Introduction
Lung cancer is the leading cause of cancer-related
deaths worldwide, accounting for 18% of all cancer deaths.
According to survey data in 2020, the incidence of lung cancer was
second only to breast cancer globally and both morbidity and
mortality rates continue to rise (1,2).
Lung cancer is categorized into two main types: Small cell lung
cancer and non-small cell lung cancer (NSCLC). NSCLC is most
prevalent in the patient population and ~85% of lung cancer
patients have NSCLC (3). The A549
cell line is derived from a primary lung tumor and is one of the
most commonly used in the study of human NSCLC cell lines. In the
face of persistently high incidence and mortality rates, the
medical treatment of lung cancer must progress and more effective
treatment methods need to be developed (4). Traditional lung cancer treatment
methods mainly include surgery and chemotherapy (5). Current therapeutic approaches and
strategies for lung cancer treatment have made progress, such as
nanodrug delivery systems, molecularly targeted therapeutic
systems, photothermal therapeutic strategies and lung cancer
immunotherapy. These approaches have provided new perspectives and
insights into the treatment of lung cancer (6). Unlike traditional cytotoxic drugs,
targeted anti-tumor drugs mainly target components of cell
signaling pathways, such as cycle-regulating proteins, immune
regulatory factors and important proteins or factors involved in
angiogenesis, with relatively high specificity (7,8).
Compared with chemotherapy, targeted therapy is safer and more
controllable (9). For example,
following the application of tyrosine kinase inhibitors (TKI) in
EGFR-positive NSCLC, overall survival is significantly increased
(10) and TKI inhibitors exhibit
fewer side effects compared with chemotherapy (11). The development of targeted
anti-tumor drugs has become the key focus of anti-tumor drug
research (12). Therefore, seeking
active ingredients with improved efficacy and lower toxicity in
natural drugs provides novel avenues of research for tumor
treatment.
Rabdoternin E is an ent-kaurane diterpene monomer
compound and is commonly found in plants of the Isodon genus
of the Lamiaceae family. It is reported that kaurene-type
diterpenoids have significant anti-tumor activities (13), however, studies on the
pharmacological activity associated with rabdoternin E have not
been reported in the current literature. Our previous preliminary
study found that rabdoternin E effectively inhibits the
proliferation of lung cancer A549 cells (14). Therefore, the present study further
investigated the inhibitory effect and underlying mechanisms of
rabdoternin E on the proliferation of A549 cells and provide basic
data for the study of the anti-tumor activity of rabdoternin E.
Materials and methods
Preparation of drugs
Rabdoternin E with >98% purity was supplied by
Henan Engineering Research Center of Medicinal and Edible Chinese
Medicine (Zhengzhou, China). Rabdoternin E was initially dissolved
in culture medium (DMEM; Beijing Solarbio Science & Technology
Co., Ltd.) at a concentration of 500 µM in vitro experiments
and was subsequently diluted to 5, 10 and 15 µM. Rabdoternin E was
dissolved in saline and formulated as a solution for oral
administration in in vivo experiments. The stock solution
concentrations of rabdoternin E in saline for in vivo use
were 0.034, 0.068 and 0.136 mg/ml and the dosage of rabdoternin E
was 0.1 ml/10 g.
Cell culture
The lung cancer cell line A549 was cultured in
Dulbecco's Modified Eagle's Medium (Beijing Solarbio Science &
Technology Co., Ltd.) supplemented with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.) at 37°C in a 5%
CO2 environment. A549 cells were cultured at a maximum
of 10 passages to ensure the integrity of the cell line. A549 cells
and MRC-5 cells were obtained from Professor Chen Suiqing at Henan
University of Chinese Medicine (Zhengzhou, China).
Cell viability assay
The cytotoxicity of rabdoternin E on A549 cells was
measured by a 3-(4,5-dimethylthiazol 2-yl)-2,5-diphenyltetrazolium
bromide (MTT; Beijing Solarbio Science & Technology Co., Ltd.)
assay. In brief, A549 cells (1×104 cells/well) were
seeded into a 96-well culture plate overnight and treated with 5,
10 and 15 µM of rabdoternin E for 24, 48 and 72 h. The cells were
incubated with MTT (5 mg/ml) for 4 h and after which 150 µl DMSO
(Beijing Solarbio Science & Technology Co., Ltd.) was added to
each well and the cells were gently shaken for 10 min to completely
dissolve the purple crystals. Finally, the absorbance values of
each well were measured at 492 nm using a microplate reader
(Infinite F50; Tecan Group, Ltd.) (15). Cell viability was calculated as the
percentage of viable cells in the A549-treated group vs. the
untreated control.
Clonogenic assay
The cells were seeded into 6-well plates (800
cells/well) and cultured for 24 h. After which, the culture medium
was replaced with medium containing different final concentrations
(0, 5, 10 and 15 µM) of rabdoternin E and incubated (37°C) for
another 24 h. The medium containing the drug was then removed and
replaced with complete culture medium for 10 days, with regular
medium changes during the culture period. After the culture period,
the cells were washed twice with PBS, fixed with 4%
paraformaldehyde (Beijing Solarbio Science & Technology Co.,
Ltd.) for 15 min at room temperature and then stained with 1%
crystal violet (Beijing Solarbio Science & Technology Co.,
Ltd.) for 5 min. Excess staining solution was removed by washing
with PBS and colonies were counted to calculate the cloning
efficiency. The cell population clones were between 0.3–1.0 mm in
size and each clone contained >50 cells.
Cell cycle analysis
Cells (3×105 cells/well) were seeded into
a 6-well culture plate overnight for attachment and treated with 0,
5 10 and 15 µM of rabdoternin E for 24 h, washed twice with cold
PBS and fixed in 70% ethanol at −4°C for 12 h. After washing the
fixed cells with 1 ml PBS and centrifuging (5 min; 85 × g; −4°C),
the supernatant was removed and 100 µl RNase A solution was added
to the cell pellet. The cells were resuspended and incubated at
37°C for 30 min. After which, 400 µl propidium iodide (PI) staining
solution was added and mixed thoroughly. The cells were incubated
in the dark at 4°C for 30 min, filtered through a 200-mesh cell
strainer and finally analyzed by flow cytometry (BD Accuri C6 Plus;
BD Biosciences) (16).
FITC annexin V/PI apoptosis assay
Cells (3×105 cells/well) were seeded onto
a 6-well culture plate overnight for attachment and treated with
rabdoternin E (0, 5, 10 and 15 µmol/l) for 24 h at 37°C with 5%
CO2. Apoptosis assay was performed using the FITC
Annexin V Apoptosis Detection Kit (Beijing Solarbio Science &
Technology Co., Ltd.). The cells were washed once with PBS,
trypsinized and resuspended in media. A total of 1×105
cells were centrifuged (5 min; 85 × g; −4°C) and the cell pellets
were washed with PBS, resuspended in 100 µl 1X binding buffer and
then incubated with 3 µl FITC Annexin V and 5 µl PI for 15 min in
the dark. Finally, all samples were analyzed by flow cytometry
analysis using a BD Accuri C6 Flow Cytometer (BD Biosciences)
(17).
Reactive oxygen species (ROS)
assay
Detection of intracellular ROS was performed using
DCEH-DA as a fluorescent probe. Cells were seeded in 6-well plates
at a density of 3×105 cells/well and incubated at 37°C
for 24 h. After treatment with 0, 5, 10 and 15 µM of rabdoternin E
for 24 h, all cells were collected, washed twice with PBS and
resuspended in 1 ml 10 µM DCEH-DA solution (loaded with probe) for
20 min at 37°C. Finally, the cells were resuspended in 500 µl PBS
and analyzed using flow cytometry (BD Accuri C6 Plus; BD
Biosciences).
Determination of malondialdehyde (MDA)
and glutathione (GSH) levels
MDA content and GSH activity were determined using
the relevant kits (MDA; cat. no. BC0025; Beijing Solarbio Science
& Technology Co., Ltd.; GSH; cat. no. BC1175; Beijing Solarbio
Science & Technology Co., Ltd.) according to the manufacturer's
instructions. After A549 cells were administered and treated for 24
h, cells were collected and lyzed by repeatedly placing the cells
in liquid nitrogen for 3–4 times. The lysed cells were centrifuged
(10 min; 8,497 × g; −4°C) and the supernatant was collected. The
MDA and GSH contents of the samples were then determined according
to the instructions of the two kits.
