPD severity (13). The present
study first investigated Angptl7 levels among patients in our
previous trial, and then measured the cord blood level of Angptl7
in very preterm neonates and its association with preterm
complications in a prospective cohort. The relationship between
Angptl7 and inflammatory cytokines, as well as VEGF-A, were further
analyzed. This clinical study showed that: i) Angptl7 levels were
higher in the ACBMNCs infusion group than those in the control
group post-intervention; ii) higher cord blood levels of Angptl7
were an independent protective factor for developing BPD; and iii)
pro-inflammatory cytokines IL-6 and MCP-1 were inversely correlated
with Angptl7 levels, whereas VEGF-A was positively correlated with
Angptl7 levels. However, it was noted that some of the correlations
were not very strong; therefore, further studies with larger
samples are required to verify the findings.
In a murine model, the present study investigated
the effects of Angptl7 intervention on systemic LPS
exposure-induced lung injury during the saccular phase of lung
development. The results revealed that Angptl7 treatment rescued
the LPS-induced loss of peripheral pulmonary blood vessels,
ameliorated lung macrophage cell infiltration and attenuated
LPS-induced inflammation. An inflammation-induced mouse model in
air containing 21% oxygen is a well-known model of BPD-like
pulmonary phenotype (41,42). The present study provides
additional evidence for the translational implementation of stem
cells and the derived cytokine Angptl7 in preventing and/or
treating BPD.
Previous studies have explored the relationship
between Angptl7 and multiple pathological processes and diseases.
Parri et al (17)
demonstrated that Angptl7 is a proangiogenic factor in
differentiated human endothelial cells and can be specifically
upregulated by hypoxia. By contrast, Toyono et al found that
Angptl7 may act as an antiangiogenic protein to maintain corneal
transparency (43). Xiao et
al reported that Angptl7 promoted the regenerative capacity of
hematopoietic stem and progenitor cells (15,16).
Angiogenesis is a complex biological process that is known to be
involved in multiple preterm diseases (44,45).
However, Angptl7 may have various effects in different disease
conditions. Until now, to the best of our knowledge, there has been
no investigation of the relationship between Angptl7 and BPD or its
underlying mechanisms.
In our previous non-randomized study, 29 patients
with very preterm infants were enrolled in the ACBMNCs infusion
group and 33 were enrolled in the control group. The severity of
BPD in survivors significantly decreased in the ACBMNCs
intervention group (13). MNCs
have been reported to exert immunomodulatory effects by reducing
the levels of inflammatory cytokines, and the association between
inflammatory responses and aberrant lung vascular development has
been well established (45,46).
A previous study showed that proangiogenic factors were decreased
in the lungs of infants dying from BPD and in animal models of BPD
induced by LPS and/or hyperoxia exposure (2,47).
Angptl7 is an important paracrine bioactive factor that serves a
role in inflammatory response regulation (37,38).
Macrophages are critical mediators of the lung inflammatory
response (31,46) and MSCs can alleviate lung
inflammation by inhibiting macrophage accumulation (10). Whether Angptl7 can alleviate the
excessive lung inflammatory response and repair lung vascular
injury is still unclear. The present study measured the
concentrations of MCP-1 and IL-6 in the cord blood of preterm
neonates; the correlation analysis results showed a negative
relationship between MCP-1, IL-6 and Angptl7. In addition, the mRNA
expression levels of inflammatory cytokines were further assessed
in the lungs of a mouse model, as were their serum concentrations.
Consistent with previous findings (26), LPS exposure increased the mRNA
expression and protein levels of pro-inflammatory cytokines, such
as MCP-1 and IL-6, which can accumulate and activate macrophages,
resulting in an augmented inflammatory cascade (47). By contrast, LPS exposure reduced
the levels of the anti-inflammatory chemokine, IL-10 (26). The present study revealed that
Angptl7 upregulated IL-10 expression, reduced MCP-1 and IL-6 mRNA
expression and serum levels, and reduced macrophage infiltration
after LPS exposure in premature lungs. This indicated that the
protective effect of Angptl7 against impaired pulmonary
angiogenesis may be associated with its anti-inflammatory
function.
Lung hypoplasia is a result of the disruption of
angiogenesis, and loss of VEGF signaling between the epithelium and
endothelium (48,49). Previous studies have reported
significantly reduced VEGF-A levels in the bronchoalveolar lavage
fluid of patients with BPD (50,51).
The present study demonstrated that VEGF-A levels were positively
correlated with Angptl7. In the LPS-induced lung injury mouse
model, Angptl7 restored lung microvascular number and diameter,
thereby enlarging the lung perfusion area. Endothelial cell
mitogens and the survival factor VEGF-A are essential for normal
blood vessel development (48).