Western blot analysis
Total proteins were extracted from cells and tumor
tissue, respectively. (RIPA lysate; cat. no. G2002; Wuhan
Servicebio Technology Co., Ltd.). Protein concentration was
assessed using a BCA assay kit (cat. no. G2026; Wuhan Servicebio
Technology Co., Ltd.). Sodium dodecyl sulfate polyacrylamide gel
electrophoresis was applied to separate proteins and the proteins
were transferred to polyvinylidene fluoride membranes. The mass of
protein per lane was 20 µg and the percentage of gel was 5%. After
which, the membranes were blocked with 5% skimmed milk for 1 h at
37°C and incubated at 4°C overnight with the following primary
antibodies: Bax, SCL7A11, glutathione peroxidase 4 (GPX4), Bcl-2,
Ki67, Cyclin A2, CDK2 and GAPDH (cat. nos. GB114122, 1:1,000;
DF12509, 1:1,000; DF12509, 1:1,000; GB113375, 1:1,000; GB11030,
1:1,000; GB11030, 1:1,000; GB112129, 1:1,000; GB15002, 1:2,000;
Wuhan Servicebio Technology Co., Ltd.). Finally, the membranes were
incubated with a goat anti-rabbit/mouse IgG HRP-linked antibody
(cat. nos. GB23301 and GB23303; 1:5,000; CST Biological Reagents
Co., Ltd.) for 2 h at room temperature. All bands were visualized
by chemiluminescence. AlphaEaseFC 4.0 (Alpha Innotech) and Adobe
PhotoShop 2024 (Adobe Systems, Inc.) were used for densitometry
(18).
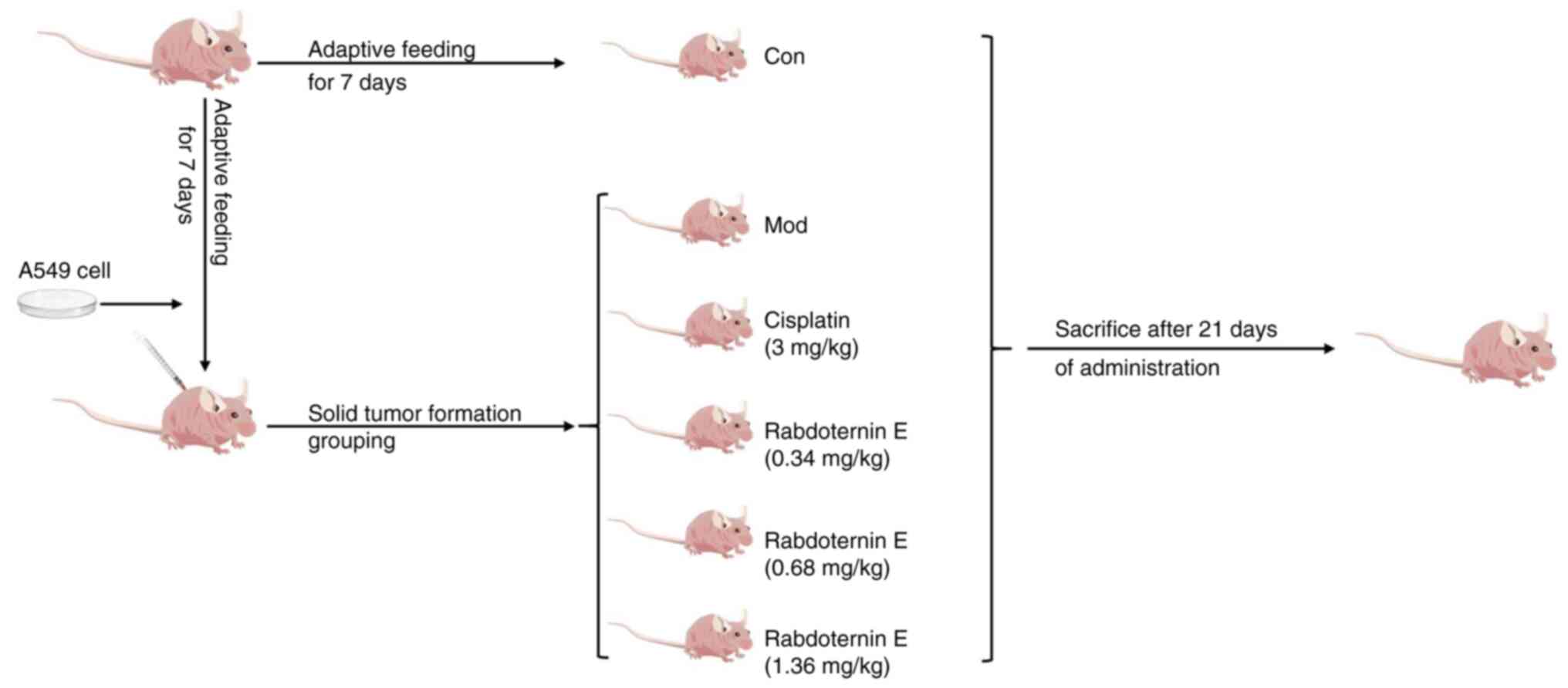
Lewis lung carcinoma mouse model
A total of 30 male BALB/c nude mice (4–6 weeks old;
18–20 g) were purchased from Beijing Vital River Laboratory Animal
Technology Co., Ltd. They were given free access to food and water
and kept in an SPF environment. The temperature in the environment
was 24±2°C, the relative humidity was 60%, and 12-h light/dark
cycle. All experimental procedures were accomplished complying with
the guidelines for the Care and Use of Laboratory Animals of Henan
University of Chinese Medicine and approved by The Animal
Experiments and Experimental Animal Management Committee from The
Henan University of Chinese Medicine (Zhengzhou, China; approval
no. DWLL202207009). BALB/c mice were injected subcutaneously with a
total of 2×106 A549 cells in the left forelimb. At 4
days after A549 inoculation, the mice were randomly divided into
five groups (n=6/per group) including: i) Model group; ii) positive
group (3 mg/kg cisplatin); iii) low rabdoternin E group (0.34
mg/kg); iv) medium rabdoternin E group (0.68 mg/kg); and v) high
rabdoternin E group (1.36 mg/kg). Drug intervention groups were
administered cisplatin or rabdoternin E by gavage for 15
consecutive days and the model group received the saline
equivalent. The mice were weighed and tumor volume was measured
with calipers every 3 days to track tumor growth and calculated
according to the following formula: 0.5× length ×
width2. The mice were sacrificed under deep anesthesia
and tumor tissue was excised. No drugs were used to sacrifice the
mice in this study. At the end of the experiment, Mice were
anesthetized with 40 mg/kg pentobarbital sodium solution by
intraperitoneal injection, blood was collected under anesthesia
through heart puncture and then the mice were sacrificed by
cervical dislocation and the collected samples were subsequently
preserved at −80°C for future use.
Histological analysis
Mouse tumor tissues were dissected and removed,
washed in saline, weighed and recorded, and then fixed in 4%
paraformaldehyde for 24 h at room temperature. Residual fixative on
the samples was rinsed with running water, and then the fixed
tissues were dehydrated in a gradient with ethanol at
concentrations of 70, 80, 90, 95 and 100% in turn, with each
concentration of ethanol solution being immersed for 2 h at room
temperature. After the tissues were dehydrated, the tissues were
placed in xylene I solution and xylene II solution and xylene II
solution, respectively, for 15 min at room temperature, in turn.
After tissue dehydration, the tissues were sequentially immersed in
xylene I solution (ethanol:xylene=1:1), xylene II solution
(ethanol:xylene=1:2) and xylene solution for 15 min at room
temperature. Then the tumor tissues were sequentially placed in
solution I (paraffin:xylene=1:1) solution II (paraffin:xylene=2:1)
and paraffin for 1.5 h at room temperature to complete the wax
infiltration. The embedded paraffin tissue was cut into sections of
3 µm thickness, transferred to slides heated at 60°C for 30 min and
stored at room temperature. Sections were placed into a staining
cylinder and stained with hematoxylin staining solution for 10 min
at room temperature. The section was removed and washed until no
further dye washed out. The cells were differentiated with 1%
hydrochloric ethanol differentiation solution for 3 sec, followed
by rinsing with water for 5 min. Then, the sections were stained
with eosin for 5 min at room temperature, followed by immersion in
water for 2 min. The sections were successively dehydrated with 85
and 95% ethanol for 2 min at room temperature. Finally, the slices
were placed in anhydrous ethanol for 5 min and placed in xylene for
5 min at room temperature. After drying the xylene, neutral resin
was added to the front side to seal the sections. The typical
histopathological changes of the tissues were observed under a
light microscope (Eclipse Ci; Nikon Corporation) following the
staining with an H&E staining kit (Wuhan Servicebio Technology
Co., Ltd., China).
Statistical analysis
All data were presented as the mean ± standard
deviation. Student's t-test was used to compare the differences
between two groups and one-way analysis of variance was used to
analyze differences among three or more groups, followed by Tukey's
post hoc analyses for between-group comparisons. Statistical
analysis was performed using the SPSS 26.0 statistical package (IBM
Corp.). P<0.05 was considered to indicate a statistically
significant difference.
Results
Rabdoternin E significantly inhibited
the proliferation of A549 cells in vitro
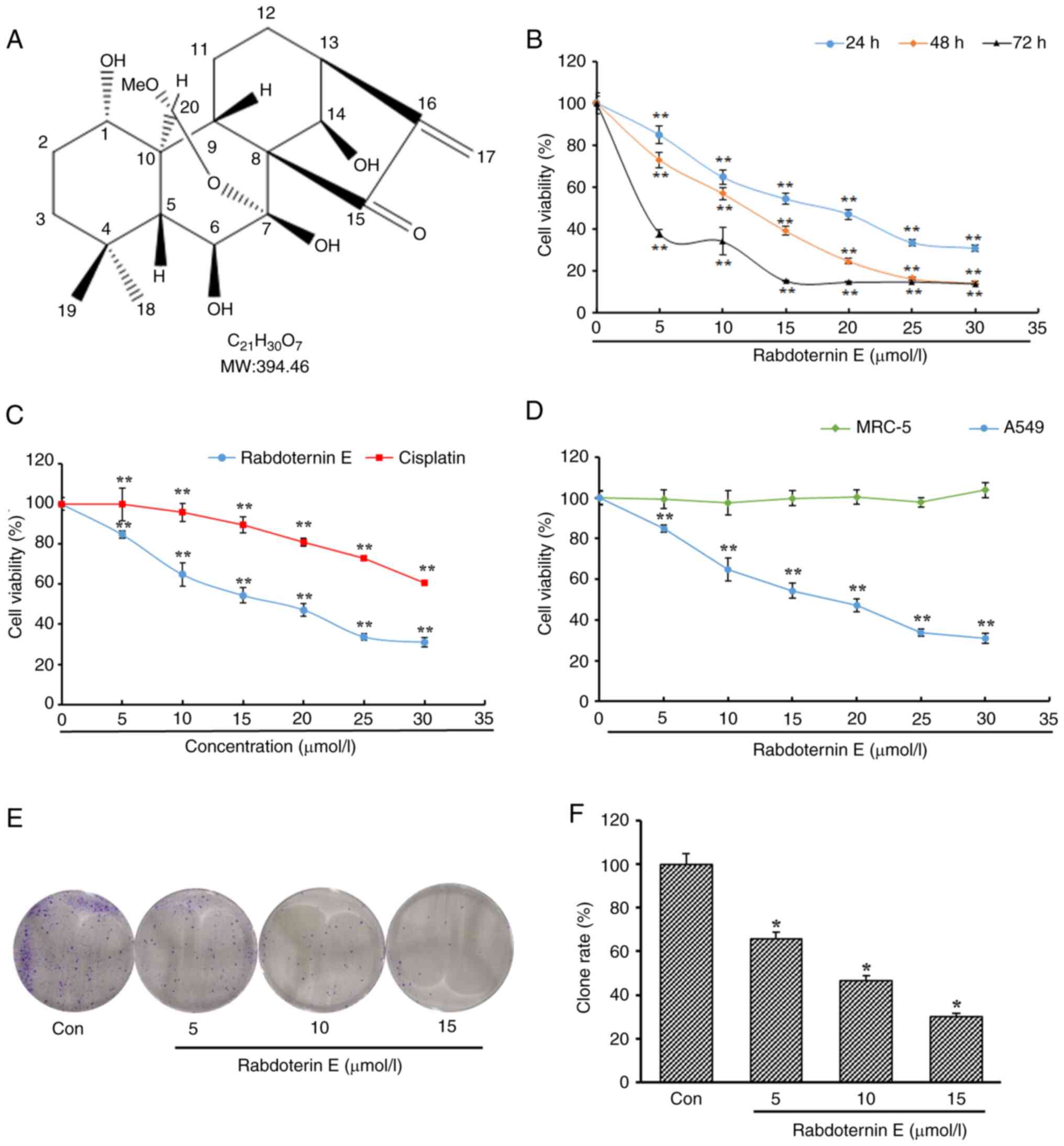
Rabdoternin E is an ent-kaurane diterpene monomer
compound (Fig. 1A). The MTT method
was used to detect the effects of rabdoternin E at different
concentrations on the proliferation of A549 cells. The
proliferation of A549 cells was significantly inhibited by
rabdoternin E intervention (Fig.
1B). Rabdoternin E inhibited A549 cell proliferation jto a
greater degree than cisplatin (positive control) during the 24-h
period with the IC50 of rabdoternin E and cisplatin
against A549 cells 16.45 and 39.52 µM, respectively (Fig. 1C). In order to verify whether
rabdoternin E has cytotoxicity on normal lung cells at tested
concentrations for 24 h, the human embryonic lung fibroblast cell
line MRC-5 was selected for cytotoxicity tests. The rabdoternin E
had no toxic effect on MRC-5 cells at the tested concentrations
(Fig. 1D). To further examine the
inhibition of rabdoternin E on the proliferation of A549 cells, a
clone formation assay was used to investigate the results. The
number of cell clones significantly declined in a dose-dependent
manner (Fig. 1E and F), which
macroscopically confirms that rabdoternin E can significantly
inhibit the proliferation of A549 cells.
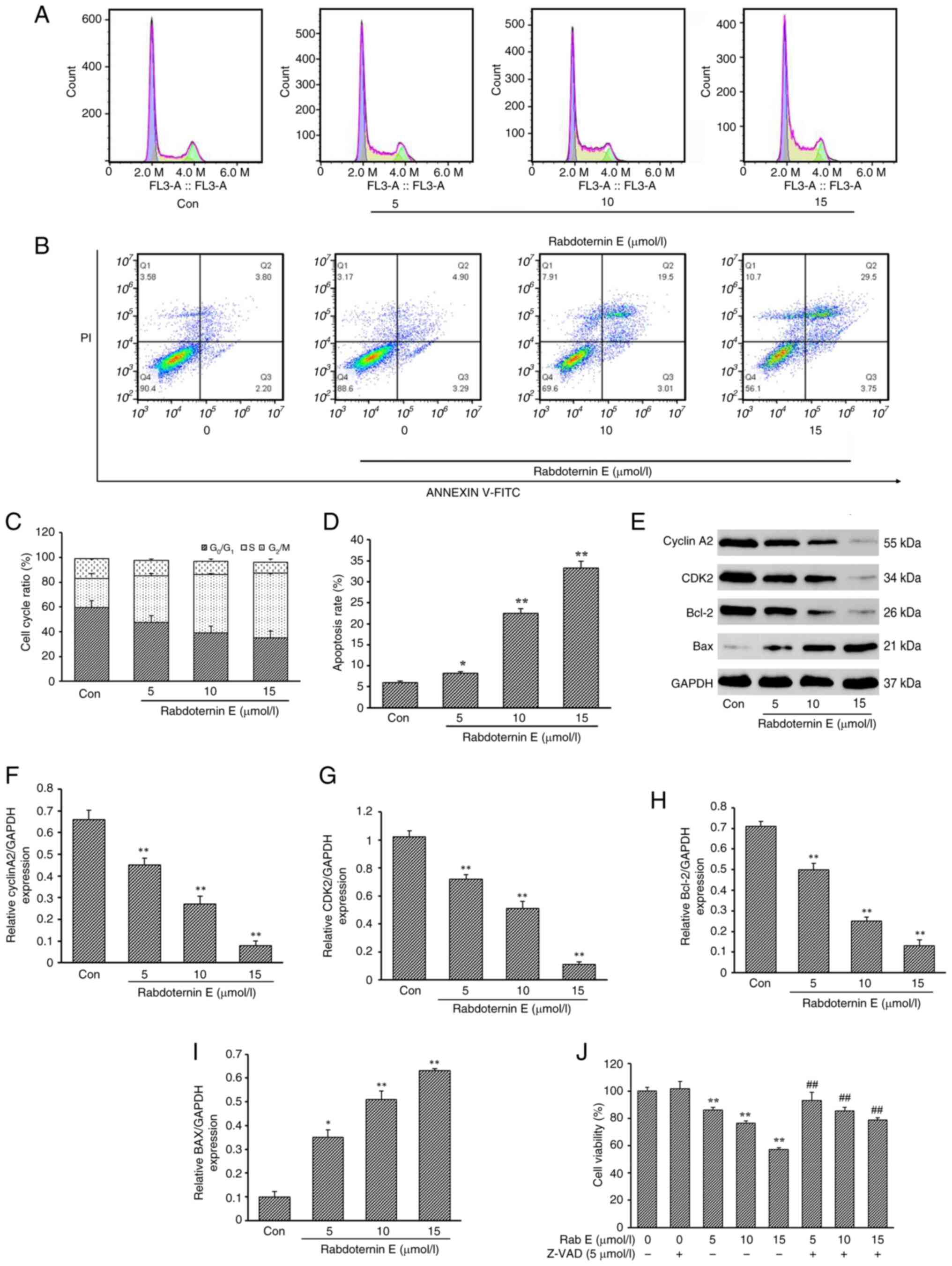
Rabdoternin E can promote S phase
arrest and induce apoptosis in A549 cells
Cell cycle progression is closely related to cell
proliferation and complete and orderly cycle progression is the key
to cell growth and proliferation (19,20).