Considering the role of Angptl7 in regulating angiogenesis, this
finding indicated that Angptl7 may have a proangiogenic effect and
could contribute to the improvement of BPD in very preterm
neonates.
The present study had several limitations. First,
among the 112 very preterm neonates (<32 gestational weeks),
only 18 had a GA of <28 weeks at birth. Further studies should
include extremely preterm infants who are at a greater risk of
developing BPD and its complications. Additionally, the
correlations between Angptl7 and inflammatory cytokines were not
very strong, further studies with larger samples were needed to
verify the findings.
Second, further in vitro studies are needed
to verify the effect of Angptl7 on the co-culture system of
macrophages and vascular endothelial cells. Third, while
inflammation-induced models in air containing 21% oxygen can cause
a BPD-like pulmonary phenotype, hyperoxia-induced lung injury
models have also been well described for mimicking BPD (25,26).
In future studies, development of an additional model by hyperoxia
stimulation could be used to verify the effect of Angptl7 on BPD.
Furthermore, an Angptl7 knock-out mouse model and an additional
control + Angptl7 group may aid in demonstrating the protective
effect of Angptl7 on inflammation-induced lung injury.
In conclusion, increased levels of Angptl7 in cord
blood are an independent protective factor against BPD development.
The anti-inflammatory and proangiogenic effects of Angptl7 may be
associated with its protective effects against BPD. These results
provide a clinical foundation for the translational application of
Angptl7 for the prevention and treatment of BPD in very preterm
neonates.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science
Foundation of China (grant nos. 82171714 and 82101817) and the
National Key R&D Program of China (grant nos. 2021YFC2701701
and 2022A1515010427).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JY, LY and ZR were involved in study conception and
design. All authors provided administrative support, and study
materials or patients. SL, JH and YY acquired and collated data.
JY, JW and CD analyzed and interpreted data. LY and JW wrote the
manuscript. JY and ZR confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Guangdong Women and Children Hospital Ethics
committee approved the present study (approval no. 202101030 for
the study involving humans; approval no. 202001031 for the animal
experiments). Written informed consent was obtained from the
parents of the infants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chawanpaiboon S, Vogel JP, Moller AB,
Lumbiganon P, Petzold M, Hogan D, Landoulsi S, Jampathong N,
Kongwattanakul K, Laopaiboon M, et al: 2019.Global, regional, and
national estimates of levels of preterm birth in 2014: A systematic
review and modelling analysis. Lancet Glob Health. 7:e37–e46. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stoll BJ, Hansen NI, Bell EF, Walsh MC,
Carlo WA, Shankaran S, Laptook AR, Sánchez PJ, Van Meurs KP,
Wyckoff M, et al: Trends in care practices, morbidity, and
mortality of extremely preterm neonates, 1993–2012. JAMA.
314:1039–1051. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cao Y, Jiang S, Sun J, Hei M, Wang L,
Zhang H, Ma X, Wu H, Li X, Sun H, et al: Assessment of Neonatal
Intensive care unit practices, morbidity, and mortality among very
preterm infants in China. JAMA Netw Open. 4:e21189042021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhu Z, Yuan L, Wang J, Li Q, Yang C, Gao
X, Chen S, Han S, Liu J, Wu H, et al: Mortality and morbidity of
infants born extremely preterm at tertiary medical centers in China
from 2010 to 2019. JAMA Netw Open. 4:e2193822021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gilfillan M, Bhandari A and Bhandari V:
Diagnosis and management of bronchopulmonary dysplasia. BMJ.
375:n19742021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thébaud B, Goss KN, Laughon M, Whitsett
JA, Abman SH, Steinhorn RH, Aschner JL, Davis PG, McGrath-Morrow
SA, Soll RF and Jobe AH: Bronchopulmonary dysplasia. Nat Rev Dis
Primers. 5:782019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Papagianis PC, Pillow JJ and Moss TJ:
Bronchopulmonary dysplasia: Pathophysiology and potential
anti-inflammatory therapies. Paediatr Respir Rev. 30:34–41.