Compared with the control (Con) group, the proportion of cells in
the S-phase in the rabdoternin E group significantly increased in a
dose-dependent manner (Fig. 2A and
C). When the dose of rabdoternin E reached 15 µM, the
proportion of cells in the S phase reached 52.2%. The apoptosis
rates in the Con group and rabdoternin E group at concentrations of
5, 10 and 15 µM for 24 h were 6.00, 8.19, 22.51 and 33.25%,
respectively (Fig. 2B and D). The
apoptosis rate in all tested groups was higher than that in the Con
group (P<0.05 or P<0.01). As shown in Fig. 2E-I, Bax and Bcl-2 were
dose-dependently up- and downregulated, respectively and the
Bax/Bcl-2 ratio increased. Moreover, the expression of S-phase
related proteins CDK2 and Cyclin A2 decreased significantly with
the increase of the rabdoternin E concentration. Finally,
rabdoternin E and the apoptosis inhibitor Z-VAD-FMK (10 µM) were
co-cultured for 24 h. Z-VAD-FMK reversed the cytotoxicity of
rabdoternin E on A549 cells by ~10% (P<0.05 or P<0.01),
indicating that rabdoternin E induces apoptosis in A549 cells
(Fig. 2J). However, apoptosis is
not the only cause of drug-induced cell death and the effects of
rabdoternin E may be caused by other forms of cell death (21,22).
Rabdoternin E induces ferroptosis in
A549 cells
In the process of cancer treatment, drug stimulation
can increase the level of ROS expression, which in turn induces
apoptosis and serves to inhibit the proliferation and spread of
cancer cells (23,24). Meanwhile, the accumulation of ROS
can induce the occurrence of intracellular ferroptosis (25,26).
In the present study, the level of ROS following rabdoternin E
treatment significantly increased in a dose-dependent manner
(Fig. 3A and B). A previous
studies showed that ROS accumulation disrupts GSH/GSSH homeostasis,
causing GSH depletion, inducing lipid peroxidation and leading to
the occurrence of ferroptosis (27,28).
Combined with the fact that the apoptosis inhibitor Z-VAD-FMK and
rabdoternin E co-culture did not completely reverse the
proliferation inhibitory effect of rabdoternin E on A549 cells,
changes in GSH and MDA (indicators associated with ferroptosis)
levels after 24 h of rabdoternin E treatment were examined.
Rabdoternin E caused intracellular GSH depletion and MDA
accumulation in a dose-dependent manner (P<0.05 or P<0.01,
respectively; Fig. 3C and D),
indicating the occurrence of ferroptosis in cells.
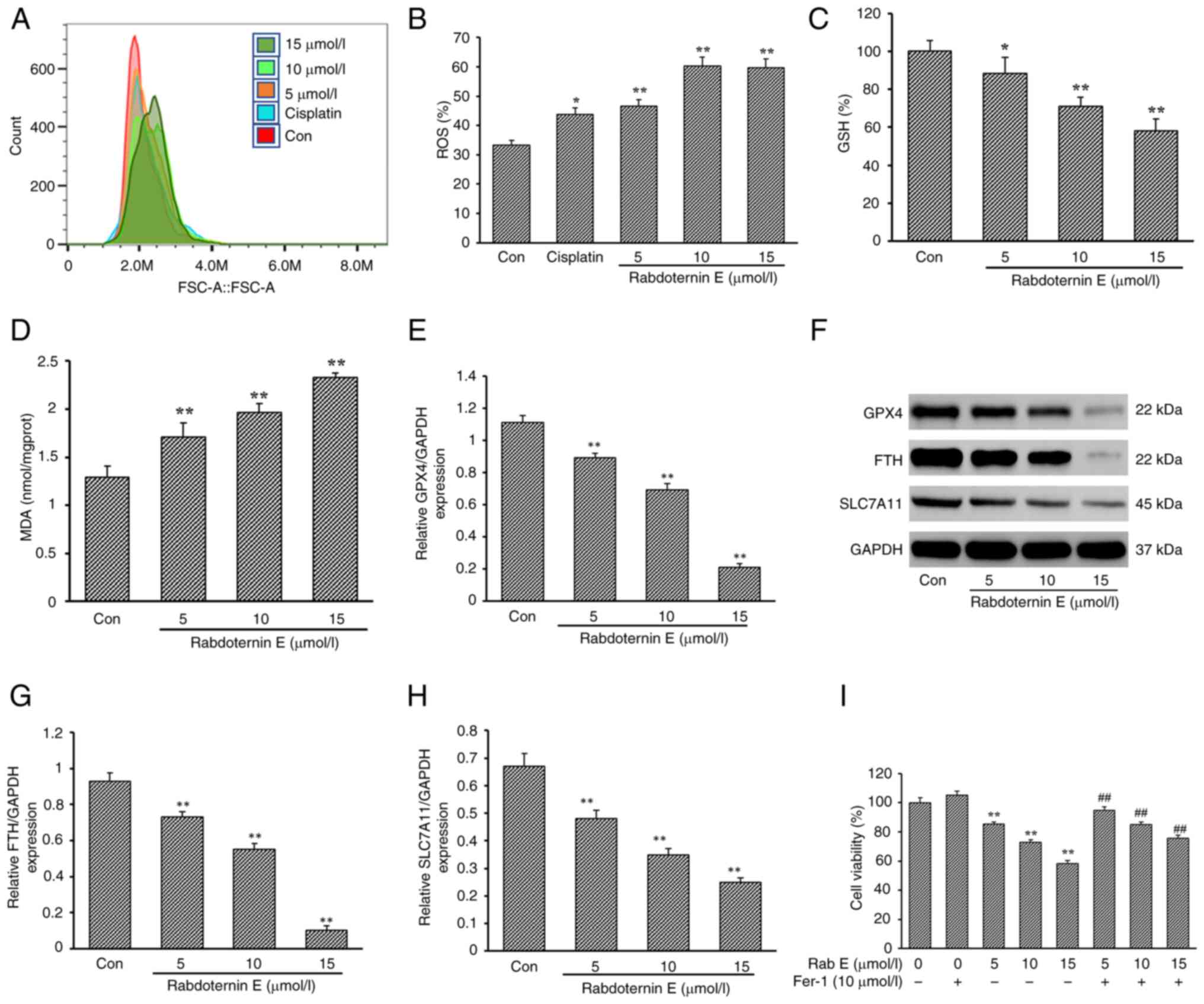 | Figure 3.Effect of Rab E on ferroptosis of
A549 cells. (A) Effects of different concentrations (5, 10 and 15
µM) of Rab E on intracellular ROS levels in A549 cells and (B)
quantitative analysis. Effect of Rab E treatment for 24 h on the
expression of (C) GSH and (D) MDA as indicators associated with
ferroptosis. (E) Protein expression of GPX4, FTH and SLC7A11 in
A549 cells treated with Rab E. (F-H) Effect of Rab E on the protein
expression of (F) GPX4, (G) FTH and (H) SLC7A11 in A549 cells. (I)
Co-culture using ferroptosis-inhibiting Fer-1 and Rab E
significantly reversed cell viability in A549 cells.
(x–±s; n=6) *P<0.05, **P<0.01, vs. Con group;
##P<0.01, vs. Fer-1 group with ferroptosis inhibitor
only. ROS, reactive oxygen species; GSH, glutathione; MDA,
malondialdehyde; GPX4, glutathione peroxidase; FTH, ferritin heavy;
SLC7A11, solute carrier family 7 member 11; Rab E, rabdoternin E;
Fer-1, ferrostatin-1; Con, control. |
Compared with the Con group, the expression of
ferroptosis marker proteins including solute carrier family 7
member 11 (SLC7A11), GPX4 and ferritin heavy (FTH) decreased in a
dose-dependent manner after rabdoternin E treatment for 24 h
(Fig. 3E-H). Furthermore, the
co-culture of rabdoternin E and a ferroptosis inhibitor
ferrostatin-1 significantly reversed the inhibitory effect of
rabdoternin E on A549 cells, suggesting that rabdoternin E induced
ferroptosis in A549 cells (Fig.
3I).
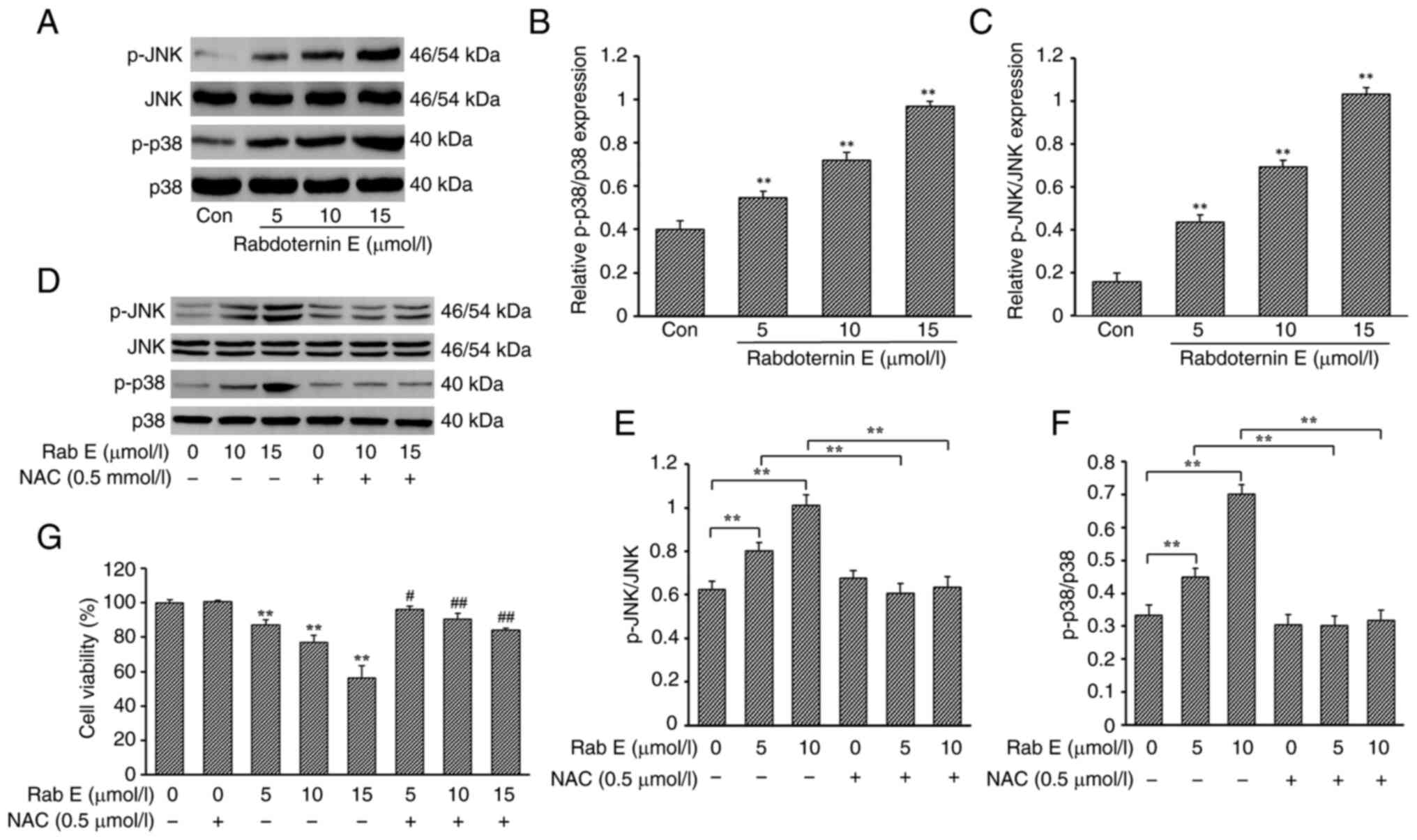
Effect of rabdoternin E on the
ROS-dependent p38 MAPK/JNK pathway
To verify whether rabdoternin E regulates apoptosis
and ferroptosis in A549 cells through the p38 MAPK/JNK pathway, the
expression of phosphorylated p38 MAPK and JNK in A549 cells
following rabdoternin E treatment was examined. The phosphorylation
levels of p38 MAPK and JNK increased in a dose-dependent manner,
indicating that rabdoternin E could effectively regulate the
transactivation of the p38 MAPK/JNK signaling pathway in A549 cells
(Fig. 4A-C). The phosphorylation
levels of p38 MAPK and JNK in cells by co-cultured experiments with
the ROS inhibitor N-acetylcysteine (NAC) and rabdoternin E were
examined. NAC treatment effectively inhibited rabdoternin
E-stimulated p38 MAPK and JNK activation (P<0.05 or P<0.01;
Fig. 4D-F). The addition of NAC
effectively reversed the cytotoxicity of rabdoternin E on A549 and
the degree of reversal was significantly stronger than the effects
of the apoptosis inhibitor or ferroptosis inhibitor (Fig. 4G). Therefore, the results indicated
that the ROS-dependent activation of the p38 MAPK/JNK pathway is
associated with rabdoternin E-induced apoptosis and ferroptosis in
A549 cells.
Rabdoternin E inhibits tumor growth
and induces tumor cell death in vivo
To verify the anti-tumor effect of rabdoternin E
in vivo using a Lewis lung carcinoma mouse model, BALB/c
mice were treated with rabdoternin E for 21 days (Fig. 5). The tumor volume and mass in the
rabdoternin E group at all doses was significantly reduced compared
with the model group of BALB/c nude mice and those of the high and
middle dosage rabdoternin E group were smaller compared with the
cisplatin group (Fig. 6A, B and
D). Moreover, all tested doses of rabdoternin E did not
significantly affect the body weight of BALB/c nude mice,
indicating that rabdoternin E had no significant toxic side effects
in the mice, but the positive dose group showed the greatest change
in body weight, with a 14.82% decrease in body weight from the
beginning of the experiment to the end of the experiment (Fig. 6C). H&E staining showed that the
tumor cells were regularly, neatly and tightly packed in the model
group, while those in the rabdoternin E groups were disorganized
with large cell gaps, severely vacuolated and the number
significantly reduced, suggesting rabdoternin E significantly
inhibited the proliferation of A549 cell (Fig. 6E). rabdoternin E treatment
significantly decreased the expressions of Bcl-2, Ki67, GPX4 and
SLC7A11 (P<0.01) and increased those of Bax and transferrin in
the tumor tissues (Fig. 6F-L),
indicating that rabdoternin E induces apoptosis and iron death via
ROS-dependent p38 MAPK/JNK pathway activation.