2019.PubMed/NCBI
|
|
8
|
Savani RC: Modulators of inflammation in
Bronchopulmonary Dysplasia. Semin Perinatol. 42:459–470. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Surate Solaligue DE, Rodríguez-Castillo
JA, Ahlbrecht K and Morty RE: Recent advances in our understanding
of the mechanisms of late lung development and bronchopulmonary
dysplasia. Am J Physiol Lung Cell Mol Physiol. 313:L1101–L1153.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Augustine S, Avey MT, Harrison B, Locke T,
Ghannad M, Moher D and Thébaud B: Mesenchymal stromal cell therapy
in bronchopulmonary dysplasia: Systematic review and Meta-Analysis
of preclinical studies. Stem Cells Transl Med. 6:2079–2093. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Walter J, Ware LB and Matthay MA:
Mesenchymal stem cells: Mechanisms of potential therapeutic benefit
in ARDS and sepsis. Lancet Respir Med. 2:1016–1026. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nitkin CR, Rajasingh J, Pisano C, Besner
GE, Thébaud B and Sampath V: Stem cell therapy for preventing
neonatal diseases in the 21st century: Current understanding and
challenges. Pediatr Res. 87:265–276. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhuxiao R, Fang X, Wei W, Shumei Y,
Jianlan W, Qiuping L, Jingjun P, Chuan N, Yongsheng L, Zhichun F
and Jie Y: Prevention for moderate or severe BPD with intravenous
infusion of autologous cord blood mononuclear cells in very preterm
infants-a prospective non-randomized placebo-controlled trial and
two-year follow up outcomes. EClinicalMedicine. 57:1018442023.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Carbone C, Piro G, Merz V, Simionato F,
Santoro R, Zecchetto C, Tortora G and Melisi D: Angiopoietin-Like
proteins in angiogenesis, inflammation and cancer. Int J Mol Sci.
19:4312018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xiao Y, Jiang Z, Li Y, Ye W, Jia B, Zhang
M, Xu Y, Wu D, Lai L, Chen Y, et al: ANGPTL7 regulates the
expansion and repopulation of human hematopoietic stem and
progenitor cells. Haematologica. 100:585–594. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xiao Y, Wei X, Jiang Z, Wang X, Ye W, Liu
X, Zhang M, Xu Y, Wu D, Lai L, et al: Loss of Angiopoietin-like 7
diminishes the regeneration capacity of hematopoietic stem and
progenitor cells. J Hematol Oncol. 8:72015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Parri M, Pietrovito L, Grandi A,
Campagnoli S, De Camilli E, Bianchini F, Marchiò S, Bussolino F,
Jin B, Sarmientos P, et al: Angiopoietin-like 7, a novel
pro-angiogenetic factor over-expressed in cancer. Angiogenesis.
17:881–896. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xia X, Ren Z, Yan L, Zheng X, Yang H, Kang
M, Yan H, Zhong Z, Xu F, Miao J, et al: Cord blood levels of
Angiopoietin-like 7 (Angptl7) in preterm infants. Biomed Res Int.
2020:18924582020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gomella TL, Cunningham MD and Eyal F:
Neonatology. 7th edition. New York: McGraw-Hill Education; 2013
|
|
20
|
Gary Cunningham F, Leveno KJ and Bloom S:
Williams Obstetrics. 25th edition. New York: McGraw-Hill Education;
2018
|
|
21
|
Jobe AH and Bancalari E: Bronchopulmonary
dysplasia. Am J Respir Crit Care. 163:1723–1729. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ren Z, Xu F, Wang J, Zhong Z, Wei W, Wen
J, Wang Q, Guocheng L and Yang J: Safety and feasibility of
umbilical cord blood collection from preterm neonates after delayed
cord clamping for the use of improving preterm complications. Am J
Transl Res. 13:4553–4560. 2021.PubMed/NCBI
|
|
23
|
Ren Z, Mo W, Yang L, Wang J, Zhang Q,
Zhong Z, Wei W, Liu Z, Wu Z, Yao Y and Yang J: Cord blood
antimicrobial peptide LL37 levels in preterm neonates and
association with preterm complications. Ital J Pediatr. 48:1112022.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals, . Guide for the Care and Use of Laboratory Animals. 8th
edition. Washington (DC); National Academies Press (US): 2011
|
|
25
|
Berger J and Bhandari V: Animal models of
bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol.
307:L936–L947. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shrestha AK, Bettini ML, Menon RT, Gopal
VYN, Huang S, Edwards DP, Pammi M, Barrios R and Shivanna B:
Consequences of early postnatal lipopolysaccharide exposure on
developing lungs in mice. Am J Physiol Lung Cell Mol Physiol.
316:L229–L244. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Baker CD and Abman SH: Impaired pulmonary
vascular development in bronchopulmonary dysplasia. Neonatology.