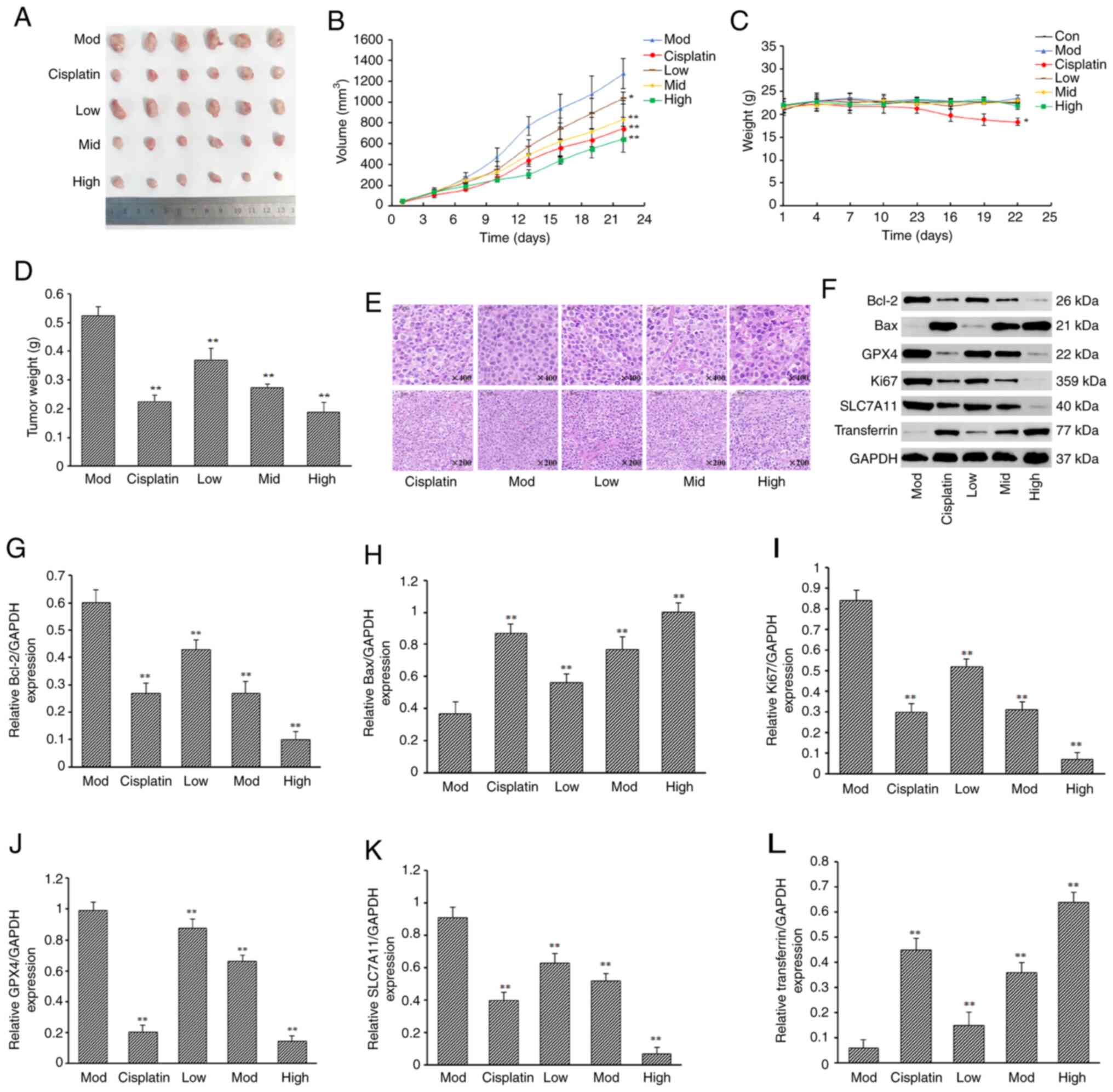 | Figure 6.Effect of rabdoternin E on the
proliferation of subcutaneous transplanted tumors in nude mice with
human lung cancer A549 cells. Plots of (A) tumor appearance, (B)
tumor size and (C) body weight of mice in each group. (D) Tumor
weight. (E) H&E staining results of tumor tissues in each
group. Magnification, ×200 and ×400. (F) Protein bands of each
experimental group. Protein expression of (G) Bcl-2, (H) Bax, (I)
Ki67, (J) GPX4, (K) SLC7A11 and (L) transferrin of each
experimental group. (x–±s; n=6) *P<0.05, **P<0.01,
vs. Mod group. GPX4, glutathione peroxidase; SLC7A11, solute
carrier family 7 member 11; Mod, model group. |
Discussion
At present, there are no literature reports on the
mechanisms of anti-tumor action of rabdoternin E. In the present
study, it was found that rabdoternin E induced apoptosis and
ferroptosis in A549 cells through the ROS/p38MAPK/JNK signaling
pathway. The MTT experiments demonstrated that rabdoternin E
significantly inhibited the proliferation of A549 cells with an
IC50 value of 16.4 µM and was not cytotoxic to normal
lung cells (MRC-5). Numerous ent-Kaurane diterpenoids have been
reported to have marked anti-lung cancer activity (14). For example, rabdoternin F and
Isorosthin O significantly inhibit A549 cell proliferation with
IC50 values of 18.1 and 18.8 µM, respectively, thus
indicating that rabdoternin E has greater inhibitory activity
against A549 cells. However, numerous ent-kaurane diterpenoids
including rabdoternin E, rabdoternin F and Isorosthin O have weak
inhibitory activity against the proliferation of SMMC7721 and SW480
cells (14).
In order to explore the mechanisms behind this
action, apoptosis and cell cycle-related analyses were conducted on
rabdoternin E-treated cells. The findings of the present study
revealed that rabdoternin E arrests the cell cycle in the S phase
and induces apoptosis. In addition, it was noted that ROS levels
increased in rabdoternin E-treated cells. It is well established
that ROS accumulation is often associated with disturbances in
cellular oxidative disorders (29,30).
Subsequent co-culture studies with rabdoternin E and Z-VAD-FMK (an
apoptosis inhibitor) showed a partial reversal of the cytotoxicity
of rabdoternin E to A549 cells. Therefore, it was hypothesized that
in addition to inducing apoptosis, rabdoternin E may contribute to
cell death through oxidative metabolic disorders. The occurrence of
ferroptosis primarily arises from intracellular GSH depletion and
reduced activity of GPX4, resulting in impaired metabolism of lipid
peroxides through GPX4-catalyzed reduction reactions (31,32).
Simultaneously, the Fenton reaction oxidizes lipids and generates a
substantial amount of ROS, thereby accelerating cell death
(33). Previous studies have
demonstrated that ferroptosis is strongly linked with the
regulation and pathophysiological processes underlying tumors,
nervous system disorders, kidney injury and hematologic diseases
(34–37).
Rabdoternin E significantly decreased the GSH
content and increased the MDA content in cells. Moreover,
ferrostatin-1 (a ferroptosis inhibitor) effectively reduced the
inhibitory effect of rabdoternin E on A549 cells. These findings
strongly suggested that rabdoternin E induced ferroptosis.
Additionally, it was found that rabdoternin E markedly
downregulated the expression of SLC7A11 and GPX4 in cells,
indicating it regulated ferroptosis through modulating the
SLC7A11/GPX4 axis. SLC7A11, as an important regulator of
ferroptosis sequestration, plays a crucial role in various
regulatory processes such as tumorigenesis, proliferation,
metastasis, prognosis and chemotherapy resistance (38,39).
Studies have demonstrated that ROS possess the
ability to facilitate signaling pathways in tumor cells and induce
oxidative stress, ultimately leading to cellular apoptosis.
Consequently, ROS has emerged as a promising target for potential
cancer therapies (40,41). The MAPK signal transduction pathway
plays a crucial role in various physiological processes and
oxidative stress (42) and the
activation of MAPK regulates cell proliferation, apoptosis,
invasion and metastasis in numerous tumor cells (43,44).
Among the MAPK signaling pathways, p38 MAPK and JNK are important
downstream effectors of ROS (45,46).
Excessive ROS can activate the downstream p38 MAPK/JNK pathway,
leading to increased expression levels of proapoptotic proteins and
induction of apoptosis (47).
Additionally, the MAPK signaling pathways are involved in
regulating ferroptosis (48).
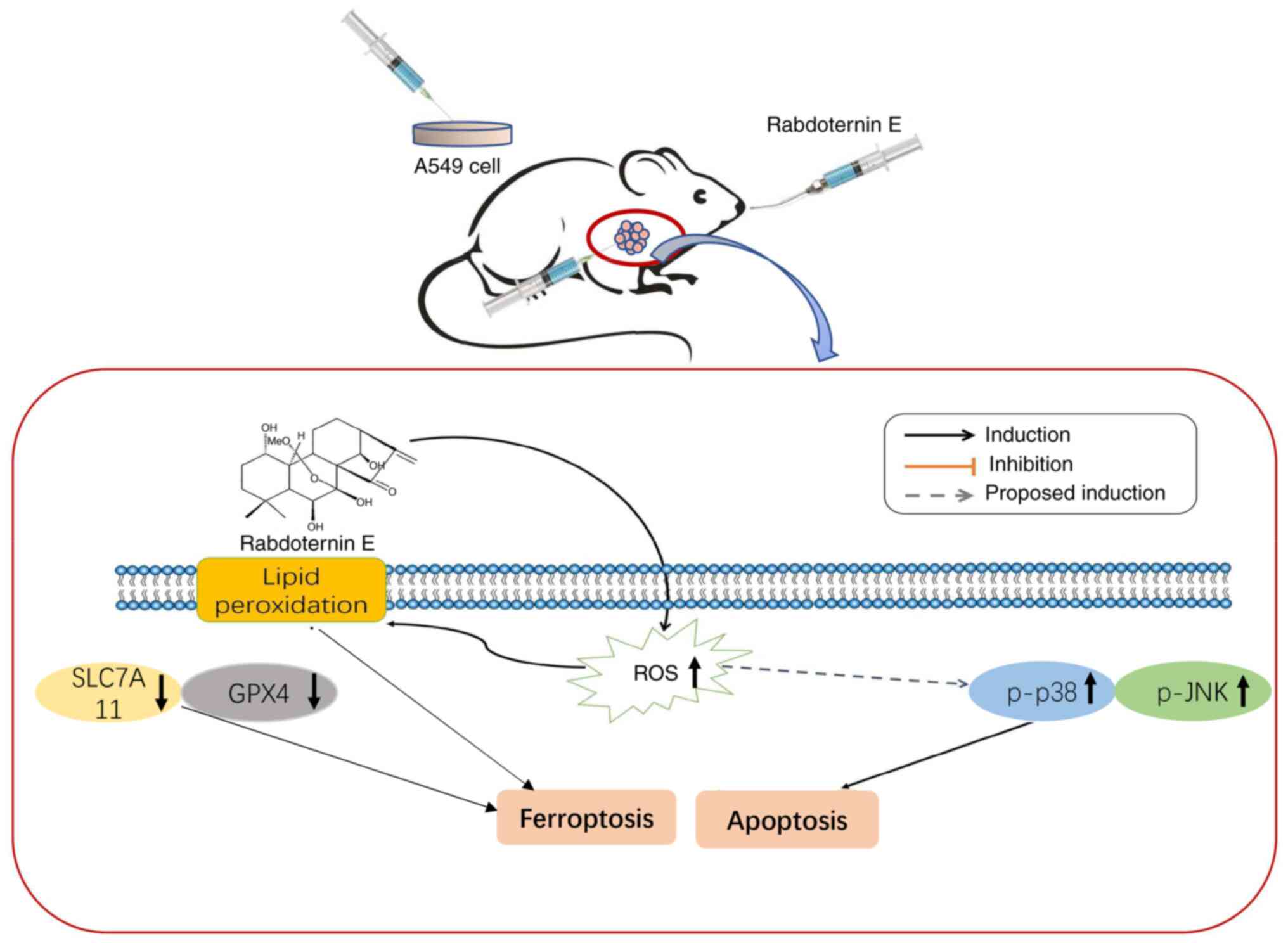
The object of the present study was to investigate
the regulatory effects of rabdoternin E on apoptosis and
ferroptosis in A549 cells through the ROS-mediated p38 MAPK/JNK
pathway (Fig. 7). It was also
found that the phosphorylation levels of p38 MAPK and JNK increased
dose-dependently following rabdoternin E treatment. Notably,
co-treatment experiments with NAC and rabdoternin E demonstrated
that the phosphorylation levels of p38 MAPK and JNK in A549 cells
and the cytotoxicity induced by rabdoternin E were markedly
reduced. The present study indicated that rabdoternin E induces
intracellular accumulation of ROS, which not only activates
downstream effectors such as p38 MAPK and JNK that promote
apoptosis and ferroptosis, but also leads to lipid peroxide
accumulation in cells, further inducing iron-dependent cell
death.
In the in vivo animal experiments, a potent
inhibitory effect of rabdoternin E on tumor growth activity was
observed. Compared with the control group, rabdoternin E
significantly slowed down the growth rate of tumors in mice,
resulting in smaller tumor volume and mass. Notably, the mice in
the administered group at the medium and high doses of rabdoternin
E had a smaller tumor volume and mass than the cisplatin group,
indicating that rabdoternin E also has strong tumor growth
inhibitory activity in vivo. Moreover, the body weight of
mice in the rabdoternin E groups were not adversely affected.
However, over the course of the present study, the body weight of
the mice in the cisplatin group significantly decreased. Western
blotting results showed that the expression content of Ki67 protein
decreased with the increase of administered dose and the weakening
of Ki67 protein expression implied the decrease of tumor
proliferation ability. At the same time, it was found that the
apoptosis-related Bax/Bcl-2 protein expression ratio increased.
Ultimately, they together promote apoptosis of tumor cells.
Meanwhile, western blotting of tumor tissues
revealed that the expression levels of SLC7A11 and GPX4 were
significantly reduced in tissues after rabdoternin E administration
compared to controls. Therefore, it was concluded that rabdoternin
E-induced downregulation of SLC7A11 and GPX4 plays a critical role
in regulating ferroptosis in A549 cells.
To conclude, in the present study rabdoternin E
effectively inhibited A549 cell proliferation in vitro
through induction of the apoptosis and ferroptosis pathways.
Acknowledgements
Not applicable.
Funding
The present study was funded by the High-Level Talents
International Training project of Henan Province (grant no.
2021-72) and Major Science and Technology Programs in Henan
Province (grant no. 221100310400).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JJ, JN and TG designed the experiments. JJ, JN and
YS performed most of the data analysis and animal and cell
experiments. XC, HW, XW and JH collected and analyzed the data. JJ
and JN wrote the manuscript. YS revised the manuscript. TG and YS
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All experiments on animals were approved by the
Subcommittee on Henan University of Traditional Chinese Medicine
Animal Experiment Center (approval no. DWLL202207009).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li C, Lei S, Ding L, Xu Y, Wu X, Wang H,
Zhang Z, Gao T, Zhang Y and Li L: Global burden and trends of lung
cancer incidence and mortality. Chin Med J (Engl). 136:1583–1590.
2023.PubMed/NCBI
|
|
2
|
Huang J, Deng Y, TinM S, Lok V, Ngai CH,
Zhang L, Lucero-Prisno DE III, Xu W, Zheng ZJ, Elcarte E, et al:
Distribution, risk factors, and temporal trends for lung cancer
incidence and mortality: A global analysis. Chest. 161:1101–1111.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tandberg DJ, Tong BC, Ackerson BG and
Kelsey CR: Surgery versus stereotactic body radiation therapy for
stage I non-small cell lung cancer: A comprehensive review. Cancer.
124:667–678. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
LeeC S, Shu CC, Chen YC, Liao KM and Ho
CH: Tuberculosis treatment incompletion in patients with lung
cancer: Occurrence and predictors. Int J Infect Dis. 113:200–206.
2021. View Article : Google Scholar
|
|
5
|
Tartour E and Zitvogel L: Lung cancer:
Potential targets for immunotherapy. Lancet Respir Med. 1:551–563.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li Y, Yan B and He S: Advances and
challenges in the treatment of lung cancer. Biomed Pharmacother.
169:1158912023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu S, Bishop WR and Liu M: Differential
effects of cell cycle regulatory protein p21(WAF1/Cip1) on
apoptosis and sensitivity to cancer chemotherapy. Drug Resist
Updat. 6:183–195. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chin EN, Sulpizio A and Lairson LL:
Targeting STING to promote antitumor immunity. Trends Cell Biol.
33:189–203. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pérez-Herrero E and Fernández-Medarde A:
Advanced targeted therapies in cancer: Drug nanocarriers, the
future of chemotherapy. Eur J Pharm Biopharm. 93:52–79. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jurisic V, Vukovic V, Obradovic J,
Gulyaeva LF, Kushlinskii NE and Djordjević N: EGFR polymorphism and
survival of NSCLC patients treated with TKIs: A systematic review
and meta-analysis. J Oncol. 2020:19732412020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Obradovic J, Todosijevic J and Jurisic V:
Side effects of tyrosine kinase inhibitors therapy in patients with
non-small cell lung cancer and associations with EGFR
polymorphisms: A systematic review and meta-analysis. Oncol Lett.
25:622022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nishimoto A: Effective combinations of
anti-cancer and targeted drugs for pancreatic cancer treatment.