107:344–351. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chang DV, Assaf SJ, Tiller CJ, Kisling JA
and Tepper RS: Membrane and capillary components of lung diffusion
in infants with bronchopulmonary dysplasia. Am J Respir Crit Care
Med. 193:767–771. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Alvira CM: Aberrant pulmonary vascular
growth and remodeling in bronchopulmonary dysplasia. Front Med
(Lausanne). 3:212016.PubMed/NCBI
|
|
31
|
Arora S, Dev K, Agarwal B, Das P and Syed
MA: Macrophages: Their role, activation and polarization in
pulmonary diseases. Immunobiology. 223:383–396. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Behnke J, Kremer S, Shahzad T, Chao CM,
Böttcher-Friebertshäuser E, Morty RE, Bellusci S and Ehrhardt H:
MSC based therapies-new perspectives for the injured lung. J Clin
Med. 9:6822020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Matthay MA, Calfee CS, Zhuo H, Thompson
BT, Wilson JG, Levitt JE, Rogers AJ, Gotts JE, Wiener-Kronish JP,
Bajwa EK, et al: Treatment with allogeneic mesenchymal stromal
cells for moderate to severe acute respiratory distress syndrome
(START study): A randomised phase 2a safety trial. Lancet Respir
Med. 7:154–162. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nitkin CR, Rajasingh J, Pisano C, Besner
GE, Thébaud B and Sampath V: Stem cell therapy for preventing
neonatal diseases in the 21st century: Current understanding and
challenges. Pediatr Res. 87:265–276. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chang YS, Ahn SY, Yoo HS, Sung SI, Choi
SJ, Oh WI and Park WS: Mesenchymal stem cells for bronchopulmonary
dysplasia: Phase 1 dose-escalation clinical trial. J Pediatr.
164:966–972.e6. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Powell SB and Silvestri JM: Safety of
intratracheal administration of human umbilical cord blood derived
mesenchymal stromal cells in extremely low birth weight preterm
infants. J Pediatr. 210:209–213.e2. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Carbone C, Piro G, Merz V, Simionato F,
Santoro R, Zecchetto C, Tortora G and Melisi D: Angiopoietin-like
proteins in angiogenesis, inflammation and cancer. Int J Mol Sci.
19:4312018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Qian T, Wang K, Cui J, He Y and Yang Z:
Angiopoietin-like protein 7 promotes an inflammatory phenotype in
RAW264.7 macrophages through the P38 MAPK signaling pathway.
Inflammation. 39:974–985. 2016.PubMed/NCBI
|
|
39
|
Zhao Y, Liu K, Yin D and Lin Z:
Angiopoietin-Like 7 contributes to angiotensin ii-induced
proliferation, inflammation and apoptosis in vascular smooth muscle
cells. Pharmacology. 104:226–234. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zepp JA and Morrisey EE: Cellular
crosstalk in the development and regeneration of the respiratory
system. Nat Rev Mol Cell Biol. 20:551–566. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cui TX, Brady AE, Fulton CT, Zhang YJ,
Rosenbloom LM, Goldsmith AM, Moore BB and Popova AP: CCR2 mediates
chronic LPS-induced pulmonary inflammation and hypoalveolarization
in a murine model of bronchopulmonary dysplasia. Front Immunol.
11:5796282020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shrestha AK, Menon RT, El-Saie A, Barrios
R, Reynolds C and Shivanna B: Interactive and independent effects
of early lipopolysaccharide and hyperoxia exposure on developing
murine lungs. Am J Physiol Lung Cell Mol Physiol. 319:L981–L996.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Toyono T, Usui T, Yokoo S, Taketani Y,
Nakagawa S, Kuroda M, Yamagami S and Amano S: Angiopoietin-like 7
is an anti-angiogenic protein required to prevent vascularization
of the cornea. PLoS One. 10:e01168382015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lu X, Lu J, Zhang L and Xu Y: Effect of
Angptl7 on proliferation and differentiation of MC3T3-E1 cells. Med
Sci Monit. 25:9524–9530. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kandasamy J, Olave N, Ballinger SW and
Ambalavanan N: Vascular endothelial mitochondrial function predicts
death or pulmonary outcomes in preterm infants. Am J Respir Crit
Care Med. 196:1040–1049. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Speer CP: Inflammation and
bronchopulmonary dysplasia: A continuing story. Semin Fetal
Neonatal Med. 11:354–362. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sahni M and Bhandari V: Recent advances in
understanding and management of bronchopulmonary dysplasia.
F1000Res. 9:F1000Faculty Rev 703, 2020. PubMed/NCBI
|
|
48
|
Lu X, Gong J, Dennery PA and Yao H:
Endothelial-to-mesenchymal transition: Pathogenesis and therapeutic
targets for chronic pulmonary and vascular diseases. Biochem
Pharmacol. 168:100–107. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tammela T, Enholm B, Alitalo K and
Paavonen K: The biology of vascular endothelial growth factors.
Cardiovasc Res. 65:550–563. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Mariduena J, Ramagopal M, Hiatt M, Chandra
S, Laumbach R and Hegyi T: Vascular endothelial growth factor
levels and bronchopulmonary dysplasia in preterm infants. J Matern
Fetal Neonatal Med. 35:1517–1522. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hendricks-Muñoz KD, Xu J and Voynow JA:
Tracheal aspirate VEGF and sphingolipid metabolites in the preterm
infant with later development of bronchopulmonary dysplasia.
Pediatr Pulmonol. 53:1046–1052. 2018. View Article : Google Scholar : PubMed/NCBI
|