World J Gastroenterol. 28:3637–3643. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fujita E, Nagao Y, Kaneko K, Nakazawa S
and Kuroda H: The antitumor and antibacterial activity of the
Isodon diterpenoids. Chem Pharm Bull (Tokyo). 24:2118–2127. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wei WJ, Zhu B, Si Y, Guo T, Kang J and Dai
L: Cytotoxic ent-kaurane diterpenoids from rabdosia rubescens. Chem
Biodivers. 19:e2022004972022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jia XB, Zhang Q, Xu L, Yao WJ and Wei L:
Lotus leaf flavonoids induce apoptosis of human lung cancer A549
cells through the ROS/p38 MAPK pathway. Biol Res. 54:72021.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jin X, Sun PP, Hong Y, Yu L and Li S:
Puerarin induces apoptosis in A549 cells. Zhongguo Ying Yong Sheng
Li Xue Za Zhi. 33:466–469. 2017.(In Chinese). PubMed/NCBI
|
|
17
|
Zhao C, Qin G, Gao W, Chen J, Liu H, Xi G,
Li T, Wu S and Chen T: Potent proapoptotic actions of
dihydroartemisinin in gemcitabine-resistant A549 cells. Cell
Signal. 26:2223–2233. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou T, Qin R, Shi S, Zhang H, Niu C, Ju G
and Miao S: DTYMK promote hepatocellular carcinoma proliferation by
regulating cell cycle. Cell Cycle. 20:1681–1691. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Oredsson SM: Polyamine dependence of
normal cell-cycle progression. Biochem Soc Trans. 31:366–370. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guarracino MR, Xanthopoulos P, Pyrgiotakis
G, Tomaino V, Moudgil BM and Pardalos PM: Classification of cancer
cell death with spectral dimensionality reduction and generalized
eigenvalues. Artif Intell Med. 53:119–125. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Efimova I, Catanzaro E, Van der Meeren L,
Turubanova VD, Hammad H, Mishchenko TA, Vedunova MV, Fimognari C,
Bachert C, Coppieters F, et al: Vaccination with early ferroptotic
cancer cells induces efficient antitumor immunity. J Immunother
Cancer. 8:e0013692020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Martinvalet D: ROS signaling during
granzyme B-mediated apoptosis. Mol Cell Oncol. 2:e9926392015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Al-Khayal K, Alafeefy A, Vaali-Mohammed
MA, Mahmood A, Zubaidi A, Al-Obeed O, Khan Z, Abdulla M and Ahmad
R: Novel derivative of aminobenzenesulfonamide (3c) induces
apoptosis in colorectal cancer cells through ROS generation and
inhibits cell migration. BMC Cancer. 17:42017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li Z, Xiao J, Liu M, Cui J, Lian B, Sun Y
and Li C: Notch3 regulates ferroptosis via ROS-induced lipid
peroxidation in NSCLC cells. FEBS Open Bio. 12:1197–1205. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang CX, Chen LH, Zhuang HB, Shi ZS, Chen
ZC, PanJ P and Hong ZS: Auriculasin enhances ROS generation to
regulate colorectal cancer cell apoptosis, ferroptosis, oxeiptosis,
invasion and colony formation. Biochem Biophys Res Commun.
587:99–106. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Son Y, Kim S, Chung HT and Pae HO:
Reactive oxygen species in the activation of MAP kinases. Methods
Enzymol. 528:27–48. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rochette L, Dogon G, Rigal E, Zeller M,
Cottin Y and Vergely C: Lipid peroxidation and iron metabolism: Two
corner stones in the homeostasis control of ferroptosis. Int J Mol
Sci. 24:4492022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Molavinia S, Dayer D, Khodayar MJ,
Goudarzi G and Salehcheh M: Suspended particulate matter promotes
epithelial-to-mesenchymal transition in alveolar epithelial cells
via TGF-β1-mediated ROS/IL-8/SMAD3 axis. J Environ Sci (China).
141:139–150. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu T, Sun L, Zhang Y, Wang Y and Zheng J:
Imbalanced GSH/ROS and sequential cell death. J Biochem Mol
Toxicol. 36:e229422022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
LiF J, Long HZ, Zhou ZW, Luo HY, Xu SG and
Gao LC: System Xc−/GSH/GPX4 axis: An important
antioxidant system for the ferroptosis in drug-resistant solid
tumor therapy. Front Pharmacol. 13:9102922022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu Y, Li Y, Li J and Chen W: Ethyl
carbamate triggers ferroptosis in liver through inhibiting GSH
synthesis and suppressing Nrf2 activation. Redox Biol.
53:1023492022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Miao Y, Chen Y, Xue F, Liu K, Zhu B, Gao
J, Yin J, Zhang C and Li G: Contribution of ferroptosis and GPX4′s
dual functions to osteoarthritis progression. EBioMedicine.
76:1038472022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ma P, Xiao H, Yu C, Liu J, Cheng Z, Song
H, Zhang X, Li C, Wang J, Gu Z and Lin J: Enhanced cisplatin
chemotherapy by iron oxide nanocarrier-mediated generation of
highly toxic reactive oxygen species. Nano Lett. 17:928–937. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nie J, Lin B, Zhou M, Wu L and Zheng T:
Role of ferroptosis in hepatocellular carcinoma. J Cancer Res Clin
Oncol. 144:2329–2337. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jia B, Li J, Song Y and Luo C:
ACSL4-mediated ferroptosis and its potential role in central
nervous system diseases and injuries. Int J Mol Sci. 24:100212023.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hou L, Li X, Su C, Chen K and Qu M:
Current status and prospects of research on ischemia-reperfusion
injury and ferroptosis. Front Oncol. 12:9207072022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li X, Zou Y, Fu YY, Xing J, Wang KY, Wan
PZ and Zhai XY: A-lipoic acid alleviates folic acid-induced renal
damage through inhibition of ferroptosis. Front Physiol.
12:6805442021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li S, Lu Z, Sun R, Guo S, Gao F, Cao B and
Aa J: The role of SLC7A11 in cancer: Friend or foe? Cancers
(Basel). 14:30592022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cheng X, Wang Y, Liu L, Lv C, Liu C and Xu
J: SLC7A11, a potential therapeutic target through induced
ferroptosis in colon adenocarcinoma. Front Mol Biosci.
9:8896882022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Moloney JN and Cotter TG: ROS signalling
in the biology of cancer. Semin Cell Dev Biol. 80:50–64. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gerami P, Kim D, Compres EV, Zhang B, Khan
AU, Sunshine JC, Quan VL and Busam K: Clinical, morphologic, and
genomic findings in ROS1 fusion Spitz neoplasms. Mod Pathol.
34:348–357. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lv C, Fu S, Dong Q, Yu Z, Zhang G, Kong C,
Fu C and Zeng Y: PAGE4 promotes prostate cancer cells survive under
oxidative stress through modulating MAPK/JNK/ERK pathway. J Exp
Clin Cancer Res. 38:242019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Guimarães LM, Coura BP, Gomez RS and Gomes
CC: The molecular pathology of odontogenic tumors: Expanding the
spectrum of MAPK pathway driven tumors. Front Oral Health.
2:7407882021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li H, Han G, Li X, Li B, Wu B, Jin H, Wu L
and Wang W: MAPK-RAP1A signaling enriched in hepatocellular
carcinoma is associated with favorable tumor-infiltrating immune
cells and clinical prognosis. Front Oncol. 11:6499802021.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pereira L, Igea A, Canovas B, Dolado I and
Nebreda AR: Inhibition of p38 MAPK sensitizes tumour cells to
cisplatin-induced apoptosis mediated by reactive oxygen species and
JNK. EMBO Mol Med. 5:1759–1774. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lu J, Yu M and Li J: PKC-δ promotes
IL-1β-induced apoptosis of rat chondrocytes and via activating JNK
and P38 MAPK pathways. Cartilage. 194760352311814462023.(Epub ahead
of print).
|
|
47
|
Zhao Q, Yu M, Li J, Guo Y, Wang Z, Hu K,
Xu F, Liu Y, Li L, Wan D, et al: GLUD1 inhibits hepatocellular
carcinoma progression via ROS-mediated p38/JNK MAPK pathway
activation and mitochondrial apoptosis. Discov Oncol. 15:82024.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang X, Tan X, Zhang J, Wu J and Shi H:
The emerging roles of MAPK-AMPK in ferroptosis regulatory network.
Cell Commun Signal. 21:2002023. View Article : Google Scholar : PubMed/NCBI
|